Abstract
Mesenchymal stem cells (MSCs) are currently thought to transdifferentiate into neural lineages under specific microenvironments. Studies have reported that the tenascin family members, tenascin-C (TnC) and tenascin-R (TnR), regulate differentiation and migration, in addition to neurite outgrowth and survival in numerous types of neurons and mesenchymal progenitor cells. However, the mechanisms by which TnC and TnR affect neuronal differentiation are not well understood. In this study, we hypothesized that different forms of tenascin might regulate the neural transdifferentiation of human bone marrow-derived mesenchymal stem cells. Human MSCs were cultured in media incorporated with soluble tenascins, or on precoated tenascins. In a qualitative polymerase chain reaction analysis, adding a soluble TnC and TnR mixture to the medium significantly enhanced the expression of neuronal and glial markers, whereas no synaptic markers were expressed. Conversely, in groups of cells treated with coated TnC, hMSCs showed neurite outgrowth and synaptic marker expression. After being treated with coated TnR, hMSCs exhibited neuronal differentiation; however, it inhibited neurite outgrowth and synaptic marker expression. A combination of TnC and TnR significantly promoted hMSC differentiation in neurons or oligodendrocytes, induced neurite formation, and inhibited differentiation into astrocytes. Furthermore, the effect of the tenascin mixture showed dose-dependent effects, and a mixture ratio of 1:1 to 1:2 (TnC:TnR) provided the most obvious differentiation of neurons and oligodendrocytes. In a functional blocking study, integrin α7 and α9β1-blocking antibodies inhibited, respectively, 80% and 20% of mRNA expression by hMSCs in the coated tenascin mixture. In summary, the coated combination of TnC and TnR appeared to regulate neural differentiation signaling through integrin α7 and α9β1 in bone marrow-derived hMSCs. Our findings demonstrate novel mechanisms by which tenascin regulates neural differentiation, and enable the use of cell therapy to treat neurodegenerative diseases.
Introduction
Transplantation of differentiated neurons from neural progenitors into adult brain tissue with functional recovery has been reported in animal models of Parkinson's disease1 and cerebral ischemia.2 However, a lack of human neural stem cells or progenitors limits the clinical application of this technique. Mesenchymal stem cells (MSCs) provide an alternative method for generating neurons.
MSCs have the ability to differentiate into osteocytes, chondrocytes, and adipocytes.3 In addition to cells of the mesoderm lineage, MSCs can transdifferentiate into other germ-layer types, including neurons.4 In previous studies, several factors including retinoic acid (RA), nerve growth factor (NGF), brain-derived neurotropic factor (BDNF), cAMP, and sonic hedgehog (SHH), have been used to induce MSC neurogenic differentiation.5–7 In addition to morphogens and growth factors, the extracellular matrix (ECM)8–10 is also critical for controlling cell differentiation, proliferation, and morphogenesis. However, interactions between the ECM from the nervous system and neurogenesis have been less extensively studied regarding effects of human mesenchymal stem cells (hMSCs).
Tenascin, an ECM glycoprotein, includes four family members: tenascin-C (TnC), tenascin-R (TnR), tenascin-W (TnW), and tenascin-Z (TnZ). All tenascin members are composed of four elements: amino-terminal heptad repeats, epidermal growth factor (EGF)-like repeats, fibronectin (FN) type III domain repeats, and a carboxyl-terminal fibrinogen-like globular domain. Heptad repeats assemble other tenascins to create trimers. Each protein member has its own number of EGF-like and FN type III repeats. FN type III repeats include alternative splicing sites to produce isoform variants.11
TnC has been reported involved in vertebrate neural, skeletal, and vascular morphogenesis during development.12 TnC appears at E10 in the mouse nervous system,13 and it is mainly expressed by astrocytes and radial glial cells.13,14 Serial studies have demonstrated that FN type III repeats of TnC regulate neurite outgrowth and guidance in rat cerebellar granule cells.15–17 Rigato et al. reported that FN type III repeats BD and D6 of TnC can elongate the length of axons in hippocampal neurons through F3/contactin.18 Furthermore, the effects of neurite outgrowth from TnC were mediated through the actions of integrin α7, α9, and β1.19,20 These studies indicate that TnC plays a central role in the regulation of neuronal differentiation in the nervous system.
The expression pattern of TnR is restricted to the central nervous system (CNS).21,22 Previous studies have demonstrated that TnR induced and regulated the process of neurite outgrowth in chick E6 tectal cells.23,24 For stem cells, TnR-overexpressing embryonic stem cells (ESC) have been shown to enhance neurogenic differentiation and increase the generation of GABAergic neurons in vitro.25 Liao et al.26 reported that EGF-like domains and FN type III domains 6–8 of TnR can promote mice neural stem cell proliferation and differentiation through β1 integrin.26
The ECM, including type I or II collagen, regulates osteogenesis and chondrogenesis in hMSCs,27–29 in addition to facilitating the delivery of MSCs to the heart.30 However, the mechanism by which the ECM controls hMSC neurogenesis is unclear. To evaluate the interaction of hMSCs with tenascins, which are abundant ECM proteins in CNS, we tested the neurogenic effects of TnC, TnR, and a mixture of TnC and TnR (TCR), either dissolved in a medium or coating a plate. Our results demonstrate that individual tenascin in the medium have no significant effects on neuronal differentiation in MSCs. However, tenascin coating modulated the morphology, neurogenesis, and gliogenesis within cells. TCR greatly increased neuronal and synaptic gene expression, both in the coatings and in the dissolved media. Our data indicate that the combination of the ECM proteins of TnC, TnR, and TCR are crucial for inducing neural differentiation, and provide a more detailed picture of neurogenic control in hMSCs.
Materials and Methods
hMSC cultivation
The hMSCs were supplied by Cambrex. The hMSCs were cultured in 10-cm dishes with low-glucose Dulbecco's modified Eagle medium (DMEM/LG; Invitrogen), 10% fetal bovine serum (FBS; Invitrogen), and a 1% penicillin-streptomycin mixture (Invitrogen). This mixture was cultured at 37°C in a humidified atmosphere with 5% CO2. The medium was refreshed thrice a week, and cells were subcultured until confluent. All experiments were performed with cells from Passages 3–6.
Neuronal induction and soluble tenascin effects
To induce neuronal transdifferentiation, 1×105 hMSCs were treated in six-well plates each with DMEM/LG that contained 1% FBS, 10 ng/mL BDNF (PeproTech), 20 ng/mL NGF-β (PeproTech), and 5 μM RA (Sigma) at 37°C in a humidified atmosphere with 5% CO2 for 1 week. The medium was refreshed thrice a week. The neurogenic medium was referred to as NGF, BDNF, and RA (NBR). First, hMSCs were treated using NBR for 7 days, and recombinant human TnC (0.5 μg/mL; Millipore), recombinant human TnR (0.5 μg/mL; R&D Systems), or TCR (0.25 μg/mL TnC+0.25 μg/mL TnR and 0.5 μg/mLTnC+0.5 μg/mLTnR) were added to the NBR to treat the hMSCs for additional 7 days. After the 14-days treatment, we collected cells for further analysis. To determine the effect of soluble tenascin in DMEM without NBR, hMSCs were treated using 0.5 μg/mL TnR (R&D Systems), 0.5 μg/mL TnC (Millipore), or TCR (0.5 μg/mLTnC+0.5 μg/mLTnR) with DMEM 1% for 7 days before mRNA analysis.
Tenascin coating effects
To determine tenascin effects on neuronal differentiation, TnC or TnR was first coated onto 24-well trays for an immunocytochemistry study and onto 6-well trays for mRNA analysis. Before cultivation, various combinations of tenascins (10 μg/mL of TnC, 10 μg/mL of TnR, 5 μg/mL of TnC and 5 μg/mL of TnR, 10 μg/mL of TnC and 10 μg/mL of TnR, 20 μg/mL of TnC and 20 μg/mL of TnR, 15 μg/mL of TnC and 5 μg/mL of TnR, 13.3 μg/mL of TnC and 6.7 μg/mL of TnR, 5 μg/mL of TnC and 15 μg/mL of TnR, and 6.7 μg/mL of TnC and 13.3 μg/mL of TnR) in phosphate-buffered saline were added to the dish at 37°C for 30 h. Then, excess solution was removed; 0.5×105 (in 24-well) or 2.0×105 (in 6-well) of hMSCs were cultivated in NBR medium on noncoated culture trays for 7 days. Then, NBR-induced cells were replated to tenascin-coated dishes with NBR medium for an additional 7 days. At the same time, to understand the effect of coated tenascin alone, 0.5, or 2.0×105 of hMSCs were cultivated in DMEM containing 1% FBS on noncoated culture dishes for 7 days. The cells were subsequently replated to tenascin-coated trays for 7 subsequent days with only DMEM.
Integrin blocking
Integrin α7 function-blocking antibody (at 5 μg/mL; Serotec) and integrin α9β1 function-blocking antibody (Chemicon) were used to inhibit the interaction between TCR and hMSCs. Blocking antibodies were added to the NBR medium before transferring the experimental hMSCs to TCR-coated six-well trays. Furthermore, the blocking antibodies were maintained in the NBR medium for the next 7 days.
RNA isolation and qualitative polymerase chain reaction
All studied cells in six-well trays were analyzed by qualitative polymerase chain reaction (qPCR), and the data of each group was obtained from three independent experiments. The total RNA from experimental hMSCs was extracted using TRIzol reagent (Sigma). A complementary cDNA synthesis was carried out using a RevertAid Premium First Strand cDNA Synthesis Kit (Thermo). The qPCR was conducted using the LightCycler 480 System (Roche Diagnostics) with LC-FastStart DNA Master SYBR Green I mix (Roche). In brief, 5 μL of each cDNA was mixed with 1 μL of the forward and reverse primers and 13 μL of the LC-FastStart DNA Master SYBR Green I mix. The amplification profile was as follows: enzyme activation at 95°C for 10 min, and annealing at 95°C for 10 s, 60°C for 5 s, and 72°C for 15 s. The specificity of the PCR products was determined by a melting-curve analysis. Forward and reverse primers of human genes were designed by LightCycler Probe Design Software 2 (Roche). Every primer pair was checked by NCBI primer-BLAST. The RNA levels were normalized to the housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA level. The data are presented as normalized RNA level divided by normalized RNA level expressed in NBR group or DMEM 10% group. The sequences of forward and reverse primers we used were in Table 1.
Table 1.
Primers for Qualitative Polymerase Chain Reaction
| Gene | Sequence (forward; reverse) | Product length (bp) |
|---|---|---|
| GAPDH (glyceraldehyde 3-phosphate dehydrogenase) | 5′-CGACCACTTTGTCAAGCTCA-3′ | 228 |
| 5′-AGGGGTCTACATGGCAACTG-3′ | ||
| MAP2 (microtubule-associated protein 2) | 5′-TTGGTGCCGAGTGAGAAGAA-3′ | 100 |
| 5′-GGTCTGGCAGTGGTTGGTTAA-3′ | ||
| NFM (neurofilament M) | 5′-GTGTCCACTCACATAAAGGT-3′ | 92 |
| 5′-TCTGTCTGTTCCTCCACAAT-3′ | ||
| GFAP (glial fibrillary acidic protein) | 5′-GATGGCGCTAGGCATAC-3′ | 90 |
| 5′-AAGAGGATGAGTCACTTCCTTA-3′ | ||
| MBP (myelin basic protein) | 5′-GAGGACGGAGATGAGGAGTA-3′ | 86 |
| 5′-TAAGCCCACACGAAACATT-3′ | ||
| SYN (synapsin 1) | 5′-GCAAACTCCACCCATCTT-3′ | 85 |
| 5′-ACACAGACACCACAGCA-3′ | ||
| ITGB1 (integrin β1) | 5′-GTAGCAAAGGAACAGCAGAGAA-3′ | 147 |
| 5′-AGTAGAGGTCAATGGGATAGTC-3′ | ||
| ITGA7 (integrin α7) | 5′-CAGCAACTCTTCTTCTCTGG-3′ | 96 |
| 5′-GGAAACCGTGACCTCATAC-3′ | ||
| ITGA9 (integrin α9) | 5′-GCTGACTCGTTCTTCGGCTA-3′ | 96 |
| 5′-GCTGTATTTGGAATCTGCCT-3′ |
Immunocytochemistry
The studied cells were fixed with 4% paraformaldehyde for 10 min, and then permeabilized with 0.02% Triton X-100 for 10 min, followed by blocking with 5% FBS for 1 h and incubation with primary antibodies for at least 1 h. Primary antibodies were as follows: rabbit anti-microtubule-associated protein-2 (MAP2) polyclonal antibody (pAb, 1:500; Chemicon); rabbit anti-synapsin-1 (SYN) pAb (1:500; Chemicon); mouse anti-neurofilament M (NFM) monoclonal antibody (mAb, 1:250; Chemicon); mouse anti-myelin basic protein (MBP) mAb (1:250; Chemicon); mouse anti-glial fibrillary acidic protein (GFAP) mAb (1:250; Chemicon); and mouse anti-β-catenin mAb (1:250; Chemicon). Cells were then incubated for 1 h with the secondary antibodies, goat anti-mouse immunoglobulin G (IgG): DyLight 488 (1:100; Serotec), or sheep anti-rabbit IgG: DyLight 488 (1:100; Serotec). Nuclear staining used 4′, 6-diamidino-2-phenylindole (DAPI; Chemicon). Images were obtained using fluorescent microscopy (Olympus). At least 1000 cells from 10 to 15 viewing fields per group were used to calculate percentages of cells. In β-catenin staining, cells with projections exceeding 30 μm were considered neurite-positive cells. The cell area and neurite length were calculated using ImageJ software. The human neuroblastoma cell line SH-SY5Y was used as the positive control, and the human osteosarcoma cell line MG63 was used as the negative control. Mouse IgG1 antibodies (Chemicon) were used as the isotype control. All control results are shown in Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/tea).
Statistical analysis
Statistical analysis of qPCR and the immunoreactivity was assessed by using the Student t-test. Values were expressed as mean±standard deviation. Significance was accepted for p<0.05 (* or #) and p<0.01 (** or ##). All statistical analyses were calculated using SigmaPlot 9.0 (Systat Software, Inc.).
Results
Regulatory effects of soluble tenascins on neural differentiation in hMSCs
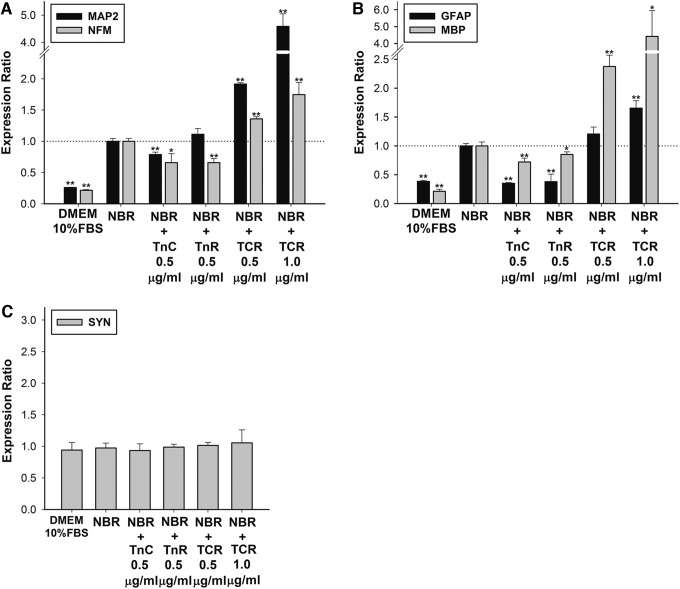
We first analyzed the qPCR for the mature neuronal markers MAP2 and NFM; the astrocyte marker GFAP; the oligodendrocyte marker MBP; and the synaptic marker SYN in tenascin-treated cells. TnC and TnR alone were not found to induce significant inhibitory effects on the mRNA expression of MAP2 or NFM. However, TCR significantly increased NFM expression over 1.36-fold, and MAP2 expression over 4.58-fold (Fig. 1A). The analysis of the oligodendrocyte marker revealed that the expression of MBP was inhibited by 15–28% when incorporated with TnC or TnR alone. Incorporation with 0.5 μg/mL TCR hMSCs did not increase GFAP expression, but enhanced MBP expression over 2.38-fold. Furthermore, incorporation with 1.0 μg/mL TCR upregulated the GFAP gene expression 1.65-fold and MBP expression 4.42-fold (Fig. 1B). The analysis of the synaptic marker revealed that soluble tenascins in the NBR medium did not change SYN expression (Fig. 1C).
FIG. 1.
Effects of soluble tenascin-C (TnC) and tenascin-R (TnR) in NBR-induced human mesenchymal stem cells (hMSCs). (A) The mRNA levels of neuron markers, MAP2 and NFM; (B) glial cell markers, GFAP and MBP; and (C) the synapse marker, synapsin (SYN) were quantified after 7 days of Tn treatment (0.5–1.0 μg/mL) in hMSCs that were pretreated with NBR for 1 week. Levels were normalized to levels observed in NBR-only treatment (set to 1.0) (presented as a dotted line). The NBR incorporated with the TnC and TnR mixture (TCR) promoted the expression of neuronal and glial cell genes, but not of synaptic genes. The data are presented as the mean±SD of an experiment that was derived from three independent experiments. *p<0.05, **p<0.01 (all vs. NBR). DMEM, Dulbecco's modified Eagle medium; GFAP, glial fibrillary acidic protein; MBP, myelin basic protein; NFM, neurofilament M; SD, standard deviation.
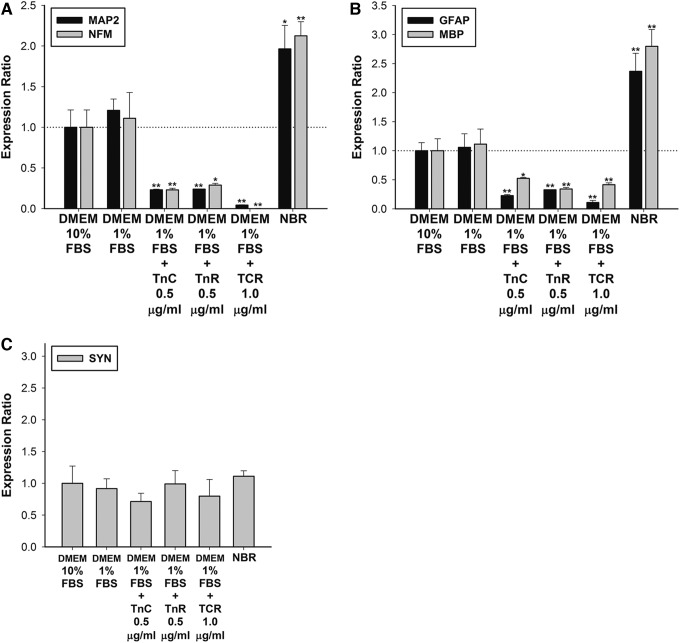
Subsequently, we determined whether soluble tenascins in DMEM medium without NBR induced neurogenic effects on hMSCs. The results showed that among TnC, TnR, or TCR, none upregulated hMSC neuronal or neuro-glial differentiation. To the contrary, tenascins inhibited MAP2, NFM, GFAP, and MBP expression by 50–99% (Fig. 2A, B). No significant change in SYN expression was found when hMSCs were treated with tenascins only (Fig. 2C). Taken together, neither a medium with a combination of soluble TnC and TnR, nor TCR alone, upregulated neurogenesis or gliogenesis in hMSCs. However, adding tenascins to the NBR medium induced regulatory effects on neuronal or neuro-glial markers with the exception of synapse expression in the hMSCs.
FIG. 2.
Effects of soluble TnC and TnR on hMSCs without NBR induction. (A) The mRNA levels of neuronal markers, MAP2 and NFM; (B) glial cell markers, GFAP and MBP; and (C) the synapse marker, synapsin (SYN) were quantified after 7 days of Tn treatment (0.5–1.0 μg/mL) in hMSCs. Levels were normalized to levels observed in DMEM with 10% FBS-only treatment (set to 1.0) (presented as a dotted line). The TnC and TnR mixture (TCR) enhanced the expression of neuronal and glial cell genes, but not of synaptic genes. TnC and TnR individually inhibited neuronal and glial marker gene expressions. The data are presented as the mean±SD of one experiment that was derived from three independent experiments. *p<0.05, **p<0.01 (all vs. DMEM and 1% FBS). DMEM, Dulbecco's modified Eagle medium; FBS, fetal bovine serum.
Regulatory effects of tenascin coating on NBR-induced neural differentiation in the hMSCs
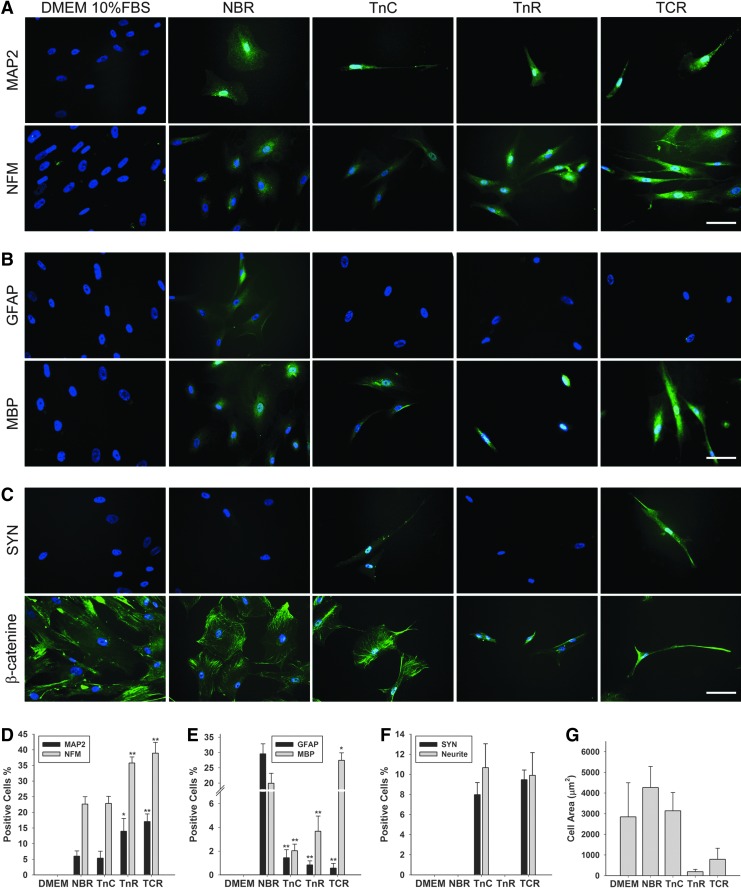
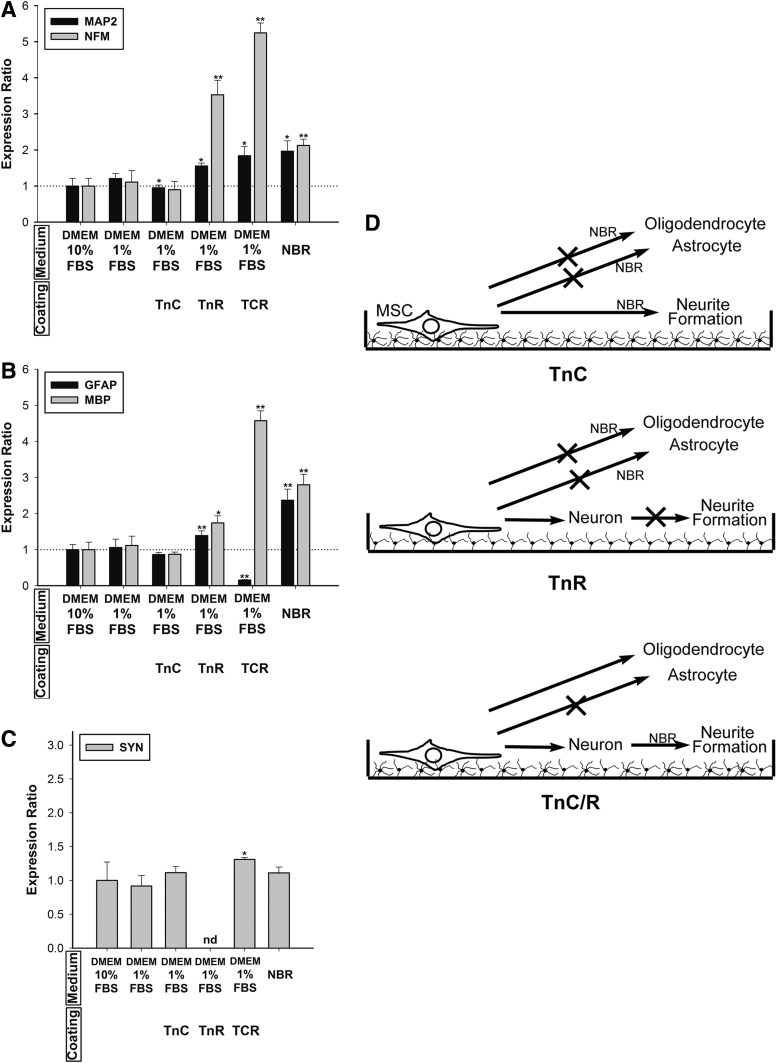
To mimic the ECM–cell interaction in the physiological environment of people, we coated tenascins onto dishes. The hMSCs were treated with NBR for 1 week, and then cultured on coated dishes for one more week. Coating TnC alone had no effect on mRNA expression of MAP2, whereas TnR-coated group increased MAP expression 2.2-fold. More significantly, in the TCR-coated groups, MSCs increased MAP2 expression 3.3-fold. Similarly, MSCs coated with TnR and TCR enhanced NFM expression 2.5-fold and 2.8-fold, respectively. TnC alone had no significant effect on neural gene expression. However, it increased neural differentiation in the combination with TnR (Fig. 3A). Furthermore, the immunocytochemical reaction of MAP2 and NFM Abs was correlated with mRNA expression (Fig. 4A). In the NBR group, the percentages of positive cells of MAP2 and NFM were 6.0% and 22.6%, respectively, and the addition of TnC did not significantly alter these percentages (5.3% and 22.8%). However, the percentages of MAP2- and NFM-positive cells increased in groups treated with TnR (13.9% and 35.7%, respectively) and TCR (17.0% and 38.9%, respectively) (Fig. 4D). To confirm the specificity of the antibodies, control tests were conducted, and the results are shown in Supplementary Figure S1.
FIG. 3.
Effects of TnC and TnR Coating on NBR-induced hMSCs. (A) The mRNA levels of neuronal markers, MAP2 and NFM; (B) glial cell markers, GFAP and MBP; and (C) the synapse marker, synapsin (SYN) were quantified after 7 days of cultivation on surfaces coated with Tns (20 μg/mL) in hMSCs pretreated with NBR for 1 week. Levels were normalized to levels observed in NBR-only treatment (set to 1.0) (presented as a dotted line). NBR-treated hMSCs on TCR-coated dishes promoted neuronal and synaptic gene expression; however, it inhibited GFAP gene expression. Data are presented as the mean±SD of one experiment that was derived from three independent experiments. **p<0.01 (all vs. NBR). nd, not determined.
FIG. 4.
Immunofluorescence of TnC and TnR coating on NBR-induced hMSCs. NBR-stimulated hMSCs cultured on Tn-coated surfaces is shown as (A) MAP2 and NFM; (B) glial cell markers, GFAP and MBP; (C) the synaptic marker, synapsin (SYN) and the cytoskeletal marker, β-catenin. The immunoreaction was examined after 7 days of cultivation on Tn-coated surfaces (20 μg/mL) in hMSCs pretreated with NBR for 1 week. DAPI (blue) was used as a counterstain. The DMEM group served as a control. When cells were cultivated on TnC- and TCR-coated surfaces, hMSCs exhibited neurite outgrowth. The white bar represents 50 μm. (D) Percentages of MAP2-positive cells and NFM-positive cells within all DAPI-positive cells; (E) percentages of GFAP-positive cells and MBP-positive cells within all DAPI-positive cells; (F) percentages of SYN-positive cells and neurite-positive cells within all DAPI-positive cells. All data are presented as the mean±SD. *p<0.05, **p<0.01 (all vs. NBR). (G) The cell areas, calculated by the β-catenin-positive cells. DAPI, 4′, 6-diamidino-2-phenylindole.
The following determination of tenascin-coating effects on neuro-glial and oligodendrocytic differentiation was shown in Figure 3B. The TnC, TnR, and TCR groups decreased GFAP expression from 86% to ∼98%. However, the oligodendrocyte marker MBP increased 1.6-fold in response to TCR coating, although neither TnC nor TnR coating alone altered MBP expression. Immunocytochemical evaluation revealed that NBR increased GFAP-positive cells by 29.5%, but TnC coating only increased GFAP-positive cells by 1.4%, and TnR coating and TCR coating only decreased GFAP-positive cells by 0.8% and 0.6%, respectively (Fig. 4B, E). Interestingly, compared with the NBR (20.0%) group, TnC (2.0%) and TnR (3.7%) decreased the percentage of MBP-positive cells, but TCR (27.4%) increased the numbers of MBP-positive cells (Fig. 4B, E). The immunocytochemical reaction of GFAP and MBP exhibited parallel mRNA expression effects in all of the groups—NBR, TnC, TnR, and TCR.
Subsequently, we observed the expression of the synaptic maker, synapsin. The hMSCs cultured with the combined TnC and TCR coating increased SYN expression 1.7- and 2.5-fold, respectively, in contrast to the complete inhibition observed in response to TnR coating (Fig. 3C). This finding further indicates that TnC acts to trigger the MSC toward mature neuronal differentiation. No immunoreactivity of SYN was found in the NBR and TnR groups, but TnC and TCR induced an increase of ∼10% in SYN-positive cells (Fig. 4C, F).
To understand and quantify the effects of tenascin-coating on morphological changes in NBR-treated hMSCs, we used the cytoskeleton protein, β-catenin, as an antitarget to calculate cell areas and numbers of neurite-positive cells (Fig. 4C, F, E). When left untreated, the hMSCs displayed a typical shape. When NBR was added to the medium on noncoated dishes, hMSCs increased in cell area, and became extended in shape, without any neurites (Fig. 4C, E). In the coated groups, NBR-treated cells on TnC-coated plates developed neurites and exhibited increased numbers of neurite-positive cells, but also exhibited enlarged cell bodies (Fig. 4C, F, E). TnR showed no neurite-positive cells, and highly decreased the cell area (Fig. 4C, F, E). The hMSCs cultured on TCR-coated plates showed neurites and a relatively small cell morphology, and the response to TCR combined the effects of TnC and TnR (Fig. 4C, F, E). The immunocytochemical reaction of neural markers was consistent with mRNA expression. These indicate that TCR enhances neuronal differentiation and induces a neuron-like morphology.
Effects of individual tenascin coating on neural differentiation in the hMSCs
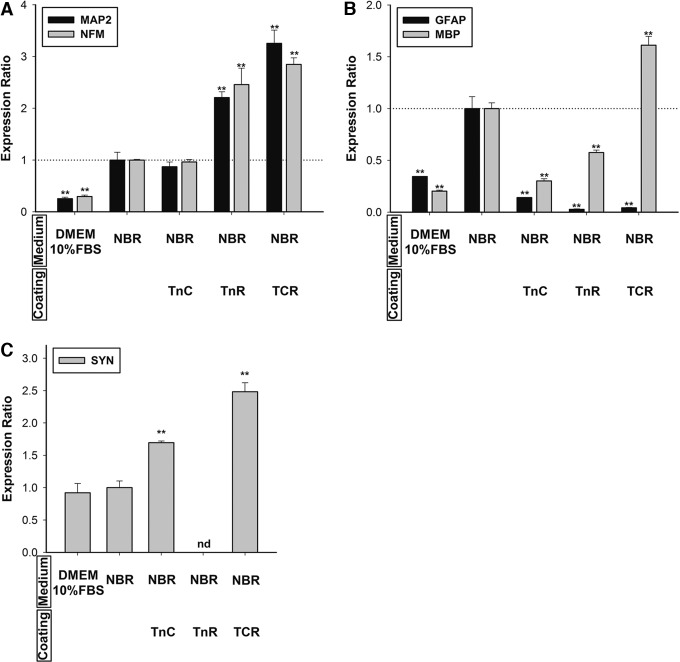
Determining the effect of NBR and each individual tenascin coating on transdifferentiation in the MSCs was our next step. TnC coating alone did not change neuronal, neuro-glial, oligodendrocytic, or synaptic differentiation (Fig. 5A–C). TnR or TCR alone increased MAP2 expression 1.55-fold and 1.84-fold, respectively. However, NFM expression showed a 3.53-fold increase in response to TnR and a 5.24-fold increase in response to TCR (Fig. 5A). TnR increased GFAP only 1.39-fold and MBP 1.74-fold. TCR dramatically triggered MBP expression (a 4.57-fold increase), but inhibited GFAP expression by 84% (Fig. 5B). We further examined the synaptic marker and found no significant change in response to TnR and TCR. However, TnR coating exhibited complete inhibition of SYN expression (Fig. 5C). TnC can trigger SYN expression with NBR treatment. TnR increases neuronal differentiation with or without NBR, but it completely inhibits neurite formation. TnR can inhibit synapse formation with or without NBR treatment. The combination of TnC and TnR not only promotes neuronal differentiation but also induces SYN expression and neurite outgrowth. Either TnC or TnR alone inhibits astrocyte or oligodendrocyte differentiation when incorporated with NBR. When TnC is combined with TnR, it significantly promotes oligodendrocyte differentiation, even while blocking astrocyte differentiation (Fig. 5D).
FIG. 5.
Effects of TnC and TnR coating on hMSCs without NBR induction. (A) The mRNA levels of neuronal markers, MAP2 and NFM; (B) glial cell markers, GFAP and MBP; and (C) the synapse marker, synapsin (SYN) were quantified after 7 days of cultivation on Tn-coated surfaces (20 μg/mL) in hMSCs. Levels were normalized to levels observed in DMEM 10% FBS-only treatment (set to 1.0) (presented as a dotted line). The hMSCs on TCR-coated dishes promoted neuronal and synaptic gene expression; however, they inhibited GFAP gene expression. TnC and TnR individually inhibited neuronal and glial marker gene expression. The data are presented as the mean±SD of one experiment that was derived from three independent experiments. *p<0.05, **p<0.01 (all vs. DMEM 1% FBS). (D) Summary of Tn coating on hMSCs. In neuronal differentiation, TnC triggers SYN expression with NBR treatment. TnR shows promotion for neuronal differentiation with or without NBR, but totally inhibits neurite formation. The combination of TnC and TnR not only promotes neuronal differentiation but also induces SYN expression and neurite outgrowth. Either TnC or TnR alone inhibits astrocyte or oligodendrocyte differentiation when incorporated with NBR. When TnC is combined with TnR, it significantly promotes oligodendrocyte differentiation, although it blocks astrocyte differentiation.
Dose effect of TCR-coating on neural differentiation in the hMSCs
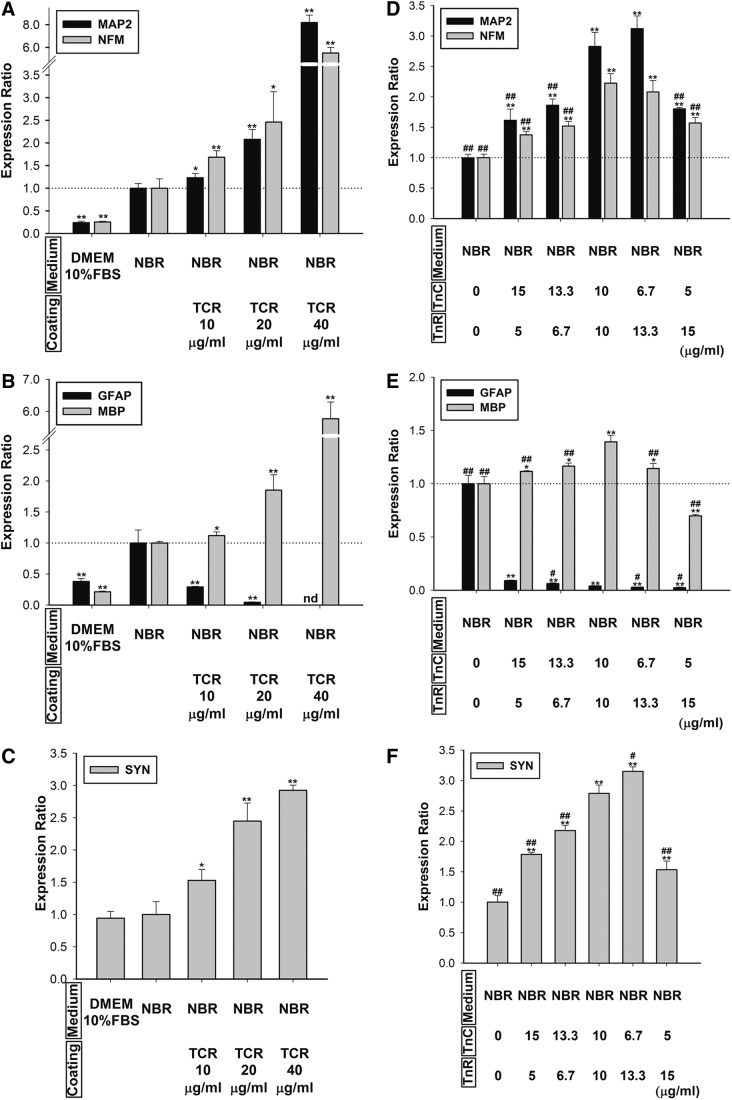
In our analysis, we determined the dose-dependent effect of TCR on neural differentiation in NBR-induced hMSCs. Compared to the NBR group, the results demonstrated that TCR promoted neuronal, synaptic, and oligodendrocyte marker expression in a dose-dependent manner (Fig. 6A–C). TCR-coating inhibited hMSC differentiation into astrocytes (Fig. 6B). The dosage effect suggests that TCR specifically induces neuronal transdifferentiation in hMSCs.
FIG. 6.
Effects of varied doses and ratios of TnC and TnR mixture (TCR) coating on NBR-induced hMSCs. (A) The mRNA levels of neuronal markers, MAP2 and NFM; (B) glial cell markers, GFAP and MBP; and (C) the synapse marker, synapsin (SYN), were quantified after 7 days of cultivation on TCR-coated surfaces (10–40 μg/mL) in hMSCs pretreated with NBR for 1 week. Levels were normalized to levels observed in NBR-only treatment (set to 1.0) (presented as a dotted line). NBR-treated hMSCs on TCR-coated dishes promoted neuronal and synaptic gene expressions, but inhibited GFAP gene expression in a dose-dependent manner. Data are presented as the mean±SD of one experiment that was derived from three independent experiments. *p<0.05, **p<0.01 (all vs. NBR). (D) The mRNA levels of neuronal markers, MAP2 and NFM; (E) glial cell markers, GFAP and MBP; and (F) the synaptic marker, synapsin (SYN), were quantified after 7 days' cultivation on TCR-coated dishes (different ratios: 3:1, 2:1, 1:1, 1:2, and 1:3, total 20 μg/mL) in NBR-induced hMSCs. Levels were normalized to levels observed in NBR-only treatment (set to 1.0) (presented as a dotted line). Data are presented as the mean±SD of one experiment that was derived from three independent experiments. *p<0.05, **p<0.01 (all vs. NBR). #p<0.05, ##p<0.01 (all vs. TCR 1:1).
Effects of different ratios of TnC and TnR-coating on neurogenic differentiation
We continued to determine five different ratios of TnC and TnR-coating (3:1, 2:1, 1:1, 1:2 and 1:3) in NBR-induced hMSCs to understand how TCR controls neurogenesis. The mRNA expressions of MAP2, NFM, MBP, and SYN showed an increase with the ratio of TnC to TnR 1:1 or 1:2 (Fig. 6D, F). In contrast, 1:3 ratios of TnC to TnR inhibited differentiation of oligodendrocytes (Fig. 6E). These results indicated that TCR-coating inhibited hMSC differentiation into astrocytes (Fig. 6E). hMSCs may differentiate into neurons or oligodendrocytes in an environment with a ratio of TnC to TnR between 1:1 to 1:2.
Effects of TCR coating mediated by integrins
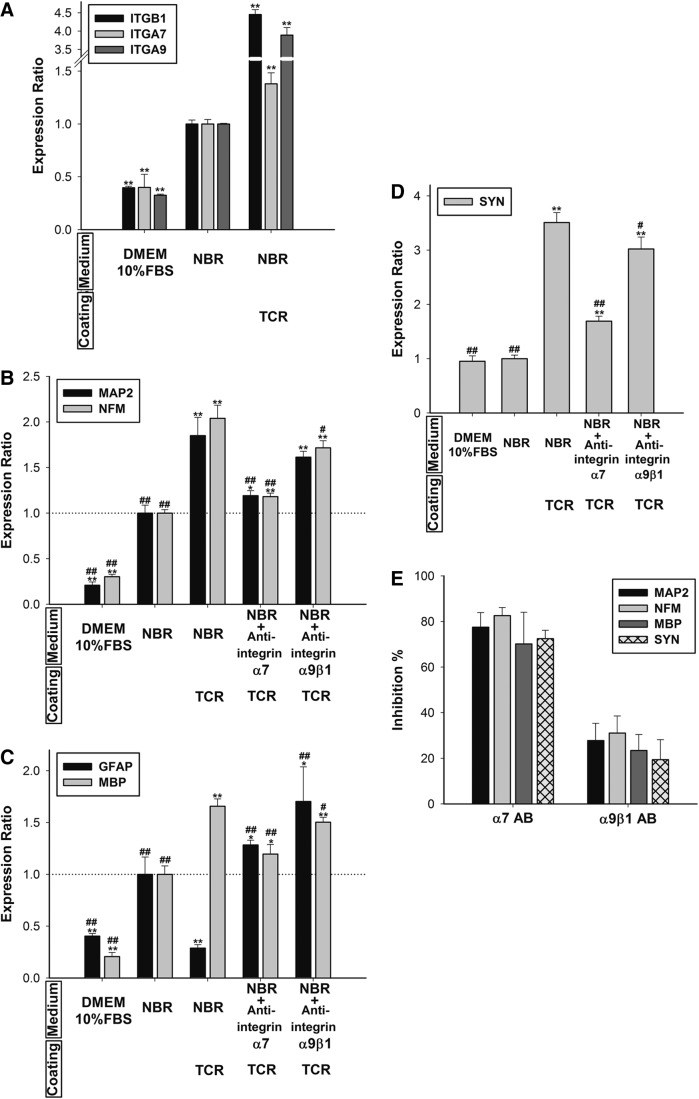
To understand how TCR regulates transdifferentiation, we investigated integrin signaling. TnC and TnR have been reported to control cell physiology through three integrins: α7, α9, and β1.19,20,26 We examined mRNA expression of integrins α7, α9, and β1 in a hMSC culture incorporated with the tenascin coatings. When TCR was added to the NBR medium, the hMSCs increased the expression of α7 by1.38-fold, α9 by 3.9-fold, and β1 by 4.45-fold (Fig. 7A). TCR-coating effects on neural differentiation might also be achieved through the signaling of these three integrins. Consequently, we used the integrin function-blocking antibodies to examine the signaling roles of TCR-coating effects. The qPCR results showed that α7 and α9β1 antibodies inhibited the expression of MAP2, NFM (Fig. 7B), MBP (Fig. 7C), and SYN (Fig. 7D). Furthermore, the α7 antibody level reduced to a greater extent (by ∼80%) than the α9β1 antibody did (∼30%) (Fig. 7E). Conversely, when cells were treated with the antibodies, the GFAP levels were restored to excess (Fig. 7C). The results demonstrated that the interaction of TCR with the α7 and α9β1 integrins is crucial for hMSC differentiation into neurons and oligodendrocytes. Furthermore, interfering with the integrin function promoted hMSC differentiation into astrocytes. Integrin α7 might act as a dominant receptor in neuronal differentiation of the MSC.
FIG. 7.
Effects of a TnC and TnR mixture (TCR) coating on NBR-induced hMSCs through the integrins, α7 and α9β1. The mRNA levels of integrins (A) were analyzed after 7 days of cultivation on TCR-coated surfaces (20 μg/mL) in hMSCs pretreated with NBR for 1 week. After adding integrin-blocking antibodies (5 μg/mL), mRNA levels of neuronal markers, (B) MAP2 and NFM; glial cell markers, (C) GFAP and MBP; and the synapse marker, (D) synapsin (SYN), were quantified after 7 days of cultivation on Tn-coated surfaces (20 μg/mL) in hMSCs pretreated with NBR for 1 week. Levels were normalized to levels observed in NBR only treatment (set to 1.0) (presented as a dotted line). Data are presented as the mean±SD of one experiment that was derived from three independent experiments. *p<0.05, **p<0.01 (all vs. NBR). #p<0.05, ##p<0.01 (all vs. NBR+TCR). (E) Inhibition percentages were calculated from (B–D).
Discussion
In this study, we found that tenascins regulate the neural differentiation in hMSCs, both in solution and in coated forms. Co-coating with TCR triggered hMSC synaptic marker expression and neurite outgrowth. These regulatory effects were mediated by the signaling of the α7 and α9β1 integrins. Two studies have revealed interactions between tenascin and neuronal differentiation in the stem (or progenitor) cell. Liao et al.26 showed that the EGF-like domain of TnR promoted mouse neuron stem cell (NSC) differentiation in neurons and oligodendrocyte progenitors, and also reduced the differentiation in astrocytes. FN6–8 inhibited NSC proliferation, whereas it promoted NSC differentiation in astrocytes and, to a lesser extent, in oligodendrocytes and neurons. TnR-overexpressing mouse ESCs also showed increased neuronal and reduced astrocytic differentiation after transplantation into the quinolinic acid-lesioned mouse striatum.25
Our data indicate that TnR-coating promoted NBR-induced neuron differentiation, but not neurite outgrowth. By itself, NBR showed upregulatory effects on neuro-glial differentiation; however, the effect was significantly inhibited in response to the addition of TnR. The regulatory functions of complete tenascin and each domain of tenascin appear to be different.31 Our results indicate that NBR might affect the different domains of TnR through unknown mechanisms, and shift the cell to an alternative differentiation outcome. We consider TnC to be significant in synapse formation and neurite outgrowth. TnC-knockout mice develop cerebral cortices that have a higher neuronal density than controls and pyramidal cells that have an abnormal dendritic morphology.32,33 Compared with wild-type mice, the CNS stem or precursor cells from TnC−/− mice exhibited accelerated differentiation into neurons and glial cells.34–36 These results suggest that TnC may not directly affect neuronal or glial differentiation, but that it may play a key role in neurite outgrowth.16,17,20 Hence, our results demonstrate the function of TnC in neuronal and glial differentiation from hMSCs.
Laminin (LM), an ECM molecule, has been shown to induce MSC neurogenesis and neurite outgrowth.37–41 The hMSCs cultured on LM1 showed an increase of MAP2 and NFM expression by immunohistochemistry and reverse transcription polymerase chain reaction.39 However, as those studies did not investigate synaptic markers or quantitative synaptic marker expression. In addition, LM is relatively lower in content within the brain42 and is absent in the perineuronal net that surrounds the cell body of various nerve cells and extends along their dendrites.43 Understanding the complete neurogenic mechanisms of ECM in hMSC differentiation is required in the clinic. Our data might link the varied differentiation signaling of ECM tenascins with the cytokines of NBR. Our qPCR results demonstrate neuronal differentiation in hMSCs. We identified morphologic changes and examined the expression of the synaptic marker, SYN. However, the signaling pathway remains unclear and identifying the signal cascades in the future is necessary.
In our study, soluble tenascin had no effect on SYN expression, but the coated form of TnC or TCR induced its expression. Conversely, the expression of the astrocyte marker, GFAP, was strongly inhibited in the tenascin-coated groups. This suggests that synapse formation and astrocyte differentiation are significantly modified by the microenvironment changes and adhesive protein interactions within the tenascin-coated plates. In a recent study, hMSCs displayed an elongated shape when cultured on nanopatterned poly(dimethylsiloxan) (PDMS), and synaptophysin was induced with or without RA.44 Jian et al. reported that aligned poly(ɛ-caprolactone) (PCL) nanofibers encapsulating 0.3 wt% RA also induced synaptic marker expression in hMSCs accompanied by an aligned and elongated cell morphology.45 These results indicate that cell morphologic changes occurring because of topography greatly modulate stem cell synapse formation. However, astrocyte differentiation appears to be uninhibited by these two artificial materials. Regardless, the nano materials increased GFAP expression by hMSCs.44,45 Among natural ECM materials, tenascin, not LM, exhibits a reduction in astrocyte differentiation.39
TnC and TnR appeared to be anti-adhesive ECM proteins.46 Another study showed that TnC inhibits the focal adhesion kinase (FAK) and RhoA signaling pathways through interactions between FN and syndecan-4.47 Compared with LM, TnC also displayed weak binding in neurons.48 Similarly, TnR inhibits FN-mediated adhesion in L cells and ganglion neurons.49 Tenascin inhibits cell adhesion and causes hMSCs to detach from the hard plastic surface and form neurites. Therefore, cell size appears smaller or maintains a cubic shape in our study. We assume that the modulation of adhesiveness plays a key role for neuronal differentiation in hMSCs.
Our results showed that the total inhibition rate of the α7 and α9 integrin antibodies was >100%. This indicates that the roles of these two integrins are more essential for MSC differentiation than NBR. Without tenascins, integrins cannot signal outward, which provides mechanical strength. Conversely, the effect of NBR may be reduced during hMSC neuronal differentiation. Blocking the α7 and α9 integrins the hMSC promotes astrocyte marker expression during differentiation into astrocytes. This further demonstrates that integrin is essential for neuronal differentiation in hMSC.
Regardless of whether it is in a soluble or in a coated form, compared to TnC or TnR only, TCR positively regulated neuronal differentiation in hMSCs. During development, TnC is expressed earlier in the morphogenesis stage of nervous system. TnR has a delayed and limited expression in the nervous system throughout the development to adulthood.50 Thus, to develop a mature neuron, neuronal stem cells and progenitor cells must grow in an environment that contains both TnC and TnR during development. As our study shows, cell cultures with TnC and TnR together appear to mimic natural physiology. Studies of tenascin-knockout mice support this point. Other studies have shown that both TnC- and TnR-knockout mice have normal lifespans and fertility without gross anatomical or histological abnormalities. However, TnC and TnR double-gene deletion might severely disrupt the nerve system morphogenesis process during development.51,52 Confirming this speculation requires further investigation.
Compared to tenascin coating on the dish, soluble tenascin had an opposite effect on hMSC neurogenesis. The difference might have been caused by a lack of support and mechanical strength when tenascin was dissolved in the medium. When tenascins were dissolved in the medium, they became soluble factors, comparable to cytokines. In other studies, TnC was reported to have an EGF-like domain binding affinity with the EGF receptor.53,54 Another study proposed TnC as an activator of Toll-like receptor 4 in arthritic joints.55 Hence, soluble tenascins trigger a different adhesive-independent pathway than that triggered by coated tenascins. Furthermore, tenascin dissolved in the medium might form an inhibitor that prevents integrin from binding to other constituents of the ECM. In addition, it affects neuronal differentiation in hMSCs. However, further investigation is necessary to determine whether TCR in the medium can significantly promote gene expression in a manner similar to TCR coating.
Conclusion
Together, soluble or coated forms of TnC, TnR, or TCR can facilitate neuronal or neuro-glial differentiation in hMSCs. This study provides evidence of the novel bioprocessing of MSCs. Precise control of tenascins in a cellular milieu could potentiate stem cell therapy for neurodegenerative diseases.
Supplementary Material
Acknowledgments
This study was funded by the National Science Council of Taiwan (grants NSC 99-2120-M-038-001 and 100-2120-M-038-002).
Disclosure Statement
No competing financial interests exist.
References
- 1.Studer L., Tabar V., and McKay R.D.Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat Neurosci 1,290, 1998 [DOI] [PubMed] [Google Scholar]
- 2.Borlongan C.V., Tajima Y., Trojanowski J.Q., Lee V.M., and Sanberg P.R.Cerebral ischemia and CNS transplantation: differential effects of grafted fetal rat striatal cells and human neurons derived from a clonal cell line. Neuroreport 9,3703, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., et al. . Multilineage potential of adult human mesenchymal stem cells. Science 284,143, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Woodbury D., Schwarz E.J., Prockop D.J., and Black I.B.Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res 61,364, 2000 [DOI] [PubMed] [Google Scholar]
- 5.Sanchez-Ramos J., Song S., Cardozo-Pelaez F., Hazzi C., Stedeford T., Willing A., et al. . Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol 164,247, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Trzaska K.A., Kuzhikandathil E.V., and Rameshwar P.Specification of a dopaminergic phenotype from adult human mesenchymal stem cells. Stem Cells 25,2797, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Seitz L.C., Abramczyk A.M., Liu L., and Chan C.cAMP initiates early phase neuron-like morphology changes and late phase neural differentiation in mesenchymal stem cells. Cell Mol Life Sci 68,863, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varghese S., Hwang N.S., Canver A.C., Theprungsirikul P., Lin D.W., and Elisseeff J.Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol 27,12, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Bi Y., Stuelten C.H., Kilts T., Wadhwa S., Iozzo R.V., Robey P.G., et al. . Extracellular matrix proteoglycans control the fate of bone marrow stromal cells. J Biol Chem 280,30481, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Armstrong S.J., Wiberg M., Terenghi G., and Kingham P.J.ECM molecules mediate both Schwann cell proliferation and activation to enhance neurite outgrowth. Tissue Eng 13,2863, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Chiquet-Ehrismann R.Tenascins. Int J Biochem Cell Biol 36,986, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Laywell E.D., Dorries U., Bartsch U., Faissner A., Schachner M., and Steindler D.A.Enhanced expression of the developmentally regulated extracellular matrix molecule tenascin following adult brain injury. Proc Natl Acad Sci U S A 89,2634, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawano H., Ohyama K., Kawamura K., and Nagatsu I.Migration of dopaminergic neurons in the embryonic mesencephalon of mice. Brain Res Dev Brain Res 86,101, 1995 [DOI] [PubMed] [Google Scholar]
- 14.Brodkey J.A., Laywell E.D., O'Brien T.F., Faissner A., Stefansson K., Dorries H.U., et al. . Focal brain injury and upregulation of a developmentally regulated extracellular matrix protein. J Neurosurg 82,106, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Meiners S., and Geller H.M.Long and short splice variants of human tenascin differentially regulate neurite outgrowth. Mol Cell Neurosci 10,100, 1997 [DOI] [PubMed] [Google Scholar]
- 16.Meiners S., Mercado M.L., Nur-e-Kamal M.S., and Geller H.M.Tenascin-C contains domains that independently regulate neurite outgrowth and neurite guidance. J Neurosci 19,8443, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meiners S., Powell E.M., and Geller H.M.Neurite outgrowth promotion by the alternatively spliced region of tenascin-C is influenced by cell-type specific binding. Matrix Biol 18,75, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Rigato F., Garwood J., Calco V., Heck N., Faivre-Sarrailh C., and Faissner A.Tenascin-C promotes neurite outgrowth of embryonic hippocampal neurons through the alternatively spliced fibronectin type III BD domains via activation of the cell adhesion molecule F3/contactin. J Neurosci 22,6596, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews M.R., Czvitkovich S., Dassie E., Vogelaar C.F., Faissner A., Blits B., et al. . Alpha9 integrin promotes neurite outgrowth on tenascin-C and enhances sensory axon regeneration. J Neurosci 29,5546, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mercado M.L., Nur-e-Kamal A., Liu H.Y., Gross S.R., Movahed R., and Meiners S.Neurite outgrowth by the alternatively spliced region of human tenascin-C is mediated by neuronal alpha7beta1 integrin. J Neurosci 24,238, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones F.S., and Jones P.L.The tenascin family of ECM glycoproteins: structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn 218,235, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Pesheva P., Spiess E., and Schachner M.J1-160 and J1-180 are oligodendrocyte-secreted nonpermissive substrates for cell adhesion. J Cell Biol 109,1765, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zacharias U., Leuschner R., Norenberg U., and Rathjen F.G.Tenascin-R induces actin-rich microprocesses and branches along neurite shafts. Mol Cell Neurosci 21,626, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Zacharias U., and Rauch U.Competition and cooperation between tenascin-R, lecticans and contactin 1 regulate neurite growth and morphology. J Cell Sci 119,3456, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Hargus G., Cui Y., Schmid J.S., Xu J., Glatzel M., Schachner M., et al. . Tenascin-R promotes neuronal differentiation of embryonic stem cells and recruitment of host-derived neural precursor cells after excitotoxic lesion of the mouse striatum. Stem Cells 26,1973, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Liao H., Huang W., Schachner M., Guan Y., Guo J., Yan J., et al. . Beta 1 integrin-mediated effects of tenascin-R domains EGFL and FN6-8 on neural stem/progenitor cell proliferation and differentiation in vitro. J Biol Chem 283,27927, 2008 [DOI] [PubMed] [Google Scholar]
- 27.Chen C.W., Tsai Y.H., Deng W.P., Shih S.N., Fang C.L., Burch J.G., et al. . Type I and II collagen regulation of chondrogenic differentiation by mesenchymal progenitor cells. J Orthop Res 23,446, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Ou K.L., Wu J., Lai W.F., Yang C.B., Lo W.C., Chiu L.H., et al. . Effects of the nanostructure and nanoporosity on bioactive nanohydroxyapatite/reconstituted collagen by electrodeposition. J Biomed Mater Res A 92,906, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Liao J., Guo X., Grande-Allen K.J., Kasper F.K., and Mikos A.G.Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials 31,8911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kouris N.A., Squirrell J.M., Jung J.P., Pehlke C.A., Hacker T., Eliceiri K.W., et al. . A nondenatured, noncrosslinked collagen matrix to deliver stem cells to the heart. Regen Med 6,569, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.To W.S., and Midwood K.S.Cryptic domains of tenascin-C differentially control fibronectin fibrillogenesis. Matrix Biol 29,573, 2010 [DOI] [PubMed] [Google Scholar]
- 32.Gurevicius K., Kuang F., Stoenica L., Irintchev A., Gureviciene I., Dityatev A., et al. . Genetic ablation of tenascin-C expression leads to abnormal hippocampal CA1 structure and electrical activity in vivo. Hippocampus 19,1232, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Irintchev A., Rollenhagen A., Troncoso E., Kiss J.Z., and Schachner M.Structural and functional aberrations in the cerebral cortex of tenascin-C deficient mice. Cereb Cortex 15,950, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Garcion E., Halilagic A., Faissner A., and ffrench-Constant C.Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development 131,3423, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Garwood J., Garcion E., Dobbertin A., Heck N., Calco V., ffrench-Constant C., et al. . The extracellular matrix glycoprotein Tenascin-C is expressed by oligodendrocyte precursor cells and required for the regulation of maturation rate, survival and responsiveness to platelet-derived growth factor. Eur J Neurosci 20,2524, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Karus M., Denecke B., ffrench-Constant C., Wiese S., and Faissner A.The extracellular matrix molecule tenascin C modulates expression levels and territories of key patterning genes during spinal cord astrocyte specification. Development 138,5321, 2011 [DOI] [PubMed] [Google Scholar]
- 37.Lee J.H., Yu H.S., Lee G.S., Ji A., Hyun J.K., and Kim H.W.Collagen gel three-dimensional matrices combined with adhesive proteins stimulate neuronal differentiation of mesenchymal stem cells. J R Soc Interface 8,998, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mruthyunjaya S., Manchanda R., Godbole R., Pujari R., Shiras A., and Shastry P.Laminin-1 induces neurite outgrowth in human mesenchymal stem cells in serum/differentiation factors-free conditions through activation of FAK-MEK/ERK signaling pathways. Biochem Biophys Res Commun 391,43, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Mruthyunjaya S., Rumma M., Ravibhushan G., Anjali S., and Padma S.c-Jun/AP-1 transcription factor regulates laminin-1-induced neurite outgrowth in human bone marrow mesenchymal stem cells: role of multiple signaling pathways. FEBS Lett 585,1915, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Neal R.A., McClugage S.G., Link M.C., Sefcik L.S., Ogle R.C., and Botchwey E.A.Laminin nanofiber meshes that mimic morphological properties and bioactivity of basement membranes. Tissue Eng Part C Methods 15,11, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qian L., and Saltzman W.M.Improving the expansion and neuronal differentiation of mesenchymal stem cells through culture surface modification. Biomaterials 25,1331, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Ruoslahti E.Brain extracellular matrix. Glycobiology 6,489, 1996 [DOI] [PubMed] [Google Scholar]
- 43.Wang D., and Fawcett J.The perineuronal net and the control of CNS plasticity. Cell Tissue Res 349,147, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Yim E.K., Pang S.W., and Leong K.W.Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Exp Cell Res 313,1820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang X., Cao H.Q., Shi L.Y., Ng S.Y., Stanton L.W., and Chew S.Y.Nanofiber topography and sustained biochemical signaling enhance human mesenchymal stem cell neural commitment. Acta Biomater 8,1290, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Orend G., and Chiquet-Ehrismann R.Adhesion modulation by antiadhesive molecules of the extracellular matrix. Exp Cell Res 261,104, 2000 [DOI] [PubMed] [Google Scholar]
- 47.Midwood K.S., Valenick L.V., Hsia H.C., and Schwarzbauer J.E.Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol Biol Cell 15,5670, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gotz B., Scholze A., Clement A., Joester A., Schutte K., Wigger F., et al. . Tenascin-C contains distinct adhesive, anti-adhesive, and neurite outgrowth promoting sites for neurons. J Cell Biol 132,681, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pesheva P., Probstmeier R., Skubitz A.P., McCarthy J.B., Furcht L.T., and Schachner M.Tenascin-R (J1 160/180 inhibits fibronectin-mediated cell adhesion—functional relatedness to tenascin-C. J Cell Sci 107 (Pt 8),2323, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Chiquet-Ehrismann R., and Tucker R.P.Tenascins and the importance of adhesion modulation. Cold Spring Harb Perspect Biol 3, pii:, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pesheva P., and Probstmeier R.The yin and yang of tenascin-R in CNS development and pathology. Prog Neurobiol 61,465, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Mackie E.J., and Tucker R.P.The tenascin-C knockout revisited. J Cell Sci 112 (Pt 22),3847, 1999 [DOI] [PubMed] [Google Scholar]
- 53.Swindle C.S., Tran K.T., Johnson T.D., Banerjee P., Mayes A.M., Griffith L., et al. . Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol 154,459, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Iyer A.K., Tran K.T., Griffith L., and Wells A.Cell surface restriction of EGFR by a tenascin cytotactin-encoded EGF-like repeat is preferential for motility-related signaling. J Cell Physiol 214,504, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Midwood K., Sacre S., Piccinini A.M., Inglis J., Trebaul A., Chan E., et al. . Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 15,774, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.