Abstract
Everything the brain knows about the content of the visual world is built from the spiking activity of retinal ganglion cells (RGCs). As the output neurons of the eye, RGCs include ~20 different subtypes, each responding best to a specific feature in the visual scene. Here we discuss recent advances in identifying where different RGC subtypes route visual information in the brain, including which targets they connect to and how their organization within those targets influences visual processing. We also highlight examples where causal links have been established between specific RGC subtypes, their maps of central connections and defined aspects of light-mediated behavior and we suggest the use of techniques that stand to extend these sorts of analyses to circuits underlying visual perception.
Introduction
Over 50 years ago, Lettvin et al. published the seminal paper ‘What the Frog’s Eye Tells the Frog’s Brain’ [1]. Lettvin described the many elaborate features encoded by the output neurons of the eye — the retinal ganglion cells (RGCs), such as edges, looming objects, or ‘bug detectors’ that respond best to small stimuli moving against a stationary background. The broad textbook model of vision nevertheless became that RGCs have simple center-surround receptive fields that are combined within the brain to generate more complex feature representations [2]. This certainly is the case for some RGCs and visual channels [3–5]. However, Lettvin also had it right: regardless of whether you examine the eye of a fish, mouse, rat, rabbit, monkey or human, you’ll find ~20 distinct subtypes of RGCs, each responding best to a specific, often highly specialized arrangement of light and dark in the visual environment [6,7•,8]. For example, some RGCs respond best to specific directions of motion [9–11] or orientations [12–14] and still others are suppressed by contrast [15] or signal the presence of looming stimuli [16]. A complete cataloging of the features encoded by different RGC subtypes is ongoing, but one thing is clear: RGCs are primed to deliver a rich set of visual information to the brain. In mammals there are also more than two-dozen brain areas that receive direct input from RGCs. Thus, the following crucial questions arise:
Where does each RGC subtype project to in the brain?
How are the visual signals encoded by different RGC subtypes integrated by local circuits within their targets?
How does the parallel organization of retinal maps influence visual perception and behavior?
In the following sections, we address recent progress toward answering these questions. We focus on four different eye-to-brain pathways, each serving a dedicated aspect of visual processing.
Intrinsically photosensitive RGCs: linking irradiance detectors to brain nuclei controlling specific non-image-forming behaviors
One of the great ongoing successes in the effort to link specific RGC subtypes and their maps in the brain to well defined visual behaviors comes from the study of intrinsically photosensitive RGCs (ipRGCs). All ipRGCs respond directly to light due to their expression of melanopsin photopigment [17–20]. Genetic labeling of ipRGCs from the melanopsin locus enabled selective mapping of ipRGC axonal projections within the brain and thereby revealed their two major targets: the supra-chiasmatic nucleus (SCN) — the hypothalamic circadian clock, and the olivary pretectal nucleus (OPN) — a midbrain nucleus involved in pupillary light reflexes [17,21]. Those maps of central projections in turn raised the hypotheses that: (i) ipRGCs serve to couple endogenously generated circadian rhythms to the ambient light-dark cycle (via their connections to the SCN) and (ii) ipRGCs drive pupillary constriction (via their inputs to the OPN). Indeed, ablation of ipRGCs abolishes both these behaviors [23•,24•,25•].
Until very recently it was unclear whether the same subtypes of ipRGCs sends irradiance information to the SCN and OPN or whether separate, designated sets of ipRGCs control circadian versus pupillary behaviors. Hattar and co-workers discovered that the transcription factor (Brn3b) is expressed by the M1 ipRGCs that target the outer shell of the OPN but not by the M1 ipRGCs that target the SCN. By crossing Melanopsin-Cre mice to mice that conditionally express a toxin from the Brn3b locus, they were able to selectively ablate only the OPN-shell projecting ipRGCs, which abolished pupil reflexes while leaving circadian entrainment intact [26••] (Figure 1). This molecular/functional isolation of a ‘labeled line’ consisting of a highly specific RGC subtype and a specialized aspect of light-mediated behavior represents an important first for the field. It also underscores the extent to which molecular signatures can be used to ‘split’ RGC populations that otherwise appear homogeneous and thereby discover their specific contributions to visual processing.
Figure 1.
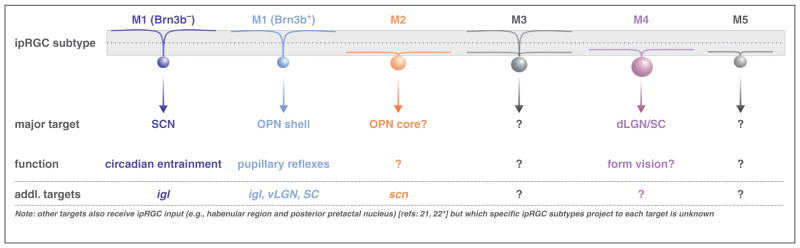
Intrinsically photosensitive retinal ganglion cell subtypes (ipRGCs), their connections in the brain and their influence on various aspects of light-mediated behaviors.
The use of Cre-based strategies for labeling ipRGCs revealed there are at least five subtypes of these cells that, collectively, project to more than a dozen central targets [22•] (Figure 1). As a general group, ipRGCs have been shown to influence mood, possibly via their inputs to the amygdala or habenula [21,27], and they have also been hypothesized to drive photic-induced migraine headache via their inputs to the posterior thalamic nuclei [28]. ipRGCs also play various developmental roles, including neonatal bright light avoidance [29], assembly of retinal vasculature [30], and patterning of early retinal activity [31,32] which in turn can influence RGC axonal refinements within the brain [32]. It is also intriguing that in both mice and primates, ipRGCs project to the dorsal lateral geniculate nucleus — the structure responsible for relaying light information to the cortex for conscious processing of visual images [22•,33,34•]. Thus, ipRGCs are poised to play diverse roles in the central processing of light information and it appears likely that each of the different M1–M5 subtypes will relate to distinct visual functions. As it stands now, however, the field lacks tools for specifically manipulating the ipRGCs that project to restricted sets of central targets other than the OPN shell. Hence, causal links between the remaining ip RGCs subtypes, their maps of central projections and discrete light-mediated behaviors, remain to be elucidated.
The superior colliculus contains functionally distinct parallel visual maps
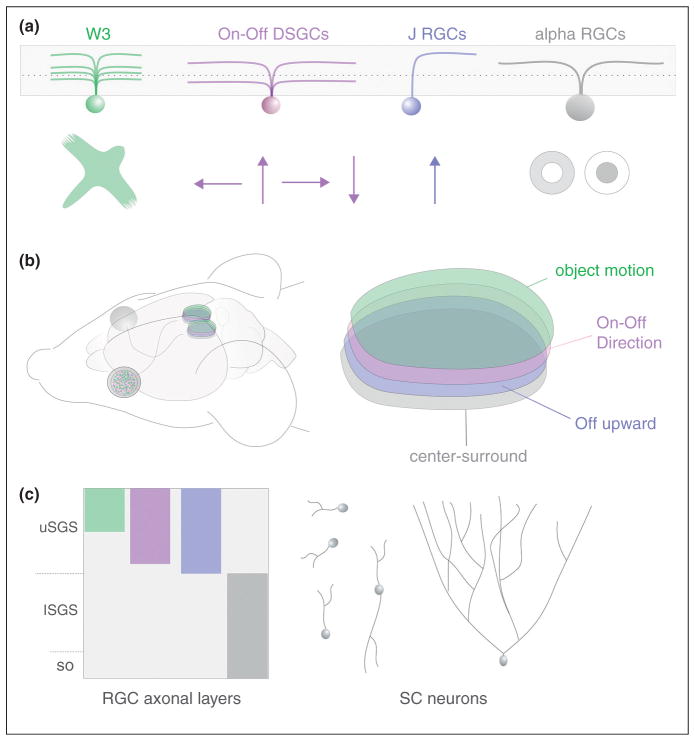
The superior colliculus (SC) is a large multimodal structure involved in directing the head and eyes to particular locations in visual space [35]. Input from the retina is delivered to the superficial-most layers of the SC where it is topographically mapped and aligned with the auditory and somatosensory maps that reside in deeper layers [36]. Recently, there has been a surge in understanding about how different RGCs and the information they encode are mapped in the SC. In large part these advances come from the discovery of transgenic mice harboring fluorescently tagged RGC subtypes. Genetic marking of Off and On-Off direction selective RGCs (DSGCs) revealed that they selectively target the superficial half of the retinorecipient SC [37••,38••,39,40•,41] along with RGCs that respond to local object motion (similar to Lettvin’s ‘bug detectors’) [41,42]. Alpha RGCs and ipRGCs —neither of which exhibit directional tuning, target the deeper portion of the retinorecipient SC [21,43•,44•]. Thus, the mouse superior colliculus receives visual signals from the retina in the form of at least four parallel retinotopically complete maps (Figure 2).
Figure 2.
Retinal ganglion cell maps in the superior colliculus identified from genetic labeling studies in the mouse. (a) Four subtypes of RGCs, each encoding a different specific feature of the visual environment. W3 RGCs encode local object motion [42]. On-Off DSGCs encode directional motion [38••,41]. J-RGCs are Off-DSGCs [37••] and alpha RGCs respond to center-surround stimuli [43•]. (b) Diagram of mouse head and brain showing the position of the two eyes, optic nerves and tracts and the two bilateral superior colliculi. On the right is a higher magnification view of one superior colliculus with the four RGC axonal maps stacked across its depth. (c) View of the four different RGC axon layers across the depth of the retinorecipient SC (comprised of upper and lower stratum griseum superficialis; uSGS, and lSGS respectively). Examples of collicular neurons that restrict their dendrites to individual or few sublaminae as well as a collilcular neuron that extends its arbor across all four RGC axon layers are shown to illustrate the possible modes of convergence for the various RGC maps.
Are the four maps of RGC input kept separate or combined within the network of collicular neurons? Each SC neuron is known to receive input from ~6 RGCs [45] but which subtypes of RGCs converge on an individual SC neuron is unknown. Many neurons in the retinorecipient SC are sensitive to directional motion [35,46] but their position, including where their dendrites stratify along the depth of the retinorecipient layers, has not been determined and thus it is unclear if they inherit their tuning directly from DSGCs. Indeed, the dendrites of retinorecipient SC neurons can span the full depth of all four RGC input-maps, suggesting they can sample these inputs in combinatorial fashion [35,47] (Figure 2). What is needed now is a thorough characterization of retinocollicular connectivity, including which RGCs connect to each SC neuron type and where those synapses arrive along the postsynaptic dendritic arbor. Monosynaptic viral tracing techniques [48,49] would greatly aid the effort to fill these gaps in knowledge, and help resolve the broader issue of how laminar-specific mapping of axons and dendrites impacts circuit function and receptive field transformations.
Zebrafish provide a window into the integration of laminar retinal maps in the tectum
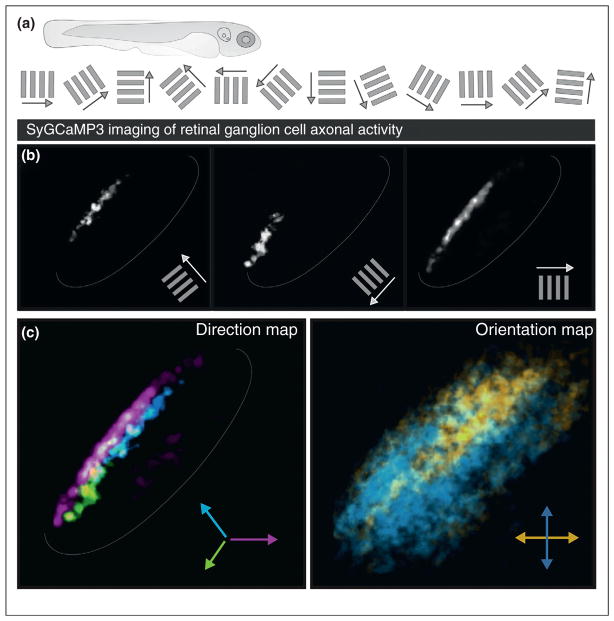
Several important steps forward were recently made in parsing how laminar maps of RGC axons relate to the response properties of their targets, using in vivo imaging of the larval zebrafish tectum (homolog of the SC). First, Baier and colleagues [50•] used ‘brainbow’ technology to label many different RGCs, each with different colors in the same animal, thereby revealing the highly stereotyped combinations of RGC types that converge their axons within individual tectal sublaminae. Second, Meyer and colleagues [51••,52•] monitored calcium activity in retinotectal terminals while presenting the fish with a range of visual stimuli, such as drifting bars of different orientations and directions. This revealed that separate populations of RGCs funnel visual information to highly restricted sublaminae within the tectum, creating three parallel maps of direction and four parallel maps of orientation (Figure 3).
Figure 3.
Calcium imaging of activity in RGC axons in the zebrafish tectum revealed direction and orientation maps. (a) Schematic of basic experimental design: larval zebrafish sequentially viewed bars moving in one of 12 different directions while the activity of RGCs was measured at the level of their axon terminals within the tectum. (b) Response maps to three different orientations and directions (see Ref. [51••] for details). (c) Composite maps of direction tuned RGC axons and orientation tuned RGC axons in the tectum, color-coded and combined.
How does the laminar-restricted delivery of visual information to the tectum relate to the response properties of neurons in this target? In an elegant study, Bollmann and colleagues [53••] compared the tuning of direction selective retinotectal axons with the tuning of genetically tagged tectal neurons. They discovered essentially two categories of direction selective tectal cells. One population was direction selective in a manner that could be directly predicted from the retinal input layer where they stratified their dendrites. The other population was also direction selective (DS) but its dendrites did not receive direct DSGC input. An additional study from Engert and co-workers [54•] identified asymmetric inhibition from local interneurons as a possible substrate for generating DS tuning in the zebrfish tectum. Together, these findings indicate that DS tuning of tectal neurons can be generated from feed-forward retinal inputs or by post-synaptic circuitry. It is important to note, however, that tectal neurons also can transform RGC inputs to create entirely new feature representations. Hunter et al. [55•] showed that some tectal neurons can exhibit directional tuning that is entirely distinct from the tuning of incoming retinal afferents. Collectively, the abovementioned studies in zebrafish illustrate that the highly restricted laminar mapping of RGC axons can instruct the tuning of target neurons, but that local circuit elements within the target can also use that information to generate receptive field properties de novo.
Cell-type-specific targeting of RGCs in the lateral geniculate nucleus
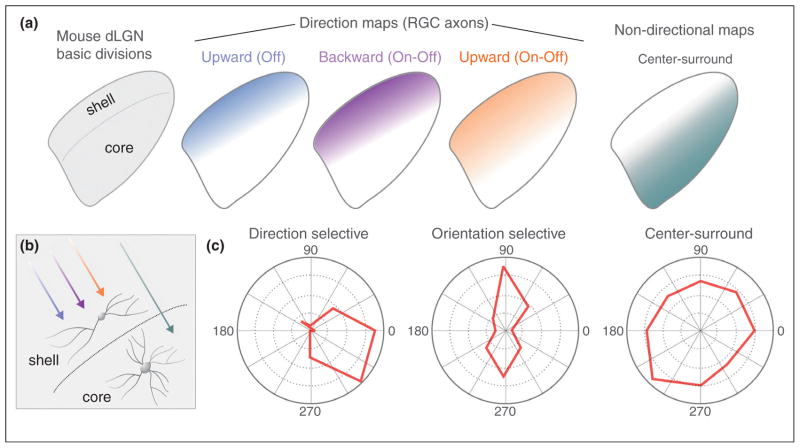
The recent advent of genetic tools for labeling specific RGC subtypes has greatly expanded understanding of how different visual channels are organized within the most famous mammalian retinorecipient target — the dorsal lateral geniculate nucleus (dLGN). Studies used transgenic labeling of specific RGC subtypes to demonstrate that the mouse dLGN contains at least two broad categories of functionally distinct retinal maps: a laminar map of direction selective RGC (DSGC) axons that resides adjacent to the optic tract and a laminar map comprising of axons from non-DSGCs located in the deeper ‘core’ compartment of dLGN (Figure 4) [37••,38••,39,40•,41,43•]. Therefore, just like the SC, there exist several distinct RGC maps in the geniculate. The question then arises: how do the different input channels to the dLGN relate to the various cell types in this target? Guido and colleagues recently discovered that the mouse harbors three relay neuron types that morphologically resemble the X, Y and W cells described in earlier studies in cats and primates [56•]. By comparing the location of each cell type they determined that W-like cells reside in the same region of the dLGN where DSGCs terminate (the shell). By contrast, Y-like cells reside in the region where alpha RGCs and other non-DSGCs terminate (the core). X cells were found in both the shell and core. In mice, each dLGN neuron receives input from ~1–3 RGCs [57], but whether individual X, Y and W cells sample visual input exclusively from DSGCs versus non-DSGCs is an important issue that still awaits resolution.
Figure 4.
Laminar specific mapping and target neuron responses in the lateral geniculate of the mouse. (a) Diagram of the mouse dLGN with its shell and core regions and the termination zones where the axons of the various genetically identified subtypes of DSGCs synapse. The terminations of axons from alpha RGCs in the core region are also shown. (b) Schematic of the RGC inputs to dLGN neurons. (c) Polar plots of direction selective, orientation selective, and center-surround neurons that were recorded from the mouse dLGN. See Ref. [59••] for details.
Two recent studies, one using in vivo calcium imaging [58••] and one using electrode recordings [59••], discovered that the shell region of the dLGN is enriched for direction-tuned and orientation-tuned neurons (Figure 4). Since the shell roughly corresponds to the same region where the axons of DSGCs terminate, several key questions emerge. First, does the orientation tuning of dLGN neurons arise from the convergence of DSGCs that respond to opposing axes of motion? This seems likely given that (i) mouse dLGN neurons often receive input from multiple RGCs and (ii) orientation-selective dLGN neurons reside postsynaptic to DSGC axons (Figure 4). However, a recent study also found evidence that even some mouse RGCs are orientation selective [60•]. Thus in order to understand the basis for orientation selectivity in the mouse dLGN it is essential to resolve whether these orientation selective RGCs project to the geniculate and if so, to which laminar compartment.
The textbook model of orientation selectivity derived from work in carnivores and primates posits that few, if any, dLGN neurons exhibit direction or orientation selectivity and that visual cortex is the first place these features are observed [61]. Are direction selective retinal and dLGN neurons unique to the mouse? After all, DSGCs have not been reported in primates. There are several reports, however, of DS-tuned geniculate neurons in rabbits, cats and primates [62–67]. Additionally, Martin and colleagues recently showed that some neurons in the koniocellular layers of the marmoset dLGN are highly orientation selective [68]. In light of this, it is key to determine whether dLGN-projecting RGCs in primates include direction-tuned or orientation-tuned RGCs and if so, whether they contribute to cortical representation of these features.
Mapping direction selective retinal inputs to brain areas that control image-stabilization
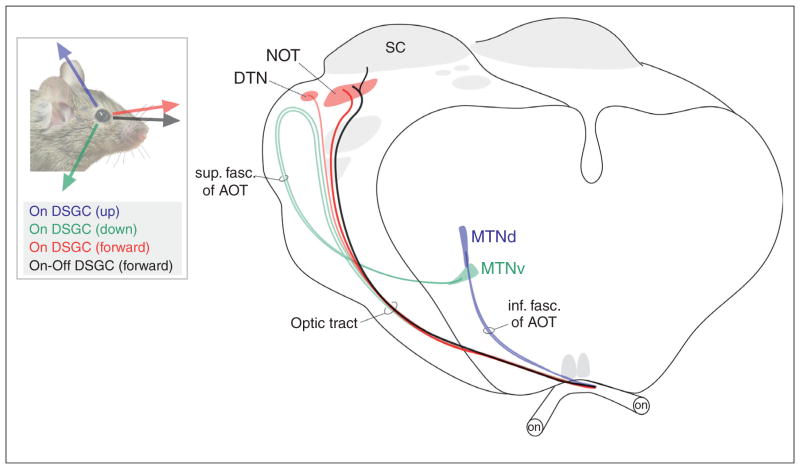
As the eyes and head move, images slip on the retina; left unchecked this would blur the image of the visual scene. A specialized set of direction selective RGCs and central targets together called the accessory optic system (AOS) generate reflexive eye movements that compensate for retinal slip [70,71,72•]. Consequently, the AOS is a powerful model for probing how the mapping of DSGCs relates to visual behavior. Classic work in rabbits [70] showed that the AOS consists of On-DSGCs that project to two distinct collections of brainstem targets: (i) the nucleus of the optic tract and dorsal terminal nucleus (NOT/DTN) which help control horizontal slip compensation and (ii) the medial and lateral terminal nuclei (MTN and LTN) which encode vertical image slip. The On-DSGCs that connect to AOS targets are tailored to the behavioral demands of this system such that their directional tuning matches the major axes of visual slip experienced by the retina whenever the head moves [70,72•]. Moreover, On-DSGCs are among the rare RGC subtypes that do not provide direct input to the superior colliculus or dLGN.
Studies in mice are starting to reveal important new principles of how DSGCs signals feed the AOS. The mouse AOS appears similar to that of rabbits and primates, based on the fact that in all these species, On-DSGCs provide major drive to this system [73••,74••]. Recent studies used genetic labeling to find, however, that the AOS also receives substantial input from On-Off DSGCs [40•,74••] (Figure 5). Moreover, the contribution of On-Off signals to the AOS appears to be target-specific. Viral labeling of the RGCs that project to the nucleus of the optic tract revealed that this target receives input from an On-Off DSGC subtype whose velocity tuning is ideally matched to the NOTs proposed role in image stabilization [74••]. By contrast, other AOS targets such as the MTN do not appear to collect input from these cells. It is now crucial to understand how the two major types of DSGCs (On and On-Off) collaborate to generate slip-compensating eye movements. It is also important to resolve whether AOS nuclei harbor sub-domains encoding different directions of retinal slip [73••]. Killing cholinergic starburst amacrine cells [75] or silencing On-bipolar cells [76] has been shown to completely eliminate slip-compensating eye movements. However, the field still awaits more refined manipulations that kill or silence the specific DSGC subtypes in order to elucidate their individual contributions to image stabilization. The fact that AOS-projecting RGCs are now genetically identified opens the door to find molecular signatures of these cells and to perform these sorts of analyses.
Figure 5.
Schematic of the accessory optic system in the mouse (adapted from [83] and [74••]). The axes of directional motion encoded by the four genetically identified subtypes of DSGCs that connect to the AOS are shown. The diagram on the right depicts the subcortical visual pathway with two optic nerves (on), optic tract and accessory optic tracts, as well as various AOS targets such as the medial terminal nucleus (MTN), nucleus of the optic tract (NOT) and dorsal terminal nucleus (DTN). The superior colliculus (SC) is also shown. The color scheme matches the different DSGC subtypes (left panel with mouse) that project along each pathway and target. Sup. fac. of AOT = superior fasciculus of the accessory optic tract and inf. fasc. of AOT = inferior fasciculus of the AOT.
Perspectives
Here we reviewed recent advances in understanding how different RGCs map visual features to the brain. In some cases such as the ipRGCs, those maps resemble ‘labeled lines’, whereas in other cases, such as the retinal inputs to the SC, they are poised to converge in a combinatorial pattern. Understanding the relevance of this convergence to visual processing and perception represents an important unmet challenge. For example, does the combined directional tuning of dLGN neurons simply mirror the tuning of the DSGCs they receive input from? Or as Levick et al. proposed [77•], do inhibitory cells within the dLGN modify and sharpen DS responses? The answers to these sorts of questions will be important for the field of visual neuroscience and more generally, they stand to reveal important general principles of how the specificity of axonal mapping can create new, richer feature representations in the brain.
It is also worth mentioning that RGC maps display various salient features whose functional relevance for visual processing remain opaque. For instance, RGC projections are heavily collateralized to multiple subcortical targets [78–80] but the functional relevance of this collateralization has not been explored. Modern tools to control neuronal activity such as optogenetic and chemical-genetic strategies [81] should greatly help resolve this and other outstanding questions. Finally, to understand the implications of such studies for human vision will require analysis of species such as fish and mice, but also primates [82]. The question of ‘what does the eye tell the brain?’ remains an important one that is certain to deliver many new and exciting findings in years to come, especially with regard to how different retinal ganglion cell types contribute to visual perception and behavior.
Acknowledgments
We thank Maureen Eztevez, David Berson, Jianhua Cang and Cris Niell and members of the Huberman Lab for helpful comments. Support was provided by Knights Templar Eye Foundation (O.S.D) and by NIH R01 EY022157-01, The McKnight Endowment Fund for Neuroscience and the Pew Charitable Trusts (A.D.H).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Lettvin JY, Maturana HR, McCulloch WS, Pitts WS. What the frog’s eye tells the frog’s brain. Proc Inst Radio Eng. 1959;47:1940–1951. [Google Scholar]
- 2.Hubel DH, Wiesel TN. Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol. 1962;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown SP, He S, Masland RH. Receptive field microstructure and dendritic geometry of retinal ganglion cells. Neuron. 2000;27:371–383. doi: 10.1016/s0896-6273(00)00044-1. [DOI] [PubMed] [Google Scholar]
- 4.Usrey WM, Reppas JB, Reid RC. Specificity and strength of retinogeniculate connections. J Neurophysiol. 1999;82:3527–3540. doi: 10.1152/jn.1999.82.6.3527. [DOI] [PubMed] [Google Scholar]
- 5.Chapman B, Zahs KR, Stryker MP. Relation of cortical cell orientation selectivity to alignment of receptive fields of the geniculocortical afferents that arborize within a single orientation column in ferret visual cortex. J Neurosci. 1991;11:1347–1358. doi: 10.1523/JNEUROSCI.11-05-01347.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dacey DM. Origins of perception: retinal ganglion cell diversity and the creation of parallel visual pathways. In: Gazzaniga MS, editor. The Cognitive Neurosciences. MIT Press; Cambridge: 2004. pp. 281–301. [Google Scholar]
- 7•.Berson DM. Retinal ganglion cell types and their central projections. In: Albright TD, Masland R, editors. The Senses: A Comprehensive Reference (Vision 1) Vol. 1. San Diego, CA: Academic Press; 2008. pp. 491–520. An excellent review on the morphological and functional classification of RGC subtypes and their axon projections across multiple species. [Google Scholar]
- 8.Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–280. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barlow HB, Hill RM. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science. 1963;139:412–414. doi: 10.1126/science.139.3553.412. [DOI] [PubMed] [Google Scholar]
- 10.Oyster CW, Barlow HB. Direction-selective units in rabbit retina: distribution of preferred directions. Science. 1967;155:841–842. doi: 10.1126/science.155.3764.841. [DOI] [PubMed] [Google Scholar]
- 11.Vaney DI, Sivyer B, Taylor WR. Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nat Rev Neurosci. 2012;13:194–208. doi: 10.1038/nrn3165. [DOI] [PubMed] [Google Scholar]
- 12.Bloomfield SA. Orientation-sensitive amacrine and ganglion cells in the rabbit retina. J Neurophysiol. 1994;71:1672–1691. doi: 10.1152/jn.1994.71.5.1672. [DOI] [PubMed] [Google Scholar]
- 13.Levick WR, Thibos LN. Analysis of orientation bias in cat retina. J Physiol. 1982;329:243–261. doi: 10.1113/jphysiol.1982.sp014301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Passaglia CL, Troy JB, Ruttiger L, Lee BB. Orientation sensitivity of ganglion cells in primate retina. Vision Res. 2002;42:683–694. doi: 10.1016/s0042-6989(01)00312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sivyer B, Taylor WR, Vaney DI. Uniformity detector retinal ganglion cells fire complex spikes and receive only light-evoked inhibition. Proc Natl Acad Sci U S A. 2010;107:5628–5633. doi: 10.1073/pnas.0909621107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Münch TA, da Silveira RA, Siegert S, Viney TJ, Awatramani GB, Roska B. Approach sensitivity in the retina processed by a multifunctional neural circuit. Nat Neurosci. 2009;12:1308–1316. doi: 10.1038/nn.2389. [DOI] [PubMed] [Google Scholar]
- 17.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt TM, Do MT, Dacey D, Lucas R, Hattar S, Matynia A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci. 2011;31:16094–16101. doi: 10.1523/JNEUROSCI.4132-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Do MT, Yau KW. Intrinsically photosensitive retinal ganglion cells. Physiol Rev. 2010;90:1547–1581. doi: 10.1152/physrev.00013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Ecker JL, Dumitrescu ON, Wong KY, Alam NM, Chen SK, LeGates T, Renna JM, Prusky GT, Berson DM, Hattar S. Melanopsin-expressing retinal ganglion-cell photoreceptors: cellular diversity and role in pattern vision. Neuron. 2010;67:49–60. doi: 10.1016/j.neuron.2010.05.023. In this study Cre-based labeling of melanopsin-expressing RGCs was used to identify several new morphologically and physiologically distinct ipRGC subtypes. The authors identified many previously unknown central targets of ipRGCs, which in turn raised new hypotheses about the potential functional roles of these cells in visual processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23•.Güler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, et al. Melanopsin cells are the principal conduits for rod–cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. Before this study it was unclear whether ipRGCs worked alone or in collaboration with other RGC types to drive various non-image-forming behaviors. This study describes a strategy involving the expression of diphtheria-toxin in melanopsin expressing RGCs, which selectively kills these neurons. Loss of ipRGCs led to severe disruptions in pupillary reflex and circadian entrainment but left pattern vision intact. In addition to verifying the crucial role of ipRGCs in these aspects of visual function, this study was among the first (also see [24•,25•]) to causally link a defined category of RGCs to behavior using a non-surgical lesion technique. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Hatori M, Le H, Vollmers C, Keding SR, Tanaka N, Buch T, Waisman A, Schmedt C, Jegla T, Panda S. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3:e2451. doi: 10.1371/journal.pone.0002451. This study independently used the same general approach and arrived at the same conclusions as [23•] but differed in the sense that the authors expressed diphtheria toxin receptor in ipRGCs and therefore were able to kill these cells by injection of DTA. This bypasses any developmental effects of ablating ipRGCs, which is important given the known roles of ipRGCs in various aspects of visual system development [29–32] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Goz D, Studholme K, Lappi DA, Rollag MD, Provencio I, Morin LP. Targeted destruction of photosensitive retinal ganglion cells with a saporin conjugate alters the effects of light on mouse circadian rhythms. PLoS ONE. 2008;3:e3153. doi: 10.1371/journal.pone.0003153. This study also arrived at the same general conclusions as [23•,24•] but here they used a saporin-based immunotoxin to target and kill ipRGCs. This is important because, in addition to bypassing developmental requirements for ipRGCs, it showed that in theory, selective ablation of ipRGCs using toxins could also be performed in other vertebrate species such as primates. Indeed, the role of ipRGCs in species other than the mouse is still unknown. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26••.Chen SK, Badea TC, Hattar S. Photoentrainment and pupillary light reflex are mediated by distinct populations of ipRGCs. Nature. 2011;476:92–95. doi: 10.1038/nature10206. In this elegant study the authors ‘split’ the type 1 (M1) population of ipRGCs into two groups by showing that Brn3b-expressing M1 ipRGCs provide dense innervation to the OPN shell but do not project to the SCN. They then used an ipRGC specific Cre line to express diphtheria toxin in Brn3b-expressing ipRGCs, thus selectively killing ipRGC inputs to the OPN while sparing ipRGC inputs to the SCN. The selective loss of OPN shell projecting ipRGCs caused a loss of the pupillary light reflex in these mice but did not affect their circadian photoentrainement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, Kirkwood A, Weber ET, Hattar S. Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature. 2012;491:594–598. doi: 10.1038/nature11673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, Burstein R. A neural mechanism for exacerbation of headache by light. Nat Neurosci. 2010;13:239–245. doi: 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson J, Wu V, Donovan M, Majumdar S, Renteria RC, Porco T, Van Gelder RN, Copenhagen DR. Melanopsin-dependent light avoidance in neonatal mice. Proc Natl Acad Sci U S A. 2010;107:17374–17378. doi: 10.1073/pnas.1008533107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rao S, Chun C, Fan J, Kofron JM, Yang MB, Hegde RS, Ferrara N, Copenhagen DR, Lang RA. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494:243–246. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkby LA, Feller MB. Intrinsically photosensitive ganglion cells contribute to plasticity in retinal wave circuits. Proc Natl Acad Sci U S A. 2013;110:12090–12095. doi: 10.1073/pnas.1222150110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renna JM, Weng S, Berson DM. Light acts through melanopsin to alter retinal waves and segregation of retinogeniculate afferents. Nat Neurosci. 2011;14:827–829. doi: 10.1038/nn.2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown TM, Gias C, Hatori M, Keding SR, Semo M, Coffey PJ, Gigg J, Piggins HD, Panda S, Lucas RJ. Melanopsin contributions to irradiance coding in the thalamo-cortical visual system. PLoS Biol. 2010;8:e1000558. doi: 10.1371/journal.pbio.1000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Dacey DM, Liao HW, Peterson BB, Robinson FR, Smith VC, Pokorny J, Yau KW, Gamlin PD. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature. 2005;433:749–754. doi: 10.1038/nature03387. The authors report that melanopsin expressing RGCs are present in human and macaque retinas. In the macaque they project to the LGN and pretectum. In addition to being intrinsically photosensitive, these RGCs are also strongly driven by rod- and cone-mediated visual signals and encode chromatic signals. [DOI] [PubMed] [Google Scholar]
- 35.May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- 36.Cang J, Feldheim DA. Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci. 2013;36:51–77. doi: 10.1146/annurev-neuro-062012-170341. [DOI] [PubMed] [Google Scholar]
- 37••.Kim IJ, Zhang Y, Yamagata M, Meister M, Sanes JR. Molecular identification of a retinal cell type that responds to upward motion. Nature. 2008;452:478–482. doi: 10.1038/nature06739. The authors discovered a previously unidentified subtype of RGC that selectively responds to the offset of light (‘off’) and to upward motion (termed J-RGCs). These cells have an unusual morphology that can explain their DS-tuning. Genetic tagging of the J-RGCs revealed that they project their axons to the superficial layers of the SC and provide input to the outer shell of the dLGN. This was also the first discovery of a RGC that signals directional motion of dark stimuli. [DOI] [PubMed] [Google Scholar]
- 38••.Huberman AD, Wei W, Elstrott J, Stafford BK, Feller MB, Barres BA. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron. 2009;62:327–334. doi: 10.1016/j.neuron.2009.04.014. This study used a novel transgenic mouse line (Drd4-GFP) in which On-Off direction selective RGCs (DSGCs) encoding posterior motion are fluorescently labeled, representing the first genetic identification of the classic DSGCs initially discovered by Barlow and Hill in the 1960s [9]. Genetic tagging of these cells revealed that their axons terminate preferentially in the superficial-most lamina of the colliculus and in a dedicated lamina adjacent to the optic tract in the dLGN. The presence of On-Off DSGC input to the mouse dLGN raised new questions about the possible origins of DS tuning in dLGN neurons and their downstream circuits, such as cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rivlin-Etzion M, Zhou K, Wei W, Elstrott J, Nguyen PL, Barres BA, Huberman AD, Feller MB. Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. J Neurosci. 2011;31:8760–8769. doi: 10.1523/JNEUROSCI.0564-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Kay JN, De la Huerta I, Kim IJ, Zhang Y, Yamagata M, Chu MW, Meister M, Sanes JR. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J Neurosci. 2011;31:7753–7762. doi: 10.1523/JNEUROSCI.0907-11.2011. Using gene-profiling analysis, mRNA in situ hybridization and immunohistochemistry, the authors identified cocaine- and amphetamine-regulated transcript (Cart) as a marker of On-Off DSGCs. They also report additional genetic markers (MMP17, Col25a1, Cdh6) that are differentially expressed by the various On-Off DSGCs encoding different preferred directions of motion. The discovery of non-transgenic markers for DSGCs opens the door for possible identification of equivalent RGC subtypes in non-rodent species such as monkeys and humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim IJ, Zhang Y, Meister M, Sanes JR. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J Neurosci. 2010;30:1452–1462. doi: 10.1523/JNEUROSCI.4779-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Kim IJ, Sanes JR, Meister M. The most numerous ganglion cell type of the mouse retina is a selective feature detector. Proc Natl Acad Sci U S A. 2012;109:E2391–E2398. doi: 10.1073/pnas.1211547109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, Barres BA. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–438. doi: 10.1016/j.neuron.2008.07.018. This study reported a transgenic mouse line (CB2-GFP) in which transient Off alpha RGCs express GFP. They found that the axonal projections of these RGCs restrict themselves to the ‘core’ of the dLGN and to the deeper retinorecipient portion of the SC. In addition, the map of transient Off alpha RGC axons is organized in a patchy columnar manner in the SC. This paper also demonstrated that the developmental emergence of columnar SC projections is dependent on early patterned spontaneous retinal activity whereas that laminar-specific targeting to the SC is refractory to activity perturbations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44•.Hong YK, Kim IJ, Sanes JR. Stereotyped axonal arbors of retinal ganglion cell subsets in the mouse superior colliculus. J Comp Neurol. 2011;519:1691–1711. doi: 10.1002/cne.22595. This study used a combination of viral and transgenic techniques in the mouse to label single retinocollicular axon arbors of distinct RGC subtypes. They found that individual RGC subtypes project to a specific laminar depth in the SC in a stereotyped manner. In addition, they found that the dendritic stratification of a given RGC subtype within the inner retina did not predict the laminar position of the RGCs axonal arbor in the SC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandrasekaran AR, Shah RD, Crair MC. Developmental homeostasis of mouse retinocollicular synapses. J Neurosci. 2007;27:1746–1755. doi: 10.1523/JNEUROSCI.4383-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang L, Sarnaik R, Rangarajan K, Liu X, Cang J. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J Neurosci. 2010;30:16573–16584. doi: 10.1523/JNEUROSCI.3305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mooney RD, Klein BG, Rhoades RW. Correlations between the structural and functional characteristics of neurons in the superficial laminae and the hamster’s superior colliculus. J Neurosci. 1985;5:2989–3009. doi: 10.1523/JNEUROSCI.05-11-02989.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Callaway EM. Transneuronal circuit tracing with neurotropic viruses. Curr Opin Neurobiol. 2008;18:617–623. doi: 10.1016/j.conb.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Packer AM, Roska B, Hausser M. Targeting neurons and photons for optogenetics. Nat Neurosci. 2013;16:805–815. doi: 10.1038/nn.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50•.Robles E, Filosa A, Baier H. Precise lamination of retinal axons generates multiple parallel input pathways in the tectum. J Neurosci. 2013;33:5027–5039. doi: 10.1523/JNEUROSCI.4990-12.2013. The authors generated a retina-specific brainbow zebrafish to examine the laminar targeting of multiple RGC axons, each labeled in a different color, in the same fish, in vivo. They found that the laminar position of different RGC subtypes is precise from the outset and invariant across early development. They also show that different tectal sublaminae can receive inputs from multiple RGC subtypes and that the combination of RGC subtypes innervating each sublaminae is highly stereotyped. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51••.Nikolaou N, Lowe AS, Walker AS, Abbas F, Hunter PR, Thompson ID, Meyer MP. Parametric functional maps of visual inputs to the tectum. Neuron. 2012;76:317–324. doi: 10.1016/j.neuron.2012.08.040. In this study the authors imaged the axon terminals of GCaMP3 expressing RGCs in the zebrafish tectum in vivo to discover that there are three subtypes of direction-selective retinal inputs and two subtypes of orientation-selective retinal inputs to the tectum. In addition, they observed that direction-selective retinal inputs were organized in a manner such that the two pathways are segregated into relatively non-overlapping laminar domains within the tectum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52•.Lowe A, Nikolaou N, Hunter PR, Thompson ID, Meyer MP. A systems-based dissection of retinal inputs to the zebrafish tectum reveals different rules for different functional classes during development. J Neurosci. 2013;33:13946–13956. doi: 10.1523/JNEUROSCI.1866-13.2013. This paper revealed the dynamic nature of the organization and tuning properties of direction-selective and orientation-selective RGC maps in the zebrafish tectum. It also probed the role of visual activity in the establishment of these maps by dark-rearing fish. They found that the establishment of direction maps does not require visual experience whereas orientation maps do. In addition this study extends the findings mentioned in Ref. [51] and describe two additional subtypes of orientation-selective retinal inputs to the tectum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Gabriel JP, Trivedi CA, Maurer CM, Ryu S, Bollmann JH. Layer-specific targeting of direction-selective neurons in the zebrafish optic tectum. Neuron. 2012;76:1147–1160. doi: 10.1016/j.neuron.2012.12.003. The authors combined Ca2+ imaging of RGC axonal activity in the tectum with targeted patch clamp recordings of genetically tagged tectal neurons. The authors identified two subtypes of interneurons that respond to opposite directions of motion. They show that these interneurons receive excitatory synaptic inputs from RGCs tuned to the same preferred direction. Furthermore, they show that dendrites of these interneurons stratify in the same lamina innervated by RGC inputs tuned to their preferred direction. [DOI] [PubMed] [Google Scholar]
- 54•.Grama A, Engert F. Direction selectivity in the larval zebrafish tectum is mediated by asymmetric inhibition. Front Neural Circuits. 2012;6:59. doi: 10.3389/fncir.2012.00059. Using a combination of two-photon calcium imaging and in vivo patch-clamp recordings, the authors report that asymmetric inhibitory inputs from interneurons generate direction selectively in some tectal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Hunter PR, Lowe AS, Thomspn ID, Meyer MP. Emergent properties of the optic tectum revealed by population analysis of direction and orientation selectivity. J Neurosci. 2013;33:13940–13945. doi: 10.1523/JNEUROSCI.1493-13.2013. The authors analyze the calcium responses of large populations of tectal neurons to different directions and orientations. They observe that orientation tuning is faithfully transferred from retinal to tectal neurons whereas direction tuning adopts new axes of motion based on the transformation of retinal information within the target. Thus, directional space encoded by tectal neurons displays emergent properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56•.Krahe TE, El-Danaf RN, Dilger EK, Henderson SC, Guido W. Morphologically distinct classes of relay cells exhibit regional preferences in the dorsal lateral geniculate nucleus of the mouse. J Neurosci. 2011;31:17437–17448. doi: 10.1523/JNEUROSCI.4370-11.2011. The authors characterized the morphology of individual dLGN relay neurons in the mouse and discovered three types that resemble the X, Y, and W cells observed in cats and primates. Although each type was morphologically distinct and resided in specific domains within the LGN, no differences were found between their intrinsic firing properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hong YK, Chen C. Wiring and rewiring of the retinogeniculate synapse. Curr Opin Neurobiol. 2011;21:228–237. doi: 10.1016/j.conb.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Marshel JH, Kaye AP, Nauhaus I, Callaway EM. Anterior-posterior direction opponency in the superficial mouse lateral geniculate nucleus. Neuron. 2012;76:713–720. doi: 10.1016/j.neuron.2012.09.021. The authors used in vivo two-photon microscopy to image visually evoked calcium responses in neurons residing in the dorsal tip of the mouse dLGN. They found that some of these dLGN neurons are tuned for single directions of motion whereas others respond to motion along opposing axes. They propose a model whereby convergence of DSGCs establishes the DS and orientation selectivity observed in these cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59••.Piscopo DM, El-Danaf RN, Huberman AD, Niell CM. Diverse visual features encoded in mouse lateral geniculate nucleus. J Neurosci. 2013;33:4642–4656. doi: 10.1523/JNEUROSCI.5187-12.2013. The authors performed extracellular multisite recordings to survey the visual response properties of dLGN neurons and recover the position of the recorded cells within the dLGN. In addition to finding predicted features such as center-surround the authors describe neurons with direction-selective and orientation-selective responses as well as neurons whose activity is suppressed by contrast. They show that the location of direction-selective and orientation-selective neurons was heavily biased toward the outer shell of the dLGN, the same region where direction-selective retinal inputs terminate. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60•.Zhao X, Chen H, Liu X, Cang JC. Orientation-selective responses in the mouse lateral geniculate nucleus. J Neurosci. 2013;33:12751–12763. doi: 10.1523/JNEUROSCI.0095-13.2013. The authors corroborate previous work [58••,59••] showing that orientation-selective neurons are present in the mouse dLGN and showed that these neurons maintain orientation-selectivity even when cortical feedback is silenced. In addition, using in vitro multi-electrode array recordings the authors document the presence of orientation-selective RGCs in the mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hubel DH. Eye, Brain, and Vision. 1995. Scientific American Library Series #22. [Google Scholar]
- 62.Shou TD, Leventhal AG. Organized arrangement of orientation-sensitive relay cells in the cat’s dorsal lateral geniculate nucleus. J Neurosci. 1989;9:4287–4302. doi: 10.1523/JNEUROSCI.09-12-04287.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soodak RE, Shapley RM, Kaplan E. Linear mechanism of orientation tuning in the retina and lateral geniculate nucleus of the cat. J Neurophysiol. 1987;58:267–275. doi: 10.1152/jn.1987.58.2.267. [DOI] [PubMed] [Google Scholar]
- 64.Stewart DL, Chow KL, Masland RH. Receptive-field characteristics of lateral geniculate neurons in the rabbit. J Neurophysiol. 1971;34:139–147. doi: 10.1152/jn.1971.34.1.139. [DOI] [PubMed] [Google Scholar]
- 65.Xu X, Ichida J, Shostak Y, Bonds AB, Casagrande VA. Are primate lateral geniculate nucleus (LGN) cells really sensitive to orientation or direction? Vis Neurosci. 2002;19:97–108. doi: 10.1017/s0952523802191097. [DOI] [PubMed] [Google Scholar]
- 66.Thompson KG, Zhou Y, Leventhal AG. Direction-sensitive X and Y cells within the A laminae of the cat’s LGNd. Vis Neurosci. 1994;11:927–938. doi: 10.1017/s0952523800003886. [DOI] [PubMed] [Google Scholar]
- 67.Scholl B, Tan AY, Corey J, Priebe NJ. Emergence of orientation selectivity in the Mammalian visual pathway. J Neurosci. 2013;33:10616–10624. doi: 10.1523/JNEUROSCI.0404-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheong SK, Tailby C, Solomon SG, Martin PR. Cortical-like receptive fields in the lateral geniculate nucleus of marmoset monkeys. J Neurosci. 2013;33:6864–6876. doi: 10.1523/JNEUROSCI.5208-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simpson JI. The accessory optic system. Annu Rev Neurosci. 1984;7:13–41. doi: 10.1146/annurev.ne.07.030184.000305. [DOI] [PubMed] [Google Scholar]
- 71.Distler C, Hoffmann K-P. The optokinetic reflex. In: Liversedge SP, Gilchrist ID, Everling S, editors. The Oxford Handbook of Eye Movements. Oxford: Oxford University Press; 2011. pp. 65–83. [Google Scholar]
- 72•.Borst A, Euler T. Seeing things in motion: models, circuits, and mechanisms. Neuron. 2011;71:974–994. doi: 10.1016/j.neuron.2011.08.031. This comprehensive review focuses on the cells, circuits and computations underlying direction-selectivity in insects and in vertebrate species, in particular the mouse and rabbit. [DOI] [PubMed] [Google Scholar]
- 73••.Yonehara K, Ishikane H, Sakuta H, Shintani T, Nakamura-Yonehara K, Kamiji NL, Usui S, Noda M. Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion. PLoS ONE. 2009;4:e4320. doi: 10.1371/journal.pone.0004320. This study described a transgenic mouse line (Spig1-GFP) in which upward preferring On-DSGCs express GFP. They used this line to show that information from On-DSGCs encoding upward and downward motion is sent to anatomically distinct compartments of the medial terminal nucleus (MTN). They reinforced those findings with experiments examining immediate early gene expression in MTN neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Dhande OS, Estevez ME, Quattrochi LE, El Danaf RN, Ngyuen PL, Berson DM, Huberman AD. Genetic dissection of retinal inputs to brainstem nuclei controlling image stabilization. 2013;33:17797–17813. doi: 10.1523/JNEUROSCI.2778-13.2013. This study reported a new transgenic mouse line (Hoxd10-GFP) with GFP expressed in the RGCs that target the nuclei of the accessory optic system. Using targeted recordings, they find that the mouse AOS is driven by three subtypes of On-DSGCs and by a subtype of anterior preferring On-Off DSGCs. Using rabies virus circuit mapping they show that brainstem centers involved in vertical retinal slip compensation obtain visual information from On-DSGCs, whereas brainstem nuclei mediating horizontal image-stabilization derive signals from both On and On-Off DSGCs. This study also reports that the On-Off DSGCs feeding the AOS are physiologically and molecularly distinct from previously described On-Off DSGCs in the mouse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoshida K, Watanabe D, Ishikane H, Tachibana M, Pastan I, Nakanishi S. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron. 2001;30:771–780. doi: 10.1016/s0896-6273(01)00316-6. [DOI] [PubMed] [Google Scholar]
- 76.Sugita Y, Miura K, Araki F, Furukawa T, Kawano K. Contributions of retinal direction-selective ganglion cells to optokinetic responses in mice. Eur J Neurosci. 2013;38:2823–2831. doi: 10.1111/ejn.12284. [DOI] [PubMed] [Google Scholar]
- 77•.Levick WR, Oyster CW, Takahashi E. Rabbit lateral geniculate nucleus: sharpener of directional information. Science. 1969;165:712–714. doi: 10.1126/science.165.3894.712. This classic study showed that direction tuned neurons in the rabbit dLGN exhibit sharper (more specific) tuning than DSGCs in the retina. To explain this transformation, the authors offer a model in which DSGCs with opposing axes of tuning (e.g. anterior and posterior preferring) can influence the same dLGN neuron through excitation or inhibition. This in essence tells the dLGN relay neuron to prefer one direction and not to prefer the opposite direction. This hypothesized circuit still awaits direct confirmation. [DOI] [PubMed] [Google Scholar]
- 78.Dhande OS, Hua EW, Guh E, Yeh J, Bhatt S, Zhang Y, Ruthazer ES, Feller MB, Crair MC. Development of single retinofugal axon arbors in normal and beta2 knock-out mice. J Neurosci. 2011;31:3384–3399. doi: 10.1523/JNEUROSCI.4899-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crook JD, Peterson BB, Packer OS, Robinson FR, Gamlin PD, Troy JB, Dacey DM. The smooth monostratified ganglion cell: evidence for spatial diversity in the Y-cell pathway to the lateral geniculate nucleus and superior colliculus in the macaque monkey. J Neurosci. 2008;28:12654–12671. doi: 10.1523/JNEUROSCI.2986-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guillery RW. Branching thalamic afferents link action and perception. J Neurophysiol. 2003;90:539–548. doi: 10.1152/jn.00337.2003. [DOI] [PubMed] [Google Scholar]
- 81.Josh Huang Z, Zeng H. Genetic approaches to neural circuits in the mouse. Annu Rev Neurosci. 2013;36:183–215. doi: 10.1146/annurev-neuro-062012-170307. [DOI] [PubMed] [Google Scholar]
- 82.Huberman AD, Niell CM. What can mice tell us about how vision works? Trends Neurosci. 2011;34:464–473. doi: 10.1016/j.tins.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pak MW, Giolli RA, Pinto LH, Mangini NJ, Gregory KM, Vanable JW., Jr Retinopretectal and accessory optic projections of normal mice and the OKN-defective mutant mice beige, beige-J, and pearl. J Comp Neurol. 1987;258:435–446. doi: 10.1002/cne.902580311. [DOI] [PubMed] [Google Scholar]