Abstract
Aim:
Galectin-3 (Gal-3) is a member of the carbohydrate-binding protein family that contributes to neoplastic transformation, tumor survival, angiogenesis, and metastasis. The aim of this study is to investigate the role of Gal-3 in human tongue cancer progression.
Methods:
Human tongue cancer cell lines (SCC-4 and CAL27) were transfected with a small-interfering RNA against Gal-3 (Gal-3-siRNA). The migration and invasion of the cells were examined using a scratch assay and BD BioCoat Matrigel Invasion Chamber, respectively. The mRNA and protein levels of β-catenin, Akt/pAkt, GSK-3β/pGSK-3β, MMP-9 in the cells were measured using RT-PCR and Western blotting, respectively.
Results:
Transient silencing of Gal-3 gene for 48 h significantly suppressed the migration and invasion of both SCC-4 and CAL27 cells. Silencing of Gal-3 gene significantly decreased the protein level of β-catenin, leaving the mRNA level of β-catenin unaffected. Furthermore, silencing Gal-3 gene significantly decreased the levels of phosphorylated Akt and GSK-3β, and suppressed the mRNA and protein levels of MMP-9 in the cells.
Conclusion:
Our data suggest that Gal-3 mediates the migration and invasion of tongue cancer cells in vitro via regulating the Wnt/β-catenin signaling pathway and Akt phosphorylation.
Keywords: tongue cancer, galectin-3, β-catenin, Wnt signaling pathway, MMP-9, RNA interference, tumor invasion, metastasis
Introduction
Squamous cell carcinoma of the tongue (TSCC) is one of the most common malignant tumors in the oral cavity and accounts for approximately 30%1 of all oral cancers. Despite advances in the therapeutic management of TSCC over the last few decades, the 5-year survival rate is still less than 50%2 due to local recurrence and metastasis. Therefore, many studies have been designed to explore agents that will inhibit tumor cell invasion and metastasis and improve the therapeutic outcome of patients with tongue cancer.
Galectin-3 (Gal-3), a member of the carbohydrate-binding protein family, contributes to neoplastic transformation, tumor survival, angiogenesis, and metastasis3,4,5. Gal-3 expression is associated with tumor invasion and metastasis in various human cancers, such as gastric, colon, prostate, pancreatic, and breast cancers6,7,8,9,10. Over-expression of Gal-3 enhances cancer cell motility and invasion6,7,8,9,10. It has been suggested that the translocation of Gal-3 from the nucleus to the cytoplasm indicates a poor prognosis for patients with tongue cancer11. Furthermore, cytoplasmic expression of Gal-3 was detected in ∼90% of tongue cancers, which correlated with severity and metastasis in tongue malignancies12. However, the precise, underlying mechanism of Gal-3 in tongue cancer cell migration and invasion remains unclear.
β-Catenin, an important factor in the canonical Wnt signaling pathway, contributes to cancer invasion and metastasis. β-Catenin enhances cancer cell adhesion and migration13 via the Wnt/β-catenin pathway or other signaling pathways (SDF-1/CXCR4 axis14 and the HSIo potassium channel15). Colon cancer metastasis is associated with activation of the Wnt/β-catenin signaling pathway through the expression of the metastasis mediator S100A416. In addition, aberrant cytoplasmic accumulation of β-catenin in the cytoplasm promotes invasion and migration of oral squamous cell carcinoma cells (OSCC) by enhancing Tcf/Lef-mediated transcriptional activity and MMP-7 expression as well as inducing epithelial-mesenchymal transition (EMT)17.
Silencing Gal-3 reduces the migration and invasion ability of pancreatic cancer cells through the degradation of β-catenin9. In contrast, Gal-3 expression increases cell motility by upregulating fascin-1 expression through the Wnt signaling pathway in gastric cancer6. Thus, previous studies indicate that the Gal-3/β-catenin axis might play an important role in tongue cancer cell migration and invasion. In the present study, the effects of Gal-3 on cell migration and invasion were examined in Gal-3-siRNA transfected tongue cancer cell lines. The role of the Wnt/β-catenin pathway (eg, β-catenin, Akt/pAkt, and GSK-3β/pGSK-3β) in cell migration and invasion was also explored.
Materials and methods
Cell culture and siRNA transfection
SCC-4 and CAL27 cell lines (provided by Shanghai Ninth People's Hospital, affiliated with Shanghai JiaoTong University, School of Medicine, China) were cultured in DMEM/F12 or DMEM (Invitrogen, Grand Island, NY, USA), respectively, and supplemented with 10% (v/v) fetal bovine serum (Hyclone, Logan, UT, USA), 100 units/mL penicillin and 100 units/mL streptomycin (GIBCO, Grand Island, NY, USA). All cells were maintained in a humidified incubator with 5% CO2 at 37 °C. Gal-3-siRNA (5′-GGGAAUGAUGUUGCCUUCCACUUUA-3′) and control-siRNA (5′-UUCUCCGAACGUGUCACGUTT-3′) were produced by GenePharma Co, Ltd (Shanghai, China). Transient transfection was performed using the Lipofectamine RNAi MAX reagent (Invitrogen, Grand Island, NY, USA) and following the manufacturer's instructions.
Western blotting
Cells were harvested following treatment with Gal-3-siRNA or control-siRNA for 48 h. Protein was extracted in lysis buffer A (50 mmol/L Tris-HCl pH 7.6, 20 mmol/L MgCl2, 200 mmol/L NaCl, 0.5% Igepal CA-630 (Sigma, Deisenhofen, Germany), 1 mmol/L DL-dithiothreitol (DTT), and protease inhibitors). Protein concentrations were measured using a BCA Protein Assay Kit. Fifty micrograms of protein was loaded on a 12% SDS-PAGE gel, followed by protein separation and electroblotting onto a polyvinylidene difluoride plus membrane (Micron Separation, Inc). The membrane was labeled with the following primary antibodies: anti-Gal-3 (1:500), anti-β-catenin (1:2000), anti-Akt (1:500), anti-phosphorylated Akt (1:250), anti-GSK-3β (1:500), anti-phosphorylated GSK-3β (1:250), anti-MMP-9 (1:1000) or anti-β-actin (1:1000). Anti-β-actin was used to monitor equal loading in each lane. Anti-mouse HRP-conjugated secondary antibodies (1:5000) were incubated in 5% BSA in PBST buffer for 1.5 h at room temperature. All antibodies were obtained from Abcam, Cambridge, UK. Immunoreactivity was detected using an enhanced chemiluminescence detection system (Amersham, England). For densitometric analysis of Western blots, Alpha Image software (Alpha Innotech, San Leandro, CA) was used. For experiments using Akt inhibitor V (Triciribine, Merck Millipore, Billerica MA, USA), cells were cultured in serum-free medium overnight and then pre-treated for 1 h with serum-free medium containing Akt inhibitor V (20 μmol/L). Total protein extracts were prepared and examined as outlined above.
Cell proliferation assay
Cell proliferation was measured by a methylthiazol tetrazolium (MTT) assay. Cancer cells (2.0×103 cells/well) were seeded in 96-well microtiter plates in a total volume of 100 μL/well. Transfection was performed when the cells were 30%–50% confluent. Following transfection, 10 μL/well of CCK-8 was added for 4 h. The absorbance of each well was determined at 450 nm using a microtiter plate reader (Molecular Devices, Sunnyvale, CA). The results are expressed as the mean±SD of three independent experiments. Measurements were made in triplicate.
Scratch assay
Cell migration was determined using a scratch assay18,19. The transfected cell lines were seeded on a 24-well plate and allowed to reach confluence. After scratching the bottom of the well with a pipette tip, the monolayer of cells was washed three times with PBS to remove the detached cells. The remaining adherent cells were incubated in medium (either DMEM/F12 or DMEM) containing 1% FBS for 24 h; this medium was then replaced with medium containing 10% FBS. Space filling by cell migration was evaluated 48 h later using bright-field microscopy. The experiments were performed in triplicate.
Cell invasion assay
Cell invasion was tested using a BD BioCoat Matrigel Invasion Chamber (Becton-Dickinson, MA). Cells were harvested 48 h after transfection. The cells were re-suspended in serum-free DMEM and then added to the upper chamber at a density of 2×105 cells/well. Cell invasion into the Matrigel was determined after 24 h of culture at 37 °C. The membrane containing invading cells was fixed using methanol and stained with HE, and invasion was quantified by light microscopy after removing the non-invading cells on the upper side of the membrane with cotton swabs.
Fluorescent distribution of β-catenin
SCC-4 monolayers grown on coverslips were transfected with Gal-3-siRNA and cultured for 48 h. These cells were fixed with 4% paraformaldehyde for 15 min, incubated with 0.02% Triton-X-100 for 15 min and blocked in 1% BSA for 30 min. The cells were then incubated with a monoclonal mouse anti-human β-catenin antibody (Abcam, Cambridge, UK) for 1 h, followed by incubation with a secondary Alexa Fluor 555-conjugated goat anti-mouse IgG antibody (Invitrogen, Grand Island, NY, USA, 1:100) for 1 h. The coverslips were washed and soaked in ddH2O for 5 s, mounted onto glass microscope slides with Vectashield mounting medium with DAPI (H-1200, Vector, Burlingame, VA, USA), and examined with an Olympus FluoView FV1000 confocal microscope. Experiments were performed in duplicate and repeated at least three independent times.
RNA isolation and RT-PCR
Total RNA was extracted from cells 48 h after transfection using Trizol. Template cDNA was synthesized from 1 μg of total RNA with a Quantscript RT Kit (TIANGEN, Shanghai, China) using random primers and a ribonuclease inhibitor. One microliter of the cDNA sample was mixed with 19 μL of a master reaction mix. PCR was performed for 40 cycles (denaturation at 95 °C for 15 s, annealing at 60 °C for 1 min and extension at 60 °C for 1 min) using a RealMasterMix Kit (SYBR Green) and a 7500 Real Time PCR System (America). The RT-PCR procedure for MMP-9 was based on a protocol described previously20. β-actin was used as an internal reference. The following primer sets were used:
Gal-3, 5′-GGCCACTGATTGTGCCTTAT-3′ (forward);
5′-TGCAACCTTGAAGTGGTCAG-3′ (reverse);
β-catenin, 5′-GCCGGCTATTGTAGAAGCTG-3′ (forward);
5′-GAGTCCCAAGGAGACCTTCC-3′ (reverse);
MMP-9, 5′-GGGCCGCTCCTACTCTGCCT-3′ (forward);
5′-TCGAGTCAGCTCGGGTCGGG-3′ (reverse); and
β-actin, 5′-CTCCTCCTGAGCGCAAGTACTC-3′ (forward);
5′-TCCTGCTTGCTGATCCACATC-3′ (reverse).
The experiments were performed in triplicate.
Statistical analysis
Statistical analyses were carried out using SPSS software (Ver 16.0, USA). The results are presented as the mean±SD, and statistical significance was determined using an ANOVA and the SNK-q test. A value of <0.05 was considered to be statistically significant.
Results
Silencing Gal-3 reduces migration and invasion of human tongue cancer cells
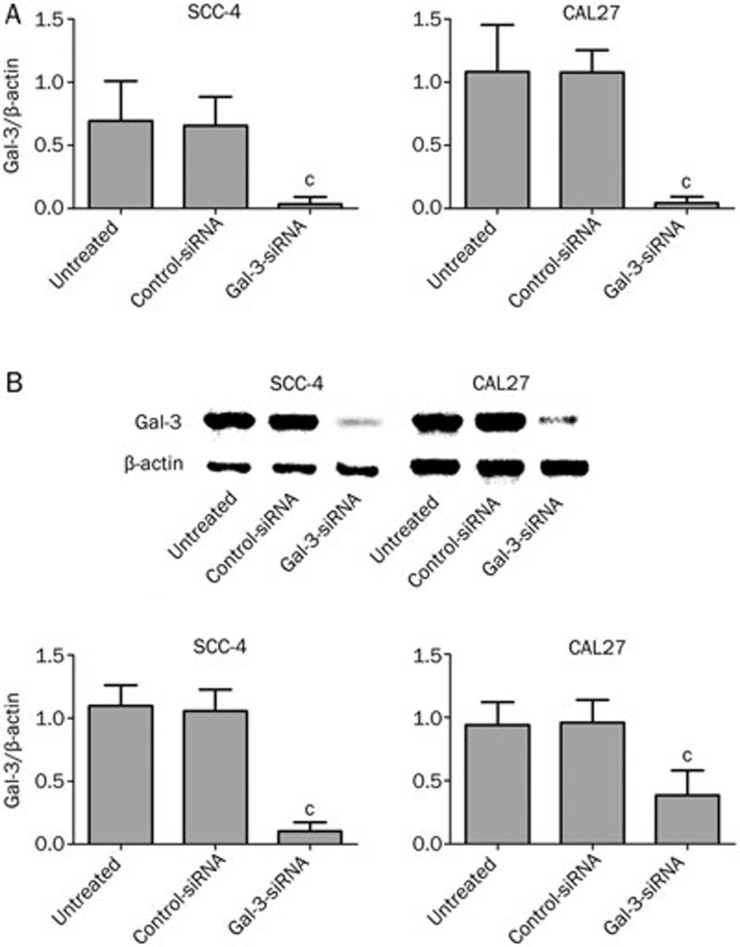
We used siRNAs to silence Gal-3 in SCC-4 and CAL27 cells. Significant inhibition of Gal-3 at the mRNA (PSCC-4=0.021; PCAL27=0.003) and protein (PSCC-4<0.01; PCAL27<0.01) level was detected 48 h following transient gene silencing of Gal-3, whereas there was no significant difference between mock-treated and control-siRNA transfected cells for either of the two cell lines (Figure 1). Gal-3 silencing caused a 99.4% knockdown in SCC-4 cells and a 98.6% knockdown in CAL27 cells.
Figure 1.
Alteration of Gal-3 expression 48 h after transfection. 48 h after transient gene silencing of Gal-3, significant inhibition of Gal-3 mRNA (A. Gal-3 siRNA vs controls, cPSCC-4=0.021; cPCAL27=0.003) as well as protein (B. Gal-3 siRNA vs controls, cPSCC-4<0.01; cPCAL27<0.01) expression was detected in experimental groups.
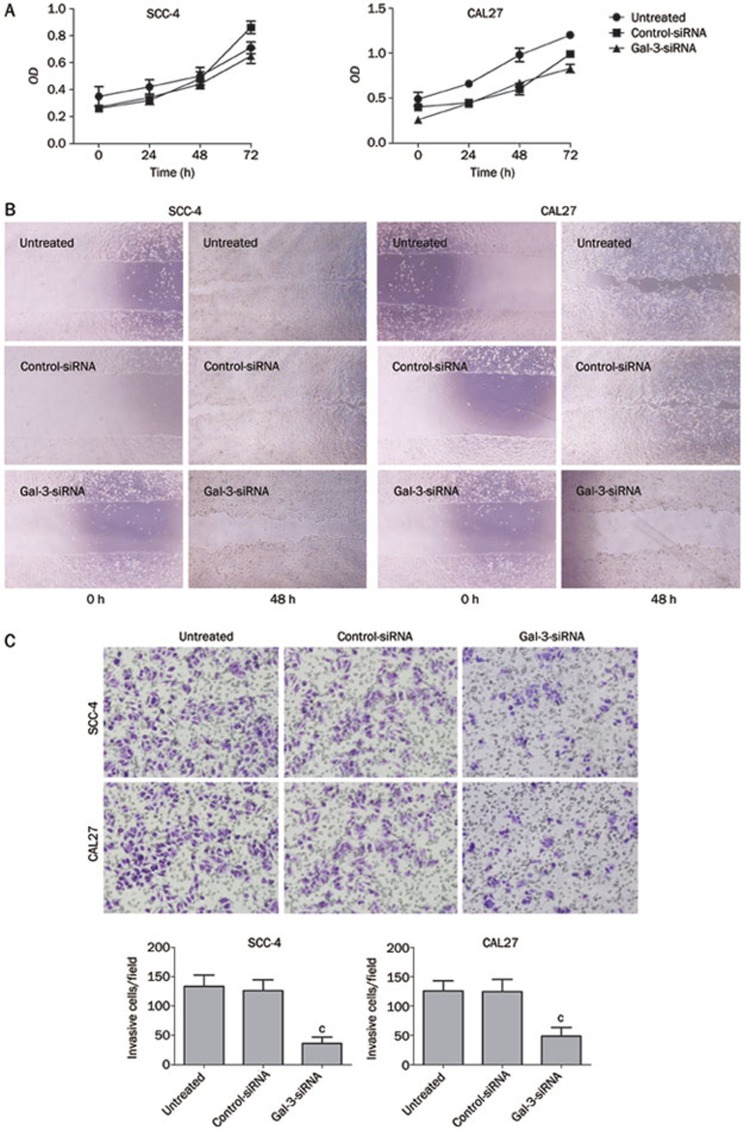
There were no significant differences in proliferation between Gal-3 and control siRNA transfected cells in either of the cell lines (Figure 2A) (PSCC-4=0.509; PCAL27=0.366). However, the migration capacity was reduced in Gal-3 siRNA transfected cells compared to the two control groups (Figure 2B). The time required for space closure by Gal-3-silenced tongue cancer cells was significantly longer than that for control cells (data not shown). Gal-3 siRNA treatment reduced the invasion capacity of both cell lines (PSCC-4<0.01; PCAL27<0.01). The number of migrating cells on the PET membrane was markedly decreased in Gal-3-siRNA treated cells compared with control cells (Figure 2C). These results indicate that Gal-3 modulates tongue cancer cell migration and invasion but not cell proliferation in vitro.
Figure 2.
Effects of Gal-3 silencing on tumor cell biological characteristics. There was no differences in cell proliferation between control and experimental groups in any of the cell lines (A. Gal-3 siRNA vs controls, PSCC-4=0.509; PCAL27=0.366). However, scratch assay demonstrated that cell migration was dramatically decreased in experimental groups after Gal-3-siRNA transfection (B). Cell invasion was decreased in cells treated with Gal-3-siRNA, when compared with the control groups (C. Gal-3 siRNA vs controls, cPSCC-4<0.01; cPCAL27<0.01).
Silencing Gal-3 reduces the level of β-catenin
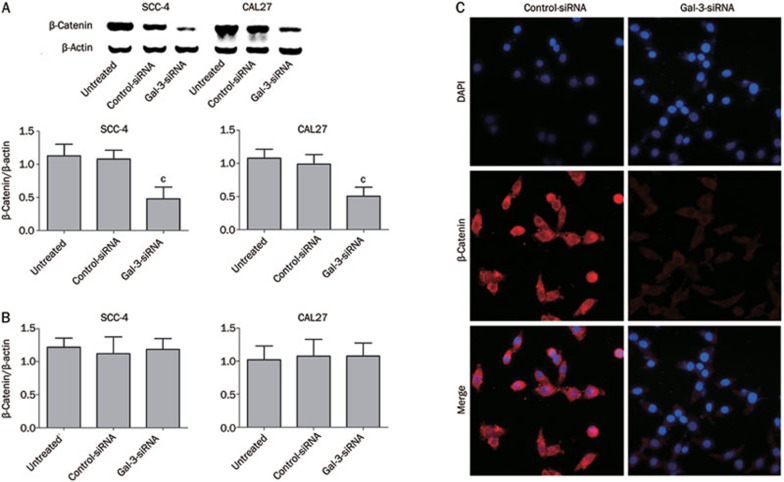
Because Gal-3 binding to β-catenin is involved in the regulation of the Wnt/β-catenin signaling pathway21,22, we analyzed the relationship between Gal-3 and β-catenin. We found that silencing the Gal-3 gene significantly reduced the protein levels of β-catenin in SCC-4 and CAL27 cells (Figure 3A) (PSCC-4<0.01; PCAL27<0.01). Interestingly, no significant change in the mRNA level of β-catenin was observed in either of the cell lines (Figure 3B) (PSCC-4=0.830; PCAL27=0.940). These results suggest that Gal-3 reduces the level of β-catenin without affecting its gene expression. The distribution of β-catenin after Gal-3 silencing in SCC-4 cells was examined by dual-fluorescence confocal microscopy. Reduced production of β-catenin (red fluorescence) was observed in the cytoplasm and in the nucleus (Figure 3C).
Figure 3.
Transfection with Gal-3-siRNA affects β-catenin expression. Inhibition of Gal-3 gene expression affected the protein expression of β-catenin in SCC-4 and CAL27 cells (A. Gal-3 siRNA vs controls, cPSCC-4<0.01; cPCAL27<0.01), while its mRNA expression was unaffected in both cell lines (B. Gal-3 siRNA vs controls, PSCC-4=0.830; PCAL27=0.940). The immunostaining of β-catenin in cytoplasm and nucleus was decreased in Gal-3 silenced cells compared with control group (C).
Silencing Gal-3 inhibits phosphorylation of Akt and GSK-3β
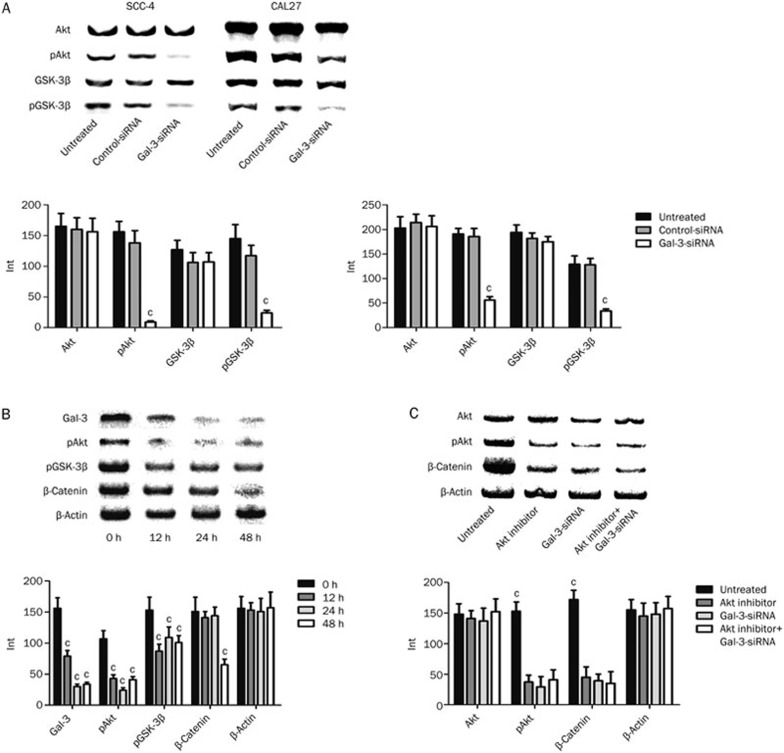
Gal-3 has been identified as a key regulator in the Wnt/β-catenin signaling pathway and influences cell invasion6,9,21,22. To investigate Gal-3-mediated regulation of β-catenin, we evaluated the levels of Akt and GSK-3β in SCC-4 and CAL27 cells treated with Gal-3-siRNA or control-siRNA. While there were no changes in total levels of the two proteins, Gal-3-siRNA treatment significantly decreased the amount of phosphorylated Akt and GSK-3β (Figure 4A). We also assessed the time dependency of phospho-protein levels in SCC-4 cells. Interestingly, we found that the decrease in both pAkt and pGSK-3β occurred 12 h after transfection, while down-regulation of β-catenin expression was detected 48 h after transfection (Figure 4B). These findings indicate that pAkt and pGSK-3β levels correlate with Gal-3 expression in tongue cancer cells and that decreased phosphorylation of Akt and GSK-3β occurs before the down-regulation of β-catenin.
Figure 4.
Regulations of Wnt/β-catenin pathway by Gal-3-siRNA. There were no significant alterations detected of Akt and GSK-3β protein in total protein level, while the expressions of phosphorylated forms of the two kinases were significantly decreased after Gal-3 silencing (A. Gal-3 siRNA vs controls, cPSCC-4<0.01; cPCAL27<0.01). The significant decreasing of both pAkt (cP<0.01) and pGSK-3β (cP<0.01) occurred at 12 h after transfection, while downregulation of β-catenin (cP<0.01) expression was detected at 48 h (B). pAkt and β-catenin expressions were decreased when Akt was inhibited or Gal-3 was silenced, or both happened (cP<0.01). No significant difference of pAkt and β-catenin alterations was detected between the three experimental groups (C).
To confirm the necessity of Akt for Gal-3-mediated Wnt signaling and β-catenin regulation, Akt inhibitor V was used in SCC-4 cells transfected with or without Gal-3-siRNA. Total Akt protein levels were unchanged for all groups. pAkt and β-catenin levels were significantly decreased when Akt was inhibited, when Gal-3 was silenced, or when both Akt and Gal-3 were inhibited; no significant difference in β-catenin levels was detected between the three groups (Figure 4C). These results suggest that Gal-3 RNAi leads to the down-regulation of β-catenin by inhibiting Akt phosphorylation.
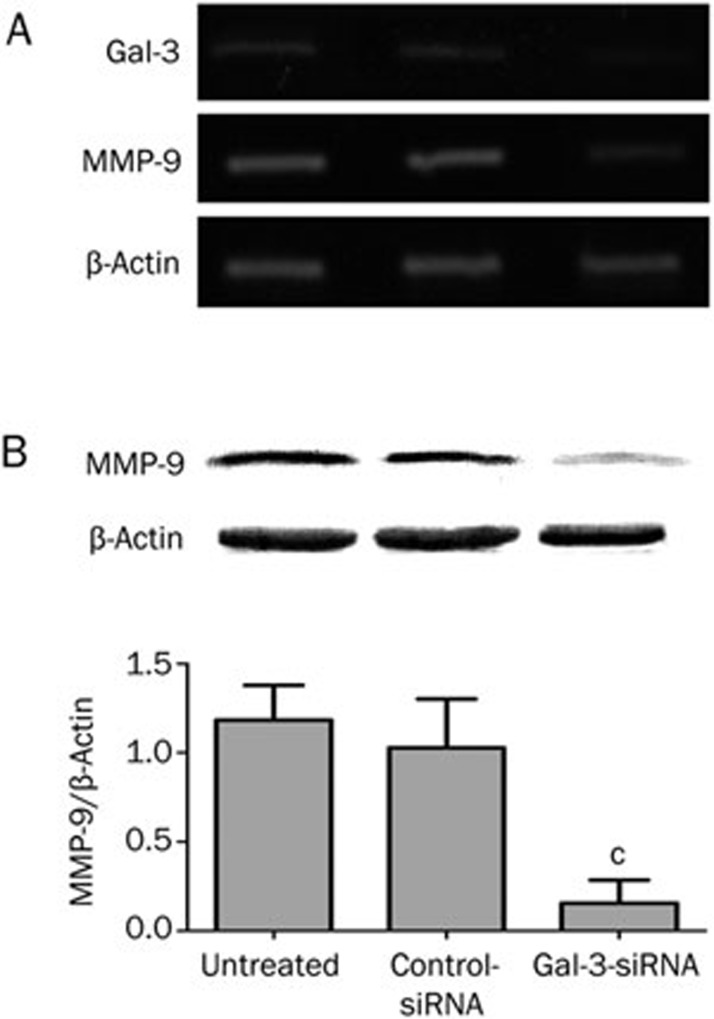
Silencing Gal-3 decreases MMP-9 expression
Matrix metallopeptidase-9 (MMP-9) is involved in the breakdown of the extracellular matrix, which is crucial for cell migration and invasion18. MMP-9 has been suggested to be an important downstream target of the β-catenin signaling pathway in nasopharyngeal carcinoma23. To test if MMP-9 might be a mediator of β-catenin-induced migration and invasion in SCC-4 cells, we analyzed MMP-9 levels by RT-PCR and Western blotting. MMP-9 mRNA and protein levels were significantly lower in Gal-3-siRNA treated cells than in the corresponding controls (Figure 5).
Figure 5.
MMP-9 mRNA expression was reduced by transfection after 48 h (A). Meanwhile, MMP-9 protein expression was significantly decreased after Gal-3 knockdown in SCC-4 cells as well (B. Gal-3 siRNA vs controls, 86.9% reduced, cP<0.01).
Discussion
In this study, we show that Gal-3 inhibition reduces the migration and invasion capacities of the tongue cancer cell lines SCC-4 and CAL27. Gal-3 is involved in the malignancy of tumor cells, including cancers of the head and neck region24. During the progression from normal cells to cancerous cells, it has been shown that Gal-3 expression markedly decreased in the nucleus and increased in the cytoplasm in tongue cancer11. A close correlation between Gal-3 expression and malignant metastasis/histological grade in tongue cancer has been demonstrated12, as well as a close correlation between Gal-3 and β-catenin in tumors9,25. These data suggest that β-catenin plays a key role in the invasion of several types of cancer3,4,5,6,7,8,9,10,13,14,15,16,17. In the SCC-4 and CAL27 cells, β-catenin was significantly inhibited at the protein level, but it was unaffected at the mRNA level. This result is consistent with previous findings in pancreatic cancer cells9. Because Gal-3 is a binding partner of β-catenin25, the reduction in β-catenin might be due to its increased degradation when Gal-3 is not bound. This result supports the finding that translocation of β-catenin from the cytoplasm into the nucleus leads to the transcription of Wnt target genes and tumor progression26,27,28. This finding further suggests that the β-catenin transcribed after Gal-3 knockdown might be degraded in the cytoplasm by its regulators, thereby causing its decreased nuclear accumulation. Gal-3 knockdown might affect molecules that are upstream regulators for β-catenin.
However, we observed diminished levels of pAkt and pGSK-3β prior to the decrease of β-catenin following Gal-3-siRNA treatment of SCC-4 cells. Investigations of other types of cancers have shown that Gal-3 stimulates Akt phosphorylation and that pAkt phosphorylates GSK-3β (a binding partner of β-catenin) at Ser-9 and inactivates this protein29,30. We hypothesize that the reduced phosphorylation of GSK-3β, mediated by the inhibition of Akt signaling, contributes to enhanced β-catenin degradation. This observation is in accordance with studies of pancreatic and colon cancer cells9,28. Inhibition of Akt activity by the down-regulation of pAkt can decrease pGSK-3β levels, while activation of Akt might phosphorylate GSK-3β31. Meanwhile, using an Akt inhibitor leads to β-catenin repression, which is similar to observations made using Gal-3-siRNA. Gal-3-siRNA cannot suppress β-catenin levels further after treatment with an Akt inhibitor, indicating that Akt plays an important role in Gal-3/β-catenin function. Our data suggest that β-catenin might be a downstream effector for Gal-3 in tongue cancer cells and that Gal-3 silencing and the inhibition of Akt and GSK-3β phosphorylation leads to β-catenin degradation; this observation is consistent with findings in pancreatic and colon cancer cells9,28.
MMP-9, a β-catenin target gene in the Wnt signaling pathway, is important for cell migration18,32. We demonstrated that MMP-9 mRNA levels and protein levels decreased with Gal-3 silencing, indicating that Gal-3 RNAi inhibits tongue cancer cell invasion and migration through the Wnt/β-catenin signaling pathway.
The PI3K/AKT pathway is a key signaling pathway involved in the regulation of cancer cell activity. pAkt may be involved in invasion by head and neck squamous cell carcinoma33,34. Gal-3-mediated cancer cell invasion occurs not only through Akt but also through other effectors that are regulated by Gal-3 and the Wnt/β-catenin signaling pathway. The precise interaction between Gal-3, PI3K/AKT, and Wnt/β-catenin pathway is unclear and will be further investigated.
Gal-3 regulates cell motility. One explanation for the role of Gal-3 in cell motility is that the expression of specific binding sites for sialylated Lewis antigens at the surface of tumor cells is altered; the presence of these sites can arrest tumor cell transmigration into the extravascular space and suppress cell migration35. Gal-3 decreases the motility potential on laminin, modifies the cytoskeleton reorganization and downregulates expression of integrins-α6/β1 (cell adhesion molecules)36. However, a controversial finding reported that the absence of Gal-3 does not interfere with the pattern of β-catenin expression and the mediation of the Wnt signaling pathway37. This conclusion is based on the finding that Gal-3 knockout mice tumors were positive for β-catenin. This observation suggests that β-catenin activity is crucial for the transformation and/or maintenance of squamous cell carcinoma of the tongue and that in the absence of Gal-3, tumor cells gain β-catenin activity by a compensatory mechanism. However, both membranous and non-membranous β-catenin staining were lower in the tumors of Gal-3 knockout mice than in wild-type mice. Furthermore, the high positivity index for non-membranous β-catenin in dysplasias and carcinomas from Gal3−/− mice might not reflect activated Wnt signaling. This finding might be due to different cancer origins and/or different biological functions of Gal-3, which may be cell- and tissue-dependent38. Interestingly, no significant difference was observed in the development and metastasis of lung tumors between Gal-3−/− and wild-type mice39. This finding might be due to other alternative molecule(s) that regulate carcinogenesis. Further in vitro and in vivo experiments are needed to clarify these contradictions.
While Gal-3 may promote cell proliferation40,41, we found that Gal-3 silencing had no effect on the proliferation of SCC-4 and CAL27; this result is consistent with findings from studies of pancreatic cancer9. We speculate that there are unknown regulators mediating cell proliferation in the Gal-3/β-catenin pathway. Such regulators may enhance cell proliferation compared to β-catenin or counteract the inhibition of cell proliferation with β-catenin silencing.
In conclusion, Gal-3 manipulates the level of β-catenin and Wnt signaling in tongue cancer. Gal-3 mediates cell migration and invasion by activating Akt, which regulates GSK-3β phosphorylation and β-catenin degradation. Understanding the underlying mechanisms may provide novel strategies for tongue cancer treatments. RNA interference of Gal-3 expression might be an effective anti-tongue cancer strategy.
Author contribution
Feng-cai WEI and Ying-wei HU designed the research; Dong ZHANG, Zheng-gang CHEN, Shao-hua LIU, and Zuo-qing DONG performed the research; Martin DALIN analyzed the data; Dong ZHANG and Martin DALIN wrote the paper; Shi-san BAO revised the paper.
Acknowledgments
This study was supported by grants from the Natural Science Foundation of Shandong (ZR2010HQ064), the Independent Innovation Foundation of Shandong University (IIFSDU, 2010TS094 and 2010TS011), and the Jinan Science and Technology Bureau, China (201004054).
References
- Ferrari D, Codeca C, Fiore J, Moneqhini L, Bosari S, Foa P. Biomolecular markers in cancer of the tongue. J Oncol. 2009;2009:412908. doi: 10.1155/2009/412908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano D, Myers JN. Metastasis of squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev. 2007;26:645–62. doi: 10.1007/s10555-007-9082-y. [DOI] [PubMed] [Google Scholar]
- Califice S, Castronovo V, Van Den Brule F. Galectin-3 and cancer. Int J Oncol. 2004;25:983–92. [PubMed] [Google Scholar]
- Nangia-Makker P, Nakahara S, Hogan V, Raz A. Galectin-3 in apoptosis, a novel therapeutic target. J Bioenerg Biomembr. 2007;39:79–84. doi: 10.1007/s10863-006-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Shimura T, Yajima T, Kubo N, Araki K, Wada W, et al. Transient silencing of galectin-3 expression promotes both in vitro and in vivo drug-induced apoptosis of human pancreatic carcinoma cell. Clin Exp Metastasis. 2011;28:367–76. doi: 10.1007/s10585-011-9376-x. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Choi IJ, Cheong TC, Lee SJ, Lotan R, Park SH, et al. Galectin-3 increase gastric cancer cell motility by up-regulating fascin-1 expression. Gastroenterology. 2010;138:1035–45. doi: 10.1053/j.gastro.2009.09.061. [DOI] [PubMed] [Google Scholar]
- Wang Y, Nangia-Makker P, Tait L, Balan V, Hogan V, Pienta KJ, et al. Regulation of prostate cancer progression by galectin-3. Am J Pathol. 2009;174:1515–23. doi: 10.2353/ajpath.2009.080816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi K, Shimura T, Masuda N, Ide M, Tsutsumi S, Yamaguchi S, et al. Galectin-3 expression in colorectal cancer: relation to invasion and metastasis Anticancer Res 200727(4B): 2289–96. [PubMed] [Google Scholar]
- Kobayashi T, Shimura T, Yajima T, Kubo N, Araki K, Tsutsumi S, et al. Transient gene silencing of galectin-3 suppresses pancreatic cancer cell migration and invasion through degradation of beta-catenin. Int J Cancer. 2011;129:2775–86. doi: 10.1002/ijc.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayoral MA, Mayoral C, Meneses A, Villalvazo L, Guzman A, Espinosa B, et al. Identification of galectin-3 and mucin-type O-glycans in breast cancer and its metastasis to brain. Cancer Invest. 2008;26:615–23. doi: 10.1080/07357900701837051. [DOI] [PubMed] [Google Scholar]
- Honjo Y, Inohara H, Akahani S, Yoshii T, Takenaka Y, Yoshida J, et al. Expression of cytoplasmic galectin-3 as a prognostic marker in tongue carcinoma. Clin Cancer Res. 2000;6:4635–40. [PubMed] [Google Scholar]
- Alves PM, Godoy GP, Gomes DQ, Medeiros AM, de Souza LB, da Silveira EJ, et al. Significance of galectins-1, -3, -4, and -7 in the progression of squamous cell carcinoma of the tongue. Pathol Res Pract. 2011;207:236–40. doi: 10.1016/j.prp.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Amin N, Vincan E. The Wnt signaling pathways and cell adhesion. Front Biosci. 2012;17:784–804. doi: 10.2741/3957. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma Q. Beta-catenin is a promising key factor in the SDF-1/CXCR4 axis on metastasis of pancreatic cancer. Med Hypotheses. 2007;69:816–20. doi: 10.1016/j.mehy.2007.01.069. [DOI] [PubMed] [Google Scholar]
- Bian S, Bai JP, Chapin H, Le Moellic C, Dong H, Caplan M, et al. Interactions between β-catenin and the HSIo potassium channel regulates HSIo surface expression. PLoS One. 2011;6:e28264. doi: 10.1371/journal.pone.0028264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein U, Arlt F, Smith J, Sack U, Herrmann P, Walther W, et al. Intervening in beta-catenin signaling by sulindac inhibits S100A4-dependent colon cancer metastasis. Neoplasia. 2011;13:131–44. doi: 10.1593/neo.101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai S, Yonekawa A, Harada C, Hamada M, Katagiri W, Nakazawa M, et al. Involvement of the Wnt-beta-catenin pathway in invasion and migration of oral squamous carcinoma cells. Int J Oncol. 2010;37:1095–103. doi: 10.3892/ijo_00000761. [DOI] [PubMed] [Google Scholar]
- Wu B, Hu K, Li S, Zhu J, Gu L, Shen H, et al. Dihydroartiminisin inhibits the growth and metastasis of epithelial ovarian cancer. Oncol Rep. 2012;27:101–8. doi: 10.3892/or.2011.1505. [DOI] [PubMed] [Google Scholar]
- Wu B, Li S, Sheng L, Zhu J, Gu L, Shen H, et al. Metformin inhibits the development and metastasis of ovarian cancer. Oncol Rep. 2012;28:903–8. doi: 10.3892/or.2012.1890. [DOI] [PubMed] [Google Scholar]
- Shin HS, Oh HY. The effect of platelet-rich plasma on wounds of OLETF rats using expression of matrix metalloproteinase-2 and -9 mRNA. Arch Plast Surg. 2012;39:106–12. doi: 10.5999/aps.2012.39.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Shin JY, Cheong TC, Choi IJ, Lee YS, Park SH, et al. Galectin-3 gemline variant at position 191 enhances nuclear accumulation and activation of β-catenin in gastric cancer. Clin Exp Metastasis. 2011;28:743–50. doi: 10.1007/s10585-011-9406-8. [DOI] [PubMed] [Google Scholar]
- Shi Y, He B, Kuchenbecker KM, You L, Xu Z, Mikami I, et al. Inhibition of Wnt-1 and galectin-3 synergistically destabilizes beta-catenin and induces apoptosis in human colorectal cancer cells. Int J Cancer. 2007;121:1175–81. doi: 10.1002/ijc.22848. [DOI] [PubMed] [Google Scholar]
- Song Y, Yang QX, Zhang F, Meng F, Li H, Dong Y, et al. Suppression of nasopharyngeal carcinoma cell by targeting β-catenin signaling pathway. Cancer Epidemiol. 2012;36:e116–21. doi: 10.1016/j.canep.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Saussez S, Decaestecker C, Mahillon V, Cludts S, Capouillez A, Chevalier D, et al. Galectin-3 upregulation during tumor progression in head and neck cancer. Laryngoscope. 2008;118:1583–90. doi: 10.1097/MLG.0b013e31817b0718. [DOI] [PubMed] [Google Scholar]
- Shimura T, Takenaka Y, Fukumori T, Tsutsumi S, Okada K, Hogan V, et al. Implication of galectin-3 in Wnt signaling. Cancer Res. 2005;65:3535–7. doi: 10.1158/0008-5472.CAN-05-0104. [DOI] [PubMed] [Google Scholar]
- Reya T, Clevers H. Wnt signaling in stem cells and cancer. Nature. 2005;434:843–50. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- Tenbaum SP, Ordonez-Moran P, Puig I, Chicote I, Arques O, Landolfi S, et al. β-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer Nat Med 2012. doi: 10.1038/nm.2772 [DOI] [PubMed]
- Song S, Mazurek N, Liu C, Sun Y, Ding QQ, Liu K, et al. Galectin-3 mediates nuclear beta-catenin accumulation and Wnt signaling in human colon cancer cells by regulation of glycogen synthase kinase-3beta activity. Cancer Res. 2009;69:1343–9. doi: 10.1158/0008-5472.CAN-08-4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek N, Sun YJ, Liu KF, Gilcrease MZ, Schober W, Nangia-Makker P, et al. Phosphorylated galectin-3 mediates tumor necrosis factor-related apoptosis-inducing ligand signaling by regulating phosphatase and tensin homologue deleted on chromosome 10 in human breast carcinoma cells. J Biol Chem. 2007;282:21337–48. doi: 10.1074/jbc.M608810200. [DOI] [PubMed] [Google Scholar]
- Xia YX, Lu L, Wu ZS, Pu LY, Sun BC, Wang XH. Inhibition of GSK-3beta ameliorates hepatic ischemia-reperfusion injury through GSK-3beta/beta-catenin signaling pathway in mice. Hepatobiliary Pancreat Dis Int. 2012;11:278–84. doi: 10.1016/s1499-3872(12)60161-1. [DOI] [PubMed] [Google Scholar]
- Maseki S, Ijichi K, Tanaka H, Fujii M, Hasegawa Y, Ogawa T, et al. Acquisition of EMT phenotype in the gefitinib-resistant cells of a head and neck squamous cell carcinoma cell line through Akt/GSK–3β/snail signaling pathway. Br J Cancer. 2012;106:1196–204. doi: 10.1038/bjc.2012.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KW, Tsai ML, Chen JC, Hsu SC, Hsia TC, Lin MW, et al. Gypenosides inhibited invasion and migration of human tongue cancer SCC4 cells through down-regulation of NF-κB and matrix metalloproteinase–9. Anticancer Res. 2008;28:1093–9. [PubMed] [Google Scholar]
- Giudice FS, Dal Vechio AM, Abrahao AC, Sperandio FF, Pinto-Junior Ddos S. Different expression patterns of pAkt, NF-κB and cyclin D1 proterins during the invasion process of head and neck squamous cell carcinoma: an in vitro approach. J Oral Pathol Med. 2011;40:405–11. doi: 10.1111/j.1600-0714.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- Hu K, Li SL, Gan YH, Wang CY, Yu GY. Epiregulin promotes migration and invasion of salivary adenoid cystic carcinoma cell line SACC–83 through activation of ERK and Akt. Oral Oncol. 2009;45:156–63. doi: 10.1016/j.oraloncology.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Hittelet A, Camby I, Nagy N, Leqendre H, Bronckart Y, Decaestecker C, et al. Binding sites for Lewis antigens are expressed by human colon cancer cells and negatively affect their migration. Lab Invest. 2003;83:777–87. doi: 10.1097/01.lab.0000073129.62433.39. [DOI] [PubMed] [Google Scholar]
- Debray C, Vereecken P, Belot N, Teillard P, Brion JP, Pandolfo M, et al. Multifaceted role of galectin-3 on human glioblastoma cell motility. Biochem Biophys Res Commun. 2004;325:1393–8. doi: 10.1016/j.bbrc.2004.10.181. [DOI] [PubMed] [Google Scholar]
- Sant'ana JM, Chammas R, Liu FT, Nonogaki S, Cardoso SV, Loyola AM, et al. Activation of the Wnt/Beta-catenin signaling pathway during oral carcinogenesis process is not influenced by the absence of galectin-3 in mice. Anticancer Res. 2011;31:2805–11. [PubMed] [Google Scholar]
- Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006;1760:616–35. doi: 10.1016/j.bbagen.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Eude-Le Parco I, Gendronneau G, Dang T, Delacour D, Thijssen VL, Edelmann W, et al. Genetic assessment of the importance of galectin-3 in cancer initiation, progression, and dessemination in mice. Glycobiology. 2009;19:68–75. doi: 10.1093/glycob/cwn105. [DOI] [PubMed] [Google Scholar]
- Jiang HB, Xu M, Wang XP. Panceratic stellate cells promote proliferation and invasiveness of human pancreatic cancer cells via galectin-3. Word J Gastroenterol. 2008;14:2023–8. doi: 10.3748/wjg.14.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Oh JS, Shin JY, Lee KD, Sung KW, Nam SJ, et al. Development of micro RNA-145 for therapeutic application in breast cancer. J Control Release. 2011;155:427–34. doi: 10.1016/j.jconrel.2011.06.026. [DOI] [PubMed] [Google Scholar]