Abstract
Background
The delta opioid receptor (DOR) is a promising target to treat multiple indications, including alcoholism, anxiety, and nonmalignant pain. The potential of the DORs has been underappreciated, in part, due to relatively low functional expression of these receptors in naïve states. However, chronic exposure to stress, opioids, and inflammation can induce a redistribution of DORs to the cell surface where they can be activated. Previously, DORs were shown to be selectively/exclusively present in spinal cord circuits mediating mechanical sensitivity but not those mediating thermal nociception under naïve conditions.
Methods
We spinally administered DOR and mu opioid receptor (MOR) selective agonists ([D-Pen2,D-Pen5]-Enkephalin, deltorphin II, SNC80, and DAMGO) and antagonists (naltriben and CTAP) and determined thermal antinociception and mechanical sensitivity in wild-type mice or mice with a genetic disruption of DOR or MOR. Thermal antinociception was measured using a radiant heat tail-flick assay; mechanical sensitivity was measured using von Frey filaments. Dose response curves were generated in naïve mice and mice exposed to ethanol in a model of voluntary consumption.
Results
We show that prolonged exposure to ethanol can promote an upregulation of functional DORs in the spinal cord in thermal pain-mediating circuits but not in those mediating mechanical sensitivity. The upregulated DORs either modulate MOR-mediated analgesia through convergence of circuits or signal transduction pathways and/or interact directly with MORs to form a new functional (heteromeric) unit.
Conclusions
Our findings suggest that DORs could be a novel target in conditions in which DORs are redistributed.
Keywords: Alcoholism, analgesia, antinociception, delta opioid receptor, mu opioid receptor, upregulation
Substance abuse and addiction are complex diseases of the brain that have a large impact on society. For example, a recent study reported that, in the United Kingdom, alcoholism has a greater societal cost than any other substance of abuse (1). The few current treatments available to alcoholics are, on average, only moderately effective (2). One of the Food and Drug Administration approved drugs used to treat alcoholism is Revia (naltrexone), an opioid receptor antagonist. Recently, we have shown that targeting the delta opioid receptor (DOR) may provide an alternate approach in treating alcoholism, especially since the DORs appear to affect not only ethanol consumption (3) but also anxiety (4). Importantly, under naïve conditions, many DORs are not functionally active due to their sequestration in large dense core vesicles (LDCVs) (5). However, several stimuli, such as chronic exposure to morphine, stress, or inflammation can induce the redistribution of DORs from LDCVs to the cell surface (6–8). Interestingly, chronic alcohol exposure has also been shown to promote an increase in DOR function in the ventral tegmental area (9). Furthermore, the effects of the DOR agonist drug TAN-67 on ethanol consumption and anxiety are increased after mice have been drinking ethanol for a period of time (3,4). Importantly, there is recent evidence that at least some of the DORs upregulated after chronic morphine (10,11) and chronic drinking (3,4) function as DOR-mu opioid receptor (MOR) heteromers. Therefore, we propose that, similar to chronic exposure to morphine, exposure to ethanol might also increase the number of DORs and potentially DOR-MOR heteromers.
A recent study suggested that MORs and DORs function in independent spinal circuits mediating thermal and mechanical nociception, respectively (12). Specifically, under naïve conditions, thermal nociception can be attenuated solely via MORs in small peptidergic neurons, whereas DORs that reside in small nonpeptidergic neurons attenuate mechanical sensation (12). However, whether this profile changes following chronic ethanol drinking has not been examined.
We hypothesized that prolonged exposure to ethanol may increase the number of DORs and/or DOR-MOR heteromers in nociceptive circuits. Using a broad selection of DOR- and MOR-selective agonists and antagonists, as well as mice with disruptions in either the DOR or MOR gene, we show that chronic ethanol introduces DORs, which can then modulate thermal nociception. This suggests that DORs may be a novel target to treat hyperalgesia observed in alcoholic neuropathy (13).
Methods and Materials
Animals and Housing
Wild-type (WT) or DOR or MOR knockout (KO) C57BL/6 mice (male, 18–23 g; Taconic, Oxnard, California) were housed (maximally five per cage) in ventilated plexiglass cages at ambient temperature (21°C) in a room maintained on a 12-hour light/12-hour dark cycle (lights on at 08:00, lights of at 20:00). Food and water were provided ad libitum. The mice were given 1 week to acclimatize before the start of the experiments. All animal procedures were preapproved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals. Mice were not deprived of food or water at any time.
Chronic Voluntary Ethanol Consumption
Mice were individually housed in ventilated Plexiglass cages at ambient temperature (21°C) in a room maintained on a reversed 12-hour light/12-hour dark cycle (lights off at 10:00, lights on at 22:00). Food and water were provided ad libitum. The mice were given 2 weeks to acclimatize to the individual housing conditions and reverse light cycle before the start of the experiments. A two-bottle limited access (4 hours/day) drinking paradigm was employed for 3 weeks to train mice to increase their intake of a 10% ethanol solution, as previously described (3). The first drug injection was given 4 days after the last exposure to ensure no ethanol was left in the mouse's circulation.
Measurement of Thermal and Mechanical Nociception
Thermal Nociception
Thermal nociception was measured using a radiant heat tail-flick assay. Before injection, a baseline measurement was performed (2–3 seconds). Based on the baseline measurement, a cutoff was set at a time of 3 × the baseline. The average of two measurements was used per mouse.
Mechanical Sensitivity
One day before testing, mice were placed in plastic chambers on a wire mesh grid to habituate for 1 hour. On test day, mice were placed in the chambers 1 hour before injection. Before injection, a baseline measurement was performed. Mechanical sensitivity was measured by stimulating the plantar surface of the hind paw of the mouse with von Frey filaments (.04, .07, .16, .4, .6, 1, 1.4, 2 g). The largest filament (2 g) was used as cutoff. The lowest force that evoked a paw withdrawal response in two out of three tests was recorded. Both paws were measured and the average was used for each animal (Figure S1 in Supplement 1).
Data are represented as the percentage maximal possible effect, which is defined as [(measurement – baseline)/(cutoff – baseline)]*100.
Intrathecal Injection
Mice were anaesthetized with 2% isoflurane 5 minutes before to injection. Intrathecal injection of an opioid solution (5 μL) was performed by direct puncture of spinal lumbar region (L4 through L6) (14) using a Luer-tipped 250 μL Hamilton (725LT) syringe (Sigma-Aldrich, St. Louis, Michigan) to which a 28.5-gauge needle was attached. Upon correct placement of the needle, a clear tail-flick response could be observed. Drug response was measured 10 minutes after intrathecal injection. Mice would generally wake up from anesthesia 2 to 3 minutes after injections and would be fully mobile and awake upon time of measurement.
Drugs
Ethanol solutions were prepared in tap water using 95% (vol/vol) ethanol (Gold Shield Chemical Company, Hayward, California). Naltriben mesylate (NTB), deltorphin II, and [D-Pen2,D-Pen5]-Enkephalin (DPDPE) were purchased from Tocris (Ellisville, Missouri). DAMGO, CTAP, and SNC80 were purchased from Sigma-Aldrich. All compounds were dissolved in saline, with the exception of NTB, which was dissolved in 5% dimethyl sulfoxide. All drugs were prepared immediately before injection and were administered intrathecally. The concentrations of NTB and CTAP were chosen based on the literature (12) and their ability to selectively block the effects of SNC80 (Figure S2A in Supplement 1) or DAMGO (Figure S2B in Supplement 1), respectively. Neither vehicle nor the antagonists alone produced any significant antinociception (Figure S2C in Supplement 1). The choice of NTB over the DOR type-1 (DOR-1) antagonist BNTX and the nonsubtype selective antagonist naltrindole was because of previous reports suggesting that BNTX displaces DOR agonists bound to MOR (3,15) and that NTB has a 10-fold lower affinity for MOR then either naltrindole or BNTX (16).
Results
Under Naïve Conditions, DOR-Selective Opioid Agonist-Induced Thermal Antinociception Is MOR-Mediated
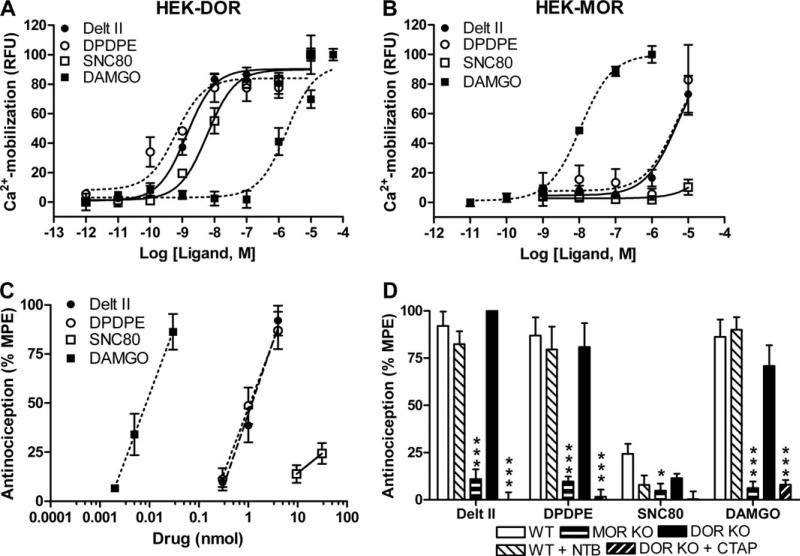
We injected three DOR-selective (SNC80, DPDPE, and deltorphin II) and one MOR-selective (DAMGO) agonist in the spinal cord of naïve C57BL/6 WT mice. The selectivity of the agonists was confirmed in vitro using a calcium mobilization assay (Figure 1A, B). For each of the four agonists, a dose response curve was obtained in the thermal nociception tail-flick assay (Figure 1C, Table 1). The three peptidic ligands DAMGO, DPDPE, and deltorphin II were more potent in reducing thermal nociception than the small molecule SNC80, which produced little thermal antinociception at even the highest dose (Figure 1C; see also Figure S3A in Supplement 1). The MOR-selective agonist DAMGO was 100-fold more potent than any of the DOR-selective agonists. In mice with a disruption of the DOR gene (DOR KO), the DOR-selective agonists DPDPE and deltorphin II still produced antinociception to the same extent as in WT mice (Figure 1D, compare white and black bars; Table 1). Similarly, the DOR antagonist NTB (.5 nmol/5 μL) was unable to block the antinociceptive effects of the DOR agonists DPDPE, deltorphin II, and SNC80 (Figure 1D). These data suggest that the thermal antinociception produced by DPDPE and deltorphin II are mediated through interactions with MOR. Consistent with this interpretation, not only the MOR but also the DOR-selective agonists were ineffective at producing thermal antinociception in mice with a disruption of MOR (MOR KO) (Figure 1D, compare white and gray bars; Table 1). Moreover, the thermal antinociceptive effects of the DOR agonists in the DOR KO mice as well as in WT mice (Figure S3B in Supplement 1) were blocked with the MOR-selective antagonist CTAP (.2 nmol/5 μL, Figure 1D). These findings corroborate previous reports of residual DPDPE-induced antinociception in DOR KO mice (15) and a reduced antinociceptive effect of deltorphin II in MOR KO mice (17).
Figure 1.
Delta opioid receptor (DOR) selective agonists produce thermal antinociception via mu opioid receptors. HEK293 cells stably expressing murine DOR (A) or murine mu opioid receptors (MOR) (B) were transiently transfected with a chimeric Gαqi4-protein. Cells were stimulated with increasing doses of a DOR-selective (deltorphin II, [D-Pen2,D-Pen5]-Enkephalin [DPDPE], or SNC80) or MOR-selective (DAMGO) agonist and calcium mobilization was measured. Experiments were performed at least three times in triplicate, representative curves are shown. (C) Wild-type (WT) C57BL/6 mice (n = 8–10) were injected inrathecally with increasing doses of a DOR-selective or MOR-selective agonist and antinociception was measured using a radiant heat tail-flick assay. (D) WT, DOR knockout (KO), and MOR KO C57BL/6 mice (n = 8–12) were injected intrathecally with agonist (deltorphin II [4 nmol], DPDPE [4 nmol], SNC80 [30 nmol], or DAMGO [30 pmol]) and thermal antinociception was measured. In WT mice, the agonist response was unaffected by co-injection of the DOR antagonist Naltriben (.5 nmol). In DOR KO mice, the agonist response was inhibited by co-injection of the MOR antagonist CTAP (.2 nmol). Data are represented as the percentage maximal possible effect, which is defined as [(measurement – baseline)/(cutoff – baseline)]*100. Significance between groups was determined by analysis of variance followed by a Newman-Keuls post hoc analysis. *p < .05; ***p < .001. Delt II, deltorphin II; HEK, HEK293; MPE, maximal possible effect; NTB, Naltriben; RFU, relative fluorescence units.
Table 1.
ED50 Values (95% Confidence Interval, nmol) for Antinociception Produced by DOR-Selective and MOR-Selective Agonists in Naïve WT, DOR KO, and MOR KO Mice and WT Mice Who Had Been Voluntarily Consuming Ethanol
| ED50 (95% CI, nmol) |
|||||
|---|---|---|---|---|---|
| Mice | Deltorphin II | DPDPE | SNC80 | DAMGO | |
| Thermal | Naïve | 1.19 (.9–1.6) | 1.18 (.9–1.7) | ND | .009 (.006–.013) |
| DOR KO | .75 (.5–1.1)a | .69 (.3–1.5) | ND | .016 (.009–.031) | |
| MOR KO | ND | ND | ND | ND | |
| Ethanol | .42 (.31–.60)b | .39 (.29–.55)b | ND | .003 (.002–.007) | |
| Mechanical | Naïve | 1.66 (1.2–2.3) | 6.37 (3.5–28.1) | 5.04 (3.1–10.1) | .024 (.016–.039) |
| DOR KO | ND | ND | ND | >.03 | |
| MOR KO | ND | ND | 6.06 (3.4–14.0) | >1 | |
| Ethanol | 2.06 (1.3–4.6) | 8.61 (5.5–24.2) | 4.06 (2.8–6.4) | .027 (.016–.046) | |
Thermal antinociception was measured using a radiant heat tail-flick assay. Mechanical sensitivity was measured using von Frey filaments. GraphPad Prism (Graphpad Software, La Jolla, California) was used to determine statistical differences between groups.
CI, confidence interval; DOR, delta opioid receptor; DPDPE, [D-Pen2,D-Pen5]-Enkephalin; ED50, effective dose 50%; KO, knockout; MOR, mu opioid receptor; ND, not detectable; WT, wild-type.
p < .05.
p < .001.
Chronic Ethanol Exposure Alters DOR but Not MOR Agonist-Induced Responses
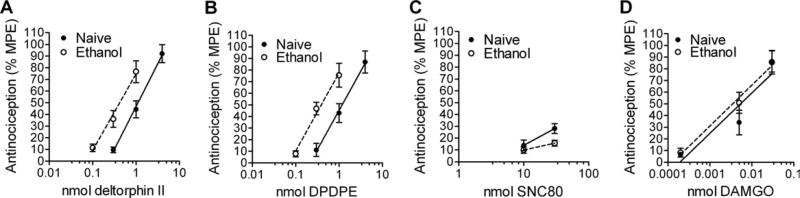
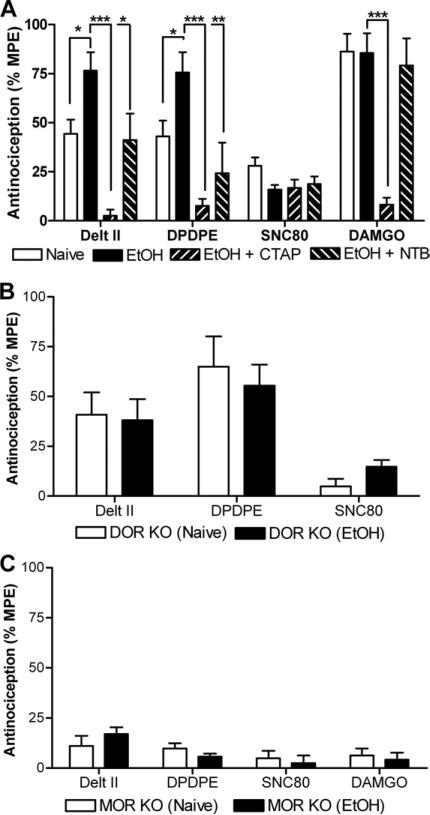
We next examined whether chronic voluntary consumption of ethanol altered the effects of DOR-selective ligands in spinal nociceptive circuits. Mice were trained to voluntarily consume ethanol ([3] and Methods and Materials). Mice who had been drinking ethanol showed a clear leftward shift in the thermal antinociceptive effects of DPDPE [F(2,46) = 10.37, p = .0002] and deltorphin II [F(2,47) = 11.97, p < 0.0001], while no changes were observed in the potencies of DAMGO [F(2,51) = .43, p = .65] and SNC80 [F(2,32) = 2.92, p = .07] (Figure 2, Table 1). The DOR-selective antagonist NTB (.5 nmol/5 μL) blocked the potentiation of the antinociceptive effects of DPDPE [F(2,22) = 11.58, p = .0004] and deltorphin II [F(2,23) = 16.48, p < .0001] on thermal nociception in the mice who had been drinking (Figure 3A), in sharp contrast to the absence of any effect of NTB on nociception to DOR agonist in ethanol-naïve mice (Figure 1D). These data suggest that the increase in potency of DOR agonists in the ethanol-drinking mice is due to an upregulation of DORs and not MORs. In support of this, there was no ethanol drinking-induced shift in DOR agonist potency in mice with a disruption in the DOR gene (Figure 3B) and no shift in the potency of DAMGO in WT mice (Figure 2D, Table 1).
Figure 2.
Chronic ethanol increases the potency of certain delta opioid receptor (DOR)-selective agonists for thermal antinociception. Naïve C57BL/6 mice (n = 8–10) or mice (n = 8–9) that had chronically self-administered ethanol (see Methods and Materials) were injected intrathecally with increasing doses of a DOR-selective (deltorphin II [A], [D-Pen2,D-Pen5]-Enkephalin [B], SNC80 [C], or mu opioid receptor-selective (DAMGO [D]) agonist and thermal antinociception was measured using a radiant heat tail-flick assay. Data are represented as the percentage maximal possible effect, which is defined as [(measurement – baseline)/(cutoff – baseline)]*100. DPDPE, [D-Pen2,D-Pen5]-Enkephalin; MPE, maximal possible effect.
Figure 3.
Both delta opioid receptor (DOR) and mu opioid receptor (MOR) are required for the ethanol-induced increase in potency of DOR-selective agonists. (A) Ethanol-drinking wild-type, C57BL/6 mice (n = 8–10) were injected intrathecally with agonist (deltorphin II [1 nmol], [D-Pen2,D-Pen5]-Enkephalin [DPDPE] [1 nmol], SNC80 [30 nmol], or DAMGO [30 pmol]) and antinociception was measured using a radiant tail-flick assay. Involvement of MOR and DOR was determined by co-injection with either the MOR-selective antagonist CTAP (.2 nmol) or the DOR-selective antagonist Naltriben (.5 nmol), respectively. Significance between groups was determined by analysis of variance followed by a Newman-Keuls post hoc analysis. (B) Naïve or ethanol-drinking C57BL/6 DOR knockout (KO) mice (n = 8–10) were injected intrathecally with agonist (deltorphin II [1 nmol], DPDPE [1 nmol], or SNC80 [30 nmol]) and thermal antinociception was measured. (C) Naïve or ethanol-drinking C57BL/6 MOR KO mice (n = 8–9) were injected intrathecally with agonist (deltorphin II [4 nmol], DPDPE [4 nmol], SNC80 [30 nmol], or DAMGO [30 pmol]) and antinociception was measured. Data are represented as the percentage maximal possible effect, which is defined as [(measurement – baseline)/(cutoff – baseline)]*100. *p < .05; ***p < .001. Delt II, deltorphin II; EtOH, ethanol; MPE, maximal possible effect; NTB, Naltriben.
Chronic Ethanol Produces Enhanced DOR-Mediated Thermal Antinociception That Is Dependent on MOR
We were intrigued by the observation that ethanol drinking produced a shift in the potency of some but not all DOR agonists (Figure 2). In particular, ethanol consumption promoted a shift in the potency of DPDPE and deltorphin II but not SNC80. In vitro, DPDPE and deltorphin II can both signal with some efficacy in cells expressing only the MOR (Figure 1B). In addition, in vivo, in naïve mice, both DPDPE and deltorphin II can induce thermal antinociception even in the absence of DOR (Figure 1D). In contrast, SNC80 shows no efficacy in cells expressing only MOR (Figure 1B) and little thermal antinociceptive effects in vivo. Together, these data suggested that the increased potency of DPDPE and deltorphin II (and no change in potency of SNC80) were dependent on MOR. In support of this hypothesis, mice with a disruption of MOR showed no ethanol consumption-induced shift in DPDPE or deltorphin II potency (Figure 3C). This suggests that the increase in antinociception observed for deltorphin II and DPDPE in ethanol-exposed WT mice requires both DOR and MOR. Since antinociception by the MOR agonist DAMGO was not affected by drinking (Figure 2D, Table 1), potentiation of DPDPE and deltorphin II potency must originate from an interaction with a target that requires both DOR and MOR, likely a heteromer. This interpretation is supported by the observation that not only NTB (.5 nmol/5 μL) but also CTAP (.2 nmol/5 μL) attenuate the effects of DPDPE and deltorphin II in the ethanol-drinking mice (Figure 3A).
Mechanical Sensitivity Is Mediated Through Both DOR and MOR
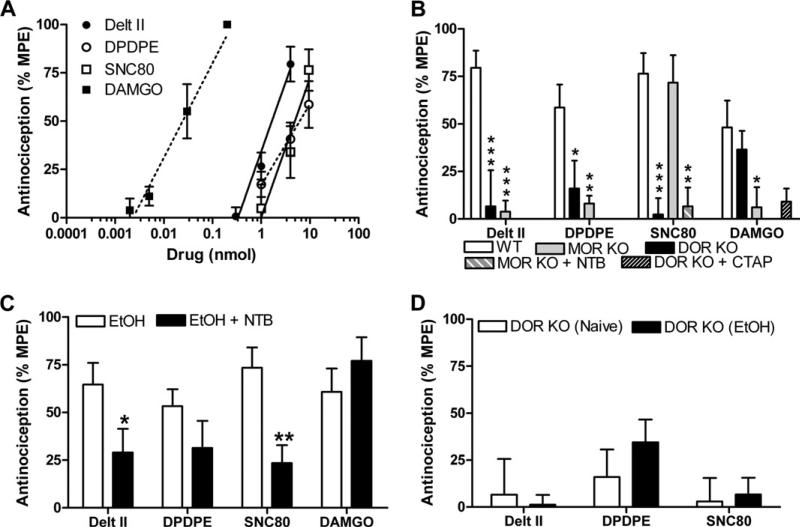
The spinal neurons that relay thermal nociceptive signals are reported to be distinct from those that transduce mechanical sensitivity (12). Thus, we next examined whether ethanol consumption altered the effects of DOR ligands in mechanical nociceptors. Whereas the DOR agonist SNC80 was ineffective in producing thermal antinociception (Figure 1C), it reduced mechanical sensitivity at a potency comparable with that of DPDPE and deltorphin II (Figure 4A, Table 1). The effects of SCN80 on mechanical sensitivity were eliminated in mice with a disruption of DOR and unaffected by the disruption of MOR (Figure 4B), suggesting that they were mediated solely by DOR. In contrast, the effects of DPDPE and deltorphin II on mechanical sensitivity were attenuated not only by disruption of DOR but also by disruption of MOR (Figure 4B). These results suggest that MORs do indeed operate in nociceptors that mediate mechanical sensitivity—even in naïve mice. In support of this hypothesis, DAMGO reduced mechanical sensitivity in naïve mice, albeit at doses much higher that those required for thermal antinociception. The mechanical antinociception produced by these high doses of DAMGO was not due to agonist effects at DOR at high doses (Figure 1) since DOR KO mice show DAMGO-induced mechanical antinociception indistinguishable from that of WT mice (Figure 4B).
Figure 4.
Mechanical sensitivity is mediated by both delta opioid receptor (DOR) and mu opioid receptor (MOR) and is not altered by chronic ethanol exposure. (A) Wild-type (WT) C57BL/6 mice (n = 8–14) were injected intrathecally with increasing doses of a DOR-selective (deltorphin II, [D-Pen2,D-Pen5]-Enkephalin [DPDPE], or SNC80) or MOR-selective (DAMGO) agonist, and mechanical sensitivity was measured using von Frey filaments. (B) WT, DOR knockout (KO), and MOR KO C57BL/6 mice (n = 8–14) were injected intrathecally with agonist (deltorphin II [4 nmol], DPDPE [10 nmol], SNC80 [10 nmol], or DAMGO [30 pmol]), and mechanical sensitivity was measured. In WT mice, the DAMGO response was unaffected by co-injection of the DOR antagonist Naltriben (NTB) (.5 nmol). In MOR KO mice, the SNC80-induced response was inhibited by co-injection of the NTB (.5 nmol). Significance between groups was determined by analysis of variance followed by a Newman-Keuls post hoc analysis. (C) Ethanol-drinking WT, C57BL/6 mice (n = 8–14) were injected intrathecally with agonist (deltorphin II [4 nmol], DPDPE [10 nmol], SNC80 [10 nmol], or DAMGO [30 pmol]), and mechanical sensitivity was measured. Involvement DOR was determined by co-injection with the DOR-selective antagonist NTB (.5 nmol), respectively. (D) Naïve or ethanol-drinking C57BL/6 DOR KO mice (n = 8–9) were injected intrathecally with agonist (deltorphin II [4 nmol], DPDPE [10 nmol], or SNC80 [10 nmol]), and mechanical sensitivity was measured. Data are represented as the percentage maximal possible effect, which is defined as [(measurement – baseline)/(cutoff – baseline)]*100. *p < .05; **p < 0.01; ***p < .001. Delt II, deltorphin II; EtOH, ethanol; MPE, maximal possible effect.
The Effects of DOR Ligands on Mechanical Antinociception Is Not Affected by Ethanol Exposure
Prolonged ethanol exposure did not affect the potencies of either the DOR or MOR agonists (Figure 4C, Table 1) for mechanical antinociception. As expected, the responses of the DOR-selective agonists in ethanol-drinking mice with disruption of the DOR gene did not differ from those in naive mice (Figure 4D), considering that all ligands require the presence of DOR to function (Figure 4B).
Discussion
The present study provides evidence for the existence of DORs in spinal neurons mediating thermal nociception after chronic voluntary ethanol consumption. These DORs are only apparent in the presence of functional MORs. In spinal neurons mediating mechanical sensitivity, we observe three populations of opioid receptor targets: a target independent of DOR (MOR homomers), a target independent of MOR (DOR homomers), and a target dependent on both DOR and MOR (DOR-MOR heteromers).
The localization and trafficking of the DOR remains a matter of great debate. Both a green fluorescent protein (GFP)-tagged DOR transgenic mouse and antibodies raised to the DOR have been used to identify neurons expressing DOR—each with their own caveats. The selectivity of the (commercially) available antibodies for the DOR is still heavily debated. Indeed, DOR antibody immunoreactivity is still present in tissue from DOR KO mice (12), although it may be possible to dilute the antibody enough to selectively label DORs (18). In part, to circumvent the selectivity issues of the DOR antibody, Scherrer et al. (19) generated a knockin mouse line carrying a N-terminally GFP-tagged DOR.
Results using the DOR antibody and the GFP-DOR have conflicting conclusions regarding the distribution of the DOR. In naïve WT mice, the DOR appears to be localized primarily in LDCVs in neurons of the dorsal root ganglion (DRG) in naïve animals (20). In the GFP-DOR mice, the receptor is expressed abundantly throughout the central nervous system and is present on the cell surface in neurons of the DRG under naïve conditions (19). Furthermore, in naive GFP-DOR mice, MORs and DORs are co-localized only rarely in DRGs (12), while, in naïve wild-type mice, DOR antibodies and in situ hybridization suggest that co-localization of MOR and DOR is more frequent (11,18). Consequently, the co-localization debate is primarily focused on whether DORs are present in the peptidergic neurons that express MOR and regulate thermal nociception and whether MORs are on nonpeptidergic neurons that mediate mechanical sensitivity.
The seemingly conflicting findings can possibly be well-resolved if one considers that the distribution of DOR is dynamically regulated; for example, if LDCVs in the peptidergic neurons in naïve mice are redistributed in response to physiological stimuli. Indeed, there is mounting evidence that numerous physiological stimuli alter the number of functional DORs (4,9,10,21–24). Our data here suggest that chronic voluntary ethanol consumption is one physiological stimulus that can change the functional expression of the DOR specifically in nociceptors that mediate thermal nociception.
Specifically, we find that in naïve mice, DAMGO, DPDPE, and deltorphin II produce thermal antinociception solely via MORs, in agreement with previous findings (12,25). However, after chronic ethanol drinking, DORs become functional in modulating thermal nociception, although their function depends on the presence of functional MORs. Interestingly, however, we find that even in naïve mice, not just DOR but also MOR modulate mechanical sensitivity, in contrast to Scherrer et al. (12), who propose that MORs play no role in mechanical sensitivity. These results can be easily reconciled since only the higher doses of DAMGO (not tested by Scherrer et al. [12]) were effective at producing mechanical antinociception in our experiments. Several reports have suggested the presence of MORs in nonpeptidergic neurons, but the expression of MOR is lower than that of DOR (26,27). This could explain why we find that DAMGO reduces mechanical sensitivity with a lower potency than it does thermal nociception.
Here, we show that the increased potency of DPDPE and deltorphin II to produce thermal antinociception in mice chronically exposed to ethanol requires both DOR and MOR. One explanation for these findings is that the DOR ligands acting on the ethanol-up-regulated DORs act in concert with antinociception produced by these DOR ligands acting on MORs. However, the response from DORs alone is not enough to cross a threshold to be antinociceptive in itself, as evidenced by the inability of SNC80 to produce strong thermal antinociception. This crosstalk could either be on the level of neurotransmitter release or intracellular at the level of downstream signal transducers. This kind of receptor crosstalk or synergism between DOR and MOR has been proposed previously (28–30). Another explanation for these findings is that the DOR agonists engage a DOR-MOR heteromer that is upregulated during drinking (31,32). Recent findings have suggested that DOR and MOR can form heteromers (31) that are upregulated after chronic morphine (10). Depending on the study, these heteromers are proposed to play either a protective/beneficial/antinociceptive role (33) or a tolerance-inducing/pronociceptive role (11). While DPDPE and deltorphin II can produce thermal antinociception in ethanol-exposed mice independently of DORs, their function is entirely dependent on both DOR and MOR in spinal neurons mediating mechanical sensitivity, suggesting that, at least in these neurons, the two receptors can interact to form functional heteromers. Importantly, we have recently shown that TAN-67 requires the presence of DORs and MORs to reduce ethanol consumption (3,4) and that this ligand is able to reduce ethanol withdrawal-induced anxiety (4). The results reported here suggest that a similar target, reliant on both DOR and MOR, is also relevant for pain and could, thereby, have the added benefit of alleviating hyperalgesia associated with alcohol abuse and alcohol withdrawal.
Supplementary Material
Acknowledgments
This work was funded by the Foundation for Alcohol Research-Alcoholic Beverage Medical Research Foundation (RMvR), the Department of Defense Grant DAMD62-10-5-071 (JLW), the National Institute on Alcohol Abuse and Alcoholism (5 P50 AA017072-03), National Institute on Drug Abuse Grants R01 DA015232 and DA019958 (JLW), and funds provided by the State of California for medical research on alcohol and substance abuse through the University of California, San Francisco (JLW).
RMvR designed and performed research, analyzed data, and wrote the paper, DIB performed research, and JLW designed research, analyzed data, and wrote the paper.
We thank Madeline Ferwerda and Li He for technical assistance.
Footnotes
The authors reported no biomedical financial interests or other potential conflicts of interest.
Supplementary material cited in this article is available online.
References
- 1.Nutt DJ, King LA, Phillips LD. Drug harms in the UK: A multicriteria decision analysis. Lancet. 2010;376:1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- 2.Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE study: A randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 3.van Rijn RM, Whistler JL. The delta(1) opioid receptor is a heterodimer that opposes the actions of the delta(2) receptor on alcohol intake. Biol Psychiatry. 2009;66:777–784. doi: 10.1016/j.biopsych.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Rijn RM, Brissett DI, Whistler JL. Dual efficacy of delta opioid receptor selective ligands for ethanol drinking and anxiety. J Pharmacol Exp Ther. 2010;335:133–139. doi: 10.1124/jpet.110.170969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng PY, Svingos AL, Wang H, Clarke CL, Jenab S, Beczkowska IW, et al. Ultrastructural immunolabeling shows prominent presynaptic vesicular localization of delta-opioid receptor within both enkephalinand nonenkephalin-containing axon terminals in the superficial layers of the rat cervical spinal cord. J Neurosci. 1995;15:5976–5988. doi: 10.1523/JNEUROSCI.15-09-05976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan J-S, Xu Z-Z, Gao H, He S-Q, Ma G-Q, Sun T, et al. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122:619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Cahill CM, Holdridge SV, Morinville A. Trafficking of delta-opioid receptors and other G-protein-coupled receptors: Implications for pain and analgesia. Trends Pharmacol Sci. 2007;28:23–31. doi: 10.1016/j.tips.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Bao L, Guan JS. Role of delivery and trafficking of delta-opioid peptide receptors in opioid analgesia and tolerance. Trends Pharmacol Sci. 2006;27:324–329. doi: 10.1016/j.tips.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Margolis EB, Fields HL, Hjelmstad GO, Mitchell JM. Delta-opioid receptor expression in the ventral tegmental area protects against elevated alcohol consumption. J Neurosci. 2008;28:12672–12681. doi: 10.1523/JNEUROSCI.4569-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A, Mulder J, Gomes I, Rozenfeld R, Bushlin I, Ong E, et al. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci Signal. 2010;3:ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He SQ, Zhang ZN, Guan JS, Liu HR, Zhao B, Wang HB, et al. Facilitation of mu-opioid receptor activity by preventing delta-opioid receptor-mediated codegradation. Neuron. 2011;69:120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Scherrer G, Imamachi N, Cao YQ, Contet C, Mennicken F, O'Donnell D, et al. Dissociation of the opioid receptor mechanisms that control mechanical and heat pain. Cell. 2009;137:1148–1159. doi: 10.1016/j.cell.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koike H, Sobue G. Alcoholic neuropathy. Curr Opin Neurol. 2006;19:481–486. doi: 10.1097/01.wco.0000245371.89941.eb. [DOI] [PubMed] [Google Scholar]
- 14.Hylden JL, Wilcox GL. Intrathecal morphine in mice: A new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- 15.Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, et al. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24:243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 16.Parkhill AL, Bidlack JM. Several delta-opioid receptor ligands display no subtype selectivity to the human delta-opioid receptor. Eur J Pharmacol. 2002;451:257–264. doi: 10.1016/s0014-2999(02)02241-0. [DOI] [PubMed] [Google Scholar]
- 17.Matthes HW, Smadja C, Valverde O, Vonesch JL, Foutz AS, Boudinot E, et al. Activity of the delta-opioid receptor is partially reduced, whereas activity of the kappa-receptor is maintained in mice lacking the mu-receptor. J Neurosci. 1998;18:7285–7295. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang HB, Zhao B, Zhong YQ, Li KC, Li ZY, Wang Q, et al. Coexpression of delta- and mu-opioid receptors in nociceptive sensory neurons. Proc Natl Acad Sci U S A. 2010;107:13117–13122. doi: 10.1073/pnas.1008382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scherrer G, Tryoen-Toth P, Filliol D, Matifas A, Laustriat D, Cao YQ, et al. Knockin mice expressing fluorescent delta-opioid receptors uncover G protein-coupled receptor dynamics in vivo. Proc Natl Acad Sci U S A. 2006;103:9691–9696. doi: 10.1073/pnas.0603359103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang HB, Guan JS, Bao L, Zhang X. Distinct subcellular distribution of delta-opioid receptor fused with various tags in PC12 cells. Neurochem Res. 2008;33:2028–2034. doi: 10.1007/s11064-008-9678-9. [DOI] [PubMed] [Google Scholar]
- 21.Cahill CM, Morinville A, Hoffert C, O'Donnell D, Beaudet A. Up-regulation and trafficking of delta opioid receptor in a model of chronic inflammation: Implications for pain control. Pain. 2003;101:199–208. doi: 10.1016/s0304-3959(02)00333-0. [DOI] [PubMed] [Google Scholar]
- 22.Bie B, Zhang Z, Cai YQ, Zhu W, Zhang Y, Dai J, et al. Nerve growth factor-regulated emergence of functional delta-opioid receptors. J Neurosci. 2010;30:5617–5628. doi: 10.1523/JNEUROSCI.5296-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hack SP, Bagley EE, Chieng BC, Christie MJ. Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci. 2005;25:3192–3198. doi: 10.1523/JNEUROSCI.4585-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chieng B, Christie MJ. Chronic morphine treatment induces functional delta-opioid receptors in amygdala neurons that project to periaqueductal grey. Neuropharmacology. 2009;57:430–437. doi: 10.1016/j.neuropharm.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 25.Scherrer G, Befort K, Contet C, Becker J, Matifas A, Kieffer BL. The delta agonists DPDPE and deltorphin II recruit predominantly mu receptors to produce thermal analgesia: A parallel study of mu, delta and combinatorial opioid receptor knockout mice. Eur J Neurosci. 2004;19:2239–2248. doi: 10.1111/j.0953-816X.2004.03339.x. [DOI] [PubMed] [Google Scholar]
- 26.Wu ZZ, Chen SR, Pan HL. Differential sensitivity of N- and P/Q-type Ca2+ channel currents to a mu opioid in isolectin B4-positive and-negative dorsal root ganglion neurons. J Pharmacol Exp Ther. 2004;311:939–947. doi: 10.1124/jpet.104.073429. [DOI] [PubMed] [Google Scholar]
- 27.Yamamoto J, Kawamata T, Niiyama Y, Omote K, Namiki A. Down-regulation of mu opioid receptor expression within distinct subpopulations of dorsal root ganglion neurons in a murine model of bone cancer pain. Neuroscience. 2008;151:843–853. doi: 10.1016/j.neuroscience.2007.11.025. [DOI] [PubMed] [Google Scholar]
- 28.Traynor JR, Elliott J. delta-Opioid receptor subtypes and cross-talk with mu-receptors. Trends Pharmacol Sci. 1993;14:84–86. doi: 10.1016/0165-6147(93)90068-u. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Z, Pan ZZ. Synaptic mechanism for functional synergism between delta- and mu-opioid receptors. J Neurosci. 2010;30:4735–4745. doi: 10.1523/JNEUROSCI.5968-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He L, Lee NM. Delta opioid receptor enhancement of mu opioid receptor-induced antinociception in spinal cord. J Pharmacol Exp Ther. 1998;285:1181–1186. [PubMed] [Google Scholar]
- 31.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang D, Sun X, Bohn LM, Sadee W. Opioid receptor homo- and heterodimerization in living cells by quantitative bioluminescence resonance energy transfer. Mol Pharmacol. 2005;67:2173–2184. doi: 10.1124/mol.104.010272. [DOI] [PubMed] [Google Scholar]
- 33.Gomes I, Gupta A, Filipovska J, Szeto HH, Pintar JE, Devi LA. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc Natl Acad Sci U S A. 2004;101:5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.