Abstract
Study Objectives
To evaluate the clinical relevance of night-to-night variability of sleep schedules and insomnia symptoms.
Methods
The sample consisted of 455 patients (193 men, mean age=48) seeking treatment for insomnia in a sleep medicine clinic. All participants received group cognitive behavioral therapy for insomnia (CBTI). Variability in sleep parameters was assessed using sleep diary data. Two composite scores were computed, a behavioral schedule composite score (BCS) and insomnia symptom composite score (ICS). The Insomnia Severity Index, the Beck Depression Inventory, and the Morningness-Eveningness Composite Scale were administered at baseline and post-treatment.
Results
Results revealed that greater BCS scores were significantly associated with younger age, eveningness chronotype, and greater depression severity (p<.001). Both depression severity and eveningness chronotype independently predicted variability in sleep schedules (p<.001). Finally, CBTI resulted in reduced sleep variability for all sleep diary variables, except bedtime. Post-treatment symptom reductions in depression severity were greater among those with high versus low baseline BCS scores (p<.001).
Conclusions
Results suggest that variability in sleep schedules predict reduction in insomnia and depressive severity following group CBTI. Schedule variability may be particularly important to assess and address among patients with high depression symptoms and those with evening chronotype.
Keywords: insomnia, sleep variability, depression, chronotype, CBTI, circadian rhythm
INTRODUCTION
Insomnia treatment outcome studies typically assess sleep onset and offset over several nights using sleep diaries and/or actigraphy. These include bed time (BT), time at lights off (LO), wake time (WT) and time out of bed (TOB). Indices of sleep disturbance are also recorded (e.g., sleep onset latency (SOL) and time awake after sleep onset (WASO) and/or derived [e.g., total sleep time (TST) and sleep efficiency (SE)]. The average value of a given sleep parameter is typically computed to obtain a more stable variable and therefore more reliable measure of an insomnia symptom than a value on a single night. However, averages of sleep parameters often fail to fully convey the nature of an individual’s sleep disturbance or sleep schedule because variability from night to night in sleep continuity, quality, duration and schedule is common among those with insomnia. Such variations in sleep constitute an important clinical feature of insomnia disorder1, 2. In fact, intra-individual variability in sleep duration and fragmentation appears to exceed differences between individuals across these measures3, 4.
With increased recognition that distress about the unpredictability of sleep may be an important determinant of sleep-related anxiety in individuals with insomnia5, 6, 7, there has been growing interest in the study of variability of sleep in individuals with insomnia. Even so, up until recently, research has primarily focused on the variability in sleep parameters that measure insomnia symptoms, such as SOL, WASO, TST, and SE, rather than sleep schedules. Night-to-night variability in insomnia symptoms is greater among people with chronic insomnia than controls8 and it is greater among individuals with insomnia related to a mental disorder than among those with primary insomnia9. Vallières et al.10 identified three clusters of sleep patterns among adults with chronic insomnia based on variability of SOL, WASO, and SE; unpredictable sleep pattern was present in approximately one third of the sample.
Variability of sleep schedules can be differentiated from variability of insomnia symptoms, which consist of sleep parameters such as BT, LO, TOB, WT, and TIB. Existing research has found greater night-to-night variability in sleep schedules among certain populations, including young adults and patients with depressive symptoms, as well as acute suicidal distress11–13. Additionally, individuals classified as evening chronotypes have greater variability in their out of bed time than those classified as morning or intermediate chronotypes14. Among adolescents and young adults, more variable sleep patterns (>2h difference between weekday and weekend sleep bouts) predict a variety of adverse outcomes, including short sleep duration, daytime sleepiness, depressive symptoms, and increased risk for obesity13, 15–17.
Among individuals with insomnia distress about the consequences of insufficient sleep, decisions about when to attempt sleep and when to get out of bed are often based on the quality of sleep in the night prior. The variability in such voluntary sleep parameters, particularly wake and rise times, is the target of stimulus control and sleep restriction therapy for insomnia; both are central components of cognitive behavioral therapy for insomnia (CBTI) that recommend regular wake and out of bed times.
Utilizing a clinical dataset, the aims of the present study were to: (1) explore the correlates of night-to-night sleep variability of insomnia symptoms and sleep schedules; (2) examine the unique contribution of depressive symptom severity and chronotype tendency to variability in sleep parameters; (3) examine whether CBTI reduces night-to-night sleep variability in sleep-related behaviors and, separately, insomnia symptoms; and (4) evaluate whether variability in sleep parameters impacts outcomes following CBTI in terms of reductions in insomnia symptoms and depressive symptom severity. We hypothesized that, at baseline, the variability of insomnia symptoms and sleep schedules would be positively correlated with measures of insomnia severity, depressive symptomatology, and evening chronotype. Based on past research, we also expected that depression symptom severity and chronotypes independently will account for a significant proportion of the variance for night-to-night sleep variability. Finally, we hypothesized that night-to-night variability for both insomnia symptoms and sleep schedules is predicted to decrease following CBTI and that individuals with high variability exhibiting a more robust treatment response compared to those with low variability.
METHODS
Participants
This study analyzes data gathered from patients who participated in group CBTI at the Stanford Sleep Disorders Clinic between 2000 and 2004. Prior to participation in the CBTI group treatment program, patients were evaluated by sleep center physicians and / or psychologists. Patients who were referred to the program presented with an initial complaint of insomnia. 455 individuals completed the self-report questionnaires at baseline and at the final group sessions (see below). All participants completed sleep diaries during the week prior to the start of treatment and after treatment. All patients who underwent group CBTI were eligible to participate in this study. Those with co-morbid psychiatric, sleep, or medical disorders as well as concomitant use of sleep or other medications were not excluded. The protocol was pre-approved by the Institutional Human Subjects Review Board at Stanford University. The protocol permitted use of de-identified, archival data collected prior to 2004.
Procedure
The most widely used non-pharmacological treatment for insomnia is CBTI. This effective treatment is currently recognized as the first-line treatment for insomnia by the National Institutes of Health (NIH) Consensus Statement20 and the British Association of Psychopharmacology21. CBTI is a multicomponent intervention that includes a combination of stimulus control treatment, sleep restriction, relaxation training exercises, and cognitive restructuring techniques. It has been found to be as effective as hypnotic medications in the short-term treatment of insomnia, and, compared to psychopharmacology, is associated with better long-term outcomes after treatment is discontinued22. Treatment for the current study consisted of seven (90-minute) sessions of group CBTI delivered in a structured sequence. The first 5 sessions were delivered weekly and the last two biweekly. Therapists were licensed psychologists. This structured intervention included psychoeducation about sleep, sleep hygiene, stimulus control instructions, sleep restriction, relaxation (deep breathing exercises and other relaxation strategies for calming the mind), and cognitive restructuring. Relapse prevention was discussed during the last session. Treatment recommendations were tailored to patients’ presentations. At each session the therapist reviewed each patient’s progress and recommended adjustments to the behavioral program as necessary.
Materials
Insomnia Severity Index (ISI)
The ISI is a 7-item self-report scale that assesses subjective symptoms of insomnia, including the degree of distress caused by this particular sleep complaint23. Each item is scored on a 0 to 4 scale, with a maximum total scale score of 28. A higher score represents greater insomnia severity. Scores above 14 are generally consistent with clinical levels of insomnia.
Beck Depression Inventory (BDI)
The BDI is a 21-item self-report inventory used to assess the severity of depressive symptoms24. Participants are asked to indicate which statement best describes the way they have been feeling over the past week. Total scores on the BDI can range from 0 to 63, with higher scores reflecting greater levels of depressive symptoms. The BDI has yielded adequate reliability estimates, and has been well validated as a measure of depressive symptomatology.
Morningness-Eveningness Composite Scale (MECS)
The MECS is a 13-item scale used to determine an individual’s preference for various activities and ease of rising in the morning (e.g. times to get up and to go to sleep, how easy to rise at 6am)25. The scale was developed using the Horne-Ostberg Morningness-Eveningness scale and the Torsvall and Akerstedt scales. The MECS has excellent internal consistency (alpha=.87) and demonstrated psychometric properties that are comparable or better than the Horne-Ostberg and Torsvall and Akerstedt scales. Since its development, the MECS has been widely used.
Based on the MECS scores in the present sample, participants were classified into morning (scores > 43), intermediate (scores 23 – 43), and evening chronotypes (scores<23). For increased power, the morning group and intermediate group were grouped together for analyses.
Sleep Diaries
Patients completed prospective sleep/wake diaries every morning for one week at baseline before receiving any specific treatment instructions for improving sleep. Nighttime diary items included BT, LO, SOL, WT, TOB, and TST. From these variables, TIB was extracted as the time between lights out and time getting out of bed and the amount of WASO was calculated as TIB – TST - SOL. Sleep diaries are routinely used for clinical and research purposes and are considered the standard of practice for measuring subjective sleep in insomnia populations26.
To assess night-to-night variability in sleep habits, we followed the recommendations proposed by Jahng et al.27, also used by Sánchez-Ortuño et al.9 in their study of night-to-night variability in insomnia symptoms. For each individual, we calculated a variability score as follows: the differences between consecutive measurement time points were squared (e.g., [value of night 2 – value of night 1]^2) and averaged for each week of data. For example, in our study, a given individual had 7 nights of observations, so there would be 6 successive differences in the variable of interest (e.g., [value of night 2 − value of night1]^2, [value of night 3 − value of night2]^2, [value of night 4 − value of night3]^2, and so forth. The advantage of this method is that it incorporates both variability across nights and temporal dependency in a time series. These computations were performed separately for BT, LO, WT, TOB, and TIB. We then derived a composite measure of the variability of sleep schedules, the behavioral schedule composite score (BCS) as the average of the z-scores of these five variables. Thus, the BCS is comprised of variables measuring behaviors that are within an individual’s control. In contrast, the average of variability in SOL, WASO, and TST constituted the insomnia symptom composite score (ICS), which represents variability in symptoms of insomnia that are outside of one’s control.
Statistical Methods
For Aim 1, we used Pearson’s correlations to examine the relationship between all sleep variability estimates and other clinical measures for continuous variables. For categorical variables, we used Spearman’s rank correlation coefficient. For Aim 2, we used multiple regression analyses with BCS (and separately ICS) as the dependent variable and BDI and MECS scores and their interaction (after centering) as independent variables. Age was also entered into the equation as a covariate, because it was highly correlated with the sleep variability measures. For Aim 3, we first performed paired t-tests comparing ICS and BCS scores pre− to post- treatment (omnibus test) and then followed with a series of t-tests on individual variables constituting each composite score that was significant.
For Aim 4, we created high and low variability groups using the median of the BCS and ICS. Median cutoff scores were used because there have been no empirically established cutoffs in the literature. To test changes in ISI and BDI scores over time we used mixed-effects modeling. Mixed-effects models are advantageous for analyzing longitudinal data because the procedure accounts for the correlations among repeated assessments within an individual and are less sensitive to missing values than other methods. Both the fixed-effects (group average effects) and random-effects (within-individual variability) were estimated. Specifically, the group effect tested baseline differences ISI and BDI scores between the high variability and low variability group. The time effect tested whether the outcome changed from pre- to post-treatment for the whole sample. The Group X Time effect tested whether the rate of change in the high variability group was significantly different from that in the low variability group.
RESULTS
Demographic and clinical data
Data were obtained for 455 participants who had completed sleep diaries for a week at baseline and again before the last session. Participants (57.6% female) ranged in age from 19 to 88, with a mean age of 48 (SD=14) years. Data on marital status, ethnicity, and education level were not collected systematically and therefore not reported. The ISI, BDI, and MECS were administered at baseline and at the end of the last group session. The average baseline scores were 21.9 (SD=3.7) for ISI and 12.8 (SD=8.7) for BDI. Based on established MECS cutoff scores, 21.1% of the sample was morning types, 56.9% were intermediate types, and 5.7% were evening Types. Tables 1 and 2 provide descriptive statistics of baseline characteristics of the sample.
Table 1.
Demographics and descriptive values at baseline and posttreatment
| Baseline | |
|---|---|
| Mean (SD) or % | |
| Age | 48.12 (14.38) |
| Gender | |
| Male | 41.8% |
| Female | 57.6% |
| ISI Baseline | 21.92 (3.72) |
| ISI Post-treatment | 13.95 (4.90) |
| BDI Baseline | 12.81 (8.75) |
| BDI Post-treatment | 4.11 (5.95) |
Abbreviations: ISI = Insomnia Severity Index; BDI = Beck Depression Inventory
Table 2.
Night-to-night variability of Sleep Parameters at Baseline
| Mean | SD |
*Range of Variability (MSSD) |
||
|---|---|---|---|---|
| Sleep schedules | Bed Time | 11:09 | 1.25 hours | 0 – 12.51 |
| Lights Out | 11:27 | 1.21 hours | 0 – 17.31 | |
| Wake Time | 7:02 | 1.67 hours | 0 – 54.41 | |
| Time out of bed | 7:40 | 1.47 hours | 0 – 58.81 | |
| Total Time In Bed | 8.21 | 1.18 hours | 0 – 54.05 | |
| Insomnia symptoms | Sleep Onset Latency | 0.61 | 0.52 hours | 0 – 58.81 |
| Wake after Sleep Onset | 1.44 | 0.91 hours | 0 – 72.21 | |
| Total Sleep Time | 6.16 | 1.35 hours | 0 – 112.36 | |
These values used mean squared successive differences (MSSD).
Among the participants in the study, 87.3% (n=397) completed treatment. Average number of sessions attended were 5.5 (±1.8) sessions. Participants who dropped out of the study did not differ with those who completed the study on BCS, ICS, ISI, BDI, or MECS.
Correlates of night-to-night sleep variability
Table 3 summarizes the correlation between sleep variables within BCS and ICS, independently. Table 4 summarizes the correlation between the composite scores of night-to-night variability and other baseline measures. BCS was significantly correlated with age (r=−.13, p=.005). Specifically, older age was associated with lower variability on BT (r=−.19, p=.001), LO (r=−.12, p=0.02), and TIB (r=−.12, p=0.02). BCS was also significantly correlated with baseline BDI (r=.26, p<.001) and MECS scores (r=.18, p<.001) but not ISI scores. Specifically, greater depressive symptom severity were associated with greater variability in LO (r=0.14, p=0.009), WT (r=0.26, p<0.001), TOB (r=0.26, p<0.001), and TIB (r=0.26, p<0.001). A greater tendency towards an evening chronotype was associated with greater variability of BT (r=.16, p=.005), LO (r=.16, p=.003), WT (r=.18, p=.001), TOB (r=.18, p=.001), and TIB (r=.13, p=.01).
Table 3.
Intercorrelation Table of Variability of Sleep Schedules and Insomnia Symptoms
| BCS | BT | LO | WT | TOB | TIB | |
| BT | * | .872** | .40** | .40** | .546** | |
| LO | * | .37** | .37** | .51** | ||
| WT | * | 1.0** | .77** | |||
| TOB | * | ,77** | ||||
| TIB | * | |||||
| ICS | SOL | WASO | TST | |||
| SOL | * | .09 | .13** | |||
| WASO | * | .39** | ||||
| TST | . | * | ||||
p<.05,
p<.01,
p<.001
Abbreviations: BCS = Behavioral Schedule Component Score; BT = Bedtime; LO = Lights Out; WT = Wake time; TOB = Time out of bed; TIB = Time in Bed; ICS = Insomnia Symptom Composite Score; SOL = Sleep Onset Latency; WASO = Wake After Sleep Onset; TST = Total Sleep Time; SE = Sleep Efficiency; ISI = Insomnia Severity Index; BDI = Beck Depression Inventory
Table 4.
Correlation Table of Variability of Sleep Schedules and Insomnia Symptoms with age, insomnia severity, depression, and chronotype
| Age | ISI | BDI | Chronotype | ||
|---|---|---|---|---|---|
| BCS | −.32 (.001)** | .01 (.88) | .26 (<.001)*** | .24 (<.001)*** | |
| BT | −.19 (.001)** | - | .10 (.06) | .16 (.005)** | |
| LO | −.12 (.02)* | - | .14 (.009)** | .16 (.003)** | |
| WT | −.07 (.14) | - | .26 (<.001)*** | .18 (.001)** | |
| TOB | −.07 (.14) | - | .26 (<.001)*** | .18 (.001)** | |
| TIB | −.12 (.02)* | - | .26 (<.001)*** | .13 (.01)* | |
| ICS | .04 (.39) | .05 (.64) | .19 (.001)** | .04 (.48) | |
| SOL | - | - | .07 (.15) | - | |
| WASO | - | - | .05 (.33) | - | |
| TST | - | - | .26 (<.001)*** | - | |
| SE | .12 (.02)* | .07 (.50) | .07 (.21) | −.06 (.25) | |
p<.05,
p<.01,
p<.001
Abbreviations: BCS = Behavioral Schedule Component Score; BT = Bedtime; LO = Lights Out; WT = Wake time; TOB = Time out of bed; TIB = Time in Bed; ICS = Insomnia Symptom Composite Score; SOL = Sleep Onset Latency; WASO = Wake After Sleep Onset; TST = Total Sleep Time; SE = Sleep Efficiency; ISI = Insomnia Severity Index; BDI = Beck Depression Inventory
ICS was correlated with BDI (r=.19, p<0.001) but not with ISI or MECS scores. Specifically, greater severity of depression symptom was associated with greater variability in TST (r=.26, p<.001) but not with other ICS variables.
Unique contribution of depressive symptom severity and chronotype tendency to variability in sleep
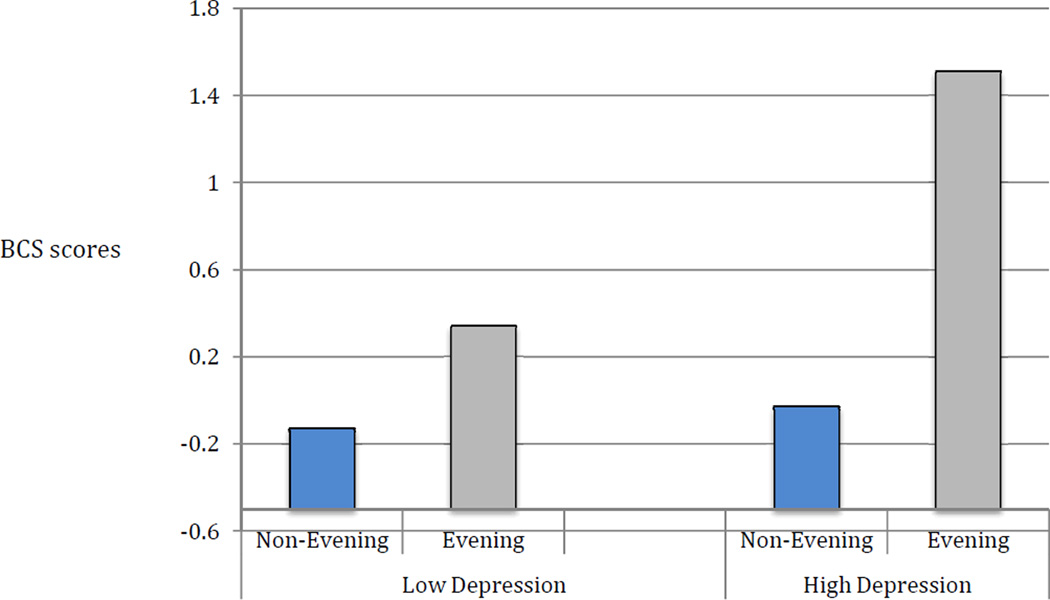
Multiple regression analyses were employed to examine the effects of depression, chronotype tendency and their interaction (after centering each) on BCS and, separately ICS, while controlling for the effects of age in each of the two models. For BCS, the model (Adjusted R2=.19) had significant effects for depression (β=.30, p<.001), chronotype tendency (β=.27, p<.001) and their interaction (β=.20, p=.003). Post-hoc analyses to explore the interaction revealed that among those with higher depression severity (BDI>14), patients with evening chronotype exhibited significantly greater variability in BCS than non-evening chronotypes (t=−2.44, p=0.03). Among those with low depressive symptoms, there was no significant difference in BCS between those with evening versus non-evening chronotype (t=−1.60, p=.016). These interactions are depicted in Figure 1. For ICS, the model (Adjusted R2=.03) had a significant effect for depression (β=.12, p=.001), but not chronotype tendency (p=.98) or their interaction (p=.06).
Figure 1. Unique contribution of depressive symptom severity and chronotype tendency to variability in sleep schedules.
* Abbreviation: BCS = Behavioral Schedule Composite Score
Change in night-to-night variability following treatment
Both BCS and ICS were significantly reduced following CBTI (p<.001). Reductions were observed in the variability of WT (t=4.09, p<0.001), TOB (t=6.23, p<0.001), and TIB (t=3.07, p=0.002), SOL (t=4.36, p<0.001), WASO (t=5.56, p<0.001), and TST (t=5.69, p<0.001). Variability for BT and LO did not change significantly following treatment. The impact of the treatment on insomnia and other measures have been published elsewhere12, 26.
Impact of night-to-night sleep variability on treatment outcomes
To explore whether variability in BCS and ICS impacted change in the severity of insomnia and depressive symptoms, we split the sample into four groups using median cutoff scores as follows. Using BCS median score (−.24), we created a high behavioral variability group (HiBCSvar, n=227) and a low behavioral variability group (LowBCSvar, n=227). Using ICS median score (−.23), we created a high ICS variability group (HiICSvar, n=225) and a low ICS variability group (LowICSvar, n=227).
Behavioral Schedule Composite Score
Mixed effects analysis with BDI scores as the dependent variable revealed a significant time X group interaction (p<.001) and a significant effect for time, reflecting a decrease in BDI scores (P<.001). Compared to the LowBCSvar group, the HiBCSvar group had significantly higher levels of depressive symptom severity at baseline but had greater reduction in depression severity from baseline to the end of treatment. The mixed effects model for ISI revealed a significant effect for time reflecting a decrease in ISI from pre to post treatment (p<.001) but the time X group interaction was not significant (p=0.29). Values for the mixed effects model for BCS are summarized in Table 5.
Table 5.
Descriptive Statistics for BCS and ICS
| Descriptive statistics for BCS | |||||
| LowBCSvar | HiBCSvar | ||||
| Mean (n= 227) |
SD | Mean (n= 227) |
SD | p-value | |
| ISI Baseline | 20.05 | 5.13 | 22.87 | 3.02 | 0.11 |
| ISI Posttreatment | 13.32 | 4.67 | 13.91 | 5.50 | 0.83 |
| BDI Baseline | 11.30 | 7.65 | 14.11 | 9.44 | 0.001** |
| BDI Posttreatment | 3.80 | 5.18 | 4.41 | 6.78 | 0.57 |
| Descriptive statistics for ICS | |||||
| LowICSvar | HiICSvar | ||||
| Mean (n=226) |
SD | Mean (n=225) |
SD | p-value | |
| ISI Baseline | 21.88 | 3.90 | 21.95 | 3.54 | 0.92 |
| ISI Posttreatment | 13.82 | 4.51 | 14.10 | 5.44 | 0.83 |
| BDI Baseline | 12.04 | 8.32 | 13.44 | 9.11 | 0.16 |
| BDI Posttreatment | 3.30 | 5.20 | 4.94 | 6.73 | 0.03* |
p<.05,
p<.01
Abbreviations: BCS = Behavioral Schedule Composite Score; ICS = Insomnia Symptom Composite Score; ISI = Insomnia Severity Index; BDI = Beck Depression Inventory
Insomnia Symptom Composite Score
Mixed effects models revealed that reductions in ISI, scores from pre- to post-treatment were significant (p<.001) but independent of variability in insomnia symptoms. Values for the mixed effects model for ICS are summarized in Table 6.
Table 6.
Mixed-effects models comparing trajectories of insomnia patients in Low Variability and High Variability Groups for BCS and ICS
| Variability Type | Outcome | Effects | Estimate | SE | t | P |
|---|---|---|---|---|---|---|
| BCS | ISI | Group | 0.83 | 0.79 | 1.05 | 0.46 |
| Time | −7.39 | 0.89 | −8.31 | p<.001 | ||
| Group × Time | −0.92 | 1.24 | −0.74 | 0.29 | ||
| BDI | Group | 4.40 | 0.74 | 5.92 | p<.001 | |
| Time | −6.99 | −6.90 | −10.54 | p<.001 | ||
| Group × Time | −3.62 | −3.62 | −3.89 | p<.001 | ||
| ICS | ISI | Group | −0.008 | 0.78 | −0.01 | 0.99 |
| Time | −7.94 | 0.85 | −9.37 | p<.001 | ||
| Group × Time | 0.04 | 1.24 | 0.03 | 0.97 | ||
| BDI | Group | 1.22 | 0.76 | 1.60 | 0.10 | |
| Time | −8.72 | 0.67 | −12.96 | p<.001 | ||
| Group × Time | 0.06 | 0.95 | 0.07 | 0.95 | ||
Abbreviations: BCS – Behavioral Schedule Composite Scale; ICS – Insomnia Symptom Composite Scale; ISI – Insomnia Severity Index; BDI – Beck Depression Inventory
DISCUSSION
The goal of this study was to investigate night-to-night sleep variability in insomnia symptoms and, separately, sleep schedules among individuals seeking treatment for insomnia in a sleep clinic. We found that greater variability in sleep schedules (BT, LO, WT, TOB, and TIB) was associated with younger age, eveningness chronotype, and greater depression severity, but not with greater insomnia severity. Effect sizes were moderate for variability in wake time, time out of bed, and time spent in bed, but small for bed time and lights out. Our findings are consistent with existing literature that documented high night-to-night variability in sleep schedules among young adults, patients with depressive symptoms, and eveningness chronotypes11, 13, 14. To the best of our knowledge, our study is the first to test a model that includes all three factors; documenting that depression severity and eveningness chronotype independently contributes to variability in sleep schedules and that those with both evening chronotypes and elevated depression severity had the greatest variability in sleep schedules. This may be because among patients with depressive symptoms, trying to sleep may be an escape from emotional pain and therefore, daily variation in mood may contribute to the patient’s decision when to get in and out of bed. Moreover, diurnal variation in mood, present in some patients with depression, may be more pronounced among patients with evening chronotype29, 30. It was surprising that there was an absence of correlation between insomnia severity and variability in sleep schedules. This may be due to a relatively restricted range of insomnia severity scores in this tertiary clinic-based sample or because eveningness chronotype is associated with greater schedule variability, even in absence of insomnia.31, 32
Patients with high schedule variability had significantly higher depressive symptom severity at baseline than those with low schedule variability, but at the end of treatment the two groups had the same level of depression. As expected, cognitive-behavioral therapy for insomnia (CBTI) reduced variability of all sleep schedule variables, except BT and LO. This is not surprising because the combination of stimulus control and sleep restriction recommends maintaining a consistent wake time and limiting time in bed but it does not recommend a fixed bed time. Instead the recommendation is to “go to bed when sleepy”. Importantly, among patients with high sleep variability, the reduction in schedule variability was associated with a reduction in depression symptom severity but this concurrent association may not be causal; for example, it may represent regression to the mean. However, one previous study found that regularizing sleep schedules among young adults with irregular sleep schedules leads to improvement in mood18. In contrast, variability in sleep schedules at baseline did not predict reductions in insomnia severity.
Similar to Edinger et al.19 we found that night-to-night variability in insomnia symptoms was reduced from baseline to post CBTI. We also found that reductions in depressive and insomnia symptom severities were similar among patients with high and low variability in ICS at baseline. BDI was significantly correlated with ICS total score and variability in TST at baseline (moderate effect sizes); greater variability in TST was associated with greater depression symptoms. One of two other studies that examined variability in TST and depression severity found greater variability in TST among patients with insomnia due to a mental disorder than among those with primary insomnia,9 but the second did not6. However, ICS did not correlate significantly with chronotype, suggesting that night-to-night variability in insomnia symptoms is independent of morningness/eveningness. This is somewhat surprising because people with eveningness chronotype tend to be in bed on weekends at times that are more congruent with their biological clocks than on weekdays (workdays) and therefore would be expected to fall asleep faster, have fewer sleep continuity disturbances, and sleep longer on weekend, thus have greater variability in these variables between on weekends and weekdays. One possible explanation for the absence of a relationship between chronotype and ICS variables is the presence of conditioned insomnia; whereby patient with eveningness tendency have difficulty sleeping even when their TIB window is congruent with their biological clocks.
Strengths of the study include the large sample size and the fact that the sample consisted of patients seeking treatment for insomnia, thus increasing external validity and the generalizability of the results to real life patients. The results of this study should nonetheless be considered exploratory and in the context of its limitations. First, this investigation was not a controlled trial and therefore we cannot rule out the possibility that the observed reduction in sleep variability may represent regression to the mean and therefore may not be definitively attributable to CBTI. Second, because the ISI and BDI were only administered at baseline and the last session we could only assess the relevance of schedule variability on outcome among treatment completers. The clinic-based sample, while a strength to external validity, limits the internal validity of the results. In other words, we cannot rule out the possibility that the heterogeneity of the sample with respect to comorbidities, concurrent treatment of comorbid conditions, and hypnotic use limit the interpretation of our results. Additionally, lack of information about the characterization of our sample, such as information about ethnicity and comorbidities, also limit the interpretations of our study. We also note that results pertaining to the relationship between sleep variability and depressive symptoms cannot be extended to a major depressive disorder (MDD) because we have not systematically assessed clinical diagnoses of MDD. Finally, it remains unclear if our findings would generalize to the delivery of CBTI using individual rather than group modality treatment or to group CBTI protocols that differ in terms of the number of sessions or the ordering of treatment components.
Despite these limitations, the present findings provide important preliminary insights about the clinical relevance of night-to-night variability in sleep schedules. We have identified two subgroups of patients for whom variability in sleep schedules may be a particularly important therapeutic target - those with elevated depressive symptoms and those with evening chronotype. We have also demonstrated that CBTI, which targets sleep variability, reduces both variability and depressive symptoms among patients with high sleep variability but the mechanism by which regularizing sleep may contributes to reduction in depressive symptom severity is unknown. Regularizing WT strengthens the underlying circadian clock and may also strengthen the social rhythms. Both rhythms seem important mood and its regulation33, 34, and therefore may be mechanistically involved.
We believe that our results and the existing literature, including evidence that sleep irregularity is associated with inflammatory markers35, highlight the clinical relevance of sleep variability and the importance of further investigation of its role in insomnia and the relationship between insomnia and other health indices, such as mood disorders and inflammatory processes.
Acknowledgment
No sponsorship was received for this research.
Footnotes
All of the authors contributed to this manuscript. Rachel Manber had full access to all of the data provided in the study. She assumes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Coates TJ, George JM, Killen JD, Marchini E, Hamilton S, Thorensen CE. First night effects in good sleepers and sleep maintenance insomniacs when recorded at home. Sleep. 1981;4:293–298. doi: 10.1093/sleep/4.3.293. [DOI] [PubMed] [Google Scholar]
- 2.Edinger JD, Marsh GR, McCall WV, Erwin CW, Lininger AW. Sleep variability across consecutive nights of home monitoring in older mixed DIMS patients. Sleep. 1991;14:13–17. [PubMed] [Google Scholar]
- 3.Wohlgemuth WK, Edinger JD, Fins AI, Sullivan RJ. How many nights are enough? The short-term stability of sleep parameters in elderly insomniacs and normal sleepers. Psychophysiology. 1999;36:233–244. [PubMed] [Google Scholar]
- 4.Knutson K, Rathouz P, Yan L, Liu K, Lauderdale D. Intra-individual daily and yearly variability in actigraphically recorded sleep measures: the CARDIA study. Sleep. 2007;30:793–796. doi: 10.1093/sleep/30.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankel FH, Orne MT. Hypnotizability and phobic behavior. Arch Gen Psychiat. 1976;33:1259–1261. doi: 10.1001/archpsyc.1976.01770100121012. [DOI] [PubMed] [Google Scholar]
- 6.Jansson-FrÖjmark M, Linton SJ. The role of psychological mechanisms to insomnia in its early phase: A focus on arousal, distress, and sleep-related beliefs. Psychol Health. 2008;23(6):691–705. doi: 10.1080/14768320701302791. [DOI] [PubMed] [Google Scholar]
- 7.Morin CM, Stone J, Trinkle D, Mercer J, Remsberg S. Dysfunctional Beliefs and Attitudes about sleep among older adults with and without insomnia complaints. Psychol Aging. 1993;8(3):463–467. doi: 10.1037//0882-7974.8.3.463. [DOI] [PubMed] [Google Scholar]
- 8.Buysse DJ, Cheng Y, Germain A, Moul DE, Franzen PL, Fletcher M, Monk TH. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11:56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sánchez-Ortuño MM, Carney CE, Edinger JD, Wyatt JK, Harris A. Moving beyond average values: assessing the night-to-night instability of sleep and arousal in DSM-IV-TR insomnia subtypes. Sleep. 2011;34:531–539. doi: 10.1093/sleep/34.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vallières A, Ivers H, Bastien CH, Beaulieu-Bonneau S, Morin CM. Variability and predictability in sleep patterns of chronic insomniacs. J Sleep Res. 2005;14:447–453. doi: 10.1111/j.1365-2869.2005.00480.x. [DOI] [PubMed] [Google Scholar]
- 11.Carney CE, Edinger JD, Meyer B, Lindman L, Istre T. Daily activities and sleep quality in college students. Chronobiol Int. 2006;23:623–637. doi: 10.1080/07420520600650695. [DOI] [PubMed] [Google Scholar]
- 12.Bernert, Joiner TE. Objectively-assessed sleep variability predicts increased suicide risk in a prospective evaluation of young adults. Sleep. 2010;33:232–233. [Google Scholar]
- 13.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69:875–887. [PubMed] [Google Scholar]
- 14.Ong JC, Huang JS, Kuo TF, Manber R. Characteristics of insomniacs with self-reported morning and evening chronotypes. J Clin Sleep Med. 2007;3:289–294. [PMC free article] [PubMed] [Google Scholar]
- 15.Hidalgo MP, Caumo W, Posser M, Coccaro SB, Camozzato AL, Chaves ML. Relationship between depressive mood and chronotype in healthy subjects. Psychiatry Clin Neurosci. 2009;63:283–290. doi: 10.1111/j.1440-1819.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- 16.Lund HG, Reider BD, Whiting AB, Prichard JR. Sleep patterns and predictors of disturbed sleep in a large population of college students. J Adolesc Health. 2010;46:124–132. doi: 10.1016/j.jadohealth.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011;127:345–352. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manber R, Bootzin RR, Acebo C, Carskadon MA. The effects of regularizing sleep-wake schedules on daytime sleepiness. Sleep. 1996;19(5):432–441. doi: 10.1093/sleep/19.5.432. [DOI] [PubMed] [Google Scholar]
- 19.Edinger JD, Hoelscher TJ, Marsh GR, Lipper S, Ionescu-Pioggia M. A cognitive-behavioral therapy for sleep-maintenance insomnia in older adults. Psychol Aging. 1992:282–289. doi: 10.1037//0882-7974.7.2.282. [DOI] [PubMed] [Google Scholar]
- 20.NIH State-of-the-Science Conference Statement on manifestations and management of chronic insomnia in adults. NIH Consens State Sci Statements. 2005;22(2):1–30. [PubMed] [Google Scholar]
- 21.Wilson SJ, Nutt DJ, Alford C, Argyropoulos SV, Baldwin DS, Bateson AN, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 24(11):1577–1601. doi: 10.1177/0269881110379307. [DOI] [PubMed] [Google Scholar]
- 22.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;17: 281(11):991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 23.Bastien CH, Vailleres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 24.Beck AT, Steer RA, Brown GK. BDI-II: Beck Depression Inventory Manual. Harcourt, Brace, Boston: 1996. [Google Scholar]
- 25.Horne J, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian thythms. Int J of Chronobio. 1976;4:97–110. [PubMed] [Google Scholar]
- 26.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29(9):1155–1173. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 27.Jahng S, Wood PK, Trull TJ. Analysis of affective instability in ecological momentary assessment: indices using successive difference and group comparison via multilevel modeling. Psychol Methods. 2008;13(4):354–375. doi: 10.1037/a0014173. [DOI] [PubMed] [Google Scholar]
- 28.Manber R, Bernert R, Suh S, Nowakowski S, Siebern A, Ong J. CBT for Insomnia with High and Low Depressive Symptom Severity: Adherence and Clinical Outcomes. J Clin Sleep Med. doi: 10.5664/jcsm.1472. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, Totterdell P, Waterhouse JM. Complex interaction of the sleep–wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997;54(2):145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- 30.Murray G, Nicholas CL, Kleiman J, Dwyer R, Carrington MJ, Allen NB, Trinder J. Nature’s clocks and human mood: The circadian system modulates reward motivation. Emotion. 2009;9(5):705–716. doi: 10.1037/a0017080. [DOI] [PubMed] [Google Scholar]
- 31.Kerkhof G. Inter-individual differences in the human circadian system: a review. Bol Psychol. 1985;20:83–112. doi: 10.1016/0301-0511(85)90019-5. [DOI] [PubMed] [Google Scholar]
- 32.Taillard J, Philip P, Bioulac B. Morningness/eveningness and the need for sleep. J Sleep Res. 1999;8:291–295. doi: 10.1046/j.1365-2869.1999.00176.x. [DOI] [PubMed] [Google Scholar]
- 33.Monk TH, Frank E, Potts JM, Kupfer DJ. A simple way to measure daily lifestyle regularity. Journal of Sleep Research. 2002;11(3):183–190. doi: 10.1046/j.1365-2869.2002.00300.x. [DOI] [PubMed] [Google Scholar]
- 34.Emens J. Circadian Misalignment in Mood Disorders. 25th meeting of Associated Professional Sleep Societies. 2011:S17. [Google Scholar]
- 35.Okun ML, Reynolds CF, III, Buysse DJ, Monk TH, Mazumdar S, Begley A, Hall M. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosomatic Medicine. 2011;73:142–150. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]