Abstract
Purpose
To provide the long-term outcomes of patients treated with fractionated conformal radiotherapy (FCRT) for presumed optic nerve sheath meningiomas (ONSMs).
Patients and Methods
Between 1995 and 2002, 9 patients with a presumed ONSM were treated with FCRT at our institution. The indications for FCRT were significant visual dysfunction at presentation, progression of visual dysfunction during a period of observation, tumor growth documented by sequential imaging, or a combination of these findings. In 2 patients, FCRT was performed as adjuvant therapy, and in 7, it was the initial and primary treatment.
Results
Of the 9 patients, 6 were women and 3 were men, with a mean age of 47 years. All 9 patients had evidence of optic nerve dysfunction in the affected eye, characterized by reduced visual acuity, a visual field defect, and a relative afferent pupillary defect. In addition, 2 patients had proptosis and 1 had diplopia. The mean follow-up period was 98 ± 31.7 months (median, 90; range, 61–151). After FCRT, the visual function improved in the 7 patients who had undergone FCRT as the primary treatment. However, 2 patients who were blind in their affected eye at FCRT remained blind. In 4 of the 7 patients with improvement, the improvement was documented within 1–3 months after FCRT. The tumor control rate was 100%. Proptosis and diplopia also regressed in 100% of patients. At 2 years after FCRT, 1 patient had developed radiation retinopathy.
Conclusion
The results of our study have shown that FCRT is a safe and effective treatment of ONSMs, affording satisfactory long-term tumor control, good functional outcome, and low treatment morbidity. FCRT should be considered the treatment of choice for patients with presumed ONSMs for whom the treatment has been deemed appropriate.
Keywords: Optic nerve sheath, Meningioma, Stereotactic fractionated radiotherapy, Anterior visual pathway
INTRODUCTION
Optic nerve sheath meningiomas (ONSMs) represent 1–2% of all meningiomas, one-third of all primary optic nerve or nerve sheath tumors, and 1.7 % of all orbital tumors (1–4). These tumors can be primary, arising from the arachnoid cap cells surrounding the orbital or canalicular portions of the optic nerve, or secondary, resulting from extension along the nerve of a tumor arising from the planum sphenoidale or tuberculum sellae (5–7). Just as with intracranial meningiomas, middle-age women are affected most often. Most ONSMs involve only one optic nerve; however, in approximately 5% of cases, usually young adults with neurofibromatosis type 2, they are bilateral (3, 8–12).
Most ONSMs exhibit a meningotheliomatous or transitional histologic type and are classified as benign according to the World Health Organization grading system. They typically show an indolent growth pattern, producing slowly progressive loss of vision associated with signs of optic neuropathy, including loss of color vision, an increasing visual field defect, a relative afferent pupillary defect (unless bilateral), and, often, mild swelling of the optic disc. Depending on the size and shape of the lesion, some patients have mild proptosis or limitations in eye movement that can be associated with diplopia, or both. With time, some patients will develop retinochoroidal (optociliary) shunt vessels, and all patients eventually develop pallor of the optic disk in the affected eye.
The optimal management of ONSMs remains somewhat controversial. Because of their intimate circumferential relationship to the optic nerve and its vascular supply, most ONSMs are impossible to resect without causing permanent blindness. Thus, until recently, a conservative approach of observation has been the preferred option, followed by resection of the tumor and the optic nerve, once severe visual loss has occurred. In recent years, fractionated radiotherapy has been recommended for presumed ONSMs (13). However, about 33% of patients who undergo this treatment develop complications, primarily visual loss from radiation retinopathy or optic neuropathy, or both (13). The advent of stereotactic three-dimensional conformal techniques has allowed the administration of optimal radiation doses to a more localized area, thus sparing unnecessary radiation to the neighboring structures (14). Although several small series using fractionated conformal radiotherapy (FCRT) have reported short-term improvement or at least stabilization of visual function and tumor size, long-term data regarding visual prognosis and complications are not available (7). We report the long-term outcomes of 9 consecutive patients with ONSMs who were treated with FCRT at a single institution.
PATIENTS AND METHODS
Patient population
Between 1995 and 2002, 9 patients with presumed ONSMs underwent FCRT at our institution. All patients were referred because of significant visual loss at presentation, progression of visual dysfunction during a period of observation, imaging evidence of an increase in tumor size or extent, or a combination of these indications.
Of the 9 patients, 6 were women and 3 were men, with a mean age of 47 years (range, 39–59). All patients had one eye involved. All 9 patients had evidence of optic nerve dysfunction in the affected eye, characterized by reduced visual acuity, a visual field defect, and a relative afferent pupillary defect. In addition, 2 patients had proptosis, 1 had diplopia, 1 had eyelid swelling, and 2 complained of orbital pain. Two patients with secondary ONSMs had undergone partial surgical resection before FCRT was performed. In these 2 patients, World Health Organization Grade I was confirmed by histologic examination. In the remaining 7 patients, the diagnosis was determined from results of a neuro-ophthalmologic examination combined with diagnostic imaging, including magnetic resonance imaging (MRI), computed tomography (CT) or both, because biopsy was considered inappropriate, given the significant risk of iatrogenic visual loss. Thus, 2 patients underwent FCRT as adjuvant treatment for histologically proven ONSM, and 7 patients underwent FCRT as the initial and primary treatment.
Treatment protocol
All patients were treated with highly conformal RT, generally using Brain Scan software, version 5.3, on a BrainLAB Treatment Planning System (BrainLAB, Feldkirchen, Germany). The neurosurgeon and radiation oncologist contoured the volume using CT fused with T1-weighted MRI with gadolinium (Figure 1). No fat suppression images were used. Daily localization was used for all patients. The BrainLAB mask and frame immobilization system was used for patients treated stereotactically, and those treated with a conformal beam were immobilized using Aquaplast masks. FCRT consisted of a 6-week course during which the patient received a median dose of 50.4 Gy in either 28 fractions of 1.8 Gy each or 30 fractions of 1.8 Gy each. One patient received a total dose of 36 Gy administered in six fractions of 6 Gy each. The doses were specified by the maximal isodose line (usually 80–98% of the isodose line) to completely encompass the tumor volume. The neurosurgeon outlined the gross tumor volume. The margins were generally 2 mm for creation of the clinical target volume and an additional 1–2 mm for the planning tumor volume. This approach was selected because of the good therapeutic ratio previously demonstrated with this dosing regimen using less conformal technologies. Our goal was to administer optimal radiation doses through the use of this fractionated regimen and conformal technology, preventing radiation toxicity from unnecessary exposure to surrounding structures and providing high long-term tumor control.
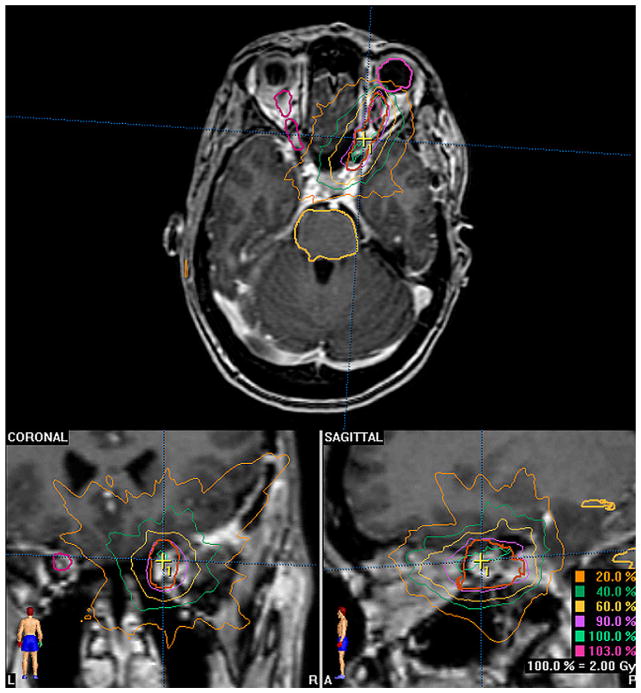
Fig. 1.
Sample treatment plan for optic nerve sheath meningioma.
Treatment evaluation
After FCRT, all patients underwent serial clinical and imaging evaluations to assess the visual status and tumor size and shape. A visual response to treatment was defined clinically by improvement in at least two measures of visual function. The best-corrected visual acuity was measured using an illuminated Snellens chart located 20 ft. from the patient. If the patients could not see the largest letters on the chart, they were moved forward until the letters were identified correctly. For patients who could not identify the letters when they were within 1 ft. of the chart, visual acuity was indicated as “finger counting,” “hand movements,” “light perception,” or “no light perception.” Color vision was determined using 10 Hardy-Rand-Rittler pseudoisochromatic plates. Visual fields were performed by static perimetry using a Humphrey automated perimeter or kinetic perimetry using a Goldmann perimetry, or both devices. Patients whose visual function was too poor to test using either of these devices were tested by asking the patient to identify the number of fingers held up in the center of the field and in the four quadrants of the field (superior temporal, superior nasal, inferior temporal, and inferior nasal). The tumor response to treatment was assessed by MRI or CT, or both. Disease progression was defined as visual deterioration or an increase in tumor size or extent on imaging.
RESULTS
All patients had ≥5 years of follow-up (range, 61–151 months; mean 98 ± 31.7; median 90). Tumor growth control at the last follow-up visit was obtained for all patients, at which point 7 had stable imaging findings and 2 had had a decrease in tumor size (Table 1).
Table 1.
Patient characteristics
| Pt. No. |
Age (y), Gender |
Eye | Clinical presentation |
VA | Treatment modality |
Radiation dose (dose @ isodose × fractions) |
Optic nerve isodose (range) |
Retinal isodose (range) |
Visual outcome |
Radiologic outcome |
Toxicity | Follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46, F | Right | VA loss | Finger counting at 3 ft. | FCRT | 6 Gy @ 80% × 6 = 36 Gy | 40–95% | 10–20% | 4/200, improved visual field and VA | Tumor regression | Eyelid edema | 151 |
| 2 | 47, F | Left | Visual field loss, diminished color vision | 20/20 | FCRT | 1.8 Gy × 30 = 54 Gy (isodose NA) | NA | NA | Improved visual field | Stable | None | 132 |
| 3 | 39, M | Left | Visual field loss | 20/20 | FCRT | 1.8 Gy @ 90% × 30 = 54 Gy | 90% (1.90–2.01 Gy) | 50% | Improved visual field | Stable | Radiation-induced retinopathy | 121 |
| 4 | 44, F | Right | Right blindness, orbital pain, headache, proptosis | — | FCRT | 1.8 Gy @ 90% × 30 = 54 Gy | 95–100% (1.90–2.0 Gy) | NA | Stable visual examination findings | Stable | None | 107 |
| 5 | 59, F | Left | Visual field and VA loss | 20/300 | FCRT | 1.8 Gy @ 98% × 30 = 54 Gy | 98% (1.81–1.84 Gy) | 98% (0.04–1.81 Gy) | 20/40, improved visual field and VA | Stable | None | 90 |
| 6 | 45, M | Left | Visual loss | 20/200 | FCRT | 1.8 Gy × 30 = 54 Gy (isodose NA) | NA | NA | Improved | Stable | None | 78 |
| 7 | 45, M | Left | Visual field loss | 20/20 | FCRT | 1.8 Gy @ 90% × 30 = 54 Gy | 90% | 98% | Improved visual field | Stable | None | 80 |
| 8 | 44, F | Left | Visual loss | 20/40 | FCRT | 1.8 Gy @ 98% × 28 = 50.4 Gy | 98% (1.83–1.85 Gy) | 72–98% (1.34–1.82 Gy) | Stable | Stable | None | 62 |
| 9 | 46, F | Right | Visual field and VA loss | 20/30 | FCRT | 1.8 Gy @ 90% × 28 = 50.4 Gy | 90% (1.58–2.10 Gy) | 40% (0.08–1.36 Gy) | 20/25, improved visual field and VA | Tumor regression | None | 61 |
Abbreviations: Pt. No. = patient number; VA = visual acuity; FCRT = functional conformal radiotherapy; NA = not available.
Seven patients (77.8%) had improvement in vision, and for 2 patients (22.2%), all visual parameters remained stable. The 2 patients without any improvement had been blind before FCRT was performed. Of these 2 patients, 1 had symptoms of headache associated with regrowth of the ONSM that had been partially resected before FCRT.
Of the 6 patients with initial visual acuity loss, 4 (66.7%) experienced improvement in visual acuity. One of these patients (Patient 5) experienced a dramatic improvement in visual acuity within 3 months after RT (pre-FCRT visual acuity, 20/300; post-FCRT visual acuity, 20/40). Of the patients whose pre-FCRT visual evaluation records were available, the visual acuity in the affected eye of most patients stabilized at a level better than at baseline, with visual acuity of 4/200 (Patient 1, pre-FCRT finger counting at 3 ft.), 20/70 (Patient 5, pre-FCRT 2/300), and 20/25 (Patient 9, pre-FCRT 20/30). One patient with a pre-FCRT visual acuity of 20/30 in the affected eye had a post-treatment visual acuity of 20/40 (Patient 8). Of the 3 patients with only visual field defects, 1 (33.3%) recovered completely, and 2 (66.7%) showed improvement at 1 and 3 months after FCRT, respectively. All other ophthalmologic signs and symptoms, including proptosis, diplopia, eyelid swelling, optic disk swelling, and orbital pain, regressed during the follow-up period, and none of the patients experienced radiation-induced visual loss in the unaffected eye.
No systemic or neurologic complications developed because of FCRT during the follow-up period; however, 1 patient (Patient 1) experienced a 3-month period of orbital swelling and pain that was treated with analgesics and systemic corticosteroids with eventual resolution, and 1 patient (Patient 3) in whom visual improvement occurred after FCRT subsequently developed radiation-induced retinopathy 2 years after FCRT. He was treated with photocoagulation, following which the retinopathy regressed; however, at the last follow-up visit 11 years after treatment, he had a reduced visual acuity of 20/200, color vision of 4/10, and a central field defect in the eye.
DISCUSSION
The options for treatment of ONSM are observation, surgery, and RT. During the latter half of the 20th century, most patients were followed up without intervention or underwent surgical resection. Surgery to decompress the affected optic nerve has been associated with a significant risk of complications and a high rate of local recurrence (2, 5, 15). In addition, attempts to remove ONSMs generally result in complete loss of vision in the affected eye. Because these lesions are not life-threatening and almost never cross the midline to affect the contralateral eye, such surgery would seem inappropriate.
Functional outcome
In an extensive review, Dutton et al. (1) reported that 9 (82%) of 11 patients with ONSMs treated with RT demonstrated stable or improved vision compared with 6% of 148 patients treated surgically and 14% of 64 patients followed up without intervention. The rare reported cases of visual improvement after attempted surgical resection of the tumor alone have been in patients with a special subset of primarily exophytic ONSMs that have penetrated the dura just posterior to the globe and have only a small intradural component (2). Also, many of these patients have experienced recurrence of their lesions over time, indicating that, even in these cases, total removal is almost never possible. Most ONSMs develop between the arachnoid and the dural sheath of the optic nerve and wrap circumferentially around the nerve, preventing complete surgical removal without compromising its vascular supply. Therefore, the role of surgical resection of ONSMs is limited to lesions presenting in patients without much functional vision, to ameliorate severe disfiguring proptosis, or to treat patients with evident intracranial extension to prevent contralateral optic nerve involvement (7, 12).
Recent reports have suggested that FCRT results in good functional outcome and tumor control (5, 16–23). Favorable (improved or stable status) visual outcomes have been reported in 85.7–100% of cases across series in the past decade, and tumor control has been achieved in all cases (5, 16–23). Turbin et al. (13) reported the long-term benefits of RT in 34 patients with an ONSM, of whom 18 (52.9%) underwent conventional RT as primary treatment and 16 (47.1%) underwent RT as adjuvant treatment after surgery. Of the 18 patients who underwent primary RT, 8 (44.4%) showed improvement in visual acuity by two lines on the Snellen chart, and 5 (31.3%) of 16 patients who had underwent adjuvant RT showed similar improvement (13). However, only 2 (8%) of 25 patients who had received no intervention had improvement in visual acuity (13).
As noted, no series has reported the long-term results of FCRT in the treatment of presumed or biopsy-proven ONSM (Table 2). Narayan et al. (20) reported a series of 14 patients treated with FCRT with a median follow-up of 51.3 months. Of 9 patients with preoperative visual field defects in that series, all experienced significant improvement (20). In contrast, 5 (45.5%) of 11 patients with initially impaired visual acuity showed significant improvement (i.e., a change by three lines on the Snellen chart) (20). In their series, stabilization of visual function was achieved in 12 (85.1%) of 14 patients (20). Similarly, Landert et al. (18) compared the visual outcome in seven eyes with a presumed ONSM treated with FCRT with six eyes of patients who received no intervention. During a follow-up period of 57 months and 61 months for the treated and untreated patients, respectively, the visual acuity in the patients treated with FCRT improved in six eyes (85.7%), with five eyes experiencing improvement by three or more lines on the Snellen chart (18). In contrast, the visual acuity deteriorated in four eyes and remained stable in two eyes of the untreated patients (18). Litre et al. (24) reported significant improvement in visual acuity in 100% of 8 patients who underwent FCRT and followed up for an average of 27 months. Liu et al. (19) reported a significant improvement in visual acuity in 80% of 5 patients with ONSM treated with FCRT, 1 of whom had been followed up for 7 years and the other 4 for 1–3 years. These data suggest that, at least in the short term, FCRT is a useful treatment option for presumed ONSMs compared with observation.
Table 2.
Findings from previous series
| Investigator | Treatment modality | Patients (n) | Dose and fractions | Visual outcome (%)
|
Radiologic outcome | Toxicity | Mean follow-up (mo) | ||
|---|---|---|---|---|---|---|---|---|---|
| Improved | Stable | Impaired | |||||||
| Liu et al. (19), 2002 | FCRT | 5 | 45–54 Gy; 1.8 Gy/fraction | 80 | 20 | 0 | Control 100% | 20% (1 transient periorbital edema) | 36 |
| Andrews et al. (5), 2002 | FCRT | 30 | 50–54 Gy; 1.8 Gy/fraction | 30 | 63.3 | 6.7 | Control 100% (decreased in 13%; stable in 87%) | 13% (2 VL, 1 RON, 1 transient OP) | 20.4* |
| Becker et al. (17), 2002 | FCRT | 39 | 54 Gy; 30 (1.8-Gy) fractions | 6 | 94 | 0 | Control 100% (decrease in 2.6%; stable in 97.4%) | 10.3% endocrinologic deficits, 25% erythema in radiation field | 34.8 |
| Narayan et al. (20), 2003 | FCRT | 14 | 50.4–56 Gy; 1.8–2 Gy/fraction | 35.7 | 50 | 14.3 | Control 100% (decreased in 7.1%; stable in 92.9%) | 35.7% (1 RR, 1 transient OP, 1 DE, 2 iritis) | 51.3* |
| Saeed et al. (23), 2003 | FCRT | 6 | 45–55 Gy; 28–30 fractions | 100 | 0 | 0 | Control 100% | 16.6% (1 cataract) | <48 |
| Baumert et al. (16), 2004 | FCRT | 23 | 45–54 Gy; 1.8–2 Gy/fraction | 72.7 | 22.7 | 4.6 | Control 100% (decreased in 4.4%; stable in 95.6%) | 4.6% (1 RR) | 20* |
| Richards et al. (21), 2005 | FCRT | 4 | Mean 43.6 Gy; 26 fractions | 100 | 0 | 0 | Control 100% | 1 radiation-induced hair loss | 30 |
| Landert et al. (18), 2005 | FCRT | 7† | 50–54 Gy; 1.7 or 1.8 Gy/ fraction | 85.7† | 0† | 14.3† | Control 100% | 0% | 57 |
| Sitathanee et al. (23), 2006 | FCRT | 12 | Mean 55.7 Gy; 1.8 Gy/fraction‡ | 60 | 40 | 0 | Control 100%§ | 1 transient VL, 1 vitreous hemorrhage 2 y after RT | 34* |
| Litre et al. (24), 2007 | FCRT | 8 | Mean 45 Gy; 25 (1.8 Gy) fractions | 37.5 | 62.5 | 0 | Control 100% | 0% | 27 |
| Present series, 2009 | FCRT | 9 | Median 51.2 Gy; 1.8–2 Gy/ fraction | 77.8 | 22.2 | 0 | Control 100% (decreased in 22.2%; stable 77.8%) | 22% (1 transient OP, 1 RR) | 98¶ |
Abbreviations: FCRT = functional conformal radiotherapy; VL = visual loss; RON = radiation optic neuropathy; OP = orbital pain; RR = radiation retinopathy; DE = dry eye; RT = radiotherapy.
Median, not mean.
Number or percentage of eyes, not patients.
One patient was treated with 15-Gy stereotactic radiosurgery.
Available for only 50% of patients.
Minimum follow-up of 60 mo.
In the present study, we found sustained improvement of visual function in 7 (77.8%) of 9 patients during a follow-up period of 61–151 months after FCRT compared with the patients who had received FCRT as an adjuvant to surgery. An unequivocal difference was found in the rate of clinical improvement between the two subgroups; however, larger studies are required to draw a conclusion on the value of adjuvant RT. These findings are consistent with those from other reported series of ONSM patients treated with either FCRT or RT. Our findings indicate that a pretreatment neurologic deficit in the postresection adjuvant treatment group might have been the consequence of irreversible microsurgical injury to critical nervous structures (5, 16–23). These data have also corroborated the findings of Leber et al. (25), who showed that pre-existing nerve compression was not correlated with an increased risk of injury after RT, even though previous reports had raised this hypothesis (26–28). Morita et al. (29) also found that a major factor predicting improvement in cranial nerve function was no previous attempt at tumor resection. In our series, the only patients without improvement after FCRT were the 2 patients who had previously undergone surgery and had no perception of light in their affected eyes before adjuvant FCRT. Thus, 100% of our patients with some visual function at FCRT showed improvement and maintained their improved vision during long-term follow-up. These long-term follow-up data seem to confirm the visual benefit of FCRT for presumed ONSM.
Tumor control
In the present study, the tumor growth control rate was 100%. These results are consistent with those from other studies of ONSM treated by FCRT, which also showed a 100% tumor growth control rate but had followed up patients for a shorter period compared with our series (5, 16–23). The tumors were treated at 80–98% of the isodose of the treatment dose in the 6 patients for whom these data were available. The optic nerve received 40–98% of the isodose of the treatment dose (Table 1). All patients in the present study were followed up for ≥5 years (mean, 98 ± 31.7 months; median, 90). Another outcome measure was imaging evidence of a tumor response. In our series, 2 patients showed a decrease in tumor size, and in 7, the tumor size and shape remained unchanged. These findings were also consistent with those from previous series, which reported imaging evidence of tumor shrinkage in <10% of cases (5, 16, 17, 20). Whether these findings represent a true lack of change in lesion size or a change in size that was less than the minimal resolution of MRI or CT is unknown. It might be appropriate to use different imaging modalities to determine tumor control. For example, Andrews et al. (5) reported the use of octreotide scintigraphy to measure meningioma activity in 6 of 30 patients in their series. They noted that, among brain tumors, high-density somatostatin receptor expression was a unique future of active meningiomas (5). This modality might be more useful in detecting meningioma progression than diagnostic tools that are only able to detect changes in size.
The results in our series have indicated that FCRT is effective against ONSMs, with beneficial effects lasting many years; however, given the potential for these tumors to grow slowly (30), it would be prudent to monitor patients who undergo FCRT for ≥10 years (6) and possibly for the life of the patient.
Toxicity
In our study, 1 patient (11%) developed radiation-induced retinopathy 2 years after FCRT. This case has been previously reported (31), and other investigators have reported similar cases (5, 20, 32, 33). These findings have indicated that although FCRT theoretically reduces the risk of radiation damage to the surrounding normal tissue, such damage can still occur. The risk of radiation-induced retinopathy or optic neuropathy secondary is greatly increased with total doses >54 Gy, fractionated doses >1.8 Gy, and single doses >6 Gy (19). In our treatment protocol, the median dose of 51.2 Gy was administered in 28–30 fractions of 1.8–2.0 Gy/fraction, each within 6 weeks. The retina received 10–98% of the isodose in all cases (Table 1). Despite this protocol, our patient, who had a presumed ONSM that involved the entire orbital and canalicular portion of the optic nerve and thus required treatment that included the posterior aspect of the globe, developed retinopathy. The retina in this patient had undergone RT at 50% of the isodose line with 1.8 Gy/ fraction (total dose, 54 Gy). A patient reported by Narayan et al. (20), who developed radiation-induced retinopathy 4 years after FCRT, consisting of a total dose <54 Gy, also had an extensive tumor extending from the posterior aspect of the globe and thus requiring treatment that included the retina in the radiation field. The patients reported by Levi (32) and Krishnan et al. (33) had similar tumors. Thus, it seems clear that patients with ONSMs that involve the proximal portion of the optic nerve adjacent to the globe are at greatest risk of developing radiation-induced retinopathy after FCRT. This potential complication should be considered when deciding whether to treat such patients.
Of concern in FCRT techniques is the question of induced carcinogenesis. Radiation-induced neoplasia is a well-documented and serious sequela of RT. In an adult population, the relative risk of a second tumor after cranial RT has been estimated to be 9.38–16 (34). The latency period of radiation-induced neoplasms precludes the estimation of its incidence in a population of patients with ONSM, because the longest mean follow-up reported in various series has been <10 years. However, this potential risk must be considered for patients undergoing FCRT.
CONCLUSION
Our experience has demonstrated that FCRT is a safe and effective long-term therapeutic option in the management of presumed or biopsy-proven ONSM. The results seem to be more favorable for patients treated for primary ONSM than for those treated with surgery and adjuvant RT. These findings strengthen those of recent studies of FCRT for ONSM. FCRT might be particularly effective in restoring vision in patients with minor visual impairment, although the potential for radiation-induced optic neuropathy must be considered in those patients with extensive tumors.
Acknowledgments
Supported by the Swenson Family Foundation, by the Monica and Hermen Greenberg Foundation, and by the Salisbury Family foundation.
Footnotes
Conflict of interest: none.
References
- 1.Dutton JJ. Optic nerve sheath meningiomas. Surv Ophthalmol. 1992;37:167–183. doi: 10.1016/0039-6257(92)90135-g. [DOI] [PubMed] [Google Scholar]
- 2.Kennerdell JS, Maroon JC, Malton M, et al. The management of optic nerve sheath meningiomas. Am J Ophthalmol. 1988;106:450–457. doi: 10.1016/0002-9394(88)90882-3. [DOI] [PubMed] [Google Scholar]
- 3.Liu JK, Forman S, Moorthy CR, et al. Update on treatment modalities for optic nerve sheath meningiomas. Neurosurg Focus. 2003;14:e7. [PubMed] [Google Scholar]
- 4.Wright JE, Call NB, Liaricos S. Primary optic nerve meningioma. Br J Ophthalmol. 1980;64:553–558. doi: 10.1136/bjo.64.8.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews DW, Faroozan R, Yang BP, et al. Fractionated stereotactic radiotherapy for the treatment of optic nerve sheath meningiomas: Preliminary observations of 33 optic nerves in 30 patients with historical comparison to observation with or without prior surgery. Neurosurgery. 2002;51:890–903. 894. doi: 10.1097/00006123-200210000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Berman D, Miller NR. New concepts in the management of optic nerve sheath meningiomas. Ann Acad Med Singapore. 2006;35:168–174. [PubMed] [Google Scholar]
- 7.Miller NR. New concepts in the diagnosis and management of optic nerve sheath meningioma. J Neuroophthalmol. 2006;26:200–208. doi: 10.1097/01.wno.0000235569.19131.ac. [DOI] [PubMed] [Google Scholar]
- 8.Bosch MM, Wichmann WW, Boltshauser E, et al. Optic nerve sheath meningiomas in patients with neurofibromatosis type 2. Arch Ophthalmol. 2006;124:379–385. doi: 10.1001/archopht.124.3.379. [DOI] [PubMed] [Google Scholar]
- 9.Landau K, Horton JC, Hoyt WF, et al. Aneurysm mimicking intracranial growth of optic nerve sheath meningioma. J Clin Neuroophthalmol. 1990;10:185–187. [PubMed] [Google Scholar]
- 10.Lindblom B, Truwit CL, Hoyt WF. Optic nerve sheath meningioma: Definition of intraorbital, intracanalicular, and intracranial components with magnetic resonance imaging. Ophthalmology. 1992;99:560–566. doi: 10.1016/s0161-6420(92)31932-3. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd GA. Primary orbital meningioma: A review of 41 patients investigated radiologically. Clin Radiol. 1982;33:181–187. doi: 10.1016/s0009-9260(82)80057-3. [DOI] [PubMed] [Google Scholar]
- 12.Miller NR. Primary tumours of the optic nerve and its sheath. Eye. 2004;18:1026–1037. doi: 10.1038/sj.eye.6701592. [DOI] [PubMed] [Google Scholar]
- 13.Turbin RE, Thompson CR, Kennerdell JS, et al. A long-term visual outcome comparison in patients with optic nerve sheath meningioma managed with observation, surgery, radiotherapy, or surgery and radiotherapy. Ophthalmology. 2002;109:890–900. doi: 10.1016/s0161-6420(02)01017-5. [DOI] [PubMed] [Google Scholar]
- 14.Lee AG, Woo SY, Miller NR, et al. Improvement in visual function in an eye with a presumed optic nerve sheath meningioma after treatment with three-dimensional conformal radiation therapy. J Neuroophthalmol. 1996;16:247–251. [PubMed] [Google Scholar]
- 15.Cristante L. Surgical treatment of meningiomas of the orbit and optic canal: A retrospective study with particular attention to the visual outcome. Acta Neurochir (Wien) 1994;126:27–32. doi: 10.1007/BF01476490. [DOI] [PubMed] [Google Scholar]
- 16.Baumert BG, Villa S, Studer G, et al. Early improvements in vision after fractionated stereotactic radiotherapy for primary optic nerve sheath meningioma. Radiother Oncol. 2004;72:169–174. doi: 10.1016/j.radonc.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Becker G, Jeremic B, Pitz S, et al. Stereotactic fractionated radiotherapy in patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2002;54:1422–1429. doi: 10.1016/s0360-3016(02)03753-7. [DOI] [PubMed] [Google Scholar]
- 18.Landert M, Baumert BG, Bosch MM, et al. The visual impact of fractionated stereotactic conformal radiotherapy on seven eyes with optic nerve sheath meningiomas. J Neuroophthalmol. 2005;25:86–91. doi: 10.1097/01.wno.0000165105.78365.22. [DOI] [PubMed] [Google Scholar]
- 19.Liu JK, Forman S, Hershewe GL, et al. Optic nerve sheath meningiomas: Visual improvement after stereotactic radiotherapy. Neurosurgery. 2002;50:950–957. doi: 10.1097/00006123-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Narayan S, Cornblath WT, Sandler HM, et al. Preliminary visual outcomes after three-dimensional conformal radiation therapy for optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2003;56:537–543. doi: 10.1016/s0360-3016(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 21.Richards JC, Roden D, Harper CS. Management of sight-threatening optic nerve sheath meningioma with fractionated stereotactic radiotherapy. Clin Exper Ophthalmol. 2005;33:137–141. doi: 10.1111/j.1442-9071.2005.00973.x. [DOI] [PubMed] [Google Scholar]
- 22.Saeed P, Rootman J, Nugent RA, et al. Optic nerve sheath meningiomas. Ophthalmology. 2003;110:2019–2030. doi: 10.1016/S0161-6420(03)00787-5. [DOI] [PubMed] [Google Scholar]
- 23.Sitathanee C, Dhanachai M, Poonyathalang A, et al. Stereotactic radiation therapy for optic nerve sheath meningioma: An experience at Ramathibodi Hospital. J Med Assoc Thai. 2006;89:1665–1669. [PubMed] [Google Scholar]
- 24.Litre CF, Noudel R, Colin P, et al. Fractionated stereotactic radiotherapy for optic nerve sheath meningioma: Eight cases. Neurochirurgie. 2007;53:333–338. doi: 10.1016/j.neuchi.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Leber KA, Bergloff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88:43–50. doi: 10.3171/jns.1998.88.1.0043. [DOI] [PubMed] [Google Scholar]
- 26.Spiegelmann R, Nissim O, Menhel J, et al. Linear accelerator radiosurgery for meningiomas in and around the cavernous sinus. Neurosurgery. 2002;51:1373–1380. [PubMed] [Google Scholar]
- 27.Parsons JT, Bova FJ, Fitzgerald CR, et al. Radiation optic neuropathy after megavoltage external-beam irradiation: Analysis of time-dose factors. Int J Radiat Oncol Biol Phys. 1994;30:755–763. doi: 10.1016/0360-3016(94)90346-8. [DOI] [PubMed] [Google Scholar]
- 28.Parsons JT, Bova FJ, Mendenhall WM, et al. Response of the normal eye to high dose radiotherapy. Oncology (Huntingt) 1996;10:837–847. 838, 851, 832. [PubMed] [Google Scholar]
- 29.Morita A, Coffey RJ, Foote RL, et al. Risk of injury to cranial nerves after gamma knife radiosurgery for skull base meningiomas: Experience in 88 patients. J Neurosurg. 1999;90:42–49. doi: 10.3171/jns.1999.90.1.0042. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura M, Roser F, Michel J, et al. The natural history of incidental meningiomas. Neurosurgery. 2003;53:62–70. 61. doi: 10.1227/01.neu.0000068730.76856.58. [DOI] [PubMed] [Google Scholar]
- 31.Subramanian P, Bressler N, Miller N. Radiation retinopathy after fractionated stereotactic radiotherapy for optic nerve sheath meningioma. Ophthalmology. 2004;111:565–567. doi: 10.1016/j.ophtha.2003.06.025. [DOI] [PubMed] [Google Scholar]
- 32.Levi L. Radiation retinopathy after therapy for meningioma. Ophthalmology. 2005;112:1484. doi: 10.1016/j.ophtha.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Krishnan R, Kumar I, Kyle G, et al. Radiation retinopathy after fractionated stereotactic conformal radiotherapy for primary intraorbital optic nerve sheath meningioma. J Neuroophthalmol. 2007;27:143–144. doi: 10.1097/WNO.0b013e318064e5b0. [DOI] [PubMed] [Google Scholar]
- 34.Brada M, Ford D, Ashley S, et al. Risk of second brain tumour after conservative surgery and radiotherapy for pituitary adenoma. BMJ. 1992;304:1343–1346. doi: 10.1136/bmj.304.6838.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]