Abstract
Purpose
BRAF is frequently activated by gene fusion or point mutation in pilocytic astrocytoma, the most common pediatric brain tumor. We investigated the functional effect of constitutive BRAF activation in normal human neural stem and progenitor cells to determine its role in tumor induction in the brain.
Experimental Design
The constitutively active BRAFV600E allele was introduced into human neurospheres, and its effects on MAPK (mitogen-activated protein kinase) signaling, proliferation, soft agarose colony formation, stem cell phenotype, and induction of cellular senescence were assayed. Immunohistochemistry was used to examine p16INK4a levels in pilocytic astrocytoma.
Results
BRAFV600E expression initially strongly promoted colony formation but did not lead to significantly increased proliferation. BRAFV600E-expressing cells subsequently stopped proliferating and induced markers of oncogene-induced senescence including acidic β-galactosidase, PAI-1, and p16INK4a whereas controls did not. Onset of senescence was associated with decreased expression of neural stem cell markers including SOX2. Primary pilocytic astrocytoma cultures also showed induction of acidic β-galactosidase activity. Immunohistochemical examination of 66 pilocytic astrocytomas revealed p16INK4a immunoreactivity in the majority of cases, but patients with tumors negative for p16INK4a had significantly shorter overall survival.
Conclusions
BRAF activation in human neural stem and progenitor cells initially promotes clonogenic growth in soft agarose, suggesting partial cellular transformation, but oncogene-induced senescence subsequently limits proliferation. Induction of senescence by BRAF may help explain the low-grade pathobiology of pilocytic astrocytoma, whereas worse clinical outcomes associated with tumors lacking p16INK4a expression could reflect failure to induce senescence or an escape from oncogene-induced senescence.
Introduction
Pilocytic astrocytoma is the most common childhood brain tumor (1). It often follows an indolent course, with waxing and waning growth kinetics, and some cases of spontaneous regression have been reported (2, 3). Tumors frequently respond to treatment with chemotherapy or radiation therapy, but they can regrow after a period of quiescence, requiring further therapy (2, 4). Several laboratories have identified constitutive activation of the RAS/RAF pathway as the primary molecular alteration in pilocytic astrocytoma (5–11). The most common alteration is a tandem duplication at chromosome 7q34 involving BRAF, an upstream regulator of the MAPK (mitogen-activated protein kinase) pathway. In most cases, the regulatory domain of BRAF is lost, and the catalytic domain is fused to KIAA1549, causing constitutive activation of downstream signaling, as measured by extracellular-signal regulated kinase (ERK) activation (8, 12). A subset of pilocytic astrocytoma lacking the KIAA1549:BRAF fusion instead contain mutations at BRAF codon 600, resulting in a single amino acid substitution (BRAFV600E) that also constitutively activates the kinase (6, 7, 9, 10, 12, 13). Finally, point mutations in KRAS, fusions involving RAF1 or small insertions activating BRAF, have been identified in rare cases (5, 7, 11, 14).
Activation of BRAF in melanocytic nevi and melanoma has been associated with a process known as "oncogene-induced senescence," in which an inciting oncogenic stimulus also limits neoplastic growth via induction of cellular senescence (15). The mechanisms by which BRAF activation leads to transformation of neural cells into pilocytic astrocytomas are not yet clear, but their often indolent growth pattern suggests that oncogene-induced senescence may play a role. To address this, we used human neural stem and progenitor cells isolated from first trimester fetal autopsy specimens and grown in long-term culture (16, 17) to examine the effects of BRAF activation on cells from which pilocytic astrocytoma are thought to arise (18). We found that expression of BRAFV600E in neurospheres derived from cerebral cortex initially promoted colony formation in soft agar, but the cells subsequently became senescent, with increased expression of acidic β-galactosidase, PAI-1 and p16INK4a. This suggests that growth of pilocytic astrocytoma may be regulated in part by the induction of senescence.
Materials and Methods
Neurosphere cell culture
Cells were obtained from first trimester human fetal autopsy specimens in concordance with German law and Ethics Board evaluation. The study was also approved by the Johns Hopkins Institutional Review Board. Cells from cerebral cortex were microdissected and processed as described previously (19) and then passaged in neurosphere media [30% Ham’s F12 medium, 70% DMEM, 5% B27 reagent (Invitrogen), 1% l-glutamine, 1% antibiotic-antimycotic, 5 µg/mL heparin, 20 ng/mL fibroblast growth factor (FGF), and 20 ng/mL endothelial growth factor (EGF)]. Cells were split at high density after incubation with Accutase (Sigma–Aldrich) and gentle trituration.
DNA constructs and viral infection
The construct encoding BRAFV600E was purchased from Addgene (Addgene plasmid 15269; ref. 20). The BRAFV600E cDNA was subcloned into the lentiviral vector pWPI (Addgene plasmid 12254; constructed and submitted by Didier Trono). Lentiviral particles were produced by transfecting 293T cells with VSVG envelope plasmid, delta 8.9 gag/pol plasmid, and the plasmid of interest, as described previously (21), using Fugene (Roche) per the manufacturer’s instructions. After 24 hours, the medium was changed to FGF/EGF neurosphere media, and supernatants were collected at 48 and 72 hours. These supernatants were pooled, passed through a 0.45-µm filter, and then frozen at −80°C until needed. Human neural stem and progenitor cells growing as neurospheres were triturated to small sphere size and then incubated with lentiviral supernatants in the presence of Polybrene for 24 hours. After approximately 1 week in culture, spheres could be identified, and they were then individually placed into wells of a 24-well plate. Spheres were visually scored for green fluorescent protein (GFP) positivity. Control cells were infected with pWPI empty vector, which constitutively expresses GFP. These cells were found to proliferate and behave identically to uninfected parental cells, and both parental and GFP cells were used as controls.
Establishment of pilocytic astrocytoma primary culture
Pilocytic astrocytoma primary cultures were established following the protocol previously described for primary glioblastoma cultures (22, 23). Freshly resected pilocytic astrocytoma tumors were placed into basal medium and dissected into 1-mm3 sections. Tumor sections were then placed in papain solution (Sigma–Aldrich) and incubated at 37°C for 15 minutes. Dissociated tissue was passed through a Pasteur pipette several times, then centrifuged, and washed once with 10% serum-containing DMEM/Ham’s F12 media. Cells were then plated in serum-containing DMEM/F12 media in tissue culture flasks.
Assays of cell proliferation, senescence, and telomere length
Bromodeoxyuridine (BrdU) assays were carried out as described previously (24). Cells were cytospun on to positively charged slides. After washing cells once with PBS, they were fixed with 4% paraformaldehyde for 15 minutes, permeabilized with 0.1% Triton/PBS, denatured with 0.1N HCl, washed in PBST (1× PBS Tween-20), and blocked with 5% normal goat serum/PBST or 5% BSA/PBST, and then incubated with the appropriate primary antibody. Anti-BrdU antibody was used as per the manufacturer’s directions (Sigma B2531) at 1:100 dilution. After washing 3 times with PBST, cells were incubated for 45 minutes in the dark with the appropriate Cy-2- or Cy-3–conjugated secondary antibody (Jackson Immunoresearch). Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), mounted with antifade (Vectastain), and visualized by fluorescence microscopy.
Acidic β-galactosidase staining was done as per the manufacturer’s instructions (Cell Signaling Technology kit #9860). Human neurosphere cells were plated on Matrigel-coated 24-well plates and incubated at 37°C for 48 hours to allow cells to extend processes before staining. Cells were then washed with PBS and fixed with a fixing solution. After 15 minutes, fixative was removed, and cells were washed twice with PBS and then incubated with staining solution. After staining, cells were washed with PBS and covered with 70% glycerol and photographed using a inverted light microscope. FISH of telomere length in GFP compared with BRAFV600E-infected cells was carried out as previously described using cytospun cells (25).
Western blotting
Western blotting was carried out as described (24). Antibodies were used as per the manufacturer’s instructions and were as follows: BRAF (Santa Cruz #SC-1660), p16INK4a (Santa Cruz #SC-56330), SOX2 (Santa Cruz #SC-17320), OLIG2 (Millipore AB9610), NESTIN (Millipore MAB 5326), glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Research Diagnostics #10R-G109a), ERK (Cell Signaling Technologies #9102), and phospho-ERK (p-ERK; Cell Signaling Technologies #4376). Blots that have had intervening lanes removed are clearly marked with a white vertical line.
Colony formation in soft agarose
A 2× concentration of the neurosphere medium was prepared and mixed 1:1 with 1% melted agarose (Invitrogen) in water to make bottom agar, which was used to coat each well of a 6-well plate. Cells were incubated in Accutase (Sigma) and triturated by pipetting through a P1000 pipette to single cell density and placed into the top agarose/soft agarose mixture (0.5%) and immediately plated into 6-well plates at a density of 20,000 cells per well in 1.5 mL of agarose. After the agarose polymerized, 2 mL of the normal growth medium was placed into each well. Media were changed every 7 days, and the cultures were incubated for 4 to 6 weeks. Colonies were visualized by staining with 0.005% cresyl violet in PBS for 3 hours at room temperature. Colonies greater than 50 µm in diameter were scanned and counted using MCID Elite software.
Clinical specimens
Pilocytic astrocytoma and control tissues were obtained from the Johns Hopkins University School of Medicine Department of Pathology, with Institutional Review Board approval. All cases from 1990 to 2009 were reviewed to confirm the diagnosis of pilocytic astrocytoma and to determine whether sufficient material was still present in the paraffin block to be used in the construction of a tissue microarray. All cases that met these criteria (a total of 77) were included in the study. One older case (from 1988) and 2 pediatric infiltrating astrocytomas (grade 2) were included in the array to yield a total of 80 tumors, but the infiltrating astrocytomas were not included in the analyses described in this article. Tumor classification and cellularity were independently confirmed by 2 neuropathologists (D.J. and C.G.E.). No tumors were of the pilomyxoid variant. The tissue array was constructed as previously described (26), with 4 cores 0.6 mm in diameter taken from each tumor. Consistent with recently published reports, for purposes of data analysis, tumor location in the hypothalamus, optic chiasm, thalamus, and brainstem was designated as unfavorable whereas all other locations including cerebellum/posterior fossa, cerebral cortex, spinal cord, optic nerve, and fornix were considered favorable (27, 28). Of the 78 tumor samples, 66 were from primary tumor resections and had sufficient material to be scored for p16INK4a immunohistochemistry.
Immunohistochemistry
Sections from the pilocytic astrocytoma microarray were stained in the Johns Hopkins Hospital Pathology Department Central Laboratory according to standard clinical protocols using prediluted p16INK4a antibody (MTM Laboratories #CMA802) and p53 antibody (Ventana #760-2542; Roche). Only tumors with 2 or more evaluable cores were scored, and staining was classified using the following scale: strong (>50% of tumor cells showing immunoreactivity), moderate (between 10% and 50% of tumor cells showing immunoreactivity), weak (up to 10% of tumor cells showing immunoreactivity), and negative (no tumor cells showing immunoreactivity).
Statistical analysis
Statistical tests for laboratory experiments were carried out using GraphPad Prism (GraphPad Software) or Excel (Microsoft). All tests were 2-sided unless otherwise indicated, and P values less than 0.05 were considered significant. Error bars in all graphs represent SEM, unless otherwise indicated.
Stata 11 (StataCorp) was used for the descriptive and multivariate clinical analyses. Baseline characteristics of study patients and their tumors were evaluated according to p16INK4a expression status. P values were calculated with the use of Fisher’s exact test for race, location, extent of resection, and therapy, the χ2 test for sex, and the t test with unequal variance for age and follow-up time. Spearman’s correlation tests were carried out to determine correlation between covariates.
An initial bivariate analysis with Fisher’s exact test was carried out to estimate the association between p16INK4a expression status and mortality. Survival comparisons were made between p16INK4a negative cases and p16INK4a positive cases, with the latter defined as samples showing weak, moderate, or strong expression. We then conducted a survival analysis using the Kaplan–Meier estimator, and the log-rank test was used to compare survival distributions across groups. A univariate and multivariate Cox proportional hazards regression analysis was carried out with overall survival as the dependent variable. Risk factors including age, sex, race, extent of resection, location of tumor, and therapy modality were considered for final model selection. The proportional hazards assumption was evaluated using complementary log–log plots and Schoenfeld residual plots.
Results
Expression of constitutively active BRAFV600E activates the MAPK pathway in neural stem and progenitor cells and promotes soft agar colony formation
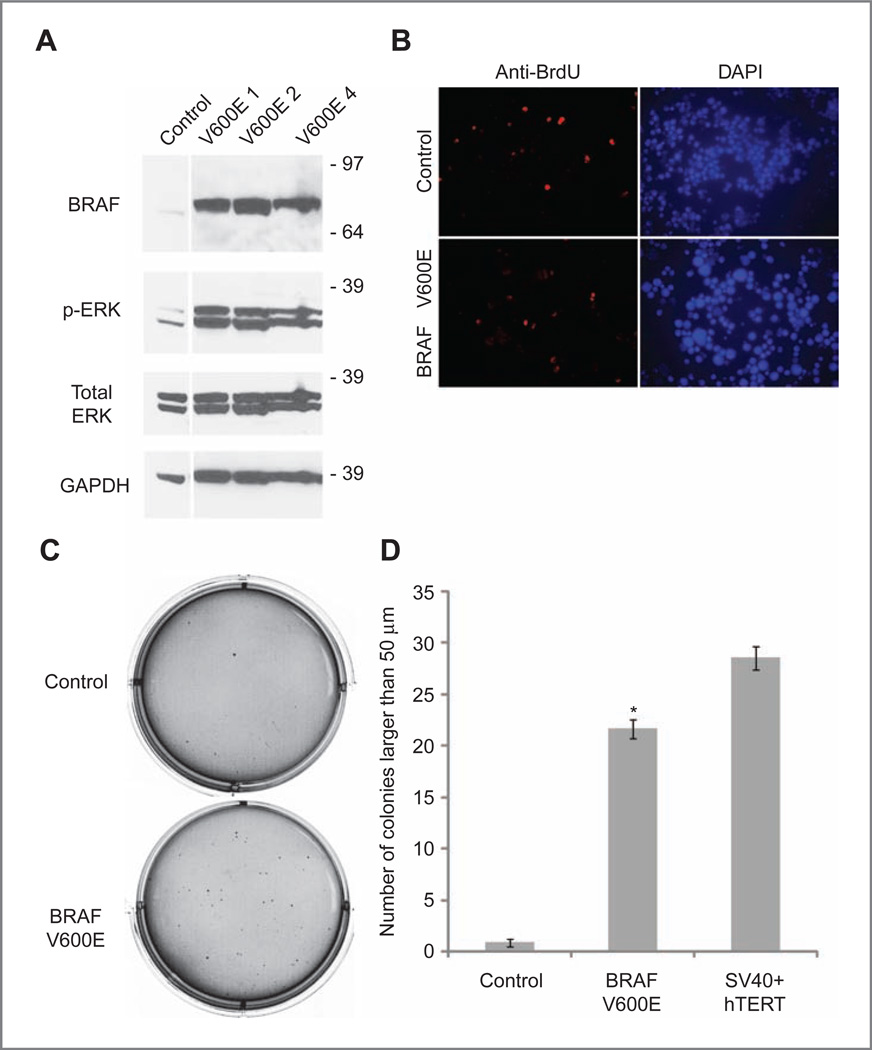
Normal neurospheres derived from developing human brain express markers for neural stem cells such as SOX2, OLIG2, and NESTIN and have the capability to differentiate into neurons, astrocytes, and oligodendroglia in vitro and in vivo (16, 17). To test the hypothesis that activation of BRAF would lead to transformation of human neural stem and progenitor cells, we infected neurospheres derived from human fetal cerebral cortex with a lentiviral construct containing constitutively active BRAFV600E. Bulk cultures and subclones derived from single neurospheres including BRAFV600E 1, 2, and 4 expressed high levels of BRAF as compared with controls (Fig. 1A). Cells transduced with BRAFV600E also showed activation of downstream signaling, as evidenced by increased levels of p-ERK (Fig. 1A). These experiments were repeated with cortical neural stemcells isolated from an additional brain with similar results (Supplementary Fig. S1A).
Figure 1.
ERK activation, cellular proliferation, and colony formation in soft agarose following BRAFV600E transduction. A, Western blot showing that normal human neural stem and progenitor cells transduced with BRAFV600E lentivirus express higher levels of activated p-ERK than control neurospheres. V600E 1–4 represent separate subclones transduced with BRAFV600E. B, proliferation as measured by BrdU incorporation is initially similar between control cells (top) and BRAFV600E-infected cells (bottom), with 11% of control cells BrdU positive and 13% of BRAFV600E cells BrdU positive, P = 0.33, Student's t test. C, soft agarose experiment, showing that control cells (top) do not form large numbers of colonies compared with BRAFV600E-transduced cells. D, quantification of soft agarose colony formation, including SV40 large T antigen and hTERT-transduced cells as positive controls. BRAFV600E-infected cells show statistically significant increase in colony formation compared with control cortex neurosphere cells. *, P < 0.0001, control versus BRAFV600E-infected cells, Student's t test.
Expression of BRAFV600E did not lead to a dramatic increase in cell proliferation (Fig. 1B), with a nonsignificant 2% increase in the percentage of cells incorporating BrdU as compared with vector-infected controls (control = 11% BrdU positive, BRAFV600E = 13% BrdU positive; P = 0.33, t test). To test the hypothesis that expression of constitutively active BRAF leads to the transformation of neural stem and progenitor cells, early-passage BRAFV600E-transduced neurospheres were placed into soft agarose. After approximately 6 weeks in culture, BRAFV600E-expressing cells formed a significantly larger number of colonies in soft agarose than in control cells (Fig.1Cand D; P < 0.001, t test). Interestingly, constitutively active BRAF was almost as effective in promoting growth in soft agar as the potent oncogenic combination of SV40 large T antigen and hTERT (Fig. 1D).
BRAFV600E expression promotes senescence in human cortical neurospheres
Although their initial proliferation rate was equal to that of control cells, after approximately 5 passages in culture, the BRAFV600E-transduced cells showed significantly decreased proliferation compared with controls (Fig. 2A and B; P < 0.05). Proliferation eventually completely ceased over the next few passages, though the cells appeared viable by light microscopy. In concert with this decreased growth, both the nuclear diameter and overall size of the cells growing in suspension increased, a change similar to the phenotype previously associated with oncogene-induced senescence (15). In contrast, at the point that the BRAFV600E-expressing cells could no longer be passaged, GFP-transduced control cultures were still proliferating and had no change in their morphology (Fig. 2A; Supplementary Fig. S2).
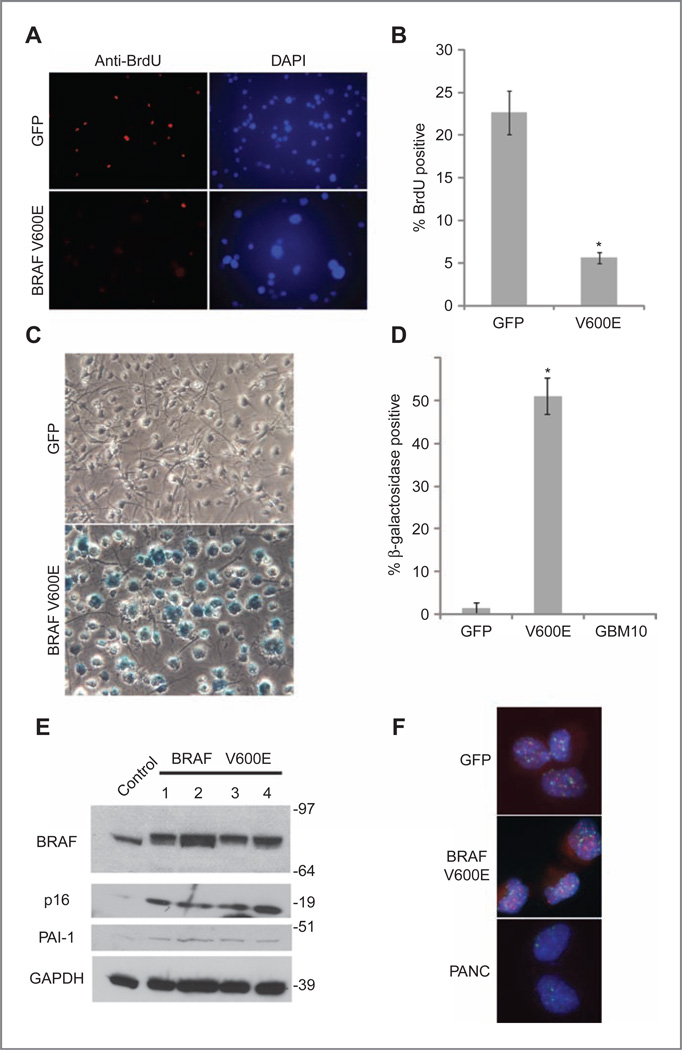
Figure 2.
BRAFV600E-expressing cells upregulate senescence markers and show decreased proliferation after several passages. A, immunofluorescence 200× photomicrographs of BrdU (red) and DAPI (blue)-stained GFP control and BRAFV600E-expressing neural stem and progenitor cells. After 5 passages, BRAFV600E-expressing neurosphere cells show increased nuclear size (DAPI) and decreased proliferation as measured by BrdU incorporation (bottom) compared with control neurospheres (top). B, quantification of BrdU positivity, showing percentage of BrdU-positive cells. *, P < 0.05, GFP versus BRAFV600E-infected cells, Student's t test. C, bright field 200× photomicrographs of acidic β-galactosidase activity in control GFP and BRAFV600E-infected neurosphere cells. Cells have been plated on an adherent substrate to allow for improved visualization of acidic β-galactosidase staining. Blue staining, indicating increased acidic β-galactosidase activity, is increased in BRAFV600E-transduced cells, which also become enlarged when compared with GFP-expressing controls. D, quantification of acidic β-galactosidase activity, comparing the mean percentage of β-galactosidase–positive cells in GFP control and BRAFV600E-transduced neural stem and progenitor cells. *, P < 0.005 GFP versus BRAFV600E-infected cells, Student's t test. E, Western blotting showing increased p16INK4a and PAI-1 expression in late-passage BRAFV600E-expressing cells compared with controls. Senescent markers p16INK4a and PAI-1 are induced in late-passage BRAFV600E-expressing neurosphere cells compared with control neurosphere cells. BRAFV600E 1–4 represent subclones isolated from the bulk infected culture. Molecular weight markers are located at the right of the blot. F, FISH analysis reveals similar telomere length in GFP control and BRAFV600E-expressing neurosphere cells. Fluorescent 1000× photomicrographs of FISH-stained nuclei from similar passage GFP and BRAFV600E neurospheres. At this passage, BRAFV600E-transduced cells were highly senescent whereas GFP-expressing neurospheres continued to proliferate normally. Telomeres are labeled red, and centromeres are labeled green. Nuclei are counterstained with DAPI. PANC is a pancreatic carcinoma cell line with shortened telomeres and corresponding decreased red telomere signal.
To test the hypothesis that these cells had undergone oncogene-induced senescence, we conducted acidic β-galactosidase staining, which has been associated with the senescent phenotype in numerous studies (15, 29). We observed diffuse acidic β-galactosidase staining in BRAFV600E-transduced cells, a 35-fold increase in percentage as compared with control cultures transduced with the same vector-only–expressing GFP (Fig. 2C and D). A human glioblastoma neurosphere culture (JHH-GBM10) grown in the same medium did not express acidic β-galactosidase (Fig. 2D), indicating that it was not a nonspecific effect of oncogenic transformation. To further confirm the induction of oncogene-induced senescence following BRAF activation, we analyzed 2 additional markers in protein lysates. Immunoblotting showed increased expression of the senescence-associated markers p16INK4a (15) and PAI-1 (30) in BRAFV600E-transduced cells as compared with controls (Fig. 2E; Supplementary Fig. S1B and C).
The passage number of the cultures used in these studies was below the threshold at which senescence due to telomere shortening generally occurs. However, to confirm that the senescent phenotype we observed was not due to effects on telomeres, we assessed their length by using FISH as previously described (25). This revealed similar telomere lengths (red fluorescence signal) between control proliferating GFP-expressing control cells and senescent BRAFV600E-transduced cells, with no sign of overall shortening in either culture (Fig. 2F). A pancreatic carcinoma cell line (PANC), which is known to have shortened telomeres, exhibited reduced red fluorescence. Quantification of the telomere length showed that BRAFV600E-transduced cells did not have shorter telomeres than GFP-transduced cells at the same passage number—in fact their telomeres were somewhat longer (Supplementary Fig. S3).
Lack of expression of the senescence marker p16INK4a in pilocytic astrocytoma is associated with shorter survival
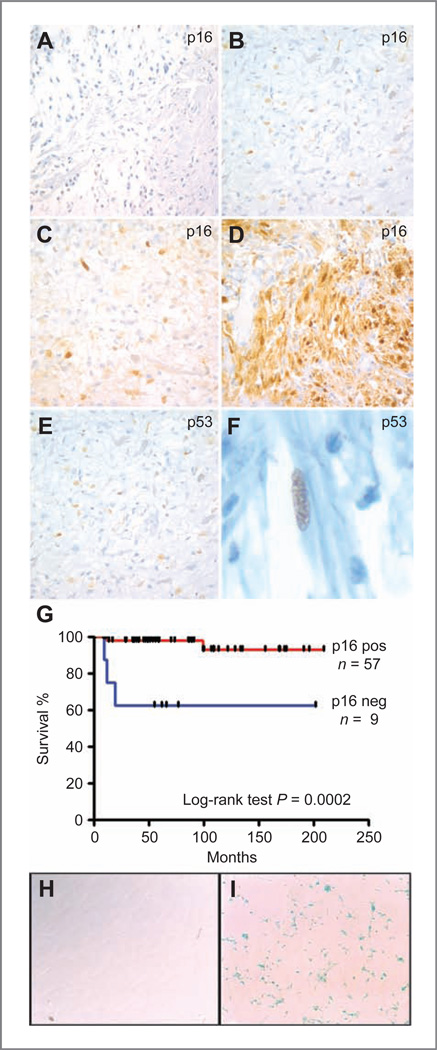
Examination of a tissue microarray containing 66 evaluable pilocytic astrocytomas showed that while 9 cases (14%) lacked p16INK4a protein (Fig. 3A), the majority (86%) exhibited weak, moderate, or strong immunoreactivity for this senescence marker (Fig. 3B–D). Table 1 summarizes the characteristics of the 66 tumors with evaluable p16INK4a staining. Most of the characteristics of the patients with p16INK4a-negative tumors, including those commonly associated with outcome, were similar to those with p16INK4a-positive tumors (location, time of follow-up, extent of resection, and need for additional therapy). There was a significant difference in the sex of the patients, with p16INK4a-negative patients being more likely to be female than p16INK4a-positive patients. There was a trend toward increasing age in the p16INK4a-negative patients, but this was not statistically significant.
Figure 3.
Pilocytic astrocytoma cells express markers of senescence in vivo and in vitro. Photomicrographs showing p16INK4a immunohistochemical staining in pilocytic astrocytoma specimens: negative (A); weak (B); moderate (C); and strong (D) p16INK4a immunohistochemical staining in pilocytic astrocytomas. E and F, immunohistochemical staining for p53 was largely negative, with only rare tumors showing scattered weakly staining cells. F, digitally expanded higher power view showing nuclear p53 staining in elongated tumor cell. (Original magnification 400× for A–F.) G, log-rank analysis of Kaplan–Meier curves showing significantly shorter overall survival in patients whose tumors were p16INK4a immunonegative (P = 0.0002). H and I, acidic β-galactosidase staining of an early-passage representative primary pilocytic astrocytoma culture. Bright field photomicrograph (100× power) showing prominent acidic β-galactosidase activity in primary pilocytic astrocytoma cells after 3 passages in culture (I), whereas human fibroblasts of equal density (H) do not stain after 10 passages.
Table 1.
Characteristics of study patients and their tumors according to p16INK4a status
| Total | p16INK4a positive | p16INK4a negative | P | |
|---|---|---|---|---|
| Patients in cohort | 66 | 57 | 9 | |
| Age, mean ± SD (range), y | 14 ± 12 (0.5–52) | 12 ± 10 (0.5–52) | 25 ± 17 (9–51) | 0.06 |
| Sex | ||||
| Male | 40 (60.6%) | 38 (66.7%) | 2 (22.2%) | 0.01a |
| Female | 26 (39.3%) | 19 (33.3%) | 7 (77.8%) | |
| Race | ||||
| White | 55 (83.3%) | 46 (80.7%) | 9 (100%) | 0.80 |
| Black | 7 (10.6%) | 7 (12.3%) | 0 (0%) | |
| Other | 4 (6.1%) | 4 (7%) | 0 (0%) | |
| Follow-up, mean ± SD (range), mo | 71 ± 55 (1–209) | 73 ± 54 (1–209) | 56 ± 62 (1–202) | 0.40 |
| Locationb | ||||
| Favorable | 40 (60.6%) | 33 (57.9%) | 7 (77.8%) | 0.50 |
| Unfavorable | 26 (39.4%) | 24 (42.1%) | 2 (22.2%) | |
| Extent of resection | ||||
| Gross total | 31 (47%) | 26 (45.6%) | 5 (55.6%) | 0.70 |
| Subtotal | 35 (53%) | 31 (54.3%) | 4 (44.4%) | |
| Therapyc | ||||
| Initial surgery only | 37 (56.1%) | 32 (56.1%) | 5 (55.6%) | 1.0 |
| Additional therapy | 29 (43.9%) | 25 (43.9%) | 4 (44.4%) | |
| Radiation | 18 (62.1%) | 15 (60%) | 3 (75%) | 0.70 |
| Repeat surgery | 24 (82.8%) | 21 (84%) | 3 (75%) | 1.0 |
| Chemotherapy | 7 (24.1%) | 6 (24%) | 1 (25%) | 1.0 |
| Outcome | ||||
| Alive | 61 (92.4%) | 55 (96.5%) | 6 (66.7%) | 0.02a |
| Died of disease | 5 (7.6%) | 2 (3.5%) | 3 (33.3%) |
NOTE: P values were calculated using Fisher's exact test for race, location, extent of resection, therapy, and outcome; χ2 test for sex; and t test with unequal variance for age and follow-up.
Statistically significant value of P < 0.05.
Unfavorable location was defined as a tumor located in the optic chiasm, thalamus, midbrain, or brain stem. All other locations were considered favorable.
Patients receiving additional therapy may have received more than 1 modality. Percentages associated with each modality are of the total receiving additional therapy.
Descriptive examination of characteristics of patients in this cohort regardless of p16INK4a staining showed positive Spearman’s rank correlation coefficients between the extent of resection and the location of tumor (Spearman ρ = 0.7, P < 0.001), the extent of resection and therapy modality (Spearman ρ = 0.59, P < 0.001), and the location of tumor and therapy modality (Spearman ρ = 0.41, P < 0.001), indicating high correlation between these variables. These correlations are similar to those previously reported in low-grade gliomas (27, 28).
We also examined expression of TP53, another marker of senescence. TP53 staining was much less intense and was detected in only 18 of 78 (23%) pilocytic astrocytoma samples (Fig. 3E and F). However, TP53 immunoreactivity correlated significantly with that of p16INK4a in the 66 cases in which both could be scored (Spearman ρ = 0.45, P = 0.0001).
p16INK4a protein expression also correlated with outcome, with bivariate analysis revealing that 33% (n = 3) of p16INK4a-negative patients died, compared with 3.6% (n = 2) of p16INK4a-positive patients (Fisher’s exact test, P = 0.02). Patients with tumors that were negative for p16INK4a also had significantly shorter overall survival when Kaplan–Meier survival curves were compared using the log-rank test (P = 0.0002; Fig. 3G). Univariate Cox proportional hazards regression analysis revealed that the relative risk of death was 13 times greater for patients with p16INK4a-negative tumors than p16INK4a-positive status (HR = 13.0, 95% CI: 2.1–80.5, P = 0.006). In multivariate analysis, we attempted to adjust for tumor location, type of resection, and therapy modality, which were highly correlated, but comparison groups included only 0 or 1 death, so it was not possible to control for these predictors given the small sample size.
Primary cultures of KIAA1549:BRAF fusion-containing pilocytic astrocytoma express markers of senescence
To further determine whether pediatric pilocytic astrocytoma undergoes oncogene-induced senescence, we examined the senescence marker acidic β-galactosidase in low-passage primary pilocytic astrocytoma cultures established from freshly resected tumors. In 2 separate cultures that were derived from tumors harboring KIAA1549:BRAF fusions, we observed expression of the senescence marker in more than half of the cells at low passage. A representative image is shown in Figure 3I.
Induction of senescence is associated with downregulation of neural stem cell markers, including SOX2
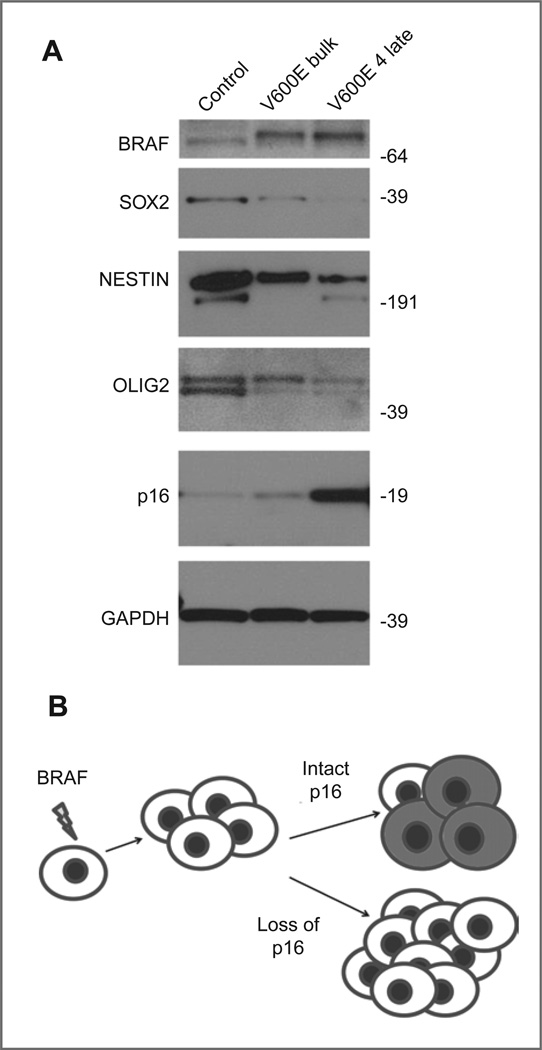
The neural stem cell and somatic cell reprogramming factor SOX2 is associated with the repression of p16INK4a-mediated senescence in multiple cell systems (31, 32). To investigate the mechanism behind the induction of senescence in BRAFV600E-transduced cells, we examined the expression of SOX2 and other markers of neural stem cells in neurospheres that had undergone senescence. Compared with GFP-infected control cells, high expression of BRAFV600E led over time to decreased expression of the neural stem cell markers SOX2, OLIG2, and NESTIN (Fig. 4A). Expression of p16INK4a increased as neural stem cell marker expression decreased. The experiment was repeated in cortex-derived neurospheres from another brain, with similar loss of SOX2 expression in late passage neurospheres (Supplementary Fig. S1B and C). While BRAFV600E-expressing neurospheres at early passage expressed SOX2 at levels comparable with GFP-infected controls (Supplementary Fig. S1B), after 5 passages, at the time when cells were exhibiting increased expression of senescent markers, the same BRAFV600E neurosphere subclone expressed less SOX2 than similar-passage GFP neurospheres (Supplementary Fig. S1C).
Figure 4.
Onset of senescence in BRAFV600E-expressing neurospheres corresponds to decreased expression of neural stem cell markers. A, Western blot showing decreased expression of neural stem cell markers in late-passage neurospheres (V600E 4) compared with the early-passage bulk culture infected with constitutively active BRAF. B, model for the formation of pilocytic astrocytoma. BRAF activation promotes initial transformation, but the induction of senescence (indicated by shaded cells) limits growth unless p16INK4a is deleted or silenced.
Discussion
Genetic alterations activating the RAS/RAF pathway, including BRAFV600E, are a hallmark of pediatric pilocytic astrocytomas. We hypothesized that introducing BRAFV600E into human neural stem and progenitor cells would lead to cellular transformation similar to that seen in pilocytic astrocytoma or other pediatric gliomas. Interestingly, although there is no significant proliferative advantage to expression of BRAFV600E in these neurospheres, neural stem and progenitor cells did show increased colony formation in soft agar, indicating that expression of BRAFV600E was sufficient to promote some aspects of oncogenic transformation. This is similar to results previously reported for activating mutations in BRAF in nonstem cells (10). However, within several passages of BRAF activation, proliferation slowed in these cells, and they subsequently stopped growing entirely.
Activation of BRAF by V600E substitution is a hallmark not only of melanoma but also of benign melanocytic nevi. In the latter, oncogene-induced senescence is thought to limit the tumorgenicity of BRAFV600E (15). The cessation of proliferation of our BRAFV600E-expressing cells in culture led us to investigate whether a similar cellular senescence phenotype might also operate in neural cells. BRAFV600E-expressing human fetal cortical neurosphere cells show increased acidic β-galactosidase expression compared with controls and have increased expression of the senescence-associated markers PAI-1 and p16INK4a. In primary human pilocytic astrocytomas, p16INK4a expression was identified using immunohistochemistry in 86% of the tumors on a tissue microarray containing 66 evaluable samples. Even in intensely stained tumors, there were piloid tumor cells without p16INK4a induction, suggesting that not all cells are uniformly senescent. This heterogeneity of p16INK4a expression may explain continued growth in a tumor that is also expressing markers of senescence.
CDKN2A locus deletion (encoding p16INK4a and p19ARF) has been reported in pediatric low-grade astrocytoma and is associated with higher-grade pediatric gliomas (27, 33, 34). Deletion of the locus is often associated with expression of activating mutations in BRAF in these tumors. Recently, homozygous deletions affecting p16INK4a were found selectively in more aggressive "anaplastic" pilocytic astrocytoma (33, 35). Consistent with this, we find that loss of p16INK4a protein expression is associated in univariate analysis with worse outcomes in the pilocytic astrocytoma patients represented on our tissue microarray. This worse outcome could not be explained by location of the tumor, because unfavorable locations (e.g., hypothalamus optic chiasm/thalamus/brainstem) and subtotal resections were not overrepresented in the p16INK4a-negative cohort (Table 1). A multivariate analysis was hampered by the relatively small size of our patient sample and the low number of deaths within the cohort. These data reveal the potential of p16INK4a expression status as a predictor of survival, although future studies with greater numbers of patients are clearly needed to further explore this association. If validated in larger cohorts, p16INK4a immunostaining may provide a tool for risk stratification in pilocytic astrocytoma. It also suggests that tumors in which cellular senescence does not occur (or tumors that escape from senescence) will behave in a more aggressive fashion.
Senescence due to telomere shortening has been investigated previously in pediatric low-grade gliomas (36). In our studies, we measured telomere length in senescent BRAFV600E-expressing neurospheres and showed that they were longer than those of nonsenescent, control GFP neurospheres of the same passage number. In our model system, shortened telomeres do not account for the senescence phenotype, although it is possible that this plays a role in vivo in pilocytic astrocytoma.
Loss of stem cell markers in our system was also temporally associated with the induction of senescence. SOX2 is a neural stem cell factor critical in somatic cell reprogramming and induced pluripotency. One of the functions of SOX2 is to suppress p16INK4a-induced cellular senescence (31, 32). In our model system, we observe downregulation of SOX2 associated with the induction of senescence, consistent with this previously reported role. We also observe downregulation of other neural stem cell markers, such as OLIG2 and NESTIN, suggesting this phenomenon may not be causally related to SOX2 but rather to depletion of stem cells in the culture.
In summary, we show that introduction of constitutively active BRAFV600E into human cortical stem and progenitor cells initially promotes clonogenic growth in soft agar but ultimately results in dramatically reduced proliferation and arrested growth of the culture. Because this coincides with the induction of senescent markers, it strongly suggests that "oncogene-induced senescence" similar to that seen in melanocytic cells can occur in neural tissues, and this may explain the often indolent growth of pilocytic astrocytoma. Furthermore, examination of p16INK4a expression in primary tumors suggests that escape from this process may underlie aggressive tumor growth. Further investigation of oncogene-induced senescence pathways may therefore provide new therapeutic opportunities in pilocytic astrocytoma.
Supplementary Material
Translational Relevance.
BRAF and the MAPK (mitogen-activated protein kinase) signaling cascade are active in the majority of pilocytic astrocytoma, but the oncogenic effects of these hallmark molecular changes are poorly understood. Using neurospheres derived from normal human fetal cortex, we found that early increases in anchorage-independent growth after BRAF activation were followed by proliferation arrest and induction of cellular senescence. We also detected the senescence markers acidic β-galactosidase and p16INK4a in pilocytic astrocytoma samples. Patients whose tumors lacked p16INK4a protein had significantly shorter survival. Immunohistochemical analysis of p16INK4a may therefore aid in therapeutic stratification. The findings presented here suggest that the indolent growth of pilocytic astrocytoma may be due to oncogene-induced senescence, similar to what is observed in melanocytic nevi. Failure to induce senescence, or an escape of the tumor from senescence, may result in more aggressive tumor biology. Understanding the regulation of oncogene-induced senescence may help develop more effective treatments of pediatric low-grade gliomas.
Acknowledgments
We thank Donata Maciaczyk for her excellent technical support.
Grant Support
This study was supported by PLGA Foundation, Children's Cancer Foundation, Pilocytic/Pilomyxoid Astrocytoma Fund, and Lauren's First and Goal (C.G. Eberhart), St. Baldrick's Foundation Fellowship (E. H. Raabe), and Comprehensive Cancer Center Freiburg (J. Maciaczyk, U. Kahlert, and G. Nikkhah).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Pfister S, Witt O. Pediatric gliomas. Recent Results Cancer Res. 2009;171:67–81. doi: 10.1007/978-3-540-31206-2_4. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunny RS, Hayward RD, Phipps KP, Harding BN, Saunders DE. Spontaneous regression of residual low-grade cerebellar pilocytic astrocytomas in children. Pediatr Radiol. 2005;35:1086–1091. doi: 10.1007/s00247-005-1546-z. [DOI] [PubMed] [Google Scholar]
- 4.Sievert AJ, Fisher MJ. Pediatric low-grade gliomas. J Child Neurol. 2009;24:1397–1408. doi: 10.1177/0883073809342005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma MK, Zehnbauer BA, Watson MA, Gutmann DH. RAS pathway activation and an oncogenic RAS mutation in sporadic pilocytic astrocytoma. Neurology. 2005;65:1335–1336. doi: 10.1212/01.wnl.0000180409.78098.d7. [DOI] [PubMed] [Google Scholar]
- 6.Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67:878–887. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 7.Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfister S, Janzarik WG, Remke M, Ernst A, Werft W, Becker N, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sievert AJ, Jackson EM, Gai X, Hakonarson H, Judkins AR, Resnick AC, et al. Duplication of 7q34 in pediatric low-grade astrocytomas detected by high-density single-nucleotide polymorphism-based genotype arrays results in a novel BRAF fusion gene. Brain Pathol. 2009;19:449–458. doi: 10.1111/j.1750-3639.2008.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacob K, Albrecht S, Sollier C, Faury D, Sader E, Montpetit A, et al. Duplication of 7q34 is specific to juvenile pilocytic astrocytomas and a hallmark of cerebellar and optic pathway tumours. Br J Cancer. 2009;101:722–733. doi: 10.1038/sj.bjc.6605179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janzarik WG, Kratz CP, Loges NT, Olbrich H, Klein C, Schafer T, et al. Further evidence for a somatic KRAS mutation in a pilocytic astrocytoma. Neuropediatrics. 2007;38:61–63. doi: 10.1055/s-2007-984451. [DOI] [PubMed] [Google Scholar]
- 12.Forshew T, Tatevossian RG, Lawson AR, Ma J, Neale G, Ogunkolade BW, et al. Activation of the ERK/MAPK pathway: a signature genetic defect in posterior fossa pilocytic astrocytomas. J Pathol. 2009;218:172–181. doi: 10.1002/path.2558. [DOI] [PubMed] [Google Scholar]
- 13.Deshmukh H, Yeh TH, Yu J, Sharma MK, Perry A, Leonard JR, et al. High-resolution, dual-platform aCGH analysis reveals frequent HIPK2 amplification and increased expression in pilocytic astrocytomas. Oncogene. 2008;27:4745–4751. doi: 10.1038/onc.2008.110. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhardt AE, Olbrich H, Roring M, Janzarik W, Van Anh TN, Cin H, et al. Functional characterization of a BRAF insertion mutant associated with pilocytic astrocytoma. Int J Cancer. 2010 Dec 28; doi: 10.1002/ijc.25893. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, Van Der Horst CM, et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature. 2005;436:720–724. doi: 10.1038/nature03890. [DOI] [PubMed] [Google Scholar]
- 16.Maciaczyk J, Singec I, Maciaczyk D, Klein A, Nikkhah G. Restricted spontaneous in vitro differentiation and region-specific migration of long-term expanded fetal human neural precursor cells after transplantation into the adult rat brain. Stem Cells Dev. 2009;18:1043–1058. doi: 10.1089/scd.2008.0346. [DOI] [PubMed] [Google Scholar]
- 17.Maciaczyk J, Singec I, Maciaczyk D, Nikkhah G. Combined use of BDNF, ascorbic acid, low oxygen, and prolonged differentiation time generates tyrosine hydroxylase-expressing neurons after long-term in vitro expansion of human fetal midbrain precursor cells. Exp Neurol. 2008;213:354–362. doi: 10.1016/j.expneurol.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 18.Gilbertson RJ, Gutmann DH. Tumorigenesis in the brain: location, location, location. Cancer Res. 2007;67:5579–5582. doi: 10.1158/0008-5472.CAN-07-0760. [DOI] [PubMed] [Google Scholar]
- 19.Braig M, Schmitt CA. Oncogene-induced senescence: putting the brakes on tumor development. Cancer Res. 2006;66:2881–2884. doi: 10.1158/0008-5472.CAN-05-4006. [DOI] [PubMed] [Google Scholar]
- 20.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129:1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 21.Woods NB, Mikkola H, Nilsson E, Olsson K, Trono D, Karlsson S. Lentiviral-mediated gene transfer into haematopoietic stem cells. J Intern Med. 2001;249:339–343. doi: 10.1046/j.1365-2796.2001.00806.x. [DOI] [PubMed] [Google Scholar]
- 22.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 23.Bar EE, Lin A, Mahairaki V, Matsui W, Eberhart CG. Hypoxia increases the expression of stem-cell markers and promotes clonogenicity in glioblastoma neurospheres. Am J Pathol. 2010;177:1491–1502. doi: 10.2353/ajpath.2010.091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raabe EH, Laudenslager M, Winter C, Wasserman N, Cole K, LaQuaglia M, et al. Prevalence and functional consequence of PHOX2B mutations in neuroblastoma. Oncogene. 2008;27:469–476. doi: 10.1038/sj.onc.1210659. [DOI] [PubMed] [Google Scholar]
- 25.Meeker AK, Hicks JL, Platz EA, March GE, Bennett CJ, Delannoy MJ, et al. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- 26.Eberhart CG, Kratz J, Wang Y, Summers K, Stearns D, Cohen K, et al. Histopathological and molecular prognostic markers in medulloblastoma: c-myc, N-myc, TrkC, and anaplasia. J Neuropathol Exp Neurol. 2004;63:441–449. doi: 10.1093/jnen/63.5.441. [DOI] [PubMed] [Google Scholar]
- 27.Horbinski C, Hamilton RL, Nikiforov Y, Pollack IF. Association of molecular alterations, including BRAF, with biology and outcome in pilocytic astrocytomas. Acta Neuropathol. 2010;119:641–649. doi: 10.1007/s00401-009-0634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stokland T, Liu JF, Ironside JW, Ellison DW, Taylor R, Robinson KJ, et al. A multivariate analysis of factors determining tumor progression in childhood low-grade glioma: a population-based cohort study (CCLG CNS9702) Neuro Oncol. 2010;12:1257–1268. doi: 10.1093/neuonc/noq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated beta-galactosidase assay. Methods Mol Biol. 2007;371:21–31. doi: 10.1007/978-1-59745-361-5_3. [DOI] [PubMed] [Google Scholar]
- 30.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–884. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brazel CY, Limke TL, Osborne JK, Miura T, Cai J, Pevny L, et al. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell. 2005;4:197–207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 32.Basu-Roy U, Ambrosetti D, Favaro R, Nicolis SK, Mansukhani A, Basilico C. The transcription factor Sox2 is required for osteoblast self-renewal. Cell Death Differ. 2010;17:1345–1353. doi: 10.1038/cdd.2010.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez EF, Scheithauer BW, Giannini C, Rynearson A, Cen L, Hoesley B, et al. PI3K/AKT pathway alterations are associated with clinically aggressive and histologically anaplastic subsets of pilocytic astrocytoma. Acta Neuropathol. 2011;121:407–420. doi: 10.1007/s00401-010-0784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schiffman JD, Hodgson JG, VandenBerg SR, Flaherty P, Polley MY, Yu M, et al. Oncogenic BRAF mutation with CDKN2A inactivation is characteristic of a subset of pediatric malignant astrocytomas. Cancer Res. 2010;70:512–519. doi: 10.1158/0008-5472.CAN-09-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez FJ, Scheithauer BW, Burger PC, Jenkins S, Giannini C. Anaplasia in pilocytic astrocytoma predicts aggressive behavior. Am J Surg Pathol. 2010;34:147–160. doi: 10.1097/PAS.0b013e3181c75238. [DOI] [PubMed] [Google Scholar]
- 36.Tabori U, Vukovic B, Zielenska M, Hawkins C, Braude I, Rutka J, et al. The role of telomere maintenance in the spontaneous growth arrest of pediatric low-grade gliomas. Neoplasia. 2006;8:136–142. doi: 10.1593/neo.05715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.