Abstract
About 25% of high-grade cervical intraepithelial neoplasias (CIN2/3) caused by human papillomavirus serotype 16 (HPV16) undergo complete spontaneous regression. However, to date, therapeutic vaccination strategies for HPV disease have yielded limited success when measured by their ability to induce robust peripheral blood T cell responses to vaccine antigen. We report marked immunologic changes in the target lesion microenvironment after intramuscular therapeutic vaccination targeting HPV16 E6/E7 antigens, in subjects with CIN2/3 who had modest detectable responses in circulating T lymphocytes. Histologic and molecular changes, including markedly (average threefold) increased intensity of CD8+ T cell infiltrates in both the stromal and epithelial compartments, suggest an effector response to vaccination. Postvaccination cervical tissue immune infiltrates included organized tertiary lymphoid-like structures in the stroma subjacent to residual intraepithelial lesions and, unlike infiltrates in unvaccinated lesions, showed evidence of proliferation induced by recognition of cognate antigen. At a molecular level, these histologic changes in the stroma were characterized by increased expression of genes associated with immune activation (CXCR3) and effector function (Tbet and IFNβ), and were also associated with an immunologic signature in the overlying dysplastic epithelium. High-throughput T cell receptor sequencing of unmanipulated specimens identified clonal expansions in the tissue that were not readily detectable in peripheral blood. Together, these findings indicate that peripheral therapeutic vaccination to HPV antigens can induce a robust tissue-localized effector immune response, and that analyses of immune responses at sites of antigen are likely to be much more informative than analyses of cells that remain in the circulation.
INTRODUCTION
On a global scale, human papillomavirus (HPV), most commonly serotype 16 (HPV16), causes about 30% of cancers attributable to infectious pathogens (1). Persistent infection with an oncogenic HPV genotype is associated with subsequent cancers of the cervix, vagina, vulva, anus, and oropharynx [reviewed in (2)]. For many reasons, HPV infections are essentially endemic. Asymptomatic transmission occurs shortly after initiation of sexual intercourse (3). Infections are anatomically restricted to mucosal epithelium, do not cause grossly visible lesions, and do not elicit systemic symptoms. The clinically indolent nature of these infections facilitates maintenance of a large herd burden of transmissible HPV.
Despite the availability of screening methods and the introduction of preventative HPV vaccines, high-grade squamous intraepithelial neoplasia, the precursor to invasive squamous cancers, remains common. Rates of preventive vaccination in eligible U.S. cohorts, young people aged 9 to 26 years, have been suboptimal; in 2010, less than half of adolescent girls aged 13 to 17 years had initiated vaccination, and the rates of vaccination are decreasing (4). Moreover, the incidence of HPV-associated cancers in anatomic sites for which screening algorithms have not yet been identified, particularly in the oropharynx, is increasing steadily and will likely bypass that of cervical cancer in the near future (2). In the end, although much has been learned about the immunobiology of HPV-associated disease, the problem of translation of this knowledge into strategies to prevent and treat disease remains unresolved.
High-grade cervical intraepithelial neoplasia (CIN2/3) is a lesion that should be susceptible to an HPV-specific immune response. The development of cervical cancer and its precursor CIN lesions is associated with integration of the HPV genome into the host genome and subsequent expression of two HPV early gene products, E6 and E7, which inactivate p53 and pRb, respectively (5, 6). Expression of both of these viral, “non-self” proteins is functionally required to initiate and maintain the transformed phenotype, thereby providing true tumor-specific antigenic targets (5, 6).
Preinvasive, intraepithelial dysplastic lesions caused by HPV are clinically indolent. Whereas all cervical squamous carcinomas arise from untreated CIN2/3, not all CIN2/3 lesions progress to invasive cancer. We and others have reported that in a time frame of 4 to 6 months, ~35% of CIN2/3 lesions undergo spontaneous regression (7, 8). Lesions resulting from mono-infection with HPV16 were less likely to undergo regression than those caused by other HPV genotypes: in this time frame, ~20 to 25% of HPV16-associated CIN2/3 lesions regressed. Regardless of whether or not lesions regressed, T cell responses to HPV16 E6 and E7 were marginal, requiring expansion by ex vivo sensitization for detection (9, 10). The fact that neither the magnitude nor the breadth of naturally occurring T cell responses detected in the blood was a robust predictor of regression of preinvasive HPV disease of the cervix raised the question of whether immune cells in the lesional mucosa would be more informative.
Indeed, both the magnitude and distribution of CD8+ T cell infiltrates in dysplastic mucosa provided prognostic information. In persistent disease, CD8+ T cells were restricted to lesional stroma and failed to access the lesional epithelium. In contrast, dysplastic lesions that “permitted” CD8+ T cell access to the epithelial compartment were significantly more likely to undergo subsequent regression (11). These observations suggested that, although CD8 T cells are likely to play a role in elimination of preinvasive, incipient HPV-associated neoplasia, access to lesional epithelium presents a critical obstacle.
Previous attempts to translate therapeutic vaccination strategies for HPV disease have yielded limited success by two standard measures of vaccine efficacy: (i) induction of robust peripheral blood T cell responses to vaccine antigen and (ii) correlation of peripheral blood immune responses with histologic regression of disease. Here, we report evidence of postvaccination immunologic changes in target lesions that suggest the induction of clinically relevant tissue-localized immune responses, despite modest detectable responses in circulating T lymphocytes. The identification of postvaccination changes in target lesions has practical implications for the design and interpretation of immunotherapeutic trials for preinvasive HPV disease.
RESULTS
Heterologous prime-boost vaccination targeting HPV16 E6/E7 is safe and tolerable in subjects with HPV16+ CIN2/3 lesions
Healthy subjects with HPV16-associated CIN2/3 underwent peripheral vaccination with a heterologous DNA prime-recombinant vaccinia vector-based boost vaccination regimen administered intramuscularly in the deltoid muscle before a standard therapeutic resection. The regimen included two priming vaccinations with a DNA vaccine expressing HPV16 E7 (DNAE7) at study weeks 0 and 4, followed by a recombinant vaccinia boost expressing HPV16 and HPV18 E6 and E7 (rVacE6E7; TA-HPV) at study week 8. At week 15, 7 weeks after the boost vaccination, subjects underwent a standard therapeutic resection of the cervical squamocolumnar junction.
A total of 12 patients in three treatment arms were evaluated (Table 1). Demographics are presented in Table 2. All subjects received 3 mg of DNAE7 at entry and at week 4. At week 8, patients received one of three rVacE6E7 doses: 1.6 × 105 plaque-forming units (PFU) (cohort DDV1; n = 3), 1.6 × 106 PFU (cohort DDV2; n = 3), or 1.6 × 107 PFU (cohort DDV3; n = 6). Peripheral blood mononuclear cells (PBMCs) were obtained at baseline and at weeks 8, 15, and 19. Rates and severity of injection site reactions during the 30 days after each vaccination and frequency of adverse events (AEs) are summarized in tables S1 and S2. All reported AEs were mild, and injection site reactions resolved without sequelae or intervention. One of three patients in each of the DDV1 and DDV2 cohorts had complete histologic regression at time of resection. In the highest dose cohort (DDV3), three of six patients had a complete histologic response.
Table 1.
Treatment cohorts. CR, complete regression.
| Treatment group | DNAE7 (mg) | VacE6E7 (PFU) | n | CR |
|---|---|---|---|---|
| DDV1 | 3 | 1.6 × 105 | 3 | 31/3 (33%) |
| DDV2 | 3 | 1.6 × 106 | 3 | 1/3 (33%) |
| DDV3 | 3 | 1.6 × 107 | 6 | 3/6 (50%) |
Table 2.
Demographics of study participants. B, black; W, white; CR, complete regression; NR, not complete regression.
| Patient ID |
HPV | Age (years) |
Race | A locus | B locus | C locus | DRB1 locus |
DRB3 | DRB4 | DRB5 | DQB1 locus |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 16 | 26 | W | CR | A* 02:01 |
A* 24:02 |
B* 13:02 |
B* 44:02 |
C* 05:01 |
C* 06:02 |
DRB1* 07:01 |
DRB4* 01:xx |
DQB1* 02:02 |
||||
| 1.2 | 16 | 24 | W | NR | A* 02:01 |
A* 29:02 |
B* 38:01 |
B* 44:03 |
C* 12:03 |
C* 16:01 |
DRB1* 04:04 |
DRB1* 07:01 |
DRB4* 01:xx |
DQB1* 02:02 |
DQB1* 03:02 |
||
| 1.3 | 16, 6, 52, 55 |
22 | W | NR | A* 03:01 |
A* 31:01 |
B* 07:02 |
B* 40:01 |
C* 03:04 |
C* 07:02 |
DRB1* 04:04 |
DRB1* 15:01 |
DRB4* 01:xx |
DRB5* 01:xx |
DQB1* 03:02 |
DQB1* 06:02 |
|
| 2.1 | 16 | 25 | B | CR | A* 29:02 |
A* 30:01 |
B* 15:03 |
B* 42:01 |
C* 02:10 |
C* 17:01 |
DRB1* 04:05 |
DRB1* 10:01 |
DRB4* 01:xx |
DQB1* 03:02 |
DQB1* 05:01 |
||
| 2.2 | 16, 62, 84 |
26 | W | NR | A* 25:01 |
B* 35:01 |
B* 44:02 |
C* 04:01 |
C* 05:01 |
DRB1* 01:01 |
DRB1* 10:01 |
DQB1* 05:01 |
|||||
| 2.3 | 16 | 26 | W | NR | A* 11:01 |
B* 07:02 |
B* 51:01 |
C* 07:02 |
C* 15:02 |
DRB1* 04:01 |
DRB1* 11:01 |
3* 02:xx |
4* 01:xx |
DQB1* 03:01 |
|||
| 3.1 | 16 | 44 | W | NR | A* 03:01 |
A* 29:02 |
B* 07:02 |
B* 27:02 |
C* 05:01 |
C* 07:02 |
DRB1* 15:01 |
DRB5* 01:xx |
DQB1* 05:03 |
DQB1* 06:02 |
|||
| 3.2 | 16, 59, 66 |
24 | W | NR | A* 02:01 |
A* 03:01 |
B* 40:01 |
B* 44:03 |
C* 03:04 |
C* 16:01 |
DRB1* 04:04 |
DRB1* 07:01 |
DRB4* 1:xx |
DQB1* 02:02 |
DQB1* 03:02 |
||
| 3.3 | 16 | 27 | W | CR | A* 02:01 |
A* 03:01 |
B* 07:02 |
B* 40:02 |
C* 02:02 |
C* 07:02 |
DRB1* 11:01 |
DRB1* 15:01 |
3* 02:xx |
5* 01:xx |
DQB1* 03:01 |
DQB1* 06:02 |
|
| 3.4 | 16, 18 | 30 | W | CR | A* 24:02 |
A* 32:01 |
B* 15:01 |
B* 40:02 |
C* 03:03 |
C* 15:02 |
DRB1* 04:01 |
DRB1* 04:04 |
4* 01:xx |
DQB1* 03:01 |
DQB1* 03:02 |
||
| 3.5 | 16 | 27 | W | NR | A* 02:66 |
A* 24:02 |
B* 07:02 |
B* 51:01 |
C* 07:02 |
C* 15:02 |
DRB1* 14:01 |
DRB1* 15:01 |
3* 02:xx |
5* 01:xx |
DQB1* 05:03 |
DQB1* 06:02 |
|
| 3.6 | 16, 62, 84 |
49 | W | CR | A* 01:01 |
B* 08:01 |
C* 07:01 |
DRB1* 03:01 |
3* 01:xx |
DQB1* 02:01 |
|||||||
Therapeutic vaccination elicits HPV-specific cellular immune responses in the blood
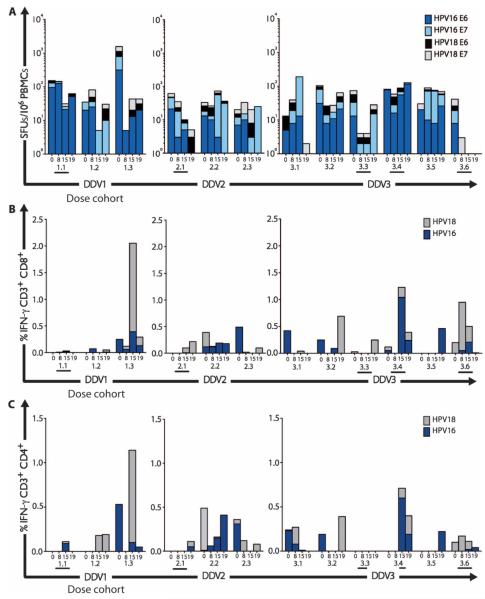
Standard interferon-γ (IFN-γ) enzyme-linked immunospot (ELISpot) assays (12, 13) were performed to determine the number of antigen-specific IFN-γ–secreting cells in response to stimulation with HPV16 or HPV18 E6 and E7 peptide pools. As shown in Fig. 1, after three vaccinations, one of three subjects who received the low-dose boost vaccination, all three subjects who received the intermediate-dose boost vaccination, and three of six subjects who received the full-dose boost vaccination mounted vaccine-induced HPV16-specific cellular immune responses. Overall, seven subjects (58%) developed cellular immune responses detectable by ELISpot. Peak responses were modest, in the range of 50 to 150 spot-forming units (SFUs) per 106 PBMCs. However, using this assay, increased responses were restricted to HPV16 E7, suggesting that the DNA vaccine, which targeted only the HPV16 E7 antigen, might indeed have a priming effect.
Fig. 1. Intramuscular immunization with DNAE7 prime, followed by recombinant vaccinia (rVacE6E7) boost, induces HPV16 E6 and E7–specific TH1 immune responses in the peripheral blood.
(A) Cellular immune responses in unfractionated PBMCs, quantified by IFN-γ ELISpot assays. All subjects received two intramuscular DNA vaccinations at weeks 0 and 4. Dose escalation of recombinant vaccinia (rVacE6E7) boost vaccination at week 8; subjects received either 1.6 × 105 PFU (DDV1), 1.6 × 106 PFU (DDV2), or 1.6 × 107 PFU (DDV3). Results are expressed as SFUs per 106 PBMCs. (B and C) Multiparametric flow cytometry was used to detect HPV16- and HPV18-specific production of IFN-γ. Bar graphs summarizing ICS and flow cytometry analyses of PBMC response to HPV16 and HPV18 peptides: (B) CD8+IFN-γ+ and (C) CD4+IFN-γ+. Subjects with complete histologic regression at week 15 are designated with a bar below the subject ID.
Intracellular cytokine staining (ICS) assays identified HPV16- and/or HPV18-specific IFN-γ–producing CD8 and CD4 T cells in one of three subjects in the low-dose boost cohort, in two of three subjects in the intermediate-dose boost cohort, and in four of six subjects in the full-dose boost cohort (Fig. 1). Sufficient cells were available to perform additional phenotyping studies on T cells cultured briefly with either HPV16 or HPV18 peptides, which demonstrated induction of T helper 1 (TH1)–biased responses in CD3+CD4+ and CD3+CD8+ cells (Fig. 1, B and C, and fig. S1). One subject (3009) in the low-dose cohort had a preexisting endogenous immune response to HPV18. The kinetics of response in this patient and in subjects in the full-dose cohort suggest that the timing of the peripheral blood draws in relationship to vaccination is an important variable in detecting responses. Most of the vaccine responses were identified in the week 15 specimens (that is, after the boost vaccination), as opposed to the earlier time point (weeks 8 to 10), which would have reflected responses after two DNA vaccinations. However, this assay detected responses to the boost vaccine antigen, HPV18, as well as to HPV16, suggesting that it may be a more sensitive indicator of immunogenicity in the blood.
Tissue CD8+ T cell infiltrates in the target lesion increase after vaccination
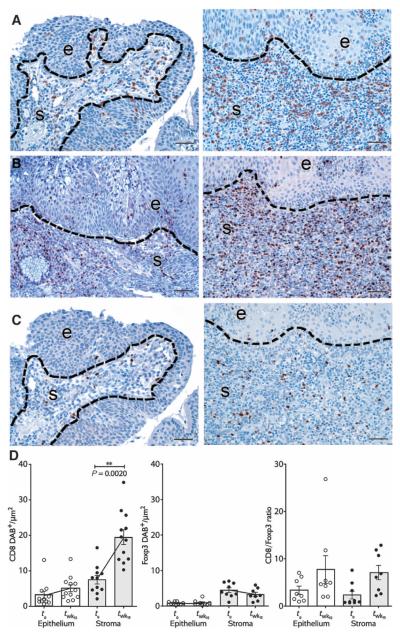
In unvaccinated persistent CIN2/3, we and others have found that tissue T cells are relatively restricted to the stromal compartment immediately subjacent to dysplastic epithelium (11, 14). This pattern of T cell distribution in unvaccinated lesions is consistent with non–antigen-specific recruitment to virally infected mucosa via a chemokine gradient. In contrast, in the postvaccination tissue specimens, the intensity of CD8+ T cell infiltration into lesions increased markedly both in the dysplastic epithelium and in the underlying stromal compartment (P = 0.0020) (Fig. 2). These infiltrates were localized in foci of residual dysplasia, not in immediately adjacent normal mucosa. Within-subject increases in tissue CD8+ T cells were significantly greater than the increases we have reported previously in unvaccinated subjects followed over the same time frame (P = 0.0300, fig. S2) (11). These infiltrates included increased absolute numbers of Tbet+ cells, suggestive of an effector T cell response. Intraepithelial CD8+ infiltrates were associated with histologic features of apoptosis in lesional epithelial cells. In contrast, the intensity of Foxp3+ infiltrates did not change significantly, resulting in an increased ratio of effector to Foxp3+ cells (P = 0.0488).
Fig. 2. Tissue CD8+ T cell infiltrates in the target lesion increase after vaccination.
(A) Representative immunohistochemical (IHC) staining for CD8 in lesional tissue before (left column) and after (right column) vaccination (patient 3009). (B) These infiltrates are Tbet+. (C) In contrast, the intensity of Foxp3+ infiltrates does not change substantially. (D) Bar graphs depicting quantitated CD8+ and Foxp3+ infiltrates, and the ratio of CD8/Foxp3+ cells in epithelium (e) and stroma (s) of CIN3, before and after vaccination, in all study subjects. Data from bar graphs are means of 3 to 10 regions of interest (ROIs) quantitated per tissue compartment per subject. Error bars show SEM. P < 0.05, **P < 0.01, Wilcoxon signed rank test. Scale bars, 50 μm.
To explore the association between the intensity of tissue T cell infiltrates and immune responses in the blood, we calculated the Pearson correlations between lesional epithelial and stromal CD8 infiltrates before and after vaccination, and peripheral blood immune responses to HPV16 E6 and E7 at baseline (before vaccination), at 8 weeks (T8), at the time of resection at week 15 (T15), and postoperatively at week 19 (T19). We found a strong association between intraepithelial CD8 infiltrates at baseline (T0) and the magnitude of T cell response to E6 in the blood after vaccination, at week 15 (r = 0.742, P = 0.0057) and at week 19 (r = 0.751, P = 0.0049). These comparisons also identified a strong correlation between the intensity of lesional stromal CD8 infiltrates at baseline and peripheral blood T cell response to E7 at week 19 (r = 0.755, P = 0.0045). Finally, in subjects who had foci of residual disease at week 15, we found that peripheral blood responses to E6 at weeks 15 and 19 correlated with increased intraepithelial CD8 infiltrates compared to baseline (week 15: r = 0.788, P = 0.0023; week 19: r = 0.76, P = 0.004). These findings suggest that detectable peripheral blood responses to vaccination in the setting of established preinvasive disease may reflect potentially effective, endogenous priming at the site of the lesion.
In vaccinated patients, the cervix is infiltrated by activated effector memory T cells with potent effector functions
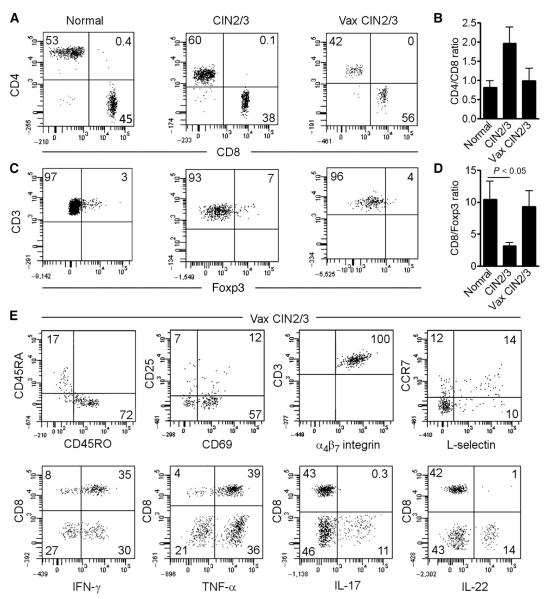
We used flow cytometry phenotyping to compare the frequencies of T cell subsets isolated from fresh tissue explants as described previously (15), in explants from normal cervical mucosa, from unvaccinated HPV16+ persistent CIN2/3, and from vaccinated HPV16+ CIN2/3. The great majority of tissue T cells in all three clinical settings had an antigen-experienced phenotype(CD3+CD45RO+) (Fig. 3 and fig. S3). Using this method, we found differences in the ratio of effector (CD8) to regulatory T (Treg) cells in these specimens that were congruent with the image analysis data. The ratio of CD8/Treg cells was lower in vaccine-naïve persistent dysplasia, compared to normal tissue, and this ratio was reversed in postvaccination resections. Cervical tissue T cells isolated from vaccinated subjects had an activated (CD69+), effector memory (CCR7−/L-selectin−) phenotype, and produced TH1 cytokines IFN-γ and tumor necrosis factor–α (TNF-α). All expressed the α4β7 homing integrin.
Fig. 3. In vaccinated patients, the cervix is infiltrated by activated effector memory T cells with potent effector functions.
(A to D) T cells were isolated from the cervix of healthy controls (normal, n = 5), patients with untreated CIN2/3 (n = 7), and patients with CIN2/3 after vaccination (n = 4). The percent of CD4 versus CD8 T cells (A), the CD4/CD8 ratio (B), the percent Foxp3+ Treg (C), and the CD8/Foxp3+ ratio (D) are shown. The CD4/CD8 ratio tended to increase in untreated CIN and normalize in vaccinated patients. The CD8/Foxp3+ ratio was lowest in untreated CIN and tended to increase after vaccination, although only the normal versus CIN2/3 group reached statistical significance. Data from bar graphs are means of cases analyzed. Error bars show SEM. P < 0.05, Wilcoxon rank sum test. (E) Representative histograms of surface phenotype and cytokine production of T cells from vaccinated patients (n = 4). Surface phenotype stains were performed on unstimulated cells, and cytokine analysis stains were performed after stimulation with phorbol 12-myristate 13-acetate and ionomycin. Most of the T cells from patients after vaccination were memory CD45RO+ T cells, many expressed the activation marker CD69, most lacked the central memory markers L-selectin/CCR7, and all expressed the gut-tropic homing receptor α4β7 integrin. Most of both CD4 and CD8 T cells produced IFN-γ, and a significant subset of CD4 T cells expressed interleukin-17 (IL-17) and/or IL-22. All histograms are gated to show CD3+ T cells; the gating strategy and representative isotype controls are included in fig. S3.
Tissue T cells in vaccinated subjects form tertiary lymphoid structures and show characteristics associated with in vivo activation via T cell receptor engagement with cognate antigen
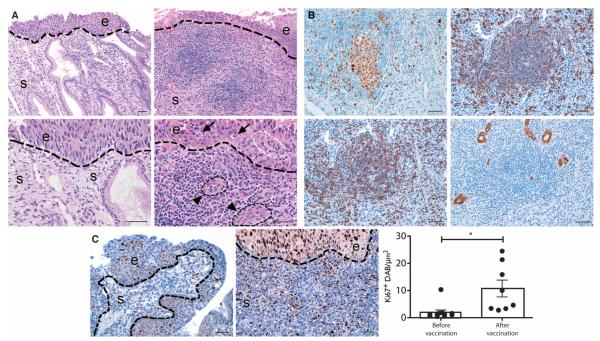
In many human solid tumors, the presence of ectopic tertiary lymphoid structures (TLSs) is associated with favorable prognosis (16–18). We found that postvaccination changes in target lesions not only included increased intensity of CD8+ T cell infiltrates in foci of residual dysplastic epithelium but also were organized in distinct lymphoid aggregates, which included, in many cases, germinal centers (Fig. 4). In unvaccinated, persistent CIN2/3, the tissue lymphoid infiltrates localized in the stroma subjacent to dysplastic epithelium are diffuse and are not commonly organized into TLSs. In the postvaccination tissue sections, the TLSs, similar to lymph nodes, were associated with high endothelial venules, structures that mediate lymphocyte recruitment, and are also associated with favorable prognosis (19).
Fig. 4. Postvaccination lymphoid neogenesis in target lesions.
(A) Hematoxylin and eosin–stained sections demonstrate organized lymphoid structures that are localized to the stroma immediately subjacent to residual dysplastic epithelium (top row, ×64 magnification; second row, ×160 magnification). Inflammatory infiltrates are accompanied by high endothelial venule–like vessels (triangle points, black dotted outline), access to dysplastic epithelium (white dotted outline), and lesional epithelial apoptosis (fat arrows). (B) TLSs in postvaccination stroma express Ki67, central CD20 (a pan-B cell marker), CD3 (a pan-T cell marker), and peripheral lymph node addressin (PNAd), which identifies vascular endothelium in high endothelial venules. (C) Representative IHC of Ki67 before (left) and after (right) vaccination, and bar graph summarizing quantitative image analysis of Ki67+ in lesional stroma before and after vaccination. Data from bar graphs are means of 3 to 10 ROIs quantitated per tissue compartment for each subject. Error bars show SEM. **P < 0.01, Wilcoxon signed rank test. Scale bars, 50 μm.
The intensity and pattern of tissue CD8+ infiltrates also suggested activation via recognition of cognate antigen. Quantitative image analyses comparing pre- and postvaccination lesional tissue in individual subjects showed that, after vaccination, tissue T cells had strongly increased expression of Ki67 (P = 0.0062), a phenotype of recent proliferation as a consequence of activation by T cell receptor (TCR) engagement (20) (Fig. 4), providing indirect evidence of activation via cognate antigen.
Histologic changes in lesional stroma in vaccinated subjects are associated with a molecular signature of immune activation in both the stromal and epithelial compartments
To assess the functional polarization of tissue T cells in postvaccination tissue lymphoid aggregates, we laser capture microdissected epithelium and subjacent stroma from tissue sections from normal cervical mucosa, from unvaccinated dysplastic mucosa, and from vaccinated subjects who had residual dysplasia. Targeted analyses of genes associated with immune cell phenotype (CD8β, CD4, Foxp3, and CCR7), activation (CD25, CD69, CD137, and CXCR3), polarization (Tbet, GATA3, Foxp3, RORγt, and BCL6), and function (PRF1, GZMB, IFN-γ, and IFN-β) were carried out on laser capture microdissected epithelium and the immediately subjacent stroma from normal cervix, unvaccinated HPV16+ CIN3, and from postvaccination HPV16+CIN2/3 sections containing TLSs (Fig. 5 and table S3). In vaccinated subjects, although this strategy resulted in stroma that was enriched for TLSs, the micro-dissected material also contained admixed stromal elements, including fibroblasts, macrophages, and vascular structures.
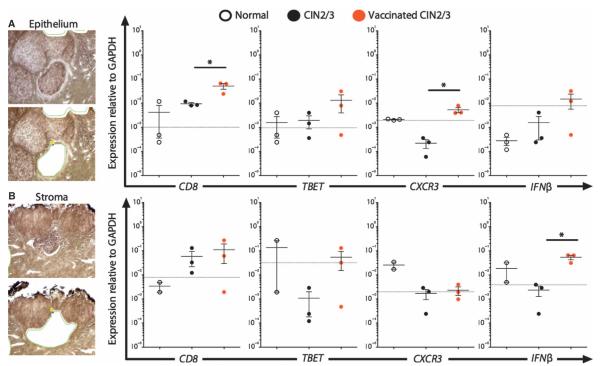
Fig. 5. Postvaccination stromal TLSs are associated with a functional signature.
Laser capture microdissection of cervical mucosal epithelium and subjacent stroma from normal (three), CIN2/3 (three), and vaccinated CIN2/3 (three) cases. (A) In lesional epithelium overlying TLSs, expression of CD8 and CXCR3 is increased in postvaccination tissue. (B) Transcripts for IFN-β are increased in the stromal compartment of vaccinated CIN2/3. Data from scatter plots are means of duplicate per case; error bars show SEM. *P < 0.05, P < 0.01, Wilcoxon rank sum test. Dotted lines indicate the threshold of sensitivity of detection. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
The relative expression of transcripts for CD8 and CD4 was internally congruent with quantitative IHC data. CD8 mRNA was increased in unvaccinated persistent CIN2/3 compared to normal mucosa and was even greater in vaccinated subjects. CD4 mRNA transcripts increased slightly in unvaccinated lesions compared to normal tissue and did not change significantly in vaccinated subjects. The consistency between groups for the intensity of infiltrates and the expression of transcripts suggested that the microdissections were accurate and that the quality of material was sufficient to draw conclusions from measures of other genes. In the stroma subjacent to persistent CIN2/3 from subjects in the unvaccinated cohort, we found down-regulated expression of genes associated with immune cell activation (CXCR3), TH1 polarization such as Tbet (TBX21), and antiviral activity (IFN-γ and IFN-β). These findings were consistent with our previously published quantitative IHC data demonstrating increased CD8+ infiltrates that were nonetheless restricted to lesional stroma in unvaccinated subjects (11). In contrast, in the postvaccination tissue sections that contained TLSs, the molecular signature suggested immune cell activation (CXCR3) and TH1 polarization (TBX21, IFN-γ, and IFN-β). Similar to the CD8 and CD4 data, the changes in Tbet and Foxp3 were internally congruent with our quantitative image analyses of IHC in the same set of specimens.
We found evidence of a postvaccination immunologic effect not only in stromal tissue but also in the overlying dysplastic epithelium. Specifically, gene transcripts for CD8β, TBX21, IFN-γ, IFN-β, and CXCR3 were up-regulated in postvaccination dysplastic epithelium compared to unvaccinated lesions. These image analysis–guided gene expression data suggest that by 7 weeks after vaccination, the epithelial compartment of dysplastic lesions is infiltrated with effector T cells that are activated and proliferating and could mediate an effect against epithelial targets expressing HPV genes.
After vaccination, clonally expanded TCRs are disproportionately represented in mucosal tissue compared to the blood
To assess the repertoire of peripheral blood and tissue T cells, we determined the amino acid sequences in the hypervariable complementarity-determining region 3 (CDR3) of the TCR β chain (TCRB) in a subset of subject-matched tissue and peripheral blood T cells by high-throughput sequencing. Genomic DNA was extracted from peripheral blood T lymphocytes and cervical tissue from three subjects whose postvaccination resection specimens had an activated immune signature in the residual lesional mucosa and from an unvaccinated subject. Amplification and sequencing of TCRB CDR3 regions was carried out on the ImmunoSEQ platform at Adaptive Biotechnologies as previously described (21).
Several measures suggested that the tissue T cells were not composed of transudate from the blood but rather reflected a process of selection, presumably mediated at least in part by tissue-localized antigens. First, the diversity of the T cell clonal repertoire in each specimen was estimated using the empirical Bayesian method described previously by Daley and Smith (21, 22). The diversity of the TCR repertoire in the tissue was highly restricted compared to the blood (Fig. 6A). We then compared the frequencies of clonally expanded TCR CDR3 sequences and the degree of overlap (that is, shared TCR sequences) in tissue and blood T cells. Although many of the clonally expanded TCRs detected in vaccinated tissue could also be found in subject-matched peripheral blood, the shared sequences comprised a greater percentage of the repertoire in the tissue compared to the blood (Fig. 6B). Tissue–peripheral blood paired specimens from vaccinated subjects also had significantly greater overlap of shared TCRs than unvaccinated persistent HPV16+ CIN2/3 (Fig. 6C). Indeed, many TCRs that either were detectable with very low frequency or were undetectable in the blood were highly expanded in the tissue (fig. S4). Conversely, many clonally expanded TCRs in the blood were not detectable in subject-matched tissue.
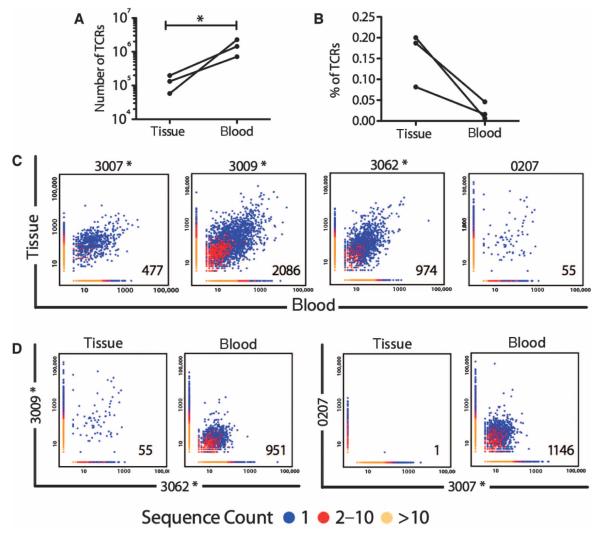
Fig. 6. Diversity and overlap of tissue and peripheral blood TCRs.
(A) The diversity of TCR in the blood greatly exceeds that in postvaccination tissue (n = 3). (B) TCRs that are shared between the tissue and blood of individual subjects who have been vaccinated comprise a greater fraction of tissue TCRs than peripheral blood TCRs. (C) Heat maps depicting the frequency of shared TCRs in tissue and peripheral blood of three vaccinated (*) subjects (3007, 3009, and 3062) and one unvaccinated subject (0207CR), all of whom had HPV16+ CIN2/3. (D) Heat maps depicting the frequency of shared TCRs in tissue and blood samples of two vaccinated subjects with shared human leukocyte antigen (HLA) alleles (3009 and 3062) and shared TCRs in tissue and blood samples of an unvaccinated (0207) and a vaccinated (3009) subject who shared HLA alleles.
Although the samples were too small to allow assessment of the specificity of the tissue T cells, we reasoned that if the lesion-localized T cell activation and proliferation was the result of stimulation by the same lesion-associated antigens, then we might find shared TCR sequences between vaccinated subjects whose residual lesions contained stromal TLSs and who shared HLA alleles. Indeed, we found shared TCR gene usage between tissue T cells of vaccinated subjects who shared HLA alleles (Fig. 6D and Table 3) as well as in the blood. In contrast, when we compared TCR sequences in an unvaccinated and a vaccinated subject who shared HLA A201, we identified overlap only in the blood. Together, these findings suggest that the T cell repertoire in the postvaccination tissue is both local and specific.
Table 3.
Shared tissue TCR between two vaccinated subjects.
| Amino acid | Percent of total |
V family | J gene | |
|---|---|---|---|---|
| 3009SA | 3062MH | |||
| CASSDSSGSTDTQYF | 6.03729 | 0.471434 | 6 | TRBJ2-3 |
| CASSQGGLAGVNEQFF | 2.02448 | 6.026706 | 3 | TRBJ2-1 |
| CASSDSTSGSNEQFF | 2.02448 | 1.083023 | 6 | TRBJ2-1 |
| CASSQVEETQYF | 0.57842 | 0.598848 | 3 | TRBJ2-5 |
| CASSLGPHNEQFF | 0.76822 | 0.586107 | 7 | TRBJ2-1 |
| CASSADTRYNEQFF | 0.61457 | 0.789970 | 2 | TRBJ2-1 |
| CASSLNSYEQYF | 0.30729 | 0.420468 | 5 | TRBJ2-7 |
| CASSLAGGYTF | 0.35248 | 0.293053 | 12 | TRBJ1-2 |
| CASSLLARGTDTQYF | 0.19883 | 0.458692 | 5 | TRBJ2-3 |
| CASSYRGTDTQYF | 0.10845 | 0.191122 | 6 | TRBJ2-3 |
| CASSVGTGNQPQHF | 0.06326 | 0.356761 | 9 | TRBJ1-5 |
| CSARDRTSGSYEQYF | 0.14461 | 0.152897 | 20 | TRBJ2-7 |
| CATSRDRAADTQYF | 0.09038 | 0.203863 | 15 | TRBJ2-3 |
| CASSPGQGAETQYF | 0.15364 | 0.063707 | 7 | TRBJ2-5 |
| CASSLAADTQYF | 0.08134 | 0.140156 | 5 | TRBJ2-3 |
DISCUSSION
Here, we report qualitative and quantitative changes in the intensity, frequency, and localization of immune cells in CIN2/3 target lesions subsequent to systemic therapeutic vaccination. By conventional measures of vaccination “success,” specifically, vaccine antigen-specific T cell responses in the blood and/or complete regression of disease, these subjects would have been considered “failures.” However, in the target lesions, we measured marked changes in immune parameters known to be associated with better prognosis, despite modest detectable T cell responses to vaccine antigen in circulating peripheral blood T cells. Vaccination was followed by increased lesional intraepithelial CD8 infiltrates composed of clonally expanded TCRs disproportionately represented compared to in the blood and were associated with TLSs, evidence of proliferation and activation mediated by TCR engagement by cognate antigen, and expression of genes associated with a TH1 response.
In unvaccinated patients with solid tumors, the quality of immune response in the tumor microenvironment has been shown to provide prognostic information that is not readily obvious in the blood (16, 26). Relevant parameters associated with improved clinical outcomes in treatment settings using conventional anticancer modalities include intratumoral CD3+CD8+ infiltrates, T cells with an antigen-experienced phenotype (CD45RO+), a TH1 molecular signature (IFNγ, IL-12, Tbet, and IRF1), cytotoxic effector molecules (granzymes, perforin, and granulysin), chemokines that play a role in recruiting effector immune cells (CX3CL1, CXCL9, CXCL10, CCL5, and CCL2), and TLSs (16, 26–28). Colocalized high endothelial venules, a vessel characteristic of lymph node structures, are also detected in tumor stroma in regions with T cell infiltrates and are also independently associated with improved overall survival (16, 29). Efforts to standardize a tissue immune “phenotype” are under way (30). The marked changes in cervical mucosal tissue in our study subjects suggested that many features associated with better prognosis had been induced by vaccination. These included a constellation of histologic changes in conjunction with molecular analyses that together suggest ongoing adaptive responses that are not only localized in tissue compared to the blood but also qualitatively and quantitatively different from unvaccinated CIN2/3 lesions.
The presence of an immune response in the tissue that is not readily detectable in the peripheral blood is likely to reflect both characteristics of the immune response to HPV E6 and E7 antigens, as well as issues associated with therapeutic vaccination. Although E6 and E7 are foreign proteins, not “self,” to date, they have not appeared to be highly immunogenic, at least in the context of most previously tested vaccine vectors. Whether this lack of “immunogenicity” reflects the underlying T cell repertoire or limitations of the processing and presentation of epitopes derived from these proteins remains to be determined. The problem of immunogenicity may be addressed in part by emerging clinical data from trials testing therapeutic DNA vaccination delivered via electroporation (31). Recent data reporting safety, tolerability, and immunogenicity of DNA vaccination targeting HPV16 and HPV18 E6 and E7, delivered using electroporation in subjects without evidence of disease, demonstrate significant induction of antigen-specific cytotoxic T cells, detectable directly ex vivo. Phase 2 studies of vaccination before resection, similar to the design of this study, are ongoing (NCT01304524).
It is worth emphasizing that therapeutic vaccination differs from prophylactic vaccination in that the antigenic target is already expressed in peripheral tissues at the time of vaccination. Our data and those from therapeutic vaccination trials in patients with vulvar intraepithelial neoplasia (VIN) suggest a model in which vaccine-induced cellular immune responses encounter antigen in peripheral tissue and are retained at the site. Two recent clinical trials testing therapeutic vaccination in subjects with HPV-induced VIN, in whom the expected rate of regression is less than 5% (23), reported complete response rates 10-fold greater (47 and 63%, respectively) than what would be expected in unvaccinated subjects (24, 25). The design of these trials differed from previously published trials in that the observation period for a clinical response was 52 weeks, which was a far longer time frame than conventional designs. However, despite encouraging clinical outcomes, immune responses to HPV E6 and E7 required ex vivo manipulation for detection and were of modest magnitude, similar to the findings from analyses of unvaccinated persons with HPV disease. Our findings of tissue-sequestered immunologic shifts may explain in part the conundrum of subjects who had clearly “responded” to systemic therapeutic vaccination, as manifested by complete regression of high-grade dysplasia, yet had systemic T cell responses that required sophisticated ex vivo approaches for detection.
Our findings suggest that there is much to be learned about the dynamics of responses to vaccination in humans, particularly in settings in which there is a tissue reservoir of the antigen being targeted. The finding that the intensity of tissue CD8+ infiltrates before vaccination was associated with the magnitude of peripheral blood responses 8 and 12 weeks after the boost vaccination was unexpected and suggested that the vaccine responses detectable in the blood could represent a boosted anamnestic response, rather than a de novo primary response to vaccination. The results also suggest that the timing of when the peripheral blood is drawn after vaccination affects the likelihood of detecting antigen-specific T cells, because the response appears to sequester significantly in the site of the persistent antigen.
Although the ability to compare pre- and postvaccination tissue specimens in vaccinated subjects did provide a valuable opportunity to query tissue sections for proof of principle, resection in this time frame essentially censored histologic endpoint analyses. Our clinical endpoints were dictated by a clear priority for patient safety. Therapeutic resections were scheduled for 7 weeks after the third vaccination because this time frame is within the standard of care for treating CIN2/3 in a noninvestigational setting. However, our findings have practical implications for the design and interpretation of clinical trials testing therapies for preinvasive HPV disease. Because intraepithelial infiltrates are associated with subsequent regression, and because an increase in intraepithelial CD8 infiltrates was associated with detectable responses to HPV in the blood, a monitoring strategy that incorporated features of a “tissue response,” such as increased intraepithelial CD8 infiltrates, in conjunction with a measure of “peripheral blood response,” such as increased ELISpot response to vaccine antigen, could potentially be used to inform clinical studies of therapeutic HPV vaccines. The quantitative image analyses we describe can be performed using reagents and methods available in any surgical pathology laboratory.
Tissue-based analyses of clinical specimens are limited by the small absolute size of available tissue, because the first principle is to not compromise the ability to make an accurate pathological diagnosis. The development of sophisticated, high-throughput analytic technologies now provides opportunities to derive a great deal of information directly ex vivo from limited specimens of unmanipulated cells. These studies hold great promise for enhancing our understanding of mechanisms of immune cell recruitment, repertoire, functional polarization, and cell retention, but must be informed and guided meticulously by tissue image analyses to minimize the odds of deriving data sets that could obscure or mask a dynamic clinical picture. In the case of our specimens, assessment of the repertoire of tissue-localized T cells was technically challenging because the absolute number of cells that could be isolated from small explants was too low to carry out conventional measures of specificity, such as ELISpot or flow cytometric analyses of cells stimulated with antigen. Although ex vivo expansion of tissue T cells would have provided more cells, published methods indicate that the cells that expanded would have represented a subset of cervical tissue T cells, in terms of repertoire, as well as phenotype and function (32, 33). The paired tissue and blood TCR sequence analyses in this sample set were carried out using genomic DNA extracted from unmanipulated cells. Data derived from carefully selected regions of tissue subsets will yield different information than those derived from whole tissue samples. These types of studies, although time-consuming and laborious, will be critical to expanding our understanding of mechanisms by which peripheral tissues may sustain relevant immune responses to peripheral manipulation, for example, vaccination, including epitope spreading (17, 34, 35). These types of studies may be especially important in the case of tumors with high rates of mutation, such as adenocarcinomas and squamous cancers of the lung (36). Tissue-based studies will also identify therapeutic barriers in the lesional microenvironment that can be targeted in concert with immune-based therapies for HPV disease.
Intraepithelial lesions associated with HPV are clinically indolent and are also directly accessible—two features that could also allow for making clinical management decisions based on tissue monitoring. Because colposcopically directed biopsies are much less invasive or destructive than a cone resection, patients whose lesional biopsies showed evidence of an effector immune response could potentially be monitored and saved from more invasive surgery, whereas patients whose lesions did not manifest an effector response could proceed to therapeutic resection. However, these kinds of treatment management decisions will be predicated on a better understanding of what constitutes a clinically meaningful tissue immune response.
MATERIALS AND METHODS
Ethics statement
The clinical trial protocols were reviewed and approved by the Institutional Review Board at Johns Hopkins Hospital (NA_00002176, NA_00023308). All study participants gave written informed consent before undergoing screening for study eligibility and enrollment. This trial is listed at http://www.clinicaltrials.gov (NCT00788164).
Patient demographics and trial design
Twelve subjects with HPV16+ CIN2/3 were enrolled in this phase 1, open-label clinical trial designed to assess the safety, tolerability, and immunogenicity of priming vaccination with DNA vaccine targeting HPV16 E7, followed by escalating doses of a boost vaccination with recombinant vaccinia targeting HPV16 and HPV18 E6 and E7 (TA-HPV). Standard therapeutic resection of lesions was performed 7 weeks after the boost vaccination. Eligibility criteria included ability to give informed consent, immune competence, HIV seronegativity, nonpregnant status, and presence of residual, visible lesion after a colposcopically directed, biopsy-confirmed diagnosis of CIN2/3. Patients included in this analysis had lesions associated with HPV16, the genotype most commonly associated with cervical cancer and its precursor lesions. Eligible subjects were assigned to a treatment cohort and received intramuscular vaccination at study weeks 0 and 4 with 3 mg of pNGVL4a-sig/E7(detox)/HSP70. At week 8, patients received one of three rVacE6E7 (TA-HPV) doses: 1.6 × 105 PFU (cohort DDV1; n = 3), 1.6 × 106 PFU (cohort DDV2; n = 3), or 1.6 × 107 PFU (cohort DDV3; n = 6), administered intramuscularly in the deltoid muscle. After vaccination with TA-HPV, the vaccination site was covered with an occlusive dressing (Opsite Flexigrid, Smith & Nephew).
Patients were observed for 30 min after each vaccination. Patients were given diary cards and instructed to record any local or systemic AEs daily for 4 weeks. All safety data, including injection site reactions and AEs, were reviewed by the institutional Data Safety and Monitoring Board and submitted for regular review to the U.S. Food and Drug Administration. Standard therapeutic resection of the cervical squamocolumnar junction was performed at week 15.
Primary endpoints included standard safety and tolerability endpoints as defined in CTCAE v4.0. Secondary endpoints included histologic regression, defined as no CIN2/3 in the resection specimen, and immune response to vaccine antigens. Immunogenicity was assessed by HPV16 and HPV18 E6/E7–specific IFN-γ ELISpot assay and flow cytometric assays for IFN-γ, granzyme B, and perforin with cryopreserved PBMCs obtained at screening (T0), at study weeks 8 to 10 (T2), at study week 15 (T3), and at study week 19 (T4).
Study vaccines
pNGVL4a-sig/E7(detox)/HSP70 is a plasmid DNA construct used previously as a stand-alone vaccination in a previous phase 1 clinical trial in a similar cohort (37). It was manufactured under Good Manufacturing Practices by the National Cancer Institute (NCI) Rapid Access to Interventional Development program and met all acceptance criteria for release. It is composed of a closed circular DNA plasmid expressing HPV16 E7 mutated at amino acids 24 and 26, linked to sequences coding for sig and for HSP70.
TA-HPV is a live attenuated recombinant vaccinia virus expressing HPV16 and HPV18 E6 and E7 (38). It is composed of fused E6 and E7 open reading frames of HPV16 and HPV18, each under the control of a vaccinia promoter, in the Wyeth strain of vaccinia. The E7 sequence is mutated so that capacity to bind to retinoblastoma protein is abrogated. The fused gene products have been shown to have no transforming activity. Vaccine was manufactured and released by Cantab (now Celtic Pharma). Production, analysis, and storage were carried out under Good Manufacturing Practice standards. Clinical trials testing this vaccine in a range of patient cohorts have been previously reported (38–41). Vaccination in estimated doses of 1.6 × 105, 1.6 × 106, or 1.6 × 107 PFU was administered intramuscularly in the deltoid muscle.
Endpoint evaluations
Colposcopy was performed at week 15, 7 weeks after the third vaccination. A standard therapeutic resection of the cervical squamocolumnar junction (either a cold knife conization or a loop electrosurgical excisional procedure) was performed. A frozen section was obtained from one radial section of the resection, and only if the pathologist did not have any suspicion of invasion, the flash-frozen tissue was banked, and an adjacent 2-mm sliver was reserved for explant cultures as previously described (11).
Immunologic analyses of PBMC samples
PBMCs were prioritized first to the IFN-γ ELISpot assay. Sufficient quantities were available to conduct assays using peptides spanning the four antigens separately, to allow estimation of individual response against each antigen. In one subject in the full-dose boost cohort, two time points were not available, because the patient was unable to keep those study visits. The remaining samples were used for ICS assays. No subject was excluded for reasons other than sample availability.
ELISpot assays were performed by the University of Pennsylvania Human Immunology Core Facility using a qualified protocol as previously described (42). The standard ELISpot protocol with 24-hour peptide stimulation was previously cross-validated across different laboratories (43) and was adapted for use with HPV-specific peptide pools. Differences between the above-referenced protocol and the protocol used in the current study relate only to the use of HPV peptides as the stimulating antigen. Specifically, the current protocol used two sets of peptides, each containing 15–amino acid residues overlapping by 8 amino acids representing the entire consensus E6/E7 fusion protein sequence of HPV16 or HPV18, and were pooled at a concentration of 2 μg/ml per peptide into two pools, spanning the length of the E6 and E7 antigens, respectively (44, 45). The average number of SFUs counted in R10 wells was subtracted from the average in individual HPV peptide wells and then adjusted to 1 × 106 PBMCs for each HPV peptide pool. To summarize the T cell ELISpot data, immune responses to each individual antigen were reported. For each time point, the mean number of SFUs from triplicate wells with PBMCs incubated with medium alone (background) was subtracted from the means of PBMCs stimulated with HPV16 or HPV18 E6 or E7 peptides. After subtracting medium control, a positive response was defined as at least 20 SFUs/106 PBMCs and greater than two times the SD of the prevaccination antigen-specific response.
Intracellular cytokine staining
ICS was performed as previously described (46) using the following markers: CD107a-PECy7, CD14–Pacific Blue, CD16–Pacific Blue, CD8-APC (allophycocyanin), CD4-PerCPCy5.5, IFN-γ–FITC (fluorescein isothiocyanate), and CD45RO-AF700 (BD Biosciences); CD19–Pacific Blue and granzyme B–PE (phycoerythrin) Texas Red (Invitrogen); CD27-PECy5 (eBioscience); and perforin-PE (Abcam). Prepared cells were acquired with an LSR II flow cytometer equipped with BD FACSDiva software (BD Biosciences). Acquired data were analyzed with FlowJo software version 7.6.3 (Tree Star). All samples used for this assay were from a preimmunization time point (all subjects tested) and from the week 8 to 10 time point, the week 15 time point, and the week 19 time point. Staining was performed once per time point per sample listed.
IHC staining of tissue sections
Tissue in paraffin blocks that was residual after histopathologic diagnoses had been finalized was prioritized first for IHC evaluation. After sections were obtained for CD8, Foxp3, and Ki67 IHC, sections were cut for laser capture microdissection from tissue blocks with sufficient residual material. Five-micrometer sections were cut from formalin-fixed, paraffin-embedded tissue from prevaccination (diagnostic biopsy) and from the postvaccination resection (7 weeks after the third vaccination). Heat-based Ag retrieval was performed for 30 min, followed by blocking endogenous peroxidase with 0.3% H2O2 and incubation with primary antibody. Primary antibodies were detected with the PowerVision+ Poly-HRP IHC Detection System (Leica Biosystems), as per the manufacturer’s instructions. After incubation with detection reagents, diaminobenzidine was used as a chromogen, and Harris hematoxylin was used as a counterstain. The following primary antibodies were used: CD8 (clone 4B11, Leica Biosystems, dilution 1:500, incubation overnight at 4°C), Tbet (clone4B10, BD Biosciences, dilution 1:500, incubation overnight at 4°C), Foxp3 (clone 236A/E7, eBioscience, dilution 1:50, incubation overnight at 4°C), Ki67 (clone MIB-1, Dako, dilution 1:400, incubation overnight at 4°C), PNAd (clone MECA-79, BD Biosciences, dilution 1:100, incubation overnight at 4°C), CD3 (clone SP7, Novus Biologicals, dilution 1:100, incubation for 4 hours at room temperature), and CD20 (clone L26, Dako, dilution 1:600, incubation for 30 min at room temperature).
Quantitation of tissue immune cells in histologic sections
Images were captured with a Nikon E600 microscope/Plan Fluor ×20/0.50 ocular and a DXM1200f Nikon digital camera. ROIs were delineated in normal epithelium, stroma immediately beneath normal epithelium, CIN2/3 epithelium, and stroma immediately beneath CIN2/3 with the NIS Elements AR3 imaging software (Nikon). Chromogen was quantitated by normalization against the area of the ROI (μm2). At least 3 and up to 10 discrete ROIs were quantified in each compartment in each case. The mean density of chromogen staining for each compartment for each case was calculated and used for statistical comparisons between groups.
Phenotype analyses of tissue T cells derived from fresh tissue explants
Primary tissue explants were obtained from surgical resection specimens. Normal mucosa obtained on banking protocol governing acquisition of residual tissue after standard diagnostic sections had been obtained. Tissue explants were obtained from standard therapeutic resections of the cervical squamocolumnar junction, and only if the surgical pathologists had no suspicion of invasive disease on a frozen section, a 2-mm section of fresh tissue immediately adjacent to the frozen section was reserved. Primary tissue explants were cultured using the method of Clark et al. (15). Flow cytometry analysis of cervical T cells was performed using directly conjugated monoclonal antibodies obtained from BioLegend (CD3, CD4, CD8, CD45RO, CD45RA, and L-selectin/CD62L), R&D Systems (CCR7), and the National Institutes of Health (NIH) AIDS Reagent Program (α4β7 and ACT-1). Analysis of flow cytometry samples was performed on BD Biosciences FACScan or FACSCanto instruments, and data were analyzed with FACSDiva software (version 5.1, BD Biosciences).
Laser capture microdissection
Laser capture microdissection was used to segregate epithelium and subjacent stroma from 10-μm sections cut from paraffin-embedded tissue. Sections were mounted onto PALM MembraneSlides that had been pretreated with RNase-ZAP and irradiated (Zeiss MembraneSlide 1.0 PEN; 415101-4401-050). Laser capture microdissection was performed with the Axiovert 200 M (PALM MicroBeam) microdissection system (Zeiss), using PALM Robo v3.2 software. ROIs were delineated by free-hand tracing and catapulted into the cap of a 0.5-ml AdhesiveCap 500 opaque tube (Zeiss 415101-4400-255).
Quantitative reverse transcription polymerase chain reaction
RNA samples were prepared from microdissected formalin-fixed sections with the AllPrep DNA/RNA FFPE kit (Qiagen). The amount and purity of RNA were verified with the NanoDrop ND-1000 spectrophotometer (Isogen) and calculated by the ratio of the readings at 260 and 280 nm (A260/A280). The average A260/A280 ratio for the samples tested was 1.92 (range, 1.6 to 2.2), and the average concentration of RNA was 10.1 ng/μl (range, 4.2 to 14.6 ng/μl). First-strand complementary DNA (cDNA) synthesis was produced with the ABI High Capacity RNA-to-cDNA Kit (Applied Biosystems). Preamplification was carried out with 300 ng of cDNA, TaqMan Custom PreAmp pools, and TaqMan PreAmp Master Mix (Applied Biosystems). The reaction conditions were as follows: denaturation step at 95°C for 10 min, followed by 14 cycles of 95°C for 15 s and 60°C for 4 min. Upon completion, samples were immediately removed from the thermal cycler, placed on ice, and diluted 1:20. Quantitative reverse transcription polymerase chain reaction (qRT-PCR) was carried out on preamplified cDNA samples in a final volume of 20 μl in 96-well plates with the Applied Biosystems Prism 7900HT TaqMan instrument. The reaction mixture was composed of diluted preamplified cDNA sample, TaqMan Gene Expression Master Mix, and TaqMan Gene Expression Assays for CD4 (Hs00181217_m1), CD8B (Hs00174762_m1), Foxp3 (Hs01085834_m1), TBX21 (Hs00203436_m1), IFNG (Hs00989291_m1), IFNB1 (Hs01077958_s1), CCR7 (Hs01013469_m1), PRF1 (Hs00169473_m1), GZMB (Hs00188051_m1), CXCR3 (Hs01847760_s1), CD25 (Hs00907777_m1), CD69 (Hs00934033_m1), CD137 (Hs00155512_m1), GATA3 (Hs00231122_m1), RORC (Hs01076112_m1), and BCL6 (Hs00277037_m1) (Applied Biosystems). Cycling conditions were as follows: denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. GAPDH was used as an internal control gene to normalize the reaction for the amount of RNA added to the reverse transcription reactions. Each real-time PCR was performed in duplicate. The entire preamplification/qRT-PCR procedure was repeated on a subset of genes for which sufficient residual material permitted, with similar results.
TCR sequencing
The sequences for both the TCRB CDR3 regions were delineated according to the definition established by the International ImMunoGeneTics collaboration (47). Sequences that did not match CDR3 sequences were removed from the analysis. A standard algorithm was used to identify which V, D, and J segments contributed to each TCRB CDR3 sequence (47). In both the blood and the tissue samples, the sample of T cells was a subsampling of a larger set of T cells. The total T cell repertoire was estimated using the unseen species model, a computational approach originally developed by Fisher et al. (48), and adapted using TCRB CDR3 sequences as individual species (21).
Statistical analyses
The sample size for the clinical protocol was based on a standard 3 + 3 dose-finding design with three dosing levels and an additional three individuals at the maximally tolerated dose. Given the low toxicity levels observed in previous DNA vaccine studies, the expected sample size was 12 patients: 9 for dose escalation and 3 for the maximally tolerated dose. Within-subject quantitative tissue analyses of immune cell subsets were assessed using Wilcoxon signed rank tests. Differences in quantitative endpoints between groups were assessed using the Wilcoxon rank sum test. Correlations were determined using Pearson correlations. Two-sided testing with a significance level of P < 0.05 was used for all analyses.
Supplementary Material
Acknowledgments
We thank Celtic Pharma for providing the recombinant vaccinia vaccine, TA-HPV, as a gift. We thank M. Salas for technical assistance.
Funding: Supported by NIH/NCI awards [1R21 CA123876 and R01 CA14269101 (C.L.T.) and 2 P50 CA098252 (T.C.W. and C.L.T.)], 2P30CA006973-49 (Cancer Center Core Grant), and a Dana Foundation Award (C.L.T.).
Footnotes
SUPPLEMENTARY MATERIALS www.sciencetranslationalmedicine.org/cgi/content/full/6/221/221ra13/DC1
Author contributions: C.L.T. designed and executed the clinical trial, image analyses, and laser capture microdissection and wrote the manuscript. R.A.C. and J.E.T. carried out explant cultures, fluorescence-activated cell sorting phenotyping, and ICS of cervical T cells. J.D.B. and M.P.M. carried out the analysis and interpretation of peripheral blood immune responses. L.M. carried out IHC and qRT-PCR experiments. T.C.W. designed the DNA vector. M.S. and I.J. carried out the DNA and RNA extractions. B.T. contributed to image analyses and to the writing of the manuscript. C.D. and H.S.R. carried out TCR sequencing experiments, analysis, and interpretation. C.W. contributed to the statistical analyses. All authors approved the final manuscript.
Competing interests: T.C.W. is a founder of Papivax LLC and owns stock. H.S.R. owns stock and consults for Adaptive Biotechnologies. C.D. is employed by and has equity ownership in Adaptive Biotechnologies. R.A.C. has stock in TremRx (a virtual company with no active products focused on vaccination to generate tissue-resident T cells) and is the scientific advisor for the Alopecia Areata Initiative, a nonprofit organization.
REFERENCES AND NOTES
- 1.de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, Plummer M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012;13:607–615. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Simard EP, Dorell C, Noone AM, Markowitz LE, Kohler B, Eheman C, Saraiya M, Bandi P, Saslow D, Cronin KA, Watson M, Schiffman M, Henley SJ, Schymura MJ, Anderson RN, Yankey D, Edwards BK. Annual Report to the Nation on the Status of Cancer, 1975–2009, featuring the burden and trends in human papillomavirus (HPV)–associated cancers and HPV vaccination coverage levels. J. Natl. Cancer Inst. 2013;105:175–201. doi: 10.1093/jnci/djs491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winer RL, Feng Q, Hughes JP, O’Reilly S, Kiviat NB, Koutsky LA. Risk of female human papillomavirus acquisition associated with first male sex partner. J. Infect. Dis. 2008;197:279–282. doi: 10.1086/524875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention (CDC) Human papillomavirus vaccination coverage among adolescent girls, 2007-2012, and postlicensure vaccine safety monitoring, 2006-2013—United States. MMWR Morb. Mortal. Wkly. Rep. 2013;62:591–595. [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson J, Bedell M, McCance D, Laiminis L. Immortalization and altered differentiation of human keratinocytes in vitro by the E6 and E7 open reading frames of human papillomavirus type 18. J. Virol. 1990;64:519–526. doi: 10.1128/jvi.64.2.519-526.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werness B, Levine A, Howley P. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science. 1990;248:76–79. doi: 10.1126/science.2157286. [DOI] [PubMed] [Google Scholar]
- 7.Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: A meta-analysis. Obstet. Gynecol. 1998;92:727–735. doi: 10.1016/s0029-7844(98)00245-2. [DOI] [PubMed] [Google Scholar]
- 8.Trimble CL, Piantadosi S, Gravitt P, Ronnett B, Pizer E, Elko A, Wilgus B, Yutzy W, Daniel R, Shah K, Peng S, Hung C, Roden R, Wu TC, Pardoll D. Spontaneous regression of high-grade cervical dysplasia: Effects of human papillomavirus type and HLA phenotype. Clin. Cancer Res. 2005;11:4717–4723. doi: 10.1158/1078-0432.CCR-04-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nakagawa M, Stites DP, Patel S, Farhat S, Scott M, Hills NK, Palefsky JM, Moscicki AB. Persistence of human papillomavirus type 16 infection is associated with lack of cytotoxic T lymphocyte response to the E6 antigens. J. Infect. Dis. 2000;182:595–598. doi: 10.1086/315706. [DOI] [PubMed] [Google Scholar]
- 10.Trimble CL, Peng S, Thoburn C, Kos F, Wu TC. Naturally occurring systemic immune responses to HPV antigens do not predict regression of CIN2/3. Cancer Immunol. Immunother. 2010;59:799–803. doi: 10.1007/s00262-009-0806-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trimble CL, Clark RA, Thoburn C, Hanson NC, Tassello J, Frosina D, Kos F, Teague J, Jiang Y, Barat NC, Jungbluth AA. Human papillomavirus 16-associated cervical intraepithelial neoplasia in humans excludes CD8 T cells from dysplastic epithelium. J. Immunol. 2010;185:7107–7114. doi: 10.4049/jimmunol.1002756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubey S, Clair J, Fu TM, Guan L, Long R, Mogg R, Anderson K, Collins KB, Gaunt C, Fernandez VR, Zhu L, Kierstead L, Thaler S, Gupta SB, Straus W, Mehrotra D, Tobery TW, Casimiro DR, Shiver JW. Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J. Acquir. Immune Defic. Syndr. 2007;45:20–27. doi: 10.1097/QAI.0b013e3180377b5b. [DOI] [PubMed] [Google Scholar]
- 13.Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN, Step Study Protocol Team Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): A double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobayashi A, Greenblatt RM, Anastos K, Minkoff H, Massad LS, Young M, Levine AM, Darragh TM, Weinberg V, Smith-McCune KK. Functional attributes of mucosal immunity in cervical intraepithelial neoplasia and effects of HIV infection. Cancer Res. 2004;64:6766–6774. doi: 10.1158/0008-5472.CAN-04-1091. [DOI] [PubMed] [Google Scholar]
- 15.Clark RA, Chong BF, Mirchandani N, Yamanaka K, Murphy GF, Dowgiert RK, Kupper TS. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J. Invest. Dermatol. 2006;126:1059–1070. doi: 10.1038/sj.jid.5700199. [DOI] [PubMed] [Google Scholar]
- 16.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman WH. J. Cadranel, Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 17.Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, Schell MJ, Sondak VK, Weber JS, Mulé JJ. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: Potential for patient selection for immunotherapy? Sci. Rep. 2012;2:765. doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, Mulé JJ. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am. J. Pathol. 2011;179:37–45. doi: 10.1016/j.ajpath.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–5687. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- 20.Scholzen T, Gerdes J. The Ki-67 protein: From the known and the unknown. J. Cell. Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 21.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, Riddell SR, Warren EH, Carlson CS. Comprehensive assessment of T-cell receptor β-chain diversity in αβ T cells. Blood. 2009;114:4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daley T, Smith AD. Predicting the molecular complexity of sequencing libraries. Nat. Methods. 2013;10:325–327. doi: 10.1038/nmeth.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Seters M, van Beurden M, de Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecol. Oncol. 2005;97:645–651. doi: 10.1016/j.ygyno.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends-van der Meer DM, Vloon AP, Essahsah F, Fathers LM, Offringa R, Drijfhout JW, Wafelman AR, Oostendorp J, Fleuren GJ, van der Burg SH, Melief CJ. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. N. Engl. J. Med. 2009;361:1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 25.Daayana S, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, Kitchener HC. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. Br. J. Cancer. 2010;102:1129–1136. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagès F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N. Engl. J. Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 27.Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 28.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 29.Byshevskii A, Galian SL, Ral’chenko IV, Alborov RG, Umutbaeva MK, Rudzevich A, Vinokurova EA. Erythrocytes and leucocytes in realization of communication between lipid peroxidation and hemostasis. Biomed. Khim. 2006;52:370–377. [PubMed] [Google Scholar]
- 30.Galon J, Pagès F, Marincola FM, Angell HK, Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, Tatangelo F, Britten CM, Kreiter S, Chouchane L, Delrio P, Arndt H, Asslaber M, Maio M, Masucci GV, Mihm M, Vidal-Vanaclocha F, Allison JP, Gnjatic S, Hakansson L, Huber C, Singh-Jasuja H, Ottensmeier C, Zwierzina H, Laghi L, Grizzi F, Ohashi PS, Shaw PA, Clarke BA, Wouters BG, Kawakami Y, Hazama S, Okuno K, Wang E, O’Donnell-Tormey J, Lagorce C, Pawelec G, Nishimura MI, Hawkins R, Lapointe R, Lundqvist A, Khleif SN, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Palmqvist R, Nagtegaal ID, Wang Y, D’Arrigo C, Kopetz S, Sinicrope FA, Trinchieri G, Gajewski TF, Ascierto PA, Fox BA. Cancer classification using the Immunoscore: A worldwide task force. J. Transl. Med. 2012;10:205. doi: 10.1186/1479-5876-10-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagarazzi ML, Yan J, Morrow MP, Shen X, Parker RL, Lee JC, Giffear M, Pankhong P, Khan AS, Broderick KE, Knott C, Lin F, Boyer JD, Draghia-Akli R, White CJ, Kim JJ, Weiner DB, Sardesai NY. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci. Transl. Med. 2012;4:155ra138. doi: 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bere A, Denny L, Hanekom W, Burgers WA, Passmore JA. Comparison of polyclonal expansion methods to improve the recovery of cervical cytobrush-derived T cells from the female genital tract of HIV-infected women. J. Immunol. Methods. 2010;354:68–79. doi: 10.1016/j.jim.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalamasz D, Long SA, Taniguchi R, Buckner JH, Berenson RJ, Bonyhadi M. Optimization of human T-cell expansion ex vivo using magnetic beads conjugated with anti-CD3 and anti-CD28 antibodies. J. Immunother. 2004;27:405–418. doi: 10.1097/00002371-200409000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Corbière V, Chapiro J, Stroobant V, Ma W, Lurquin C, Lethé B, van Baren N, Van den Eynde BJ, Boon T, Coulie PG. Antigen spreading contributes to MAGE vaccination-induced regression of melanoma metastases. Cancer Res. 2011;71:1253–1262. doi: 10.1158/0008-5472.CAN-10-2693. [DOI] [PubMed] [Google Scholar]
- 35.Cipponi A, Mercier M, Seremet T, Baurain JF, Théate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 36.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, Zhang Q, Wendl MC, Lawrence MS, Larson DE, Chen K, Dooling DJ, Sabo A, Hawes AC, Shen H, Jhangiani SN, Lewis LR, Hall O, Zhu Y, Mathew T, Ren Y, Yao J, Scherer SE, Clerc K, Metcalf GA, Ng B, Milosavljevic A, Gonzalez-Garay ML, Osborne JR, Meyer R, Shi X, Tang Y, Koboldt DC, Lin L, Abbott R, Miner TL, Pohl C, Fewell G, Haipek C, Schmidt H, Dunford-Shore BH, Kraja A, Crosby SD, Sawyer CS, Vickery T, Sander S, Robinson J, Winckler W, Baldwin J, Chirieac LR, Dutt A, Fennell T, Hanna M, Johnson BE, Onofrio RC, Thomas RK, Tonon G, Weir BA, Zhao X, Ziaugra L, Zody MC, Giordano T, Orringer MB, Roth JA, Spitz MR, Wistuba II, Ozenberger B, Good PJ, Chang AC, Beer DG, Watson MA, Ladanyi M, Broderick S, Yoshizawa A, Travis WD, Pao W, Province MA, Weinstock GM, Varmus HE, Gabriel SB, Lander ES, Gibbs RA, Meyerson M, Wilson RK. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, Pardoll D, Wu TC. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin. Cancer Res. 2009;15:361–367. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, Evans AS, Adams M, Stacey SN, Boursnell ME, Rutherford E, Hickling JK, Inglis SC. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 39.Kaufmann AM, Stern PL, Rankin EM, Sommer H, Nuessler V, Schneider A, Adams M, Onon TS, Bauknecht T, Wagner U, Kroon K, Hickling J, Boswell CM, Stacey SN, Kitchener HC, Gillard J, Wanders J, Roberts JS, Zwierzina H. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin. Cancer Res. 2002;8:3676–3685. [PubMed] [Google Scholar]
- 40.Baldwin PJ, van der Burg SH, Boswell CM, Offringa R, Hickling JK, Dobson J, Roberts JS, Latimer JA, Moseley RP, Coleman N, Stanley MA, Sterling JC. Vaccinia-expressed human papillomavirus 16 and 18 E6 and E7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clin. Cancer Res. 2003;9:5205–5213. [PubMed] [Google Scholar]
- 41.Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, McVey RJ, Dobson J, Roberts JS, Hickling J, Kitchener HC, Stern PL. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003;63:6032–6041. [PubMed] [Google Scholar]
- 42.Boyer JD, Robinson TM, Kutzler MA, Parkinson R, Calarota SA, Sidhu MK, Muthumani K, Lewis M, Pavlakis G, Felber B, Weiner D. SIV DNA vaccine co-administered with IL-12 expression plasmid enhances CD8 SIV cellular immune responses in cynomolgus macaques. J. Med. Primatol. 2005;34:262–270. doi: 10.1111/j.1600-0684.2005.00124.x. [DOI] [PubMed] [Google Scholar]
- 43.Janetzki S, Panageas KS, Ben-Porat L, Boyer J, Britten CM, Clay TM, Kalos M, Maecker HT, Romero P, Yuan J, Kast WM, Hoos A, Elispot Proficiency Panel of the CVC Immune Assay Working Group Results and harmonization guidelines from two large-scale international Elispot proficiency panels conducted by the Cancer Vaccine Consortium (CVC/SVI) Cancer Immunol. Immunother. 2008;57:303–315. doi: 10.1007/s00262-007-0380-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan J, Reichenbach DK, Corbitt N, Hokey DA, Ramanathan MP, McKinney KA, Weiner DB, Sewell D. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine. 2009;27:431–440. doi: 10.1016/j.vaccine.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan J, Harris K, Khan AS, Draghia-Akli R, Sewell D, Weiner DB. Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine. 2008;26:5210–5215. doi: 10.1016/j.vaccine.2008.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morrow MP, Yan J, Pankhong P, Shedlock DJ, Lewis MG, Talbott K, Toporovski R, Khan AS, Sardesai NY, Weiner DB. IL-28B/IFN-λ3 drives granzyme B loading and significantly increases CTL killing activity in macaques. Mol. Ther. 2010;18:1714–1723. doi: 10.1038/mt.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yousfi Monod M, Giudicelli V, Chaume D, Lefranc MP. IMGT/JunctionAnalysis: The first tool for the analysis of the immunoglobulin and T cell receptor complex V–J and V–D–J JUNCTIONs. Bioinformatics. 2004;20(Suppl. 1):i379–i385. doi: 10.1093/bioinformatics/bth945. [DOI] [PubMed] [Google Scholar]
- 48.Fisher RA, Corbet AS, Williams CB. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 1943;12:42–58. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.