Abstract
Age-related hearing loss (AHL) is a universal feature of mammalian aging and is the most common sensory disorder in the elderly population. Experimental evidence suggests that mitochondrial dysfunction associated with reactive oxygen species (ROS) plays a central role in the aging process of cochlear cells. Although it is well established that mitochondria are the major source of ROS in the cell, specific molecular mechanisms of aging induced by ROS remain poorly characterized. Here we review the evidence that supports a central role for Bak-mediated mitochondrial apoptosis in AHL. We also propose that this mechanism may be of general relevance to age-related cell death in long-lived post-mitotic cells of multiple tissues, providing an opportunity for a targeted therapeutic intervention in human aging.
Keywords: Aging, Age-related hearing loss, Mitochondria, Oxidative stress, Antioxidants, Apoptosis, Bak, Cochlea, Alpha-lipoic acid, Coenzyme Q10
1. Introduction (age-related hearing loss and mitochondria)
Age-related hearing loss (AHL) is a universal feature of mammalian aging and is the most common sensory disorder in the elderly population. AHL affects more than 40% of people over 65 years of age in the US and is projected to afflict 28 million Americans by 2030 (Aging, 2009; Gates and Mills, 2005; Yamasoba et al., 2007). AHL is associated with an age-dependent loss of sensory hair cells, spiral ganglion (SG) neurons, and stria vascularis cells in the inner ear (Gates and Mills, 2005; Yamasoba et al., 2007). The progressive loss of these cells eventually leads to AHL in mammals because hair cells and cochlear neurons do not regenerate in these organisms.
AHL is thought to be the result of aging, oxidative damage, mitochondrial impairment, and environmental factors (Kokotas et al., 2007; Liu and Yan, 2007). Noise is the most well-documented environmental factor causing hearing loss. Outer hair cells are the primary lesion from noise exposure, and the accumulated effect of noise is thought to contribute to AHL (Liu and Yan, 2007). Ototoxic substances such as aminoglycoside antibiotics also increase susceptibility to AHL as these drugs can damage hair cells (Liu and Yan, 2007). Assuming that AHL is mechanistically similar between short-lived and long-lived mammals, it should be possible to understand basic mechanisms of aging in long-lived post-mitotic cells by understanding the molecular features that account for the different rates of progression of AHL in different species. The relatively early onset of AHL in rodents, its slow progression, and our ability to monitor its progression non-invasively, make AHL an ideal system to study basic mechanisms of aging and age-related diseases.
2. Human mitochondrial diseases associated with hearing loss
A central role for mitochondrial dysfunction in AHL is supported by the finding that a large number of genetic syndromes associated with hearing loss are due to defects in mitochondria (Kokotas et al., 2007). This observation suggests that cochlear cells are exquisitely sensitive to disturbances in energy metabolism, and that the well-reported mitochondrial decay associated with aging may selectively impact the cochlea. Diseases associated with a primary mitochondrial defect can be due to mutations in the mtDNA, or mutations in nuclear genes that encode factors that function in the mitochondria (Kujoth et al., 2007). Both types of mutations have been associated with progressive hearing loss (Table 1).
Table 1.
Human genetic disorders associated with both mitochondrial dysfunction and deafness.
| Disease | Gene (reference) | Age of onset (years) |
|---|---|---|
| mtDNA metabolism | ||
| ADOAD | OPA1, dynamin-related GTPase (Liguori et al., 2008; Mancuso et al., 2004) | ~30 |
| PEO | POLG1, mitochondrial DNA Polymerase (Hudson et al., 2008) | ~28 |
| Mitochondrial function | ||
| Wolfram syndrome 2 | ZCD2 (Amr et al., 2007) | ~10 |
| mtDNA mutations | ||
| MELAS/MERRF | A3243G, tRNALeu (Deschauer et al., 2001; Chinnery et al., 2000) | ~3 |
| MIDD | A3243G, tRNALeu (Laloi-Michelin et al., 2009) | ~48 |
| Non-syndromic deafness | A1555G, 12SrRNA (Malik et al., 2003; Prezant et al., 1993) | ~6 |
| Pigmentary retinopathy | G12183A, tRNAHis (Crimi et al., 2003) | ~11 |
MIDD, maternally inherited diabetes and deafness; MELAS, myoclonic epilepsy, lactic acidosis, and stroke-like episodes, PEO, progressive external ophtalmoplegia; ADOAD, autosomal dominant optic atrophy and deafness; MERRF, myoclonic epilepsy with ragged red fibers.
It is estimated that 20% of inherited post-lingual hearing loss is caused by mutations in the mtDNA genome (Kokotas et al., 2007) and only a few percent of cases of inherited deafness display maternal inheritance (Marazita et al., 1993). Mitochondrial genome screening in a large collection of French maternally inherited non-syndromic hearing loss suggests that approximately 30% of such cases are associated with inherited mtDNA mutations (Leveque et al., 2007). Importantly, although only a minority of cases of inherited deafness is likely to result from a primary mitochondrial defect, it is clear that mitochondrial dysfunction can lead to deafness, and presumably to AHL.
Mutations that impact mtDNA genomic stability, such as defects in the DNA polymerase γ (POLG) that maintains mtDNA replication fidelity (Filosto et al., 2003; Hudson et al., 2008), or the OPA1 gene (Liguori et al., 2008; Mancuso et al., 2004), which is involved in mitochondrial fission, lead to premature hearing loss. Wolfram Syndrome, a recessive autosomal disorder associated with diabetes, optic atrophy and deafness, has been recently shown to be due to mutations in ZCD2 (Amr et al., 2007). Mutation in Cisd2 in the mouse leads to the onset of Wolfram Syndrome 2 clearly associated with mitochondrial dysfunction, suggesting that the encoded protein, which localizes to the mitochondria, is important for mitochondrial function in affected tissues (Chen et al., 2009). There are also a number of well-characterized multisystem syndromes due to inherited mtDNA point mutations that are associated with deafness. These include MIDD (Laloi-Michelin et al., 2009), MELAS (Deschauer et al., 2001) and MERRF (Chinnery et al., 2000; Fischel-Ghodsian, 2003), which are each associated with multiple clinical phenotypes. However, similar to most phenotypes observed in mtDNA genetic disorders, deafness is not an obligatory clinical feature. Possibly, nuclear modifying genes, as well as the level of heteroplasmy of the mtDNA mutation in various tissues determine the range of clinically relevant phenotypes in an affected individual. Importantly, these genetic observations strongly suggest that alterations in mitochondrial function with age have the potential to play a major role in AHL.
3. Animal models of mitochondrial diseases associated with hearing loss
Most inbred mouse strains display at least some degree of AHL, and the age of onset of AHL is known to vary from 3 months in DBA/2J mice to over 20 months in CBA/CaJ mice (Zheng et al., 1999). The C57BL/6J mouse strain, which is widely used for aging research, displays the classic pattern of AHL by 12–15 months of age (Hunter and Willott, 1987; Keithley et al., 2004). Strains susceptible to early onset AHL are known to carry a specific mutation in the cadherin 23 gene (Cdh23), which encodes a component of the hair cell tip link (Keithley et al., 2004; Noben-Trauth et al., 2003; Ohlemiller, 2006). Johnson et al. (2001) screened reciprocal backcrosses of three inbred mouse strains, A/J, NOD/LtJ and SKH2/J that display AHL, and determined that mtDNA derived from the A/J strain exerted detrimental effects on hearing as compared with mtDNA from the CAST/Ei strain. The effect was only observed in mice homozygous for the A/J allele at the Ahl locus, and sequencing of mtDNA revealed that the effect was due to a single nucleotide insertion in the mtDNA tRNAArg gene. However, we note that inherited mtDNA mutations are only one factor in the development of AHL in A/J mice, since most of the early onset-hair cell loss in this strain appears to be due to a genetic interaction between the Cdh23 and Ahl4 loci (Zheng et al., 2009).
Direct evidence for mitochondrial dysfunction in AHL comes from the observation that mice engineered to carry a mutation (D257A) that disrupts the exonuclease domain of the mitochondrial DNA Polymerase γ show early onset of AHL (Kujoth et al., 2005; Someya et al., 2008). Mice carrying this mutator allele of POLG display a several hundred-fold increase in the level of point mutations in mtDNA (Vermulst et al., 2007), and this increased load of point mutations increases the levels of apoptotic cells in multiple tissues (Kujoth et al., 2005). DNA microarray analysis of cochleae from mitochondrial mutator mice was associated with transcriptional alterations consistent with impairment of energy metabolism, induction of apoptosis, cytoskeletal dysfunction, and hearing dysfunction (Someya et al., 2008). TUNEL staining and caspase-3 immunostaining analysis revealed that the levels of apoptotic markers were significantly increased in the cochleae of mitochondrial mutator mice compared to age-matched controls (Someya et al., 2008). DNA microarray analysis of the cochleae of DBA/2J mice, which show severe hearing loss by 8 months of age, is also consistent with a profound downregulation of genes involved in mitochondrial energy metabolism in AHL (Someya et al., 2007a). AHL-correlated genes that change in expression in the cochleae of 8-month-old DBA/2J mice were representative of several Gene Ontology categories linked to mitochondrial function, including the mitochondrial electron transport chain and oxidative phosphorylation. A striking observation was the downregulation of 31 genes encoding components of the mitochondrial respiratory chain complexes I, II, III, IV, and V in the cochlea (Someya et al., 2007a). Taken as a whole, our observations lead us to propose a model of how accumulation of mtDNA mutations impact cochlear function, whereby mtDNA mutations lead to mitochondrial dysfunction resulting in an associated impairment of energy metabolism, and the induction of an apoptotic program that leads to death of hair cells and neurons (Someya et al., 2009).
4. Evidence for a causal role of mitochondrial ROS in AHL
There is a growing body of evidence suggesting mitochondrial ROS contributes to AHL that is age-dependent and has no defining genetic basis. The free radical theory of aging postulates that aging is the result of accumulated oxidative damage caused by ROS (Beckman and Ames, 1998; Finkel and Holbrook, 2000; Harman, 1956; Shigenaga et al., 1994). It is now widely accepted that mitochondria are a major source of ROS and a major site of ROS-induced oxidative damage, and that ROS production increases with age (Balaban et al., 2005; Beckman and Ames, 1998; Shigenaga et al., 1994; Wallace, 2005). This theory is supported by the observations that over-expressing the mitochondrial antioxidant gene Sod2 (Sun et al., 2002) or the mitochondrial iron regulator protein frataxin (Runko et al., 2008) significantly increases longevity in Drosophila, while over-expressing a mitochondrially-targeted catalase gene (MCAT) results in reduced age-related pathology and moderately increases lifespan in mice (Schriner et al., 2005).
Oxidative damage caused by ROS has also been postulated to play a causal role in AHL (Darrat et al., 2007; Liu and Yan, 2007; Seidman, 2000; Seidman et al., 2000; Someya et al., 2009; Van Eyken et al., 2007; Yamasoba et al., 2007). Several studies have shown that ROS are generated in cochleae exposed to high intensity noise (Jacono et al., 1998; Ohlemiller et al., 1999). Age-related cochlear hair cell loss is enhanced in mice lacking the antioxidant enzyme Sod1 (McFadden et al., 1999), while mice lacking the antioxidant enzymes Gpx1 or Sod1 show enhanced susceptibility to noise-induced hearing loss (Fortunato et al., 2004; Ohlemiller et al., 2000). Moreover, oxidative protein damage increases with age in the cochleae of CBA mice (Jiang et al., 2007; Staecker et al., 2001). We have recently shown that overexpression of mitochondrially-targeted catalase results in reduced cochlear cell damage in mice (Someya et al., 2009). Specifically, we reported that the mean ABR hearing thresholds of middle-aged MCAT transgenic mice were significantly lower than those of age-matched wild-type mice at all the frequencies tested (Someya et al., 2009). In agreement with the ABR results, cell counting demonstrated that catalase overexpression reduced outer hair cell and inner hair cell loss. Furthermore, cochlear oxidative DNA damage increased during aging, and oxidative damage to DNA was reduced by mitochondrially-targeted catalase overexpression (Someya et al., 2009). Collectively, these findings suggest that mitochondrial ROS may play a causal role in AHL in mammals.
5. Evidence for a causal role of mitochondrial apoptosis in AHL
There is a growing body of evidence suggesting that an apoptosis program contributes to aging and age-related degenerative diseases (Dirks et al., 2006; Dirks and Leeuwenburgh, 2004; Kujoth et al., 2007; Someya et al., 2008, 2009). Apoptosis can occur through two major pathways: the intrinsic pathway, also known as the mitochondrial pathway, is initiated when the outer mitochondrial membrane loses its integrity, while the extrinsic pathway is initiated through ligand binding to cell surface receptors (Lindsten et al., 2000; Youle and Strasser, 2008). In mammals, mitochondria play a major role in apoptosis that is regulated by Bcl-2 family members (Youle and Strasser, 2008). Of the Bcl-2 family members, the pro-apoptotic proteins Bak and Bax have been proposed to play a central and sometimes redundant role in promoting mitochondrial-mediated apoptosis (Lindsten et al., 2000; Takeuchi et al., 2005).
Caloric restriction (CR), the only intervention known to retard several aspects of the aging process in multiple species (Sohal and Weindruch, 1996; Walford et al., 1987; Weindruch and Sohal, 1997), may retard aging in part by preventing apoptosis. In an animal model of Parkinson’s disease, CR lowers symptom severity and levels of apoptosis in neurons (Mattson, 2000). CR also reduces levels of caspase-3 and caspase-9 in the brain of aged rats, suggesting that CR is neuroprotective and that apoptosis may contribute to brain aging (Shelke and Leeuwenburgh, 2003). CR has also been proposed to promote the long-term survival of irreplaceable cells by SIRT1-mediated deacetylation of the DNA repair factor Ku70, causing it to sequester the pro-apoptotic factor Bax away from mitochondria (Cohen et al., 2004). The findings that the pro-apoptotic protein Bak is upregulated in the aging human brain (Kitamura et al., 1998), as well as in the hippocampus of Alzheimer’s disease patients (Obonai et al., 1998), suggest that neurons may be an important target of an age-related mitochondrial apoptotic program.
Several studies have shown that aging is associated with increased expression of apoptosis-associated genes such as the Bcl-2 family members Bak, Bax, and Bim in the cochlea of several strains of mice (Someya et al., 2007a, 2008; Tadros et al., 2008). Previous studies have also demonstrated that TUNEL-positive hair cells and SG neurons were distinctly evident in the cochlea of aged gerbils (Zheng et al., 1998) and mice (Usami et al., 1997). Moreover, CR slows the onset of AHL in mice, reduces the levels of apoptosis, and reduces the expression of the mitochondrial apoptosis activator gene Bak in the aged cochlea (Someya et al., 2007b), suggesting that Bak-mediated mitochondrial apoptosis may contribute to AHL.
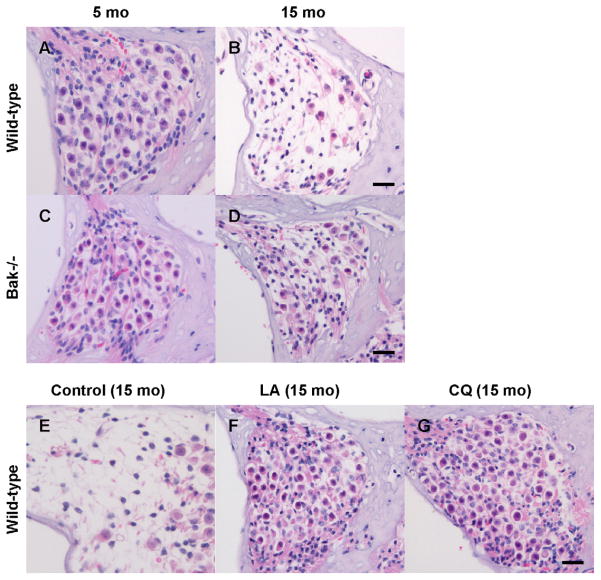
We have shown recently that deletion of the mitochondrial pro-apoptotic gene Bak prevents AHL in mice (Someya et al., 2009). We found that the ABR hearing thresholds of middle-aged Bak−/− mice were significantly lower than those of age-matched wild-type mice at all frequencies tested, but were not significantly different from those of young wild-type mice at the middle and high frequencies, indicating that Bak is required for the development of AHL (Someya et al., 2009). In agreement with the ABR results, cell counting demonstrated that Bak deficiency increased cochlear SG neuron survival (Fig. 1A–D) and outer hair cell survival. We also investigated whether age-related cochlear cell death was apoptotic and found that aging resulted in increased levels of TUNEL-positive cells in the wild-type cochlea, while levels of TUNEL-positive cells in the Bak−/− cochlea did not increase with age. Paraquat (PQ) is known to damage neurons and cochlear cells by generating ROS (Fei et al., 2008; Nicotera et al., 2004). We also reported that primary cochlear cells isolated from mice lacking Bak were resistant to PQ-induced cell death at all PQ concentrations tested (Someya et al., 2009). Furthermore, PQ-induced oxidative stress increased the expression of Bak mRNA in wild-type cells (Someya et al., 2009). Taken together, these findings suggest that mitochondrial apoptosis may play a causal role in AHL in mammals. We note that these findings do not exclude a role for the extrinsic apoptosis pathways or other pathways such as ER stress, because AHL is a multifactorial process.
Fig. 1.
Bak deficiency or antioxidant supplementation reduces cochlear neurodegeneration. (A–D) Neurons in the basal cochlear regions of wild-type and Bak−/− mice at 5 and 15 months of age. (E–G) Neurons in the basal cochlear regions of 15-month-old C57BL/6J mice fed control diet or diets supplemented with LA (alpha-lipoic acid), or CQ (coenzyme Q10) (Someya et al., 2009). Scale bar = 200 μm.
6. Dietary antioxidant supplementation interventions prevents AHL
If ROS plays a causal role in AHL, then it is likely that enhancing antioxidant defenses through antioxidant supplementation can reduce oxidative cochlear cell damage and delay the onset of AHL. In support of this hypothesis, supplementation with the antioxidant alpha-lipoic acid (LA) significantly delays the onset of AHL in Fisher 344 rats (Seidman et al., 2000) and in DBA/2J mice (Ahn et al., 2008). Several studies have also shown that supplementation with the antioxidant acetyl-L-carnitine delays the onset of AHL in Fisher 344 rats (Seidman et al., 2000) and Wistar rats (Derin et al., 2004). Seidman investigated the effects of vitamin C and vitamin E on AHL in Fisher 344 rats and found that supplementation with both vitamin C and E slows the progression of AHL in this animal model (Seidman, 2000).
We have recently investigated the effects of 17 antioxidant compounds including LA, acetyl-L-carnitine, beta-carotene, carnosine, coenzyme Q10 (CQ), curcumin, d-alpha-tocopherol, epigallocatechin gallate, gallic acid, lutein, lycopene, melatonin, N-acetyl-L-cysteine (NAC), proanthocyanidin, quercetin, resveratrol, and tannic acid on AHL in C57BL/6J mice (Someya et al., 2009). All animals were fed the antioxidants orally under conditions of controlled caloric intake, and the dietary regimen was maintained for 11 months starting from 4 months of age. We found that the mean ABR hearing thresholds from mice fed LA, CQ, or NAC were significantly lower at the high frequency than those of control diet-fed mice, and that these interventions prevented age-related cochlear SG neuron death (Fig. 1E–G). LA and NAC are thiol compounds that have been shown to reduce mitochondrial ROS production and associated mitochondrial dysfunction (Banaclocha, 2001; Hart et al., 2004; Palaniappan and Dai, 2007), while CQ is an essential component of the mitochondrial electron transfer chain and acts as a mitochondrial antioxidant (Sohal and Forster, 2007). Interestingly, we found that antioxidants that do not selectively target mitochondria did not delay the onset of AHL at all the frequencies tested (Someya et al., 2009). Together, these results suggest that a diet rich in specific antioxidants that selectively improves the mitochondrial antioxidant defense system can prevent cochlear neuron loss and retard AHL.
7. A model for the central mechanism of ROS in age-related cell death and aging
We have proposed a model of AHL that involves ROS-induced and Bak-mediated mitochondrial apoptosis, which may be of wide relevance to the aging process of multiple tissues in mammals (Someya et al., 2009) (Fig. 2). There is a growing body of evidence suggesting that oxidative damage and associated cell death contributes to the development of Parkinson’s disease, Alzheimer’s disease, and other age-associated neurodegenerative diseases (Darrat et al., 2007; Liu and Yan, 2007; Mattson, 2000; Sohal and Weindruch, 1996; Someya et al., 2009; Van Eyken et al., 2007; Weindruch and Sohal, 1997; Yamasoba et al., 2007). As discussed earlier, tissues such as brain and cochlea which consist of post-mitotic cells are particularly susceptible to oxidative damage since extensive cell loss in these non-regenerating tissues leads to permanent tissue dysfunction.
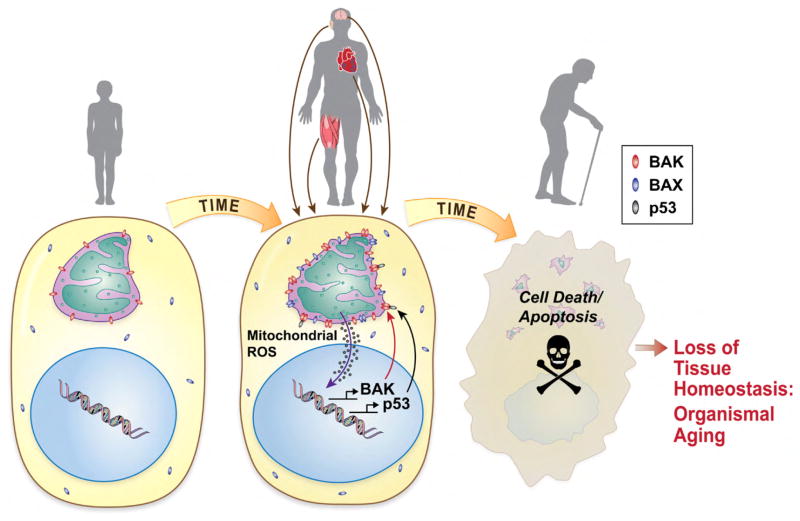
Fig. 2.
Potential role of mitochondrial apoptosis in aging of long-lived cells. During aging, mitochondrial ROS production steadily increases, leading to DNA damage and the activation of a p53-mediated transcriptional response. p53 transcriptional targets include pro-apoptotic genes such as Bak and Bax. p53 also directly triggers mitochondrial apoptosis by binding to and promoting the oligomerization of pro-apoptotic Bak protein in the outer mitochondrial membrane. Chronic activation of this pathway is likely to negatively impact tissues dependent on non-regenerating long-lived cells, such as the cochlea, brain, and heart.
We also propose that a nuclear, pro-apoptotic signaling pathway is likely to play a key role in this pathway. It is well known that the nuclear transcription factor p53 is activated by DNA damage and that activation of p53 can trigger apoptosis in a wide range of cell types including neurons (Culmsee and Mattson, 2005). In response to cell stress, p53 rapidly translocates to mitochondria (Erster et al., 2004) and directly binds to Bak and induces its oligomerization, leading to cytochrome c release and eventually to cell death (Leu et al., 2004; Mihara et al., 2003). We have previously reported that p53-induced apoptotic genes are induced in multiple tissues with aging (Edwards et al., 2007). In agreement with the role for p53 in aging, p53 is activated in hair cells following ototoxic drug exposure (Zhang et al., 2003), while deletion of p53 protects hair cells from the same drug-induced cell death (Cheng et al., 2005). Therefore, we propose that in response to oxidative DNA damage caused by mitochondria-derived ROS in the aged cochlea and other target tissues, p53 may translocate to mitochondria and activate Bak, leading to Bak-mediated mitochondrial apoptosis (Fig. 2).
An important conclusion derived from our studies of the role of ROS and mitochondrial apoptosis in AHL is that cells may not need to be irreversibly damaged by ROS in order to enter the mitochondrial apoptotic program. This key conclusion is supported by the observation that Bak−/− mice do not display cochlear cell loss and display normal hearing at middle age, despite the fact that these animals have no evidence of reduced ROS (Someya et al., 2009). Presumably, the level of ROS that is produced in cochlear cells during aging is sufficient to trigger the Bak-mediated apoptotic program, but not sufficient to impair cellular function. Thus, the cell loss associated with AHL is an active process that can be blocked by Bak inhibition, in the absence of deleterious effects to the target tissue. If this paradigm is applicable to other tissues impacted by cell loss during aging, a significant component of the aging process may be pharmacologically blocked by improving the mitochondrial antioxidant defense system and by blocking mitochondrial apoptosis.
References
- Administration on Aging, A.o. Administration on Aging, Aging Statistics Web Site. 2009. [Google Scholar]
- Ahn JH, Kang HH, Kim TY, Shin JE, Chung JW. Lipoic acid rescues DBA mice from early-onset age-related hearing impairment. Neuroreport. 2008;19:1265–1269. doi: 10.1097/WNR.0b013e328308b338. [DOI] [PubMed] [Google Scholar]
- Amr S, Heisey C, Zhang M, Xia XJ, Shows KH, Ajlouni K, Pandya A, Satin LS, El-Shanti H, Shiang R. A homozygous mutation in a novel zinc-finger protein, ERIS, is responsible for Wolfram syndrome 2. Am J Hum Genet. 2007;81:673–683. doi: 10.1086/520961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Banaclocha MM. Therapeutic potential of N-acetylcysteine in age-related mitochondrial neurodegenerative diseases. Med Hypotheses. 2001;56:472–477. doi: 10.1054/mehy.2000.1194. [DOI] [PubMed] [Google Scholar]
- Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- Chen YF, Kao CH, Chen YT, Wang CH, Wu CY, Tsai CY, Liu FC, Yang CW, Wei YH, Hsu MT, et al. Cisd2 deficiency drives premature aging and causes mitochondria-mediated defects in mice. Genes Dev. 2009;23:1183–1194. doi: 10.1101/gad.1779509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13:343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- Chinnery PF, Elliott C, Green GR, Rees A, Coulthard A, Turnbull DM, Griffiths TD. The spectrum of hearing loss due to mitochondrial DNA defects. Brain. 2000;123 (Pt 1):82–92. doi: 10.1093/brain/123.1.82. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;16:305. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Crimi M, Galbiati S, Perini MP, Bordoni A, Malferrari G, Sciacco M, Biunno I, Strazzer S, Moggio M, Bresolin N, Comi GP. A mitochondrial tRNA (His) gene mutation causing pigmentary retinopathy and neurosensorial deafness. Neurology. 2003;8:1200. doi: 10.1212/01.wnl.0000055865.30580.39. [DOI] [PubMed] [Google Scholar]
- Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem Biophys Res Commun. 2005;331:761–777. doi: 10.1016/j.bbrc.2005.03.149. [DOI] [PubMed] [Google Scholar]
- Darrat I, Ahmad N, Seidman K, Seidman MD. Auditory research involving antioxidants. Curr Opin Otolaryngol Head Neck Surg. 2007;15:358–363. doi: 10.1097/MOO.0b013e3282efa641. [DOI] [PubMed] [Google Scholar]
- Derin A, Agirdir B, Derin N, Dinc O, Guney K, Ozcaglar H, Kilincarslan S. The effects of L-carnitine on presbyacusis in the rat model. Clin Otolaryngol Allied Sci. 2004;29:238–241. doi: 10.1111/j.1365-2273.2004.00790.x. [DOI] [PubMed] [Google Scholar]
- Deschauer M, Muller T, Wieser T, Schulte-Mattler W, Kornhuber M, Zierz S. Hearing impairment is common in various phenotypes of the mitochondrial DNA A3243G mutation. Arch Neurol. 2001;58:1885–1888. doi: 10.1001/archneur.58.11.1885. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Hofer T, Marzetti E, Pahor M, Leeuwenburgh C. Mitochondrial DNA mutations, energy metabolism and apoptosis in aging muscle. Ageing Res Rev. 2006;5:179–195. doi: 10.1016/j.arr.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol Med. 2004;36:27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Edwards MG, Anderson RM, Yuan M, Kendziorski CM, Weindruch R, Prolla TA. Gene expression profiling of aging reveals activation of a p53-mediated transcriptional program. BMC Genomics. 2007;8:80. doi: 10.1186/1471-2164-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erster S, Mihara M, Kim RH, Petrenko O, Moll UM. In vivo mitochondrial p53 translocation triggers a rapid first wave of cell death in response to DNA damage that can precede p53 target gene activation. Mol Cell Biol. 2004;24:6728–6741. doi: 10.1128/MCB.24.15.6728-6741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei Q, McCormack AL, Di Monte DA, Ethell DW. Paraquat neurotoxicity is mediated by a Bak-dependent mechanism. J Biol Chem. 2008;283:3357–3364. doi: 10.1074/jbc.M708451200. [DOI] [PubMed] [Google Scholar]
- Filosto M, Mancuso M, Nishigaki Y, Pancrudo J, Harati Y, Gooch C, Mankodi A, Bayne L, Bonilla E, Shanske S, et al. Clinical and genetic heterogeneity in progressive external ophthalmoplegia due to mutations in polymerase gamma. Arch Neurol. 2003;60:1279–1284. doi: 10.1001/archneur.60.9.1279. [DOI] [PubMed] [Google Scholar]
- Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Fischel-Ghodsian N. Mitochondrial deafness. Ear Hear. 2003;24:303–313. doi: 10.1097/01.AUD.0000079802.82344.B5. [DOI] [PubMed] [Google Scholar]
- Fortunato G, Marciano E, Zarrilli F, Mazzaccara C, Intrieri M, Calcagno G, Vitale DF, La Manna P, Saulino C, Marcelli V, Sacchetti L. Paraoxonase and superoxide dismutase gene polymorphisms and noise-induced hearing loss. Clin Chem. 2004;50:2012–2018. doi: 10.1373/clinchem.2004.037788. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hart AM, Terenghi G, Kellerth JO, Wiberg M. Sensory neuroprotection, mitochondrial preservation, and therapeutic potential of N-acetyl-cysteine after nerve injury. Neuroscience. 2004;125:91–101. doi: 10.1016/j.neuroscience.2003.12.040. [DOI] [PubMed] [Google Scholar]
- Hudson G, Amati-Bonneau P, Blakely EL, Stewart JD, He L, Schaefer AM, Griffiths PG, Ahlqvist K, Suomalainen A, Reynier P, et al. Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain. 2008;131:329–337. doi: 10.1093/brain/awm272. [DOI] [PubMed] [Google Scholar]
- Hunter KP, Willott JF. Aging and the auditory brainstem response in mice with severe or minimal presbycusis. Hear Res. 1987;30:207–218. doi: 10.1016/0378-5955(87)90137-7. [DOI] [PubMed] [Google Scholar]
- Jacono AA, Hu B, Kopke RD, Henderson D, Van De Water TR, Steinman HM. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear Res. 1998;117:31–38. doi: 10.1016/s0378-5955(97)00214-1. [DOI] [PubMed] [Google Scholar]
- Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28:1605–1612. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KR, Zheng QY, Bykhovskaya Y, Spirina O, Fischel-Ghodsian N. A nuclear-mitochondrial DNA interaction affecting hearing impairment in mice. Nat Genet. 2001;27:191–194. doi: 10.1038/84831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keithley EM, Canto C, Zheng QY, Fischel-Ghodsian N, Johnson KR. Age-related hearing loss and the ahl locus in mice. Hear Res. 2004;188:21–28. doi: 10.1016/S0378-5955(03)00365-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Shimohama S, Kamoshima W, Ota T, Matsuoka Y, Nomura Y, Smith MA, Perry G, Whitehouse PJ, Taniguchi T. Alteration of proteins regulating apoptosis, Bcl-2, Bcl-x, Bax, Bak, Bad, ICH-1 and CPP32, in Alzheimer’s disease. Brain Res. 1998;780:260–269. doi: 10.1016/s0006-8993(97)01202-x. [DOI] [PubMed] [Google Scholar]
- Kokotas H, Petersen MB, Willems PJ. Mitochondrial deafness. Clin Genet. 2007;71:379–391. doi: 10.1111/j.1399-0004.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- Kujoth GC, Bradshaw PC, Haroon S, Prolla TA. The role of mitochondrial DNA mutations in mammalian aging. PLoS Genet. 2007;3:e24. doi: 10.1371/journal.pgen.0030024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- Laloi-Michelin M, Meas T, Ambonville C, Bellanne-Chantelot C, Beaufils S, Massin P, Vialettes B, Gin H, Timsit J, Bauduceau B, et al. The clinical variability of maternally inherited diabetes and deafness is associated with the degree of heteroplasmy in blood leukocytes. J Clin Endocrinol Metab. 2009;94:3025–3030. doi: 10.1210/jc.2008-2680. [DOI] [PubMed] [Google Scholar]
- Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- Leveque M, Marlin S, Jonard L, Procaccio V, Reynier P, Amati-Bonneau P, Baulande S, Pierron D, Lacombe D, Duriez F, et al. Whole mitochondrial genome screening in maternally inherited non-syndromic hearing impairment using a microarray resequencing mitochondrial DNA chip. Eur J Hum Genet. 2007;15:1145–1155. doi: 10.1038/sj.ejhg.5201891. [DOI] [PubMed] [Google Scholar]
- Liguori M, La Russa A, Manna I, Andreoli V, Caracciolo M, Spadafora P, Cittadella R, Quattrone A. A phenotypic variation of dominant optic atrophy and deafness (ADOAD) due to a novel OPA1 mutation. J Neurol. 2008;255:127–129. doi: 10.1007/s00415-008-0571-x. [DOI] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Yan D. Ageing and hearing loss. J Pathol. 2007;211:188–197. doi: 10.1002/path.2102. [DOI] [PubMed] [Google Scholar]
- Malik SG, Pieter N, Sudoyo H, Kadir A, Marzuki S. Prevalence of the mitochondrial DNA A1555G mutation in sensorineural deafness patients in island Southeast Asia. J Hum Genet. 2003;48:480–483. doi: 10.1007/s10038-003-0056-9. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Filosto M, Bellan M, Liguori R, Montagna P, Baruzzi A, DiMauro S, Carelli V. POLG mutations causing ophthalmoplegia, sensorimotor polyneuropathy, ataxia, and deafness. Neurology. 2004;62:316–318. doi: 10.1212/wnl.62.2.316. [DOI] [PubMed] [Google Scholar]
- Marazita ML, Ploughman LM, Rawlings B, Remington E, Arnos KS, Nance WE. Genetic epidemiological studies of early-onset deafness in the U.S. school-age population. Am J Med Genet. 1993;46:486–491. doi: 10.1002/ajmg.1320460504. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Nicotera TM, Ding D, McFadden SL, Salvemini D, Salvi R. Paraquat-induced hair cell damage and protection with the superoxide dismutase mimetic m40403. Audiol Neurootol. 2004;9:353–362. doi: 10.1159/000081284. [DOI] [PubMed] [Google Scholar]
- Noben-Trauth K, Zheng QY, Johnson KR. Association of cadherin 23 with polygenic inheritance and genetic modification of sensorineural hearing loss. Nat Genet. 2003;35:21–23. doi: 10.1038/ng1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obonai T, Mizuguchi M, Takashima S. Developmental and aging changes of Bak expression in the human brain. Brain Res. 1998;783:167–170. doi: 10.1016/s0006-8993(97)01361-9. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK. Contributions of mouse models to understanding of age- and noise-related hearing loss. Brain Res. 2006;1091:89–102. doi: 10.1016/j.brainres.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol. 2000;1:243–254. doi: 10.1007/s101620010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- Palaniappan AR, Dai A. Mitochondrial ageing and the beneficial role of alpha-lipoic acid. Neurochem Res. 2007;32:1552–1558. doi: 10.1007/s11064-007-9355-4. [DOI] [PubMed] [Google Scholar]
- Prezant TR, Agapian JV, Bohlman MC, Bu X, Oztas S, Qiu WQ, Arnos KS, Cortopassi GA, Jaber L, Rotter JI, et al. Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet. 1993;4:289. doi: 10.1038/ng0793-289. [DOI] [PubMed] [Google Scholar]
- Runko AP, Griswold AJ, Min KT. Overexpression of frataxin in the mitochondria increases resistance to oxidative stress and extends lifespan in Drosophila. FEBS Lett. 2008;582:715–719. doi: 10.1016/j.febslet.2008.01.046. [DOI] [PubMed] [Google Scholar]
- Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- Seidman MD. Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope. 2000;110:727–738. doi: 10.1097/00005537-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Khan MJ, Bai U, Shirwany N, Quirk WS. Biologic activity of mitochondrial metabolites on aging and age-related hearing loss. Am J Otol. 2000;21:161–167. doi: 10.1016/s0196-0709(00)80003-4. [DOI] [PubMed] [Google Scholar]
- Shelke RR, Leeuwenburgh C. Lifelong caloric restriction increases expression of apoptosis repressor with a caspase recruitment domain (ARC) in the brain. Faseb J. 2003;17:494–496. doi: 10.1096/fj.02-0803fje. [DOI] [PubMed] [Google Scholar]
- Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Forster MJ. Coenzyme Q, oxidative stress and aging. Mitochondrion. 2007;7 (Suppl):S103–S111. doi: 10.1016/j.mito.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Xu J, Kondo K, Ding D, Salvi RJ, Yamasoba T, Rabinovitch PS, Weindruch R, Leeuwenburgh C, Tanokura M, Prolla TA. Age-related hearing loss in C57BL/6J mice is mediated by Bak-dependent mitochondrial apoptosis. Proc Natl Acad Sci USA. 2009 doi: 10.1073/pnas.0908786106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Kujoth GC, Pugh TD, Weindruch R, Tanokura M, Prolla TA. The role of mtDNA mutations in the pathogenesis of age-related hearing loss in mice carrying a mutator DNA polymerase gamma. Neurobiol Aging. 2008;29:1080–1092. doi: 10.1016/j.neurobiolaging.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Prolla TA, Tanokura M. Genes encoding mitochondrial respiratory chain components are profoundly down-regulated with aging in the cochlea of DBA/2J mice. Brain Res. 2007a;1182:26–33. doi: 10.1016/j.brainres.2007.08.090. [DOI] [PubMed] [Google Scholar]
- Someya S, Yamasoba T, Weindruch R, Prolla TA, Tanokura M. Caloric restriction suppresses apoptotic cell death in the mammalian cochlea and leads to prevention of presbycusis. Neurobiol Aging. 2007b;28:1613–1622. doi: 10.1016/j.neurobiolaging.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Staecker H, Zheng QY, Van De Water TR. Oxidative stress in aging in the C57B16/J mouse cochlea. Acta Otolaryngol. 2001;121:666–672. doi: 10.1080/00016480152583593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zhu X, Frisina RD. Apoptosis-related genes change their expression with age and hearing loss in the mouse cochlea. Apoptosis. 2008;13:1303–1321. doi: 10.1007/s10495-008-0266-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ. Essential role of BAX, BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci USA. 2005;102:11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usami S, Takumi Y, Fujita S, Shinkawa H, Hosokawa M. Cell death in the inner ear associated with aging is apoptosis? Brain Res. 1997;747:147–150. doi: 10.1016/s0006-8993(96)01243-7. [DOI] [PubMed] [Google Scholar]
- Van Eyken E, Van Camp G, Van Laer L. The complexity of age-related hearing impairment: contributing environmental and genetic factors. Audiol Neurootol. 2007;12:345–358. doi: 10.1159/000106478. [DOI] [PubMed] [Google Scholar]
- Vermulst M, Bielas JH, Kujoth GC, Ladiges WC, Rabinovitch PS, Prolla TA, Loeb LA. Mitochondrial point mutations do not limit the natural lifespan of mice. Nat Genet. 2007;39:540–543. doi: 10.1038/ng1988. [DOI] [PubMed] [Google Scholar]
- Walford RL, Harris SB, Weindruch R. Dietary restriction and aging: historical phases, mechanisms and current directions. J Nutr. 1987;117:1650–1654. doi: 10.1093/jn/117.10.1650. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Sohal RS. Seminars in medicine of the Beth Israel Deaconess Medical Center. Caloric intake and aging. N Engl J Med. 1997;337:986–994. doi: 10.1056/NEJM199710023371407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasoba T, Someya S, Yamada C, Weindruch R, Prolla TA, Tanokura M. Role of mitochondrial dysfunction and mitochondrial DNA mutations in age-related hearing loss. Hear Res. 2007;226:185–193. doi: 10.1016/j.heares.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience. 2003;120:191–205. doi: 10.1016/s0306-4522(03)00286-0. [DOI] [PubMed] [Google Scholar]
- Zheng QY, Ding D, Yu H, Salvi RJ, Johnson KR. A locus on distal chromosome 10 (ahl4) affecting age-related hearing loss in A/J mice. Neurobiol Aging. 2009;30:1693–1705. doi: 10.1016/j.neurobiolaging.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res. 1999;130:94–107. doi: 10.1016/s0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Ikeda K, Nakamura M, Takasaka T. Endonuclease cleavage of DNA in the aged cochlea of Mongolian gerbil. Hear Res. 1998;126:11–18. doi: 10.1016/s0378-5955(98)00138-5. [DOI] [PubMed] [Google Scholar]