Abstract
Background
Allergic bronchopulmonary aspergillosis (ABPA) is a complicating factor in cystic fibrosis (CF), affecting 2–15% of patients. We hypothesized that sensitization/challenge of CFTR−/− mice with an Aspergillus fumigatus (Af) extract will affect eicosanoid pathway gene expression, impacting ABPA and CF.
Methods
FABP-hCFTR+/−-CFTR−/− mice were sensitized/challenged with an Af extract and gene expression of lung mRNA was evaluated for >40 genes, with correlative data in human CF (IB3.1) and CFTR-corrected (S9) bronchoepithelial cell lines.
Results
Pla2g4c, Pla2g2c, Pla2g2d and Pla2g5 were induced in response to Af in CFTR−/− mice. Interestingly, PLA2G2D was induced by LPS, IL-2, IL-6, IL-13, and Af only in CFTR-deficient human IB3.1 cells. Prostanoid gene expression was relatively constant, however, several 12/15-lipoxygenase genes were induced in response to Af. Numerous cytokines also caused differential expression of ALOX15 only in IB3.1 cells.
Conclusions
The distinct regulation of PLA2G4C, PLA2G2D and ALOX15 genes in Aspergillus sensitization and/or cystic fibrosis could provide new insights into diagnosis and treatment of ABPA and CF.
Keywords: Allergy, Lung, Lipid Mediators, Inflammation
1. Introduction
The eicosanoid pathway plays a defining role in inflammation, producing bioactive lipids which modulate both the onset of inflammation and its resolution. Hydrolytic cleavage of arachidonic acid (AA) from the phospholipid membrane is followed by metabolism of AA by the cyclooxygenases and lipoxygenases leading to the production of downstream prostaglandins (PGs) and leukotrienes (LTs), respectively. These pro- and anti-inflammatory lipid mediators orchestrate many aspects of inflammation including vasodilation, vascular permeability, bronchoconstriction, chemotaxis, and the transcription of pro-inflammatory enzymes; thus they are critical to several diseases such as asthma and cystic fibrosis (CF) (1–3). Specifically in CF, the fatty acid profile is skewed toward AA, though it is not yet clear whether this is due to increased production of this precursor or decreased metabolism into the downstream products (2, 3).
Within these pathophysiological events, the PGs and LTs regulate many aspects of airway inflammation and reactivity. Prostaglandins normally maintain a balance in airway responsiveness; both PGD2 and thromboxane A2 are bronchoconstrictors, while PGE2 and prostacyclin serve in bronchoprotection (1). On the other hand, the action of LTs appears to be principally pro-inflammatory, with LTB4 possessing potent chemoattractive activity for neutrophils and eosinophils in the airways (4). Furthermore, the cysteinyl leukotrienes (cysLTs), LTC4, LTD4 and LTE4, elicit bronchoconstriction and increased endothelial membrane permeability leading to airway edema and enhanced mucosal secretion, which are also physiological hallmarks of asthma (5).
Due to this complex balance and the changes in eicosanoid metabolism that occur in CF, we hypothesized that a more detailed evaluation of eicosanoid gene expression in an animal model of asthma superimposed on CF would be valuable in predicting other therapeutic targets in eicosanoid metabolism. We developed a model of allergic bronchopulmonary aspergillosis (ABPA)/allergic asthma which employs crude Aspergillus fumigatus (Af) extract as the sensitizing and challenging agent and evaluated this in a cystic fibrosis transmembrane conductance regulator knockout (FABP-hCFTR+/−-CFTR−/−) mouse (6). A CFTR−/− mouse model was used because asthma and Af sensitization are both potential complications in CF patients due to the inability to effectively clear secretions leading to infection and increased inflammation (7, 8). Af-sensitized/challenged mice develop a Th2 mediated allergic inflammatory response, including elevated levels of the Th2 cytokines, IL-4, IL-5 and IL-13, increased total serum IgE, goblet cell hyperplasia and airway eosinophilia (6). More specifically, an increase in Af-specific IgE and IgG are found in the serum of sensitized mice, demonstrating an Af-specific immune response (6). In the present study, the gene expression levels of numerous enzymes involved in eicosanoid metabolism are analyzed by real-time RT-PCR after Af sensitization and challenge in CFTR−/− mice with comparisons in human CF derived and normal bronchial epithelial cell lines. Our data demonstrate alterations in gene expression for a unique subset of eicosanoid pathway enzymes which may provide relevant alternative targets in the development of markers and therapeutic regimens for CF.
2. Material and methods
2.1. Mouse strains
The original CFTR−/− mice were generated from the embryonic stem cell line, E14TGa, derived from 129/Ola mice and maintained on a C57BL/6 background (9). Zhou et al. corrected the intestinal CFTR deficits by generating a transgenic mouse expressing the human CFTR gene (hCFTR) driven by the intestinal specific fatty acid binding protein (FABP) promoter (10). These corrected mice, FABP-hCFTR+/−-CFTR−/− mice have a mixed background of 129/Ola, C57BL/6 and FVB/N (6, 9, 10). In this manuscript, CFTR−/− refers to the mixed background intestinally corrected mice. CFTR−/−, C57BL/6, and FVB mice were housed in the SPF facility of the University of Florida according to NIH guidelines. All experiments were approved by the IACUC of the University of Florida.
2.2. Sensitization and challenge
Animals were sensitized with a single lot of Aspergillus fumigatus extract (Af) (XPM3D3A4, Greer Laboratories) by intraperitoneal injections on days 0 and 14 and subsequently aerosol challenged in an acrylic chamber with nebulized Af extract on days 28, 29 and 30 as previously described (6). A subset of mice was similarly sensitized and challenged with ovalbumin (OVA) while non-sensitized mice were mock-sensitized with PBS and similarly challenged with Af.
2.3. Lung mRNA extraction and real-time RT-PCR
Whole lungs from mice were flash frozen in liquid nitrogen and total RNA was isolated as per the Chomczynski and Sacchi method with modifications (11, 12). cDNA was produced with the SuperScript first-strand synthesis Kit (Invitrogen) and subsequently utilized for real-time RT-PCR with 1x SYBR Green (Applied Biosystems, Bio-Rad). Reactions were carried out in an ABI Sequence Detection System 7000 and relative fold changes were determined using the ΔΔCT method normalized to cyclophilin A. Briefly, the difference between crossing threshold (CT) values of the target and control genes represent ΔCT value. The difference between the ΔCT value for any given sample and the control sample generates the ΔΔCT value. 2−ΔΔCT provides the relative fold change for each sample compared to the control, which is normalized to 1. Primers used are listed in Supplemental Table 1. Human and mouse gene symbols, based on HUGO and Mouse Genome Informatics guidelines, used throughout this study are detailed in Supplemental Table 2.
2.4. Tissue Culture
SV40 T-antigen transformed human bronchial epithelial cells from a CF patient (IB3.1) (13) and IB3.1 cells corrected through the expression of an AAV inserted functional CFTR gene (S9) (14) were maintained in Ham’s F12K medium (Life Technologies) with 10% fetal bovine serum, 10μg/mL penicillin G, 0.1mg/mL streptomycin and 0.25μg/mL amphotericin B at 37 °C in 5% CO2. Cells were grown to 70–75% confluency before 12 h of treatment with pharmacologic agents and/or cytokines. Treatment concentrations were as follows: Af, 100μg/mL; LPS, 0.5μg/mL; IFNγ, 5ng/mL; TNF-α, 10ng/mL; IL-1β, 2ng/mL; IL-2, 5ng/mL; IL-3, 5ng/mL; IL-4, 20ng/mL; IL-6 10ng/mL; IL-10, 20ng/mL; IL-13, 20ng/mL.
2.5. Statistical Analysis
The data from each group of mice were pooled and analyzed by two-tailed unpaired Student’s t-tests. Cell line studies were performed based on animal data and were analyzed by one-tailed paired Student’s t-tests. Statistical significance was determined as p ≤ 0.05.
3. Results
3.1. Allergic and inflammatory model in CF
We previously evaluated Af extract as an allergen in C57BL/6 and CFTR−/− mice as a model of allergic asthma demonstrating that Af-sensitized mice develop a Th2-mediated response as seen in asthma with increases in total serum IgE, goblet cell hyperplasia and airway eosinophilia (6). As indicated in 2.1. Material and methods, we chose a mixed background mouse strain, FABP-hCFTR+/−-CFTR−/− (6, 9, 10), which may be more analogous to a genetically and immunologically diverse human population. We have performed similar analyses in two normal inbred mouse strains, C57BL/6 and FVB (summarized in Table 2), due to their contribution to the mixed background of the FABP-hCFTR+/−-CFTR−/− mice (6, 9, 10). It should be noted that even if a gene responds differently in the knockout mouse compared to each of the background strains, it is impossible to conclude whether differences are due to the CFTR gene ablation or a combination of factors arising from the mix strain background. Therefore, any response due to the lack of a functional CFTR was further substantiated in human cell lines. Specifically, we have utilized bronchoepithelial cells isolated from a cystic fibrosis patient and immortalized with a hybrid virus, adeno-12-SV40 expressing SV40 T-antigen, IB3.1 (13). As a control, we provide results from the S9 cell line, derived from the IB3.1 cell line by introduction of a wild-type CFTR gene harbored in an adeno-associated viral vector (15). Differences between the CF cell line, IB3.1, and the corrected S9 cells have been previously highlighted electrophysiologically (16) and in the inflammatory response (17), with the consistent implication that S9 cells behave similarly to normal bronchoepithelial cells. In this way, the mixed background of the mice allows for observations that may be linked to the CFTR gene, while subsequent studies in the human cell lines allow for a direct comparison in which the only difference is the presence of a functional CFTR.
Table 2.
Fold change of phospholipase A2 and lipoxygenase gene expression in Af sensitization/challenge, compared to PBS sensitized mice
| CFTR−/− | C56BL/6 | FVB | |
|---|---|---|---|
| Pla2g2c | 2.3 ± 0.4* | 0.9 ± 0.1a | - |
| Pla2g2d | 3.2 ± 0.9* | 1.4 ± 0.3a | 0.7 ± 0.2 |
| Pla2g2e | 1.2 ± 0.2 | 1.7 ± 0.2*a | 1.5 ± 0.3 |
| Pla2g5 | 3.6 ± 0.5* | 1.9 ± 0.3*a | 0.9 ± 0.2 |
| Pla2g10 | 1.1 ± 0.3 | 1.0 ± 0.2a | - |
| Pla2g12b | 1.1 ± 0.2 | 0.5 ± 0.1*a | - |
| Pla2g4a | 1.4 ± 0.4 | 1.2 ± 0.1 | 1.0 ± 0.2 |
| Pla2g4b | 1.2 ± 0.2 | 0.9 ± 0.1 | - |
| Pla2g4c | 6.4 ± 2.2* | 25.0 ± 2.8* | 2.6 ± 0.7 |
| Ptgs1 | 1.4 ± 0.2 | 0.9 ± 0.3 | - |
| Ptgs2 | 1.5 ± 0.5 | 0.7 ± 0.3 | - |
| Ptges | 1.8 ± 0.6 | 2.2 ± 0.7 | - |
| Tbxas1 | 1.0 ± 0.3 | 1.1 ± 0.3 | - |
| Ptgds | 1.0 ± 0.5 | 0.3 ± 0.1 | - |
| Ptgis | 1.1 ± 0.5 | 0.7 ± 0.3 | - |
| Aloxe3 | 1.2 ± 0.3 | 0.7 ± 0.1* | - |
| Alox5 | 0.9 ± 0.1 | 1.2 ± 0.2 | - |
| Alox5ap | 1.2 ± 0.3 | 1.9 ± 0.4 | - |
| Alox12 | 0.6 ± 0.1 | 0.5 ± 0.1* | 0.4 ± 0.1* |
| Alox12b | 1.7 ± 0.3* | 0.7 ± 0.1 | - |
| Alox15 | 4.2 ± 0.6* | 30.1 ± 6.1* | 3.1 ± 0.8* |
| Alox8 | 3.6 ± 0.8* | 4.7 ± 0.6* | 0.8 ± 0.3 |
| Alox12eb | 9.2 ± 1.8* | 20.0 ± 2.7* | 3.7 ± 1.1* |
Values represent means of 2−ΔΔCT ± SEM.
p ≤ 0.05 by Student’s t-test
Originally published in Reference (20).
mouse ortholog of the human pseudogene ALOX12P
Our previous study evaluated the induction of specific cytokines and chemokines in Af sensitized/challenged C57BL/6 and CFTR−/− mice (6) with similar results in FVB mice (data not shown). To further demonstrate the effectiveness of Af in lung inflammation, we studied the induction of similar inflammatory mediators in response to Af compared to the more commonly used sensitizing antigen, ovalbumin (OVA). These results demonstrated a similar response with only a difference in magnitude between Af and OVA-treated animals (Supp. Fig. 1). As a clinically relevant antigen, this comparison with OVA further validates the use of Af as an experimentally relevant allergen.
3.2. Gene Expression of the Phospholipase A2s
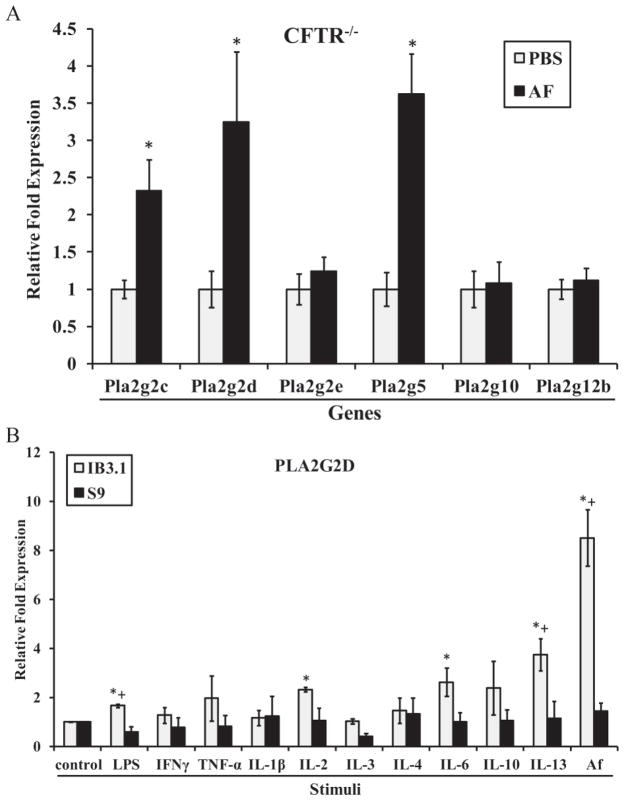
Given the importance of eicosanoids in lung disease (18), including CF (3), we next studied changes in lung gene expression for a large repertoire of enzymes involved in the eicosanoid pathway in Af sensitization/challenge in CFTR−/− mice. This pathway (Supp. Fig. 2) was targeted due to the compounding clinical manifestations of asthma in some CF patients with linkage to Aspergillus fumigatus (19). Supplemental Table 2 summarizes all the genes analyzed for lung expression providing both the mouse and human gene names and symbols with corresponding NCBI accession numbers. We first evaluated the phospholipase A2 (PLA2) family, composed of several groups of secretory PLA2s and two groups of cytosolic PLA2s, groups IV (cPLA2s) and VI (iPLA2s), which is responsible for the initial release of the primary metabolite, arachidonic acid (AA). Lung gene expression was increased for three secretory phospholipases, Pla2g2c, Pla2g2d and Pla2g5, in Af sensitization/challenge CFTR−/− mice compared to PBS (Fig. 1A). We previously studied these genes in C57BL/6 mice, where only Pla2g2e and Pla2g5 were induced. All of these results and data from C57BL/6 (20) and FVB mice are summarized in Table 2.
Figure 1.
Secretory phospholipase A2 steady state mRNA levels in mouse lungs and human cell lines. (A) CFTR−/− mice were sensitized/challenged with PBS or Af. Real-time RT-PCR was performed from whole lung mRNA for group II, V, X and XII PLA2s: Pla2g2c, Pla2g2d, Pla2g2e, Pla2g5, Pla2g10 and Pla2g12b. Data points are the means of 2−ΔΔCT ± SEM (7≤n≤10). * indicate p≤0.05 as compared to PBS-sensitized mice. (B) Real-time RT-PCR of PLA2G2D following a 12h treatment of S9 and IB3.1 cells with the indicated inflammatory stimuli. Data points are the means of 2−ΔΔCT ± SEM (n=3). * and + indicate p≤0.05 as compared to IB3.1 control and treated S9 cells, respectively.
Due to the significant response seen by Pla2g2d and Pla2g5 in CFTR−/− mice, we then studied the homologous genes in human IB3.1 and S9 cells. Of these two cell lines, only IB3.1 demonstrated an induction of PLA2G2D in response to a panel of stimuli (Fig. 1B). In the case of PLA2G5, we observed an induction in both cell lines only in response to TNF-α (data not shown). Of note, we have also previously demonstrated that various cytokines, including IL-6, IL-10, IFNγ, and TNF-α, are elicited from murine primary alveolar macrophages in response to the Af extract, providing additional relevance to these stimuli (20).
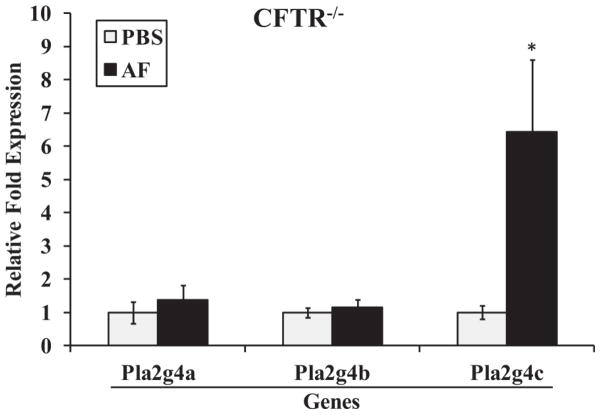
We next evaluated the expression levels of three members of the Group IV cytosolic phospholipases (cPLA2), Pla2g4a, Pla2g4b, and Pla2g4c, with only Pla2g4c expression displaying a significant increase in Af-sensitized/challenged CFTR−/− mice (Fig. 2). We observed a similar response in C57BL/6 (20) and FVB mice (Table 2), as well as an analogous induction of Pla2g4c mRNA levels by OVA-sensitization/challenge, in support of the experimental relevance of Af as a sensitizing agent (Supp. Fig. 3). We similarly tested the expression of PLA2G4C in IB3.1 cells which displayed substantial inductions by the pro-inflammatory stimuli TNF-α and IL-1β (Table 1), with no significant differences as compared to our results in S9 cells (20).
Figure 2.
Group IV cytosolic phospholipase A2s steady state mRNA levels in mouse lungs. CFTR−/− mice were sensitized/challenged with PBS or Af as described in Fig. 1. Real-time RT-PCR was then used to determine the steady state mRNA levels from whole lungs for the group IV PLA2s, Pla2g4a, Pla2g4b and Pla2g4c. * indicates p≤0.05 as compared to PBS-sensitized mice.
Table 1.
Fold change of PLA2G4C in S9 and IB3.1 cells
| LPS | IFNγ | TNFα | IL-1β | IL-2 | IL-3 | IL-4 | IL-6 | IL-10 | IL-13 | Af | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S9a | 1.2 ± 0.1 | 0.9 ± 0.1 | 6.4 ± 1.0* | 3.0 ± 0.2* | 1.3 ± 0.1 | 1.4 ± 0.3 | 1.1 ± 0.1 | 1.4 ± 0.1* | 1.2 ± 0.1* | 1.0 ± 0.1 | 1.0 ± 0.1 |
| IB3.1 | 1.1 ± 0.1 | 0.8 ± 0.1 | 5.7 ± 1.7* | 2.9 ± 0.1* | 1.4 ± 0.3 | 1.4 ± 0.2 | 1.0 ± 0.1 | 1.3 ± 0.0* | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1* |
p ≤ 0.05 by Student’s t-test as compared to respective untreated cells.
Originally published in Reference (20) - PLA2G4C data in S9 cells.
3.3. Gene Expression Analysis of Enzymes Involved in Prostanoid Synthesis
Eicosanoid biosynthesis following the liberation of AA from membrane phospholipids can result in the metabolism of AA by cyclooxygenases (COXs) and specific downstream synthases or lipoxygenases (LOXs). The relative expression levels of cyclooxygenases (Ptgs1 and Ptgs2), microsomal prostaglandin E synthase-1 (Ptges), thromboxane A2 synthase (Tbxas1), lipocalin-type prostaglandin D synthase (Ptgds) and prostaglandin I2 synthase (Ptgis) were analyzed. We observed a small upward trend in the expression of Ptgs1, Ptgs2 and Ptges in the CFTR−/− mice (Fig. 3). This small increase in Ptges was also observed in C57BL/6 mice (Supp. Fig. 4) and summarized in Table 2. With no statistical significance to these trends, follow-up studies were not performed in human cell lines.
Figure 3.
Gene expression analysis of enzymes involved in prostanoid synthesis in mouse lungs. CFTR−/− mice were sensitized/challenged as described in Fig. 1, and real-time RT-PCR on whole lung mRNA was performed for Ptgs1, Ptgs2, Ptges, Tbxas1, Ptgds, and Ptgis. Data points are the means of 2−ΔΔCT ± SEM (7≤n≤10).
3.4. Gene Expression Analysis of Enzymes Involved in Leukotriene (LT), HPETE and HETE Synthesis
The alternate fate of AA is peroxidation by the lipoxygenase (LOX) family of enzymes leading to the production of lipoxins and leukotrienes (LTs). The LTs have physiological roles in innate immune responses in the lung and in the pathology of inflammatory diseases, such as asthma, allergic rhinitis and atherosclerosis (21). Figure 4A illustrates that the gene expression levels for Aloxe3, Alox5 and Alox5ap are not elevated in the CFTR−/− mice. We also evaluated other members of the LOX family: Alox12 (platelet 12(S)-LOX); Alox12b (Epidermis-type,12(R)-LOX); Alox15 (12-LOX Leukocyte-type, human ortholog 15-LOX-A); Alox8 (8-LOX, human ortholog 15-LOX-B); and Alox12e (human ortholog pseudogene ALOX12P2) (Fig. 4A). These results demonstrate significant increases in the expression of most of the 12 and 15 LOXs in response to Af sensitization/challenge. The results in the CFTR−/− mice are consistent with our observations in C57BL/6 (Supp. Fig. 5A–B) and FVB mice as summarized in Table 2.
Figure 4.

Gene expression analysis of enzymes in the lipoxygenase pathway in mouse lungs and human cell lines. (A) CFTR−/− mice were sensitized/challenged as with PBS or Af, and whole lung mRNA was analyzed by real-time RT-PCR for lipoxygenase gene expression (Aloxe3, Alox5, Alox5ap, Alox12, Alox12b, Alox15, Alox8, and Alox12e). Data points are the means of 2−ΔΔCT ± SEM (7≤n≤10). * indicate p≤0.05 as compared to PBS-sensitized mice. (B) Steady state mRNA levels of human ALOX15 in response to the indicated stimuli in S9 or IB3.1 cells. Data points are the means of 2−ΔΔCT SEM (n=3). (C) Steady state mRNA levels of human ALOX15B in response to the indicated stimuli in S9 or IB3.1 cells. Data points are the means of 2−ΔΔCT ± SEM (n=3). * and + indicate p≤0.05 as compared to control and S9 cells, respectively.
Based on these findings, we next evaluated the human orthologs, ALOX15 (Fig. 4B) and ALOX15B (Fig. 4C), in IB3.1 and S9 cells in response to various inflammatory mediators. We did not study Alox12e because this gene only exists in humans as a pseudogene (ALOX12P). Of note, several stimuli caused an upward trend in ALOX15 gene expression which occurred only in the IB3.1 cells (Fig. 4B). Similarly, we observed upward trends for various stimuli for ALOX15B gene expression primarily in the IB3.1 cells (Fig. 4C). The extremely low cellular abundance of both ALOX15 and ALOX15B mRNA in these cells leads to a high variance between levels of induction. Thus, despite the lack of statistical significance, we believe that these trends represent a true induction based on our experience with low abundance genes by using multiple PCR primer sets and corroboration by northern analysis.
4. Discussion
Eicosanoids constitute a diverse family of physiologically active fatty acids involved in regulating airway inflammation and reactivity, which are linked to diseases such as asthma and CF (1–3). Recent studies have linked the ubiquitous fungus Aspergillus fumigatus (Af) with an increased prevalence in both asthmatics (22, 23) and cystic fibrosis patients (24). Therefore, we employed a mouse model that could mimic the pathology of allergen-induced asthma using an Af extract for sensitization and challenge. We hypothesized that potentially distinct enzymes in the eicosanoid pathway may display altered levels of gene expression in response to sensitization/challenge to this environmentally relevant allergen and the resulting inflammatory response, thus highlighting potentially novel therapeutic targets.
Our examination of the phospholipases (PLA2s) provides new insights into the role these enzymes may play in asthma and CF. To support our observations in the CFTR−/− mice, observed changes were validated in a human CF cell line alongside a cell line containing a functional CFTR as the only difference. Our data highlight potential new roles for Pla2g2c, Pla2g2d and Pla2g5 in allergen sensitization/challenge, based on the selective induction of these sPLA2s in the CFTR−/− mice (Fig. 1A). We have also corroborated the CF specific induction of PLA2G2D by demonstrating that a number of inflammatory mediators can preferentially induce expression in the IB3.1 cells (LPS, IL-2, IL-6, IL-13, Af) (Fig. 1B). The unique expression in IB3.1 cells versus the corrected S9 may provide a relevant linkage to CF pathophysiology (Fig. 1B). Furthermore, the induction of PLA2G2D by IL-6 and IL-13 is a new observation in any cell type, since the only stimuli documented to induce this gene are IFN-γ and LPS (25).
We next examined the group IV PLA2s, which have been directly linked to the liberation of AA as a consequence of the inflammatory response (26). The vast majority of studies have focused on PLA2G4A/cPLA2α, which responds to a variety of stimuli and is regulated both at the transcriptional and post-translational levels (27). Most surprisingly, we observed an induction of Pla2g4c in all mouse strains with no effect on Pla2g4a or Pla2g4b (Fig. 2 and Table 2). Furthermore, confirming that this allergic response is not antigen specific, Pla2g4c was also induced by OVA (Supp Fig. 3). This gene was similarly induced in both the IB3.1 and S9 cell lines by TNF-α and IL-1β (Table 1), implicating its potential importance to the inflammatory response. We believe that PLA2G4C responds to the allergic asthmatic response, with no appreciable contributions to this induction from the presence of the CFTR gene. We have also recently demonstrated that the induction of PLA2G4C specifically involves a promoter proximal element mediated by the transcription factors ATF-2/cJun, p65, and USF1/2 (20). To date, the precise physiological role for PLA2G4C/cPLA2γ has not been elucidated; it was first identified by orthology to PLA2G4A, to which it has ~30% overall sequence identity (28–32). In addition to its PLA2 activities, Yamashita et al. (29) have also reported that this enzyme displays coenzyme A (CoA)–independent transacylation and lysophospholipid (LPL) dismutase (LPLase/transacylase) activities and have suggested a possible role in fatty acid remodeling of phospholipids and the clearance of toxic lysophospholipids. Our new data in both allergic asthma and inflammation add impetus to further studies of this gene.
Another interesting result is the lack of a significant response from the enzymes on the cyclooxygenase branch of AA metabolism (Fig. 3 and Supp. Fig. 4), which, at least at the transcriptional level, implies that the prostanoids may have a limited role in this allergy model. These data directed our attention to the lipoxygenase branch where the literature (21, 33) and the significant pharmaceutical investment in anti-LT therapies would argue that alterations in the expression of either Alox5 (5-LOX) or Alox5ap (FLAP) in response to Af could be expected. As with the prostanoid enzymes, we saw no changes with either of these enzymes or in the levels of Aloxe3 (Fig. 4A).
Af sensitization/challenge did, however, cause a significant increase in Alox15, Alox8 and the mouse specific Alox12e. Consistent with these data was the induction, only in IB3.1 cells, of the human ALOX15 (ortholog of mouse Alox15) by TNF-α, IL-3, and Af (Fig. 4B) and ALOX15B (ortholog of mouse Alox8) by IL-13 (Fig. 4C). The lack of response in S9 cells may be indicative of a more complex interplay between CFTR and ALOX15. ALOX15 has been previously shown to be regulated by IL-4 and IL-13 (34, 35), however, no previous studies have linked induction of ALOX15 to TNF-α or IL-3. Our mouse data for Alox15 induction are also in line with recent studies by Andersson et al. (36) which demonstrated that Alox15−/− mice in a systemic OVA sensitization model had impaired airway inflammation, reduced levels of eosinophils, lymphocytes and macrophages in BAL fluid along with lower levels of the Th2 cytokines (IL-4, IL-5 and IL-13) and tissue eosinophils. Our data and that of Andersson et al. (36) would imply that Alox15 inhibition may provide an alternative therapeutic target for asthma and CF patients. This would also be consistent with studies that have demonstrated an increased level of 15-LOX metabolites in asthmatics, where 15(S)-HETE levels in BALF were elevated and associated with tissue eosinophil numbers, sub-membrane thickness and the observation that severe asthmatics presenting with persistent airway eosinophils exhibit high levels of 15(S)-HETE in BALF (37, 38).
Other than evidence that the ALOX15B gene may be regulated by its own product (39), very little is known about this enzyme’s regulation, thus making the induction in the Af-treated mice and by IL-13 in the IB3.1 cells an intriguing observation. In addition to its role in the synthesis of pro-inflammatory products, 15-lipoxygenases also participate through transcellular biosynthesis in the production of anti-inflammatory bioactive lipid mediators of resolution, the lipoxins (40–43). Lipoxins, LXA4 and LXB4 being the main components, are lipid mediators generated from AA that act to reduce inflammation and promote resolution. Lipoxins are generated through the combined action of 5- and 15-lipoxygenases during cell-cell interactions. Recent data have also demonstrated that lipoxin concentrations in CF patient’s airway fluid are significantly suppressed when compared to other inflammatory lung conditions (40).
We therefore hypothesize that the coupled induction of both Pla2g4c and the lipoxygenases in our mouse models of Af sensitization/challenge provide for the generation of both arachidonate and downstream lipoxygenase metabolites as potent mediators in the pathology of ABPA, asthma and CF. To this end, we have identified a number of genes in the eicosanoid pathway that display altered gene expression that may be associated with ABPA, allergic asthma or CF. The results have also helped to highlight a PLA2G4C→lipoxygenase axis where the downstream metabolites can act as potentially important mediators in the inflammatory response in these diseases. Furthermore, the altered expression of these genes provide potential identifiers and therapeutic targets that are specifically linked to Aspergillus sensitization and/or cystic fibrosis.
Supplementary Material
Acknowledgments
We would like to thank the other members of the Nick and Flotte labs for helpful suggestions and enthusiastic support throughout this work. These studies were supported by grants from the National Institutes of Health to HSN, [R37HL067456] and [RO1HL39593].
Role of the funding source
The funding source had no role in the study design, in the writing of the report or in the decision to submit the paper for publication.
Abbreviations
- LTs
leukotrienes
- LTB4
leukotriene B4
- cysLTs
cysteinyl leukotrienes
- PLA2
phospholipase A2
- sPLA2
secretory PLA2
- cPLA2
group IV PLA2
- HPETEs
hydroperoxyeicosatetranoic acids
- HETEs
hydroxyeicosatetraenoic acids
- CF
cystic fibrosis
- Af
Aspergillus fumigatus
- CFTR
cystic fibrosis transmembrane conductance regulator
- AA
arachidonic acid
- COXs
cyclooxygenases
- mPGES
microsomal prostaglandin E synthase
- L-PGDS
lipocalin-type prostaglandin D synthase
- LOX
lipoxygenase
- FLAP
5-LOX activating protein
Footnotes
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bloemen K, Verstraelen S, Van Den Heuvel R, Witters H, Nelissen I, Schoeters G. The allergic cascade: review of the most important molecules in the asthmatic lung. Immunol Lett. 2007 Oct 31;113(1):6–18. doi: 10.1016/j.imlet.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Freedman SD, Blanco PG, Zaman MM, Shea JC, Ollero M, Hopper IK, et al. Association of cystic fibrosis with abnormalities in fatty acid metabolism. N Engl J Med. 2004 Feb 5;350(6):560–9. doi: 10.1056/NEJMoa021218. [DOI] [PubMed] [Google Scholar]
- 3.Worgall TS. Lipid metabolism in cystic fibrosis. Curr Opin Clin Nutr Metab Care. 2009 Mar;12(2):105–9. doi: 10.1097/mco.0b013e32832595b7. [DOI] [PubMed] [Google Scholar]
- 4.Smith MJ, Ford-Hutchinson AW, Bray MA. Leukotriene B: a potential mediator of inflammation. J Pharm Pharmacol. 1980 Jul;32(7):517–8. doi: 10.1111/j.2042-7158.1980.tb12985.x. [DOI] [PubMed] [Google Scholar]
- 5.Montuschi P. Leukotrienes, antileukotrienes and asthma. Mini Rev Med Chem. 2008 Jul;8(7):647–56. doi: 10.2174/138955708784567395. [DOI] [PubMed] [Google Scholar]
- 6.Muller C, Braag SA, Herlihy JD, Wasserfall CH, Chesrown SE, Nick HS, et al. Enhanced IgE allergic response to Aspergillus fumigatus in CFTR−/− mice. Lab Invest. 2006 Feb;86(2):130–40. doi: 10.1038/labinvest.3700379. [DOI] [PubMed] [Google Scholar]
- 7.Virnig C, Bush RK. Allergic bronchopulmonary aspergillosis: a US perspective. Curr Opin Pulm Med. 2007 Jan;13(1):67–71. doi: 10.1097/MCP.0b013e328010c812. [DOI] [PubMed] [Google Scholar]
- 8.Kanthan SK, Bush A, Kemp M, Buchdahl R. Factors effecting impact of Aspergillus fumigatus sensitization in cystic fibrosis. Pediatr Pulmonol. 2007 Sep;42(9):785–93. doi: 10.1002/ppul.20656. [DOI] [PubMed] [Google Scholar]
- 9.Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992 Aug 21;257(5073):1083–8. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994 Dec 9;266(5191):1705–8. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- 11.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–9. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 12.Aiken KJ, Bickford JS, Kilberg MS, Nick HS. Metabolic regulation of manganese superoxide dismutase expression via essential amino acid deprivation. J Biol Chem. 2008 Apr 18;283(16):10252–63. doi: 10.1074/jbc.M709944200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeitlin PL, Lu L, Rhim J, Cutting G, Stetten G, Kieffer KA, et al. A cystic fibrosis bronchial epithelial cell line: immortalization by adeno-12-SV40 infection. Am J Respir Cell Mol Biol. 1991 Apr;4(4):313–9. doi: 10.1165/ajrcmb/4.4.313. [DOI] [PubMed] [Google Scholar]
- 14.Egan M, Flotte T, Afione S, Solow R, Zeitlin PL, Carter BJ, et al. Defective regulation of outwardly rectifying Cl- channels by protein kinase A corrected by insertion of CFTR. Nature. 1992 Aug 13;358(6387):581–4. doi: 10.1038/358581a0. [DOI] [PubMed] [Google Scholar]
- 15.Flotte TR, Afione SA, Solow R, Drumm ML, Markakis D, Guggino WB, et al. Expression of the cystic fibrosis transmembrane conductance regulator from a novel adeno-associated virus promoter. J Biol Chem. 1993 Feb 15;268(5):3781–90. [PubMed] [Google Scholar]
- 16.Tabary O, Boncoeur E, de Martin R, Pepperkok R, Clement A, Schultz C, et al. Calcium-dependent regulation of NF-(kappa)B activation in cystic fibrosis airway epithelial cells. Cell Signal. 2006 May;18(5):652–60. doi: 10.1016/j.cellsig.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Chaudhary N, Datta K, Askin FB, Staab JF, Marr KA. Cystic fibrosis transmembrane conductance regulator regulates epithelial cell response to Aspergillus and resultant pulmonary inflammation. Am J Respir Crit Care Med. 2012 Feb 1;185(3):301–10. doi: 10.1164/rccm.201106-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charbeneau RP, Peters-Golden M. Eicosanoids: mediators and therapeutic targets in fibrotic lung disease. Clin Sci (Lond) 2005 Jun;108(6):479–91. doi: 10.1042/CS20050012. [DOI] [PubMed] [Google Scholar]
- 19.Kurup VP, Knutsen AP, Moss RB, Bansal NK. Specific antibodies to recombinant allergens of Aspergillus fumigatus in cystic fibrosis patients with ABPA. Clin Mol Allergy. 2006;4:11. doi: 10.1186/1476-7961-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bickford JS, Newsom KJ, Herlihy JD, Mueller C, Keeler B, Qiu X, et al. Induction of group IVC phospholipase A2 in allergic asthma: transcriptional regulation by TNFalpha in bronchoepithelial cells. The Biochemical journal. 2012 Feb 15;442(1):127–37. doi: 10.1042/BJ20111269. [DOI] [PubMed] [Google Scholar]
- 21.Peters-Golden M, Henderson WR., Jr Leukotrienes. N Engl J Med. 2007 Nov 1;357(18):1841–54. doi: 10.1056/NEJMra071371. [DOI] [PubMed] [Google Scholar]
- 22.Hogaboam CM, Carpenter KJ, Schuh JM, Buckland KF. Aspergillus and asthma--any link? Med Mycol. 2005 May;43(Suppl 1):S197–202. doi: 10.1080/13693780400025211. [DOI] [PubMed] [Google Scholar]
- 23.Maurya V, Gugnani HC, Sarma PU, Madan T, Shah A. Sensitization to Aspergillus antigens and occurrence of allergic bronchopulmonary aspergillosis in patients with asthma. Chest. 2005 Apr;127(4):1252–9. doi: 10.1378/chest.127.4.1252. [DOI] [PubMed] [Google Scholar]
- 24.Shoseyov D, Brownlee KG, Conway SP, Kerem E. Aspergillus bronchitis in cystic fibrosis. Chest. 2006 Jul;130(1):222–6. doi: 10.1378/chest.130.1.222. [DOI] [PubMed] [Google Scholar]
- 25.Lindbom J, Ljungman AG, Tagesson C. Interferon gamma-induced gene expression of the novel secretory phospholipase A2 type IID in human monocyte-derived macrophages is inhibited by lipopolysaccharide. Inflammation. 2005 Apr;29(2–3):108–17. doi: 10.1007/s10753-006-9007-x. [DOI] [PubMed] [Google Scholar]
- 26.Ghosh M, Tucker DE, Burchett SA, Leslie CC. Properties of the Group IV phospholipase A(2) family. Prog Lipid Res. 2006 Nov;45(6):487–510. doi: 10.1016/j.plipres.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Gijon MA, Spencer DM, Leslie CC. Recent advances in the regulation of cytosolic phospholipase A(2) Adv Enzyme Regul. 2000;40:255–68. doi: 10.1016/s0065-2571(99)00031-x. [DOI] [PubMed] [Google Scholar]
- 28.Vitale A, Perlin J, Leonelli L, Herr J, Wright P, Digilio L, et al. Mouse cPLA2gamma, a novel oocyte and early embryo-abundant phospholipase A2 gamma-like protein, is targeted to the nuclear envelope during germinal vesicle breakdown. Dev Biol. 2005 Jun 15;282(2):374–84. doi: 10.1016/j.ydbio.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Yamashita A, Kamata R, Kawagishi N, Nakanishi H, Suzuki H, Sugiura T, et al. Roles of C-terminal processing, and involvement in transacylation reaction of human group IVC phospholipase A2 (cPLA2gamma) J Biochem (Tokyo) 2005 May;137(5):557–67. doi: 10.1093/jb/mvi067. [DOI] [PubMed] [Google Scholar]
- 30.Mancuso P, Canetti C, Gottschalk A, Tithof PK, Peters-Golden M. Leptin augments alveolar macrophage leukotriene synthesis by increasing phospholipase activity and enhancing group IVC iPLA2 (cPLA2gamma) protein expression. Am J Physiol Lung Cell Mol Physiol. 2004 Sep;287(3):L497–502. doi: 10.1152/ajplung.00010.2004. [DOI] [PubMed] [Google Scholar]
- 31.Asai K, Hirabayashi T, Houjou T, Uozumi N, Taguchi R, Shimizu T. Human group IVC phospholipase A2 (cPLA2gamma). Roles in the membrane remodeling and activation induced by oxidative stress. J Biol Chem. 2003 Mar 7;278(10):8809–14. doi: 10.1074/jbc.M212117200. [DOI] [PubMed] [Google Scholar]
- 32.Pickard RT, Strifler BA, Kramer RM, Sharp JD. Molecular cloning of two new human paralogs of 85-kDa cytosolic phospholipase A2. J Biol Chem. 1999 Mar 26;274(13):8823–31. doi: 10.1074/jbc.274.13.8823. [DOI] [PubMed] [Google Scholar]
- 33.Drazen JM. Leukotrienes in asthma. Adv Exp Med Biol. 2003;525:1–5. doi: 10.1007/978-1-4419-9194-2_1. [DOI] [PubMed] [Google Scholar]
- 34.Chen B, Tsui S, Boeglin WE, Douglas RS, Brash AR, Smith TJ. Interleukin-4 induces 15-lipoxygenase-1 expression in human orbital fibroblasts from patients with Graves disease. Evidence for anatomic site-selective actions of Th2 cytokines. The Journal of biological chemistry. 2006 Jul 7;281(27):18296–306. doi: 10.1074/jbc.M603484200. [DOI] [PubMed] [Google Scholar]
- 35.Liu C, Xu D, Sjoberg J, Forsell P, Bjorkholm M, Claesson HE. Transcriptional regulation of 15-lipoxygenase expression by promoter methylation. Exp Cell Res. 2004 Jul 1;297(1):61–7. doi: 10.1016/j.yexcr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Andersson CK, Claesson HE, Rydell-Tormanen K, Swedmark S, Hallgren A, Erjefalt JS. Mice Lacking 12/15-Lipoxygenase have Attenuated Airway Allergic Inflammation and Remodeling. Am J Respir Cell Mol Biol. 2008 May 29; doi: 10.1165/rcmb.2007-0443OC. [DOI] [PubMed] [Google Scholar]
- 37.Chu HW, Balzar S, Westcott JY, Trudeau JB, Sun Y, Conrad DJ, et al. Expression and activation of 15-lipoxygenase pathway in severe asthma: relationship to eosinophilic phenotype and collagen deposition. Clin Exp Allergy. 2002 Nov;32(11):1558–65. doi: 10.1046/j.1365-2222.2002.01477.x. [DOI] [PubMed] [Google Scholar]
- 38.Nasser SM, Lee TH. Products of 15-lipoxygenase: are they important in asthma? Clin Exp Allergy. 2002 Nov;32(11):1540–2. doi: 10.1046/j.1365-2222.2002.01529.x. [DOI] [PubMed] [Google Scholar]
- 39.Subbarayan V, Krieg P, Hsi LC, Kim J, Yang P, Sabichi AL, et al. 15-Lipoxygenase-2 gene regulation by its product 15-(S)-hydroxyeicosatetraenoic acid through a negative feedback mechanism that involves peroxisome proliferator-activated receptor gamma. Oncogene. 2006 Sep 28;25(44):6015–25. doi: 10.1038/sj.onc.1209617. [DOI] [PubMed] [Google Scholar]
- 40.Karp CL, Flick LM, Park KW, Softic S, Greer TM, Keledjian R, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat Immunol. 2004 Apr;5(4):388–92. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 41.Parkinson JF. Lipoxin and synthetic lipoxin analogs: an overview of anti-inflammatory functions and new concepts in immunomodulation. Inflamm Allergy Drug Targets. 2006 Apr;5(2):91–106. doi: 10.2174/187152806776383125. [DOI] [PubMed] [Google Scholar]
- 42.Romano M, Recchia I, Recchiuti A. Lipoxin receptors. ScientificWorldJournal. 2007;7:1393–412. doi: 10.1100/tsw.2007.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005 Sep-Oct;73(3–4):141–62. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.