Abstract
We investigated the ability of autologous adipose-derived cells injected into cryoinjured rabbit urethras to improve urinary continence and explored the possible mechanisms by which it occurred. Adipose tissue was harvested from the perivesical region of nine 10-week-old female New Zealand White rabbits and cultured for 7 days. Immediately after harvesting the tissue, we injured the internal urethral orifice by spraying liquid nitrogen for 20 s. The cultured cells expressed the mesenchymal cell marker STRO1, but not muscle cell markers myoglobin or smooth muscle actin (SMA). Just before implantation, the adipose-derived cells were labeled with the PKH26 fluorescent cell linker. Autologous 2.0×106 adipose-derived cells (five rabbits) or a cell-free control solution (four rabbits) was injected around the cryoinjured urethras at 7 days after injury. Fourteen days later, the leak point pressure (LPP) was measured, and the urethras were harvested for immunohistochemical analyses. At 14 days after implantation, LPP of the cell-implanted group was significantly higher compared with the cell-free control group (p<0.05). In immunohistochemical examination, the reconstructed skeletal and smooth muscle areas in the cell-implanted regions were significantly more developed than those in controls (p<0.01). Implanted PKH26-labeled adipose-derived cells were immunohistochemically positive for myoglobin, SMA, and Pax7 antibodies, which are markers for skeletal muscles, smooth muscles, and myoblast progenitor cells, respectively. In addition, these implanted cells were positive for the nerve cell markers, tubulin β3, S100, and the vascular endothelial cell marker, von Willebrand factor. Furthermore, some of the implanted cells were positive for the transforming growth factor β1, nerve growth factor, and vascular endothelial growth factor. In conclusion, implantation of autologous adipose-derived cells into the cryoinjured rabbit urethras promoted the recovery of urethral function by myogenic differentiation, neuroregeneration, and neoangiogenesis of the implanted cells and/or the surrounding tissues as well as by bulking effects. Thus, treatment of human radical prostatectomy-related stress urinary incontinence by adipose-derived cell implantation could have significant therapeutic effects.
Introduction
Postsurgical-related urinary incontinence can occur as a result of radical prostatectomy1,2 or bladder neck surgery.3 It is characterized by severely decreased urethral closure pressure due to malfunction of the closure mechanism, and it results in intractable urinary incontinence.4 Under these circumstances, improvement of urinary continence requires increased urethral closure pressure. Surgical and/or conservative treatment strategies are widely accepted to achieve this. Surgical treatment options include retropubic suspension,5,6 midurethral7 and pubovaginal slings,8,9 and the implantation of an artificial urinary sphincter.10 Conservative treatment options include pelvic physiotherapy, pharmacological therapy, and urethral bulking agents.7,11 However, these treatments do not provide good success rates for long term.7 Recently, adipose-derived cells were used successfully to treat radical prostatectomy-related stress urinary incontinence in humans.12 However, there is little evidence regarding the ability of cell therapy, especially in humans, to reconstruct the tissues associated with functional recovery of urethral sphincters.
Previously, we showed that implantation of autologous bone marrow-derived cells could reconstruct functional urethral sphincters in a rabbit cryoinjury model.13 As sources for cell therapy, skeletal muscles14–16 and other somatic (stem) cells have also been investigated to treat for urinary incontinence. These cell sources for regenerative medicine would be in great demand if they could be easily isolated in high quality and large quantities without donor-site morbidity or discomfort. The adipose-derived cells have the potential to overcome these problems.17,18 In this study, we produced cryoinjured rabbit urethral sphincters according to our previous report13 and determined that autologous adipose-derived cells could reconstruct functional urethral sphincters. Based on those results, we explored the possible mechanisms by which the implanted cells regenerated the urethral tissue.
Materials and Methods
Animals
Nine female New Zealand White rabbits (2.0–2.5 kg; Japan SLC, Inc., Shizuoka, Japan) at postnatal week 10 were used in this study. All experimental procedures were approved by the Animal Ethics Committee of Shinshu University School of Medicine and performed in accordance with the National Institutes of Health Animal Care Guidelines. Throughout the study period, all rabbits were given food and tap water ad libitum and maintained in a temperature and humidity controlled room on a 12-h light/12-h dark cycle.
Isolation and culture of adipose-derived cells
To harvest adipose tissues, the rabbits were anesthetized by inhalation of 4% sevoflurane (Sevofrane®; Abbot Japan Co., Ltd., Tokyo, Japan). Following a midline abdominal incision, ∼1 g of adipose tissue was harvested from the perivesical region. The adipose tissue was washed with ice-cold phosphate-buffered saline (PBS, pH 7.4) and then minced into small pieces. Following that, the tissue was digested with 0.25% collagenase type I (Wako Pure Chemical Industries, Ltd., Osaka, Japan) for 90 min at 37°C with moderate shaking. After shaking, the cell suspension was centrifuged at 1000 rpm for 4 min, and then, the supernatant was discarded. The pellet was suspended with 20 mL of the culture medium composed of 4.5 g/L glucose–Dulbecco's modified Eagle's medium (Life Technologies, Carlsbad, CA) supplemented with 15% regular fetal bovine serum (FBS; Biowest, Paris, France) and 0.1% penicillin–streptomycin (Life Technologies). The suspended cells were filtered with 75-μm nylon mesh filters (BD Biosciences, San Jose, CA). Finally, 5 mL of the cell suspension was seeded into type I collagen-coated 60-mm culture dishes (Iwaki; Ashai Techno Glass, Chiba, Japan). The cells were incubated at 37°C in humid air with 5% CO2 for 7 days. The medium was changed every day to wash off nonattached cells.
Seven days after seeding, we simply harvested the attached and proliferated cells. Briefly, the cells were detached with 0.25% trypsin–ethylenediaminetetraacetic acid solution and washed by centrifugation at 1000 rpm for 5 min through a medium without FBS. To identify the adipose-derived cells after implantation into the cryoinjured urethras, just before implantation, the harvested cultured cells were labeled with the PKH26 fluorescent cell linker (Sigma Aldrich, St. Louis, MO), following the manufacturer's protocol. The harvested cells were suspended with the PKH26 solution and incubated at 25°C for 5 min. The labeling reaction was stopped by adding an equal volume of FBS and incubated for 1 min. The number of labeled cells and the viability of the cells determined by trypan blue exclusion were estimated with an automated cell counter (Countess®; Life Technologies) according to the manufacturer's protocol. Following that, we resuspended the labeled cells in the complete medium at 1.0×106 cells/mL.
Production of cryoinjured urethral sphincter model
Immediately after harvesting the adipose tissues, we sprayed liquid nitrogen to injure the urethral sphincters of the same rabbits according to our previous report.13 Briefly, the urinary bladder was exposed through the midline abdominal incision, and then the anterior wall of the bladder was incised. The internal urethral sphincter, located at the inferior end of the bladder and the proximal end of the urethra, was sprayed with liquid nitrogen for 20 s (Cryo Pro®; Cortex Technology, Hudsund, Denmark). The bladder was surgically closed and returned to the pelvic cavity. The incision was closed in two layers by suture and anesthesia was weaned. Following the surgeries, the rabbits were allowed to recover and kept for 7 days.
Implantation of autologous adipose-derived cells
Seven days after cryoinjury and culture, the internal urethral orifice was exposed in anesthetized rabbits as above. Using a stereomicroscope, we implanted 0.5×106 autologous adipose-derived cells suspended with 500 μL into the injured regions at 3-, 6-, 9-, and 12-o'clock positions with a 29-gauge syringe (n=5 rabbits). The total cell number was 2.0×106. For the cell-free control group (n=4), 500 μL of cell-free culture medium was similarly injected.
Measurement of leak point pressure
At 14 days after cell implantation and cell-free control injections, the leak point pressure (LPP) of each rabbit was measured. The rabbits were anesthetized as above, and then an 8 Fr catheter (SAFEED® Nelaton Catheter; Terumo, Tokyo, Japan) was inserted into the bladder through the urethra. The bladder was emptied and the catheter was connected to a three-way stopcock that allowed connection to a microinjection pump (Model 200; Muromachi-Kikai, Tokyo, Japan) and to a pressure transducer (P23 DC; Statham, Oxnard, CA). The bladder was filled at a continuous rate of 100 mL/h saline maintained at room temperature. The internal bladder pressure was recorded on a pen oscilligraph (10 mm/min recording speed, Recti-Horiz-8K; NEC San-ei Instruments, Tokyo, Japan). The internal bladder pressure sufficient to cause leakage from the urethra was recorded as the LPP. The average LPP of each rabbit was calculated after three voiding cycles.
Immunohistochemistry
Just after LPP measurements, the rabbits were euthanized with an overdose of phenobarbital injected through the inferior vena cava. Following that, the lower urinary tracts, including the urinary bladder and urethra, were quickly harvested for immunohistochemistry. Tissue samples were fixed in 4% paraformaldehyde for 12 h at 4°C. For analysis of the cell-implanted regions, we trimmed the fixed tissues to identify the 3-, 6-, 9-, and 12-o'clock positions of urethras. The treated samples were embedded in paraffin and then cut into 5-μm-thick serial sections. The urethral sections were deparaffinized with xylene, rehydrated with ethanol, rinsed three times with PBS, immersed in 10 mM sodium citrate (pH 6.0), and microwaved at 100°C for 10 min for antigen retrieval. The sections were blocked with 1.5% normal donkey serum and 1.5% nonfat milk in PBS for 1 h at 4°C.
To detect muscle structures, the sections were incubated with antibodies for myoglobin (1:200, rabbit polyclonal; Spring Bioscience, Inc., Pleasanton, CA), smooth muscle actin (SMA, 1:100, mouse monoclonal; Progen Biotechnik GmbH, Heidelberg, Germany), or the satellite cell marker Pax7 (1:1000, rabbit; LifeSpan Bioscience, Inc., Seattle, WA) for 12 h at 4°C. To detect nerve cells, the sections were incubated with antibodies for the common nerve cell markers S100 (1:50, mouse monoclonal; Abcam, Cambridge, United Kingdom) or tubulin β3 (1:50, rabbit monoclonal; Novus Biologicals, Inc., Littleton, CO) for 12 h at 4°C. In addition, other sections were incubated with antibodies for von Willebrand factor (vWF, 1:200, rabbit polyclonal; Abcam), transforming growth factor β1 (TGF β1, 1:100, rabbit polyclonal; Abcam), nerve growth factor (NGF, 1:500, rabbit polyclonal; Abcam), or vascular endothelial growth factor (VEGF, 1:100, rabbit polyclonal; Bioss, Woburn, MA) for 12 h at 4°C.
After rinsing the sections with PBS, they were incubated with the respective secondary antibodies consisting of donkey anti-rabbit or anti-mouse IgG conjugated with Alexa Fluor 488 (1:250; Molecular Probes, Eugene, OR) for 1 h at 4°C. Following rinsing, they were counterstained with 4′,6-diamidino-phenylindole dihydrochloride (5 μg/mL; Molecular Probes) and mounted with the Fluorescent Mounting Medium (Dako Cytomation, Carpinteria, CA). The slides were observed and photographed with a Leica DAS Microscope (Leica Microsystems GmbH, Wetzlar, Germany). Other sections from each sample were stained by Masson's trichrome stain. These slides were observed and photographed with a common optical microscope.
To characterize the cultured PKH26-labeled adipose-derived cells, they were double stained with antibodies for the mesenchymal marker STRO1 (CD34; R&D System, Inc., Minneapolis, MN) and either myoglobin or SMA as above.
Quantification of muscle area
The images of myoglobin- and SMA-stained samples were used to semiquantitatively evaluate the proportion of skeletal muscle and smooth muscle layers in each slide. The cross-sectional areas of both skeletal and smooth muscle were determined using a computerized digital morphometric analysis system (Image-Pro® Plus; Media Cybernetics, Inc., Bethesda, MD). The percentage of myoglobin- and SMA-positive areas of the internal urethral sphincters in each slide was quantitatively estimated.
Statistical analysis
Data were entered into a database and analyzed using GraphPad Prism (version 5.0f; GraphPad Software, San Diego, CA). Results are expressed as means±standard deviations, and the differences between the groups were assessed by unpaired t-tests. Throughout the analysis, two-tailed p-values<0.05 were considered to be statistically significant.
Results
Implantation of adipose-derived cells into cryoinjured urethral sphincter
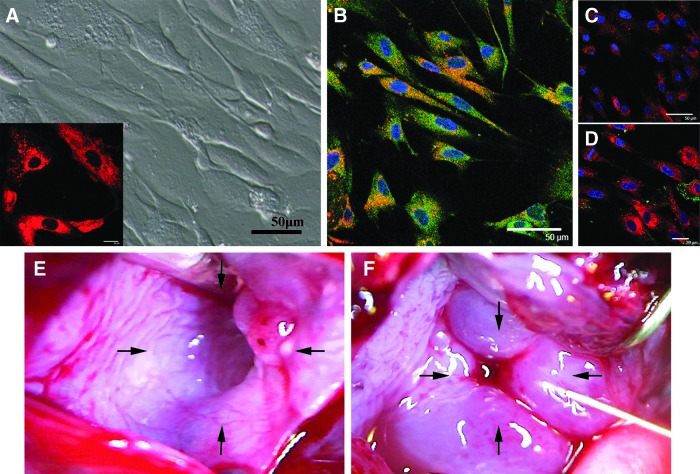
Immediately after seeding in collagen-coated dishes, the population of cells harvested from the adipose tissue was composed of rounded, polygonal cells along with red blood cells. By phase microscopy, most of the cultured cells were spindle shaped, but varied in size after 7 days in culture (Fig. 1A). At that time, they were labeled with PKH26 (Fig. 1A inset), and autologously implanted into the injured urethral sphincters. The cultured cells were characterized by staining for the mesenchymal cell marker STRO1, skeletal muscle marker myoglobin, or smooth muscle marker SMA. The cells were uniformly positive for STRO1 (Fig. 1B), while they were negative for myoglobin (Fig. 1C) and SMA (Fig. 1D).
FIG. 1.
Cultured autologous adipose-derived cells and cryoinjured urethral sphincters. (A) At 7 days after culture, the adhered and proliferating adipose-derived cells were spindle shaped. Just before implantation, the cells were labeled with PKH26 (inset). Scale bar: 50 and 20 μm. (B) The cultured cells were positive for STRO1 (yellow). Blue: nuclei. Scale bar: 50 μm. (C, D) The cultured cells (red) were negative for myoglobin (C) and smooth muscle actin (SMA) antibody (D). Blue: nuclei. Scale bar: 50 and 20 μm. (E) At 7 days after injury, the cryoinjured urethra remained opened (arrows). (F) The adipose-derived cells were implanted around the cryoinjured urethra at 3-, 6-, 9-, and 12-o'clock positions (arrows).
Just before implantation, the 7-day-old cryoinjured internal urethral orifices appeared to be relaxed, creating a large orifice (Fig. 1E). Immediately after implantation, the regions were closed with small swellings formed with the injections of the suspended adipose-derived cells (Fig. 1F).
Effect of adipose-derived cell implantation on LPP and urethral histology
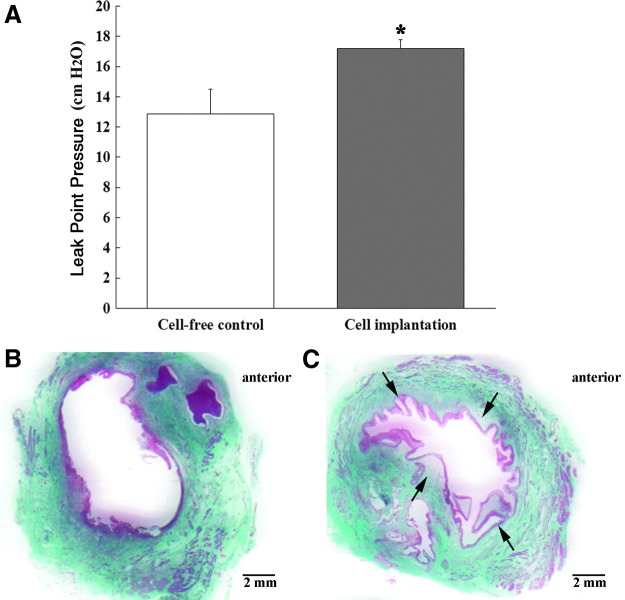
At 14 days after cell implantation or cell-free control injection, the effects on urethral sphincter structure and function were estimated by LPP measurements and histological analysis. The LPP of the cell-implantation group, 17.19±0.60 cm H2O, was significantly higher compared with the cell-free control group, 12.86±1.64 cm H2O (p<0.05, Fig. 2A). In histological investigations, the cell-free control injected urethras showed that the cryoinjured internal urethral orifices maintained the relaxed state, creating a large orifice (Fig. 2B). In contrast, the internal orifices of the implanted urethras were slightly closed, possibly due to the bulking effects produced by cell implantation (Fig. 2C).
FIG. 2.
Measurements of leak point pressure (LPP) values and histology of urethral sphincters at 14 days after cell-free control and adipose-derived cell implantation. (A) At 14 days, the LPP of the cell-implantation group was significantly higher compared with the cell-free control group (*p<0.05). (B) The cell-free control injected urethra remained gaped opened and flaccid with loss of muscle tissues. (C) The injured internal urethral orifices with implanted cells were slightly closed due to bulking effects of the implanted cells (arrows).
Reconstruction of layered muscle structures in cryoinjured urethral sphincters
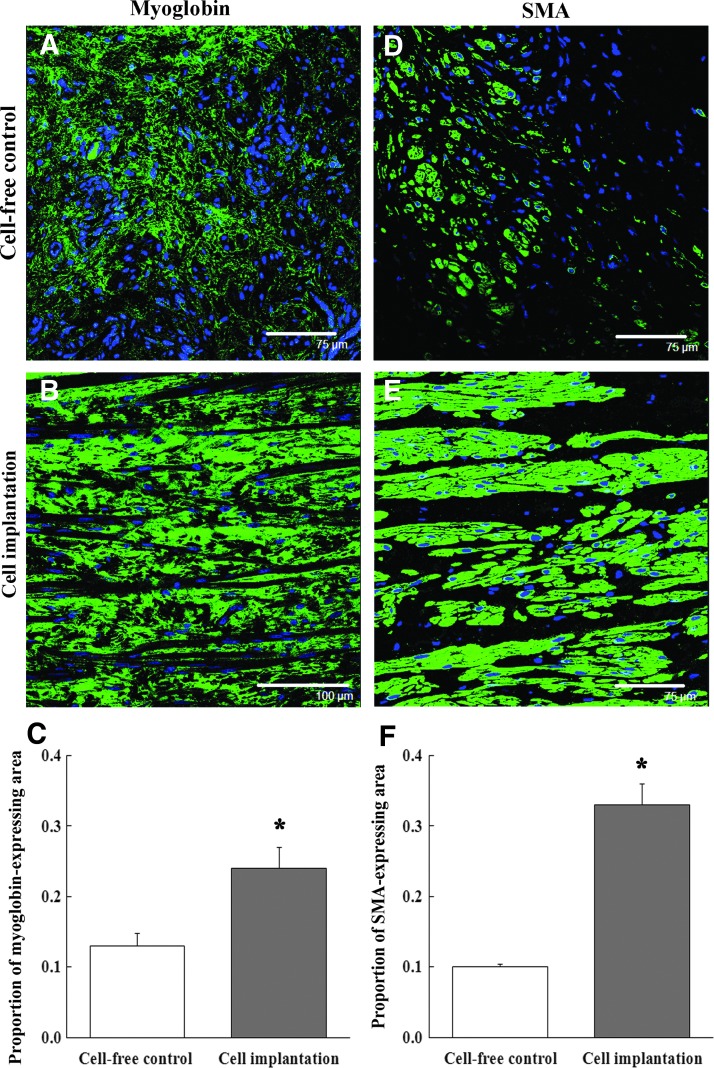
At 14 days after implantation, the cell-free control injection group had few myoglobin-expressing areas in the injured urethras (Fig. 3A). In contrast, the cell implantation group had numerous myoglobin-positive areas (Fig. 3B). The proportion of myoglobin-expressing areas in the cell-implanted regions was significantly higher compared with the cell-free control regions (Fig. 3C, p<0.01). Similarly, the cell-free control injection group had very few SMA-positive cells (Fig. 3D), while the cell implanted regions were composed of numerous SMA-positive areas (Fig. 3E). The proportion of SMA-expressing areas in the cell implantation group was significantly higher than cell-free controls (Fig. 3F, p<0.01). The myoglobin- and SMA-positive cells were both organized into layered skeletal and smooth muscle structures.
FIG. 3.
Reconstruction of muscle layers composed of skeletal muscle or smooth muscle cells. (A) At 14 days after cell-free control injection, the urethral sphincters had few myoglobin-positive areas (green). Blue: nuclei. Scale bar: 75 μm. (B) At the same time, the cell-implanted urethral sphincters had numerous myoglobin-positive areas. Blue: nuclei. Scale bar: 100 μm. (C) The proportion of myoglobin-expressing areas in the cell-implanted urethral sphincters was significantly higher than in the cell-free control group (*p<0.01). (D) At 14 days, the cell-free control injected urethral sphincters had few layered SMA-positive muscle structures (green). Blue: nuclei. Scale bar: 75 μm. (E) At the same time, the cell-implanted urethral sphincters had numerous SMA-positive muscle layer structures. Blue: nuclei. Scale bar: 75 μm. (F) The proportion of SMA-expressing areas in the cell-implanted urethral sphincters was also significantly higher than in the cell-free control group (*p<0.01).
Myogenesis, neurogenesis, and angiogenesis from implanted adipose-derived cells
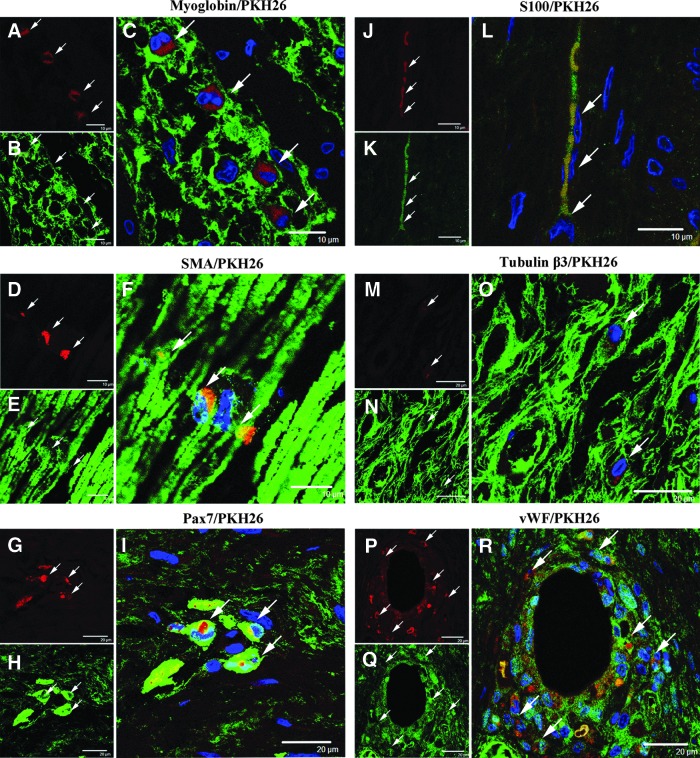
Within the implanted regions, we found numerous cells that were identified by the presence of conjugated PKH26 (red). Some of the PKH26-labeled cells (Fig. 4A, D) were positive for the myoglobin (Fig. 4B) and SMA (Fig. 4E) antibody. This showed that the implanted cells differentiated into skeletal muscle-like (Fig. 4C) and smooth muscle-like cells (Fig. 4F). These differentiated cells were integrated into the reconstructed muscle layers. Similarly, some PKH26-labeled implanted cells were positive for Pax7 antibodies, which suggested differentiation into myoblast progenitor-like cells (Fig. 4G–I). The differentiated Pax7-positive cells were widely distributed within the reconstructed muscle layers.
FIG. 4.
Differentiation of adipose-derived cells into skeletal muscle-, smooth muscle-, myoblast-, nerve-, and vascular endothelial-like cells. For each triad of images, the PKH26-labeled cells are in the upper left micrograph, the cell-specific marker stain is in the lower left micrograph, and the merged images are in the larger micrograph to the right side of each group. The PKH26-labeled implanted cells were positive for myoglobin (A–C, arrows, scale bar: 10 μm), SMA (D–F, arrows, scale bar: 10 μm), and Pax7 (G–I, arrows, scale bar: 20 μm). Some PKH26-labeled cells were positive for S100 (J–L, arrows, scale bar: 10 μm) and tubulin β3 (M–O, arrows, scale bar: 20 μm). Also, some of the implanted cells were positive for von Willebrand factor (vWF) around the blood vessels (P–R, arrows, scale bar: 20 μm). Blue: nuclei.
In addition, some of the PKH26-labeled implanted cells (Fig. 4J, M) were positive for S100 (Fig. 4K) and tubulin β3 (Fig. 4N). These showed that the implanted cells differentiated into nerve-like cells (Fig. 4L, O). In addition, the PKH26-labeled implanted cells were positive for vWF, an endothelial cell marker, suggesting that these cells supported angiogenesis within the cryoinjured urethral tissues (Fig. 4P–R).
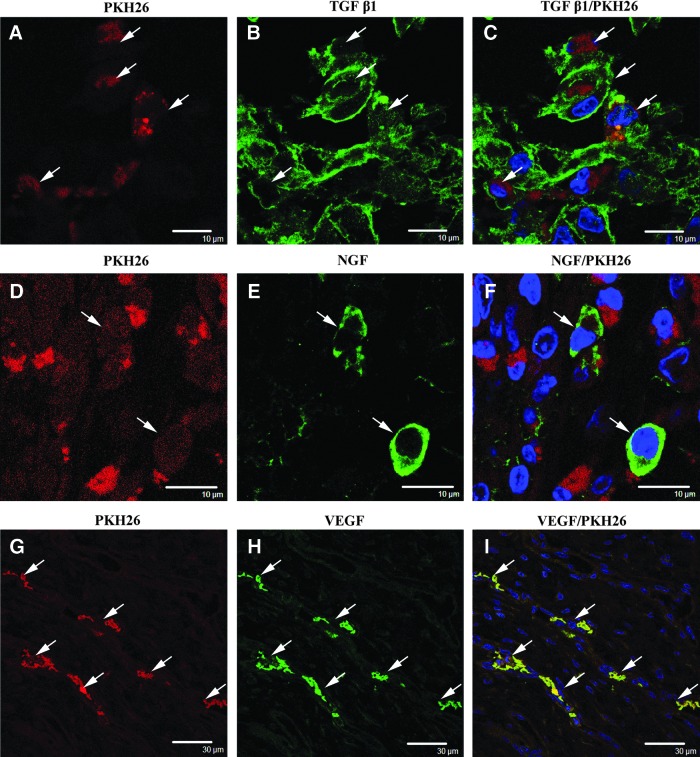
Paracrine growth factors in implanted adipose-derived cells
The implanted PKH26-labeled cells were investigated by immunohistochemistry to determine if they expressed growth factors that had the potential to support reconstruction of urethral sphincters and/or differentiation of themselves. Some of the PKH26-labeled cells were positive for the TGF β1 antibody (Fig. 5A–C). Other PKH26-labeled cells were positive for the NGF antibody (Fig. 5D–F). In addition, the implanted PKH26-labeled cells near the blood vessel structures were positive for the VEGF antibody (Fig. 5G–I).
FIG. 5.
Expression of growth factors in adipose-derived cells at 14 days after implantation. PKH26 labeling is shown in the left image of each row. Growth factor antibody-positive cells are shown in the middle image of each row, and the merged micrographs are shown in the right image of each row. (A–C) Some PKH26-labeled implanted cells (A, arrows) were positive for transforming growth factor β1 (TGF β1) (B, arrows) within the muscle layers. Scale bar: 10 μm. (D–F) Other PKH26-labeled implanted cells (F, arrows) were positive for nerve growth factor (NGF). Scale bar: 10 μm. (G–I) Near the blood vessel structures, the PKH26-labeled implanted cells (G, arrows) were positive for vascular endothelial growth factor (VEGF). Scale bar: 30 μm. Blue: nuclei.
Discussion
Cell therapy using adipose-derived cells for radical prostatectomy-related stress urinary incontinence has shown promising therapeutic effects.12 However, it is difficult to estimate the amount of tissue reconstruction that was associated with the recovery of the functional urethral sphincters. Previously, we developed the cryoinjured urethral sphincter models in rabbits to investigate the ability of autologous bone marrow-derived cell implantation to reconstruct the urethral sphincter.13 At 7 days after cryoinjury, the LPP values of rabbits with cryoinjured urethral sphincters were significantly lower compared with the intact sphincters. We concluded that the decreased LPP values were caused by flaccid and gaping internal urethral orifices. The injured sphincters exhibited loss of muscle mass and relative disorganization of the remaining muscle tissue.13 Our current study investigated the ability of implanted autologous adipose-derived cells to restore the structure and function of the injured urethral sphincters.
In this study, we simply used the adipose-derived cells that were attached and proliferating on type I collagen-coated dishes. The cells were not sorted by the use of any marker antibodies that characterized them as adipose-derived cells. Nevertheless, we confirmed that the adipose-derived cells were positive for the mesenchymal marker STRO1, but were not positive for muscle differentiation markers myoglobin and SMA. These results suggested that the cultured adipose-derived cells consisted of a heterogeneous cell population of which the majority were mesenchymal cells. This is consistent with at least one other report that adipose-derived cells are capable of differentiation into various cell types in vitro and in vivo.19
At 14 days after implantation, the LPP values of the cell-implanted rabbits were significantly higher than those of the cell-free control rabbits. We examined histologically the improvement of LPP in cell-implanted urethras. The implantation of the cells might have provided a bulking effect that increased the urethral closure pressure. Immediately after injection of the cells, we confirmed the presence of small swellings that formed with the injections of the adipose-derived cells. In addition, at 14 days after implantation, we observed a significant number of implanted PKH26-labeled adipose-derived cells within the urethra. We could not exactly estimate the number of cells that was attached, survived, and proliferated, nor could we determine the roles of the differentiation marker-negative cells within the tissues; however, it is possible that the formation of the small swellings and/or the presence of the PKH26-labeled implanted cells might have continued to provide a bulking effect to increase LPP values.
At 14 days after implantation, the proportion of both the myoglobin- and SMA-expressing areas of the cell-implanted regions was significantly greater compared with cell-free controls. Some myoglobin- and SMA-positive cells that differentiated from the implanted adipose-derived cells were present within these positive areas. In addition, the myoglobin- and SMA-positive cells were both organized into layered skeletal and smooth muscle structures. Therefore, we suggest that the recovery of the muscle structures also contributes to the increased LPP values in the cell-implantation group.
Our data also show possible mechanisms by which the recovery of functional sphincters occurs due to the implantation of the adipose-derived cells. By immunohistochemistry, we demonstrated the presence of the growth factors TGF β1, NGF, and/or VEGF within the implanted PKH26-labeled adipose-derived cells. Secretion of these growth factors may have a dual role. First, these growth factors, if secreted by the cells, can actively contribute to the environment by both autocrine and paracrine signaling. The paracrine secretion may provide a supportive role in differentiation, cytoprotection, and migration of adipose-derived cells in our study. Second, these secreted growth factors are likely to influence tissue microenvironments by creating favorable conditions that can enhance cell survival, endogenous repair, and tissue regeneration.20 We also need to investigate the possible roles of growth factors produced from the surrounding intact regions in addition to those of the implanted cells. Our results suggest that the produced growth factors might support and/or promote the recovery of the muscle structures.
This study showed significant findings regarding other differentiated cells derived from the adipose-derived cells within the reconstructed regions. The presence of Pax7-positive implanted cells with satellite cell-like properties was detected. Although the mechanisms behind satellite cell activation and myogenic differentiation are beyond the scope of our study,21–23 the presence of Pax7-positive cells might have long-term clinical significance. If the tissue in the newly regenerated layers died by apoptosis, the presence of these Pax7-expressing implanted adipose-derived cells might support further myogenic differentiation and repair the tissues.
In the present study, the presence of S100- and tubulin β3-positive implanted cells was also detected. Whereas we could not determine if the differentiated nerve cells formed neural networks, our results suggest that the neuroregeneration might support the recovery of continence. In addition, this study demonstrated numerous vWF-positive implanted cells around vessel-like structures. This suggested that implanted adipose-derived cells differentiated into endothelial-like cells and promoted neoangiogenesis in the cryoinjured urethras. Whereas we did not show that the formed vessel-like structures reconstructed the microcirculation with the uninjured remaining blood vessels, neoangiogenesis might support the recovery of muscle structures. Thus collectively, the myogenic differentiation, neuroregeneration, and neoangiogenesis events may work together to support and/or promote recovery of the muscle structures.
This current study had two major limitations that need to be considered. First, we could not estimate exactly the number of attached, surviving, proliferating, and differentiated cells within the implanted regions. Second, the cryoinjury did not cause irreversible damage. The cryoinjured urinary sphincters exhibited self-healing induced from the surrounding uninjured intact regions. Thus, we could not explore the long-term effects of the implanted adipose-derived cells. Consequently, we could not entirely separate the implanted cell effects and from the self-healing effects. Therefore, this study demonstrated the limited results of a short-term, 2-week period after implantation.
In conclusion, we showed that autologous adipose-derived cell implantation into the cryoinjured rabbit urethras promoted the recovery of urethral function. The implanted cells provided a bulking effect that probably increased the urethral pressure. The populations of both myoglobin- and SMA-expressing areas in the cell-implanted regions were significantly larger than those in the control regions. Some cells differentiated into muscle-, nerve-, and endothelial-like cells that were identified by the expression of specific marker proteins. The differentiated cells were incorporated into portions of the reconstructed tissue structures. Additionally, the implanted cells produced several growth factors that could have promoted the myogenic differentiation, neuroregeneration, and neoangiogenesis for themselves and/or surrounding tissues. This current study suggests that these effects might support the reconstruction of functional urethral sphincters. Therefore, the implantation of adipose-derived cells for human radical prostatectomy-related stress urinary incontinence could provide a beneficial therapeutic effect.
Disclosure Statement
No competing financial interests exist.
References
- 1.Bauer R.M., Bastian P.J., Gozzi C., and Stief C.G.Postprostatectomy incontinence: all about diagnosis and management. Eur Urol 55,322, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Romano S.V., Metrebian S.E., Vaz F., Muller V., D'Ancona C.A., Costa De Souza E.A., et al. . An adjustable male sling for treating urinary incontinence after prostatectomy: a phase III multicentre trial. BJU Int 97,533, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Huang W.C., and Yang J.M.Bladder neck funneling on ultrasound cystourethrography in primary stress urinary incontinence: a sign associated with urethral hypermobility and intrinsic sphincter deficiency. Urology 61,936, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Fritel X., Fauconnier A., and Pigné A.Circumstances of leakage related to low urethral closure pressure. J Urol 180,223, 2008 [DOI] [PubMed] [Google Scholar]
- 5.McGuire E.J.Pathophysiology of stress urinary incontinence. Rev Urol 6Suppl 5,S11, 2004 [PMC free article] [PubMed] [Google Scholar]
- 6.Cundiff G.W.The pathophysiology of stress urinary incontinence: a historical perspective. Rev Urol 6Suppl 3,S10, 2004 [PMC free article] [PubMed] [Google Scholar]
- 7.Shah S.M., and Gaunay G.S.Treatment options for intrinsic sphincter deficiency. Nat Rev Urol 9,638, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Marshall V.F., Marchetti A.A., and Krantz K.E.The correction of stress incontinence by simple vesicourethral suspension. Surg Gynecol Obstet 88,509, 1949 [PubMed] [Google Scholar]
- 9.Burch J.C.Urethrovaginal fixation to Cooper's ligament for correction of stress incontinence, cystocele, and prolapse. Am J Obstet Gynecol 81,281, 1961 [DOI] [PubMed] [Google Scholar]
- 10.Nilsson C.G., Kuuva N., Falconer C., Rezapour M., and Ulmsten U.Long-term results of the tension-free vaginal tape (TVT) procedure for surgical treatment of female stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 12Suppl 2,S5, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Chaikin D.C., Rosenthal J., and Blaivas J.G.Pubovaginal fascial sling for all types of stress urinary incontinence: long-term analysis. J Urol 160,1312, 1998 [PubMed] [Google Scholar]
- 12.Yamamoto T., Gotoh M., Kato M., Majima T., Toriyama K., Kamei Y., et al. . Periurethral injection of autologous adipose-derived regenerative cells for the treatment of male stress urinary incontinence: report of three initial cases. Int J Urol 19,652, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Imamura T., Ishizuka O., Kinebuchi Y., Kurizaki Y., Nakayama T., Ishikawa M., et al. . Implantation of autologous bone-marrow-derived cells reconstructs functional urethral sphincters in rabbits. Tissue Eng Part A 17,1069, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Carr L.K., Steele D., Steele S., Wagner D., Pruchnic R., Jankowski R., et al. . 1-year follow-up of autologous muscle-derived stem cell injection pilot study to treat stress urinary incontinence. Int Urogynecol J Pelvic Floor Dysfunct 19,881, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Mitterberger M., Marksteiner R., Margreiter E., Pinggera G.M., Colleselli D., Frauscher F., et al. . Autologous myoblasts and fibroblasts for female stress incontinence: a 1-year follow-up in 123 patients. BJU Int 100,1081, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Mitterberger M., Pinggera G.-M., Marksteiner R., Margreiter E., Fussenegger M., Frauscher F., et al. . Adult stem cell therapy of female stress urinary incontinence. Eur Urol 53,169, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Mizuno H.Adipose-derived stem and stromal cells for cell-based therapy: current status of preclinical studies and clinical trials. Curr Opin Mol Ther 12,442, 2010 [PubMed] [Google Scholar]
- 18.Mizuno H., Tobita M., and Uysal A.C.Concise review: adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 30,804, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., et al. . Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng 7,211, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Imamura T., Yamamoto T., Ishizuka O., Gotoh M., and Nishizawa O.The microenvironment of freeze-injured mouse urinary bladders enables successful tissue engineering. Tissue Eng Part A 15,3367, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Relaix F., and Zammit P.S.Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development 139,2845, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Kadi F., Charifi N., Denis C., Lexell J., Andersen J., Schjerling P., et al. . The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Arch Eur J Physiol 451,319, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Collins C.A., Olsen I., Zammit P.S., Heslop L., Petrie A., Partridge T.A., et al. . Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122,289, 2005 [DOI] [PubMed] [Google Scholar]