Abstract
Background
Imatinib has been found to substantially improve outcomes in patients with chronic myeloid leukemia (CML) compared with previously available therapies. However, its use is complicated by development of resistance or drug intolerance, prompting dose escalation or a trial of dasatinib or nilotinib, the second-generation tyrosine kinase inhibitors (TKIs).
Objectives
This article reviews the mechanisms of TKI resistance; discusses the tolerability and efficacy of high-dose imatinib, dasatinib, and nilotinib; and provides background for the rational use of second-line treatment options.
Methods
MEDLINE (1966–December 2009) and EMBASE (1993–December 2009) were searched for pertinent English-language publications using search terms that included, but were not limited to, chronic myeloid leukemia, imatinib, dasatinib, nilotinib, and clinical trial. Abstracts from American Society of Hematology annual meetings (2005–2009) were also reviewed. There were no prespecified inclusion or exclusion criteria.
Results
Major and complete cytogenetic responses (MCyR and CCyR, respectively) to second-line treatment with high-dose (600–800 mg/d PO) imatinib were restricted to CML patients who had achieved a CyR to standard-dose imatinib: >90% of patients without a previous CyR failed to respond. The expected durability of the response to this approach remains unclear. Grade 3/4 thrombocytopenia, neutropenia, and anemia occurred in 14%, 39%, and 8%, respectively, of patients receiving high-dose imatinib. In patients who failed first-line treatment with imatinib, dasatinib (70 mg BID PO) was associated with higher rates of CCyR at 2 years compared with imatinib (44% vs 18%, respectively; P = 0.003), as well as higher estimated rates of progression-free survival at 2 years (86% vs 65%; P = 0.001). Dasatinib use was complicated by grade 3/4 thrombocytopenia and neutropenia in 57% and 63% of patients, respectively, and pleural effusion in 5%. Nilotinib treatment was effective in patients who were resistant to or unable to tolerate imatinib, with 46% and 58% achieving a CCyR and MCyR, respectively, at 2 years. Nilotinib use was complicated by grade 3/4 thrombocytopenia and neutropenia in 28% and 40% of patients, respectively, and QTc-interval prolongation in 1% to 10% of patients. Neither agent was clinically effective in patients with the common T315I mutation.
Conclusion
Dasatinib and nilotinib were effective and generally well tolerated as second-line treatments for CML patients with a suboptimal response to standard doses of imatinib or imatinib intolerance.
Keywords: chronic myeloid leukemia, chronic myelogenous leukemia, CML, imatinib, dasatinib, nilotinib, tyrosine kinase inhibitors
INTRODUCTION
Chronic myeloid leukemia (CML) accounts for ~20% of all adult leukemias.1 It is characterized by the Philadelphia (Ph) chromosome, formed after a reciprocal translation between chromosomes 9 and 22.2,3 The juxtaposition of chromosomes 9 and 22 results in fusion of the BCR and ABL genes to form BCR-ABL, which codes for a constitutively active tyrosine kinase (TK). The activity of this BCR-ABL TK, including its antiapoptotic effects, underpins the pathophysiology of CML.4–6 Untreated, CML progresses from a chronic phase (CP) through an accelerated phase (AP) characterized by increasing splenomegaly, peripheral blood or bone marrow blasts (<20%), basophilia (>20%), and thrombocytopenia (<1 × 105) or thrombocytosis (>1 × 106), to a terminal blast phase (BP) with >20% peripheral blood or bone marrow blasts, typically over a period of 3 to 5 years.1,7
Introduction of TK inhibitors (TKIs) targeting BCR-ABL dramatically changed the treatment of CML. Imatinib* was the first TKI to become available for the treatment of CML.8,9 It was approved by the US Food and Drug Administration (FDA) as first-line therapy for CP CML after the release of data from IRIS (International Randomized Study of Interferon and STI571).10 After 18 months of treatment, imatinib 400 mg/d PO was associated with unprecedented rates of hematologic and cytogenetic response (CyR) and a significant improvement in the estimated rate of freedom from progression compared with interferon (IFN)-α plus cytarabine (96.7% vs 91.5%, respectively; P < 0.001). Eight-year follow-up of the original patient cohort from IRIS reported overall survival (OS) rates of 85% (93% when only CML-related deaths were considered).11
However, imatinib use is complicated by the development of resistance or intolerance.10–14 Primary resistance leads to either a suboptimal response (with reconsideration of the treatment strategy) or treatment failure, as defined by National Comprehensive Cancer Network (NCCN)7 and European LeukemiaNet (ELN)15 criteria (Table I). As a result of primary resistance, 24% of patients in IRIS failed to achieve a complete CyR (CCyR) after 18 months,10 which represented treatment failure according to NCCN and ELN criteria. IRIS also found evidence of the emergence of secondary drug resistance, manifested as relapsed disease in ~17% of patients and progressive disease in 7%.13 Inability to tolerate first-line treatment with imatinib because of adverse events (AEs) led to discontinuation of this therapy in ~6% of patients in IRIS at 8 years.11
Table I.
European LeukemiaNet (ELN)15 and National Comprehensive Cancer Network (NCCN)7 criteria for suboptimal response (ELN)/reconsideration of treatment strategy (NCCN)* and treatment failure with imatinib therapy in patients with newly diagnosed chronic-phase chronic myeloid leukemia.
| Assessment Time |
|||||
|---|---|---|---|---|---|
| Term/Source | 3 Mo | 6 Mo | 12 Mo | 18 Mo | Any Time |
| Suboptimal response | |||||
| ELN | No CHR | No MCyR | No CCyR | No MMR | Secondary chromosomal changes, loss of MMR, or BCR-ABL mutation |
| NCCN | – | No CyR (Ph+ ≤90%) | No CCyR | – | – |
| Treatment failure | |||||
| ELN | No HR (stable disease or disease progression) | No CHR or no CyR (Ph+ ≤95%) | No MCyR | No CCyR | Imatinib-resistant BCR-ABL mutations, loss of CHR or CCyR |
| NCCN | No CHR or hematologic relapse | No CyR (Ph+ ≤90%) or cytogenetic relapse | No MCyR or cytogenetic relapse | No CCyR or cytogenetic relapse | BCR-ABL mutation or disease progression |
CHR = complete hematologic response (platelet count <450 × 109 cells/L, white blood cell count <10 × 109 cells/L, differential with <5% basophils and no immature granulocytes, and nonpalpable spleen); MCyR = major cytogenetic response (≤35% Philadelphia-chromosome positive [Ph+] cells); CCyR = complete cytogenetic response (0% Ph+ cells); MMR = major molecular response (BCR-ABL transcript level ≤0.1 compared with a standardized control gene [ie, a 3-log lower level]); HR = hematologic response.
Hereafter included in suboptimal response.
Second-generation TKIs targeting BCR-ABL are now available. Dasatinib† and nilotinib‡ are approved by the FDA for the treatment of patients with CP or AP CML who developed resistance to or were unable to tolerate previous imatinib therapy.16,17 Dasatinib is also approved for use in patients with BP CML and Ph+ acute lymphoblastic leukemia (ALL).16
This paper reviews the mechanisms of TKI resistance; discusses the tolerability and efficacy of high-dose imatinib, dasatinib, and nilotinib in patients with CML; and provides background for the rational use of second-line treatment options.
METHODS
MEDLINE (1966–December 2009) and EMBASE (1993–December 2009) were searched for pertinent English-language publications using search terms that included, but were not limited to, chronic myeloid leukemia, imatinib, dasatinib, nilotinib, and clinical trial. Abstracts from American Society of Hematology annual meetings (2005–2009) were also reviewed. There were no prespecified inclusion or exclusion criteria.
IMATINIB
Imatinib was approved by the FDA in 2002. The pharmacologic properties of imatinib, potential drug interactions, and recommendations for use of imatinib in patients with renal or hepatic impairment are summarized in Table II.
Table II.
Pharmacokinetics and drug interactions for tyrosine kinase inhibitors approved by the US Food and Drug Administration.
| Variable | Imatinib9 | Dasatinib16 | Nilotinib17 |
|---|---|---|---|
| Metabolism | CYP3A4* | CYP3A4* | CYP3A4* |
| Drug interactions | Avoid warfarin; systemic exposure to acetaminophen increases | Drug levels decreased by antacids, histamine2-receptor antagonists, and proton pump inhibitors | Avoid agents known to prolong QT interval† and strong CYP3A4 inhibitors |
| Contraindications | None | None | Hypokalemia, hypomagnesemia, long QT syndrome (risk of sudden death) |
| Elimination | Feces | Feces | Feces |
| Hepatic impairment | 25% Dose reduction with severe impairment | No dose adjustment | Consider alternative therapy if possible Child-Pugh class A and B: starting dose 400 mg PO morning/200 mg PO evening Child-Pugh class C: starting dose 200 mg PO BID |
| Renal impairment | CrCI 20–39 mL/min: 50% dose reduction (dose >400 mg not recommended) CrCI 40–59 mL/min: dose >600 mg not recommended |
Patients with impaired renal function excluded from studies | Patients with impaired renal function (Cr >1.5) excluded from studies |
| Use in pregnancy | Category D (avoid use) | Category D (avoid use) | Category D (avoid use) |
CYP = cytochrome P450; CrCI = creatinine clearance.
Concomitant strong CYP3A4 inducers (eg, dexamethasone, phenytoin, carbamazepine, rifampin, rifabutin, phenobarbital) may decrease plasma concentrations; concomitant strong CYP3A4 inhibitors (eg, ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, voriconazole) may increase plasma concentrations and should be used with caution.
Amiodarone, disopyramide, procainamide, quinidine, sotalol, chloroquine, halofantrine, clarithromycin, haloperidol, methadone, moxifloxacin, bepridil, and pimozide.
First-Line Treatment
Imatinib is the only TKI currently indicated for the first-line treatment of CML. The recommended starting doses in adults are imatinib 400 mg/d PO for CP CML and 600 mg/d PO for AP/BP CML.9
In a 7-year follow-up to IRIS, the best observed rates of major CyR (MCyR) (defined as 0%–35% Ph+ metaphases on cytogenetic analysis) and CCyR (defined as no Ph+ metaphases on cytogenetic analysis) were 89% and 82%, respectively.12 At 8-year follow-up, estimated rates of event-free and progression-free survival (PFS) were 81% and 92%, respectively; the best observed major molecular response (MMR) (defined as BCR-ABL: control gene ratio <0.1%) was 86%.11 In the 360 patients who crossed over to imatinib after first receiving IFN-α plus cytarabine, 86% achieved an MCyR and 81% achieved a CCyR after 54 months of follow-up; the estimated rates of freedom from progression and OS were 91% and 89%, respectively, after 48 months.18 At 8 years of follow-up, 55% of patients remained on imatinib per protocol; the reasons for discontinuation or crossover included lack of efficacy and/or progression in 16% of patients.11 In a 7-year update in patients with AP CML who were receiving imatinib 600 mg/d, the cumulative best rates of MCyR and CCyR were 30% and 21%, respectively; rates of PFS and event-free survival were 36.5% and 15.0%, respectively.19
Imatinib resistance appears to be multifactorial.20,21 Approximately half of clinically resistant cases are associated with mutations in the BCR-ABL TK domain that inhibit imatinib's ability to bind to ABL. These mutations, found in 36% to 90% of patients with imatinib resistance, may arise spontaneously or as a result of the selective pressure of imatinib.21–23 The most frequently occurring mutations (36%–40%) fall within the adenosine triphosphate–binding loop (P-loop) of the ABL TK domain22–24 and are associated with a 70- to 100-fold decrease in sensitivity to imatinib compared with native BCR-ABL. Treatment of these patients with imatinib alone has been associated with poorer response rates and survival: among patients with CP or AP CML with mutations in the BCR-ABL TK domain, 12 of 13 with P-loop mutations died at a median follow-up of 4.5 months after detection of the mutation, compared with 3 of 14 with mutations outside the P-loop over a similar duration of follow-up (P = 0.002).22 Similarly, among 40 patients with CP CML who had cytogenetic resistance to imatinib, 8 of 9 with P-loop mutations progressed to AP/BP CML at a median of 9 months after detection of the mutation and 6 died, compared with 3 of 9 with mutations outside the P-loop who progressed to AP/BP CML (P = 0.032) and 1 who died (P = 0.045).25 The contact-point T315I mutation, also associated with a poor prognosis,26 is unique in its resistance to all approved BCR-ABL inhibitors.27–31
The remaining cases of clinical imatinib resistance generally involve one of several potential mechanisms. These include increased production of BCR-ABL through genomic amplification or overexpression, development of secondary clonal abnormalities, constitutive activation of downstream signaling molecules (eg, Src-family kinases [SFKs]), increased production of imatinib-binding plasma proteins or transmembrane transport molecules, and insufficient plasma levels of imatinib resulting from pharmacokinetic variability.20,32
Intolerance is another reason for discontinuation of imatinib, with ~4% of patients in the IRIS study discontinuing imatinib at 6 years due to AEs.13,33 The most commonly reported AEs with imatinib are consistent with those in the IRIS study,10 which were primarily hematologic, with 45% to 61% of patients developing anemia, neutropenia, and/or thrombocytopenia (all grades) after a median follow-up of 19 months. Grade 3/4 neutropenia, thrombocytopenia, and anemia were reported in 14%, 8%, and 3% of patients, respectively. The most common nonhematologic AEs associated with imatinib (all grades) were superficial edema (56%), nausea (44%), muscle cramps (38%), and rash (34%); other grade 3/4 nonhematologic AEs included musculoskeletal pain (3%), joint pain (2%), abdominal pain (2%), and rash (2%).10 In general, most AEs can be managed by dose interruption/reduction and appropriate supportive measures, although rash, fluid retention, and liver toxicity may prompt discontinuation.7,9
The prescribing information for imatinib contains numerous warnings regarding both early and long-term toxicities.9 One of the more controversial warnings concerns the potential for severe congestive heart failure (CHF) and left ventricular dysfunction. These events are relatively rare; in a study in 1276 patients treated with imatinib, ≤1% of patients had cardiac events associated with the drug, and the incidence of CHF increased with increasing age.34 Moreover, patients with these events were more likely to have preexisting conditions predisposing them to CHF. In addition, the prescribing information carries warnings regarding edema and fluid retention (grade 3/4 superficial edema in 2%–6% of patients with CML), cytopenias, hepatotoxicity (severe elevations in transaminases or bilirubin in ~5% of patients with CML), hemorrhage (grade 3/4 hemorrhage in 1.8% of patients with CML), gastrointestinal perforation (rare), and bullous dermatologic reactions.9 Based on the findings of animal studies, renal toxicity and immuno-suppression may occur with long-term use.9
High-Dose First-Line Treatment
Clinical investigations of high-dose imatinib as first-line therapy for CML are ongoing. The RIGHT (Rationale and Insight for Gleevec High-Dose Therapy) trial35 was a multicenter, open-label, single-arm, Phase II investigation of imatinib 400 mg PO BID in 115 patients with newly diagnosed CP CML, of whom 83 completed the study (10 discontinued due to AEs and 6 due to unsatisfactory effect). An MMR was achieved in 48% of patients at 6 months, 54% at 12 months (vs 39% with standard-dose imatinib, based on historical data from the IRIS trial), and 63% at 18 months; the corresponding rates of complete molecular response were 39%, 44%, and 55%. Although the reduction in tumor burden was more rapid compared with historical controls, most patients were low risk based on the Sokal score (70%).
A Phase III comparison of imatinib 400 and 800 mg/d PO in high-risk patients with CP CML found no significant difference between groups in rates of CCyR at 12 months (58% and 64%, respectively).36 There were no significant differences in rates of MMR between the 2 arms, nor were there differences in the frequency of AEs.
The TOPS (Tyrosine Kinase Inhibitor Optimization and Selectivity) study,37 a prospective, randomized Phase III study, compared rates of MMR at 12 months in patients treated with imatinib 400 or 800 mg PO. MMR and CCyR occurred more rapidly in patients treated with imatinib 800 mg, with significantly higher response rates at 6 months compared with imatinib 400 mg (MMR: 33.5% vs 17.0%, respectively; P < 0.001; CCyR: 56.7% vs 44.6%; P = 0.015). However, there was no statistically significant difference between groups in either measure at 12 months.
The German CML–Study IV38 also reported higher rates of MMR at 12 months with imatinib 800 mg PO (29%) compared with imatinib 400 mg PO (52%; P < 0.001) or imatinib 400 mg/IFN-α (34%; P = 0.01), although the clinical significance of the differences is unknown. Another study found that reducing the dose from 800 to 400 mg/d PO had no effect on rates of event-free survival.39 However, event-free survival was reported to be significantly worse if the dose reduction occurred before achievement of a CCyR.
High-Dose second-Line Treatment
High-dose imatinib (600–800 mg/d PO) is approved for use in patients in whom imatinib resistance developed after receipt of standard-dose imatinib.7,9 The rationale for the use of higher doses is based on findings that some BCR-ABL mutations may result in only intermediate resistance to imatinib, suggesting that higher doses may be able to overcome this resistance.15,40,41 In addition, overproduction of BCR-ABL, another mechanism of resistance to standard-dose imatinib, may respond to an increased dose.42,43
In studies in patients who had failed imatinib treatment (ELN/NCCN criteria), dose escalation was associated with CCyR rates of 11% to 39% and MCyR rates of 26% to 52%.44–49 It is important to note that escalation of the imatinib dose has not been found to offer benefit in most patients who fail to achieve a CyR to first-line imatinib therapy; studies have reported that 93% of patients had no improvement.44,45 The original IRIS data suggest that up to 22% of patients may not benefit from escalation of the imatinib dose, with failure to achieve a CyR at 6 months.10
The expected durability of the response to imatinib dose escalation remains unclear. Early studies suggested that the best attained CyRs were subsequently lost in 43% to 50% of patients.46,47,50 However, data from a more recent study with a median follow-up of 61 months indicated that 40% of patients who met the criteria for failure with standard-dose imatinib achieved a CCyR, and that 88% of patients with an MCyR had a sustained response beyond 2 years.51 A retrospective analysis of data from IRIS also suggested a high rate of PFS (89%) after 3 years in 106 patients undergoing imatinib dose escalation; PFS was similar among patients who underwent dose escalation according to either IRIS-defined or ELN criteria.52 Further data and longer durations of follow-up will help clarify the utility of high-dose imatinib in patients with resistance to standard doses. In addition, it will be important to gain greater understanding of the specific mechanism(s) of imatinib resistance, as the clinical effect of high-dose imatinib is likely to be limited to patients with resistance to standard-dose imatinib who do not have a P-loop or T315I mutation.
On the other hand, patients who have a suboptimal hematologic response or CyR (ELN criteria) to initial standard-dose imatinib appear to respond well to escalation of the imatinib dose. After 6 to 20 months of follow-up, 50% to 100% of patients in 2 studies had a durable CCyR; yet, as expected, patients subsequently identified as having BCR-ABL mutations often failed to achieve even an MCyR.53,54 There are ongoing efforts to incorporate molecular response criteria into clinical trials to improve understanding of the impact of imatinib dose escalation in patients with suboptimal responses; however, it has been suggested that fewer than half of patients with suboptimal molecular responses will achieve an MMR to this treatment strategy.54
The frequency of AEs in patients receiving high-dose imatinib is summarized in Table III. Among AEs of all grades,55 the most common were fluid retention (43%), superficial edema (43%), and nausea (33%). However, grade 3/4 thrombocytopenia, neutropenia, and anemia occurred in a respective 14%, 39%, and 8% of patients receiving high-dose imatinib. Dose interruption, reduction, or discontinuation was necessary in a substantial proportion (31%–63%) of patients taking high-dose imatinib; grade 3/4 hematologic AEs were the cause in 24% to 31% of these patients.44,45,47 Other AEs that contributed to a change in treatment in those taking high-dose imatinib included abdominal pain, diarrhea, vomiting, fatigue, pain, joint effusion, rash, bone ache, dizziness, and blisters.44,45
Table III.
Incidence (%) of treatment-related adverse events in patients exposed to second-line treatments for chronic-phase chronic myeloid leukemia.
| Imatinib 800 mg (n = 49)55 |
Dasatinib 70 mg BID (n = 101)55 |
Dasatinib 100 mg Once Daily (n = 165)16 |
Nilotinib 400 mg BID (n = 318)17 |
|||||
|---|---|---|---|---|---|---|---|---|
| Adverse Event | All Grades | Grade 3/4 | All Grades | Grade 3/4 | All Grades | Grade 3/4 | All Grades | Grade 3/4 |
| Thrombocytopenia | NR | 14 | NR | 57 | NR | 23 | NR | 28 |
| Neutropenia | NR | 39 | NR | 63 | NR | 36 | NR | 28 |
| Anemia | NR | 8 | NR | 20 | NR | 13 | NR | 8 |
| Fluid retention | 43 | 0 | 39 | 7 | 34 | 4 | NR | NR |
| Superficial edema | 43 | 0 | 20 | 1 | 18 | 0 | NR | NR |
| Peripheral edema | NR | NR | NR | NR | 3 | 0 | 11 | 0 |
| Pleural effusion | 0 | 0 | 25 | 5 | 18 | 2 | NR | NR |
| Diarrhea | 29 | 2 | 37 | 3 | 27 | 2 | 22 | 3 |
| Fatigue | 22 | 4 | 33 | 3 | 24 | 2 | 28 | 1 |
| Headache | 10 | 2 | 26 | 2 | 33 | 1 | 31 | 3 |
| Nausea | 33 | 0 | 24 | 0 | 18 | 1 | 31 | 1 |
| Dyspnea | 4 | 0 | 23 | 5 | 20 | 2 | 11 | 1 |
| Rash | 20 | 0 | 18 | 0 | 17 | 2 | 33 | 2 |
| Pyrexia | 10 | 0 | 14 | 0 | 5 | 1 | 14 | 1 |
| Asthenia | 4 | 0 | 15 | 0 | NR | NR | 14 | 0 |
| Vomiting | 24 | 0 | 10 | 0 | 7 | 1 | 21 | <1 |
| Pain in extremity (musculoskeletal pain) | 12 | 2 | 21 | 1 | 19 | 2 | 13 | 1 |
NR = not reported.
DASATINIB
Approved by the FDA in 2006, dasatinib is the first of the second-line, or second-generation, TKIs. The approved dosages are 100 mg PO once daily for patients with CP CML and 70 mg BID for patients with AP/BP CML.16
Dasatinib binds both the active and inactive conformations of BCR-ABL, and has in vitro activity against all currently described imatinib-resistant mutations except T315I, supporting its use as second-line treatment.28,29,31 It has 325-fold greater in vitro activity against native BCR-ABL compared with imatinib,29 suggesting that it may mitigate imatinib resistance caused by increased BCR-ABL expression.28,29,56,57 Furthermore, dasatinib potently inhibits SFKs associated with BCR-ABL–independent imatinib resistance,28,29 and it is not a substrate for organic cation transporter-1 (OCT-1), which is likely to be largely responsible for low in vitro sensitivity to imatinib.58–60 However, dasatinib-insensitive BCR-ABL mutations have been identified, indicating that development of clinical dasatinib resistance is possible when patients with CML are treated with this second-line agent. Although 9 dasatinib-resistant mutants affecting 6 residues were detected in an in vitro mutagenesis study, only the F317V and T315I mutations emerged at intermediate drug concentrations, and only the T315I mutation persisted at the maximum achievable plasma concentrations.61
The pharmacologic properties of dasatinib and dosing recommendations for patients with renal or hepatic impairment are summarized in Table II.
The START Program Trials
The START (SRC/ABL Tyrosine Kinase Inhibition Activity Research Trials of Dasatinib) program was a series of multicenter, open-label, Phase II clinical studies in patients with CML who were resistant to imatinib (NCCN criteria) or unable to tolerate it.45,62,63 These studies led to the approval of dasatinib as second-line treatment across all phases of CML.
In an update to studies of dasatinib in CP CML (START-A and START-R), 44% of patients with resistance to imatinib had achieved a CCyR with dasatinib 70 mg at 24 months of follow-up, and 55% achieved an MCyR.64 Among those with a CCyR or MCyR, 86% and 84%, respectively, maintained the response at 24 months; PFS was 75% at this time point. Among patients who were unable to tolerate imatinib, 82% and 78% achieved a CCyR and MCyR at 24 months; PFS was 94% in this subgroup. This update indicated high rates and durability of responses in patients who were resistant to or unable to tolerate imatinib. Two-year follow-up data from START-C and a Phase III randomized trial indicated that rates of CCyR and OS in patients with hematologic imatinib intolerance were 43% and 88%, respectively, compared with 79% and 98% in those with nonhematologic imatinib intolerance.65
In START-R, Kantarjian et al55 conducted a prospective comparison of dasatinib 70 mg BID and high-dose imatinib (800 mg/d) in patients who had failed first-line treatment with imatinib. After a minimum follow-up of 2 years, dasatinib was associated with higher rates of MCyR compared with high-dose imatinib (53% vs 33%, respectively; P = 0.017), as well as higher rates of CCyR (44% vs 18%; P = 0.003) and MMR (29% vs 12%; P = 0.028). In patients with no previous CyR to imatinib, the corresponding rates of MCyR were 51% and 7%. Estimated PFS at 2 years was 86% for dasatinib and 65% for imatinib (P = 0.001). Rates of discontinuation due to toxicity were comparable between dasatinib and imatinib (23% and 20%).
Dasatinib has also been reported to have activity in patients with AP/BP CML. In START-A, after 14 months of follow-up, dasatinib was associated with achievement of a CCyR and MCyR in a respective 32% and 39% of patients with AP CML; PFS was 66% at 12 months.66 In patients with BP CML (START-B and START-L), response rates differed depending on whether patients had myeloid blast phase (MBP) or lymphoid blast phase (LBP) disease. After 12 months of follow-up, 26% of MBP patients achieved a CCyR, compared with 46% of LBP patients; a respective 33% and 52% of MBP and LBP patients achieved an MCyR. However, PFS was 6.7 months in those with MBP, compared with 3.0 months in those with LBP.67 The durability of these responses is under investigation.
The majority of AEs seen with dasatinib 70 mg BID in the START program were hematologic, with grade ¾ thrombocytopenia and neutropenia occurring in 57% and 63% of patients, respectively (Table III).55 In general, grade ¾ nonhematologic AEs were rare. To date, there are no data on cumulative toxicity with long-term therapy.63 The main nonhematologic AE of concern with dasatinib is development of pleural effusion (all grades, 18%–25%). Traditionally, imatinib has been associated with superficial edema and fluid retention, whereas effusions have been more commonly described with dasatinib.55 The etiology of fluid retention and effusions with these agents is unclear.
As might be predicted, the frequency of AEs was increased in those with advanced disease. Rates of grade ¾ thrombocytopenia and neutropenia in AP CML (START-A) were 82% and 76%, respectively.66 The dasatinib toxicity profile appeared to be comparable in imatinib-resistant and imatinib-intolerant patients, suggesting little to no cross-intolerance with imatinib and overall good treatment compliance.62,63,68 Most AEs, including cytopenias and pleural effusions, may be managed by dose interruption and reduction.7,16,69 The prescribing information for dasatinib carries warnings concerning myelosuppression, bleeding-related events, fluid retention, and QT-interval prolongation.16
Treatment of Patients With BCR-ABL Mutations
Dasatinib has been reported to be effective in patients with imatinib-resistant BCR-ABL mutations (except T315I).62,70,71 A recent analysis in 1043 patients with CP CML recruited into Phase II/III trials of dasatinib supported the efficacy and durability of dasatinib in patients with baseline BCR-ABL mutations relative to those without baseline mutations.72 After 2 years, dasatinib treatment in imatinib-resistant patients with or without preexisting mutations was associated with comparable rates of CCyR (43% and 47%, respectively), MCyR (55% and 58%), PFS (70% and 80%), and OS (88% and 92%). High proportions of patients with highly resistant imatinib mutations also achieved a CCyR and MCyR: rates were 40% and 67%, respectively, in patients with L248 mutations; 61% and 65% in those with Y253 mutations; 36% and 56% in those with E255K mutations; 60% and 66% in those with F359C mutations; and 39% and 52% in those with H396 mutations. Poor responses were seen in those with F317L and T315I mutations; rates of CCyR and MCyR were 7% and 14%, respectively, in those with the former mutation and 0% and 10% in those with the latter mutation.
This research group reported the emergence of dasatinib resistance and new mutations (T315I/A, F317L, V299L, M351T, L248V, G250E, K271R, and Y320C) in patients who had previously been resistant only to imatinib (Table IV).72,73 T315I mutations were the most frequent of these new mutations. Of 47 patients with new mutations, 24 experienced disease progression. Dasatinib resistance has also been associated with F486S and E507G mutations in patients with advanced disease.74
Table IV.
Relationship of specific mutations to the efficacy of dasatinib and nilotinib in patients with chronic-phase chronic myeloid leukemia (CP CML).*
| Rates of CCyR, n/N (%) |
||
|---|---|---|
| Mutation | Dasatinib | Nilotinib |
| Any mutation | 158/369 (42.8) | 18/77 (23.4) |
| P-loop mutations | 61/141 (43.3) | NR |
| L248V | NR | 0/2 |
| G250E | 19/51 (37.3) | 1/4 (25.0) |
| Y253F/H | 12/23 (52.2) | 0/8 |
| E255K/V | 8/24 (33.3) | 0/6 |
| T315I | 0/20 | 0/4 |
| F317L | 1/14 (7.1) | NR†‡ |
| F359C/V | 14/27 (51.9) | 0/10† |
CCyR = complete cytogenetic response; NR = not reported.
Dasatinib data are based on 1093 patients with CP CML enrolled in clinical trials of dasatinib.73 Nilotinib data are based on 280 patients with CP CML enrolled in a Phase II clinical trial of nilotinib.82
Considered a nilotinib-sensitive mutation (40.0% [18/45] of patients harboring nilotinib-sensitive mutations achieved a CCyR).100
Phase III Dose-Optimization study
A Phase III study was conducted to evaluate optimal dosing regimens of dasatinib in CP CML.75 The dosing schedules that were compared were dasatinib 100 mg once daily, 50 mg BID, 140 mg once daily, and 70 mg BID. The rationale for comparing dasatinib 100 mg with the approved dose of 140 mg arose from the Phase II program, in which the median delivered dose in patients with CP CML was ~100 mg/d at 8-month follow-up.63 The dose-optimization study found similar rates of CCyR (50%–54%) and MCyR (61%–63%) across all 4 dosing arms.75 After 24 months of follow-up, 89% and 87% of responding patients who received dasatinib 100 mg maintained a CCyR and MCyR, respectively; PFS at 24 months was 80% in this subgroup.
Early results from this study also suggested an improvement in the toxicity profile, with the 100-mg arm having significantly lower rates of grade ¾ thrombocytopenia compared with the other 3 arms combined (23% vs 36%–41%, respectively; P = 0.003).75 There was also a significantly lower incidence of grade ¾ pleural effusion (2% vs 4%–5%; P = 0.049). The 100-mg arm had the lowest incidence of treatment interruption and discontinuation (12%) relative to the other 3 dosing arms (16%–21%). Overall, the dasatinib 100-mg regimen appeared to have a better safety profile and comparable efficacy compared with the other regimens studied.
The results of the previous study prompted adjustment of the recommended starting dose for patients with CP CML to 100 mg once daily.16 The recommended starting dose for patients with advanced CML and Ph+ ALL remains unchanged at 70 mg BID. However, more recent reports suggested comparable efficacy and an improved safety profile in patients with AP/BP CML or Ph+ ALL receiving dasatinib 140 mg once daily compared with 70 mg BID.76–79
First-Line Treatment
Based on its reported rates of efficacy and encouraging safety profile, dasatinib 100 mg/d has been investigated in previously untreated patients with CP CML. In an ongoing Phase II study, patients were randomized to receive dasatinib 100 mg/d given as either a single or divided dose.80 After a median 24 months of follow-up, data from 50 patients suggested both rapid and clinically meaningful responses: rates of CCyR at 3 months (82%), 6 months (94%), 12 months (98%), 24 months (84%), and 30 months (83%) compared favorably with historical data from newly diagnosed patients treated with standard-dose imatinib (37%, 54%, 65%, 67%, and 67%, respectively) or high-dose imatinib (63%, 85%, 89%, 88%, and 89%). Overall, 98% of evaluable patients achieved a CCyR; at 24 months, 71% of patients achieved an MMR. The toxicity profile at 24 months continued to support the tolerability of dasatinib in these previously untreated patients. The most common grade ¾ toxicities were hematologic in nature, including neutropenia (21%), thrombocytopenia (10%), and anemia (6%). Pleural effusions occurred in 13% of evaluable patients, including 2% with grade ¾ effusions.80 A multicenter, randomized, Phase III study is being conducted to compare dasatinib with imatinib as first-line agents.81
NILOTINIb
Nilotinib, approved in 2007 for the second-line treatment of CML, is an imatinib analogue that is 10- to 50-fold more potent against native BCR-ABL than is its parent compound.30 The approved dosing schedule in CP and AP CML is 400 mg BID PO.17 Food is not to be consumed from 2 hours before to 1 hour after nilotinib dosing because of increased drug bioavailability after food consumption and concerns about QTc-interval prolongation and possible cardiac AEs.17
Nilotinib's increased potency relative to imatinib may allow it to override some forms of imatinib resistance. It binds to the inactive conformation of the ABL protein, is active against all imatinib-resistant BCR-ABL mutations except T315I,30 and is not a substrate of OCT-1.58 However, as with dasatinib, nilotinib-insensitive BCR-ABL mutations have been identified in vitro.61 Two mutations of the P-loop region, Y253H and E255V, have been identified at intermediate drug concentrations29,61; as with dasatinib, only the T315I mutation was isolated at maximum achievable plasma nilotinib concentrations.61 Nilotinib does not have measurable activity against SFKs and, therefore, may not be clinically active in imatinib-resistant CML with noted SFK escape mechanisms.
The pharmacologic properties of nilotinib are summarized in Table II.
second-Line Treatment
Nilotinib 800 mg/d was approved based on data from an open-label, Phase II study in patients with CP/AP CML who had failed initial imatinib therapy (NCCN criteria) or were unable to tolerate it.17,82 In a recent 2-year follow-up of patients with CP CML, the overall rate of MMR was 28%; rates of CCyR and MCyR were 46% and 58%, respectively83,84; and rates of PFS and OS at 24 months were 64% and 87%, respectively. The responses were durable, with 84% of patients maintaining a CCyR at 24 months. Data from 1422 patients in the Phase III ENACT (Expanding Nilotinib Access in Clinical Trials)85 study were similar, with overall CCyR and MCyR rates of 36% and 45%, respectively.
As anticipated based on results for both imatinib and dasatinib, the efficacy of nilotinib appears to be lower in advanced disease. In patients with AP CML, rates of CCyR and MCyR were 19% and 32%, respectively.86 In patients with BP CML, only 25% of patients achieved a complete hematologic response.87
AEs with nilotinib have been predominantly mild to moderate in severity, and were generally managed with dose reduction or interruption and appropriate supportive care.7,17 In the pivotal Phase II study, the most frequent grade 3/4 AEs in patients with CP and AP CML included bone marrow suppression (neutropenia: 30% and 40%, respectively; thrombocytopenia: 28% and 40%; anemia: 10% and 25%) and asymptomatic serum lipase elevations (16% and 17%).83,86 In a subanalysis of the Phase II study, cross-intolerance with imatinib (defined as recurrence of a grade 3/4 AE during nilotinib treatment leading to discontinuation of imatinib) was observed in 51% of patients.88 In general, cross-intolerance was limited to hematologic toxicity (often a recurrence of thrombocytopenia), and only 4% of patients experienced nonhematologic toxicities similar to those occurring with previous imatinib treatment.88 Overall, 15% of patients discontinued treatment as a result of AEs.83
The prescribing information for nilotinib carries a black box warning regarding the risk of QTc-interval prolongation and sudden death.17 Sudden deaths were reported in 0.6% of patients in the initial clinical studies and were noted at a similar frequency in the expanded-access study.17 QTc-interval prolongation and palpitations have been reported in 1% to 10% of patients.17 As a result, the prescribing information recommends monitoring for and correction of hypokalemia and hypomagnesemia, as well as monitoring of ECGs initially and after any dose adjustments. Nilotinib should be avoided in those with long QT syndrome, as well as in those taking drugs known to prolong the QT interval (antiarrhythmics) or to inhibit cytochrome P450 (CYP) 3A4.17 For that reason, the prescribing information also carries a warning against consumption of foods such as grapefruit and grapefruit juice, which are known to inhibit CYP3A4. It is unclear why cardiac AEs were observed more frequently with nilotinib therapy than with imatinib or dasatinib therapy. The prescribing information for nilotinib also carries warnings regarding myelosuppression, elevations in serum lipases, hepatic abnormality or impairment, and electrolyte abnormalities.
Treatment of Patients With BCR-ABL Mutations
Although the pivotal Phase II study found that nilotinib was active in patients with all BCR-ABL mutations (except T315I) associated with imatinib resistance and in patients with BCR-ABL–independent resistance,82 evidence of the emergence of nilotinib-insensitive mutations has since become available. Among the mutations that develop most frequently during nilotinib therapy, the P-loop mutations Y253H and E255K/V are often associated with disease progression.89 In a subanalysis in patients with CP CML enrolled in the pivotal Phase II trial of nilotinib, the Y253H, E255K/V, and F359C mutations were reported in 14% of imatinib-resistant patients, but were associated with disease progression in 69% of patients harboring these mutations.90,91 Possession of the E255K/V and F359V mutations was associated with high rates of disease progression (86% [6/7] and 82% [9/11], respectively).90,92 The association between individual mutations and disease progression also held true for patients with AP CML harboring the Y253H, E255K/V, and F359C mutations, in whom the rate of progression was 64%.91 However, the mutants most frequently associated with progression during nilotinib therapy were T315I (60%), F359V (80%), and M244V (80%).91 The poor prognosis associated with development of a T315I mutation during imatinib treatment has already been established.26 Compared with P-loop mutations, development of non–P-loop mutations (including F359V and M244V) during imatinib treatment is associated with a lower rate of disease progression and better survival.22,25,26
First- and Third-Line Treatment
Nilotinib is currently being investigated as a first-line treatment in a Phase III study comparing nilotinib 600 and 800 mg/d with standard-dose imatinib in patients with newly diagnosed CP CML. A recent 12-month update reported higher rates of MMR in patients receiving nilotinib 300 and 400 mg BID compared with imatinib 400 mg once daily (44% and 43% vs 22%, respectively; both comparisons, P ≤ 0.001).93 Rates of CCyR were higher in those receiving nilotinib 300 and 400 mg BID compared with imatinib (80% and 78% vs 65%; P ≤ 0.001 and P = 0.001, respectively), with comparable rates of discontinuation in the 3 arms (7%, 11%, and 9%). This study was based on data from the Phase II pilot study, in which 98% of patients followed for at least 3 months achieved a CCyR and 63% achieved an MMR.94 At the 3-month time point, the rate of CCyR appeared to be higher than in historical data for initial imatinib therapy (37%)94 and similar to that for dasatinib (79%).95 In a separate study of nilotinib in previously untreated patients with CML, rates of CCyR and MMR were 96% and 85%, respectively, after a median follow-up of 15 months.96
Nilotinib is the only approved TKI that is reported to have activity in patients with CML who have failed to respond to or were unable to tolerate both imatinib and dasatinib.87,97 After a median nilotinib exposure of 7.3 months, 32% of study patients with CP CML attained a CCyR and 38% attained an MCyR.97 Rates of grade 3/4 neutropenia, thrombocytopenia, and anemia were 38%, 24%, and 5%, respectively.97 At 4 months of follow-up, 23% of nilotinib-treated patients with AP disease and 5% of nilotinib-treated patients with BP disease had a return to CP disease.87 In a study in 14 patients receiving third-line nilotinib after the failure of imatinib and dasatinib, and 34 patients receiving third-line dasatinib after the failure of imatinib and nilotinib, 6 achieved a CCyR and 7 achieved an MMR; however, the responses were not durable, except in 3 patients with CP disease, who had responses lasting >12 months.98
DISCUSSION
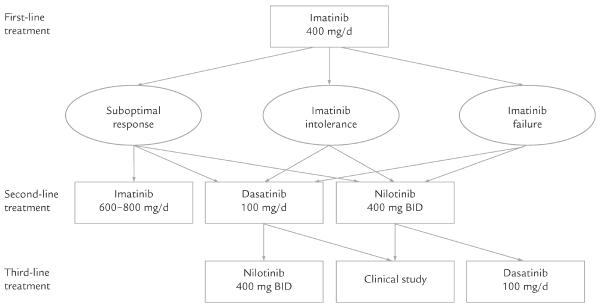
At a dose of 400 mg/d, imatinib is the only TKI currently approved by the FDA for the first-line treatment of CML.9 In cases of resistance or intolerance to standard-dose imatinib, several possible options are available, including escalation of the imatinib dose (to 600 or 800 mg/d), a switch to dasatinib (100 mg once daily or 70 mg BID), or a switch to nilotinib (400 mg BID) (Figure).15 Although the data are accumulating with ongoing follow-up, the evidence is limited, as few randomized comparisons of these agents have been completed.
Figure.
Possible sequence of tyrosine kinase inhibitors in the treatment of patients with chronic myeloid leukemia who are resistant, intolerant, or suboptimal responders to imatinib.
This review included 4 studies of first-line therapy with high-dose imatinib.35–38 Although one of these studies was limited by its single-arm design,35 the 3 randomized studies were limited by a lack of clinically relevant results.36–38 Although more patients receiving imatinib 800 mg achieved a CCyR or MCyR at 6 months compared with those receiving imatinib 400 mg in the TOPS study, there were no significant differences in rates of response at 12 months.37 The studies of high-dose imatinib as a second-line agent in patients who failed standard-dose imatinib therapy were also limited by having a single-arm or retrospective design, a lack of direct comparisons, or a limited duration of follow-up.44–54,56 Furthermore, the durability of response is unclear and appears to be restricted to patients who have achieved a previous cytogenetic response to standard doses.44,53,54,99 In the only randomized, prospective study that directly compared dose escalation of imatinib with an alternative therapy (dasatinib) in patients who had failed first-line imatinib therapy, dasatinib was associated with higher rates of CyR and better tolerability compared with imatinib.55 Finally, toxicity (primarily hematologic) limited the use of high doses of imatinib.
Both dasatinib and nilotinib have been reported to be efficacious as first-line treatments in patients with newly diagnosed CML. However, evidence supporting the use of dasatinib is currently limited to a prospective, Phase II study that compared 2 dasatinib regimens in 50 patients.80 Guidance will likely come from the results of a multicenter, randomized study that is currently under way.81 The use of nilotinib is supported by the results of a Phase III, multicenter, randomized study comparing 2 nilotinib dosing schedules with standard-dose imatinib93; however, the findings are limited to 1 year of follow-up (compared with 8 years for standard-dose imatinib11). In a randomized study of second-line treatment in those with imatinib resistance or intolerance, dasatinib was associated with higher response rates compared with high-dose imatinib.55 The evidence for the efficacy of nilotinib as a second-line agent is currently limited to Phase II studies, although the data are accumulating.
The approach to the use of second-generation TKIs must be individualized based on patients' comorbid conditions and ability to tolerate previous imatinib therapy. While both dasatinib and nilotinib have shown hematologic cross-intolerance with imatinib, each agent has a unique nonhematologic toxicity profile that may guide the treatment decision.16,17 For example, dasatinib may be preferred in patients at risk for hepatic and pancreatic disorders, diabetes, electrolyte abnormalities, renal impairment, high eosinophil levels, and cardiac dysrhythmias. Nilotinib may be preferred in patients at risk for bleeding-related events, fluid retention (including those with CHF), and lung disease.16,17 Both agents have effects on QTc-interval prolongation, but only the prescribing information for nilotinib carries a black box warning concerning QTc-interval prolongation and sudden death.17 Patients at highest risk for QT-interval prolongation may be considered for a clinical trial of a novel agent without such toxicity.
The expanded use of testing for BCR-ABL mutations may help direct the choice of a second-generation TKI. Patients with T315I-mutated BCR-ABL should be considered for clinical trials of compounds with activity against these hard-to-treat mutations. Dasatinib may be considered for patients with P-loop mutations, such as Y253H, E255K/V, and F359C/V, in whom high rates of disease progression have been noted with nilotinib.90,91,100 Nilotinib may offer the best chance of a clinical response in patients with F317L, V299L, and T315A mutations, in whom dasatinib appears to be less clinically effective.90,91,100 Emergence of dasatinib- and nilotinib-resistant mutations may complicate the use of these agents.
Finally, patients failing second-line treatment with dasatinib may be considered for nilotinib treatment or for entry into a clinical trial, and patients failing second-line treatment with nilotinib may be directed to appropriate clinical trials. In addition, those without a previous CyR to imatinib, those with anemia, and those with a high disease burden (>90% Ph+ metaphases) are expected to have a poor response to second-generation TKIs (MCyR 14%), and alternative therapy should be considered in these patients.101 A number of compounds are currently under clinical development, including bosutinib, INNO-406, AZD530, and PHA-739358.21 However, development of another compound, MK-0457, was halted in light of concerns about cardiotoxicity.102
This review had some limitations, perhaps the most important being that it was not a systematic review. Thus, although the search was comprehensive, data extraction and review were not systematic, a qualitative synthesis of study designs and analyses was not performed, and the heterogeneity of the studies was not quantified. Although an attempt was made to review the most current clinical information, a large part of the data came from Phase II and III studies published in abstract form only, which often lacked detailed statistical information. Restriction to the English language and publication bias may have further limited this review.
CONCLUSIONS
While imatinib is associated with improvements in the natural history of CP CML, its use has been limited by intolerance and development of resistance in some patients. The second-generation TKIs dasatinib and nilotinib generally have been found to be effective and well-tolerated alternatives. Future randomized studies will clarify the optimal first- and second-line agents for the treatment of patients with newly diagnosed CML and those who are unable to tolerate or development resistance to imatinib.
ACKNOWLEDGMENTS
Bristol-Myers Squibb Company funded writing and editing support on this manuscript, which was provided by StemScientific, Secaucus, New Jersey. Bristol-Myers Squibb had no influence on the content of the manuscript, nor did the authors receive any financial compensation. The authors participated in all aspects of the development of the manuscript, including its planning, writing, and editing. They take full responsibility for its content and confirm that it reflects their viewpoints and medical expertise.
Dr. Stein is supported by a National Institutes of Health K12 Institutional Research and Academic Career Development Award. Dr. Smith has served as a consultant for Bristol-Myers Squibb and Novartis Pharmaceuticals Corporation. The authors have indicated that they have no other conflicts of interest with regard to the content of this article.
Footnotes
Trademark: Gleevec® (Novartis Pharmaceuticals Corporation, East Hanover, New Jersey).
Trademark: Sprycel® (Bristol-Myers Squibb Company, New York, New York).
Trademark: Tasigna® (Novartis Pharmaceuticals Corporation).
REFERENCES
- 1.Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340:1330–1340. doi: 10.1056/NEJM199904293401706. [DOI] [PubMed] [Google Scholar]
- 2.Nowell PC, Hungerford DA. A minute chromosome in human chronic granulocytic leukemia. Science. 1960;132:1497. doi: 10.1126/science.144.3623.1229. [DOI] [PubMed] [Google Scholar]
- 3.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 4.Bartram CR, de Klein A, Hagemeijer A, et al. Translocation of c-ab1 oncogene correlates with the presence of a Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1983;306:277–280. doi: 10.1038/306277a0. [DOI] [PubMed] [Google Scholar]
- 5.Groffen J, Stephenson JR, Heisterkamp N, et al. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984;36:93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- 6.Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247:1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network [Accessed December 30, 2009];NCCN Clinical Practice Guidelines in Oncology. Chronic myelogenous leukemia V.2 2010. doi: 10.6004/jnccn.2009.0065. http://www.nccn.org/professionals/physician_gls/PDF/cml.pdf. [DOI] [PubMed]
- 8.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 9.Gleevec (imatinib mesylate) [prescribing information] Novartis Pharmaceuticals Corporation; East Hanover, NJ: 2009. [Google Scholar]
- 10.O'Brien SG, Guilhot F, Larson RA, et al. IRIS Investigators Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 11.Deininger M, O'Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: Sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood (ASH Annual Meeting Abstracts) 2009;114:1126. [Google Scholar]
- 12.O'Brien SG, Guilhot F, Goldman JM, et al. International randomized study of interferon versus STI571 (IRIS) 7-year follow-up: Sustained survival, low rate of transformation and increased rate of major molecular response (MMR) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CMLCP) treated with imatinib (IM) Blood (ASH Annual Meeting Abstracts) 2008;112:186. [Google Scholar]
- 13.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 14.Hochhaus A, O'Brien SG, Guilhot F, et al. IRIS Investigators Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 15.Baccarani M, Saglio G, Goldman J, et al. European LeukemiaNet Evolving concepts in the management of chronic myeloid leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 16.Sprycel (dasatinib) [prescribing information] Bristol-Myers Squibb Company; Princeton, NJ: 2009. [Google Scholar]
- 17.Tasigna (nilotinib) [prescribing information] Novartis Pharmaceuticals Corporation; East Hanover, NJ: 2007. [Google Scholar]
- 18.Guilhot F, Druker B, Larson RA, et al. High rates of durable response are achieved with imatinib after treatment with interferon alpha plus cytarabine: Results from the International Randomized Study of Interferon and STI571 (IRIS) trial. Haematologica. 2009;94:1669–1675. doi: 10.3324/haematol.2009.010629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palandri F, Castagnetti F, Alimena G, et al. The long-term durability of cytogenetic responses in patients with accelerated phase chronic myeloid leukemia treated with imatinib 600 mg: The GIMEMA CML Working Party experience after a 7-year follow-up. Haematologica. 2009;94:205–212. doi: 10.3324/haematol.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kantarjian HM, Talpaz M, Giles F, et al. New insights into the pathophysiology of chronic myeloid leukemia and imatinib resistance. Ann Intern Med. 2006;145:913–923. doi: 10.7326/0003-4819-145-12-200612190-00008. [DOI] [PubMed] [Google Scholar]
- 21.Weisberg E, Manley PW, Cowan-Jacob SW, et al. Second generation inhibitors of BCR-ABL for the treatment of imatinib-resistant chronic myeloid leukaemia. Nat Rev Cancer. 2007;7:345–356. doi: 10.1038/nrc2126. [DOI] [PubMed] [Google Scholar]
- 22.Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–283. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- 23.Jabbour E, Cortes J, Giles F, et al. Preliminary activity of nilotinib (AMN107), a novel selective potent oral bcr-abl tyrosine kinase inhibitor, in newly diagnosed Philadelphia chromosome (Ph)-positive chronic phase chronic myelogenous leukemia (CML-CP) Blood (ASH Annual Meeting Abstracts) 2006;108:2172. [Google Scholar]
- 24.Hochhaus A, Erben P, Ernst T, Mueller MC. Resistance to targeted therapy in chronic myelogenous leukemia. Semin Hematol. 2007;44(Suppl 1):S15–S24. doi: 10.1053/j.seminhematol.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Soverini S, Martinelli G, Rosti G, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: A study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23:4100–4109. doi: 10.1200/JCO.2005.05.531. [DOI] [PubMed] [Google Scholar]
- 26.Nicolini FE, Corm S, Lê QH, et al. Mutation status and clinical outcome of 89 imatinib mesylate-resistant chronic myelogenous leukemia patients: A retrospective analysis from the French intergroup of CML (Fi(phi)-LMC GROUP) Leukemia. 2006;20:1061–1066. doi: 10.1038/sj.leu.2404236. [DOI] [PubMed] [Google Scholar]
- 27.La Rosée P, Corbin AS, Stoffregen EP, et al. Activity of the Bcr-Abl kinase inhibitor PD180970 against clinically relevant Bcr-Abl isoforms that cause resistance to imatinib mesylate (Gleevec, STI571) Cancer Res. 2002;62:7149–7153. [PubMed] [Google Scholar]
- 28.Shah NP, Tran C, Lee FY, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 29.O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 30.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl [published correction appears in Cancer Cell. 2005;7:399] Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Tokarski JS, Newitt JA, Chang CY, et al. The structure of Dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res. 2006;66:5790–5797. doi: 10.1158/0008-5472.CAN-05-4187. [DOI] [PubMed] [Google Scholar]
- 32.Mauro M. Imatinib resistance: Current concepts in detection and management. Hematol Oncol. In press. [Google Scholar]
- 33.Hochhaus A, Druker BJ, Larson RA, et al. IRIS 6-year follow-up: Sustained survival and declining annual rate of transformation in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib. Blood (ASH Annual Meeting Abstracts) 2007;110:25. [Google Scholar]
- 34.Atallah E, Durand JB, Kantarjian H, Cortes J. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood. 2007;110:1233–1237. doi: 10.1182/blood-2007-01-070144. [DOI] [PubMed] [Google Scholar]
- 35.Cortes JE, Kantarjian HM, Goldberg SL, et al. High-dose imatinib in newly diagnosed chronic-phase chronic myeloid leukemia: High rates of rapid cytogenetic and molecular responses. J Clin Oncol. 2009;27:4754–4759. doi: 10.1200/JCO.2008.20.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baccarani M, Rosti G, Castagnetti F, et al. Comparison of imatinib 400 mg and 800 mg daily in the front-line treatment of high-risk, Philadelphia-positive chronic myeloid leukemia: A European LeukemiaNet Study. Blood. 2009;113:4497–4504. doi: 10.1182/blood-2008-12-191254. [DOI] [PubMed] [Google Scholar]
- 37.Cortes JE, Baccarani M, Guilhot F, et al. Phase III, randomized, open-label study of daily imatinib mesylate 400 mg versus 800 mg in patients with newly diagnosed, previously untreated chronic myeloid leukemia in chronic phase using molecular end points: Tyrosine kinase inhibitor optimization and selectivity study. J Clin Oncol. 2010;28:424–430. doi: 10.1200/JCO.2009.25.3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hehlmann R, Jung-Munkwitz S, Lauseker M, et al. German CML Study Group Randomized comparison of imatinib 800 mg vs. imatinib 400 mg +/− IFN in newly diagnosed BCR/ABL positive chronic phase CML: Analysis of molecular remission at 12 months; The German CML-Study IV. Blood (ASH Annual Meeting Abstracts) 2009;114:339. [Google Scholar]
- 39.Jain N, Kantarjian HM, Fava C, et al. Imatinib dose can be safely reduced after complete cytogenetic response (CCyR) in patients (pts) with chronic myeloid leukemia (CML) in early chronic phase (CP) treated with high-dose imatinib. Blood (ASH Annual Meeting Abstracts) 2007;110:1043. [Google Scholar]
- 40.Corbin AS, La Rosée P, Stoffregen EP, et al. Several Bcr-Abl kinase domain mutants associated with imatinib mesylate resistance remain sensitive to imatinib. Blood. 2003;101:4611–4614. doi: 10.1182/blood-2002-12-3659. [DOI] [PubMed] [Google Scholar]
- 41.Redaelli S, Piazza R, Rostagno R, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009;27:469–471. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- 42.Roumiantsev S, Shah NP, Gorre ME, et al. Clinical resistance to the kinase inhibitor STI-571 in chronic myeloid leukemia by mutation of Tyr-253 in the Abl kinase domain P-loop. Proc Natl Acad Sci U S A. 2002;99:10700–10705. doi: 10.1073/pnas.162140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Azam M, Latek RR, Daley GQ. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003;112:831–843. doi: 10.1016/s0092-8674(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 44.Kantarjian HM, Talpaz M, O'Brien S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473–475. doi: 10.1182/blood-2002-05-1451. [DOI] [PubMed] [Google Scholar]
- 45.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: A randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 46.Marin D, Goldman JM, Olavarria E, Apperley JF. Transient benefit only from increasing the imatinib dose in CML patients who do not achieve complete cytogenetic remissions on conventional doses. Blood. 2003;102:2702–2703. doi: 10.1182/blood-2003-06-2042. Letter. [DOI] [PubMed] [Google Scholar]
- 47.Zonder JA, Pemberton P, Brandt H, et al. The effect of dose increase of imatinib mesylate in patients with chronic or accelerated phase chronic myelogenous leukemia with inadequate hematologic or cytogenetic response to initial treatment. Clin CancerRes. 2003;9:2092–2097. [PubMed] [Google Scholar]
- 48.Jabbour E, Kantarjian H, Atallah E, et al. Impact of imatinib mesylate dose escalation on resistance and sub-optimal responses to standard-dose therapy in patients (pts) with chronic myeloid leukemia (CML) Blood (ASH Annual Meeting Abstracts) 2007;110:1035. [Google Scholar]
- 49.Breccia M, Stagno F, Latagliata R, et al. Results of imatinib dose escalation after 36 months of follow-up in chronic myeloid leukemia patients with failure or sub-optimal response according to 2006 European LeukemiaNet (ELN) criteria. Blood (ASH Annual Meeting Abstracts) 2009;114:3302. [Google Scholar]
- 50.Cortes J, Kantarjian H. Response: Dose escalation of imatinib may improve responses in patients with CML who fail standard-dose imatinib. Blood. 2003;102:2703. [Google Scholar]
- 51.Jabbour E, Kantarjian HM, Jones D, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood. 2009;113:2154–2160. doi: 10.1182/blood-2008-04-154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kantarjian HM, Larson RA, Guilhot F, et al. International Randomized Study of Interferon and STI571 (IRIS) Investigators Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase. Cancer. 2009;115:551–560. doi: 10.1002/cncr.24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim DY, Kim HJ, Jeong JS, et al. Efficacy of imatinib mesylate dose-escalation in CML patients showing suboptimal response to standard dose. Blood. 2007;110:871a. Abstract 2967. [Google Scholar]
- 54.Rea D, Etienne G, Corm S, et al. Imatinib dose escalation for chronic phase-chronic myelogenous leukaemia patients in primary suboptimal response to imatinib 400 mg daily standard therapy. Leukemia. 2009;23:1193–1196. doi: 10.1038/leu.2009.32. [DOI] [PubMed] [Google Scholar]
- 55.Kantarjian H, Pasquini R, Lévy V, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: Two-year follow-up of a randomized phase 2 study (START-R) Cancer. 2009;115:4136–4147. doi: 10.1002/cncr.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 57.Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- 58.White DL, Saunders VA, Dang P, et al. OCT-1-mediated influx is a key determinant of the intracellular up-take of imatinib but not nilotinib (AMN107): Reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108:697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- 59.Giannoudis A, Davies A, Lucas CM, et al. Unlike imatinib, dasatinib up-take into chronic myeloid leukaemia cells is independent of hOCT1 expression. Blood (ASH Annual Meeting Abstracts) 2007;110:3458. [Google Scholar]
- 60.Hiwase DK, White DL, Saunders VA, et al. In contrast to imatinib, OCT-1 mediated influx has minimal impact on cellular uptake of dasatinib in CML patients at diagnosis. Blood (ASH Annual Meeting Abstracts) 2007;110:1937. [Google Scholar]
- 61.Bradeen HA, Eide CA, O'Hare T, et al. Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: High efficacy of drug combinations. Blood. 2006;108:2332–2338. doi: 10.1182/blood-2006-02-004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cortes J, Rousselot P, Kim DW, et al. Dasatinib induces complete hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in blast crisis. Blood. 2007;109:3207–3213. doi: 10.1182/blood-2006-09-046888. [DOI] [PubMed] [Google Scholar]
- 63.Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy [published correction appears in Blood. 2007;110:1438] Blood. 2007;109:2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 64.Baccarani M, Rosti G, Saglio G, et al. Dasatinib time to and durability of major and complete cytogenetic response (MCyR and CCyR) in patients with chronic myeloid leukemia in chronic phase (CML-CP) Blood (ASH Annual Meeting Abstracts) 2008;112:450. [Google Scholar]
- 65.Khoury HJ, Mauro MJ, Matloub Y, et al. Dasatinib is well-tolerated and efficacious in imatinib-intolerant patients with chronic-phase chronic myeloid leukemia (CP-CML) Blood (ASH Annual Meeting Abstracts) 2009;114:1128. [Google Scholar]
- 66.Apperley JF, Cortes JE, Kim DW, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: The START a trial. J Clin Oncol. 2009;27:3472–3479. doi: 10.1200/JCO.2007.14.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cortes J, Kim DW, Raffoux E, et al. Efficacy and safety of dasatinib in imatinib-resistant or -intolerant patients with chronic myeloid leukemia in blast phase. Leukemia. 2008;22:2176–2183. doi: 10.1038/leu.2008.221. [DOI] [PubMed] [Google Scholar]
- 68.Guilhot F, Apperley J, Kim DW, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–4150. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- 69.Rousselot P, Bergeron A, Réa D, et al. Pleural and pulmonary events in patients treated with dasatinib for chronic myeloid leukemia in chronic phase. Haematologica. 2007;92(Suppl 2):203. Abstract 0546. [Google Scholar]
- 70.Stone RM, Kantarjian HM, Baccarani M, et al. Efficacy of dasatinib in patients with chronic-phase chronic myelogenous leukemia with resistance or intolerance to imatinib: 2-Year follow-up data from START-C (CA180-013) Blood (ASH Annual Meeting Abstracts) 2007;110:734. [Google Scholar]
- 71.Guilhot F, Apperley JF, Kim DW, et al. Efficacy of dasatinib in patients with accelerated-phase chronic myelogenous leukemia with resistance or intolerance to imatinib: 2-Year follow-up data from START-A (CA180-005) Blood (ASH Annual Meeting Abstracts) 2007;110:470. [Google Scholar]
- 72.Müller MC, Cortes JE, Kim D, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: Analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009;114:4944–4953. doi: 10.1182/blood-2009-04-214221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mueller MC, Erben P, Ernst T, et al. Molecular response according to type of preexisting BCR-ABL mutations after second line dasatinib therapy in chronic phase CML patients. Blood (ASH Annual Meeting Abstracts) 2007;110:319. [Google Scholar]
- 74.Müller MC, Erben P, Schenk T, et al. Response to dasatinib after imatinib failure according to type of preexisting BCR-ABL mutations. Blood (ASH Annual Meeting Abstracts) 2006;108:748. [Google Scholar]
- 75.Shah NP, Kim DW, Kantarjian HM, et al. Dasatinib dose-optimization in chronic phase chronic myeloid leukemia (CML-CP): Two-year data from CA180-034 show equivalent long-term efficacy and improved safety with 100 mg once daily dose. Blood (ASH Annual Meeting Abstracts) 2008;112:3225. [Google Scholar]
- 76.Kantarjian HM, Kim DW, Dorlhiac-Llacer P, et al. Dasatinib 140 mg once daily (QD) demonstrates equivalent efficacy and improved safety compared with 70 mg twice daily (BID) in patients with accelerated phase chronic myeloid leukemia (CML-AP): 2-Year follow-up data from CA180-035. Blood (ASH Annual Meeting Abstracts) 2008;112:3224. [Google Scholar]
- 77.Larson RA, Ottmann OG, Shah NP, et al. Dasatinib 140 mg once daily (QD) has equivalent efficacy and improved safety compared with 70 mg twice daily (BID) in patients with imatinib-resistant or -intolerant Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL): 2-Year data from CA180-035. Blood (ASH Annual Meeting Abstracts) 2008;112:2926. [Google Scholar]
- 78.Saglio G, Kantarjian HM, Hochhaus A, et al. Dasatinib 140 mg once daily (QD) demonstrates equivalent effi cacy and improved safety compared with 70 mg twice daily (BID) in patients with chronic myeloid leukemia in blast phase (CML-BP): 2-Year data from CA180-035. Blood (ASH Annual Meeting Abstracts) 2008;112:3226. [Google Scholar]
- 79.Kantarjian H, Cortes J, Kim DW, et al. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-Month median follow-up. Blood. 2009;113:6322–6329. doi: 10.1182/blood-2008-11-186817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cortes J, Borthakur G, O'Brien S, et al. Efficacy of dasatinib in patients (pts) with previously untreated chronic myelogenous leukemia (CML) in early chronic phase (CMLCP) Blood (ASH Annual Meeting Abstracts) 2009;114:338. [Google Scholar]
- 81. [Accessed April 5, 2010];A phase III study of dasatinib vs. imatinib in patients with newly diagnosed chronic phase CML (DASISION) ClinicalTrials.gov Identifier NCT00481247. ClinicalTrials.gov http://www.clinical trials.gov/ct2/show/NCT00481247?term=NCT00481247&rank=1.
- 82.Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN 107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 83.Kantarjian HM, Giles F, Bhalla KN, et al. Nilotinib in chronic myeloid leukemia patients in chronic phase (CMLCP) with imatinib resistance or intolerance: 2-Year follow-up results of a phase 2 study. Blood (ASH Annual Meeting Abstracts) 2008;112:3238. [Google Scholar]
- 84.Kantarjian HM, Giles FJ, Bhalla KN, et al. Update on imatinib-resistant chronic myeloid leukemia patients in chronic phase (CML-CP) on nilotinib therapy at 24 months: Clinical response, safety, and long-term outcomes. Blood (ASH Annual Meeting Abstracts) 2009;114:1129. [Google Scholar]
- 85.Powell BL, Khoury HJ, Lipton JH, et al. Nilotinib responses and tolerability confirmed in North American patients with chronic myeloid leukemia (CML) from ENACT (expanding nilotinib access in clinical trials) Blood (ASH Annual Meeting Abstracts) 2009;114:3295. [Google Scholar]
- 86.le Coutre PD, Giles F, Hochhaus A, et al. Nilotinib in chronic myeloid leukemia patients in accelerated phase (CML-AP) with imatinib resistance or intolerance: 2-Year follow-up results of a phase 2 study. Blood (ASH Annual Meeting Abstracts) 2008;112:3229. [Google Scholar]
- 87.Giles FJ, le Coutre P, Bhalla KN, et al. Nilotinib therapy after dasatinib failure in patients with imatinib-resistant or -intolerant chronic myeloid leukemia (CML) in chronic phase (CP), accelerated phase (AP) or blast crisis (BC) Blood (ASH Annual Meeting Abstracts) 2007;110:1029. [Google Scholar]
- 88.Jabbour E, Kantarjian HM, Baccarani M, et al. Minimal cross-intolerance between nilotinib and imatinib in patients with imatinib-intolerant chronic myeloid leukemia in chronic phase (CML-CP) or accelerated phase (CML-AP) Blood (ASH Annual Meeting Abstracts) 2008;112:3215. [Google Scholar]
- 89.Branford S, Shou Y, Lawrence R, et al. The P-Loop mutations Y253H and E255K/V may develop more frequently than T315I during nilotinib therapy after imatinib failure and are associated with progression in patients with Ph-positive leukemia. Haematologica. 2007;92(Suppl 2):337. Abstract 0904. [Google Scholar]
- 90.Hughes T, Saglio G, Branford S, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009;27:4204–4210. doi: 10.1200/JCO.2009.21.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Saglio G, Kim DW, Hochhaus A, et al. Correlation of clinical response to nilotinib with BCR-ABL mutation status in advanced phase chronic myelogenous leukemia (CML-AP) patients with imatinib-resistance or intolerance. Blood (ASH Annual Meeting Abstracts) 2007;110:1940. [Google Scholar]
- 92.Hochhaus A, Kim D, Martinelli G, et al. Nilotinib efficacy according to baseline BCR-ABL mutations in patients with imatinib-resistant chronic myeloid leukemia in chronic phase (CML-CP) Blood (ASH Annual Meeting Abstracts) 2008;112:3216. [Google Scholar]
- 93.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib demonstrates superior efficacy compared with imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase: Results from the International Randomized Phase III ENESTnd trial. Blood (ASH Annual Meeting Abstracts) 2009;114:1. [Google Scholar]
- 94.Cortes J, O'Brien S, Jones D, et al. Efficacy of nilotinib in patients (pts) with newly diagnosed, previously untreated Philadelphia chromosome (Ph)-positive chronic myelogenous leukemia in early chronic phase (CML-CP) Blood (ASH Annual Meeting Abstracts) 2009;114:341. [Google Scholar]
- 95.Cortes J, O'Brien S, Jones D, et al. Efficacy of dasatinib in patients (pts) with previously untreated chronic myelogenous leukemia (CML) in early chronic phase (CML-CP) Blood (ASH Annual Meeting Abstracts) 2007;110:30. [Google Scholar]
- 96.Rosti G, Palandri F, Castagnetti F, et al. GIMEMA CML Working Party Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114:4933–4938. doi: 10.1182/blood-2009-07-232595. [DOI] [PubMed] [Google Scholar]
- 97.Giles F, le Coutre PD, Bhalla KN, et al. Efficacy and tolerability of nilotinib in chronic myeloid leukemia patients in chronic phase (CML-CP) who failed prior imatinib and dasatinib therapy: Updated results of a phase 2 study. Blood (ASH Annual Meeting Abstracts) 2008;112:3234. [Google Scholar]
- 98.Garg RJ, Kantarjian H, O'Brien S, et al. The use of nilotinib or dasatinib after failure to 2 prior tyrosine kinase inhibitors: Long-term follow-up. Blood. 2009;114:4361–4368. doi: 10.1182/blood-2009-05-221531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kantarjian HM, Druker BJ, Guilhot F, et al. Imatinib dose escalation is effective in patients with chronic myeloid leukemia in chronic phase (CML-CP) Blood (ASH Annual Meeting Abstracts) 2007;110:1047. [Google Scholar]
- 100.Hughes T, Saglio G, Martinelli G, et al. Responses and disease progression in CML-CP patients treated with nilotinib after imatinib failure appear to be affected by the BCR-ABL mutation status and types. Blood (ASH Annual Meeting Abstracts) 2007;110:320. [Google Scholar]
- 101.Jabbour E, Kantarjian H, O'Brien S, et al. Predictive factors for response and outcome in patients (pts) treated with second generation tyrosine kinase inhibitors (2-TKI) for chronic myeloid leukemia in chronic phase (CML-CP) post imatinib failure. Blood (ASH Annual Meeting Abstracts) 2009;114:509. [Google Scholar]
- 102. [Accessed October 6, 2009];Vertex Pharmaceuticals. Vertex's collaborator Merck suspends patient enrollment in clinical trials of MK-0457 (VX-680) pending full analysis of clinical data. http://files.shareholder.com/downloads/VRTX/0x0x145756/5991297b-3934-487f-8a4a-c1aef639f13f/VRTX_News_2007_11_20_General.pdf.