Abstract
Purpose
Patients with hormone receptor–negative breast cancer generally do not benefit from endocrine-targeted therapies. However, a subset with androgen receptor (AR) expression is predicted to respond to antiandrogen therapies. This phase II study explored bicalutamide in AR-positive, estrogen receptor (ER), and progesterone receptor (PgR)-negative metastatic breast cancer.
Experimental Design
Tumors from patients with ER/PgR-negative advanced breast cancer were tested centrally for AR [immunohistochemistry (IHC) > 10% nuclear staining considered positive]. If either the primary or a metastatic site was positive, patients were eligible to receive the AR antagonist bicalutamide at a dose of 150 mg daily. Clinical benefit rate (CBR), the primary endpoint, was defined as the total number of patients who show a complete response (CR), partial response (PR), or stable disease (SD) > 6 months; secondary endpoints included progression-free survival (PFS) and toxicity. Correlative studies included measurement of circulating endocrine markers and IHC surrogates for basal-like breast cancer.
Results
Of 424 patients with ER/PgR-negative breast cancer, 12% tested AR-positive. The 6-month CBR was19%[95% confidence interval (CI), 7%–39%]for bicalutamide. The median PFS was 12 weeks (95% CI, 11–22 weeks). Bicalutamide was well-tolerated with no grade 4/5 treatment-related adverse events observed.
Conclusion
AR was expressed in 12% of patients with ER/PgR-negative breast cancer screened for this trial. The CBR of 19% observed with bicalutamide shows proof of principle for the efficacy of minimally toxic androgen blockade in a select group of patients with ER/PgR-negative, AR-positive breast cancer.
Introduction
Estrogen and the estrogen receptor (ER) have been well-recognized and highly effective targets for the treatment of ER [and progesterone receptor (PgR)]-positive breast cancers. Yet patients with breast cancer who truly lack expression of the ER and PgR have not traditionally derived benefit from conventional endocrine therapies such as selective ER modulators or aromatase inhibitors. For those patients with triple-negative breast cancer (TNBC), whose tumors also lack overexpression or amplification of HER2, standard palliative systemic treatment options are limited to cytotoxic chemotherapy agents. Patients diagnosed with advanced TNBCs may respond initially to chemotherapy but the duration of response is often short and there is a higher likelihood of visceral metastases, rapidly progressive disease, and inferior survival outcomes compared to the other subtypes (1–3).
A comprehensive molecular analysis of 99 archived primary breast tumors at Memorial Sloan-Kettering Cancer Center (MSKCC; New York, NY) previously identified a subset of ER/PgR-negative cancers that associated with ER (+) tumors and expressed a molecular profile suggestive of active hormonal signaling without expression of ER or PgR. This subset represented about 22% of ER/PgR(−) cancers and had a transcription profile that resembled molecular apocrine or luminal AR (4–7). Further evaluation confirmed the absence of ER and revealed overexpression of the androgen receptor (AR). The functional role of AR was established by the AR-dependent, estrogen-independent growth observed in response to synthetic androgen, estrogen, and ER antagonist exposure using an MDA-MB-453 cell line representative of this subset of ER/PgR-negative breast cancer (4).
On the basis of the observations above, we hypothesized that AR inhibition would have antitumor activity for patients with AR(+) ER/PgR(−) advanced breast cancer. Bicalutamide is an oral, nonsteroidal, AR antagonist that is approved by the U.S. Food and Drug Administration for use in combination with luteinizing hormone-releasing hormone (LHRH) analogs for the treatment of metastatic prostate cancer. Data for the use of antiandrogens inwomen have been reported from small studies when used as treatment of hirsutism, polycystic ovarian syndrome, or ovarian cancer in women with elevated ovarian androgen production at baseline. However, these data about the effect of antiandrogens on circulating androgens and estrogens are limited and, in one case, confounded by coadministration of an LHRH agonist (8–11).
We conducted a multicenter phase II, proof-of-concept trial testing bicalutamide for the treatment of women with AR(+) ER/PgR(−) metastatic breast cancer (MBC). We measured serial levels of free and total testosterone, estradiol, and sex hormone–binding globulin (SHBG). Cytokeratin-5/6 and EGF receptor (EGFR) were tested to apply Nielsen criteria as a surrogate for basal-like breast cancer (12).
Materials and Methods
Study design
This open-label, single-arm study was initially opened at MSKCC and later expanded to 7 additional centers through the Translational Breast Cancer Research Consortium (TBCRC). The primary objective was to evaluate the efficacy of oral bicalutamide, 150 mg/d for the treatment of women with AR+ER/PgR-negative MBC. The primary endpoint was clinical benefit rate (CBR), defined as the total number of patients who show a complete response (CR), partial response (PR), or stable disease (SD) > 6 months. Secondary endpoints included progression-free survival (PFS), safety and toxicity assessments, and correlative science studies. Enrollment required 2 steps: (i) consent to determine AR status, which was permitted while on another therapy for breast cancer, followed by (ii) consent to the therapeutic portion of the trial for patients with centrally confirmed AR (+) ER/PgR(−) MBC.
Patient eligibility
Four hundred and fifty-two patients with histologically confirmed ER/PgR-negative [immunohistochemistry (IHC) ≤10%] MBC consented for AR testing at participating TBCRC institutions from March 2007 through January 2012. Patients were eligible for the therapeutic portion of the trial if they had ER/PgR-negative unresectable locally advanced or metastatic disease and formalin-fixed, paraffin-embedded tumor from either the primary or a metastatic site tested positive for AR (IHC > 10% nuclear staining) using a commercially available antibody (Dako, AR 441; dilution: 1:300). Initially local testing at study sites was permitted with central confirmation at MSKCC. Nine of 43 patients who elected to have local AR testing were AR(+) locally. Four of the 9 patients were AR(−) on central testing. Given this discordance, as of August 2010, all testing was conducted centrally at MSKCC for standardization of methods.
Additional eligibility criteria included measurable or nonmeasurable disease per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0, Eastern Cooperative Oncology Group performance status < 2, and adequate hepatic, renal, and hematologic function. There was no limit on prior therapies, except prior trastuzumab was required for patients with HER2-positive disease (IHC 3+ or FISH > 2.0). Exclusion criteria included chemotherapy within 2 weeks and investigational therapy within 3 weeks. The institutional review boards of the participating centers approved this protocol. All patients gave written informed consent (NCT00468715).
Treatment
Bicalutamide 150 mg was administered orally on a continuous daily schedule. Patients were treated until disease progression or unacceptable adverse events. A maximum of 2 dose reductions for grade ≥3 toxicity were allowed (100 and 50 mg). A maximum of 2 weeks was permitted for treatment delays due to toxicity.
Patient evaluation
Patients were evaluated for toxicity at the time of each 4-week treatment cycle, according to the National Cancer Institute Common Toxicity Criteria, version 3.0 (NCI-CTC v3.0). Radiographic response was evaluated every 12 weeks with radiographic scans that were reviewed at each site by a designated study radiologist, according to RECIST.
Statistical analysis
This study was designed as a single-stage, phase II which required a total of 28 patients to discriminate between true CBRs of ≤5% and ≥20% at a type I error of 5% and a type II error of 16%. If 4 or more patients had a CR, PR, or SD > 6 months, bicalutamide would be considered to have activity in this patient population and would merit further clinical study.
PFS was defined from start of therapy to progression of disease or last date of follow-up and analyzed using Kaplan–Meier methods. Response rates were calculated with 95% exact confidence intervals (CI). Toxicities were summarized using NCI-CTC v3.0, and the maximum grade per patient was used as the summary measure.
Correlative studies
Peripheral blood was obtained at baseline, start of cycle 2 (C2) and end of study (EOS) to measure total and free testosterone (T), estradiol (E), and SHBG using a commercially available assay. Summary statistics such as mean, median, and proportions were calculated for these values. Wilcoxon signed-rank test was conducted to compare baseline to C2 and EOS values. Percent change for each endocrine biomarker from baseline was examined to account for variations in baseline values.
Unstained slides or tissue blocks representative of malignant AR(+), ER/PR(−) tissue were stained for CK5/6 (Dako D5/16 B4; dilution 1:200) and EGFR (Invitrogen 31G7; dilution 1:100) using standard immunoperoxidase techniques by the core facility at Memorial Sloan-Kettering Cancer Center. Staining intensity was reviewed and scored by the study pathologist as follows: percent cells staining and the intensity of staining (0, no staining; 1+, mild; 2+, moderate; and 3+, strong intensity). Data will be presented in tabular form and analysis is primarily exploratory and hypothesis generating.
Results
Patient demographics
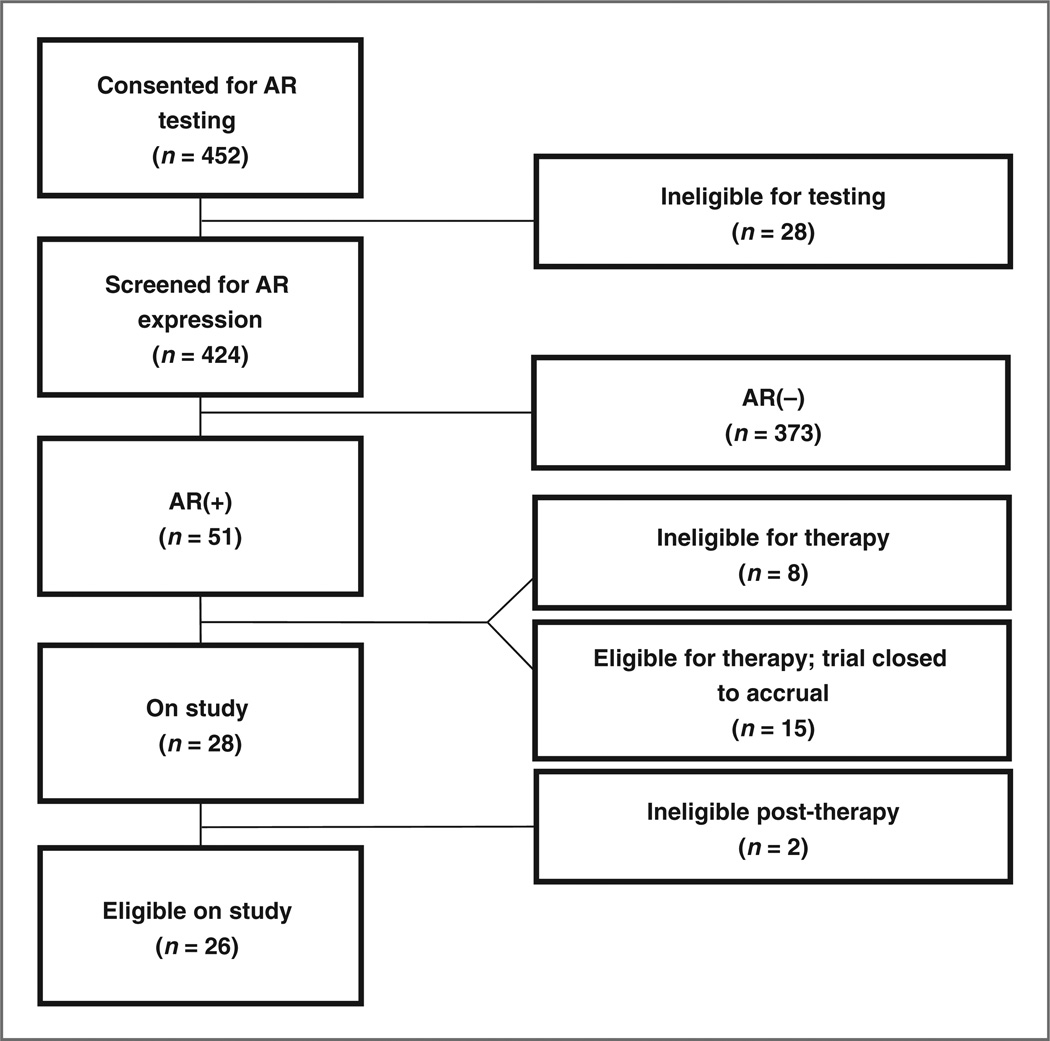
Twelve percent (51 of 424) of screened patients tested AR (+). Fewer than 10 of the patients who tested negative for AR expression had staining in the range from 1% to 10% by IHC. Thirty-two of the 424 patient samples tested were HER2-positive (7.5%) and 7 were positive for both AR and HER2. Of the 452 patients consented for testing, 28 did not undergo testing as they had either insufficient tissue (n = 17), tested ER(+) (n = 4), died before testing (n = 2), withdrew consent (n = 3), consented after the close of trial (n = 1), or did not have confirmed metastatic disease (n = 1). Eight patients whose tumors tested AR+ were ineligible for treatment due to testing ER(+) centrally (2), developing a second primary cancer (1), not meeting all of the prespecified eligibility criteria (2), or having a significant decline in performance status (3). Fifteen patients were eligible for bicalutamide but continued on effective treatment and thus were not enrolled on the therapeutic portion of the trial before it reached its accrual goal. Twenty-eight patients were treated on study (Fig. 1). Two patients who initiated bicalutamide were later found to be ER(+) and were removed from study—one at the time of PD and the other at the time of central confirmation. Patient characteristics are shown in Table 1. Study participants had a median age of 66 years (range, 41–83) and ECOG performance status (median, 0; range, 0–1). The majority had visceral metastases and received a median of 1 (0–8) prior line of chemotherapy for metastatic disease.
Figure 1.
CONSORT diagram.
Table 1.
Characteristics of patients with AR(+) tumors
| Eligible patients treated with bicalutamide (N = 26) |
Patients not treated with bicalutamide/ineligible (N = 25) |
|
|---|---|---|
| Characteristics | No. of pts (n) | No. of pts (n) |
| ER/PgR | ||
| 0% | 22 | 16 |
| 1%–10% | 4 | 5 |
| >10% | NA | 4 |
| HER2-positive | 1 | 6 |
| Site of AR testing | ||
| Primary | 17 | 14 |
| Metastatic | 9 | 11 |
| Median age (range), y | 66 (41–83) | 58 (30–76) |
| Median ECOG PS (range) | 0 (0–1) | NAa |
| Sites of metastases | ||
| Visceral metastases | 15 | NAa |
| Thoracic/pleural (9), hepatic (8), gastric (1), adrenal (2) | ||
| Bone/soft tissue/lymph node metastases | 21 | NAa |
| Measurable | 22 | NAa |
| Nonmeasurable | 4 | NAa |
| Prior chemotherapy for early-stage breast cancer | ||
| Adjuvant | 13 | NAa |
| Neoadjuvant | 7 | NAa |
| Number of patients with de novo MBC | 2 | NAa |
| Prior chemotherapy for MBC | ||
| Median number of regimens (range) | 1 (0–8) | NAa |
NOTE: HER2-positive defined as IHC 3+ or FISH > 2.
TBCRC011 involved a 2-step enrollment process that allowed patients with ER/PgR(−) breast cancer that was metastatic to be tested for AR without enrolling in the therapeutic portion of the trial. Therefore, the patients who tested AR-positive but did not consent to therapy did not have clinical data collected aside from ER, PR, HER2, age, and site of disease tested for AR.
Efficacy
Twenty-six study participants with AR(+) ER/PgR(−) MBC were evaluable for the primary endpoint. Five patients had stable disease >6 months (number of cycles completed: 6, 8, 10+, 13, 57+) as their best response on treatment. There were no confirmed complete or partial responses yielding a CBR of 19% (95% CI, 7%–39%) in the target population (n = 26). In an intention-to-treat analysis, a CBR of 18% (95% CI, 6%–37%) was observed. One patient with unresectable, locally advanced, ER/PgR/HER2-negative breast cancer following neoadjuvant anthracycline- and taxane-based therapy had stable disease per RECIST after 6 months on study but was then able to undergo curative breast surgery. At the time of mastectomy, she was found to have HER2(+) cancer and subsequently received 1 year of trastuzumab. Clinicopathologic features of the 5 patients deriving clinical benefit from therapy are shown in Table 2. Two patients had stable disease < 6 months, and 19 patients had disease progression as best response. We delivered a median of 3 cycles of therapy (2–57+) and 2 patients remain on treatment after 57+ and 11+ cycles. Median PFS was 12 weeks (95% CI, 11–22 weeks; Fig. 2).
Table 2.
Characteristics of patients with clinical benefit
| Pts with clinical benefit on bicalutamide |
AR% | ER% | PgR% | HER2 | Site of testing |
Site of metastases |
Prior therapy LABC/MBC |
DOR on prior therapy, wks |
DOR on bicalutamide, wks |
|---|---|---|---|---|---|---|---|---|---|
| #1 | 10–20 | 1 | 0 | Neg | P | LN | 0 | NA | 231+ |
| #2 | >80 | 3 | 0 | Neg | Met | GI | 0 | NA | 54 |
| #3 | >80 | 0 | 0 | −/+ | P | Breast LN | 1 | NR | 25 |
| #4 | >90 | 0 | 0 | Neg | P | LN Bone | 1 | 158 | 35 |
| #5 | >50 | 0 | 0 | Neg | P | LN Bone | 1 | 15 | 43+ |
Abbreviations: DOR, duration of response; GI, gastrointestinal; LABC, locally advanced breast cancer; LN, lymph node; Met, metastasis; NA, not applicable; NR, no response; P, primary tumor.
Figure 2.
PFS on oral daily bicalutamide 150 mg.
Adverse events
The most common, possibly drug-related toxicities of any grade were fatigue (6 of 28), hot flashes (6 of 28), limb edema (6 of 28), aspartate aminotransferase (AST) elevation (7 of 28), and alkaline aminotransferase (ALT) elevation (6 of 28). Grade 1toxicities reported in more than 10% of patients on study are shown in Table 3. There were few grade 2 or 3 adverse events associated with bicalutamide (Table 3). All grade 3 liver enzyme abnormalities (elevation in AST, bilirubin, and alkaline phosphatase) were documented in 1 patient with known liver metastases who had progressed on therapy. Thus, it remains unclear whether these laboratory findings were attributable to bicalutamide therapy or disease progression. There were no grade 4 or 5 events or treatment-related serious adverse events. Two patients had dose delays as a result of grade 2 AST elevations later determined to be related to disease progression in the liver. One patient had a protocol-stipulated dose reduction to 100 mg for cerebrovascular ischemia that was later determined to be related to poorly controlled hypertension rather than study drug; she remains on therapy with stable disease.
Table 3.
Bicalutamide-related adverse events per NCI CTCAE version 3
| Toxicity | Grade 1 (n) |
Grade 2 (n) |
Grade 3 (n) |
|---|---|---|---|
| AST | 5 | 1 | 1 |
| ALT | 5 | 1 | |
| Hyperbilirubinemia | 1 | ||
| Alkaline phosphatase | 1 | 1 | |
| Nausea | 1 | ||
| Pain | |||
| Headache, back, other | 3 | ||
| Breast | 3 | ||
| Limb | 3 | ||
| Constipation | 1 | ||
| Anorexia | 3 | 1 | |
| Fatigue | 5 | 1 | |
| Hemoglobin | 1 | ||
| Vaginal dryness | 1 | ||
| Diarrhea | 3 | ||
| Hot flashes | 6 | ||
| Limb edema | 6 |
NOTE: Grade 1 toxicities reported in >10% of patients (n = 28). No grade 4 or 5 events or treatment-related serious adverse events observed.
Correlative endpoints
Serum hormone levels
Ninety-two percent of evaluable patients at baseline were postmenopausal. Evaluable patients were numbered 26, 26, and 19 at baseline, C2, and EOS, respectively. Median free and total T, E, and SHBG are shown in Supplementary Fig. S1 as boxplots. Nodiscernible patterns of change in serum hormone levels were observed in response to bicalutamide therapy. There was no difference in median percent change observed across time points for each endocrine biomarker examined (Supplementary Table S1 and Supplementary Fig. S2).
IHC characterization of basal-like breast cancer
Four patients (15%) had sufficient invasive tumor adequate for correlative IHC following testing of ER, PR, and AR. The results of CK5/6, EGFR, HER2, and ER staining are shown in Table 4. One of the 4 patients had clinical benefit to bicalutamide and lacked expression of basal-like cytokeratins and EGFR. Two nonresponders showed expression of both CK5/6 and EGFR, suggestive of a basal-like subtype rather than an AR-dependent one.
Table 4.
Use of Nielsen criteria as a surrogate for basal-like breast cancer
| ER | HER2 | CK5/6 | EGFR | Best response |
|
|---|---|---|---|---|---|
| Patient 1 | 0% | 1+ | 0% | 0% | POD < 3 mo |
| Patient 2 | 0% | 0 | 0% | 0% | SD > 6 mo |
| Patient 3 | 0% | 0 | 10% | 10% | POD 3 mo |
| Patient 4 | 0% | 0 | 25% | <10% | POD 3 mo |
NOTE: Absence of basal-like markers may correlate with response to antiandrogen therapy.
Discussion
On the basis of the results of the screening stage of this trial, AR is expressed in 12% of patients with ER/PgR-negative breast cancer. Our patient population largely represented TNBC, with the majority of patients having HER2 normal cancers. In this selected subset of patients with AR (+) ER/PgR(−) MBC, this study met its prespecified endpoint, showing a CBR of 19% for bicalutamide 150 mg by mouth daily. This therapy was well-tolerated with the most common treatment-related adverse events including fatigue, hot flashes, limb edema, and transaminase elevations.
This is the first clinical trial to report activity of antiandrogen therapy in advanced breast cancer and establishes the potential of targeting AR in AR-dependent, ER-independent disease. Previous studies that examined the use of flutamide, an oral antiandrogen, for the treatment of MBC concluded a lack of meaningful antitumor activity. However, these small phase II trials were conducted in unselected patient populations irrespective of AR, ER, or PgR (13, 14).
Overall, AR is expressed in about 77% of breast cancers and coexpression with ER is common (15–17). In contrast, the literature suggests expression ofARin about20%to50% of ER-negative breast cancers (4, 15). This wide range may be attributed to the retrospective nature of these studies and the biases inherent to this type of analysis, variability in patient selection from archival specimens (i.e., primary tumors vs. metastases; coexpression of HER2), differing assays for AR testing, or other factors not yet realized. Our prospective screening experience in this study of more than 450 patients with ER/PgR(−) cancers found that about 12% expressed nuclear staining of AR in excess of 10%. The IHC methods on study used the same commercially available antibody from the preclinical studies that informed the design of our trial. Although the observed AR rate of 12% is lower than that previously reported, it is consistent with more recent reports from a triple-negative breast cancer dataset in which 10% of more than 170 breast cancer primary tumors tested AR(+) (18).
We observed clinical features in this population of patients with AR(+) ER/PgR(−) breast cancers that differ from those that typically characterize TNBC. The median age of 66 years was higher than the mean age at diagnosis for patients with TNBC, which is usually more than a decade earlier (~53 years of age; ref. 3). Sites of metastases in our study often included nodal, soft tissue and bone, whereas TNBCs have been noted to have patterns of spread preferentially to brain, lungs, and other viscera (19–21).
While correlative science studies are ongoing to investigate potential genomic predictors of response to antiandrogen therapy, we observed that those patients who derived clinical benefit from bicalutamide received treatment in the first- or second-line setting. All patients had substantial AR expression, measuring 20% to >90%. One patient who had prolonged stable disease for >12 months had weak ER expression measuring 3%. At the time of study accrual, ASCO/CAP guidelines had not yet lowered the threshold defining ER positivity to its current level of 1% or greater (22). We elected to maintain eligibility criteria as previously specified due to the recognized heterogeneity within TNBCs. Preclinical cell line models from Doane and colleagues showed estrogen independence in this molecular subtype; therefore, the impact of weak ER expression for this 1 patient is unclear. In addition, recent preclinical data showed that bicalutamide did not inhibit estrogen-mediated proliferation of ER+ breast cancer cells (23).
Recent reports suggest that TNBCs may be divided into as many as 6 subtypes based on molecular profiling, 1 of which is defined as luminal AR and marked by hormone-regulated pathways with expression of higher levels of AR mRNA than the other subtypes (6). We hypothesized that the absence of basal-like breast cancer IHC markers would predict for response to antiandrogen therapy as this AR-dependent subtype of breast cancer is distinct from the basal-like subtypes (BL1 and BL2) described by Lehmann and colleagues (6). Our findings are consistent with this hypothesis, albeit limited by the small numbers available for analysis. One patient with prolonged stable disease lacked expression of CK5/6, EGFR, and HER2, whereas two-thirds of the nonresponders expressed the Nielsen criteria suggestive of the basal-like subtype.
One of the patients with response to therapy had unresectable locally advanced ER/PgR(−), HER2 1–2+/FISH 1.1 breast cancer following neoadjuvant anthracycline and taxane-based therapy. After 6 months of study treatment, she had tumor reduction sufficient to enable definitive breast surgery but did not meet RECIST for partial response. At the time of mastectomy, she was found to have HER2 overexpression (IHC 3+) and went on to receive 1 year of adjuvant trastuzumab off-study. Interestingly, AR expression has been reported in about 50% to 60% of HER2-positive breast cancers (15, 24–26), and others have found a significant number of ER(−)/HER2(+) breast tumors that express AR and exhibit androgen-dependent growth. It has also been shown that androgen stimulates tumor cell growth through Wnt and HER2 signaling pathways via AR-dependent upregulation of WNT7B and HER3 (27). Functionally significant cross-talk between AR and HER2 signaling pathways in ER(−) breast cancer has also been shown in molecular apocrine cell lines by inhibition of heregulin-mediated growth with the use of flutamide. Synergy with combined use of flutamide and the anti-ErbB2 AG825 has been shown with respect to cell proliferation and apoptosis, suggesting a potential clinical advantage to combination therapy for AR(+), ER(−), HER2(+) breast cancers (28).
Median PFS in this study was 12 weeks, a rate comparable to that reported for single-agent or combination chemotherapy in multiple recent trials conducted in the triple-negative population (29–31). The disease stabilization observed in 5 patients on this study is encouraging and suggests a signal of activity for androgen blockade in AR (+) ER/PgR(−) breast cancer. However, given the generally aggressive clinical course associated with TNBCs, these findings may alternatively reflect identification of a more indolent subtype of the disease characterized by AR expression. This possibility is supported by the observation that the clinical characteristics of this cohort appeared to differ from the traditional TNBC clinical features as described above. This remains an area of investigation to be answered by future trial designs.
This study highlights the challenges of drug development in the era of "precision medicine." Targets may be rare, responses may not meet criteria per RECIST, and individual centers may not be able to complete such studies. At the same time, this study is proof of concept for the use of targeting AR when positive in patients with ER/PgR(−) breast cancers. Although this subgroup represents a small percentage of all breast cancers (15% of breast cancers are triple-negative, 12% of these are AR+, meaning that only 2% of all patients have tumors in this subset), the absolute numbers are nonetheless clinically meaningful. Two percent of the more than 200,000 women diagnosed with breast cancer in the United States in 2012 yields 4,000 potential patients (32). For the subset of these who develop metastatic disease, the opportunity to receive minimally toxic, oral, endocrine therapy with clinical benefit is a new treatment option.
There are additional challenges to the development of AR targeting agents in women with MBC. As in other settings, there are no validated biomarkers of response to antiandrogen therapy. To that point, our exploration of serum hormone levels did not appear to offer use as a pharmacodynamic marker of bicalutamide activity (33). The full potential of targeting AR in both ER(−) and ER(+) breast cancers is not yet explored, and the possibility of dual pathway inhibition of androgen and HER2, MEK, or PI3K/AKT as suggested by preclinical trials is unexplored clinically (6, 27, 28, 34, 35). Perhaps equally challenging will be the feasibility of drug development through conventional randomized phase III trials for the small population of patients expressing this target. To this end, trials are ongoing to test the safety and feasibility of next-generation, novel androgen-targeted therapies such as enzalutamide (NCT01597193) in this patient population, but new regulatory approaches to establishing their efficacy may be needed.
Supplementary Material
Translational Relevance.
Genome-wide transcriptional analysis identified a subset of androgen receptor (AR) positive, estrogen receptor (ER)/progesterone receptor (PgR)-negative breast cancers. In vitro studies confirmed the functional role of AR and showed that growth could be abrogated by antiandrogens. We conducted this multicenter phase II trial of the oral AR inhibitor bicalutamide in patients with AR(+) ER/PR(−) metastatic breast cancer to test the hypothesis that androgen blockade could benefit patients with androgen-dependent, estrogen-independent cancer. This is the first clinical trial to report activity of antiandrogen therapy in breast cancer and establishes the potential of targeting AR in ER(−) disease.
Acknowledgments
V. Stearns has received research funding from Pfizer, Novartis, Abraxis, and Merck. M. Danso owns stock in ImmunoGen, Inc. and has received an honorarium from Amgen.
Grant Support
This trial was supported in part by the Translational Breast Cancer Research Consortium, Breast Cancer Alliance, and AstraZeneca.
Footnotes
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions
Conception and design: A. Gucalp, V. Stearns, A.S. Doane, M. Danso, M.E. Moynahan, S. Patil, C.A. Hudis, T.A. Traina
Development of methodology: A. Gucalp, D. Giri, C.A. Hudis, T.A. Traina
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): A. Gucalp, S.J. Isakoff, J.N. Ingle, M.C. Liu, L.A. Carey, K.L. Blackwell, H. Rugo, A. Forero-Torres, L.F. Momen, D. Giri, K.N. Feigin, C.A. Hudis, T.A. Traina
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): A. Gucalp, K.L. Blackwell, H. Rugo, A. Forero-Torres, A.S. Doane, D. Giri, S. Patil, C.A. Hudis, T.A. Traina
Writing, review, and/or revision of the manuscript: A. Gucalp, S. Tolaney, S.J. Isakoff, J.N. Ingle, M.C. Liu, L.A. Carey, K.L. Blackwell, H. Rugo, A. Forero-Torres, V. Stearns, A.S. Doane, M. Danso, M.E. Moynahan, J.M. Gonzalez, D. Giri, S. Patil, C.A. Hudis, T.A. Traina
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): K.L. Blackwell, L. Nabell, L.F. Momen, J.M. Gonzalez, A. Akhtar, D. Giri, C.A. Hudis, T.A. Traina
Study supervision: A. Gucalp, S. Tolaney, K.L. Blackwell, L. Nabell, L.F. Momen, A. Akhtar, C.A. Hudis, T.A. Traina
References
- 1.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109:1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 2.Kassam F, Enright K, Dent R, Dranitsaris G, Myers J, Flynn C, et al. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin Breast Cancer. 2009;9:29–33. doi: 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- 3.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 4.Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25:3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- 5.Farmer P, Bonnefoi H, Becette V, Tubiana-Hulin M, Fumoleau P, Larsimont D, et al. Identification of molecular apocrine breast tumours by microarray analysis. Oncogene. 2005;24:4660–4671. doi: 10.1038/sj.onc.1208561. [DOI] [PubMed] [Google Scholar]
- 6.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121:2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanga S, Broom BM, Cristini V, Edgerton ME. Gene expression meta-analysis supports existence of molecular apocrine breast cancer with a role for androgen receptor and implies interactions with ErbB family. BMC Med Genomics. 2009;2:59. doi: 10.1186/1755-8794-2-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levine D, Park K, Juretzka M, Esch J, Hensley M, Aghajanian C, et al. A phase II evaluation of goserelin and bicalutamide in patients with ovarian cancer in second or higher complete clinical disease remission. Cancer. 2007;110:2448–2456. doi: 10.1002/cncr.23072. [DOI] [PubMed] [Google Scholar]
- 9.De Leo V, Lanzetta D, D'Antona D, la Marca A, Morgante G. Hormonal effects of flutamide in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83:99–102. doi: 10.1210/jcem.83.1.4500. [DOI] [PubMed] [Google Scholar]
- 10.Muderris II, Bayram F, Ozcelik B, Guven M. New alternative treatment in hirsutism: bicalutamide 25 mg/day. Gynecol Endocrinol. 2002;16:63–66. [PubMed] [Google Scholar]
- 11.Bahceci M, Tuzcu A, Canoruc N, Tuzun Y, Kidir V, Aslan C. Serum C-reactive protein (CRP) levels and insulin resistance in non-obese women with polycystic ovarian syndrome, and effect of bicalutamide on hirsutism, CRP levels and insulin resistance. Horm Res. 2004;62:283–287. doi: 10.1159/000081973. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
- 13.Perrault DJ, Logan DM, Stewart DJ, Bramwell VH, Paterson AH, Eisenhauer EA. Phase II study of flutamide in patients with metastatic breast cancer. A National Cancer Institute of Canada Clinical Trials Group study. Invest New Drugs. 1988;6:207–210. doi: 10.1007/BF00175399. [DOI] [PubMed] [Google Scholar]
- 14.Zhao TP, He GF. A phase II clinical trial of flutamide in the treatment of advanced breast cancer. Tumori. 1988;74:53–56. doi: 10.1177/030089168807400109. [DOI] [PubMed] [Google Scholar]
- 15.Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses' Health Study. Mod Pathol. 2011;24:924–931. doi: 10.1038/modpathol.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez-Angulo AM, Stemke-Hale K, Palla SL, Carey M, Agarwal R, Meric-Berstam F, et al. Androgen receptor levels and association with PIK3CA mutations and prognosis in breast cancer. Clin Cancer Res. 2009;15:2472–2478. doi: 10.1158/1078-0432.CCR-08-1763. [DOI] [PubMed] [Google Scholar]
- 17.Loibl S, Muller BM, von Minckwitz G, Schwabe M, Roller M, Darb-Esfahani S, et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;130:477–487. doi: 10.1007/s10549-011-1715-8. [DOI] [PubMed] [Google Scholar]
- 18.Gucalp A, Gupta G, Patil S, Wen Y, Akram M, Brogi E, et al. P4-02-04: androgen receptor (AR) expression in a cohort of patients (pts) with triple negative breast cancer (TNBC) Cancer Res. 2012;71 P4-02-4. [Google Scholar]
- 19.Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113:2638–2645. doi: 10.1002/cncr.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin NU, Vanderplas A, Hughes ME, Theriault RL, Edge SB, Wong Y, et al. Clinicopathological features and sites of recurrence according to breast cancer subtype in the National Comprehensive Cancer Network (NCCN) ASCO Meeting Abstracts. 2009;27:543. [Google Scholar]
- 21.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–448. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 22.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cochrane D, Bernales S, Jacobsen B, D'Amato N, Guerrero J, Gomez F, et al. Preclinical evaluation of enzalutamide in breast cancer models. Cancer Res. 2012;72 P2-14-02. [Google Scholar]
- 24.Park S, Koo J, Park HS, Kim JH, Choi SY, Lee JH, et al. Expression of androgen receptors in primary breast cancer. Ann Oncol. 2010;21:488–492. doi: 10.1093/annonc/mdp510. [DOI] [PubMed] [Google Scholar]
- 25.Park S, Koo JS, Kim MS, Park HS, Lee JS, Kim SI, et al. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol. 2011;22:1755–1762. doi: 10.1093/annonc/mdq678. [DOI] [PubMed] [Google Scholar]
- 26.Yu Q, Niu Y, Liu N, Zhang JZ, Liu TJ, Zhang RJ, et al. Expression of androgen receptor in breast cancer and its significance as a prognostic factor. Ann Oncol. 2011;22:1288–1294. doi: 10.1093/annonc/mdq586. [DOI] [PubMed] [Google Scholar]
- 27.Ni M, Chen Y, Lim E, Wimberly H, Bailey ST, Imai Y, et al. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20:119–131. doi: 10.1016/j.ccr.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naderi A, Hughes-Davies L. A functionally significant cross-talk between androgen receptor and ErbB2 pathways in estrogen receptor negative breast cancer. Neoplasia. 2008;10:542–548. doi: 10.1593/neo.08274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baselga J, Gomez P, Awada A, Greil R, Braga S, Climent MA, et al. The addition of cetuximab to cisplatin increases overall response rate (ORR) and progression-free survival (PFS) in metastatic triple-negative breast cancer (TNBC): results of a randomized phase II study (BALI-1) Ann Oncol. 2010;21:viii96–viii121. [Google Scholar]
- 30.Carey LA, Rugo HS, Marcom PK, Mayer EL, Esteva FJ, Ma CX, et al. TBCRC 001: randomized phase II study of cetuximab in combination with carboplatin in stage IV triple-negative breast cancer. J Clin Oncol. 2012;30:2615–2623. doi: 10.1200/JCO.2010.34.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med. 2011;364:205–214. doi: 10.1056/NEJMoa1011418. [DOI] [PubMed] [Google Scholar]
- 32.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 33.Gucalp A, Tolaney S, Isakoff S, Ingle J, Liu M, Carey L, et al. Endocrine biomarkers in response to AR-inhibition with bicalutamide for the treatment of AR(+), ER/PR(−) metastatic breast cancer (MBC) (TBCRC011) Cancer Res. 2012;72 P6-05-2. [Google Scholar]
- 34.Naderi A, Chia KM, Liu J. Synergy between inhibitors of androgen receptor and MEK has therapeutic implications in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13:R36. doi: 10.1186/bcr2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naderi A, Liu J. Inhibition of androgen receptor and Cdc25A phosphatase as a combination targeted therapy in molecular apocrine breast cancer. Cancer Lett. 2010;298:74–87. doi: 10.1016/j.canlet.2010.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.