Abstract
A novel cardiac scaffold comprised of decellularized porcine heart matrix was investigated for use as a biodegradable patch with a potential for surgical reconstruction of the right ventricular outflow tract. Powdered heart matrix solution was blended with chitosan and lyophilized to form three-dimensional scaffolds. For this investigation, we examined the influence of different blending ratios of heart matrix to chitosan on porosity and mechanical properties, then gene expression and electrophysiological function of invading neonatal rat ventricular myocytes (NRVM) compared to type-A gelatin/chitosan composite scaffolds. Heart matrix/chitosan-blended hydrogels (1.6 mg/mL heart matrix) had similar porosity (109±34 μm), and elastic modulus (13.2±4.0 kPa) as previously published gelatin/chitosan scaffolds. Heart matrix/chitosan hydrogels maintained>80% viability and had higher NRVM retention (∼1000 cells/mm2) than gelatin/chitosan scaffolds. There was a significant increase in α-myosin heavy chain and connexin-43 expression in NRVM cultured on heart matrix/chitosan scaffolds after 14 days compared with gelatin/chitosan scaffolds. Further, heart matrix/chitosan scaffolds had significantly higher conduction velocity (12.6±4.9 cm/s) and contractile stress (0.79±0.13 mN/mm2) than gelatin/chitosan scaffolds. In summary, NRVM cultured on heart matrix scaffold showed improvements in contractile and electrophysiological function.
Introduction
Tetralogy of Fallot, the most common cyanotic heart defect, occurs in 3 to 6 of every 10,000 live births in the United States and generally requires surgical placement of a patch or baffle across the right ventricular outflow tract (RVOT) in an area that normally consists of contractile myocardial tissue.1,2 Various types of biocompatible polymers or decellularized matrices are clinically used for RVOT patch applications, including Dacron, Gore-Tex®, and autologous or bovine pericardium.3–7 However, these constructs have significant drawbacks as a cardiac tissue replacement. Studies have found that the body reacts to each of these materials with an inflammatory foreign body response, resulting in encasement of the material in a fibrous scar-like tissue that does not degrade, does not grow with the child's heart, does not provide contractile force, disrupts electrical conduction, and can serve as a nexus for calcification.5,6 As a result, patients with heart patches have an increased risk of heart failure, arrhythmias, infection, and aneurysm.8,9 Previously, we reported on a suturable multilayered cardiac patch that facilitates cardiac cell invasion and degrades over time. This patch is made from a chitosan and gelatin composite hydrogel supported by a polycaprolactone (PCL) core. The PCL core provided sufficient tensile strength for use as a cardiac patch, while the gelatin–chitosan composite hydrogel provides an extracellular matrix (ECM) environment for cardiac cell attachment and for eventual degradation of the patch and incorporation into native tissue.10,11 In this study, we tested whether substitution of gelatin with a cardiac specific decellularized matrix that mimics the chemical composition of native ECM results in improved contractile and electrophysiological function for cardiac patch applications.
Studies have demonstrated that preserved micro-architecture and mechanical properties of native ECM promote an optimal environment for cell immigration, maturation, and tissue formation.12–14 Hence, decellularized matrices have been investigated as a cell delivery system for various applications including tracheal,15 corneal,16 hepatic,13 and cardiac17 therapies. Despite significant findings in the use of decellularized matrix, there are significant drawbacks in the use of unprocessed (or raw) decellularized matrix for cardiac patch applications, including structural disruption,18 lower mechanical strength after the decellularization process,19 and inability to form into desired shapes or volumes. Injectable decellularized cardiac matrix was introduced to treat myocardial infarction (MI), and several studies from the Christman group at the University of California, San Diego demonstrated that injection of this material in a rat MI model increases endogenous cardiomyocytes in the infarct area and the ability of the matrix to promote arteriole formation.12,20,21 Further, when infarcted pigs were treated with percutaneous transendocardial injections of the myocardial matrix hydrogel, they found improvement in cardiac function and ventricular volumes, and a significantly larger zone of cardiac muscle in the endocardium in matrix-injected pigs.22
We hypothesized that the incorporation of heart matrix would improve cardiac cell attachment and maturation in addition to physiologic functions including conduction velocity and contractile force compared with type-A gelatin/chitosan composite scaffolds. To test this, we incorporated decellularized heart matrix into a suturable hydrogel by blending it with chitosan solution and gelling it around a PCL core. The microstructural, mechanical, biological, and physiological properties of novel heart matrix/chitosan scaffolds were compared with properties of type-A gelatin/chitosan scaffolds. Additionally, we incorporated neonatal rat ventricular myocytes (NRVM) in those three-dimensional (3D) scaffolds and evaluated cell viability, markers of cardiomyocytes maturation, conduction velocity, and contractile force.
Materials and Methods
Solutions and hydrogel scaffolds
Hydrogels were made using previously described procedures11,23 with minor modifications. In brief, 10% (wt/v) PCL solutions in glacial acetic acid (Pharmco Products, Inc.) were pipetted into a custom-made Teflon mold containing 2 mL of water and formed solid matrices (diameter=16 mm). Chitosan (20 mg/mL; Sigma Aldrich) solution was prepared in distilled water with 0.5 M acetic acid. Decellularized porcine heart left ventricular myocardium (6.7 mg/mL, from Dr. Karen L. Christman) and type-A gelatin (G) (20 mg/mL w/v; Sigma Aldrich) solutions were prepared in distilled water. Mixtures of heart myocardium+chitosan and gelatin+chitosan solutions were mixed with different concentrations and emulsified using a sonicator (Fisher Scientific FS20D) at 37°C for 30 min. Blended solutions (250 μL) were poured into custom-made Teflon molds around PCL matrices and lyophilized at −50°C for 24 h followed by freezing with dry ice. Formed scaffolds were neutralized using 100% ethanol and rehydrated using phosphate-buffered saline (PBS).
Measurement of porous characteristics
Pore diameter and circularity were analyzed to evaluate whether hydrogels had cell-sized pores to promote cell invasion and whether pores had a symmetric structure. Briefly, lyophilized samples were allowed to dry overnight in a vacuum desiccator at room temperature. The diameter and thickness of dry matrices were measured to calculate the volume, and then samples were attached to aluminum stubs with carbon tape and sputter-coated with gold for 1 min. Surface architecture of the scaffolds was analyzed using a scanning electron microscope (JEOL 6360; Jeol USA, Inc.) at an accelerating voltage of 15 kV. Images were analyzed for the average pore diameter, truss thickness, and circularity (n=15 on four different samples) using ImageJ according to the instructions on the NIH ebsite (NIH). Briefly, images were converted to gray scale; then, major and minor axes of pores were measured. Since there was no significant difference between the major and minor axes, the averages of axes were considered as pore diameters. Circularity was calculated using an ImageJ plug-in for the Measure command (version 1.29 or later) that calculates object circularity using the following formula:
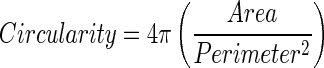 |
To evaluate whether liquid content and diffusion, the ability of the hydrogels to absorb water was measured by determining volumes of dry and wet samples. Total volumes of dry scaffolds (Vt) were measured using an analytical balance. The samples were then submerged in a graduated cylinder containing absolute ethanol. Samples were removed from the cylinder and the remaining amount of liquid was recorded to determine the uptake of alcohol by the hydrogel (Vu). The liquid content was calculated as
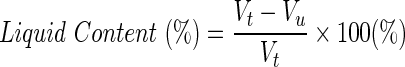 |
Measurement of compressive modulus
The compressive modulus of hydrogels was also measured to find the effect of different concentrations of heart matrix (3.3, 2.2, 1.6, and 1.3 mg/mL) using a previously described procedure with a minor modification.11 Samples were neutralized using 100% ethanol and rehydrated using PBS, then hydrogels were preconditioned in high-serum plating media (Dulbecco's modified Eagle's media, 17% M199, 10% horse serum, 5% fetal bovine serum, 100 U/mL penicillin, and 50 mg/mL streptomycin) at 37°C for 24 h. Hydrogels (n=5) were placed in stainless steel platens in an Instron 5942 and compressed up to 3% strain (∼100 μm) at 1 mm/s of load speed. The load displacement data were converted into compression stress.
NRVM isolation and culture
All studies involving experimental animals were approved by the Institutional Animal Care and Use Committees of both Rice University and Baylor College of Medicine. NRVM were isolated from 1- to 3-day-old Sprague-Dawley rat hearts as previously described with minor modifications.24 Briefly, rats were anesthetized with isoflurane, decapitated, and the hearts were removed. Blood vessels and atria were trimmed, leaving only the ventricles. Ventricular cardiomyocytes were isolated using enzymatic digestion with an isolation kit (Cellutron). Isolated cells were preplated in Petri dishes for 2 h to remove fibroblasts and endothelial cells. Unattached cells at 0.5–3×106 cells in 2 mL high-serum plating media were seeded onto each hydrogel. After 24 h, cell seeded samples were transferred to a low serum media (Dulbecco's modified Eagle's media, 18.5% M199, 5% horse serum, 1% fetal bovine serum, and antibiotics). Cell cultures were maintained at 37°C and 5% CO2/95% air and fresh maintenance media was added every day. All culture media was purchased from Invitrogen Corp. and serum was purchased from PAA Laboratories.
Cell seeding and culturing
Cardiomyocyte adhesion, morphology, and viability on hydrogels were analyzed. Cells were seeded at 0.5×106/mL onto hydrogels and cultured for 4 to 14 days, maintained in 5% CO2/95% air at 37°C with medium changed every 24 h. Cells were then washed in cold (4°C) PBS and fixed in 4% paraformaldehyde (Electron Microscopy Sciences) for 20 min at 4°C. Cells were washed with PBS and made permeable with 0.5% Triton-X 100 (Sigma). Cells were again washed in PBS and stained with Alexa Fluor® 488 Phalloidin (Invitrogen Corp.) at a 1:1000 dilution in 1% bovine serum albumin (BSA; Gemini Bioproducts) overnight at 4°C. Cells were then counterstained with DAPI-containing Vecta Shield (Vector). Images were obtained using a DMI 6000B (Leica Microsystems, Bannockburn, IL) fluorescence microscope to analyze cell adhesion and morphology. Primary and secondary antibodies of cardiac cell markers were also used to characterize NRVM morphologies. Monoclonal anti-α-actinin (1:400; Sigma-Aldrich) and anti-connexin-43 (Cx-43)/GJA1 (1:400; Abcam) were used to visualize sarcomeres and gap junctions respectively. Antibodies were used at a 1:400 dilution in 1% BSA and incubated for 1 h at room temperature. Secondary antibodies of DyLight™ 488-conjugated goat anti-mouse (1:400; Jackson ImmunoResearch Laboratories) and 549-conjugated goat anti-rabbit were used at 1:400 dilutions in 1% BSA and incubated for 30 min at room temperature followed by four 1% BSA washes. Additionally, NRVM were cultured on multilayered scaffolds for 7 days and stained with Live/Dead assay reagents (Invitrogen Corp.) to determine cell viability.
Measurement of gene expression
Gene expression of cells cultured on hydrogels was evaluated using a previously described procedure with modifications.25 Briefly, NRVM were cultured on gelatin (10 mg/mL) or heart matrix (1.6 mg/mL) scaffolds for 1, 4, 7, and 14 days. RNA was extracted from scaffolds using 1 mL of TRIzol (Invitrogen Corp.) for each scaffold and homogenized using a power homogenizer. cDNA was synthesized from 2 μg total RNA, reverse transcribed in a 20 μL reaction mixture using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Relative quantification (RQ) of α-myosin heavy chain (Myh6, TaqMan Gene Expression Assay; Rn00691721_g1), β-myosin heavy chain (Myh7, Rn01488777_g1) and Cx-43 (Rn01433957_m1) was analyzed using quantitative real-time polymerase chain reaction (qRT-PCR) (Applied Biosystems) and gene expression was normalized to GAPDH (Hs99999905_m1). Next, 100 μg cDNA was amplified using TaqMan Gene Expression Master Mix with 250 nM TaqMan probe. Data were obtained using the standard program for 40 cycles on an ABI ViiA 7 Real-Time PCR System and analyzed using the comparative CT method with software from Applied Biosystems. All samples were normalized to GAPDH. Western blot analysis was performed using Image J by comparing the intensity of bands according to the instructions on the NIH Website (NIH).
Measurement of protein content
Western blotting was performed to determine the level of proteins in heart matrix versus gelatin scaffolds using a previously described procedure with modifications.26 After 7 days in culture, total protein lysate was isolated using 1 mg/mL of type 2 collagenase (Worthington). Lysate concentrations were analyzed using a bicinchoninic acid kit (BCA; Thermo Scientific). Extracts were denatured using β-mercaptoethanol and boiled for 5 min, then diluted to equal concentrations of total protein. The samples were electrophoresed by 0.1% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 100 V for 2 h and transferred onto nitrocellulose membranes at 30 V overnight. Membranes were washed in Tris-buffered saline with 0.05% Tween-20 (TBST), then blocked with 5% goat serum in TBST to reduce nonspecific binding. Membranes were incubated for 1 h at room temperature with primary antibodies: Cx-43 (Abcam) and GAPDH (Abcam). Membranes were washed with TBST and incubated for 30 min at 25°C with secondary antibodies conjugated to horseradish peroxidase (Jackson ImmunoResearch Laboratories). A 1-min Luminol reagent exposure was used to provide chemiluminescence, and images were developed using high-sensitivity X-ray film. Western blots were normalized to GAPDH expression and analysis was performed using Image J.
Measurement of conduction velocity
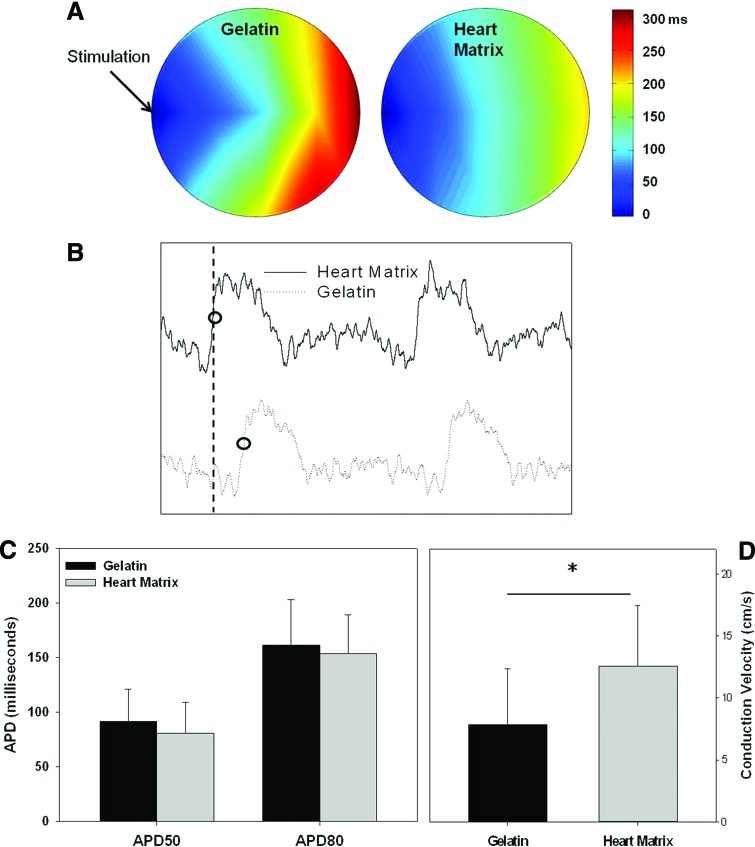
Conduction velocity of NRVM on gelatin or heart matrix scaffolds was analyzed using the voltage sensitive dye Di-8 ANEPPS (Enzo Life Science) and an IonOptix system (IonOptix LLC) followed by manufacturer's and previously described procedures with modifications.27,28 Briefly, spontaneously beating scaffolds with 7 days cultured NRVM were transferred to a 35 mm Petri dish and covered with 2 mL of warm Tyrode's solution (pH=7.4) of following composition: 130 NaCl, 5.4 KCl, 1 MgCl2, 0.3 Na2HPO4, 5.5 D-Glucose, 10 HEPES, and 2 CaCl2 in mM. Then, 2 mL of Di-8 ANEPPS (2 mM) in DMSO was added to the Petri dish and scaffolds were incubated for 13 min after being covered with aluminium foil. Stained scaffolds were transferred to a custom-built chamber that maintains the temperature of Tyrode's solution at 37°C. Cells were stimulated though platinum electrodes on the side of the scaffold with the following settings: 0.5–1 Hz, 5V, 10 ms duration, and bipolar. Action potential signals at 11 different positions were measured while electrodes were fixed in the same position though the experiment. Conduction velocity was calculated by measuring the activation time to 50% of action potential magnitude and the distance between electrodes and the positions where signals were obtained. Action potential duration (APD50 and APD80) was determined by measuring the repolarization time to 50% and 80% of return to baseline amplitude.
Contractile force measurement
Contractile forces of NRVM cultured on hydrogels were measured using a previously described procedure with modifications.29–31 Briefly, ring-shaped scaffolds (outer diameter=13 mm, thickness=4 mm) were prepared using custom-made Teflon molds. NRVM were cultured at 4×106 cells/mL for 7 days. Spontaneously beating scaffolds were transferred into a custom-made chamber equipped with parallel electrodes in Tyrode's buffer solution at 37°C. One end was attached to a post and the opposite end was attached to an isometric force transducer (Radnoti). An electrical impulse of 5 V magnitude with a pulse width of 10 ms and a frequency of 0.5–1 Hz was used. Then, force tracings were recorded until waveform was uniform using a LabVIEW data acquisition system (National Instruments Corporation). Obtained data were analyzed using MATLAB software. Fresh and warm (37°C) Tyrode's buffer solution was replaced for new samples.
Statistical analysis
Cell culture experiments were repeated three to six times with quadruplicate samples. Mechanical testing was repeated four or more times. Specific repeat numbers are noted in figure captions. Results are reported as mean±standard deviation. Significant differences between two groups were evaluated using analysis of variance with 95% or 99% confidence intervals; then, paired differences were tested with a post hoc t-test with a Bonferroni-Dunn correction for multiple comparisons. When p<0.05, the differences were considered to be statistically significant.
Results
Porous characteristics
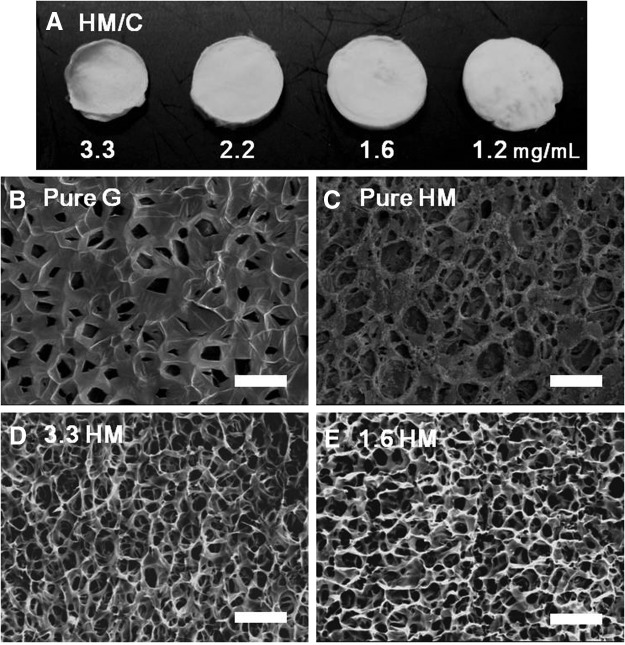
The effects of altering the concentration of heart matrix on pore diameter, porosity, volume, and pore shape were analyzed using Scanning Electron Microscopy and ImageJ. Macroscopic and SEM images showed that heart matrix hydrogels were significantly thinner than gelatin containing hydrogels (Fig. 1A and Table 1), and heart matrix hydrogels had closed, disconnected pores, in contrast to open, interconnected pores in gelatin hydrogels. To improve the porous structure, heart matrix solution was blended with chitosan. The concentration of heart matrix was varied by decreasing the amount of heart matrix and increasing the amount of chitosan to give the same final concentration of blends. Hydrogels with heart matrix/chitosan had open pores similar to gelatin/chitosan hydrogels (Fig. 1B–E). In addition, both heart matrix and gelatin-based hydrogels had an anisotropic and symmetric porous structure (Fig. 1B–E). Although the same volume (250 μL) of each solution and the same dimensions of molds were used to make all hydrogels, heart matrix/chitosan hydrogels had a smaller diameter, thickness, and volume after the lyophilization process compared with gelatin hydrogels (Table 1). However, there was no statistically significant effect of different materials and concentration on truss thickness (p>0.05; n=4) (data not shown).
FIG. 1.
Macro- and microscopic structure of hydrogels. (A) Macrograph of heart matrix hydrogels with different concentrations, and (B–E) SEM micrographs of pure gelatin, pure heart matrix, and heart matrixes with different concentrations. Open pores and interconnected three-dimensional structures of heart matrix containing hydrogels were formed by the presence of chitosan similar to gelatin containing hydrogels. Scale bars are 100 μm. SEM, scanning electron microscopy.
Table 1.
Comparison of Surface Architectures of Heart Matrix or Gelatin Containing Hydrogels
| Gelatin | Heart matrix | ||||
|---|---|---|---|---|---|
| Concentration (mg/mL) | 10 | 3.3 | 2.2 | 1.6 | 1.3 |
| Diameter (mm) | 12.2±0.1 | 9.9±0.2 | 11.1±0.2 | 11.85±0.2 | 12.0±0.1 |
| Thickness (mm) | 1.33±0.06 | 0.21±0.05 | 0.63±0.13 | 1.38±0.15 | 1.52±0.10 |
| Circularity (%) | 84±8 | 82±6 | 81±6 | 82±7 | 82±7 |
Heart matrix hydrogels had smaller diameter, thickness, and volume after the lyophilization process compared with gelatin containing hydrogels. There was significant changes in diameter and thickness of heart matrix composite hydrogels as amounts of heart matrix decreased (p>0.05, n=8). There was no significant effect of different concentrations on circularity of heart matrix hydrogels (p>0.05, n=8).
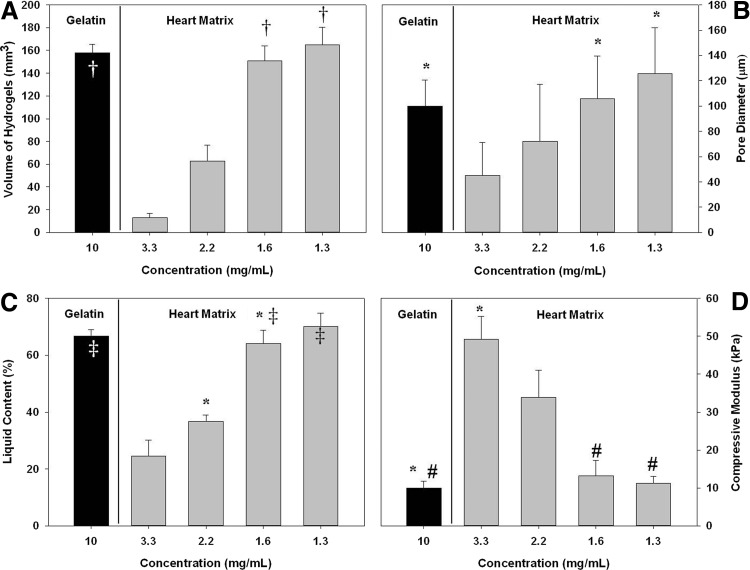
The volume of heart matrix/chitosan increased significantly when the proportion of chitosan was increased (13±4 in 3.3 mg/mL heart matrix and 165±15 in 1.3 mg/mL heart matrix in mm3; p<0.05, n=8). In addition, quantified results (Fig. 2B) showed an increase in pore diameter with increasing amounts of chitosan and 1.6 and 1.3 mg/mL heart matrix hydrogels had a similar pore diameter as gelatin hydrogels (109±34 in 1.6 mg/mL heart matrix, 125±36 in 1.3 mg/mL heart matrix, and 100±20 in gelatin in μm; p>0.05 n=5). Even though there were significant changes in pore diameter and volume, there was no significant effect of different heart matrix concentrations on the circularity of heart matrix hydrogels (p>0.05, n=8) (Table 1).
FIG. 2.
Effects of different concentrations on (A) volume, (B) pore diameter, (C) liquid content, and (D) compressive modulus. The volume of heart matrix hydrogels significantly increased as the proportion of chitosan increased (†p<0.05, n=8). Heart matrix hydrogels had an increase in pore diameter as amounts of chitosan increases, whereas gelatin hydrogels had a reduction in pore diameter. However, 1.6 and 1.3 mg/mL heart matrix has a pore diameter similar to gelatin hydrogels (*p>0.05 n=5). Liquid content significantly increased at reduced concentrations of heart matrix samples (*p<0.05, n=4). Heart matrix containing hydrogels had a significantly higher elastic modulus than gelatin containing hydrogels (*p<0.05, n=4). Elastic modulus of heart matrix hydrogels decreased with the decrease of heart matrix concentration. About 1.6 and 1.3 mg/mL heart matrix hydrogels and gelatin hydrogels had no significant difference in elastic modulus (#p=0.195, n=4).
Liquid content significantly increased at higher amounts of chitosan for heart matrix hydrogels (p<0.05, n=4); 3.3 mg/mL heart matrix hydrogels absorbed 25%±6% of the liquid volume in the scaffold and 2.2 mg/mL heart matrix samples absorbed 37%±3%, while 1.6 mg/mL heart matrix samples absorbed 64%±5% and 1.3 mg/mL heart matrix samples absorbed 70%±5%. In addition, 1.6 mg/mL heart matrix and 1.3 mg/mL heart matrix hydrogels had similar liquid contents to gelatin hydrogels (p=0.146, n=4) (Fig. 2C).
Compressive modulus
Heart matrix containing hydrogels had a significantly higher elastic modulus than gelatin containing hydrogels at the same blending ratio (p<0.05, n=4) (Fig. 2D). The elastic modulus of both types of hydrogels decreased with the decrease concentration of heart matrix (49.2±6.1 in 3.3 mg/mL heart matrix, 11.2±1.8 in 1.3 mg/mL heart matrix and 10.0±1.8 in gelatin, in kPa). The same influence of chitosan on hydrogel elastic modulus has been previously reported.11 1.6 mg/mL heart matrix and gelatin hydrogels had no significant difference in elastic modulus (13.2±4.0 in 1.6 mg/mL heart matrix and 10.0±1.8 in gelatin in kPa; p=0.195, n=4).
Viability, morphology, and retention of NRVMs
Since 1.6 mg/mL heart matrix containing hydrogels (referred to as heart matrix) had a similar structure and mechanical strength with 10 mg/mL gelatin containing hydrogels, which showed the best results in terms of cell spreading and viability and scaffold integrity previously,11 NRVMs were seeded on hydrogels of those blending ratios to evaluate cellular activity. NRVM morphology, retention, and viability were evaluated after 4 or 7 days in culture. NRVMs on all hydrogels were dispersed and attached to the hydrogels after 4 days in culture (Fig. 3A, B). Further, NRVMs on all hydrogels had clear sarcomeres and gap junctions with no difference in their morphology and connectivity after 7 days in culture (Fig. 3C, D). However, a significantly higher number of cells attached on heart matrix hydrogels compared with gelatin hydrogels (970±130 on heart matrix and 780±110 on gelatin in cells/mm2; p<0.05, n=10) (Fig. 3E).
FIG. 3.
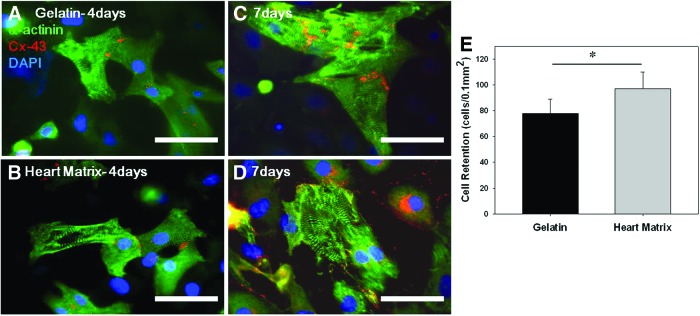
Attachment and morphology of neonatal rat ventricular myocytes (NRVM) on hydrogels. (A–D) Representative fluorescent images of NRVM stained for sarcomeres (Anti-α-actinin, green), gap junctions (anti-connexin-43 [Cx-43], red), and DNA (DAPI, blue) after 4 and 7 days in culture. (E) Quantified NRVM retention. NRVM on all hydrogels showed apparent sarcomeres and gap junctions with no difference in their morphology and connectivity after 7 days in culture. However, significantly higher number of cells was attached on heart matrix scaffolds compared with gelatin scaffolds (*p<0.05, n=10). Scale bars are 50 μm. Color images available online at www.liebertpub.com/tea
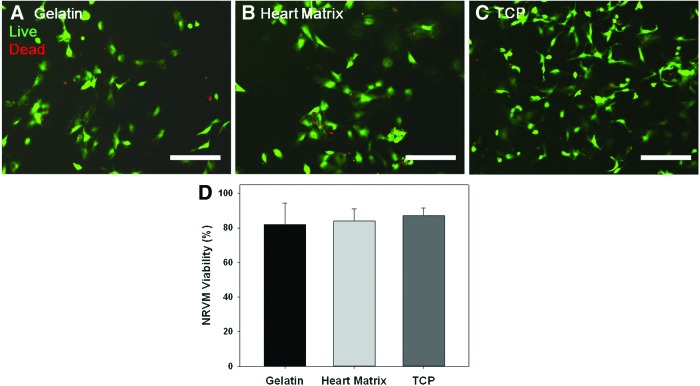
NRVM viabilities on hydrogels and tissue culture plastic (TCP) were quantified after 4 days in culture. NRVM cultured on heart matrix hydrogels were spindle shaped (Fig. 4A–C) and had no significant differences in cell viability compared to NRVM cultured on gelatin hydrogels and TCP (p=0.510, n=10). Both heart matrix and gelatin samples had viabilities of higher than 80% and no significant difference compared to TCP (p>0.05, n=10) (Fig. 4D).
FIG. 4.
Live cells were stained with green fluorescence by polyanionic dye calcein, and dead cells with red fluorescence were stained with EthD-1 after 4 days in samples of (A) gelatin, (B) heart matrix, and (C) tissue culture plastic (TCP). (D) Quantified NRVM viability. Both heart matrix and gelatin containing hydrogels had viabilities greater than 80% and no significant difference compared to TCP (p>0.05, n=10). Scale bars are 100 μm. Color images available online at www.liebertpub.com/tea
Gene and protein expression
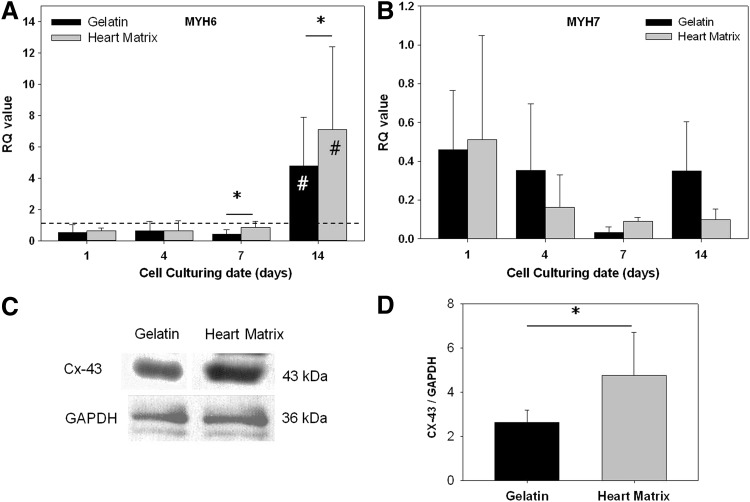
To verify the effect of heart matrix as a scaffold on levels of cardiac muscle-specific gene expression, MYH6, and MYH7 genes were evaluated after 1, 4, 7, and 14 days in culture using qRT-PCR. Gene expression levels were quantified as RQ values that represent fold changes of expression levels. Results (Fig. 5A) showed that MYH6 expression levels in both heart matrix and gelatin hydrogels were significantly increased after 14 days in culture (p<0.05, n=6). Further, MYH6 levels in heart matrix hydrogels were significantly higher than levels in gelatin hydrogels after 14 days in culture (7.1±5.3 of heart matrix and 4.7±3.1 of gelatin; p<0.05, n=6). In addition, MYH7 expression levels in both samples significantly decreased with time from day 1 to 7. However, no notable differences in MYH7 expression levels were observed in between heart matrix and gelatin hydrogels (Fig. 5B).
FIG. 5.
Cardiac muscle-specific gene expression, (A) α-myosin heavy chain (MYH6) and (B) β-myosin heavy chain (MYH7) genes were evaluated after 1, 4, 7, and 14 days in culture using quantitative real-time polymerase chain reaction. (C) The level of Cx-43 was determined by western blotting and (D) normalized to GAPDH expression. Significant increases of MYH6 expression levels in both heart matrix and gelatin scaffolds were observed after 14 days in culture (*p<0.05, n=6). MYH6 levels in heart matrix scaffolds were significantly higher than levels in gelatin after 14 days in culture (#p<0.05, n=6). MYH7 expression levels in both scaffolds significantly decreased with time from day 1 to 7. There were no significant differences in MYH7 expression levels in between heart matrix and gelatin scaffolds. GAPDH normalized Cx-43 expression levels in heart matrix scaffolds were significantly higher than in gelatin scaffold after 7 days in culture (*p<0.05, n=6).
Western blot analysis (Fig. 5C, D) showed that GAPDH normalized Cx-43 expression level in heart matrix samples was significantly higher than in gelatin samples after 7 days in culture (4.8±1.9 in heart matrix and 2.6±0.6 in gelatin; p<0.05, n=6).
Conduction velocity
Action potential characteristics of NRVM cultured on heart matrix and gelatin containing hydrogels were evaluated using voltage sensitive dye (Di-8 ANEPPS) after 7 days in culture. Figure 6A illustrates optical maps of activation time propagations (Fig. 6A). Figure 6B presents examples of individual action potential signals from NRVM on different hydrogels. The quantified result (Fig. 6D) showed that the average conduction velocity of NRVM on heart matrix hydrogels was significantly faster than on gelatin hydrogels (12.6±4.9 in heart matrix and 7.9±4.4 in gelatin in cm/s; p<0.05, n=11). There was no significant difference in either APD50 or APD80 (APD50; 81±28 in heart matrix and 92±29 in gelatin in milliseconds; APD80; 154±35 in heart matrix and 161±41 in gelatin in ms; p>0.05, n=4 for both APD50 and APD80) (Fig. 6C).
FIG. 6.
NRVM cultured on heart matrix and gelatin containing hydrogels were stained with Di-8 ANEPPS after 7 days in culture to evaluate action potential characteristics. (A) Optical maps of activation time, (B) action potential profile, (C) action potential duration (APD) at 50% and 80% of return to baseline amplitude, and (D) conduction velocity (CV). The average conduction velocity of heart matrix scaffolds was significantly faster in gelatin scaffolds (*p<0.05, n=11). There was no significant difference in both APD50 and APD80 (p>0.05, n=4). Color images available online at www.liebertpub.com/tea
Contractile stress
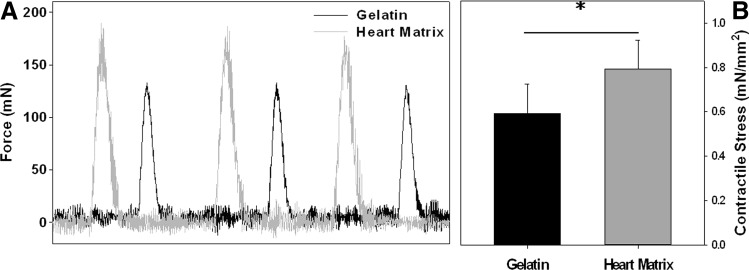
The contractile stress of NRVM cultured on heart matrix and gelatin containing hydrogels was compared after 7 days in culture. A regular train of contractions from NRVM was observed in response to the stimulation with varying frequencies from 0.5 Hz to 1 Hz. The contractile forces of cells on both types of hydrogels were in the range of 110 to 200 mN (Fig. 7A). The contractile stress of cells on heart matrix hydrogels was significantly higher than on gelatin hydrogels (0.79±0.13 of heart matrix and 0.59±0.13 of gelatin in mN/mm2; p<0.05, n=5) (Fig. 7B).
FIG. 7.
Contractile force of NRVM cultured on heart matrix and gelatin containing hydrogels were compared after 7 days in culture: (A) Representative contractile force profile and (B) contractile stress (force/area). A regular train of contractions from NRVM was observed in response to the stimulation with varying frequencies from 0.5 to 1 Hz. The contractile stresses of cells on both types of hydrogels were in the range of 110 and 200 mN. The contractile stress of cells on heart matrix hydrogels was significantly higher than on gelatin hydrogels (*p<0.05, n=5).
Discussion
In this study, we designed a 3D cardiac patch using decellularized porcine heart matrix, chitosan, and a PCL core for a surgical use. Chitosan allowed control of degradation as previous studies have shown that chitosan-gelatin blends of higher than 50% gelatin degraded prior to the end of the tissue regeneration process.32–34 PCL provided suturability (1.8 N of stitch tension), ultimate tensile strength (2 MPa), and biodegradability (10% of weight loss after 50 days) that allow the hydrogel to be used as a cardiac patch for full-thickness RVOT repair.11 Porous characteristics and compressive modulus were evaluated at various concentrations of heart matrix (3.3, 2.2, 1.6, and 1.3 mg/mL of heart matrix).
Even though the same volume (250 μL) of solutions were used to make all hydrogels, heart matrix containing hydrogels were thinner than gelatin containing hydrogels while there was no significant difference in the diameter. In addition, although heart matrix hydrogels had significantly larger pore diameter than gelatin hydrogels, heart matrix hydrogels had lower liquid content ratio and volume than gelatin hydrogels. This suggests that higher amounts of heart matrix (>3.3 mg/mL) tend to form denser hydrogels than gelatin. SEM analysis also showed that pure heart matrix hydrogels had completely closed pores in contrast to pure gelatin samples. The concentration of heart matrix had no effect on either diameter or circularity. However, the thickness and pore diameter of heart matrix hydrogels were increased by decreasing the concentration of the heart matrix. An increase in thickness and pore diameter also demonstrated increased volume and liquid content of heart matrix hydrogels. Pore size, circularity, and density have significant roles in forming 3D tissue with cells, since larger and more open pores allow immigration; interconnectivity of cells; and easy transport of nutrients, wastes, and cellular signals for proper tissue regeneration.35–37 Previous studies reported that porous characteristics were altered by mixing chitosan solution with gelatin or PCL.11,36,37 Heart matrix hydrogels with a concentration of 1.6 mg/mL or lower had open pores and significantly increased average pore diameters compared with higher concentrations of heart matrix hydrogels. In addition, heart matrix samples with a concentration of 1.6 mg/mL or lower showed similar hydrogel volume and pore diameter to gelatin containing samples (10 mg/mL gelatin), which was found to have the best scaffold integrity with cell viability and spreading in previous study.11 Elastic moduli of both heart matrix/chitosan and gelatin/chitosan hydrogels increased as the chitosan concentration increased. Our previous article reported the same influence of chitosan on hydrogels. About 1.6 mg/mL heart matrix containing hydrogels had a similar elastic modulus to native heart matrix.38 In addition, previous research reported that there was no significant effect of hydrogel coating on tensile strength in multilayered hydrogels (1.89±0.14 MPa).11 Hence, the tensile strength of heart matrix hydrogels was not tested in this study.
Since 1.6 mg/mL heart matrix containing hydrogels had similar structural and mechanical properties as 10 mg/mL gelatin containing hydrogels, which showed the best results in terms of cell spreading and viability and scaffold integrity previously,11 biological and physiological properties of 1.6 mg/mL heart matrix containing hydrogels (referred to as heart matrix) were compared to properties of 10 mg/mL gelatin containing hydrogel (referred to as gelatin). Significant amounts of myosin heavy chain in both heart matrix scaffold and gelatin scaffold were detected after 14 days in culture. There was a significant increase in α-MYH levels of NRVM cultured on heart matrix hydrogels after 14 days compared with gelatin hydrogels. There was no difference in α-MYH expression levels between heart matrix scaffold and gelatin scaffold from 1 to 7 days in culture. However, there was no effect on β-MYH gene expression levels for the 14 day culture period. Previous studies have reported that the myosin heavy chain composition of the ventricular myocardium of rodent models has more than 90% α-MYH39,40 during postnatal maturation, whereas humans had 95% β-MYH.41,42 Further, in rodent models, α-MYH level is increased by increase of thyroid hormone secretion and exercise, whereas β-MYH is increased by aging, cardiomyopathy, and reduction of thyroid hormone.40,43 A previous study reported that the percentage of α-MYH expression of 10 day-old rats was significantly increased compared with 3 day-old rats and reached maximum levels at about 20-day-old rats.44
Since Cx-43-mediated gap junctions provide the pathways for intercellular current flow, enabling coordinated action potential propagation, these play a critical role in determining conduction velocity in cardiac tissue.45,46 Our results demonstrated that NRVM cultured on heart matrix containing hydrogels had a significantly faster conduction velocity than NRVM cultured on gelatin containing hydrogels. Previous studies measured conduction velocities of NRVM on different biomaterials or native tissues: ∼15 cm/s when cultured on poly(glycolic acid),47 ∼14 cm/s when cultured on collagen sponge,48 and ∼27 cm/s in native neonatal (10 days old) cardiac tissues.49 Furthermore, Western blot analysis found a significant higher level of Cx-43 in heart matrix/containing hydrogels compared with gelatin containing hydrogels after 7 days in culture. Both APD50 and APD80 of NRVM on heart matrix hydrogels were similar to gelatin hydrogels. Our results were comparable with previous reports that measured the APDs of NRVM (APD70=134±5 ms50; APD90=140±12 ms27).
The average contractile stress of NRVM on heart matrix hydrogels was significantly higher than on gelatin hydrogels. This might be due to higher cell retention rates and connectivity of NRVMs on heart matrix hydrogels. In these hydrogels, the contractile stress was higher than or comparable with previously reported results of average contractile stress of NRVM on different types of hydrogels; 0.42 mN/mm2 of collagen/matrigel,51 0.7 mN/mm2 of collagen/fibrin,52 and 1.1–2.1 mN/mm2 pure fibrin27 hydrogels.
In conclusion, these results demonstrate that a heart patch incorporating heart matrix can support cardiomyocytes and generates higher contractile stress and conduction velocity than a hydrogel containing gelatin, demonstrating improved function as myocardial tissue used as a full-thickness defect patch in RVOT repair for Tetralogy of Fallot and other heart defects. Future research will involve testing this multilayered scaffold in vivo in a full-thickness defect right ventricle patch in a rat model.
Acknowledgments
We would like to thank Dr. Karen L. Christman in the Department of Bioengineering at University of California, San Diego for providing decellularized porcine heart myocardium and Dr. Antonios Mikos in the Department of Bioengineering at Rice University for the use of lyophilizer. This work was supported by the NIH R21NL110330 (to J.G.J.) and Texas Children's Hospital.
Disclosure Statement
No competing financial interests exist.
References
- 1.Jenkins K.J., Correa A., Feinstein J.A., Botto L., Britt A.E., Daniels S.R., et al. Noninherited risk factors and congenital cardiovascular defects: current knowledge. Circulation 115,2995, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Reller M.D., Strickland M.J., Riehle-Colarusso T., Mahle W.T., and Correa A.Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr 153,807, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang D., Shadrin I.Y., Lam J., Xian H.-Q., Snodgrass H.R., and Bursac N.Tissue-engineered cardiac patch for advanced functional maturation of human ESC-derived cardiomyocytes. Biomaterials 34,5813, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarig U., and Machluf M.Engineering cell platforms for myocardial regeneration. Expert Opin Biol Ther 11,1055, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Santibanez-Salgado J.A., Olmos-Zuniga J.R., Perez-Lopez M., Aboitiz-Rivera C., Gaxiola-Gaxiola M., Jasso-Victoria R., et al. Lyophilized glutaraldehyde-preserved bovine pericardium for experimental atrial septal defect closure. Eur Cells Mater 19,158, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Tudorache I., Kostin S., Meyer T., Teebken O., Bara C., Hilfiker A., et al. Viable vascularized autologous patch for transmural myocardial reconstruction. Eur J Cardiothorac Surg 36,306, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Park H., Radisic M., Lim J.O., Chang B.H., and Vunjak-Novakovic G.A novel composite scaffold for cardiac tissue engineering. In Vitro Cell Dev Biol Anim 41,188, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Mirensky T.L., and Breuer C.K.The development of tissue-engineered grafts for reconstructive cardiothoracic surgical applications. Pediatr Res 63,559, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Mayer J.E., Jr., Uses of homograft conduits for right ventricle to pulmonary artery connections in the neonatal period. Semin Thorac Cardiovasc Surg 7,130, 1995 [PubMed] [Google Scholar]
- 10.Pok S.Design of synthetic scaffolds for tissue regeneration applications [PhD Dissertation]. Chemical Engineering, Oklahoma State University, 2012 [Google Scholar]
- 11.Pok S., Myers J.D., Madihally S.V., and Jacot J.G.A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater 9,5630, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arenas-Herrera J.E., Ko I.K., Atala A., and Yoo J.J.Decellularization for whole organ bioengineering. Biomed Mater 8,2013 [DOI] [PubMed] [Google Scholar]
- 13.Soto-Gutierrez A., Zhang L., Medberry C., Fukumitsu K., Faulk D., Jiang H., et al. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods 17,677, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pok S., and Jacot J.G.Biomaterials advances in patches for congenital heart defect repair. J Cardiovasc Transl Res 4,646, 2011 [DOI] [PubMed] [Google Scholar]
- 15.Zang M., Zhang Q., Chang E.I., Mathur A.B., and Yu P.Decellularized tracheal matrix scaffold for tissue engineering. Plast Reconstr Surg 130,532, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Ponce Marquez S., Saez Martinez V., Ambrose W.M., Wang J., Garagorri Gantxegui N., Schein O., et al. Decellularization of bovine corneas for tissue engineering applications. Acta Biomater 5,1839, 2009 [DOI] [PubMed] [Google Scholar]
- 17.McDade J.K., Brennan-Pierce E.P., Ariganello M.B., Labow R.S., and Michael Lee J.Interactions of U937 macrophage-like cells with decellularized pericardial matrix materials: Influence of crosslinking treatment. Acta Biomater 9,7191, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Crapo P.M., Gilbert T.W., and Badylak S.F.An overview of tissue and whole organ decellularization processes. Biomaterials 32,3233, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evans D.W., Moran E.C., Baptista P.M., Soker S., and Sparks J.L.Scale-dependent mechanical properties of native and decellularized liver tissue. Biomech Model Mechanobiol 12,569, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Singelyn J.M., and Christman K.L.Injectable materials for the treatment of myocardial infarction and heart failure: the promise of decellularized matrices. J Cardiovasc Transl Res 3,478, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singelyn J.M., DeQuach J.A., Seif-Naraghi S.B., Littlefield R.B., Schup-Magoffin P.J., and Christman K.L.Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials 30,5409, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seif-Naraghi S.B., Singelyn J.M., Salvatore M.A., Osborn K.G., Wang J.J., Sampat U., et al. Safety and efficacy of an injectable extracellular matrix hydrogel for treating myocardial infarction. Sci Transl Med 5,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pok S.W., Wallace K.N., and Madihally S.V.In vitro characterization of polycaprolactone matrices generated in aqueous media. Acta Biomater 6,1061, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jacot J.G., McCulloch A.D., and Omens J.H.Substrate stiffness affects the functional maturation of neonatal rat ventricular myocytes. Biophys J 95,3479, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Connell J.P., Augustini E., Moise K.J., Jr., Johnson A., and Jacot J.G.Formation of functional gap junctions in amniotic fluid-derived stem cells induced by transmembrane co-culture with neonatal rat cardiomyocytes. J Cell Mol Med 17,774, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benavides O.M., Petsche J.J., Moise K.J., Johnson A., and Jacot J.G.Evaluation of endothelial cells differentiated from amniotic fluid-derived stem cells. Tissue Eng Part A 18,1123, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sondergaard C.S., Mathews G., Wang L., Jeffreys A., Sahota A., Wood M., et al. Contractile and electrophysiologic characterization of optimized self-organizing engineered heart tissue. Ann Thorac Surg 94,1241, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zong X.H., Bien H., Chung C.Y., Yin L.H., Fang D.F., Hsiao B.S., et al. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials 26,5330, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Baar K., Birla R., Boluyt M.O., Borschel G.H., Arruda E.M., and Dennis R.G.Self-organization of rat cardiac cells into contractile 3-D cardiac tissue. FASEB J 19,275, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Birla R.K., Borschel G.H., Dennis R.G., and Brown D.L.Myocardial engineering in vivo: formation and characterization of contractile, vascularized three-dimensional cardiac tissue. Tissue Eng 11,803, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Yuan Ye K., Sullivan K.E., and Black L.D.Encapsulation of cardiomyocytes in a fibrin hydrogel for cardiac tissue engineering. J Vis Exp 19,2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghasemi-Mobarakeh L., Prabhakaran M.P., Morshed M., Nasr-Esfahani M.-H., and Ramakrishna S.Electrospun poly([var epsilon]-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials 29,4532, 2008 [DOI] [PubMed] [Google Scholar]
- 33.Hu M., Kurisawa M., Deng R., Teo C.-M., Schumacher A., Thong Y.-X., et al. Cell immobilization in gelatin-hydroxyphenylpropionic acid hydrogel fibers. Biomaterials 30,3523, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Huang Y., Onyeri S., Siewe M., Moshfeghian A., and Madihally S.V.In vitro characterization of chitosan-gelatin scaffolds for tissue engineering. Biomaterials 26,7616, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Pok S., Dhane D.V., and Madihally S.V.Computational simulation modelling of bioreactor configurations for regenerating human bladder. Comput Methods Biomech Biomed Eng 16,840, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Yang C., Xu L., Zhou Y., Zhang X., Huang X., Wang M., et al. A green fabrication approach of gelatin/CM-chitosan hybrid hydrogel for wound healing. Carbohydr Polym 82,1297, 2010 [Google Scholar]
- 37.Zhong X., Ji C., Chan A.K., Kazarian S.G., Ruys A., and Dehghani F.Fabrication of chitosan/poly(epsilon-caprolactone) composite hydrogels for tissue engineering applications. J Mater Sci Mater Med 22,279, 2011 [DOI] [PubMed] [Google Scholar]
- 38.Jacot J.G., Martin J.C., and Hunt D.L.Mechanobiology of cardiomyocyte development. J Biomech 43,93, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mercadier J.J., Lompre A.M., Wisnewsky C., Samuel J.L., Bercovici J., Swynghedauw B., et al. Myosin isoenzyme changes in several models of rat cardiac hypertrophy. Circ Res 49,525, 1981 [DOI] [PubMed] [Google Scholar]
- 40.Miyata S., Minobe W., Bristow M.R., and Leinwand L.A.Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res 86,386, 2000 [DOI] [PubMed] [Google Scholar]
- 41.Schwartz K., Lecarpentier Y., Martin J.L., Lompre A.M., Mercadier J.J., and Swynghedauw B.Myosin isoenzymic distribution correlates with speed of myocardial contraction. J Mol Cell Cardiol 13,1071, 1981 [DOI] [PubMed] [Google Scholar]
- 42.Tsuchimochi H., Sugi M., Kuro-o M., Ueda S., Takaku F., Furuta S., et al. Isozymic changes in myosin of human atrial myocardium induced by overload. Immunohistochemical study using monoclonal antibodies. J Clin Invest 74,662, 1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swynghedauw B.Developmental and functional adaptation of contractile proteins in cardiac and skeletal-muscles. Physiol Rev 66,710, 1986 [DOI] [PubMed] [Google Scholar]
- 44.Cappelli V., Bottinelli R., Poggesi C., Moggio R., and Reggiani C.Shortening velocity and myosin and myofibrillar ATPase activity related to myosin isoenzyme composition during postnatal development in rat myocardium. Circ Res 65,446, 1989 [DOI] [PubMed] [Google Scholar]
- 45.Jongsma H.J., and Wilders R.Gap junctions in cardiovascular disease. Circ Res 86,1193, 2000 [DOI] [PubMed] [Google Scholar]
- 46.Rohr S.Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res 62,309, 2004 [DOI] [PubMed] [Google Scholar]
- 47.Bursac N., Loo Y., Leong K., and Tung L.Novel anisotropic engineered cardiac tissues: studies of electrical propagation. Biochem Biophys Res Commun 361,847, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radisic M., Fast V.G., Sharifov O.F., Iyer R.K., Park H., and Vunjak-Novakovic G.Optical mapping of impulse propagation in engineered cardiac tissue. Tissue Eng Part A 15,851, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun L.S., Legato M.J., Rosen T.S., Steinberg S.F., and Rosen M.R.Sympathetic innervation modulates ventricular impulse propagation and repolarization in the immature rat-heart. Cardiovasc Res 27,459, 1993 [DOI] [PubMed] [Google Scholar]
- 50.den Haan A.D., Veldkamp M.W., Bakker D., Boink GJJ, Janssen R.B., de Bakker J.M.T., et al. Organ explant culture of neonatal rat ventricles: a new model to study gene and cell therapy. PLoS One 8,2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leontyev S., Schlegel F., Spath C., Schmiedel R., Nichtitz M., Boldt A., et al. Transplantation of engineered heart tissue as a biological cardiac assist device for treatment of dilated cardiomyopathy. Eur J Heart Fail 15,23, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Boudou T., Legant W.R., Mu A., Borochin M.A., Thavandiran N., Radisic M., et al. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Eng Part A 18,910, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]