Abstract
Adenosine is a neuromodulator with its level increasing up to 100-fold during ischemic events, and attenuates the excitotoxic neuronal injury. Adenosine is produced both intracellularly and extracellularly, and nucleoside transport proteins transfer adenosine across plasma membranes. Adenosine levels and receptor-mediated effects of adenosine are regulated by intracellular ATP consumption, cellular release of ATP, metabolism of extracellular ATP (and other adenine nucleotides), adenosine influx, adenosine efflux and adenosine metabolism. Recent studies have used genetically modified mice to investigate the relative contributions of intra- and extracellular pathways for adenosine formation. The importance of cortical or hippocampal neurons as a source or a sink of adenosine under basal and hypoxic/ischemic conditions was addressed through the use of transgenic mice expressing human equilibrative nucleoside transporter 1 (hENT1) under the control of a promoter for neuron-specific enolase. From these studies, we conclude that ATP consumption within neurons is the primary source of adenosine in neuronal cultures, but not in hippocampal slices or in vivo mice exposed to ischemic conditions.
Keywords: adenosine, cerebral ischemia, ATP, nucleoside transport, hENT1 transgenic mice, hippocampus
Introduction
Adenosine is a neuromodulator with receptor-mediated effects. De novo synthesis of adenosine in brain is low1; thus, adenosine is primarily derived from dephosphorylation of ATP. In physiological conditions, cells salvage adenosine and other nucleosides for nucleotide synthesis. However, in ischemic conditions ATP concentrations drop and adenosine levels rise. Since basal adenosine levels are in the nanomolar range and basal ATP levels are in the low millimolar range, a small percentage drop in ATP can translate into a several fold increase in adenosine levels2. From these considerations adenosine was termed a “retaliatory metabolite”3; however, it is evident that adenosine functions in other roles as well.
The purpose of this brief review is to discuss recent findings from mice genetically modified to increase or decrease nucleoside transporter expression. We conclude that the levels and actions of adenosine are influenced by nucleoside transporter expression; however, the experimental preparation and the experimental conditions used modulate the influence of transporter abundance.
Adenosine – not merely a retaliatory metabolite
A common view of adenosine is that it is a retaliatory metabolite and is of particular relevance during hypoxia and ischemia when ATP levels are low3. Adenosine has effects through activation of members of a family of G-protein coupled receptors, termed A1, A2A, A2B, and A3. In particular, adenosine A1 receptor activity most closely corresponds to that of a retaliatory metabolite, as this receptor produces inhibition of neurotransmitter release secondary to inhibition of calcium channel opening and, in addition, causes post-synaptic inhibition by promoting potassium channel opening2. The concept of adenosine as a retaliatory metabolite includes the vasodilatation that can result from the activation of adenosine A2A receptors on vascular smooth muscle, an effect that would serve to enhance delivery of oxygen and glucose to metabolically stressed cells. However, since activation of adenosine A2A receptors on striatal neurons is associated with enhanced ischemic injury, the view of adenosine as a retaliatory metabolite is insufficient to describe all its actions4,5. Furthermore, as illustrated by the pharmacological effects of caffeine, a non-selective antagonist of adenosine receptors, it is apparent that adenosine's effects are observed in conditions of physiological levels of oxygen and glucose and not just during conditions of high ATP consumption, such as hypoxia and ischemia.
As a retaliatory metabolite, adenosine shares the stage with AMP. There is abundant evidence that AMP is an intracellular sensor of energy depletion. As ATP levels fall, AMP levels rise and AMP dependent kinase (AMPK) is activated6. AMPK is activated by phosphorylation (pAMPK) and it, in turn, phosphorylates a wide range of substrates to activate catabolic pathways and inhibit anabolic pathways7. AMPK is expressed in neurons and pAMPK is increased in neurons in ischemic brain where it persists during several hours of reperfusion6. Both neuroprotective and deleterious effects of AMPK inhibition have been reported in stroke studies6,8. During hypoxia and ischemia, and in tissues with abundant adenosine A1 receptors, it may be that both AMP and adenosine act as retaliatory metabolites, with AMP acting intracellularly via AMPK and adenosine acting extracellularly via its A1 receptors.
The view of adenosine as primarily a retaliatory metabolite is also being revised in light of the expanding volume of information about purinergic P2 receptors that utilize ATP and other nucleotides as agonists. The prevalence of these signalling pathways has led to the hypothesis that the effects of adenosine at its receptors are secondary to the effects of nucleotides at P2 receptors9,10,11. Depending on the receptor subtype expressed, ATP enhances or inhibits glutamate neurotransmission12. Thus, it has been demonstrated that ATP can produce inhibitory or excitatory effects, via P2X or P2Y receptors, with subsequent inhibitory effects via A1 receptors or excitatory effects via A2A receptors after metabolism to adenosine10,11.
Nucleoside transporters regulate extracellular adenosine levels
Nucleoside transporters facilitate the movement of adenosine, and other physiological and chemotherapeutic nucleosides, across biological membranes. Transporter-mediated cellular influx or efflux of adenosine attenuates or enhances, respectively, extracellular levels of adenosine and adenosine receptor activity. Two families of nucleoside transporters have been described, equilibrative and concentrative, with the former expressed by all cell types and the latter localized primarily in absorptive tissues such as epithelial cells13. Concentrative nucleoside transporters (CNTs) are members of the solute carrier 28 (SLC28) gene family. They are sodium symporters and, thus, mediate cellular influx of nucleosides in the presence of an inwardly directed sodium gradient. Three CNTs (CNT1-3) have been described and CNT2 and CNT3 accept adenosine as a permeant; however, to date a role for CNTs in regulating adenosine levels and adenosine receptor activity has not been demonstrated in CNS tissue13. Four members of the equilibrative nucleoside transporter (ENT) family, also known as SLC29, have been identified: ENT1-4. ENT1 and ENT2 are bidirectional and, thus, facilitate net cellular influx or efflux of nucleosides, including adenosine, according to the prevailing concentration gradient of permeants. Virtually all cells appear to express ENT1 and/or ENT2. A potent inhibitor, S-(p-nitrobenzyl)-6-thioinosine (NBTI), is available as a pharmacological tool and as a radioligand for ENT1. No similar highly selective and high affinity probe is available for ENT2; however, non-selective inhibition of ENT1 and ENT2, leading to potentiation of the vasodilatory actions of adenosine, is the pharmacological mechanism of action of the coronary dilators dipyridamole and dilazep. ENT3 and ENT4 are less studied members of the ENT family and their importance in regulating adenosine levels and adenosine receptor activity is poorly characterized. ENT3 has an intracellular localization and is, thus, unlikely to affect adenosine levels and adenosine receptor activity14. ENT4 is also known as plasma membrane monoamine transporter (PMAT). It is expressed in heart and brain and has relatively low affinity for transport of adenosine and serotonin15. Interestingly, ENT4 has enhanced activity at acidic pH relative to physiological pH; thus, it may be of importance in acidotic conditions of ischemia15.
ENT1 knock out mice were developed to investigate the role of adenosine as a mediator of the pharmacological effects of ethanol16. These mice exhibited decreased motor-incoordination and hypnosis after ethanol administration, which were associated with reduced basal levels of extracellular adenosine and reduced basal activity of adenosine A1 receptors in nucleus accumbens16,17. In contrast to these decreases in adenosine levels in brain, increased blood levels of adenosine were observed in ENT1 knock out mice, relative to wild type mice18; in further studies, ENT1 knock out mice exhibited ischemic cardioprotection, consistent with enhanced adenosine A1 receptor activity19.
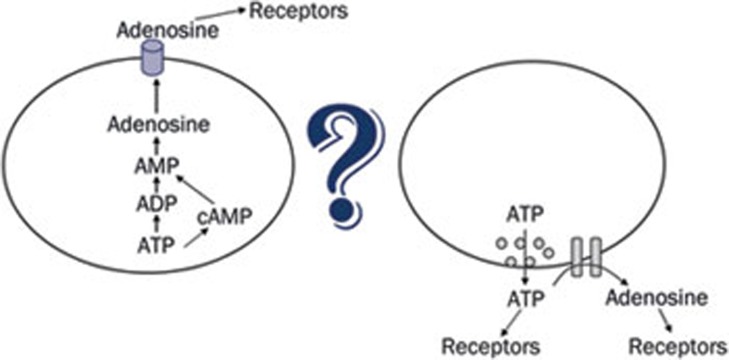
Considering that extracellular adenosine levels in ENT1 knock out mice appear to be decreased in brain, suggesting intracellular adenosine formation, yet increased peripherally, suggesting extracellular adenosine formation, it appears the pathway for adenosine formation is highly dependent upon the experimental conditions and cells or tissues under investigation. To address the question of whether, or to what extent, adenosine is formed from ATP, the intracellular energy molecule, or from ATP, the extracellular signalling molecule (Figure 1), we have used three different experimental preparations derived from brain: primary cultures of neurons and astrocytes, hippocampal slices and anesthetized mice. Adenosine levels, adenosine A1 receptor activity and ischemic infarct sizes were used as outcomes to ascertain whether intracellular or extracellular ATP was the primary source of adenosine during physiological and hypoxic/ischemic conditions.
Figure 1.
Extracellular adenosine produces receptor-mediated effects. The relative contributions of adenosine formed intracellularly, from ATP the energy molecule, and adenosine formed extracellularly, from ATP the signalling molecule, to the receptor-mediated effects of adenosine are under investigation.
Cultured neurons, but not astrocytes, release adenosine via ENTs
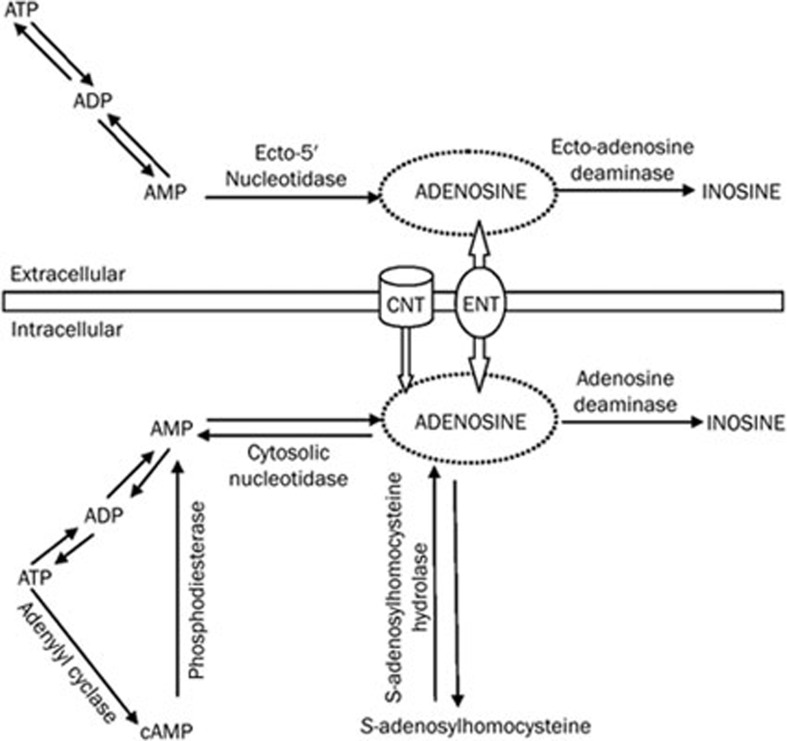
To mimic ischemia, rat cortical neurons were treated with N-methyl-D-aspartate (NMDA), hypoxia, or oxygen-glucose deprivation and increases in extracellular levels of adenosine were measured20,21,22. The appearance of adenosine in the extracellular media was inhibited by the equilibrative nucleoside transport (ENT) inhibitor dipyridamole, indicating that adenosine was formed intracellularly during these ATP-depleting conditions, and released from cells via a nucleoside transport system (Figure 2). In contrast, similar experiments were performed with rat cortical astrocytes, but dipyridamole did not affect the appearance of adenosine in the extracellular media20,21,22. Instead, α,β-methylene adenosine diphosphate (AOPCP) decreased the appearance of adenosine20,21,22. As AOPCP is an inhibitor of ecto-5'-nucleotidase, these data indicated that astrocytes release adenine nucleotides that are metabolized to adenosine in the extracellular environment.
Figure 2.
Pathways for adenosine formation. Intracellularly, adenosine is primarily formed by dephosphorylation of ATP, although cAMP and S-adenosyl homocysteine are alternative precursors for adenosine formation. Extracellularly, adenosine is thought to be a product of ATP that is metabolized by a series of ecto-enzymes. Nucleoside transport processes mediate cellular uptake or release of adenosine and other nucleosides, including inosine. Concentrative nucleoside transporters (CNT) and equilibrative nucleoside transporters (ENT) are highlighted.
Co-cultures of rat cortical astrocytes and cortical neurons were tested for the presence of extracellular adenosine following treatment of co-cultures with NMDA, to induce excitotoxicity21,22. Although NMDA receptors are more abundant on neurons than astrocytes, and NMDA treatment of neurons evokes adenosine release via nucleoside transporters, NMDA treatment of co-cultures induced adenosine release that was inhibited by AOPCP21,22. These data indicate that the extracellular pathway is predominant when both cell types are present.
Human equilibrative nucleoside transporter 1 (hENT1) transgenic mice
Given that during excitotoxic conditions, adenosine appears to be released selectively from neurons via a nucleoside transporter mechanism, we developed a transgenic mouse model to further explore adenosine release mechanisms23. A transgene consisting of the rat promoter region for neuron specific enolase was coupled to the coding sequence of human equilibrative nucleoside transporter 1 (hENT1) and used to develop mice with neuron specific expression of hENT1. We reasoned that ENT facilitate adenosine efflux during events where adenosine receptor mediated effects are a result of intracellular adenosine formation, subsequent to ATP utilization, yet facilitate adenosine influx during events where adenosine receptor mediated effects are a result of extracellular adenosine formation (Figure 2). Since neurons are more sensitive to ischemic injury than astrocytes and show more rapid depletion of intracellular ATP20, and since in cell culture rat neurons appear to release adenosine per se during stroke-like conditions22, we created mice with neuron specific expression of hENT123.
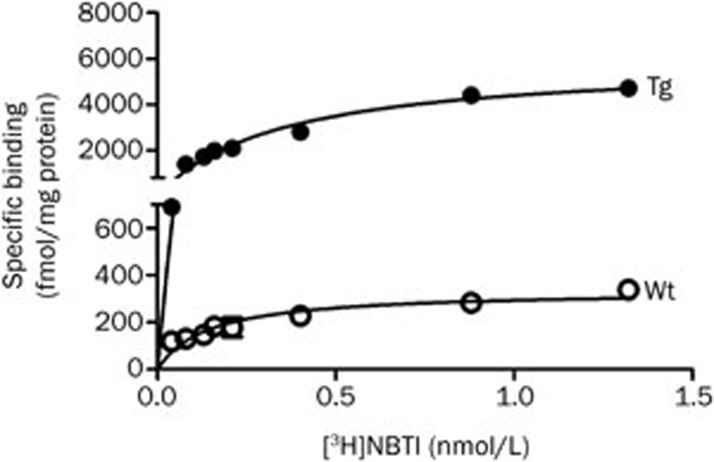
Using the high affinity and highly selective radioligand [3H]NBTI to radiolabel mouse ENT1 and hENT1, we found high expression of ENT1 proteins in synaptosomes prepared from cortex (Figure 3) and hippocampus24. Maximum binding site density (Bmax) was increased more than 15-fold in synaptosomes from transgenic mice (Figure 3)24. Affinity constants (Kd values) were increased 2-fold in samples from transgenic mice.
Figure 3.
Abundance of human equilibrative nucleoside transporter 1 (hENT1) determined from radioligand binding assays utilizing the selective radioligand [3H] S-(p-nitrobenzyl)-6-thioinosine ([3H]NBTI; 0.05–1.2 nmol/L) and synaptosomal preparations from cortex of heterozygous hENT1 transgenic mice (Tg) and wild type littermates (Wt). Assays with cortical synaptosomes were performed using the methods previously published for hippocampal synaptosomes and similar results were obtained24.
Cultured neurons, from hENT1 transgenic mice, release adenosine via ENTs
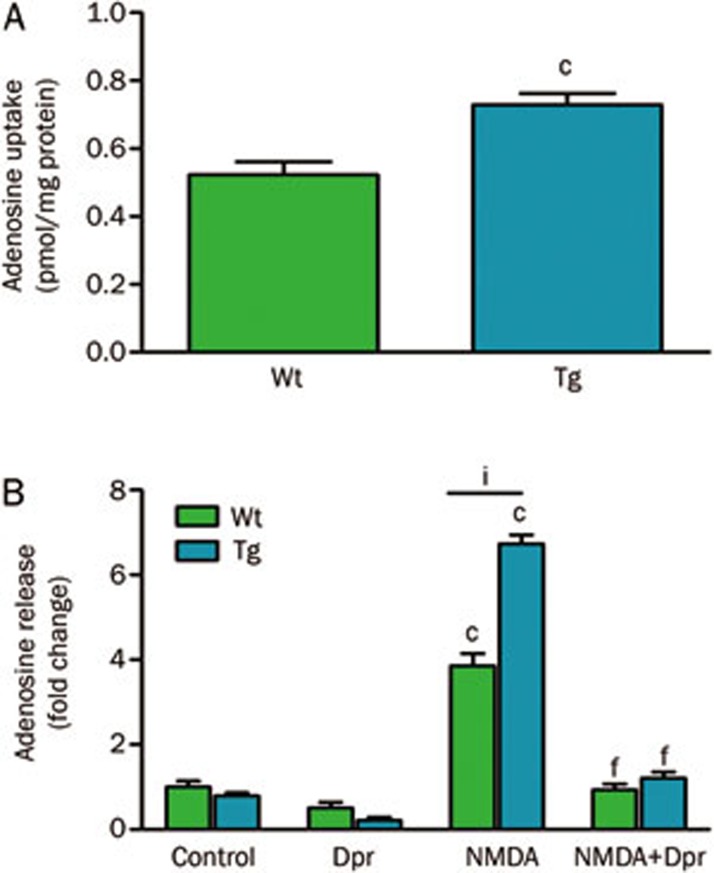
Using cortical neurons cultured from wild type or transgenic hENT1 mice, [3H]adenosine uptake into hENT1 neurons was greater than uptake into wild type neurons (Figure 4A). It is of interest that, while there was greater than 15-fold increase in ENT1 density in synaptosomal membranes, this led to only a 40% increase in [3H]adenosine uptake into cultured neurons. However, cellular accumulation of adenosine is determined by transport across the plasma membrane followed by intracellular trapping, with the latter as the rate limiting process. Low activity of adenosine kinase, which has been reported to be an astrocytic enzyme25, is likely to limit the intracellular accumulation of [3H]adenosine by neurons.
Figure 4.
Adenosine uptake (A) and release (B) from cultured cortical neurons from wild type (Wt) or hENT1 transgenic (Tg) mice. (A) [3H]Adenosine (1 μmol/L; 0.1 μCi) uptake was performed at 22 °C using primary cultures of neurons (11–15 d in vitro) using an assay volume of 0.5 mL and an uptake interval of 1 min. Non-specific uptake was determined with dipyridamole (Dpr; 30 μmol/L). For further details on methods, see36. cP<0.01; t-test. (B) Neurons were incubated for 30 min at 37 °C with [3H]adenine to radiolabel intracellular ATP, then treated with buffer, Dpr (30 μmol/L), N-methyl-D-aspartate (NMDA; 100 μmol/L) or the combination of Dpr and NMDA for 30 min at 37 °C to evoke release of purines. [3H]Purines were separated by thin layer chromatography and quantified by scintillation spectroscopy. For additional details on methods, see22. Data are mean±SEM (n≥8) and are expressed as pmol/mg protein (A) or fold change, relative to wild type controls (B). Data were analyzed by two-way ANOVA. cP<0.01 vs controls. fP<0.01 vs NMDA. iP<0.01 vs wild type NMDA.
Wild type and hENT1 transgenic neurons were treated with an excitotoxic condition, 100 μmol/L NMDA for 30 min, and extracellular adenosine levels were determined (Figure 4B). NMDA treatment of hENT1 transgenic neurons led to a 75% increase in extracellular adenosine levels, relative to the same treatment of wild type neurons. As previously observed with rat neurons22, this increase in adenosine was abolished by treatment with dipyridamole, indicating that nucleoside transporters mediated adenosine release from neurons. Again, it was noted that there was a mismatch between the 15-fold increase in ENT1 density in synaptosomal membranes and the 75% increase in adenosine release from cultured neurons evoked by NMDA. This is largely explained by the finite quantities of intracellular purines; treatment with NMDA causes release of upwards of 20% of radiolabelled purines from these cells.
Hippocampal slice electrophysiology as a bioassay for adenosine production
Hippocampal slices from rats and mice have been used to record excitatory post-synaptic potentials for many years. Using this method, it has been demonstrated that basal adenosine levels inhibit synaptic activity via activation of adenosine A1 receptors26. Exogenous adenosine and receptor agonists decrease synaptic activity whereas receptor antagonists increase synaptic activity26.
Hypoxia and oxygen-glucose deprivation decrease synaptic activity in hippocampal slices27,28. Adenosine A1 receptor antagonists attenuate this synaptic inhibition28. This synaptic inhibition is also attenuated in slices from A1 receptor knock out mice29. Thus, conditions such as hypoxia increase extracellular adenosine levels and promote adenosine A1 receptor activity. Using an enzyme-based biosensor, Frenguelli and colleagues have demonstrated that extracellular adenosine levels rapidly increase in hippocampal slices exposed to ischemic conditions; increases in extracellular ATP were also observed, but were slower and more modest than the increases in adenosine30. The effect of calcium on adenosine and ATP release evoked by ischemic conditions was examined. Adenosine release was increased, but ATP release was reduced, in calcium-free aCSF containing EGTA, relative to physiological aCSF. Furthermore, ischemia-evoked ATP release was enhanced, but adenosine release was not significantly altered, by treatment with kynurenic acid, a non-selective antagonist of ionotropic glutamate receptors. In sum, these data suggest that the ischemia-evoked release of adenosine is largely independent of ATP release30.
Nucleoside transport inhibitors produce synaptic inhibition that is blocked by adenosine A1 receptor antagonists26 and these inhibitors were shown to increase adenosine release without an effect on ATP release from ischemic slices30, indicating that these transporters are more important for adenosine uptake than adenosine release during physiological and hypoxic/ischemic conditions.
Neuronal ENTs in hippocampus mediate adenosine uptake not release during hypoxia/ischemia
In light of research showing that nucleoside transport inhibitors enhance the actions of adenosine in hippocampal slices24,26 yet in neuron cultures these inhibitors prevent evoked release of adenosine18,20 (Figure 4), we tested adenosine-dependent responses to basal, hypoxic or ischemia-like conditions in hippocampal slices from hENT1 transgenic, relative to wild type, mice.
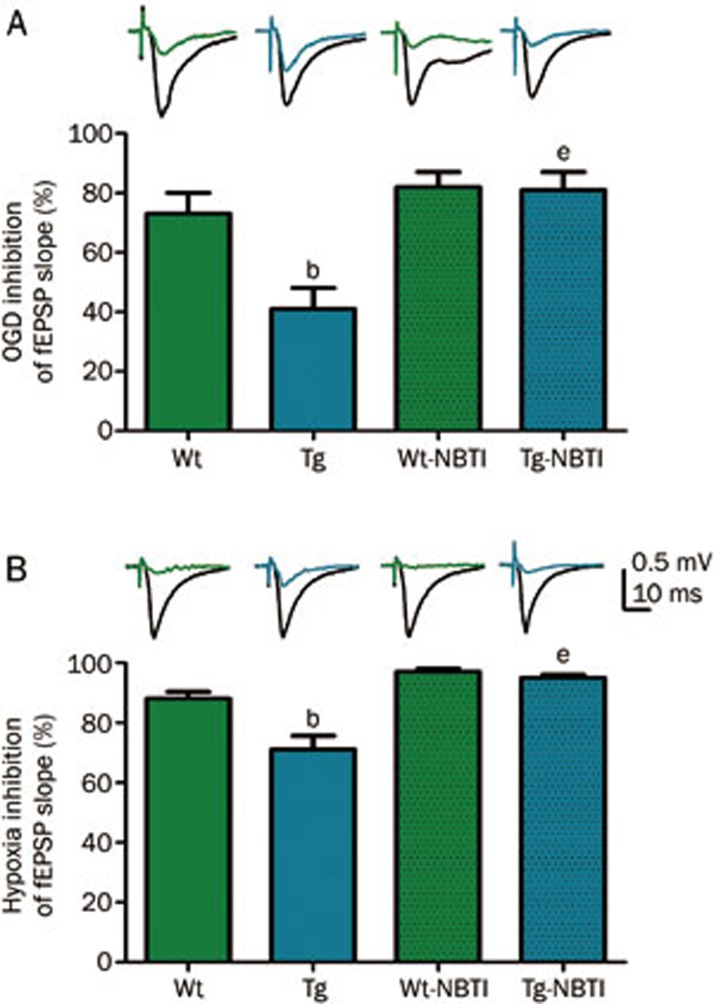
Using hippocampal slices from wild type mice, it was noted that exogenous adenosine inhibited synaptic transmission with an IC50 value of approximately 25 μmol/L; however, using slices from hENT1 transgenic mice, adenosine had an IC50 value of greater than 50 μmol/L24. In contrast, the adenosine A1 receptor agonist, N6-cyclopentyladenosine had similar inhibitory potency in slices from wild type and hENT1 transgenic slices. Furthermore, the A1 receptor antagonist 8-cyclopentyl-1,3-dipropyl-xanthine increased synaptic activity in wild type slices but had no stimulatory effect in hENT1 transgenic slices, indicating that basal adenosine levels were reduced in transgenic slices. In slices exposed to hypoxic or oxygen-glucose deprivation conditions, hENT1 transgenic slices showed less synaptic inhibition than wild type slices (Figure 5). NBTI (100 nmol/L) decreased synaptic activity in slices from both wild type and hENT1 transgenic mice and enhanced the synaptic depression produced by hypoxia or oxygen-glucose deprivation. This effect was greater in slices from hENT1 transgenic mice (Figure 5) as, in the presence of NBTI, wild type and hENT1 transgenic slices responded similarly to hypoxia or oxygen-glucose deprivation. Together, these data indicate that hENT1 expression in hippocampal neurons was associated with reduced extracellular adenosine levels, increased clearance of extracellularly applied adenosine, and reduced adenosine receptor activation in response to hypoxia or ischemia-like conditions.
Figure 5.
Extracellular post-synaptic excitatory potentials (fEPSP) were recorded from electrically stimulated hippocampal slices from wild type (Wt) or hENT1 transgenic (Tg) mice. Synaptic responses were evoked by stimulation of the Schaffer collateral/commissural pathway with a concentric bipolar stimulating electrode with 0.1 ms pulse width at 30 s intervals. Extracellular field excitatory postsynaptic potentials (fEPSPs) were recorded in striatum radiatum of CA1 hippocampus using glass microelectrodes (1–2 Ω) filled with aCSF. Slices were continuously superfused with aCSF or with 100 nmol/L S-(p-nitrobenzyl)-6-thioinosine (NBTI) in aCSF at a flow rate of 1.5 mL/min (32.5 °C). (A) Slices were exposed to glucose-free hypoxic aCSF (oxygen-glucose deprivation; OGD) for 3 min. (B) Slices were exposed to hypoxic aCSF for 10 min. Representative wave forms show fEPSP response before (black) or during OGD (A) or hypoxia (B) from Wt (green) or Tg (blue) slices. Bars are mean±SEM (n≥3) of maximally inhibited fEPSP responses, calculated as the difference between black and colored waveforms. Data were analyzed by one-way ANOVA and Bonferroni post-tests. bP<0.05 vs Wt. eP<0.05 vs Tg. Data are adapted from24; for additional details on methods, see24.
Neuronal hENT1 expression is associated with larger endothelin-1 induced cerebral infarcts
Endothelin-1 is a potent vasoconstrictor that has been used to produce ischemic infarcts31. Previously, it has been determined that reduction of blood flow to 20% of normal flow results in ischemic injury32. We found that an injection of endothelin-1 (400 pmol) into cerebral cortex produced a long-lasting decrease in blood flow at the site of injection33. Using perfusion-weighted magnetic resonance imaging, cerebral blood flow was determined at 4 and 48 h post-injection and found to be less than 15% and 50%, respectively, relative to pre-injection values. Volume matched saline injections produced transient decreases in cerebral blood flow that returned to pre-injection values within 48 h.
Interestingly, while cerebral blood flow responses were similar between wild type and hENT1 transgenic mice, cerebral infarct sizes were 60% larger, as determined by T2-weighted magnetic resonance imaging, in hENT1 transgenic mice33. This genotype difference in infarct sizes was not evident in mice that had received the adenosine receptor antagonist caffeine (25 mg/kg, ip) 30 min prior to intracerebral injection of endothelin-1, indicating that a genotype difference in ischemic adenosine levels and adenosine receptor activity was responsible for the differences in ischemic infarct sizes between wild type and hENT1 transgenic mice33.
Conclusions from ENT1 null and hENT1 transgenic mice
Adenosine dependent activation of A1 receptors is beneficial in cerebral ischemia34. It has been repeatedly demonstrated that activation of A1 receptors causes a rapid and profound decrease in synaptic activity with hypoxia or oxygen-glucose deprivation26,28,35,36. Nevertheless, the origin of this adenosine is still under investigation.
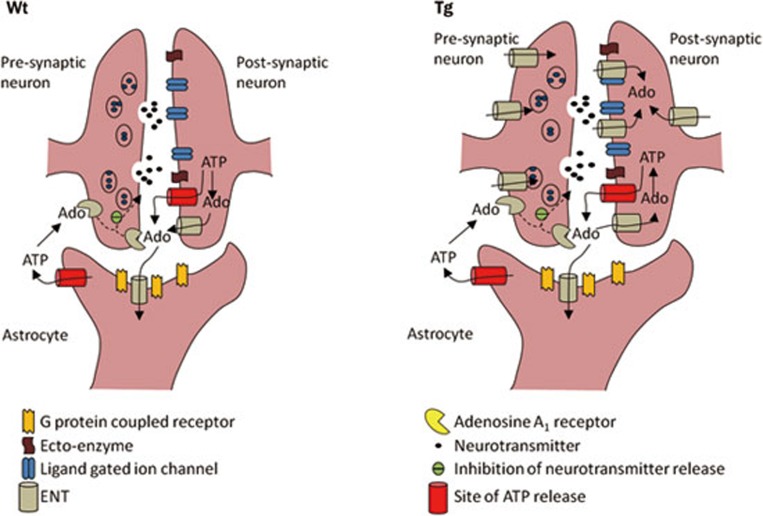
ENT1 is an equilibrative transporter that transports adenosine across cell membranes in a direction dictated by its concentration gradient. We proposed that, in neurons and brain tissues from mice with enhanced neuronal expression of ENT1, elevated extracellular adenosine levels and enhanced adenosine A1 receptor activity would be observed if adenosine was primarily produced intracellularly, in accordance with the traditional view of adenosine acting as a retaliatory metabolite. Interestingly, our studies reveal a difference between cultured neurons and hippocampal slices. The neuronal cultures exhibited nucleoside transporter mediated adenosine release during conditions of excitotoxicity but the hippocampal slices exhibited neuronal transporter mediated adenosine uptake during hypoxia/ischemia. The in vivo stroke model was consistent with the hippocampal slice experiments, as stroke injury was enhanced in hENT1 transgenic mice, which would be expected if adenosine levels and adenosine A1 receptor activity were reduced (Figure 6).
Figure 6.
A schematic diagram illustrating the effect of neuronal expression of human equilibrative nucleoside transporter 1 (hENT1). While mouse ENT1 and ENT2 are located on neurons and astrocytes, hENT1 is selectively expressed in neurons. From the data obtained with hippocampal slice electrophysiology and endothelin-1 injections into cerebral cortex, it appears that hENT1 expression in neurons leads to increased neuronal uptake of adenosine during hypoxic-ischemic conditions and decreased extracellular adenosine levels leading to decreased adenosine A1 receptor activity. Ado, adenosine. Wt: wild type littermates. Tg: hENT1 transgenic mice.
From our results, we conclude that when fetal neurons are cultured in isolation of other cell types, they appear to efflux cytoplasmic adenosine, produced from intracellular ATP that is rapidly degraded during conditions of hypoxia or ischemia. However, this is not the primary source of adenosine in in vitro hippocampus or in vivo cerebral cortex, derived from adult animals. Interestingly, adenosine levels and adenosine receptor activity were decreased in nucleus accumbens from ENT1 knock out mice, indicating that ENT1 mediates net adenosine efflux in this brain region16,17. Further research is required to resolve these differences between pathways that produce adenosine in cultured neurons and in different regions of adult brain. As cytosolic formation of adenosine in neurons is not a primary source of adenosine under basal, hypoxic or ischemic experimental conditions in adult cerebral cortex or hippocampus, current studies are underway to evaluate alternative sources of extracellular adenosine, including ATP, or other adenine nucleotides, released from neurons or glia and metabolized to adenosine extracellularly.
Acknowledgments
Research in the authors' laboratories is supported by the Canadian Institutes for Health Research, the Heart and Stroke Foundation of Manitoba, the Natural Sciences and Engineering Research Council of Canada and the St Boniface General Hospital Research Foundation. BCA is a Research Affiliate at the University of Manitoba's Centre on Aging and the Everett Endowment Fund Chair.
References
- Camici M, Micheli V, Ipata PL, Tozzi MG. Pediatric neurological syndromes and inborn errors of purine metabolism. Neurochem Int. 2010;56:367–78. doi: 10.1016/j.neuint.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Newby AC, Worku Y, Holmquist CA. Adenosine formation. Evidence for a direct biochemical link with energy metabolism. Adv Myocardiol. 1985;6:273–84. [PubMed] [Google Scholar]
- Phillis JW. The effects of selective A1 and A2a adenosine receptor antagonists on cerebral ischemic injury in the gerbil. Brain Res. 1995;705:79–84. doi: 10.1016/0006-8993(95)01153-6. [DOI] [PubMed] [Google Scholar]
- Melani A, Pantoni L, Bordoni F, Gianfriddo M, Bianchi L, Vannucchi MG, et al. The selective A2A receptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res. 2003;959:243–50. doi: 10.1016/s0006-8993(02)03753-8. [DOI] [PubMed] [Google Scholar]
- McCullough LD, Zeng Z, Li H, Landree LE, McFadden J, Ronnett GV. Pharmacological inhibition of AMP-activated protein kinase provides neuroprotection in stroke. J Biol Chem. 2005;280:20493–502. doi: 10.1074/jbc.M409985200. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zeng Z, Viollet B, Ronnett GV, McCullough LD. Neuroprotective effects of adenosine monophosphate-activated protein kinase inhibition and gene deletion in stroke. Stroke. 2007;38:2992–9. doi: 10.1161/STROKEAHA.107.490904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–6. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Wang HK, Ye CQ, Ge W, Chen Y, Jiang ZL, et al. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 2003;40:971–82. doi: 10.1016/s0896-6273(03)00717-7. [DOI] [PubMed] [Google Scholar]
- Huxtable AG, Zwicker JD, Poon BY, Pagliardini S, Vrouwe SQ, Greer JJ, et al. Tripartite purinergic modulation of central respiratory networks during perinatal development: the influence of ATP, ectonucleotidases, and ATP metabolites. J Neurosci. 2009;29:14713–25. doi: 10.1523/JNEUROSCI.2660-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–90. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Damaraju VL, Graham K, Yao SYM, Baldwin SA, Cass CE, et al. Molecular biology of nucleoside transporters and their distributions and functions in the brain. Curr Top Med Chem. 2011;11:948–72. doi: 10.2174/156802611795347582. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Yao SY, Hyde RJ, Ng AM, Foppolo S, Barnes K, et al. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem. 2005;280:15880–7. doi: 10.1074/jbc.M414337200. [DOI] [PubMed] [Google Scholar]
- Barnes K, Dobrzynski H, Foppolo S, Beal PR, Ismat F, Scullion ER, et al. Distribution and functional characterization of equilibrative nucleoside transporter-4, a novel cardiac adenosine transporter activated at acidic pH. Circ Res. 2006;99:510–9. doi: 10.1161/01.RES.0000238359.18495.42. [DOI] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, et al. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–61. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Nam HW, Lee MR, Zhu Y, Wu J, Hinton DJ, Choi S, et al. Type 1 equilibrative nucleoside transporter regulates ethanol drinking through accumbal N-methyl-D-aspartate receptor signaling. Biol Psychiatry. 2011;69:1043–51. doi: 10.1016/j.biopsych.2011.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JB, Naydenova Z, Bang A, Ramadan A, Klawitter J, Schram K, et al. Absence of equilibrative nucleoside transporter 1 in ENT1 knockout mice leads to altered nucleoside levels following hypoxic challenge. Life Sci. 2011;89:621–30. doi: 10.1016/j.lfs.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Rose JB, Naydenova Z, Bang A, Eguchi M, Sweeney G, Choi DS, et al. Equilibrative nucleoside transporter 1 plays an essential role in cardioprotection. Am J Physiol Heart Circ Physiol. 2010;298:H771–7. doi: 10.1152/ajpheart.00711.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W. Stimulus- and cell-type-specific release of purines in cultured rat forebrain astrocytes and neurons. J Neurochem. 2004;88:1305–12. doi: 10.1046/j.1471-4159.2003.02266.x. [DOI] [PubMed] [Google Scholar]
- Zamzow CR, Xiong W, Parkinson FE. Adenosine produced by neurons is metabolized to hypoxanthine by astrocytes. J Neurosci Res. 2008;86:3447–55. doi: 10.1002/jnr.21789. [DOI] [PubMed] [Google Scholar]
- Zamzow CR, Xiong W, Parkinson FE. Astrocytes affect the profile of purines released from cultured cortical neurons. J Neurosci Res. 2008;86:2641–9. doi: 10.1002/jnr.21718. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W, Zamzow CR, Chestley T, Mizuno T, Duckworth ML. Transgenic expression of human equilibrative nucleoside transporter 1 in mouse neurons. J Neurochem. 2009;109:562–72. doi: 10.1111/j.1471-4159.2009.05991.x. [DOI] [PubMed] [Google Scholar]
- Zhang D, Xiong W, Albensi BC, Parkinson FE. Expression of human equilibrative nucleoside transporter 1 in mouse neurons regulates adenosine levels in physiological and hypoxic-ischemic conditions. J Neurochem. 2011;118:4–11. doi: 10.1111/j.1471-4159.2011.07242.x. [DOI] [PubMed] [Google Scholar]
- Etherington LA, Patterson GE, Meechan L, Boison D, Irving AJ, Dale N, et al. Astrocytic adenosine kinase regulates basal synaptic adenosine levels and seizure activity but not activity-dependent adenosine release in the hippocampus. Neuropharmacology. 2009;56:429–37. doi: 10.1016/j.neuropharm.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Diao L. Extracellular adenosine concentrations in hippocampal brain slices and the tonic inhibitory modulation of evoked excitatory responses. J Pharmacol Exp Ther. 1994;268:537–45. [PubMed] [Google Scholar]
- Frenguelli BG, Llaudet E, Dale N. High-resolution real-time recording with microelectrode biosensors reveals novel aspects of adenosine release during hypoxia in rat hippocampal slices. J Neurochem. 2003;86:1506–15. doi: 10.1046/j.1471-4159.2003.01957.x. [DOI] [PubMed] [Google Scholar]
- Pearson T, Damian K, Lynas RE, Frenguelli BG. Sustained elevation of extracellular adenosine and activation of A receptors underlie the post-ischaemic inhibition of neuronal function in rat hippocampus in vitro. J Neurochem. 2006;97:1357–68. doi: 10.1111/j.1471-4159.2006.03823.x. [DOI] [PubMed] [Google Scholar]
- Johansson B, Halldner L, Dunwiddie TV, Masino SA, Poelchen W, Gimenez-Llort L, et al. Hyperalgesia, anxiety, and decreased hypoxic neuroprotection in mice lacking the adenosine A1 receptor. Proc Natl Acad Sci U S A. 2001;98:9407–12. doi: 10.1073/pnas.161292398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem. 2007;101:1400–13. doi: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windle V, Szymanska A, Granter-Button S, White C, Buist R, Peeling J, et al. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp Neurol. 2006;201:324–34. doi: 10.1016/j.expneurol.2006.04.012. [DOI] [PubMed] [Google Scholar]
- Siesjo BK. Pathophysiology and treatment of focal cerebral ischemia. Part I: Pathophysiology. J Neurosurg. 1992;77:169–84. doi: 10.3171/jns.1992.77.2.0169. [DOI] [PubMed] [Google Scholar]
- Soylu H, Zhang D, Buist R, Martin M, Albensi BC, Parkinson FE. Intracortical injection of endothelin-1 induces cortical infarcts in mice: effect of neuronal expression of an adenosine transporter. Exp Transl Stroke Med. 2012;4:4. doi: 10.1186/2040-7378-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Adenosine and brain ischemia. Cerebrovasc Brain Metab Rev. 1992;4:346–69. [PubMed] [Google Scholar]
- Masino SA, Diao L, Illes P, Zahniser NR, Larson GA, Johansson B, et al. Modulation of hippocampal glutamatergic transmission by ATP is dependent on adenosine a(1) receptors. J Pharmacol Exp Ther. 2002;303:356–63. doi: 10.1124/jpet.102.036731. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W, Zamzow CR. Astrocytes and neurons: different roles in regulating adenosine levels. Neurol Res. 2005;27:153–60. doi: 10.1179/016164105X21878. [DOI] [PubMed] [Google Scholar]