Abstract
ATP-sensitive potassium (KATP) channels are weak, inward rectifiers that couple metabolic status to cell membrane electrical activity, thus modulating many cellular functions. An increase in the ADP/ATP ratio opens KATP channels, leading to membrane hyperpolarization. KATP channels are ubiquitously expressed in neurons located in different regions of the brain, including the hippocampus and cortex. Brief hypoxia triggers membrane hyperpolarization in these central neurons. In vivo animal studies confirmed that knocking out the Kir6.2 subunit of the KATP channels increases ischemic infarction, and overexpression of the Kir6.2 subunit reduces neuronal injury from ischemic insults. These findings provide the basis for a practical strategy whereby activation of endogenous KATP channels reduces cellular damage resulting from cerebral ischemic stroke. KATP channel modulators may prove to be clinically useful as part of a combination therapy for stroke management in the future.
Keywords: ATP-sensitive potassium channel (KATP), Kir6.2, SUR subunit, stroke, nociception, neuropathic pain, neuroprotection
Introduction
Potassium (K+) channels are the most ubiquitously distributed ion channels and are found virtually in all types of cells1,2. Because K+ ions have a negative equilibrium potential across the cell membrane, the opening of K+ channels stabilizes the membrane potential by hyperpolarizing the cell closer to the K+ equilibrium potential (EK). Thus, K+ channels play a major role in setting the resting membrane potential and the duration of action potentials. These channels also play a role in repetitive firing frequency, thereby suppressing the excitability of a cell1,2. Selective K+ permeability was originally described in nerve cells3,4. To date, more than 80 genes for K+ channel subunits have been identified in mammalian cells, and multiple K+ channel subtypes are expressed in a single cell to control its K+ permeability1,2. Inwardly rectifying K+ (Kir) channels conduct the inward rectifier current, thus hyperpolarizing the membrane potential5.
Adenosine triphosphate (ATP)-sensitive K+ (KATP) channels are members of the Kir superfamily and were originally described in the heart6. These channels were later identified in many other tissues, including the skeletal muscle7, smooth muscle8, brain9,10,11,12, pituitary gland13,14, kidney15, and in pancreatic beta cells16,17,18,19. KATP channels conduct inward-rectifier potassium currents that are inhibited by intracellular ATP and couple the cellular metabolic status to the electrical activity of the cell membrane6,16,20,21. An increase in the ATP/ADP ratio closes KATP channels (leading to depolarization), whereas a decrease in the ATP/ADP ratio opens KATP channels (leading to hyperpolarization). Thus, KATP channels modulate many of the cellular events and functions under physiological and pathophysiological conditions. Recently, it has been proposed that KATP channels are one of the non-glutamate mechanisms for stroke22,23. Broad reviews of KATP channels in the heart21,24 and pancreatic cells20,21,25 have been presented elsewhere. The specific role of KATP channels in the neurovascular unit in stroke has also been recently reviewed26. This review focuses on neuronal KATP channels and the neuroprotective role of KATP channels in cerebral ischemia.
General description of KATP channels
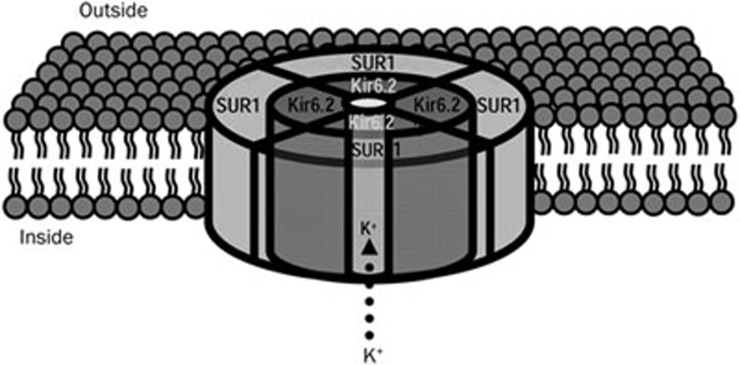
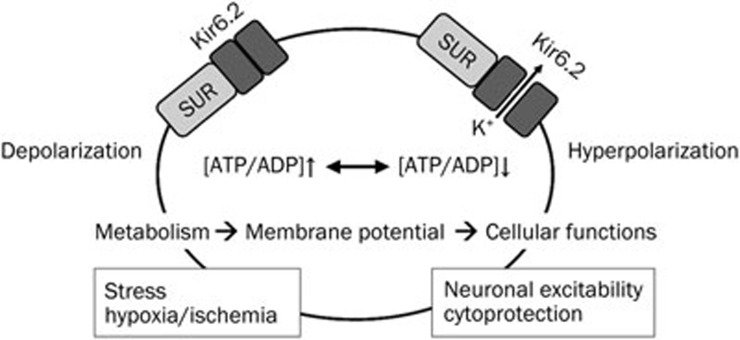
KATP channels are heteromultimers of Kir6 and sulfonylurea receptor (SUR) subunits27 (Figure 1). They are activated by energy depletion and conduct a weak inwardly rectifying K+ current, thus playing important roles in the regulation of cellular function by linking cellular metabolism to the electrical activity of cell membranes20,27,28,29 (Figure 2).
Figure 1.
A schematic illustration showing the proposed structure of a KATP channel in neurons. The KATP channel is a hetero-octamer comprising two subunits: four pore-forming Kir6.2 subunits and four regulatory sulfonylurea receptor (SUR) subunits.
Figure 2.
Schematic illustration of KATP channels function in neurons. KATP channels serve as metabolic sensors to couple electrical activity of neurons. Under normal physiological conditions, KATP channels remain closed due to the high ATP/ADP ratio. Reducing the ATP/ADP ratio by either decreasing ATP levels or enhancing ADP levels opens the channel and allows K+ ions to exit the cells, thus hyperpolarizing the neurons. SUR, sulfonylurea receptor.
KATP channel genes
The Kir6 subfamily is a member of the weak inward rectifier family and has two members, Kir6.1 and Kir6.2. The Kir6.2 gene (KCNJ11) in humans is located on chromosome 11. Kir6.2 was cloned from a human genomic library and shares 96% amino acid identity with mouse and rat Kir6.230. Kir6.1 genes (KCNJ8) are mainly expressed in the mitochondria12,31,32. Two SUR subunits, SUR1 and SUR2, have been identified as the regulatory subunits of KATP channels33. The SUR1 subunit is encoded by the ABCC8 gene, which is located on chromosome 11p15.1 and is mainly found in pancreatic beta cells and neurons33,34,35. The SUR2 subunit is encoded by the ABCC9 gene, which is located on chromosome 12p12.1 and is expressed mainly in the heart and skeletal muscles, as well as in some neurons5,36. SUR2 has two alternative splice variants, SUR2A and SUR2B, which have major differences in the last 42 C-terminal amino acid residues (C42)37. The C42 of SUR2B shows a higher homology to the C42 of SUR137.
Structure of functional KATP channels
KATP channels are heteromultimers of the Kir6 and SUR subunits. These subunits are in a 1:1 ratio of stoichiometry. The Kir6 subunit is the pore-forming subunit of KATP channels30,38. Kir6.2 mediates the inhibitory effect of ATP on the KATP channels. The SUR subunits are members of the ATP-binding cassette (ABC) protein superfamily39. The SUR subunits are sensitive to the adenine nucleotides, Mg-ADP20,27,29,40. Reducing the intracellular concentration of ATP or the ATP/ADP ratio gates the KATP channel.
A functional KATP channel is assembled by four Kir6.x and four SUR subunits, forming a hetero-octameric complex (SUR/Kir)4. The functional diversity of the KATP channels results from the assembly of different subtypes of the Kir6.x and SUR subunits. Kir6.1 genes are mainly expressed in the mitochondria. Kir6.1-based channels have a smaller unitary conductance than Kir6.2 subtype channels27. Kir6.2 subunits are found in plasmalemmal membranes (cell surface membranes)[12, 20, 27, 29, 32]. Most functional KATP channels contain the Kir6.2 subunit; thus, the heterogeneity observed between different KATP channels mainly arises from the differential expression of the SUR regulatory subunits. SUR1- and SUR2-based channels are differentiated by their selective sensitivity to sulfonylureas drugs (such as glibenclamide), whereas SUR2A- and SUR2B-based channels are differentiated by their selective affinity to diazoxide27.
Gating properties
KATP channels exhibit fast and long interburst closing kinetics41,42,43. The fast gating kinetics of the channels is determined by an intrinsic structure within the pore-loop that is near the selectivity filter of the Kir6.2 subunit41,44. The long last interburst closing kinetics requires ATP binding to the TM2 helices42,45. The conformational modeling describes one open and multiple closed states of the channel36,43. A current conducting channel requires all four Kir6.2 subunits in the open conformation, and ATP binding to any one of the four subunits will shift the channel to a closed state. SUR subunits are considered the gatekeepers of the ATP-inhibited Kir6.2 pores because they couple their N-terminal bundle of five transmembrane helices (TMD0-L0) to the outer helix and N-terminus of Kir6.2. This coupling bidirectionally modulates channel gating34,46.
Nucleotide sensitivities of KATP channels
KATP channels have distinctive sensitivities to the nucleotides ATP and ADP27. ATP binds to the cytoplasmic domain of the Kir6.2 subunit44,47,48 to inhibit the channel49. Each Kir6.2 subunit provides one ATP binding pocket, which is constituted by residues R50, I182, K185, R201, and G3345,36. The interference of ATP binding releases channel inhibition and results in channel activation5,36. Mg-ADP is the endogenous activator of KATP channels via the SUR regulatory subunits5. Each SUR subunit has two nucleotide binding domains (NBD1 and NBD2) and 17 putative transmembrane domains, including an N-terminal hydrophobic region (TMD0) containing five TM helices and two repeats of six TM helices (TMD1 and TMD2). The NBDs are found with the Walker A and Walker B motifs, which are located at the large cytosolic loops following the TMD1 and TMD2 repeats. Dimerization of the two NBDs generates the catalytic sites for ATP hydrolysis and thus is essential for transducing the effect of ADP to Kir6.250,51,52. Mutations that disrupt NBD dimerization reduce the ADP-mediated activation of KATP channels49. These mutations include G1479R40,53 in the NBD2 of SUR1 and V187D54 in the TMD0 of SUR1. Under normal physiological ATP levels, the probability that there will be open KATP channels is less than 0.1% if SUR regulatory subunits are absent5,36.
Pharmacology
SUR subunits are major pharmacological targets. KATP channels have distinctive pharmacology27. Sulfonylureas are a type of potassium channel blocker that works by binding to SUR subunits27,55. The most common sulfonylureas include glibenclamide, acetohexamide, tolbutamide, glipizide, and glimepiride. A single serine residue (S1237) located at the C-terminus of the TMs of the SUR1 subunit is critical for the high affinity binding (Ki=2 μmol/L) of tolbutamide and glibenclamide to Kir6.2/SUR1 KATP channels56. A bivalent structure in the glibenclamide binding site requires the cytoplasmic loop 3 (between TM5 and TM6) and cytoplasmic loop 8 (between TM15-TM16) regions of the SUR1 subunit57. The low affinity binding site (Ki=1.8 mmol/L) for tolbutamide is located on Kir6.258.
KATP channels can be activated by a group of drugs called potassium channel openers. These drugs include diazoxide, cromakalim, pinacidil, nicorandil and minoxidil sulfate. Diazoxide binds to SUR1 subunits and enhances K+ efflux through the Kir6.2/SUR1 channels in pancreatic beta-cells, resulting in cell membrane hyperpolarization59. In contrast, the drugs pinacidil, nicorandil and cromakalim have a high sensitivity to Kir6.2/SUR1 channels, thus leading to stronger effects on the Kir6.2/SUR2A subunits of cardiac KATP channels24,60. The binding site for cromakalim, pinacidil and nicorandil is located within the TM2 domain of SUR2. The nucleotides L1249 and T1253 in SUR2A and T1286 and M1290 in SUR2B are necessary and sufficient for the channel opener effects61,62,63. KATP channels in smooth muscle respond to all of these drugs. Recently, several new KATP channel openers, such as iptakalim, have been developed. Iptakalim showed cytoprotective effects64 via activating the SUR subunits65. However, it is unknown whether iptakalim acts by regulating Kir6.2-KATP and/or mitoKATP channels66.
Cellular regulation of KATP channels
KATP channel activity is regulated by phosphatidylinositol 4,5-biphosphate (PIP2) via interaction with the cytoplasmic domain that is close to the ATP binding site of Kir6.2 (including residues R54, R176, R177, and R206)28,67,68. PIP2 decreases the ATP sensitivity of the channels by preventing the channel from closing, thus stabilizing the open state. KATP channel activity is also regulated by protein kinase A (PKA) in smooth muscle cells69,70 and cytoskeletal actin in the cardiac atrium71. KATP channel activity is suppressed by a SNARE protein, syntaxin 1A, via protein-protein interactions with the SUR subunits72,73,74,75. Syntaxin 1A decreases the activity76 and the membrane expression level of KATP channels77. Syntaxin 1A binding to the SUR1 subunit also attenuates the effect of K+ channel openers, such as diazoxide, NNC55-0462, P1075, and cromakalim78,79.
KATP channels in the central nervous system
KATP channels are extensively expressed in various regions of the mammalian brain9,11,80, including the substantia nigra (SNr)81,82, neocortex83, hippocampus84, and hypothalamus85,86. They have been detected in many cell types, such as glial cells84,87 and neurons in the hippocampus88, dorsal vagus89, hypothalamus85,86,90, and SNr91. Single cell RT-PCR analysis showed that Kir6.2 mRNA is predominantly expressed in interneurons and pyramidal, granule and neuroglial cells of the hippocampus in the brains from young rats aged 10–13 d84. In the adult brain, Kir6.2 subunits have been found in hippocampal87,92, cortical93, and hypothalamic neurons94, as well as in the SNr pars reticulata95. Immunohistochemical studies showed that Kir6.2 subunits are mainly located in the somata and dendrites of the central neurons92,93,96. Mitochondrial KATP channels are also found in the rat brain, and the expression level of Kir6.1 (per milligram of mitochondrial protein) is six to seven times higher than that in the heart and liver12. Radioligand binding studies showed that regional expression of KATP channels in the brain showed different affinities to sulfonylureas97.
Function of neuronal KATP channels
KATP channels in the hypothalamus play a critical role in glucose homeostasis by regulating the secretion of counter-regulatory hormones, including glucagon and catecholamines, via the autonomic nervous system98. However, the primary role of KATP channels in many other central neurons is not glucosensing84,90,99. For instance, basal activity of KATP channels can affect neuronal excitability in non-glucosensing neurons100,101. In the dentate granule neurons in the mouse hippocampus, KATP channels are expressed with a high density84,99. The single channel conductance of KATP channels is suppressed by strophanthidin, which is a blocker of the Na+–K+ ATPase. Moderate action potential firing can evoke KATP channel opening via Na influx and ATP depletion. The ketone body R-beta-hydroxybutyrate can enhance neuronal electrical activity by opening KATP channels102. Similarly, single channel recordings in brainstem inspiratory neurons show that bursts of single KATP channel openings are in synchrony with the respiratory firing rhythm. The probability of open channels (Popen) is increased by approximately 60% after a strong burst of action potentials103. Blocking the Na+–K+ ATPase reduces the increased Popen of KATP channels. Thus, ATP consumption in response to Na+ influx from action potentials regulates the opening of KATP channels under physiological conditions.
KATP channels in dorsal root ganglia (DRG) neurons may involve nociception and neuropathic pain
KATP channels are also expressed in DRG neurons104,105. The subunits Kir6.2, SUR1 and SUR2, but not Kir6.1, are identified in the DRG neurons using immunohistochemistry and electrophysiology104,105. The neuronal injury in axotomy decreases the KATP channel current in the primary afferent neurons in the DRG, which is mediated by CaMKII106. The suppressed KATP channels in axotomized DRG neurons are still responsive to the KATP channel blocker glibenclamide and opener diazoxide. In addition, KATP channels are activated by CaMKII activators. The neuroprotective role of these CaMKII-dependent KATP channels may inhibit excitotoxic cell injury. KATP-mediated neuroprotection may be suppressed in axotomy. Thus, opening of the DRG neuronal KATP channels decreases neuronal excitability, inhibits neurotransmission and possibly suppresses hyperalgesia. The KATP channels in the DRG neurons are potential therapeutic targets for antihyperalgesia in neuropathic pain.
KATP channels in the forebrain involving neuroprotection against seizure
Overexpression of SUR1 in the forebrain, including the cortex, hippocampus and striatum, results in resistance to kainic acid-induced seizures107. Mice that overexpress SUR1 exhibit normal brain anatomy and morphology, as well as normal locomotor and cognitive behaviors. The regional transgenic overexpression of the SUR1 subunit of KATP channels in the forebrain protects mice against kainic acid-induced seizure and hippocampal neuronal cell death. This observation indicates that the neuronal KATP channels are important mediators of neuroprotection in the brain and may have potential applications in protecting neurons against hyperexcitability and excitotoxicity during seizure and epileptic insults.
KATP channels in the substantia nigra region involving neuroprotection against hypoxia-induced seizures
The SNr plays a crucial role in the propagation of seizures108, which can be evoked by insults such as hypoxia and hypoglycemia. The KATP channels expressed in the SNr region are composed of the Kir6.2/SUR1 subunits109, which display high affinity binding to sulfonylureas97,110. KATP channels are predominantly expressed in GABAergic neurons11. A decrease in glucose levels leads to the opening of presynaptic KATP channels and suppression of GABA release11. KATP channels are involved in protecting the brain against seizures and mediating ischemic preconditioning in the brain11. A brief hypoxia (90 s) hyperpolarizes the membrane potential and decreases the firing rate by 30% in wild-type SNr neurons. In contrast, hypoxic conditions depolarize the membrane potential and increase the firing rate in the Kir6.2−/− neurons, suggesting that the KATP channel-mediated suppressive effect on SNr activity is sufficient to reverse hypoxia-induced superactivity of the neurons. In addition, neurons deficient for Kir6.2 show more susceptibility to hypoxic damage than their wild-type counterparts95. The Kir6.2−/− mice exhibit generalized seizures in response to the same period of hypoxia, whereas wild-type mice revive normally95. KATP channels suppress the neuronal activity in the SNr. This suppression may determine the seizure threshold under hypoxic conditions95. SUR1−/− mice also exhibit hypersensitivity to hypoxic insult111. Thus, the KATP channels in SNr neurons act as a metabolic sensor to mediate hypoxic hyperpolarization, and in turn, prevent seizure propagation during hypoxic stress. This may have implications in human epilepsy112,113.
KATP channels and their neuroprotective role in cerebral ischemia
Similar to that observed in cardiac ischemia55,60,114, a neuroprotective role of KATP channels has also been suggested in focal and global ischemia models twenty years ago115. KATP channels in a large number of central neurons remain closed, except under conditions of severe metabolic deprivation, such as anoxia or ischemia (Figure 2).
Activation of mitochondrial KATP channels initiates ischemic pre-conditioning and prevents the mitochondrial dysfunction associated with Ca2+ overload during ischemic reperfusion in the heart116,117,118. In adult animals, application of mitochondrial KATP openers, such as diazoxide or BMS-191095, reduces neuronal death (rats:116,119,120,121; mice:122), whereas a selective mitochondrial KATP channel blocker, 5-hydroxydecanoate, prevents preconditioning-induced neuronal protection in middle cerebral artery occlusion (MCAO) focal cerebral ischemia123. In contrast, xenon-induced preconditioning is not associated with the mitochondrial channels, but rather, is mediated by plasmalemma KATP channels124. The mechanisms underlying the mitochondrial KATP channel-related neuroprotective effects remain unclear. However, SUR1 subunits may not be directly involved because adult SUR1 knockout mice exhibit preconditional ischemic tolerance124.
Activation of the plasmalemmal KATP channels hyperpolarizes neurons (Figure 3) and may stabilize the resting membrane potential during ischemic insults and stress125, which, in turn, can protect neurons against neuronal damage and neurodegeneration that is caused by anoxic membrane depolarization and excitotoxicity115,126. Expression of KATP channel genes and proteins have been detected by PCR84 and immunohistochemistry in different neuronal populations in the hippocampus of adult rats87. KATP channels are preferentially expressed in the interneurons, CA3 neurons, and granule cells that are resistant to ischemic injury, especially in global ischemia127,128. However, the underlying mechanism is not yet understood.
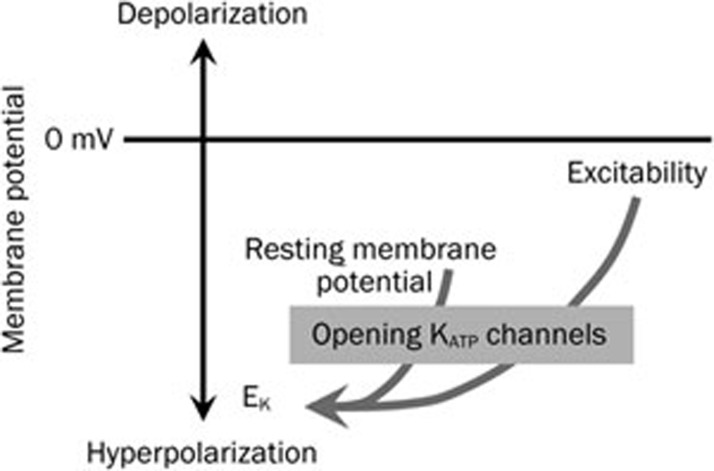
Figure 3.
Opening of KATP channels lead to K+ ion efflux and hyperpolarization of the cell membrane, resulting in a decrease in cell excitability. This hyperpolarization occurs by making the membrane potential more negative, which brings it towards the potassium equilibrium potential (EK). Hyperpolarization suppresses and inhibits cell excitability.
Under normal physiological conditions, neuronal KATP channels are presumably closed due to high intracellular ATP levels (Figure 2). During the initial phase of hypoxia and ischemia, energy failure reduces the ATP/ADP ratio, which activates neuronal KATP channels, causing hyperpolarization of the neuronal cell membrane and suppression of neuronal activity (Figure 2). Similar to previous reports88,95,129,130, we reported that membrane hyperpolarization was induced during the first 5 min of ischemia in both hippocampal and cortical neurons92,93. Using Kir6.2 knockout mice, we further reported that hippocampal CA1 neurons that were deficient for Kir6.2 revealed rapid depolarization and increased neuronal activity in response to hypoxia. In contrast, neuronal suppression occurred in wild-type neurons under similar conditions92. Hypoxic hyperpolarization in wild-type hippocampal CA1 neurons could be prevented by the KATP channel blocker tolbutamide. Depending on the length of the hypoxic challenge, wild-type neurons initially demonstrate hypoxic hyperpolarization, which is then followed by speedy recovery and stabilization of the cell membrane potential. Our in vivo study also showed that cortical neurons lacking the Kir6.2 gene are more vulnerable than wild-type neurons to ischemic insults by middle cerebral artery occlusion93. Our findings provide the first convincing evidence that Kir6.2 containing KATP channels are important for neuroprotection against cerebral ischemia.
An independent group, using knock-in (KI) mice overexpressing Kir6.2-containing KATP channels, further confirmed the crucial role of Kir6.2 in neuroprotection against hypoxia-ischemia131. Specifically, overexpression of Kir6.2 reduced the spontaneous electrical activity recorded in hippocampal and cortical neurons. In this study, the resting membrane potentials for both animals were not described. In response to hypoxia-ischemia challenge, the infarction in Kir6.2 overexpressing animals was smaller compared to that of the wild-type animals. The findings using overexpression of Kir6.2 in Kir6.2 KO mice strongly support the notion that Kir6.2 is neuroprotective against ischemia.
In addition to neurons, KATP channels are also found in astrocytes and microglial cells. Recent studies show that the SUR1 receptor is upregulated in response to pro-inflammatory signals, and the KATP channel blocker, glibenclamide, exerts neuroprotective effects through its action on non-neuronal cells in the MCAO model in rat132,133. Because glibenclamide also has non-KATP channel effects, mechanisms underlying the effect of glibenclamide remain to be further investigated.
Summary and therapeutic aspects
Kir6.2 of KATP channels is ubiquitously expressed in the brain. Studies using Kir6.2 knock-in or knock-out mouse models indicate that the opening of KATP channels shifts the cell membrane potential more negatively (hyperpolarization) towards the EK, leading to suppression of neuronal activity and excitability. Thus, opening KATP channels under metabolic stress can protect neurons against neuronal injury during cerebral ischemia and stroke. While the functional roles of Kir6.1, SUR1 and SUR2 in neuroprotection necessitate further evaluation, it has been suggested that KATP channels may be involved in the remodeling of neurovascular units in stroke26. Kir6.2-containing KATP channels regulate the membrane potential and contribute to the pathophysiological hyperexcitability of neurons induced by hypoxia and ischemia incidents. Thus, Kir6.2-containing KATP channels may have therapeutic potential as a target for stroke. It is anticipated that KATP channel modulators will be useful in the treatment of stroke.
Abbreviations
ABC, ATP-binding cassette protein; ABCC8, ATP-binding cassette C8 gene; CaMKII, calmodulin kinase II; DRG, dorsal root ganglia; KATP, ATP sensitive potassium channels; KCN, gene name for potassium channel; KCNJ11, potassium channel J11 gene; Kir, inward rectifier potassium channels; MCAO, iddle cerebral artery occlusion; NBD, nucleotide binding domain; PIP2, phosphatidylinositol 4,5-biphosphatel; RT-PCR, reverse transcription polymerase chain reaction; SNr, substantia nigra; SUR, sulfonylurea receptor; TM, transmembrane region; TMD, transmembrane domain.
Acknowledgments
This work was supported by a Grant-In-Aid operating grant from the Heart and Stroke Foundation of Canada to HSS. ZPF holds a New Investigator Award from the Heart and Stroke Foundation of Canada.
References
- Miller C. An overview of the potassium channel family. Genome Biol. 2000;1:REVIEWS0004. doi: 10.1186/gb-2000-1-4-reviews0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B.Potassium Channels and Chloride Channels in Ion channels of excitable membranes3ed. Sinauer Associates, Inc, Sunderkabd, Massachusetts USA, 2001. p131–68.
- Hodgkin AL, Huxley AF. Potassium leakage from an active nerve fibre. Nature. 1946;158:376. doi: 10.1038/158376b0. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952;116:449–72. doi: 10.1113/jphysiol.1952.sp004717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–8. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Spruce AE, Standen NB, Stanfield PR. Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature. 1985;316:736–8. doi: 10.1038/316736a0. [DOI] [PubMed] [Google Scholar]
- Standen NB, Quayle JM, Davies NW, Brayden JE, Huang Y, Nelson MT. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989;245:177–80. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Ashford ML, Sturgess NC, Trout NJ, Gardner NJ, Hales CN. Adenosine-5′-triphosphate-sensitive ion channels in neonatal rat cultured central neurones. 1988;412:297–304. doi: 10.1007/BF00582512. [DOI] [PubMed] [Google Scholar]
- Bernardi H, Fosset M, Lazdunski M. Characterization, purification, and affinity labeling of the brain [3H]glibenclamide-binding protein, a putative neuronal ATP-regulated K+ channel. Proc Natl Acad Sci U S A. 1988;85:9816–20. doi: 10.1073/pnas.85.24.9816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoroso S, Schmid-Antomarchi H, Fosset M, Lazdunski M. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990;247:852–4. doi: 10.1126/science.2305257. [DOI] [PubMed] [Google Scholar]
- Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P. Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J Biol Chem. 2001;276:33369–74. doi: 10.1074/jbc.M103320200. [DOI] [PubMed] [Google Scholar]
- Bernardi H, De W, Jr, Epelbaum J, Mourre C, Amoroso S, Slama A, et al. ATP-modulated K+ channels sensitive to antidiabetic sulfonylureas are present in adenohypophysis and are involved in growth hormone release. Proc Natl Acad Sci U S A. 1993;90:1340–4. doi: 10.1073/pnas.90.4.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Tsuura Y, Namba N, Masuda K, Gonoi T, Horie M, et al. Cloning and functional characterization of a novel ATP-sensitive potassium channel ubiquitously expressed in rat tissues, including pancreatic islets, pituitary, skeletal muscle, and heart. J Biol Chem. 1995;270:5691–4. doi: 10.1074/jbc.270.11.5691. [DOI] [PubMed] [Google Scholar]
- Hunter M, Giebisch G. Calcium-activated K-channels of Amphiuma early distal tubule: inhibition by ATP. Pflugers Arch. 1988;412:331–3. doi: 10.1007/BF00582517. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–8. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- Cook DL, Hales CN. Intracellular ATP directly blocks K+ channels in pancreatic B–cells. Nature. 1984;311:271–3. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Trube G. Glucose dependent K+-channels in pancreatic beta-cells are regulated by intracellular ATP. Pflugers Arch. 1985;405:305–9. doi: 10.1007/BF00595682. [DOI] [PubMed] [Google Scholar]
- Kakei M, Kelly RP, Ashcroft SJ, Ashcroft FM. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett. 1986;208:63–6. doi: 10.1016/0014-5793(86)81533-2. [DOI] [PubMed] [Google Scholar]
- Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu Rev Physiol. 1999;61:337–62. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog. Biophys. Mol Biol. 2003;81:133–76. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- Besancon E, Guo S, Lok J, Tymianski M, Lo EH. Beyond NMDA and AMPA glutamate receptors: emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol Sci. 2008;29:268–75. doi: 10.1016/j.tips.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Tymianski M. Emerging mechanisms of disrupted cellular signaling in brain ischemia. Nat Neurosci. 2011;14:1369–73. doi: 10.1038/nn.2951. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kurachi Y. Function, regulation, pharmacology, and molecular structure of ATP-sensitive K+ channels in the cardiovascular system. J Cardiovasc Electrophysiol. 1997;8:1431–46. doi: 10.1111/j.1540-8167.1997.tb01040.x. [DOI] [PubMed] [Google Scholar]
- Huopio H, Shyng SL, Otonkoski T, Nichols CG. KATP channels and insulin secretion disorders. Am J Physiol Endocrinol Metab. 2002;283:E207–16. doi: 10.1152/ajpendo.00047.2002. [DOI] [PubMed] [Google Scholar]
- Sun XL, Hu G. ATP-sensitive potassium channels: a promising target for protecting neurovascular unit function in stroke. Clin Exp Pharmacol Physiol. 2010;37:243–52. doi: 10.1111/j.1440-1681.2009.05190.x. [DOI] [PubMed] [Google Scholar]
- Babenko AP, guilar-Bryan L, Bryan J. A view of sur/KIR6.X, KATP channels. Annu Rev Physiol. 1998;60:667–87. doi: 10.1146/annurev.physiol.60.1.667. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, et al. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–4. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Miki T, Nagashima K, Seino S. The structure and function of the ATP-sensitive K+ channel in insulin-secreting pancreatic beta-cells. J Mol Endocrinol. 1999;22:113–23. doi: 10.1677/jme.0.0220113. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Namba N, Inazawa J, Gonzalez G, et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–70. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244–7. doi: 10.1038/352244a0. [DOI] [PubMed] [Google Scholar]
- Garlid KD. Opening mitochondrial KATP in the heart — what happens, and what does not happen. Basic Res Cardiol. 2000;95:275–9. doi: 10.1007/s003950070046. [DOI] [PubMed] [Google Scholar]
- Aguilar-Bryan L, Nichols CG, Wechsler SW, Clement JP, Boyd AE, III, Gonzalez G, et al. Cloning of the beta cell high-affinity sulfonylurea receptor: a regulator of insulin secretion. Science. 1995;268:423–6. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Bryan J. Sur domains that associate with and gate KATP pores define a novel gatekeeper. J Biol Chem. 2003;278:41577–80. doi: 10.1074/jbc.C300363200. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, et al. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–7. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–6. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, et al. A novel sulfonylurea receptor forms with BIR (Kir6.2) a smooth muscle type ATP-sensitive K+ channel. J Biol Chem. 1996;271:24321–4. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- Ammala C, Moorhouse A, Gribble F, Ashfield R, Proks P, Smith PA, et al. Promiscuous coupling between the sulphonylurea receptor and inwardly rectifying potassium channels. Nature. 1996;379:545–8. doi: 10.1038/379545a0. [DOI] [PubMed] [Google Scholar]
- Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, Gonzalez G, et al. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–7. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- Proks P, Capener CE, Jones P, Ashcroft FM. Mutations within the P–loop of Kir6.2 modulate the intraburst kinetics of the ATP-sensitive potassium channel. J Gen Physiol. 2001;118:341–53. doi: 10.1085/jgp.118.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. The open pore conformation of potassium channels. Nature. 2002;417:523–6. doi: 10.1038/417523a. [DOI] [PubMed] [Google Scholar]
- Enkvetchakul D, Nichols CG. Gating mechanism of KATP channels: function fits form. J Gen Physiol. 2003;122:471–80. doi: 10.1085/jgp.200308878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain P, Li L, Wang J. KATP channel inhibition by ATP requires distinct functional domains of the cytoplasmic C terminus of the pore-forming subunit. Proc Natl Acad Sci U S A. 1998;95:13953–8. doi: 10.1073/pnas.95.23.13953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drain P, Geng X, Li L. Concerted gating mechanism underlying KATP channel inhibition by ATP. Biophys J. 2004;86:2101–12. doi: 10.1016/S0006-3495(04)74269-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babenko AP, Bryan J. SUR-dependent modulation of KATP channels by an N-terminal KIR6.2 peptide. Defining intersubunit gating interactions. J Biol Chem. 2002;277:43997–4004. doi: 10.1074/jbc.M208085200. [DOI] [PubMed] [Google Scholar]
- Antcliff JF, Haider S, Proks P, Sansom MS, Ashcroft FM. Functional analysis of a structural model of the ATP-binding site of the KATP channel Kir6.2 subunit. EMBO J. 2005;24:229–39. doi: 10.1038/sj.emboj.7600487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong K, Tang LQ, MacGregor GG, Leng Q, Hebert SC. Novel nucleotide-binding sites in ATP-sensitive potassium channels formed at gating interfaces. EMBO J. 2005;24:1318–29. doi: 10.1038/sj.emboj.7600626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Kurachi Y. The nucleotide-binding domains of sulfonylurea receptor 2A and 2B play different functional roles in nicorandil-induced activation of ATP-sensitive K+ channels. Mol Pharmacol. 2004;65:1198–207. doi: 10.1124/mol.65.5.1198. [DOI] [PubMed] [Google Scholar]
- Campbell JD, Proks P, Lippiat JD, Sansom MS, Ashcroft FM. Identification of a functionally important negatively charged residue within the second catalytic site of the SUR1 nucleotide-binding domains. Diabetes. 2004;53:S123–7. doi: 10.2337/diabetes.53.suppl_3.s123. [DOI] [PubMed] [Google Scholar]
- Masia R, Nichols CG. Functional clustering of mutations in the dimer interface of the nucleotide binding folds of the sulfonylurea receptor. J Biol Chem. 2008;283:30322–9. doi: 10.1074/jbc.M804318200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergani P, Lockless SW, Nairn AC, Gadsby DC. CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains. Nature. 2005;433:876–80. doi: 10.1038/nature03313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Crane A, Gonzalez G, Bryan J, Aguilar-Bryan L. Familial hyperinsulinism and pancreatic beta-cell ATP-sensitive potassium channels. Kidney Int. 2000;57:803–8. doi: 10.1046/j.1523-1755.2000.00918.x. [DOI] [PubMed] [Google Scholar]
- Otonkoski T, Ammala C, Huopio H, Cote GJ, Chapman J, Cosgrove K, et al. A point mutation inactivating the sulfonylurea receptor causes the severe form of persistent hyperinsulinemic hypoglycemia of infancy in Finland. Diabetes. 1999;48:408–15. doi: 10.2337/diabetes.48.2.408. [DOI] [PubMed] [Google Scholar]
- Misler S, Giebisch G. ATP-sensitive potassium channels in physiology, pathophysiology, and pharmacology. Curr Opin Nephrol Hypertens. 1992;1:21–33. doi: 10.1097/00041552-199210000-00005. [DOI] [PubMed] [Google Scholar]
- Ashfield R, Gribble FM, Ashcroft SJ, Ashcroft FM. Identification of the high-affinity tolbutamide site on the SUR1 subunit of the KATP channel. Diabetes. 1999;48:1341–7. doi: 10.2337/diabetes.48.6.1341. [DOI] [PubMed] [Google Scholar]
- Mikhailov MV, Mikhailova EA, Ashcroft SJ. Molecular structure of the glibenclamide binding site of the beta-cell KATP channel. FEBS Lett. 2001;499:154–60. doi: 10.1016/s0014-5793(01)02538-8. [DOI] [PubMed] [Google Scholar]
- Gribble FM, Tucker SJ, Ashcroft FM. The interaction of nucleotides with the tolbutamide block of cloned ATP-sensitive K+ channel currents expressed in Xenopus oocytes: a reinterpretation. J Physiol. 1997;504:35–45. doi: 10.1111/j.1469-7793.1997.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft FM, Gribble FM. New windows on the mechanism of action of KATP channel openers. Trends Pharmacol Sci. 2000;21:439–45. doi: 10.1016/s0165-6147(00)01563-7. [DOI] [PubMed] [Google Scholar]
- Terzic A, Jahangir A, Kurachi Y. Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+ channel-opening drugs. Am J Physiol. 1995;269:C525–45. doi: 10.1152/ajpcell.1995.269.3.C525. [DOI] [PubMed] [Google Scholar]
- Moreau C, Jacquet H, Prost AL, D'hahan N, Vivaudou M. The molecular basis of the specificity of action of KATP channel openers. EMBO J. 2000;19:6644–51. doi: 10.1093/emboj/19.24.6644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau C, Gally F, Jacquet-Bouix H, Vivaudou M. The size of a single residue of the sulfonylurea receptor dictates the effectiveness of KATP channel openers. Mol Pharmacol. 2005;67:1026–33. doi: 10.1124/mol.104.008698. [DOI] [PubMed] [Google Scholar]
- Moreau C, Prost AL, Derand R, Vivaudou M. SUR, ABC proteins targeted by KATP channel openers. J Mol Cell Cardiol. 2005;38:951–63. doi: 10.1016/j.yjmcc.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang YL, Tang XC, Feng HS, Hu G. Targeting ischemic stroke with a novel opener of ATP-sensitive potassium channels in the brain. Mol Pharmacol. 2004;66:1160–8. doi: 10.1124/mol.104.003178. [DOI] [PubMed] [Google Scholar]
- Hu LF, Wang S, Shi XR, Yao HH, Sun YH, Ding JH, et al. G ATP-sensitive potassium channel opener iptakalim protected against the cytotoxicity of MPP+ on SH-SY5Y cells by decreasing extracellular glutamate level. J Neurochem. 2005;94:1570–9. doi: 10.1111/j.1471-4159.2005.03306.x. [DOI] [PubMed] [Google Scholar]
- Costa AD. Iptakalim: a new or just another KCO. Cardiovasc Res. 2009;83:417–8. doi: 10.1093/cvr/cvp193. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Cukras CA, Harwood J, Nichols CG. Structural determinants of PIP(2) regulation of inward rectifier KATP channels. J Gen Physiol. 2000;116:599–608. doi: 10.1085/jgp.116.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Barbieri A, Gumusboga A, Cukras C, Pike L, Davis JN, et al. Modulation of nucleotide sensitivity of ATP-sensitive potassium channels by phosphatidylinositol-4-phosphate 5-kinase. Proc Natl Acad Sci U S A. 2000;97:937–41. doi: 10.1073/pnas.97.2.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res. 2004;94:1359–66. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, et al. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1205–14. doi: 10.1152/ajpregu.00337.2007.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wagoner DR. Mechanosensitive gating of atrial ATP-sensitive potassium channels. Circ Res. 1993;72:973–83. doi: 10.1161/01.res.72.5.973. [DOI] [PubMed] [Google Scholar]
- Kang Y, Leung YM, Manning-Fox JE, Xia F, Xie H, Sheu L, et al. Syntaxin-1A inhibits cardiac KATP channels by its actions on nucleotide binding folds 1 and 2 of sulfonylurea receptor 2A. J Biol Chem. 2004;279:47125–31. doi: 10.1074/jbc.M404954200. [DOI] [PubMed] [Google Scholar]
- Pasyk EA, Kang Y, Huang X, Cui N, Sheu L, Gaisano HY. Syntaxin-1A binds the nucleotide-binding folds of sulphonylurea receptor 1 to regulate the KATP channel. J Biol Chem. 2004;279:4234–40. doi: 10.1074/jbc.M309667200. [DOI] [PubMed] [Google Scholar]
- Chao C, Liang T, Kang Y, Lin X, Xie H, Feng ZP, et al. Syntaxin-1A inhibits KATP channels by interacting with specific conserved motifs within sulfonylurea receptor 2A. J Mol Cell Cardiol. 2011;51:790–802. doi: 10.1016/j.yjmcc.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Chang N, Liang T, Lin X, Kang Y, Xie H, Feng ZP, et al. Syntaxin-1A interacts with distinct domains within nucleotide-binding folds of sulfonylurea receptor 1 to inhibit beta-cell ATP-sensitive potassium channels. J Biol Chem. 2011;286:23308–18. doi: 10.1074/jbc.M111.217950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui N, Kang Y, He Y, Leung YM, Xie H, Pasyk EA, et al. H3 domain of syntaxin 1A inhibits KATP channels by its actions on the sulfonylurea receptor 1 nucleotide-binding folds-1 and -2. J Biol Chem. 2004;279:53259–65. doi: 10.1074/jbc.M410171200. [DOI] [PubMed] [Google Scholar]
- Chen PC, Bruederle CE, Gaisano HY, Shyng SL. Syntaxin 1A regulates surface expression of {beta}-cell ATP-sensitive potassium channels. Am J Physiol Cell Physiol. 2011;300:C506–16. doi: 10.1152/ajpcell.00429.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng B, Kang Y, Elias CL, He Y, Xie H, Hansen JB, et al. The actions of a novel potent islet beta-cell specific ATP-sensitive K+ channel opener can be modulated by syntaxin-1A acting on sulfonylurea receptor 1. Diabetes. 2007;56:2124–34. doi: 10.2337/db07-0030. [DOI] [PubMed] [Google Scholar]
- Ng B, Kang Y, Xie H, Sun H, Gaisano HY. Syntaxin-1A inhibition of P-1075, cromakalim, and diazoxide actions on mouse cardiac ATP-sensitive potassium channel. Cardiovasc Res. 2008;80:365–74. doi: 10.1093/cvr/cvn210. [DOI] [PubMed] [Google Scholar]
- Bernardi H, Fosset M, Lazdunski M. ATP/ADP binding sites are present in the sulfonylurea binding protein associated with brain ATP-sensitive K+ channels. Biochemistry. 1992;31:6328–32. doi: 10.1021/bi00142a023. [DOI] [PubMed] [Google Scholar]
- Roper J, Ashcroft FM. Metabolic inhibition and low internal ATP activate KATP channels in rat dopaminergic substantia nigra neurones. Pflugers Arch. 1995;430:44–54. doi: 10.1007/BF00373838. [DOI] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Electrophysiological investigation of adenosine trisphosphate-sensitive potassium channels in the rat substantia nigra pars reticulata. Neuroscience. 1996;74:499–509. doi: 10.1016/0306-4522(96)00151-0. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Yamamoto C. Identification of an ATP-sensitive K+ channel in rat cultured cortical neurons. Pflugers Arch. 1992;422:260–6. doi: 10.1007/BF00376211. [DOI] [PubMed] [Google Scholar]
- Zawar C, Plant TD, Schirra C, Konnerth A, Neumcke B. Cell-type specific expression of ATP-sensitive potassium channels in the rat hippocampus. J Physiol. 1999;514:327–41. doi: 10.1111/j.1469-7793.1999.315ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford ML, Boden PR, Treherne JM. Tolbutamide excites rat glucoreceptive ventromedial hypothalamic neurones by indirect inhibition of ATP-K+ channels. Br J Pharmacol. 1990;101:531–40. doi: 10.1111/j.1476-5381.1990.tb14116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford ML, Boden PR, Treherne JM. Glucose-induced excitation of hypothalamic neurones is mediated by ATP-sensitive K+ channels. Pflugers Arch. 1990;415:479–83. doi: 10.1007/BF00373626. [DOI] [PubMed] [Google Scholar]
- Zhou M, Tanaka O, Suzuki M, Sekiguchi M, Takata K, Kawahara K, et al. Localization of pore-forming subunit of the ATP-sensitive K(+)-channel, Kir6.2, in rat brain neurons and glial cells. Brain Res Mol Brain Res. 2002;101:23–32. doi: 10.1016/s0169-328x(02)00137-7. [DOI] [PubMed] [Google Scholar]
- Fujimura N, Tanaka E, Yamamoto S, Shigemori M, Higashi H. Contribution of ATP-sensitive potassium channels to hypoxic hyperpolarization in rat hippocampal CA1 neurons in vitro. J Neurophysiol. 1997;77:378–85. doi: 10.1152/jn.1997.77.1.378. [DOI] [PubMed] [Google Scholar]
- Trapp S, Ballanyi K. KATP channel mediation of anoxia-induced outward current in rat dorsal vagal neurons in vitro. J Physiol. 1995;487:37–50. doi: 10.1113/jphysiol.1995.sp020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Rawson NE, Levin BE. Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res. 1998;814:41–54. doi: 10.1016/s0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- Liss B, Roeper J. A role for neuronal KATP channels in metabolic control of the seizure gate. Trends Pharmacol Sci. 2001;22:599–601. doi: 10.1016/s0165-6147(00)01861-7. [DOI] [PubMed] [Google Scholar]
- Sun HS, Feng ZP, Miki T, Seino S, French RJ. Enhanced neuronal damage after ischemic insults in mice lacking Kir6.2-containing ATP-sensitive K+ channels. J Neurophysiol. 2006;95:2590–601. doi: 10.1152/jn.00970.2005. [DOI] [PubMed] [Google Scholar]
- Sun HS, Feng ZP, Barber PA, Buchan AM, French RJ. Kir6.2-containing ATP-sensitive potassium channels protect cortical neurons from ischemic/anoxic injury in vitro and in vivo. Neuroscience. 2007;144:1509–15. doi: 10.1016/j.neuroscience.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–12. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, et al. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292:1543–6. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- Thomzig A, Laube G, Pruss H, Veh RW. Pore-forming subunits of KATP channels, Kir6.1 and Kir6.2, display prominent differences in regional and cellular distribution in the rat brain. J Comp Neurol. 2005;484:313–30. doi: 10.1002/cne.20469. [DOI] [PubMed] [Google Scholar]
- Mourre C, Ben AY, Bernardi H, Fosset M, Lazdunski M. Antidiabetic sulfonylureas: localization of binding sites in the brain and effects on the hyperpolarization induced by anoxia in hippocampal slices. Brain Res. 1989;486:159–64. doi: 10.1016/0006-8993(89)91288-2. [DOI] [PubMed] [Google Scholar]
- Taborsky GJ, Jr, Ahren B, Havel PJ. Autonomic mediation of glucagon secretion during hypoglycemia: implications for impaired alpha-cell responses in type 1 diabetes. Diabetes. 1998;47:995–1005. doi: 10.2337/diabetes.47.7.995. [DOI] [PubMed] [Google Scholar]
- Karschin C, Ecke C, Ashcroft FM, Karschin A. Overlapping distribution of KATP channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Lett. 1997;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Bischoff AM, Richter DW. ATP-sensitive K+ channels are functional in expiratory neurones of normoxic cats. J Physiol. 1996;494:399–409. doi: 10.1113/jphysiol.1996.sp021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TG, Brown DA. Modulation of the excitability of cholinergic basal forebrain neurones by KATP channels. J Physiol. 2004;554:353–70. doi: 10.1113/jphysiol.2003.055889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner GR, Lutas A, Martinez–Francois JR, Yellen G. Single KATP channel opening in response to action potential firing in mouse dentate granule neurons. J Neurosci. 2011;31:8689–96. doi: 10.1523/JNEUROSCI.5951-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller M, Mironov SL, Karschin A, Richter DW. Dynamic activation of K(ATP) channels in rhythmically active neurons. J Physiol. 2001;537:69–81. doi: 10.1111/j.1469-7793.2001.0069k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busija DW, Lacza Z, Rajapakse N, Shimizu K, Kis B, Bari F, et al. Targeting mitochondrial ATP-sensitive potassium channels — a novel approach to neuroprotection. Brain Res Rev. 2004;46:282–94. doi: 10.1016/j.brainresrev.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Zoga V, Kawano T, Liang MY, Bienengraeber M, Weihrauch D, McCallum B, et al. KATP channel subunits in rat dorsal root ganglia: alterations by painful axotomy. Mol Pain. 2010;6:6. doi: 10.1186/1744-8069-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano T, Zoga V, Gemes G, McCallum JB, Wu HE, Pravdic D, et al. Suppressed Ca2+/CaM/CaMKII-dependent KATP channel activity in primary afferent neurons mediates hyperalgesia after axotomy. Proc Natl Acad Sci U S A. 2009;106:8725–30. doi: 10.1073/pnas.0901815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Sanchez C, Basile AS, Fedorova I, Arima H, Stannard B, Fernandez AM, et al. Mice transgenically overexpressing sulfonylurea receptor 1 in forebrain resist seizure induction and excitotoxic neuron death. Proc Natl Acad Sci U S A. 2001;98:3549–54. doi: 10.1073/pnas.051012898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadarola MJ, Gale K. Substantia nigra: site of anticonvulsant activity mediated by gamma-aminobutyric acid. Science. 1982;218:1237–40. doi: 10.1126/science.7146907. [DOI] [PubMed] [Google Scholar]
- Yamada K, Inagaki N. Neuroprotection by KATP channels. J Mol Cell Cardiol. 2005;38:945–9. doi: 10.1016/j.yjmcc.2004.11.020. [DOI] [PubMed] [Google Scholar]
- Hicks GA, Hudson AL, Henderson G. Localization of high affinity [3H]glibenclamide binding sites within the substantia nigra zona reticulata of the rat brain. Neuroscience. 1994;61:285–92. doi: 10.1016/0306-4522(94)90231-3. [DOI] [PubMed] [Google Scholar]
- Munoz A, Nakazaki M, Goodman JC, Barrios R, Onetti CG, Bryan J, et al. Ischemic preconditioning in the hippocampus of a knockout mouse lacking SUR1-based KATP channels. Stroke. 2003;34:164–70. doi: 10.1161/01.str.0000048215.36747.d1. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Potassium channels: brief overview and implications in epilepsy. Neurology. 2009;72:664–9. doi: 10.1212/01.wnl.0000343739.72081.4e. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–49. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- Ashcroft FM. Adenosine 5′–triphosphate-sensitive potassium channels. Annu Rev Neurosci. 1988;11:97–118. doi: 10.1146/annurev.ne.11.030188.000525. [DOI] [PubMed] [Google Scholar]
- Heurteaux C, Bertaina V, Widmann C, Lazdunski M. K+ channel openers prevent global ischemia-induced expression of c-fos, c-jun, heat shock protein, and amyloid beta-protein precursor genes and neuronal death in rat hippocampus. Proc Natl Acad Sci U S A. 1993;90:9431–5. doi: 10.1073/pnas.90.20.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asemu G, Papousek F, Ostadal B, Kolar F. Adaptation to high altitude hypoxia protects the rat heart against ischemia-induced arrhythmias. Involvement of mitochondrial K(ATP) channel. J Mol Cell Cardiol. 1999;31:1821–31. doi: 10.1006/jmcc.1999.1013. [DOI] [PubMed] [Google Scholar]
- Testai L, Rapposelli S, Calderone V. Cardiac ATP-sensitive potassium channels: a potential target for an anti-ischaemic pharmacological strategy. Cardiovasc Hematol Agents Med Chem. 2007;5:79–90. doi: 10.2174/187152507779315831. [DOI] [PubMed] [Google Scholar]
- Kim KO, Choe G, Chung SH, Kim CS. Delayed pharmacological pre-conditioning effect of mitochondrial ATP-sensitive potassium channel opener on neurologic injury in a rabbit model of spinal cord ischemia. Acta Anaesthesiol Scand. 2008;52:236–42. doi: 10.1111/j.1399-6576.2007.01534.x. [DOI] [PubMed] [Google Scholar]
- Mayanagi K, Gaspar T, Katakam PV, Busija DW. Systemic administration of diazoxide induces delayed preconditioning against transient focal cerebral ischemia in rats. Brain Res. 2007;1168:106–11. doi: 10.1016/j.brainres.2007.06.071. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Lacza Z, Rajapakse N, Horiguchi T, Snipes J, Busija DW. MitoK(ATP) opener, diazoxide, reduces neuronal damage after middle cerebral artery occlusion in the rat. Am J Physiol Heart Circ Physiol. 2002;283:H1005–11. doi: 10.1152/ajpheart.00054.2002. [DOI] [PubMed] [Google Scholar]
- Mayanagi K, Gaspar T, Katakam PV, Kis B, Busija DW. The mitochondrial KATP channel opener BMS-191095 reduces neuronal damage after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2007;27:348–55. doi: 10.1038/sj.jcbfm.9600345. [DOI] [PubMed] [Google Scholar]
- Liu D, Lu C, Wan R, Auyeung WW, Mattson MP. Activation of mitochondrial ATP-dependent potassium channels protects neurons against ischemia-induced death by a mechanism involving suppression of Bax translocation and cytochrome c release. J Cereb Blood Flow Metab. 2002;22:431–43. doi: 10.1097/00004647-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Liu D, Slevin JR, Lu C, Chan SL, Hansson M, Elmer E, et al. Involvement of mitochondrial K+ release and cellular efflux in ischemic and apoptotic neuronal death. J Neurochem. 2003;86:966–79. doi: 10.1046/j.1471-4159.2003.01913.x. [DOI] [PubMed] [Google Scholar]
- Bantel C, Maze M, Trapp S. Neuronal preconditioning by inhalational anesthetics: evidence for the role of plasmalemmal adenosine triphosphate-sensitive potassium channels. Anesthesiology. 2009;110:986–95. doi: 10.1097/ALN.0b013e31819dadc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross GJ, Fryer RM. Sarcolemmal versus mitochondrial ATP-sensitive K+ channels and myocardial preconditioning. Circ Res. 1999;84:973–9. doi: 10.1161/01.res.84.9.973. [DOI] [PubMed] [Google Scholar]
- Blondeau N, Plamondon H, Richelme C, Heurteaux C, Lazdunski M. KATP channel openers, adenosine agonists and epileptic preconditioning are stress signals inducing hippocampal neuroprotection. Neuroscience. 2000;100:465–74. doi: 10.1016/s0306-4522(00)00304-3. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Johansen FF. Interneurons in rat hippocampus after cerebral ischemia. Morphometric, functional, and therapeutic investigations. Acta Neurol Scand Suppl. 1993;150:1–32. [PubMed] [Google Scholar]
- Yamamoto S, Tanaka E, Higashi H. Mediation by intracellular calcium-dependent signals of hypoxic hyperpolarization in rat hippocampal CA1 neurons in vitro. J Neurophysiol. 1997;77:386–92. doi: 10.1152/jn.1997.77.1.386. [DOI] [PubMed] [Google Scholar]
- Garcia de AS, Franke H, Pissarek M, Nieber K, Illes P. Neuroprotection by ATP-dependent potassium channels in rat neocortical brain slices during hypoxia. Neurosci Lett. 1999;273:13–6. doi: 10.1016/s0304-3940(99)00603-5. [DOI] [PubMed] [Google Scholar]
- Heron-Milhavet L, Xue-Jun Y, Vannucci SJ, Wood TL, Willing LB, Stannard B, et al. Protection against hypoxic-ischemic injury in transgenic mice overexpressing Kir6.2 channel pore in forebrain. Mol Cell Neurosci. 2004;25:585–93. doi: 10.1016/j.mcn.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Simard JM, Chen M, Tarasov KV, Bhatta S, Ivanova S, Melnitchenko L, et al. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat Med. 2006;12:433–40. doi: 10.1038/nm1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega FJ, Gimeno-Bayon J, Espinosa-Parrilla JF, Carrasco JL, Batlle M, Pugliese M, et al. ATP-dependent potassium channel blockade strengthens microglial neuroprotection after hypoxia-ischemia in rats. Exp Neurol. 2012;235:282–96. doi: 10.1016/j.expneurol.2012.02.010. [DOI] [PubMed] [Google Scholar]