Abstract
To examine the role of neuropeptide Y (NPy) in the first luteinizing hormone (LH) surge of puberty. The effect of passive immunoneutralization of NPY with antiserum against NPY (anti-NPY) injected centrally (third ventricle) or peripherally (jugular vein) was studied in pubertal female rats on the day of first proestrus. Both peripheral and central anti-NPY administration reduced the magnitude of the LH surge during the afternoon of first proestrus; however, the central route of administration appeared to be most effective. Centrally administered anti-NPY also reduced the magnitude of proestrous LH-releasing hormone (LHRH) release into pituitary portal blood in these rats. These results suggest that endogenous NPY plays a facilitatory role in the generation of the LHRH surge necessary for preovulatory gonadotropin release and puberty.
Keywords: Neuropeptide Y, Luteinizing hormone, Luteinizing hormone-releasing hormone, Puberty, Preovulatory luteinizing hormone surge, Female rat
Puberty, the culmination of a multitude of developmental processes at the hypothalamic, pituitary and gonadal levels, is manifested by the preovulatory gonadotropin surge leading to the first ovulation [1]. Maturation of the luteinizing hormone (LH)-releasing hormone (LHRH) surge center is indispensable and is achieved by integration of various functional components [1–3]. Neuropeptide Y (NPY) has been reported to modulate the secretion of LH from the pituitary gland, possibly by affecting the secretion of LHRH from the hypothalamus and the sensitivity of the pituitary gland to LHRH [4–8], We have recently shown that there is a striking similarity between the developmental changes of postnatal NPY and LHRH content in the hypothalamus and preoptic area and that the preovulatory surge of LHRH on puberty is accompanied with a surge of NPY in hypophysial portal blood [9], These findings, along with the histological data showing NPY innervation of LHRH-positive neural elements [10], prompted us to examine the hypothesis that NPY plays a significant role in the initiation of the first LHRH surge leading to the onset of puberty. In this study, we examined the effect of passive immunoneutralization of NPY on the preovulatory LH surge in peripheral plasma and LHRH release in pituitary portal plasma in pubertal female rats.
Materials and Methods
Female Sprague-Dawley rats, weighing 80 g, were obtained from the Laboratory Animal Resources Center at Washington State University, housed in a controlled environment (22 °C, light on 05.00–19.00 h) and provided with food and water ad libitum. The vaginal orifice of these rats was surgically opened with a fine glass pipette, and the vaginal smears were inspected daily until the day of first estrus. Surgical opening of the vagina followed by taking of vaginal smears daily did not affect the timing of the first estrus (operated, n = 12, 39.2 ± 0.7 days; control, n = 11, 38.5 ± 0.6 days).
Experiment 1
About a week prior to the experiment, a stainless-steel (27-gauge) cannula was inserted into the third ventricle (intracerebroventricular) and fixed to the skull of the pubertal rats under halothane anesthesia. On the day of first proestrus, 30 μl of anti-NPY raised in rabbit against porcine NPY (No. 2–7; Dr. M. Brown, University of California at San Diego, La Jolla, Calif., USA; antiserum No. 2–7 was obtained 2 weeks later from the same animal as was No. 2–6; it has binding characteristics similar to No. 2–6 in radioimmunoassay [11], was infused (2 μl/min) intracerebroventricularly at 09.30–10.00 h. The control rats received the same amount of normal rabbit serum (NRS) intracerebroventricularly or received no treatment Serial blood specimens (400 μl) were collected at 1-hour intervals between 12.00 and 18.00 h through the indwelling right atrial cannula placed 2–4 days prior to the experiment, and replaced with red cells suspended in saline. Plasma was immediately separated and stored at −70 °C until assayed. The next morning (the day of first estrus), ovulation was examined by laparotomy and the number of ova in the fallopian tubes counted.
Experiment 2
The pubertal rats were implanted with an indwelling cannula in the right atrium 2–4 days prior to the experiment under halothane anesthesia. On the day of first proestrus. 300 μl of anti-NPY or NRS was administered to the rat through the atrial cannula at 09.30–10.00 h. Serial blood samples were collected on the day of first proestrus and ova were examined the next morning as in experiment 1.
Experiment 3
On the day of first proestrus, the rats were anesthetized with Saffan (Glaxo, Research Triangle Park, N.C., USA; 4 ml/kg body weight, i.p.) at 10.00 h and the pituitary stalk of the rat was exposed as described previously [2]. Additional amounts of Saffan were infused (0.2 ml/kg/h, i.v.) continuously. At 11.00 h, a 27-gauge stainless-steel needle was inserted into the third ventricle through the median eminence and 30 μl of anti-NPY or NRS was administered for 15 min by infusion pump. The pituitary stalk was cut at approximately 14.00 h, and pituitary portal blood samples were collected during 4 consecutive 60-min periods between 14.00 and 18.00 h.
LH concentrations were determined by a double-antibody radioimmunoassay using kits supplied by the NIADDK. The reference standard for LH used was RP-2. The pituitary portal plasma was extracted with HCl-methanol, and LHRH concentration in the plasma extract measured by double-antibody radioimmunoassay using synthetic LHRH standard and LHRH antiserum (No. CRRIIB73; provided by Dr. Ramirez, University of Illinois, Urbana, Ill., USA). The intra- and interassay coefficients of variation for LH were 3.8 and 9.7%, respectively, and for LHRH they were 3.9 and 10.2%, respectively. The data are expressed as mean ± SEM. Statistical differences between multiple groups were determined by analysis of variance and Duncan’s multiple-range test. The difference between the two groups was determined by t test. A p value of less than 0.05 was considered significant.
Results and Discussion
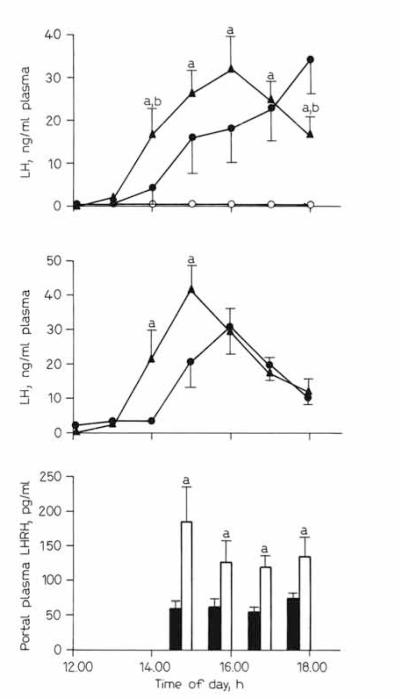
The systemic LH levels of the untreated animals exhibited the expected preovulatory surge on the afternoon of first proestrus (n = 5; ng/ml: 12.00 h, 0.32 ± 0.07; 13.00 h, 3.7 ± 3.1; 14.00 h, 24.6 ± 15.1; 15.00 h, 41.6 ± 10.5; 16.00 h, 41.2 ± 10.4; 17.00 h, 27.5 ± 6.6; 18.00 h, 19.5 ± 5.7). Administration of NRS did not change the LH levels significantly during the afternoon of the first proestrus as compared to rats without treatment regardless of the route of injection (fig. 1a, b); therefore, NRS was used as a control for an anti-NPY injection.
Fig. 1.
Effect of NPY immunoneutralization on LH and LHRH secretion during the first proestrus in rats, a Peripheral plasma LH profiles in rats infused with 30 μl/15 min of NRS (▴ n = 10) or rabbit anti-NPY serum (○, ● n = 12) into the third ventricle (intracerebroventriclarly) on the day of first proestrus. Animals treated with anti-NPY showed either a delayed (significant) LH surge (● n = 6) or no significant increase in plasma LH (○; n = 6). ap<0.05 compared with anti-NPY (○) at respective times; bp < 0.05 compared with anti-NPY (●) at respective times, b Peripheral plasma LH profiles in rats that received intravenous injection of 300 μl of NRS (▴ n = 7) or anti-NPY (● n = 9). ap < 0.05 vs. anti-NPY-treated rats at respective times, c Pituitary portal plasma LHRH concentration in rats treated with intracerebroventricular infusion of NRS ( ; n = 8) or anti-NPY (
; n = 8) or anti-NPY ( ; n = 6). ap < 0.05 vs. anti-NPY-treated rats at respective times.
; n = 6). ap < 0.05 vs. anti-NPY-treated rats at respective times.
Figure la represents the plasma LH profiles on the day of first proestrus in rats which received an intracerebroventricular injection of anti-NPY or NRS. All the rats given NRS showed an LH surge during the afternoon of the day of first proestrus with the peak appearing at 15.00–16.00 h. Among the rats given anti-NPY intracerebroventricularly, the mean plasma LH levels were significantly lower between 14.00 and 17.00 h than those in control animals. Inspection of plasma LH profiles in individual animals revealed that one population of rats (n = 6) maintained low levels of LH throughout the collection period, while another population of rats (n = 6) showed a delayed LH surge (fig. 1a). The LH values at 16.00–18.00 h between these two subpopulations of responders were significantly different (p < 0.05). A possible explanation for the presence of two populations is that the effects of anti-NPY were different according to the amount of the antibody which reached the site responsible for LH secretion.
Ovulation occurred in all of the control and anti-NPY-treated rats on the next morning, except for 1 anti-NPY-treated rat. The number of ova were not different in these two groups (control vs. anti-NPY-treated, 10.2 ± 0.57 vs. 11.0 ± 0.52). The presence of ovulation in the group of anti-NPY-treated rats which did not show the preovulatory LH surge during the observation period indicated the possible occurrence of an LH surge after 18.00 h. An alternative explanation of ovulation in the anti-NPY-treated rats is the occurrence of a small LH surge that could not be detected by our experimental procedures, since it is known that an LH surge of only 20% of the magnitude of a full-strength surge is enough to produce ovulation.
When anti-NPY was administered to the rat peripherally through the atrial cannula on the morning of the day of first proestrus, the LH release was significantly reduced at 14.00 and 15.00 h as compared to control rats given NRS (fig. 1b). The levels of LH in anti-NPY- and NRS-treated rats were similar between 16.00 and 18.00 h. Ovulation occurred in all control and anti-NPY-treated rats the next morning and the number of ova were not different in these groups (control vs. anti-NPY-treated, 11.2 ± 0.92 vs. 12.8 ± 1.3).
In order to clarify whether the reduction of LH surge induced by the central administration of anti-NPY was the result of suppression of LHRH secretion from the hypothalamus, we measured the concentration of LHRH in the pituitary portal plasma on the day of first proestrus after intracerebroventricular injection of anti-NPY. We have previously shown that, like plasma LH levels, portal plasma levels of LHRH increase 4- to 5-fold on the afternoon of first proestrus (156 pg/ml) as compared to those on the afternoon of the day before first proestrus (31 pg/ml) and the day of first estrus (41 pg/ml) in the pubertal rats [2], As shown in figure 1c, the level of LHRH observed in control rats treated with NRS in this study is comparable to that observed previously in untreated control rats on the day of first proestrus [2]. Figure 1c also shows that the concentration of LHRH in the pituitary portal plasma was lower in anti-NPY-treated rats than in NRS-treated controls during the observation period. This finding indicates that the neutralization of the central NPY suppressed LHRH secretion at least until 18.00 h.
The prominent effect of intracerebroventricular anti-NPY administration on the LH surge (as opposed to peripheral administration) and the fact that LHRH secretion was suppressed by the antibody treatment suggest that the facilitatory role of NPY on the first preovulatory LH surge is mainly attributed to the enhancement of LHRH secretion. These results agree with the finding that the estrogen/progesterone-induced LH surge in ovariectomized adult female rats was inhibited by the anti-NPY administered centrally [12], Since NPY has been shown to augment the pituitary LH response to LHRH [5], it is possible that peripherally administered anti-NPY affects the pituitary gland by decreasing the responsiveness of LH secretion to LHRH. It is also possible that the peripherally administered anti-NPY acted at the circumventricular organ such as the median eminence or the organum vasculosum of the lamina terminal is, and thereby affected LH secretion via LHRH.
We have previously observed that after birth the content of NPY increases in parallel with that of LHRH in the hypothalamus and preoptic area until stabilizing around puberty, and that the preovulatory LH surge is preceded by a surge of NPY in the pituitary portal blood in the pubertal female rat [9]. Furthermore, it has been shown that the effect of exogenous NPY on the secretion of LH and LHRH is variable in adult rats, depending on the steroidal milieu. NPY increases plasma LH levels and the in vitro secretion of LHRH from the medial basal hypothalamus of estrogen-treated ovariectomized rats but not of vehicle-treated ovariectomized rats [6–8]. A sufficient maturation of the ovarian follicles and the associated secretion of estrogen are required both to develop the surge center and to sensitize the pituitary in order to elicit the first preovulatory LH surge in pubertal rat [1]. The steroidal milieu during the afternoon of first proestrus in pubertal rats is consistent with a stimulatory action of NPY on LHRH. We, therefore, propose that in the prepubertal period, under the increasing influence of estrogen, NPY enhances the secretion of LHRH and augments the effect of LHRH on LH secretion from the pituitary gland to induce the preovulatory LHRH and LH surges which result in pubertal onset.
Acknowledgments
We gratefully acknowledge Dr. Marvin Brown for NPY antiserum, Dr. V. Ramirez for LHRH antiserum, Dr. A.F. Parlow, Pituitary Hormones and Antisera Center, and the NIADDK for LH radioimmunoassay kits and Glaxo for Saffan. This work was supported by NIH grants HD 20498 and AG05453 to D.K.S. and HD 13527 to P.M.P.
References
- 1.Ojeda SR, Urbanski HF, Ahmed CE. The onset of female puberty: Studies in the rat; in Greep RO (ed): Recent Progress in Hormone Research. Vol. 42. Academic Press; London: 1986. pp. 385–440. [DOI] [PubMed] [Google Scholar]
- 2.Sarkar DK, Fink G. Mechanism of the first spontaneous gonadotrophin surge and that induced by pregnant mare serum and effects of neonatal androgen in rats. J Endocrinol. 1979;83:339–354. doi: 10.1677/joe.0.0830339. [DOI] [PubMed] [Google Scholar]
- 3.Sarkar DK, Smith GC, Fink G. Effect of manipulating central catecholamines on puberty and the surge of luteinizing hormone and gonadotropin releasing hormone induced by pregnant mare serum gonadotropin in female rats. Brain Res. 1981;213:335–349. doi: 10.1016/0006-8993(81)90239-0. [DOI] [PubMed] [Google Scholar]
- 4.McDonald JK, Lumpkin MD, Samson WK, et al. Neuropeptide Y affects secretion of luteinizing hormone and growth hormone in ovariectomized rats. Proc Natl Acad Sci USA. 1985;82:561–564. doi: 10.1073/pnas.82.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowley WR, Hassid A, Kalra SP. Neuropeptide Y enhances the release of luteinizing hormone (LH) induced by LH-releasing hormone. Endocrinology. 1987;120:941–945. doi: 10.1210/endo-120-3-941. [DOI] [PubMed] [Google Scholar]
- 6.Khorram O, Francis Pau K-Y, Spies HG. Bimodal effects of neuropeptide Y on hypothalamic release of gonadotropin-releasing hormone in conscious rabbits. Neuroendocrinology. 1987;45:290–297. doi: 10.1159/000124743. [DOI] [PubMed] [Google Scholar]
- 7.Crowley WR, Kalra SP. Neuropeptide Y stimulates the release of luteinizing hormone-releasing hormone from medial basal hypothalamus in vitro: Modulation by ovarian hormones. Neuroendocrinology. 1987;46:97–103. doi: 10.1159/000124804. [DOI] [PubMed] [Google Scholar]
- 8.Sabatino FD, Collins P, McDonald JK. Neuropeptide-Y stimulation of luteinizing hormone-releasing hormone secretion from the median eminence in vitro by estrogen-dependent and extracellular Ca2+-independent mechanisms. Endocrinology. 1989;124:2089–2098. doi: 10.1210/endo-124-5-2089. [DOI] [PubMed] [Google Scholar]
- 9.Sutton SW, Mitsugi N, Plotsky PM, et al. Neuropeptide Y(NPY): A possible role in the initiation of puberty. Endocrinology. 1988;123:2152–2154. doi: 10.1210/endo-123-4-2152. [DOI] [PubMed] [Google Scholar]
- 10.Guy J, Li S, Pelletier G. Studies on the physiological role and mechanism of action of neuropeptide Y in the regulation of luteinizing hormone secretion in the rat. Regul Pept. 1988;23:209–216. doi: 10.1016/0167-0115(88)90028-6. [DOI] [PubMed] [Google Scholar]
- 11.Sutton SW, Toyama TT, Otto S, et al. Evidence that neuropeptide Y (NPY) released into the hypophysial-portal circulation participates in priming gonadotropes to the effects of gonadotropin releasing hormone (GnRH) Endocrinology. 1988;123:1208–1210. doi: 10.1210/endo-123-2-1208. [DOI] [PubMed] [Google Scholar]
- 12.Wehrenberg WB, Corder R, Gaillard RC. A physiological role for neuropeptide Y in regulating the estrogen/progesterone induced luteinizing hormone surge in ovariectomized rats. Neuroendocrinology. 1989;49:680–682. doi: 10.1159/000125188. [DOI] [PubMed] [Google Scholar]