Abstract
Aim:
Constraint-induced movement therapy (CIMT), which forces use of the impaired arm following unilateral stroke, promotes functional recovery in the clinic but animal models of CIMT have yielded mixed results. The aim of this study is to develop a refined endothelin-1 (ET-1) model of focal ischemic injury in rats that resulted in reproducible, well-defined lesions and reliable upper extremity impairments, and to determine if an appetitively motivated form of rehabilitation (voluntary forced use movement therapy; FUMT) would accelerate post-ischemic motor recovery.
Methods:
Male Sprague Dawley rats (3 months old) were given multiple intracerebral microinjections of ET-1 into the sensorimotor cortex and dorsolateral striatum. Sham-operated rats received the same surgical procedure up to but not including the drill holes on the skull. Functional deficits were assessed using two tests of forelimb placing, a forelimb postural reflex test, a forelimb asymmetry test, and a horizontal ladder test. In a separate experiment ET-1 stroke rats were subjected to daily rehabilitation with FUMT or with a control therapy beginning on post-surgery d 5. Performance and post-mortem analysis of lesion volume and regional BDNF expression were measured.
Results:
Following microinjections of ET-1 animals exhibited significant deficits in contralateral forelimb function on a variety of tests compared with the sham group. These deficits persisted for up to 20 d with no mortality and were associated with consistent lesion volumes. FUMT therapy resulted in a modest but significantly accelerated recovery in the forelimb function as compared with the control therapy, but did not affect lesion size or BDNF expression in the ipsilesional hemisphere.
Conclusion:
We conclude that refined ET-1 microinjection protocols and forcing use of the impaired forelimb in an appetitively motivated paradigm may prove useful in developing strategies to study post-ischemic rehabilitation and neuroplasticity.
Keywords: stroke, brain ischemia, endothelin-1, rehabilitation therapy, voluntary forced use movement therapy (FUMT), BDNF, neuroplasticity
Introduction
Upper extremity impairment represents one of the most debilitating and pervasive functional deficits following ischemic stroke, and proper function of the arm and hand is important for achieving independence following stroke. Chronic hemiparesis of the arm is likely due in part to the phenomenon of learned non-use, which results from repeated failure with the impaired arm immediately following injury, increased compensation with the less affected arm, and eventual deterioration of representation to the affected arm1.
A promising rehabilitative technique aimed at improving upper extremity impairment presently emerging is to constrain use of the unaffected arm, thereby forcing patients to use their affected limb that would normally become neglected1,2,3,4,5,6. The mechanisms responsible for reinstatement of function in 'constraint induced movement therapy' (CIMT) are not well understood, but likely involve discouraging learned non-use1, thereby modulating post-ischemic neuroplasticity. Further investigation into this phenomenon is warranted, and mechanistic studies of CIMT, learned non-use, and post-ischemic neuroplasticity require appropriate animal models.
Previous attempts to model CIMT in rodents have resulted in discrepant results7,8,9,10, probably due to a combination of the varied stroke models used and confounding effects of stress when forelimb function is constrained in rodents. Models of middle cerebral artery occlusion (MCAo), in which the MCA is exposed and occluded permanently or temporarily have conventionally been used to study post-ischemic sensorimotor impairment. However these methods often result in large, inconsistently placed lesions and are often associated with a relatively high mortality rate11, unless accompanied by labour-intensive post-operative care12,13, rendering them inadequate for studying specifically localized deficits and/or early interventions. To gain more specific control of lesion location, reproducibility, and marked deficits to forelimb function, different rat models of focal ischemic stroke have been developed14,15,16,17,18. Permanent methods such as devascularization16 and photo-irradiation of blood vessels that supply the forelimb region18 affect surface tissue only (without accompanying damage to subcortical regions) and do not allow for restoration of blood flow to the ischemic region. An alternative method is to biochemically and reversibly constrict local vessels through administration of a vasoconstrictor such as endothelin-1 (ET-1). Endothelin-1 reduces local blood flow to produce ischemic injury, either when injected proximal to the MCA19, applied topically to the cortical surface20, injected intracerebrally15, or when used as a combination of intracerebral and topical application21. Intracerebral injection of ET-1 permits the induction of ischemic injury to the forelimb sensorimotor cortex as well as subcortical regions15,17,22 allows for gradual reperfusion of circulation over the course of several hours20,22,23, and is characterized by a low mortality rate compared to MCAo22,24, making this method an attractive option for studying post-ischemic rehabilitation.
The objectives of the current study were 1) to further develop the endothelin-1 model of focal ischemic injury in rats, resulting in reproducible, well-defined lesions and reliable and long-lasting upper extremity impairments associated with stroke, and 2) to test whether a novel appetitively motivated model of therapy (voluntary forced use movement therapy; FUMT) would accelerate post-ischemic motor recovery. We found that by using microinjections of ET-1 in a previously unreported set of stereotaxic coordinates we were able to create reproducible lesions and deficits, and that the novel approach of voluntary forced use results in modest but consistent acceleration in functional recovery. The application of these methods may prove useful in developing strategies to study post-ischemic rehabilitative-dependent neuroplasticity.
Materials and methods
Experimental animals
Adult male Sprague-Dawley rats (Rattus norvegicus; n=20 for Experiment 1; n=46 for Experiment 2) were purchased from Charles River Laboratories (Montreal, Canada) and single housed on a 12 h light/dark cycle. All procedures in Experiment 2 took place during the dark cycle, as activity levels affected animals' participation in the rehabilitation. Animals had ad libitum access to food and water, and weighed between 300–350 g at the time of surgery. All procedures were conducted in accordance with the guidelines of the Canadian Council for Animal Care and were approved in advance by the University of Prince Edward Island Animal Care Committee.
Surgical procedures
Prior to surgery, rats were allocated to sham or stroke group, wherein there was no difference in preoperative performance on any of the behavioural tests to be used (data not shown). Forelimb sensorimotor coordinates for ET-1 injections were determined using the Rat Brain Atlas25 (Figure 1A). Prior to the study, injection placement was verified using injections of cresyl violet dye (not shown). Rats were placed into an induction chamber filled with 3.5% isoflurane in oxygen for 8 min; anesthesia was maintained during surgery with 2% isoflurane. Animals were mounted on a stereotaxic apparatus (David Kopf Instruments, USA), with the skull level between bregma and lambda. Local anesthetic (Xylocaine, AstraZeneca, Canada) was applied topically, a midline scalpel incision was made, and the scalp was retracted with clamps. Small holes were drilled at injection locations (Table 1) using a stereotaxically mounted dental drill (Foredom Motor; Stoelting Co, IL, USA). At each location, a 26 gauge 10 μL stereotaxically mounted syringe was lowered into position and left undisturbed in the brain for 1 min. Endothelin-1 (CalBioChem; 400 pmol/μL dissolved in sterile water) was then injected 1 μL at a flow rate of 0.5 μL/min, after which the needle was left undisturbed for 4 min prior to being slowly retracted from the brain. At the conclusion of surgery, the scalp was sutured and the incision site was treated with topical anesthetic. Body temperature was maintained at 36±0.2 °C for the duration of surgery using a heating pad. Following surgery, animals were given subcutaneous injections of butorphanol (2.0 mg/kg; Experiment 2 only), returned to their home cage, and allowed to recover. A heating pad remained under the home cage for 2 h post-surgery. Sham-operated rats received the same surgical procedure up to but not including the drill holes, and remained anesthetized for the same amount of time as the ischemic animals to serve as a control for all aspects of the experimental surgery.
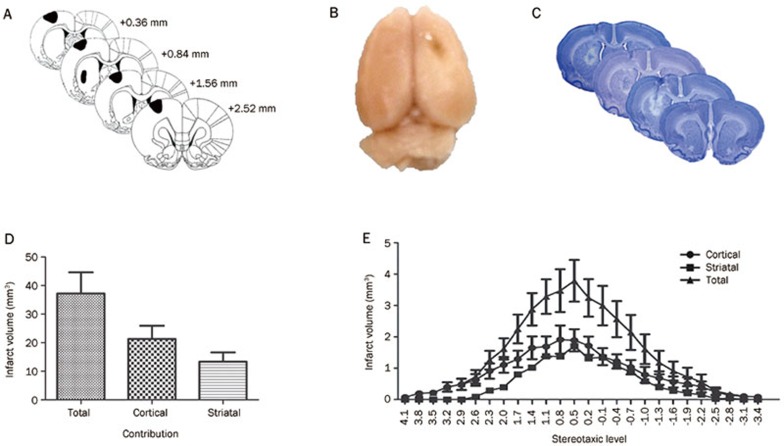
Figure 1.
Overview of the surgical procedure. (A) Approximate targeted infarct regions based on microinjection locations (in black; adapted from the Rat Brain Atlas25). (B) Gross assessment of the lesion. (C) Typical damage produced at targeted stereotaxic levels. (D) Volume of the total infarct, as well as the cortical and striatal contribution. (E) Infarct distribution across stereotaxic levels. Error bars represent SEM. n=14 (ischemia), n=6 (sham).
Table 1. Coordinates for cortical and striatal microinjections.
| Injection |
Stereotaxic coordinates (mm from bregma) |
||
|---|---|---|---|
| AP | ML | DV | |
| Cortex injection 1 | +2.5 | −2.8 | −2.5 |
| Cortex injection 2 | +1.6 | −2.8 | −2.5 |
| Cortex injection 3 | +0.9 | −2.8 | −2.5 |
| Cortex injection 4 | +0.4 | −2.8 | −2.5 |
| Striatum injection | +0.9 | −3.7 | −6.0 |
AP=anterior/posterior; ML=medial/lateral; DV=dorsal/ventral.
Behavioural tests of sensorimotor function
Several behavioural tests commonly employed to determine sensorimotor deficits were used. For three weeks prior to surgery, animals were handled daily and acclimated to all tests, then pre-surgery scores were recorded. Following surgery, animals were tested regularly until the end of the study at post surgery day (PSD) 21. When using behavioural assessments, 'deficit' was defined as a post-surgical performance significantly worse than sham control animals and 'recovery' was considered the point at which the animals returned to sham level performance. In any instance where animals had recovered at a particular time point, then had subsequent testing days with impaired performance, 'recovery' was considered the latest time point at which they would perform consistently at sham level. All performance evaluations were conducted by an experimenter blind to the surgical condition.
Forelimb placing tests
Two tests of forelimb placing were employed daily: tactile-stimulated forelimb placing (TFP) and vibrissae-stimulated forelimb placing (VFP). Rats were held by their torso, with 3 limbs secured and the forelimb to be tested hanging free. The distal portion of the unrestrained forelimb or the corresponding vibrissae was gently brushed against the edge of a table. For each forelimb, the deficit was determined based on the number of correct forelimb placements on the table top out of 5 attempts. Attempts that missed the tabletop or did not occur within 2 s of stimulation were considered unsuccessful.
Forelimb postural reflex test
To test for deficits in forelimb postural reflex position daily following surgery, animals were suspended by the base of the tail 50 cm above their home cage, and slowly lowered. The position of the contralesional forelimb was scored on a scale of 0 (forelimbs extending directly toward the cage; no deficit), 1 (extension of the forelimb at an angle; moderate deficit), or 2 (extension of the forelimb combined with twisting of the torso; severe deficit).
Forelimb asymmetry test
Animals were tested for limb preference and asymmetry in postural weight support during exploratory activity using the Schallert cylinder test26 on PSD 1, 3, 6, 10, 14, 18, and 21 (for Experiment 2, the testing on PSD 3 was omitted). Rats were placed inside a clear open-ended cylinder (25 cm×30 cm) and allowed to rear and place their forepaws on the cylinder wall 15 times. A camera was stationed above the top of the cylinder to record forepaw usage during rearing and exploratory behaviour. Videos were then analyzed to determine the number of forelimb wall contacts made. The percentage use of the contralesional forelimb was determined using the following formula:
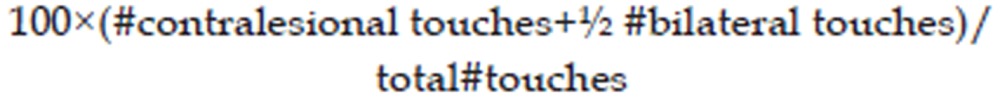 |
Horizontal ladder test
The horizontal ladder test was used (Experiment 2) as a measure of forelimb coordination on PSD 1, 6, 10, 14, 18, and 21. Animals ran on the 1.5 m long ladder containing unevenly spaced rungs (1–3 cm apart) for 3 trials. The average number of foot slips made with the contralesional forelimb was determined by video analysis.
Forced use movement therapy
Animals were acclimated to and trained to use commercially available clear plastic pet activity balls (29 cm diameter; Super Pet, USA). Prior to the initiation of the study, ability to move the balls was assessed to verify that all ball movement (initiating, propelling, changing direction, and stopping) required the use of both forelimbs. Therefore, animals were forced to evoke use of the impaired forelimb when engaging in post-surgical rehabilitation. Training took place with six exposures over two weeks, and began with exposure to stationary balls, gradually progressing to increasingly non-stationary periods of time, and resulting in a final assessment of ability to engage in the rehabilitation. A small number of animals (n=4, ie, 8.7%) were not able to manipulate the balls following this training regimen and were subsequently disqualified from being assigned to a FUMT group. These animals were randomly assigned to receive either Sham or Stroke followed by control therapy. Following surgery, rats were randomly assigned to receive FUMT (n=11 stroke; n=12 sham), or Control (n=11 stroke; n=12 sham) beginning on PSD 5. Rehabilitation sessions lasted for 30 min each day (during the dark phase of the light/dark cycle), during which the animals were free to move voluntarily around an enclosed 8 m2 arena. Sessions were video recorded and analysed to determine the amount of time spent moving during each 30 min period. Videos were scored by an experimenter blind to the surgical condition and to the test day of each animal. Control therapy consisted of placing rats into stationary balls for 30 min each day.
Histology and infarct quantification
Following behavioural testing, rats were deeply anesthetised and decapitated. For infarct quantification only (Experiment 1), brains were quickly removed to 250 mL of 10% phosphate buffered neutral formalin for 72 h, sectioned (150 μm) using a Vibratome 1000 plus (Vibratome Co, St Louis, USA), mounted on slides and stained using 0.1% cresyl violet. Photographs (Canon EOS DS6041 camera) of every second section (ie, 300 μm apart) were then assessed for infarct damage using Image J software (Image J, National Institutes of Health, USA) by overlaying each experimental section on a corresponding template section and tracing the area of injury. Ischemic injury was defined as pallor, abnormal architecture of the brain tissue, and apparent neuronal necrosis determined by a lack of cresyl violet staining27. The volume of brain injury was determined by summing the area of damaged tissue recorded from each section (both striatal and cortical) and multiplying that value by the distance between the measured sections28.
For infarct quantification accompanied by immunohistochemistry (Experiment 2), rats were perfused transcardially with phosphate buffered saline (PBS), followed by 4% paraformaldehyde. Brains were extracted, post-fixed for a further 24 h in 4% paraformaldehyde, then permeated with 30% sucrose in PBS for 72 h (or until they were no longer floating in the solution). They were then cryoprotected using Cryomatrix (Thermo Scientific, USA), and stored at −80 °C until use. Some brains (n=4/group) were sectioned (10 μm) for immunohistochemical analysis with sections taken from intact tissue caudal and rostral to the damage, as well as anterior, middle, and posterior sections of the infarct itself (at +4.1, +2.3, +0.5, -1.3, and -3.1 mm from bregma). Remaining sections were taken at 100 μm and used for cresyl violet staining. All other brains were completely sectioned at 100 μm for cresyl violet staining only. Tissue was sectioned using a Cryostat (Thermo Scientific, USA) and mounted on slides, dried overnight, and stored at −20 °C until use. Infarct quantification was performed as described above.
Immunohistochemistry
Expression of brain derived neurotrophic factor (BDNF) was determined as follows. Slides were subjected to antigen retrieval (90 °C for 20 min) then washed in PBS, quenched with 3% H2O2 for 20 min, and blocked (5% goat serum) for 2 h. They were then incubated with primary antibody against BDNF for 48 h (diluted 1:500; AB1779; Millipore, USA), washed in PBS (3×15 min), and incubated with secondary antibody (BA1000; Vector Laboratories, USA) for 1 h. Slides were treated with avidin biotin complex (ABC; Vector Laboratories, USA) and visualized using diaminobenzidine solution (DAB; Vector Laboratories, USA). Negative controls were prepared by omitting the primary antibody. Following immunohistochemical staining, slides were counterstained with hematoxylin to visualize the cell nuclei.
BDNF expression in various regions of interest in the ipsilesional and contralesional hemisphere (and corresponding regions of sham sections) was quantified by calculating the percentage of cells in each region expressing the protein of interest [(#BDNF positive cells/total#cells)×100]. Sections were examined in 8 regions: three along the edge of the cortical infarct, one in the area of the striatal infarct border, and the 4 equivalent regions of the contralesional hemisphere.
Statistical analyses
Statistical analyses were performed using GraphPad Prism (v5.00). Data were analyzed with multiple factor [sham versus stroke; post surgery day; FUMT versus Control (Experiment 2 only)] ANOVAs with subsequent planned Bonferroni post-hoc comparisons. In Experiment 2, Sham groups receiving FUMT and Control did not differ in any outcome measure and were therefore combined for analyses. In both experiments 'recovery' was considered the point at which the animals returned to sham level performance. In any instance where animals had recovered at a particular time point, then had subsequent testing days with impaired performance, 'recovery' was considered the latest time point at which they would perform consistently at sham level. Comparisons of lesion volume were performed with t-tests. Significance was considered P-value below 0.05.
Results
Experiment 1: Development and validation of the model
Histology
A total of five intracerebral injections of ET-1 were administered in this study, targeting brain regions previously shown to be responsible for forelimb sensorimotor function, including sensorimotor cortex and dorsolateral striatum (Figure 1A). Gross anatomy of the brains upon removal from the skull revealed visible infarcts on the sensorimotor region (Figure 1B). Brains were then sectioned for complete analysis of the infarct at various stereotaxic levels; a representation of typical damage at each level is presented in Figure 1C. The average volume of ischemic damage resulting from this injection protocol was 21.31±4.81 mm3 (cortical) and 13.36±3.36 mm3 (striatal), giving a combined total damage volume of 37.23±7.69 mm3 (Figure 1D). The infarct spread and concentration at various stereotaxic levels are presented in Figure 1E.
Behavioural tests
Forelimb placing tests
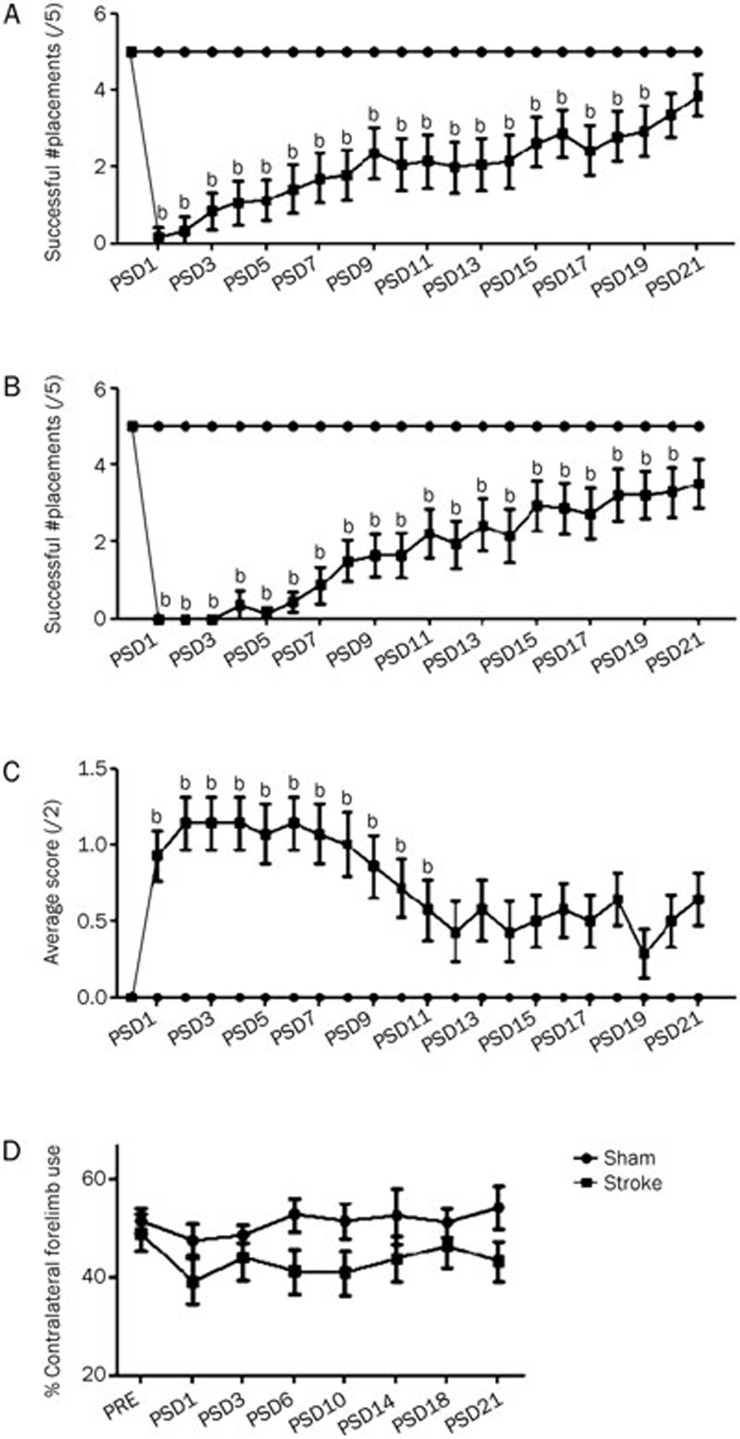
Prior to surgery, all animals were able to successfully place 5/5 times in response to both tactile and vibrissae stimulation. Following ET-1 surgery, the ipsilateral placing response remained intact (not shown) and there was a significant impairment in contralateral tactile-stimulated placing [PSD1=0.2±0.2 (SEM)] until PSD 19 (P<0.05) compared to sham animals (Figure 2A). Similarly ET-1 surgery resulted in a significant impairment in contralateral forelimb placing in response to vibrissae stimulation [PSD1=0±0 (SEM)] compared to sham control animals until PSD 20 (Figure 2B).
Figure 2.
Effects of the ET-1 microinjection protocol on various tests of contralesional forelimb function in Experiment 1. (A) Tactile-stimulated forelimb placing test of reflexive paw placement following physical stimulation. (B) Vibrissae-stimulated forelimb placing test of reflexive paw placement following physical-sensory stimulation. (C) Forelimb postural reflex test of reaching for a flat surface during descent. (D) Cylinder test of forelimb use for upright support. Error bars represent SEM. n=14 (stroke), n=6 (sham); bP<0.05 relative to sham.
Forelimb postural reflex
Prior to surgery, stroke and sham animals all received a score of 0 in the forelimb postural reflex test. Following surgery there was a deficit in postural reflex in stroke animals [PSD1=0.93±0.16 (SEM)], which remained significant for 11 d post surgery (Figure 2C).
Cylinder test of asymmetry
To assess differences in the use of the contralateral forelimb, the cylinder test was used to evoke and analyze percent forelimb usage. Sham and stroke groups performed similarly prior to surgery, relying on the contralesional forelimb for exploring and weight bearing approximately half of the time [51.5%±2.8% (SEM) and 49.1%±3.8%, respectively]. Following ET-1 surgery, animals that received stroke demonstrated a consistent tendency to rely more on their ipsilateral forelimb [PSD1=39.1%±4.7%], although this difference was not significant (P>0.05; Figure 2D).
Experiment 2: Effects of FUMT rehabilitation
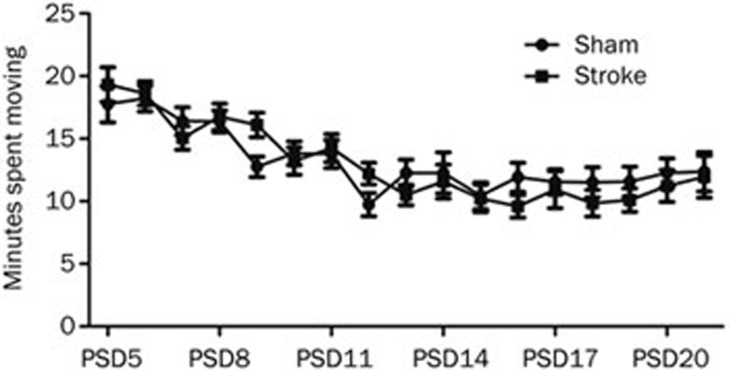
While FUMT was the main independent variable in the present study, we were interested in evaluating whether the activity level of ET-1 stroke animals would be influenced by ischemia. All animals spent an equal amount of time moving during the 30 min rehabilitation sessions on any given day (P>0.05), however the amount of time declined steadily over the course of the study from 18 min on PSD 5 to 12 min by PSD 21 in both groups (P<0.0001; Figure 3).
Figure 3.
Rehabilitation intensity. Mean (±SEM) time (min) spent moving the exercise ball each day during 30 min of rehabilitation. On PSD 5, Sham animals spent 18±1.5 min (SEM) engaging in the voluntary forced movement, while ET-1 animals spent 19±1.5 min (SEM) moving. At PSD 21, both groups spent 12±1.7 min moving. n=11 (stroke); n=12 (sham).
Behavioural tests
Forelimb placing tests
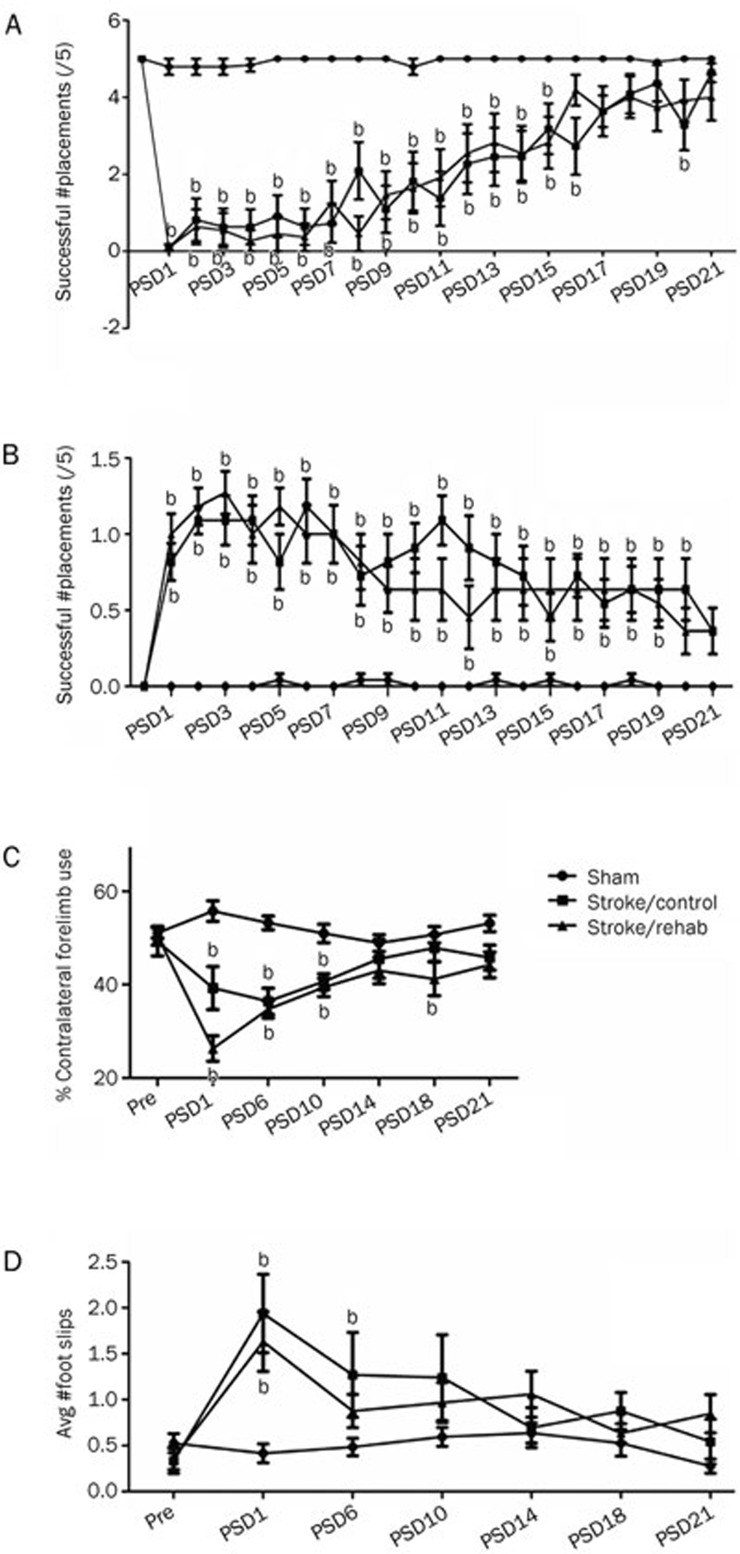
Prior to surgery, all animals had equivalent performance on both the tactile-stimulated placing test and the vibrissae-stimulated placing test, scoring 5/5 without error. Following surgery, all ischemic animals had a significant functional deficit compared to sham animals beginning on PSD 1 [0.09±.09 (SEM) for both groups on tactile-stimulated placing; 0.45±0.48 Control and 0.09±0.09 for FUMT on vibrissae-stimulated placing]. Rats receiving FUMT recovered (ie, were not significantly different from shams; see Methods above) 5 d sooner than animals receiving control therapy (Control) (PSD 16 vs 21, respectively) on the tactile-stimulated test (Figure 4A), but not on the vibrissae-stimulated test (PSD 21 vs 18; data not shown).
Figure 4.
Effects of the ET-1 microinjection protocol on various tests of contralesional forelimb function in Experiment 2. (A) Tactile-stimulated forelimb placing test. (B) Forelimb postural reflex test. (C) Cylinder test. (D) Horizontal ladder test. Error bars represent SEM. n=11 (stroke groups), n=24 (combined sham group); bP<0.05 relative to sham.
Forelimb postural reflex
Prior to surgery, all animals scored '0' or no deficit on the forelimb postural reflex test. Following surgery, animals from both ischemic groups showed a significant deficit [Stroke/Control=0.9±0.1 (SEM); Stroke/FUMT=1.0±0.1; P<0.001]. Animals in the FUMT group were significantly impaired until PSD 19, while those from the Control group were impaired until PSD 20 (Figure 4B).
Cylinder test of asymmetry
Before surgery, all animals used the contralesional forelimb for approximately 50% of exploratory movement while rearing in the cylinder. Following surgery, ischemic animals showed a significant decrease in the percent usage of the contralateral limb [Stroke/Control=26.3%±2.9% (SEM); Stroke/FUMT=39.3%±4.8%; P<0.05] that recovered (ie, was not significantly different from sham performance) by PSD 14 in FUMT rats, while control therapy animals did not recover until PSD 21 (Figure 4C).
Horizontal ladder test
Prior to surgery all animals were able to cross the ladder with minimal number of foot slips [0.4±0.4 (SEM)]. Following surgery, the number of foot slips with the contralateral forelimb was significantly greater in both the ischemia groups [Stroke/Control=1.9±0.4; Stroke/FUMT=1.6±0.3; P<0.001) relative to sham. This impairment had recovered by PSD 6 in FUMT animals, but not until PSD 10 in Control animals (Figure 4D).
Histology and immunohistochemistry
Cresyl violet histology for infarct quantification revealed that there was no significant difference in the total infarct volume between groups. Animals receiving control therapy had infarcts measuring 12.2±3.0 mm3 while FUMT animals had infarcts measuring 10.7±2.0 mm3 (P>0.05; data not shown).
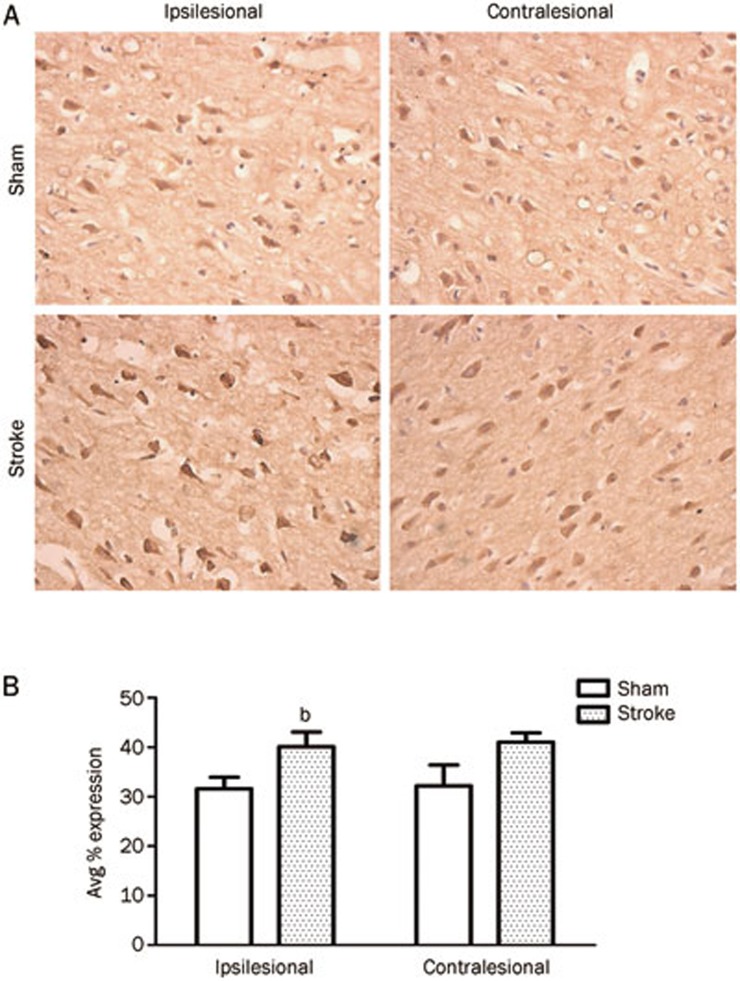
The proportion of cells expressing BDNF was determined from a total of 8 areas of interest encompassing both the ipsilesional and contralesional hemispheres (see Materials and methods). In the ipsilesional hemisphere, there was an increase in the percent cells expressing BDNF in animals that had undergone the ET-1 surgery [Sham=31.6%±2.7% (SEM) compared to Stroke=40.1%±3.4%; P<0.05; Figure 5], however this ratio remained unaffected by rehabilitative therapy. In the contralesional hemisphere there were no significant differences in percent BDNF expression although there was a tendency for stroke animals to have greater percent expression [Sham=32.2%±4.8% (SEM); Stroke=41.0%±2.2%; P>0.05; Figure 5].
Figure 5.
BDNF expression. (A) Representative images of BDNF expression in the ipsi- and contralesional hemisphere. (B) Quantification of the percent cells expressing BDNF in stroke and sham treated rats. Following surgery, Sham animals showed BDNF expression from 31.6%±2.7% (SEM) cells, compared to Stroke animals with 40.1%±3.4% expressing the protein. In the contralesional hemisphere there were no significant differences in percent BDNF expression, although there was a tendency for stroke animals to have greater percent expression [Sham=32.2%±4.8% (SEM); Stroke=41.0%±2.2%; P>0.05). n=4; bP<0.05.
Discussion
Mechanistic studies of neurorehabilitation require the use of a stroke model that results in consistent, well-defined lesions, and that preserves sufficient neural substrate to support neuroplastic events. This study was performed to determine whether multiple, low volume injections of ET-1 at previously unreported coordinates would result in appropriate lesions for this purpose. We then used this injection protocol to study a novel, appetitively motivated model of forced use movement therapy to rehabilitate upper extremity impairment.
Relative to more traditional models of focal ischemia, the ET-1 multi-injection model is an understudied method for inducing experimental stroke. Recent studies have begun to evaluate the use of this model to generate permanent histopathological damage to forelimb regions of the brain as well as impairment of forelimb function on a number of established sensorimotor tests15,17,21,24. While several labs have employed distinct ET-1 injection protocols to produce ischemic damage to forelimb regions of the brain15,17,21, no surgical standard currently exists. In the current experimental series, our first objective was to evaluate a modified ET-1 microinjection protocol using tests of forelimb sensorimotor function and histological assessments of the resulting infarct. Our results demonstrated that the ET-1 multi-injection protocol employed produced reproducible lesions to brain areas that control forelimb function (Figure 1), specifically, the forelimb sensorimotor cortex and lateral striatum. Resulting infarcts were highly reproducible, were notably smaller than those previously reported using similar methods22 while still producing reasonably long-lasting functional deficits (Figure 2), thereby preserving more neural tissue to support potential neuroplastic changes. It is possible that a larger stroke injury would have produced longer-lasting deficits that would improve opportunities for detecting accelerated recovery, but on the other hand a larger infarct could compromise rehabilitation by destroying excess neural substrate. We believe, therefore, that the current protocol represents a good compromise of these variables and has considerable potential as a surgical standard for post-ischemic neuroplasticity and rehabilitation studies.
Upper extremity impairment following stroke represents one of the most common disabilities in stroke survivors29,30. A presently emerging rehabilitation therapy, CIMT, results in marked functional recovery in stroke patients. To better understand the mechanisms underlying recovery following CIMT, a number of experimental studies have attempted to force use of the impaired forelimb using animal models. Following photothrombosis, forced use of the affected arm by plaster casting did not accelerate motor recovery10, perhaps due to the lack of a strong behavioural pressure to use the free limb that CIMT patients encounter during treatment. Similarly, constraint of the unaffected forelimb as a singular approach following striatal hemorrhagic stroke was insufficient to accelerate recovery, however when combined with exercises that forced use of the unconstrained, impaired forelimb, functional recovery could be promoted7. Moreover, exposure of rats to task-specific7,31,32 or nonspecific10,33,34,35,36 forelimb activity post-ischemia can improve functional outcomes. Notably, some experimental models of forced limb use in rodents have resulted in increased damage and worsened functional recovery8,37,38,39. The reasons for these discrepancies are unclear, but probably involve interactions between the stroke model used, the intensity of forced use, and the stress inherent in restraint. In an attempt to overcome these problems we combined the new ET-1 stroke model with a form of rehabilitation that is appetitively motivated rather than aversive. To our knowledge this is the first time that activity balls (or equivalents) have been used following stroke or as a form of appetitively motivated forced use. Although not described in detail, pet activity balls were employed by Moroz et al40 to measure ipsilateral rotation induced by amphetamine administration following 6-hydroxydopamine lesions, but the use of activity balls for post-ischemic rehabilitation has not been previously described.
The behavioural tests used in the second experiment revealed that the voluntary FUMT caused a modest significant acceleration in functional recovery using several assessments of forelimb function (Figure 4), without exacerbating damage. In addition to the tests presented herein, it would be interesting to evaluate the effect of FUMT on skilled forelimb reaching, for example by using a pellet-retrieval task. Rehabilitation, however, did not affect the expression of the neurotrophin BDNF (Figure 5). When we evaluated the average amount of time rats engaged in movement therapy (Figure 3), it was clear that while rats performed well in the task at first, there was declining engagement in the therapy throughout the duration of the study [from Sham=18±1.5 min (SEM) and Stroke=19±1.5 min (SEM) at PSD 5 to 12±1.7 min for both groups at PSD 21] . This may explain why recovery was only mildly affected. Rehabilitation is believed to be most effective when it is of increasing intensity, both in clinical41 and experimental33 studies, and rats given free access to running wheels for several hours per day naturally increase their activity over time42,43,44,45,46. It is possible that as animals in the present study became increasingly accustomed to the rehabilitation, they became disinterested during the daily 30 min sessions. Increasing stimulation around the room during the sessions, and/or breaking the movement sessions into multiple shorter sessions, might increase participation. It was interesting to note, however, that the amount of time spent rolling the exercise ball did not differ between ischemic and control rats (Figure 3). Navigation of the ball around the arena requires the use of all four limbs. Thus, despite marked deficits on contralesional forelimb function on multiple tests (Figures 2 and 4) ischemic rats could use that limb during rehabilitation. The reasons for this apparent contradiction are unclear and warrant further investigation.
In summary, we have reported that the use of a new endothelin-1 microinjection protocol results in reproducible lesions and deficits that are appropriate for studying rehabilitation of upper extremity impairment. Furthermore, a novel approach to voluntary forced use therapy in rats results in modest but consistent accelerations in functional recovery. The further refinement and application of these methods may prove useful in developing strategies to study post-ischemic rehabilitation and neuroplasticity.
Author contribution
All authors were involved with the design of the research. Andrew Wilson HUME and Jessica Mary Livingston-THOMAS performed research and analysed data; Andrew Wilson HUME, Jessica Mary Livingston-THOMAS, and Richard Andrew TASKER prepared the manuscript.
Acknowledgments
Jessica Mary Livingston-THOMAS is supported by a post-graduate scholarship from the PEI Prosperity Strategy. Research funding was provided by Atlantic Innovation Fund grant 193639 to Richard Andrew TASKER.
References
- Taub E, Uswatte G, Mark VW, Morris DM. The learned nonuse phenomenon: implications for rehabilitation. Eura Medicophys. 2006;42:241–56. [PubMed] [Google Scholar]
- Sawaki L, Butler AJ, Xiaoyan L, Wassenaar PA, Mohammad YM, Blanton S, et al. Constraint-induced movement therapy results in increased motor map area in subjects 3 to 9 months after stroke. Neurorehabil Neural Repair. 2008;22:505–13. doi: 10.1177/1545968308317531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterr A, Elbert T, Berthold I, Kolbel S, Rockstroh B, Taub E. Longer versus shorter daily constraint-induced movement therapy of chronic hemiparesis: an exploratory study. Arch Phys Med Rehabil. 2002;83:1374–7. doi: 10.1053/apmr.2002.35108. [DOI] [PubMed] [Google Scholar]
- Page SJ, Levine P, Leonard AC. Modified constraint-induced therapy in acute stroke: a randomized controlled pilot study. Neurorehabil Neural Repair. 2005;19:27–32. doi: 10.1177/1545968304272701. [DOI] [PubMed] [Google Scholar]
- Wolf SL, Thompson PA, Winstein CJ, Miller JP, Blanton SR, Nichols-Larsen DS, et al. The EXCITE stroke trial: comparing early and delayed constraint-induced movement therapy. Stroke. 2010;41:2309–15. doi: 10.1161/STROKEAHA.110.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberg GF, Chen R, Ishii K, Bushara KO, Eckloff S, Croarkin E, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- DeBow SB, Davies ML, Clarke HL, Colbourne F. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 2003;34:1021–6. doi: 10.1161/01.STR.0000063374.89732.9F. [DOI] [PubMed] [Google Scholar]
- DeBow SB, McKenna JE, Kolb B, Colbourne F. Immediate constraint-induced movement therapy causes local hyperthermia that exacerbates cerebral cortical injury in rats. Can J Physiol Pharmacol. 2004;82:231–7. doi: 10.1139/y04-013. [DOI] [PubMed] [Google Scholar]
- Muller HD, Hanumanthiah KM, Diederich K, Schwab S, Schabitz WR, Sommer C. Brain-derived neurotrophic factor but not forced arm use improves long-term outcome after photothrombotic stroke and transiently upregulates binding densities of excitatory glutamate receptors in the rat brain. Stroke. 2008;39:1012–21. doi: 10.1161/STROKEAHA.107.495069. [DOI] [PubMed] [Google Scholar]
- Schabitz WR, Berger C, Kollmar R, Seitz M, Tanay E, Kiessling M, et al. Effect of brain-derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke. 2004;35:992–7. doi: 10.1161/01.STR.0000119754.85848.0D. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Gribkoff VK, Donlan NA, Jones TA. Long-lasting functional disabilities in middle-aged rats with small cerebral infarcts. J Neurosci. 2003;23:10913–22. doi: 10.1523/JNEUROSCI.23-34-10913.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun HS, Doucette TA, Liu Y, Fang Y, Teves L, Aarts M, et al. Effectiveness of PSD95 inhibitors in permanent and transient focal ischemia in the rat. Stroke. 2008;39:2544–53. doi: 10.1161/STROKEAHA.107.506048. [DOI] [PubMed] [Google Scholar]
- Ryan CL, Doucette TA, Gill DA, Langdon KD, Liu Y, Perry MA, et al. An improved post-operative care protocol allows detection of long-term functional deficits following MCAo surgery in rats. J Neurosci Methods. 2006;154:30–7. doi: 10.1016/j.jneumeth.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Adkins DL, Voorhies AC, Jones TA. Behavioral and neuroplastic effects of focal endothelin-1 induced sensorimotor cortex lesions. Neuroscience. 2004;128:473–86. doi: 10.1016/j.neuroscience.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Gilmour G, Iversen SD, O'Neill MF, Bannerman DM. The effects of intracortical endothelin-1 injections on skilled forelimb use: implications for modelling recovery of function after stroke. Behav Brain Res. 2004;150:171–83. doi: 10.1016/j.bbr.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Gonzalez CL, Kolb B. A comparison of different models of stroke on behaviour and brain morphology. Eur J Neurosci. 2003;18:1950–62. doi: 10.1046/j.1460-9568.2003.02928.x. [DOI] [PubMed] [Google Scholar]
- Hewlett KA, Corbett D. Delayed minocycline treatment reduces long-term functional deficits and histological injury in a rodent model of focal ischemia. Neuroscience. 2006;141:27–33. doi: 10.1016/j.neuroscience.2006.03.071. [DOI] [PubMed] [Google Scholar]
- Shanina EV, Schallert T, Witte OW, Redecker C. Behavioral recovery from unilateral photothrombotic infarcts of the forelimb sensorimotor cortex in rats: role of the contralateral cortex. Neuroscience. 2006;139:1495–506. doi: 10.1016/j.neuroscience.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Macrae IM, Todd M, Reid JL, McCulloch J. Reduction of local cerebral blood flow to pathological levels by endothelin-1 applied to the middle cerebral artery in the rat. Neurosci Lett. 1990;118:269–72. doi: 10.1016/0304-3940(90)90644-o. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Bjelke B, Andbjer B, Grahn H, Rimondini R, Agnati LF. Endothelin-1 induced lesions of the frontoparietal cortex of the rat. A possible model of focal cortical ischemia. Neuroreport. 1997;8:2623–9. doi: 10.1097/00001756-199707280-00040. [DOI] [PubMed] [Google Scholar]
- Soleman S, Yip P, Leasure JL, Moon L. Sustained sensorimotor impairments after endothelin-1 induced focal cerebral ischemia (stroke) in aged rats. Exp Neurol. 2010;222:13–24. doi: 10.1016/j.expneurol.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle V, Szymanska A, Granter-Button S, White C, Buist R, Peeling J, et al. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp Neurol. 2006;201:324–34. doi: 10.1016/j.expneurol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Nikolova S, Moyanova S, Hughes S, Bellyou-Camilleri M, Lee TY, Bartha R. Endothelin-1 induced MCAO: dose dependency of cerebral blood flow. J Neurosci Methods. 2009;179:22–8. doi: 10.1016/j.jneumeth.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Windle V, Corbett D. Fluoxetine and recovery of motor function after focal ischemia in rats. Brain Res. 2005;1044:25–32. doi: 10.1016/j.brainres.2005.02.060. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C.The Rat Brain in Stereotaxic Coordinates6th ed. San Deigo: Academic Press, 2007
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Luke LM, Allred RP, Jones TA. Unilateral ischemic sensorimotor cortical damage induces contralesional synaptogenesis and enhances skilled reaching with the ipsilateral forelimb in adult male rats. Synapse. 2004;54:187–99. doi: 10.1002/syn.20080. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Davidson C, Sharp FR. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 1990;10:290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part II: Time course of recovery. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:406–12. doi: 10.1016/s0003-9993(95)80568-0. [DOI] [PubMed] [Google Scholar]
- Jorgensen HS, Nakayama H, Raaschou HO, Vive-Larsen J, Stoier M, Olsen TS. Outcome and time course of recovery in stroke. Part I: Outcome. The Copenhagen Stroke Study. Arch Phys Med Rehabil. 1995;76:399–405. doi: 10.1016/s0003-9993(95)80567-2. [DOI] [PubMed] [Google Scholar]
- Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil Neural Repair. 2008;22:250–61. doi: 10.1177/1545968307308551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Corbett D. Enriched rehabilitative training promotes improved forelimb motor function and enhanced dendritic growth after focal ischemic injury. J Neurosci. 2001;21:5272–80. doi: 10.1523/JNEUROSCI.21-14-05272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Z, Yip SP, Li L, Zheng XX, Tong KY. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS One. 2011;6:e16643. doi: 10.1371/journal.pone.0016643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin R, Williams A, Hale S, Burge B, Mense M, Bauman R, et al. The effect of voluntary exercise exposure on histological and neurobehavioral outcomes after ischemic brain injury in the rat. Physiol Behav. 2003;80:167–75. doi: 10.1016/j.physbeh.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Ohlsson AL, Johansson BB. Environment influences functional outcome of cerebral infarction in rats. Stroke. 1995;26:644–9. doi: 10.1161/01.str.26.4.644. [DOI] [PubMed] [Google Scholar]
- Johansson BB, Ohlsson AL. Environment, social interaction, and physical activity as determinants of functional outcome after cerebral infarction in the rat. Exp Neurol. 1996;139:322–7. doi: 10.1006/exnr.1996.0106. [DOI] [PubMed] [Google Scholar]
- Kozlowski DA, James DC, Schallert T. Use-dependent exaggeration of neuronal injury after unilateral sensorimotor cortex lesions. J Neurosci. 1996;16:4776–86. doi: 10.1523/JNEUROSCI.16-15-04776.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humm JL, Kozlowski DA, James DC, Gotts JE, Schallert T. Use-dependent exacerbation of brain damage occurs during an early post-lesion vulnerable period. Brain Res. 1998;783:286–92. doi: 10.1016/s0006-8993(97)01356-5. [DOI] [PubMed] [Google Scholar]
- Bland ST, Schallert T, Strong R, Aronowski J, Grotta JC, Feeney DM. Early exclusive use of the affected forelimb after moderate transient focal ischemia in rats : functional and anatomic outcome. Stroke. 2000;31:1144–52. doi: 10.1161/01.str.31.5.1144. [DOI] [PubMed] [Google Scholar]
- Moroz IA, Pecina S, Schallert T, Stewart J. Sparing of behavior and basal extracellular dopamine after 6-hydroxydopamine lesions of the nigrostriatal pathway in rats exposed to a prelesion sensitizing regimen of amphetamine. Exp Neurol. 2004;189:78–93. doi: 10.1016/j.expneurol.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Carr JH, Shepherd RB. Enhancing physical activity and brain reorganization after stroke. Neurol Res Int. 2011;2011:515938. doi: 10.1155/2011/515938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leasure JL, Grider M. The effect of mild post-stroke exercise on reactive neurogenesis and recovery of somatosensation in aged rats. Exp Neurol. 2010;226:58–67. doi: 10.1016/j.expneurol.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Shyu BC, Andersson SA, Thoren P. Spontaneous running in wheels. A microprocessor assisted method for measuring physiological parameters during exercise in rodents. Acta Physiol Scand. 1984;121:103–9. doi: 10.1111/j.1748-1716.1984.tb07435.x. [DOI] [PubMed] [Google Scholar]
- Risedal A, Mattsson B, Dahlqvist P, Nordborg C, Olsson T, Johansson BB. Environmental influences on functional outcome after a cortical infarct in the rat. Brain Res Bull. 2002;58:315–21. doi: 10.1016/s0361-9230(02)00796-7. [DOI] [PubMed] [Google Scholar]
- Makatsori A, Duncko R, Schwendt M, Moncek F, Johansson BB, Jezova D. Voluntary wheel running modulates glutamate receptor subunit gene expression and stress hormone release in Lewis rats. Psychoneuroendocrinology. 2003;28:702–14. doi: 10.1016/s0306-4530(02)00062-8. [DOI] [PubMed] [Google Scholar]
- Persson AI, Naylor AS, Jonsdottir IH, Nyberg F, Eriksson PS, Thorlin T. Differential regulation of hippocampal progenitor proliferation by opioid receptor antagonists in running and non-running spontaneously hypertensive rats. Eur J Neurosci. 2004;19:1847–55. doi: 10.1111/j.1460-9568.2004.03268.x. [DOI] [PubMed] [Google Scholar]