Abstract
Background
Following ACL reconstruction, there is significant atrophy of quadriceps muscles which can limit full recovery and place athletes at risk for recurrent injury with return to play. The etiology of this muscle atrophy is not fully understood.
Hypothesis
We hypothesized that circulating levels of pro-atrophy, pro-inflammatory and cartilage turnover cytokines and biomarkers would increase following ACL reconstruction.
Study Design
Descriptive laboratory study.
Methods
Subjects (N=18, mean age 28±2.4 years) underwent surgical reconstruction of the ACL following non-contact athletic injury. Circulating levels of biomarkers were measured along with SF-12, IKDC and objective knee strength measures preoperatively, and at 6 postoperative visits. Differences were tested using repeated measures one-way ANOVA tests.
Results
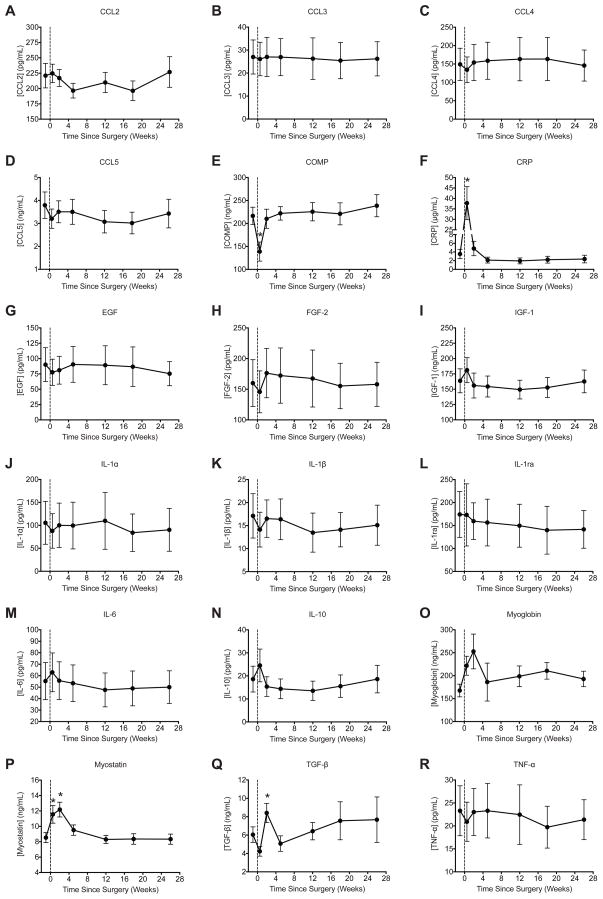
Myostatin, TGF-β and CRP levels were significantly increased in the early postoperative period, and returned to baseline. COMP levels decreased immediately after surgery and then returned to baseline. CCL2, CCL3, CCL4, CCL5, EGF, FGF-2, IGF-1, IL-10, IL-1α, IL-1β, IL-1ra, IL-6, myoglobin and TNF-α were not different over the course of the study.
Conclusions
An increase in potent atrophy-inducing cytokines and corresponding changes in knee strength and functional scores were observed following ACL reconstruction.
Clinical Relevance
Although further studies are necessary, the therapeutic inhibition of myostatin may help prevent the muscle atrophy that occurs following ACL reconstruction and provide an accelerated return of patients to sport.
Key Terms: myostatin, transforming growth factor-β, cartilage oligomeric matrix protein, c-reactive protein, muscle atrophy, ACL reconstruction
Introduction
ACL tears are among the most frequent knee injuries in physically active individuals, with tear rates in the US up to 250,000 per year 19. Despite improvements in postoperative rehabilitation, many patients that suffer from ACL tears have a persistent atrophy and weakness of their quadriceps muscles following ACL reconstruction (ACL-R). Several studies have reported a persistent weakness exceeding 20% in quadriceps muscles following ACL-R 32. This weakness appears to occur for all types of ACL grafts, including hamstring and patellar tendon autografts as well as allografts 32. In addition to reducing physical performance and increasing the susceptibility to repeated injuries 24, many studies have indicated this loss of strength can alter knee kinematics in a way that promotes the development of early-onset osteoarthritis (OA) in younger patients 28, 35, 42. Surgical reconstruction of torn ACLs, while helpful in restoring some joint kinematics and proprioception, does not appear to modify the likelihood of development of OA in ACL-R patients 35. Developing new therapeutic interventions to prevent muscle atrophy and weakness following ACL reconstruction is likely to reduce post-injury performance deficits, protect from re-injury, and possibly reduce the likelihood of developing OA.
The etiology of muscle atrophy and persistent dysfunction after ACL-R has not been fully characterized. Several studies have suggested loss of proprioception and impaired neuromuscular control after ACL-R to be responsible for persistent muscle activation deficits and atrophy 8, 44. Changes in neurological function, however, provide only a partial explanation of the observed strength deficits following ACL surgery 32. In many different types of neuromuscular diseases and injuries, there is an atrophy and weakness of muscle fibers that reflects alterations in signaling pathways that regulate muscle protein synthesis and degradation 36. Identifying changes in atrophy-inducing or hypertrophy-inducing signaling molecules that occur after ACL tear could provide new pharmacological options to prevent weakness and enhance the recovery and safe return to sport for patients who suffer ACL tears.
Several cytokines and signaling molecules are known to control muscle fiber growth and strength, but little is known about the role these molecules play in regulating the muscle atrophy that occurs following ACL tear. One of the most widely studied atrophy-inducing signaling molecules is myostatin (GDF-8). Myostatin is a member of the transforming growth factor-β (TGF-β) superfamily of cytokines and induces muscle atrophy by activating the ubiquitin-proteasome pathway 36. Closely related to myostatin is TGF-β, which also potently induces muscle atrophy and weakness via activation of the ubiquitin-proteasome pathway 29. Insulin-like growth factor 1 (IGF-1), which activates the Akt/mTOR protein synthesis pathway 36, is among the most well studied hypertrophy-inducing factors. It is not known, however, if myostatin, TGF-β and IGF-1 levels change in patients in patients that are undergoing ACL-R.
We hypothesized that myostatin and TGF-β would increase immediately following ACL-R and remain elevated during the early post-operative period, and IGF-1 levels would decrease following surgery and remain depressed in the early post-operative period. We measured circulating levels of myostatin, TGF-β and IGF-1 in patients with ACL tears immediately before surgery, and at six post-operative clinical follow-up visits until discharge to return to full activity. In addition to myostatin, TGF-β and IGF-1, we also measured other circulating signaling molecules that are known regulators of muscle atrophy or hypertrophy that act either by directly signaling in muscle fibers or indirectly via the recruitment of macrophages or modulation of muscle stem cell activity 9, 22, 25, 31, 36. The atrophy-related factors measured include chemokine (C-C motif) ligands 2, 3, 4 and 5 (CCL2, CCL3, CCL4 and CCL5), interleukin 1 α (IL-1α), interleukin 1 β (IL-1β), interleukin 1 receptor antagonist (IL-1ra), interleukin 6, and tumor necrosis factor α (TNF-α). The hypertrophy-related factors evaluated were epidermal growth factor (EGF), fibroblast growth factor 2 (FGF-2) and interleukin 10 (IL-10). Additional circulating proteins that were measured include myoglobin which is a marker of muscle fiber damage, C-reactive protein (CRP) which is an acute phase protein that increases due to inflammation, and cartilage oligomeric matrix protein (COMP) which is a marker of cartilage degradation 7, 34, 40. Short Form 12 (SF-12) and International Knee Documentation Committee (IKDC) surveys and knee strength measurements were performed to assess subjective and objective function.
Materials and Methods
Subjects
This study was approved by the University of Michigan Medical School Institutional Review Board. All subjects provided written informed consent to participate in this study. Subjects who were 16–55 years of age (written parental consent was obtained for subjects less than 18 years of age), who had unilateral complete ACL tears and consented to surgical repair by an orthopaedic surgeon were eligible to participate in the study. Patients who were undergoing revision ACL reconstruction, who had gone through menopause, had a previous injury to their involved knee, or a history of myopathy or a rheumatologic disorder were excluded from participation in the study.
Study Design
An overview of the study design is presented in Figure 1. Approximately one week prior to surgery, subjects reported to our clinic for a preoperative history and physical screening, and initial study measures were performed at this time. Subjects were asked to complete the International Knee Documentation Committee (IKDC) survey to evaluate knee function 1 and the Short Form-12 (SF-12) survey to measure general health-related quality of life 43. After surveys were completed, approximately 4 mL of venous blood was collected from an antecubital vein into K2EDTA tubes (BD, Franklin Lakes, NJ) and spun down at 1000×g for 10 minutes. Plasma was immediately collected from the tubes, placed in 150μL aliquots and stored at −80°C for further analysis. Following the blood draw, knee strength measurements were performed.
Figure 1.
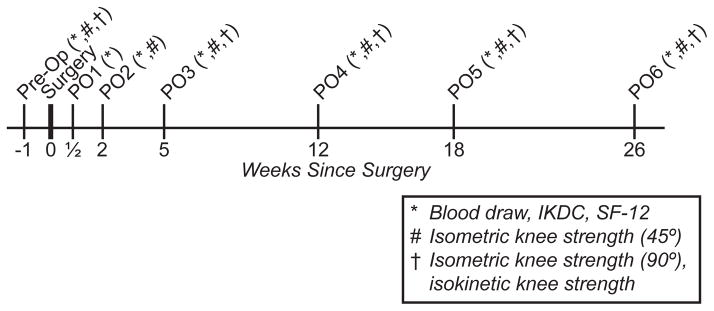
Overview of the study design.
Surgical Repair of ACL Tears
All patients sustained noncontact, traumatic ACL injury during athletic activity and desired to return to their respective sport. All patients underwent preoperative rehabilitation to improve range-of-motion and control effusion before surgical reconstruction for a period lasting approximately 6–8 weeks. Graft choice for ACL reconstruction was bone-patellar-tendon-bone (BTB) autograft in 15 patients, Achilles tendon allograft in 2 patients, and a tibialis anterior tendon allograft in 1 patient. ACL reconstructions were performed by a single, fellowship-trained sports medicine surgeon using a single-bundle, anatomic reconstruction to recapitulate native ligament footprints and ligament obliquity. An anteromedial portal reaming technique was used for preparation of the femoral socket. All grafts were tensioned and fixed in 10 degrees of flexion. BTB grafts were fixed with co-linear, interference screws to achieve aperture fixation. Allografts were fixed with suspensory fixation on the femoral side (EndoButton, Smith & Nephew, Andover, MA) and co-linear, interference fixation of the tibial side (IntraFix, DePuy-Mitek, Raynham, MA).
Following ACL reconstruction surgery, subjects were seen in our clinic for post-operative follow-up appointments three days after surgery (post-op 1), two weeks after surgery (post-op 2), five weeks after surgery (post-op 3), twelve weeks after surgery (post-op 4), eighteen weeks after surgery (post-op 5) and twenty six weeks after surgery (post-op 6). Study measures were repeated for all post-operative follow-up visits with the exception of isometric strength measures at the first post-operative visit, and isokinetic strength measurements at the first and second postoperative visits. Subjects participated in a standardized, accelerated closed chain exercise ACL rehabilitation program beginning after the first post-operative clinic visit 5. Patients were discharged to full activities at the sixth post-operative visit based on return to play criteria, which included absence of an effusion, full range of motion, leg press test demonstrating comparable strength to the uninvolved contralateral lower extremity, and completion of an agility and proprioceptive training program 4.
Measurement of Knee Strength
Bilateral strength measurements were performed seated in a dynamometer (BioDex System 3, Shirley, NY). Maximum isometric knee flexion and extension force measurements were performed at 45° of and 90° of knee flexion, and isokinetic knee flexion and extension force measurements were performed over a range from 0° to 90° of knee flexion for each knee at each time point. Due to subject safety and comfort, isometric measurements at 45° of knee flexion were deferred at the first post-operative visit, and isometric measurements at 90° of knee flexion and isokinetic measurements were deferred at the first and second post-operative visits. For each measurement, the highest force from a series of five repetitions was used. All force data of the involved side was normalized to the uninvolved side at each visit.
Measurement of Biomarkers
The concentration of circulating biomarkers from plasma samples was analyzed in duplicate for each time point, and manufacturer instructions were followed for each assay. The coefficient of determination for all standards was greater than 0.99. Any replicate samples that had greater than 3% variation between values were analyzed again. Plasma was selected over serum, as platelets contain high levels of many of the proteins evaluated in this study. A Luminex-based Milliplex multiplex assay (Millipore, Billerica, MA) was used to measure circulating levels of CCL2, CCL3, CCL4, CCL5, EGF, FGF-2, IL-1α, IL-1β, IL-1ra, IL-6, IL-10 and TNF-α in a MagPIX system (Luminex, Austin, TX). ELISAs were performed to measure circulating levels of COMP (R&D Systems, Minneapolis, MN), high sensitivity CRP (Calbiotech, Spring Valley, CA), IGF-1 (R&D Systems), myoglobin (Calbiotech), myostatin (Alpco, Salem, NH) and TGF-β (R&D Systems). ELISA plates were read in a SpectraMax microplate reader (Molecular Devices, Sunnyvale, CA).
Statistical Analyses
Results are presented as mean±SE. Prism 6.0 software (GraphPad Software, La Jolla, CA) was used to conduct statistical analyses. Differences between time points were tested using a repeated measures one-way ANOVA (α=0.05) followed by Dunnett’s post-hoc sorting to identify differences from the pre-operative visit.
Results
There were 18 subjects (12 males, 6 females) that completed the study. The age of the subjects was 28±2.4 years (range 16 to 50 years) and BMI was 26±0.7 kg/m2 (range 20 to 32 kg/m2). All subjects in the study tolerated the surgical reconstruction well and progressed along the accelerated rehabilitation plan without complications. For the SF-12 physical component summary (PCS), scores decreased immediately following surgery, returned to pre-operative values by the third post-operative visit, and had values significantly higher than the pre-operative scores by the fifth post-operative visit (Figure 2A). A nearly identical relationship was observed for IKDC scores, except values became significantly higher than the pre-operative score by the fourth post-operative visit (Figure 2C). No differences in SF-12 mental component summary (MCS) scores were detected throughout the study (Figure 2B).
Figure 2.
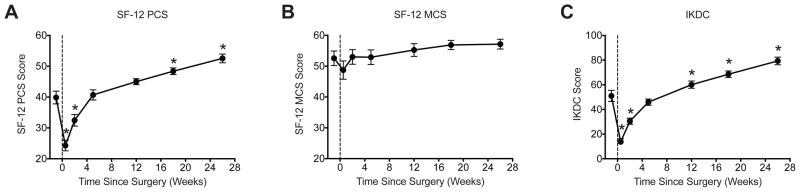
Changes in outcomes scores over the course of the study. (A) SF-12 physical component summary (PCS) score, (B) SF-12 mental component summary (MCS) score and (C) IKDC score. Vertical dashed line indicates the time of surgery. Values are mean±SE. N=18 subjects at each time point. *, significantly different from the pre-operative time point (P<0.05).
Strength values also followed an expected pattern of change (Figure 3). Isometric knee flexion and extension values at 45° of knee flexion decreased immediately after surgery, and were significantly greater than pre-operative values by the time of discharge (Figures 3A–B). For isometric values at 90°, knee flexion strength returned to pre-operative levels by the fourth post- operative visit, while knee extension strength returned at the six post-operative visit (Figures 3C–D). Isokinetic knee flexion force values were significantly higher than pre-operative values by the fifth post-operative visit, and the strength of the involved knee nearly returned to the strength of the uninvolved knee (Figure 3E). For isokinetic knee extension, strength values returned to pre-operative levels by the fifth post-operative visit, but patients were discharged with a strength deficit of 29% relative to the uninvolved limb (Figure 3F).
Figure 3.
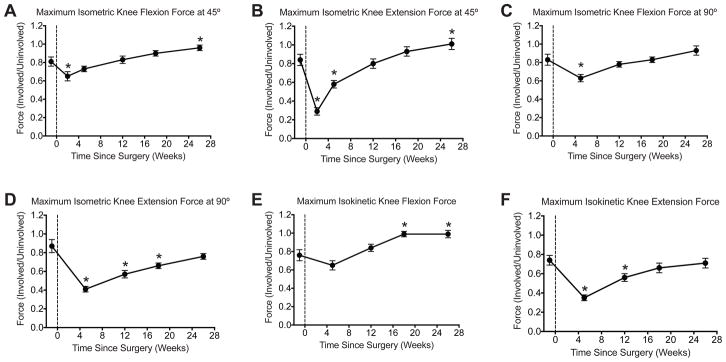
Changes in isometric and isokinetic strength over the course of the study. (A) Maximum isometric knee flexion force at 45° of knee flexion, (B) Maximum isometric knee extension force at 45° of knee flexion, (C) Maximum isometric knee flexion force at 90° of knee flexion, (D) Maximum isometric knee extension force at 90° of knee flexion, (E) Maximum isokinetic knee flexion force, and (F) Maximum isometric knee extension force. All force values of the involved limb were normalized to the uninvolved limb at each time point. Vertical dashed line indicates the time of surgery. Values are mean±SE. N=18 subjects at each time point. *, significantly different from the pre-operative time point (P<0.05).
The panel of biomarkers evaluated in this study are presented in Figure 4. The acute phase pro-inflammatory protein CRP was highly elevated immediately after surgery, but then returned to pre-operative levels by the second post-operative visit (Figure 4F). For atrophy- related biomarkers, myostatin levels were elevated at the first post-operative visit, and both myostatin and TGF-β were elevated at the second post-operative visit, but both cytokines returned to pre-operative levels by the third post-operative visit (Figures 4P–Q). Other atrophy related biomarkers, CCL2, CCL3, CCL4, CCL5, IL-1α, IL-1β, IL-1ra, IL-6 and TNF-α, did not change throughout the study (Figures 4A–D, J–M, R). No differences in the hypertrophy-related biomarkers IGF-1, EGF, FGF-2 and IL-10 were observed (Figures 4G–I, N). The cartilage turnover biomarker COMP decreased immediately after surgery but then returned to pre- operative levels (Figure 4E). The intramyocellular protein myoglobin, used as a marker of physical muscle damage, did not change over the course of the study (Figure 4O).
Figure 4.
Changes in plasma levels of biomarkers over the course of the study. (A) CCL2, (B) CCL3, (C) CCL4, (D) CCL5, (E) COMP, (F) CRP, (G) EGF, (H) FGF-2, (I) IGF-1, (J) IL-1α, (K) IL-1β, (L) IL-1ra, (M) IL-6, (N) IL-10, (O) myoglobin, (P) myostatin, (Q) TGF-β, and (R) TNF- α. Vertical dashed line indicates the time of surgery. Values are mean±SE. N=18 subjects at each time point. *, significantly different from the pre-operative time point (P<0.05).
Discussion
The purpose of this study was to measure circulating levels of biomarkers involved in muscle atrophy and hypertrophy, inflammation, and cartilage turnover during the postoperative recovery following ACL reconstruction. In the current study, myostatin and TGF-β levels significantly increased in the early postoperative period following ACL reconstructive surgery. Patients perceived a return of knee function as their IKDC and SF-12 PCS scores returned to their preoperative baseline by 5 weeks postoperatively. Despite the improvement in subjective function, however, patients continued to have a substantial deficit in force production compared to their uninvolved limb. The results of this study suggest that the etiology of muscle atrophy after ACL surgery may be multifactorial, and that elevated levels of myostatin and TGF-β postoperatively may significantly contribute to the delayed return of strength and dynamic joint stability. Furthermore, the study supports the notion that objective improvements in strength and muscle function lag behind patient-perceived functional recovery.
The mechanisms that induce muscle atrophy after ACL-R have not been fully characterized. Many studies have evaluated knee proprioception and neuromuscular control in patients with ACL injuries, but little is known about the role of cytokines or hormones that may directly or indirectly regulate muscle mass change after ACL-R. Myostatin is a cytokine that potently induces muscle atrophy. Mice, dogs, cattle, pigs, sheep, trout and humans with mutations in myostatin have marked increases in muscle mass 39, and systemic administration of myostatin in mice leads to profound muscle atrophy and weakness 46. Intramuscular levels of myostatin are elevated following hindlimb unloading 11, and in a rat model of ACL injury, increases in intramuscular myostatin levels correlated with tear-induced quadriceps atrophy 14. Myostatin and its primary receptor, ActRIIB, are expressed in ACLs and recombinant myostatin can increase cultured ACL fibroblast proliferation and type I collagen expression 16, but the role of myostatin in the ACL graft integration and ligamentization process is unknown. TGF-β is a cytokine closely related to myostatin, and also directly induces muscle atrophy and dramatically reduces muscle force production 29. Consistent with the elevation of myostatin and TGF-β in the immediate post-operative period, there was a reduction in knee strength, SF-12 PCS, and IKDC scores. While increases in myostatin and TGF-β were anticipated and confirmed after ACL-R, of interesting note are several factors that did not demonstrate significantly different plasma levels after ACL reconstruction. IL-1α, IL-1β, IL-6 and TNF-α can directly cause muscle atrophy by activating proteolytic systems in muscle 20, 26, 27, and levels of these cytokines increase in synovial fluid acutely following ACL tears 6, 10, 13, 23. However, no differences in serum levels of IL-1α, IL-1β, IL-6, TNF-α or the IL-1 regulatory protein IL-1ra were detected at any time point in the current study. The chemokine (C-C motif) ligands play central roles in recruiting macrophages to skeletal muscle where they can directly or indirectly induce muscle atrophy 15, 25, but no changes in CCL2, CCL3, CCL4 or CCL5 were observed.
We also evaluated changes in circulating factors that induce muscle hypertrophy. Studies in cultured muscle cells and animal models have identified IGF-1 as a major regulator of muscle growth. IGF-1 activates protein synthesis within muscle fibers and also promotes the proliferation of muscle stem cells, which are critical to enhance the growth of atrophied muscle 22, 36. EGF and FGF-2 also stimulate muscle growth through similar mechanisms 12, 22, 38, 45. IL-10 is an anti-inflammatory cytokine that deactivates pro-inflammatory, atrophy-inducing macrophages in skeletal muscle 41. We anticipated that hypertrophy-associated factors would be reduced in the immediate post-operative period when muscle atrophy is greatest, and slowly increase as patients progress through rehabilitation and regain strength. Surprisingly, we found no change in circulating levels of IGF-1, EGF, FGF-2 or IL-10 throughout the course of the study. It is possible that these factors do not play a role in determining changes in muscle size after ACL-R, or that local changes of these factors within the muscle are not reflected in the circulation.
In addition to atrophy- and hypertrophy-associated factors, we evaluated circulating levels of myoglobin, CRP and COMP. Myoglobin is a protein found within the cytosol of muscle fibers, and elevations in circulating levels of myoglobin occur as a result of physical injury to muscle fibers and disruption of the sarcolemma 40. Myoglobin levels did not change over the course of the study, suggesting that minimal direct injury to muscle tissue occurred during the ACL-R surgical procedure or secondary to use of a lower extremity tourniquet. CRP is an acute phase protein that plays a central role in initiating the systemic response to inflammation 7, and acute increases in CRP have been reported following hip and knee arthroplasty 3, 37. Normative values have yet to be established for most of the biomarkers evaluated in this study, but for CRP values between 1 and 3 μg/mL are considered average by American Heart Association and Centers for Disease Control and Prevention guidelines 33. In the current study, CRP levels went up over 10 fold immediately after surgery, and then returned to normal levels by the third postoperative visit. COMP is a glycoprotein found in articular cartilage that helps to stabilize and align type II collagen molecules 17. When articular cartilage is broken down, COMP is released into the circulation which makes it a useful marker of cartilage degeneration 17. COMP is commonly used as a biomarker to track the progression of OA, and elevations in COMP are associated with the formation of knee osteophytes and joint space narrowing 18. As COMP is a sensitive biomarker for OA progression, and ACL tears are associated with an elevated risk of developing early onset OA 35, we anticipated a slow and steady increase in COMP levels over time. However, we observed a significant decrease in COMP immediately after surgery, but otherwise COMP levels were not different from the pre-operative time point. The reason for this acute decrease in COMP levels is not known but may be due to irrigation of the joint and dilution during arthroscopic ACL reconstruction. Given the association between OA and ACL tear, it is also not clear why COMP levels were not significantly elevated, but it is possible that the baseline preoperative levels are already high secondary to the traumatic joint injury from the index event.
There are several limitations to this study. While substantial muscle atrophy is known to occur after ACL reconstruction 21, 44, we did not directly measure muscle volume in our subjects. We were not able to enroll subjects in the study immediately after they suffered an ACL tear, but instead enrolled patients at their pre-operative time point after they had successfully completed a standard prehabilitation regimen. Patients were not followed past their six month time point, and it is likely that if we evaluated subjects past this point increases in COMP levels might be observed. While most patients are able to return to sport after ACL-R, the level at which patients can compete varies greatly 24, and it would be interesting to evaluate whether biomarkers of muscle atrophy and inflammation could identify which patients have greater deficits in athletic performance after return to play. Although it is likely that the muscles in the involved limb were the source of the increased myostatin and TGF-β observed in the circulation, we did not directly measure biomarker levels within the muscle secondary to potential morbidity associated with direct and serial muscle biopsy in athletes. Additional insight would be gained in future studies by looking at the changes in biomarkers immediately after suffering an ACL tear, looking at changes across a wide variety of grafts, age ranges and genders, evaluating changes in biomarkers in the context of EMG and muscle activation patterns, following patients for at least two years of follow-up and exploring local changes in biomarkers via muscle biopsy.
Despite the limitations of our study, our results demonstrated significant increases in myostatin in the immediate post-operative period, and these changes corresponded to marked decreases in muscle strength and SF-12 PCS and IKDC scores. While it would be necessary to determine if intramuscular changes in myostatin levels occur in a similar fashion to what was observed in plasma, targeted inhibition of myostatin may have the potential to prevent muscle atrophy in the acute post-operative period following ACL repair. In a recent phase 1 clinical trial, the soluble decoy myostatin receptor ACE-031 was generally well tolerated, and administration of a single dose resulted a small increase in lean mass one to two months after injection 2. However, there are some concerns within the sports medicine community as to the potential use of myostatin inhibitors as doping agents, although studies from mice suggest that myostatin inhibitors are not likely to be effective doping agents. The muscle fibers from otherwise healthy mice with a targeted inhibition of myostatin were bigger but not stronger than those from wild type mice 30. As myostatin induces the expression of the main E3-ubiquitin ligases that serve as rate limiting steps in protein degradation in muscle, the reason for an increase in fiber size with no change in force production was postulated to be due to an accumulation of non-functional proteins that would otherwise be targeted for degradation by the ubiquitin proteasome 30. For pathological conditions of muscle atrophy in which myostatin is elevated, the targeted inhibition of myostatin remains a potentially attractive target. If intramuscular levels of myostatin also increase after ACL-R, the pharmacological inhibition of myostatin may offer a way to safely restore patients to their pre-injury physical abilities and accelerate the overall rehabilitation process.
What is known about the subject
Several studies in animal models, and some limited human studies, have indicated myostatin and TGF-β as important mediators of muscle atrophy. It was unknown whether levels of these cytokines changed after ACL reconstruction.
What this study adds to existing knowledge
Our results are the first to explore changes in myostatin and TGF-β levels in an orthopaedic injury context in patients. The results provide support for further studies looking at local regulation of myostatin in specific muscles, and are encouraging for a potential clinical trial of a myostatin inhibitor in patients undergoing ACL reconstruction.
Acknowledgments
The authors would like to thank Dr. Christopher Walsh for assistance with data collection. This study was funded by grant AR058920 from NIAMS.
References
- 1.Anderson AF, Irrgang JJ, Kocher MS, Mann BJ, Harrast JJ, Committee I. The International Knee Documentation Committee Subjective Knee Evaluation Form: normative data. Am J Sports Med. 2006;34(1):128–135. doi: 10.1177/0363546505280214. [DOI] [PubMed] [Google Scholar]
- 2.Attie KM, Borgstein NG, Yang Y, et al. A single ascending-dose study of muscle regulator ACE-031 in healthy volunteers. Muscle & nerve. 2012 doi: 10.1002/mus.23539. [DOI] [PubMed] [Google Scholar]
- 3.Bergin PF, Doppelt JD, Kephart CJ, et al. Comparison of minimally invasive direct anterior versus posterior total hip arthroplasty based on inflammation and muscle damage markers. J Bone Joint Surg Am. 2011;93(15):1392–1398. doi: 10.2106/JBJS.J.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beynnon BD, Johnson RJ, Abate JA, Fleming BC, Nichols CE. Treatment of anterior cruciate ligament injuries, part 2. American Journal of Sports Medicine. 2005;33(11):1751–1767. doi: 10.1177/0363546505279922. [DOI] [PubMed] [Google Scholar]
- 5.Beynnon BD, Uh BS, Johnson RJ, et al. Rehabilitation after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind comparison of programs administered over 2 different time intervals. American Journal of Sports Medicine. 2005;33(3):347–359. doi: 10.1177/0363546504268406. [DOI] [PubMed] [Google Scholar]
- 6.Bigoni M, Sacerdote P, Turati M, et al. Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res. 2012 doi: 10.1002/jor.22208. [DOI] [PubMed] [Google Scholar]
- 7.Black S, Kushner I, Samols D. C-reactive Protein. J Biol Chem. 2004;279(47):48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 8.Brown TN, Palmieri-Smith RM, McLean SG. Sex and limb differences in hip and knee kinematics and kinetics during anticipated and unanticipated jump landings: implications for anterior cruciate ligament injury. Br J Sports Med. 2009;43(13):1049–1056. doi: 10.1136/bjsm.2008.055954. [DOI] [PubMed] [Google Scholar]
- 9.Brunelli S, Rovere-Querini P. The immune system and the repair of skeletal muscle. Pharmacol Res. 2008;58(2):117–121. doi: 10.1016/j.phrs.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Cameron M, Buchgraber A, Passler H, et al. The natural history of the anterior cruciate ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med. 1997;25(6):751–754. doi: 10.1177/036354659702500605. [DOI] [PubMed] [Google Scholar]
- 11.Carlson CJ, Booth FW, Gordon SE. Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am J Physiol. 1999;277(2 Pt 2):R601–606. doi: 10.1152/ajpregu.1999.277.2.r601. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Murphy J, Toth R, Campbell DG, Morrice NA, Mackintosh C. Complementary regulation of TBC1D1 and AS160 by growth factors, insulin and AMPK activators. Biochem J. 2008;409(2):449–459. doi: 10.1042/BJ20071114. [DOI] [PubMed] [Google Scholar]
- 13.Cuellar VG, Cuellar JM, Golish SR, Yeomans DC, Scuderi GJ. Cytokine profiling in acute anterior cruciate ligament injury. Arthroscopy. 2010;26(10):1296–1301. doi: 10.1016/j.arthro.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Delfino GB, Peviani SM, Durigan JLQ, et al. Quadriceps Muscle Atrophy After Anterior Cruciate Ligament Transection Involves Increased mRNA Levels of Atrogin-1, Muscle Ring Finger 1, and Myostatin. Am J Phys Med Rehabil. 2012 doi: 10.1097/PHM.0b013e3182643f82. [DOI] [PubMed] [Google Scholar]
- 15.Dumont N, Lepage K, Côté CH, Frenette J. Mast cells can modulate leukocyte accumulation and skeletal muscle function following hindlimb unloading. J Appl Physiol. 2007;103(1):97–104. doi: 10.1152/japplphysiol.01132.2006. [DOI] [PubMed] [Google Scholar]
- 16.Fulzele S, Arounleut P, Cain M, et al. Role of myostatin (GDF-8) signaling in the human anterior cruciate ligament. J Orthop Res. 2010 doi: 10.1002/jor.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garvican ER, Vaughan-Thomas A, Clegg PD, Innes JF. Biomarkers of cartilage turnover. Part 2: Non-collagenous markers. Vet J. 2010;185(1):43–49. doi: 10.1016/j.tvjl.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Golightly YM, Marshall SW, Kraus VB, et al. Biomarkers of incident radiographic knee osteoarthritis: do they vary by chronic knee symptoms? Arthritis Rheum. 2011;63(8):2276–2283. doi: 10.1002/art.30412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34:1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 20.Haddad F, Zaldivar F, Cooper DM, Adams GR. IL-6-induced skeletal muscle atrophy. J Appl Physiol. 2005;98(3):911–917. doi: 10.1152/japplphysiol.01026.2004. [DOI] [PubMed] [Google Scholar]
- 21.Hasegawa S, Kobayashi M, Arai R, Tamaki A, Nakamura T, Moritani T. Effect of early implementation of electrical muscle stimulation to prevent muscle atrophy and weakness in patients after anterior cruciate ligament reconstruction. J Electromyogr Kinesiol. 2011;21(4):622–630. doi: 10.1016/j.jelekin.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91(2):534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 23.Hayward AL, Deehan DJ, Aspden RM, Sutherland AG. Analysis of sequential cytokine release after ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2011;19(10):1709–1715. doi: 10.1007/s00167-011-1486-0. [DOI] [PubMed] [Google Scholar]
- 24.Ingersoll CD, Grindstaff TL, Pietrosimone BG, Hart JM. Neuromuscular consequences of anterior cruciate ligament injury. Clin Sports Med. 2008;27(3):383–404. vii. doi: 10.1016/j.csm.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Koh TJ, Pizza FX. Do inflammatory cells influence skeletal muscle hypertrophy? Front Biosci (Elite Ed) 2009;1:60–71. doi: 10.2741/E7. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Moylan JS, Chambers MA, Smith J, Reid MB. Interleukin-1 stimulates catabolism in C2C12 myotubes. AJP - Cell Physiology. 2009;297(3):C706–714. doi: 10.1152/ajpcell.00626.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y-P, Chen Y, John J, et al. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19(3):362–370. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 29.Mendias CL, Gumucio JP, Davis ME, Bromley CW, Davis CS, Brooks SV. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve. 2012;45(1):55–59. doi: 10.1002/mus.22232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendias CL, Kayupov E, Bradley JR, Brooks SV, Claflin DR. Decreased specific force and power production of muscle fibers from myostatin-deficient mice are associated with a suppression of protein degradation. J Appl Physiol. 2011;111(1):185–191. doi: 10.1152/japplphysiol.00126.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murton AJ, Greenhaff PL. Physiological control of muscle mass in humans during resistance exercise, disuse and rehabilitation. Current Opinion in Clinical Nutrition and Metabolic Care. 2010;13(3):249–254. doi: 10.1097/MCO.0b013e3283374d19. [DOI] [PubMed] [Google Scholar]
- 32.Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med. 2008;27(3):405–424. vii–ix. doi: 10.1016/j.csm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 34.Punzi L, Oliviero F, Plebani M. New biochemical insights into the pathogenesis of osteoarthritis and the role of laboratory investigations in clinical assessment. Crit Rev Clin Lab Sci. 2005;42(4):279–309. doi: 10.1080/10408360591001886. [DOI] [PubMed] [Google Scholar]
- 35.Roos EM. Joint injury causes knee osteoarthritis in young adults. Current opinion in rheumatology. 2005;17(2):195–200. doi: 10.1097/01.bor.0000151406.64393.00. [DOI] [PubMed] [Google Scholar]
- 36.Sandri M. Signaling in muscle atrophy and hypertrophy. Physiology (Bethesda, Md) 2008;23:160–170. doi: 10.1152/physiol.00041.2007. [DOI] [PubMed] [Google Scholar]
- 37.Shen H, Zhang N, Zhang X, Ji W. C-reactive protein levels after 4 types of arthroplasty. Acta orthopaedica. 2009;80(3):330–333. doi: 10.3109/17453670903066596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith C, Kruger MJ, Smith RM, Myburgh KH. The inflammatory response to skeletal muscle injury: illuminating complexities. Sports Med. 2008;38(11):947–969. doi: 10.2165/00007256-200838110-00005. [DOI] [PubMed] [Google Scholar]
- 39.Stinckens A, Georges M, Buys N. Mutations in the myostatin gene leading to hypermuscularity in mammals: indications for a similar mechanism in fish? Anim Genet. 2011;42(3):229–234. doi: 10.1111/j.1365-2052.2010.02144.x. [DOI] [PubMed] [Google Scholar]
- 40.Vassallo JD, Janovitz EB, Wescott DM, Chadwick C, Lowe-Krentz LJ, Lehman-McKeeman LD. Biomarkers of drug-induced skeletal muscle injury in the rat: troponin I and myoglobin. Toxicol Sci. 2009;111(2):402–412. doi: 10.1093/toxsci/kfp166. [DOI] [PubMed] [Google Scholar]
- 41.Villalta SA, Rinaldi C, Deng B, Liu G, Fedor B, Tidball JG. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Human Molecular Genetics. 2011;20(4):790–805. doi: 10.1093/hmg/ddq523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ware JE. SF-12v2 Health Survey: Admin Guide for Clinical Trial Investigators. QualityMetric, Incorporated; 2010. [Google Scholar]
- 44.Williams GN, Snyder-Mackler L, Barrance PJ, Buchanan TS. Quadriceps femoris muscle morphology and function after ACL injury: a differential response in copers versus non- copers. J Biomech. 2005;38(4):685–693. doi: 10.1016/j.jbiomech.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 45.Yamada S, Buffinger N, DiMario J, Strohman RC. Fibroblast growth factor is stored in fiber extracellular matrix and plays a role in regulating muscle hypertrophy. Medicine and science in sports and exercise. 1989;21(5 Suppl):S173–180. [PubMed] [Google Scholar]
- 46.Zimmers TA, Davies MV, Koniaris LG, et al. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296(5572):1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]