Abstract
Background
This Phase II study was designed to determine response to chemotherapy and survival after response-based radiation (RT) in children with CNS germ cell tumors.
Procedure
Children with germinomas and normal markers received cisplatin 100 mg/m2 + etoposide, alternating with vincristine + cyclophosphamide (CPM) 2 g/m2/d, for four cycles. Children with nongerminomatous tumors or with abnormal markers received doubled doses of cisplatin and CPM. For germinoma patients in complete response (CR), RT was decreased from 50.4 to 30.6 Gy. High-risk patients received neuraxis RT: 50.4 Gy local + 30.6 Gy neuraxis in CR; 54 Gy local + 36 Gy if less than CR.
Results
Of 12 germinoma patients, 4 had cerebrospinal fluid (CSF) human chorionic gonadotropin (HCG) 6.9-21 mIU/ml. Of 14 nongerminomatous patients, HCG in serum or CSF was > 50 mIU/ml in 9, α-fetoprotein (AFP) abnormal in 9. Four germinoma patients attained CR, six PR, one SD, one not evaluable after resection. Two nongerminomatous patients had CR, three PR, three SD, one PD, four not evaluable after resection; one inadequately treated patient had progressive disease (PD). Both PD patients died; one SD patient died during a seizure. Eleven germinoma patients are PF at median 66 months; one patient in CR refused RT, had PD at 10 months, received RT, and was PF at 56 months. Eleven of 14 nongerminomatous patients were PF at median 58 months.
Conclusion
Response (germinoma, 91%; nongerminomatous, 55%) and survival are encouraging after this regimen plus response-based RT.
Keywords: brain tumors, chemotherapy, germinoma
INTRODUCTION
Germ cell tumors of the brain include pure germinomas, which may be successfully treated in 85–100% of patients with radiation therapy, and the more diverse nongerminomatous germ cell tumors, which tend to recur after standard radiation (20–45% reported 5-year survivals) [1–5]. The biologic disparity between pure germinomas and nongerminomatous tumors is also supported by the difference in mean age at diagnosis (adolescent versus middle school years), with nongerminomatous tumors having a more heterogeneous appearance with spread to distant structures [1–5]. With the goal of reducing potential late sequelae of cranial radiation [6–11], platinum-based regimens have sought to decrease radiation dose by administering chemotherapy before reduced radiation [12–16]. It is not yet known whether this approach will lead to similar cure rates with decreased toxicity.
Nongerminomatous tumors are usually mixed tumors with elements of choriocarcinoma, endodermal sinus tumor (also called yolk-sac tumor), embryonal carcinoma, teratoma with malignant germ cell elements, and include germinomas with choriocarcinomatous elements (defined herein by b-human chorionic gonadotropin (HCG) >50 mIU/ml in serum or cerebrospinal fluid (CSF)). Studies in new and relapsed patients found responses and/or improved survival after platinum-containing regimens [17–25], with one study suggesting synergy with etoposide [26]. Alkylating agents, such as cyclophosphamide (CPM) also were added with apparent efficacy in some trials [12,14,23].
In 1996, the Pediatric Oncology Group (now part of the Children's Oncology Group (COG)) opened the first national CNS germ cell trial of chemotherapy followed by radiation therapy to evaluate:
whether cranial radiation could be reduced in good-risk germinoma patients after complete response (CR) to four courses of cisplatin (CDDP) plus etoposide (VP-16), alternating with vincristine (VCR) plus CPM;
whether progression-free survival (PFS) could be improved in high-risk children with nongerminomatous germ cell tumors after the same regimen with doubled CDDP and CPM plus full craniospinal radiotherapy (RT);
whether accrual to a groupwide trial would be adequate and representative for future national Phase III trials.
METHODS
Patients
Children 3- to 21-year old were eligible for POG 9530 if pathology of their tumor specimen on central review by Dr. Burger showed CNS germinoma or other malignant germ cell variant, such as embryonal carcinoma, yolk-sac tumor, endodermal sinus tumor, mixed germ cell tumor, or malignant teratoma. No histologic diagnosis was required if HCG or α-fetoprotein (AFP) was elevated in serum or CSF (HCG > 50 mIU/ml; AFP > institutional normal). Patients were to be registered within 22 days of diagnosis and untreated except for corticosteroids. Normal organ function was required: Hgb > 10 g/dl, ANC > 1,500/μl, platelets > 100,000/l, creatinine < 1.2 mg/dl or creatinine clearance ≥ 70 ml/min/1.75 m2, ALT < 1.5 of normal, bilirubin < 1.5 mg/dl. Written informed consent was obtained according to National Cancer Institute guidelines after local Institutional Review Board approval, in accordance with the Declaration of Helsinki.
Treatment Plan
Low-risk patients had tumor pathology showing pure germinoma with normal AFP and HCG < 50 mIL/ml in serum and CSF. These patients received four alternating courses of chemotherapy (ABAB) every 3 weeks before response assessment at week 12. Course A: etoposide 100 mg/m2/day IV for 5 days plus CDDP 20 mg/m2/day IV for 5 days. Course B: CPM 1 g/m2/day IV for 2 days plus VCR 1.5 mg/m2 IV push (maximum dose 2 mg) days 1, 8, and 15.
High-risk patients had tumor pathology that showed mixed germinoma or other malignant variant, and/or elevated HCG or AFP in serum or CSF (HCG > 50 mIU/ml; AFP above institutional normal). These patients received the same chemotherapy with doubled doses of CDDP (40 mg/m2/day IV for 5 days) and CPM (2 g/m2/day IV for 2 days).
Cisplatin was given over 1 hr in twice maintenance D5NS that contained mannitol 3 g/m2 with twice maintenance fluids before and after infusion. CMP was given with mesna. Patients received supplementary IVor oral magnesium, oral trimethoprim/sufisoxazole prophylaxis against pneumocystis carinii, and high-risk patients received G-CSF 5 μg/kg/day subcutaneously until the post-nadir ANC was ≥1,500/μl for two consecutive days. Dexamethasone to decrease intracranial pressure was tapered as rapidly as possible.
The post-chemotherapy week 12 response evaluation required repeat craniospinal MRI, and if initially positive, CSF cytology and serum and CSF AFP and HCG. Response was evaluated as indicated in Table I using criteria standard at that time (HCG and AFP measurements were assessed but were not included in response criteria). All patients had enhancing lesions, which were measured for response in central review of scans. RT began during week 13 as determined by risk group, dissemination, and response status for low-risk patients (see Table I of treatment schema and Table II). Treatment for low-risk patients was to the primary site only (CR: 30.6 Gy; <CR: 50.4 Gy) based upon size at diagnosis and with a margin of 2 cm (conventional planning), or a margin of 0.5 cm (fractionated stereotactic RT). Low-risk patients with dissemination received the same primary site doses with the neuraxis dose also reduced for CR (CR: 30.6 Gy; <CR: 36 Gy). High-risk patients received primary site RT (CR: 50.4 Gy; <CR: 54 Gy) plus neuraxis RT (CR: 30.6 Gy; <CR: 36 Gy). For either risk group, patients with multiple midline tumors (two primary sites) were considered disseminated.
TABLE I.
Schema For Low-Risk Disease (Germinoma β-HCG ≤50 mIU/ml, and Normal αFP) and High-Risk Disease (NG-GCT or Germinoma With β-HCG >50 mIU/ml, or Elevated αFP)
| Treatment schema | ||||
|---|---|---|---|---|
| Assess extent of disease | Week 0 | Week 3 | Week 6 | Week 9 |
| Low-risk disease | CDDP/VP-16 | V/CPM | CDDP/VP-16 | V/CPM |
| High-risk patients | HDCDDP/VP-16 | V/HDCPM | HDCDDP/VP-16 | V/HDCPM |
HCG, β subunit of human chorionic gonadotrophin; AFP, α-fetoprotein; CDDP, cisplatin 20 mg/m2/day IV for 5 days; VP-16, etoposide 100 mg/m2/day IV for 5 days; V, vincristine 1.5 mg/m2 IVon days 1, 8, 15; CPM, cyclophosphamide 1 g/m2/day IV for 2 days; CR, complete response; Gy, gray; HDCDDP, cisplatin 40 mg/m2/day IV for 5 days; HDCPM, cyclophosphamide 2 g/m2 day for 2 days. Patients in CR received 50.4 Gy to local disease, or 54 Gy if not in CR. All patients received 54 Gy to spine + 36 Gy neuraxis.
Patients with localized disease then received 30.6 Gy if in CR, or 50.4 Gy if <CR.
Patients with dissemination received added 23.4 Gy neuraxis if in CR, or 45 Gy to spine + 36 Gy neuraxis not in CR.
TABLE II.
Definition of Response
| Complete response (CR) | Disappearance of all radiographically discernible lesions with negative CSF cytology |
| Partial response (PR) | ≥50% reduction of all lesions, as measured by the sum of the products of the maximum perpendicular diameters, with negative CSF cytology |
| Stable disease (SD) | <50% reduction in tumor size, persistently negative or positive CSF cytology |
| Progressive disease (PD) | >25% increase in tumor size, new lesions, or newly positive CSF cytology |
CSF, cerebrospinal fluid.
Statistical Considerations
In this pilot study there was no planned statistical methodology to assess comparative response and survivals. Grade 4 nonhematologic toxicities were monitored by the study coordinator. The low-risk stratum was to be closed if their three patients showed progressive disease (PD) during chemotherapy, and the high-risk stratum if there were five PDs during chemotherapy.
RESULTS
Thirty patients were enrolled from February 1997 through February 1999, of whom two were ineligible. One patient was diagnosed incorrectly and did not begin chemotherapy, and one began therapy as a low-risk germinoma but had no CSF sample sent for tumor marker analysis or cytology and was declared ineligible. Two other patients with germinoma were inevaluable because the parents refused chemotherapy after registration or because the patient developed PD on the second day of chemotherapy and began immediate RT.
Patient Characteristics
Patient data are shown in Tables III and IV. The median age of the 12 children with germinoma was significantly older (15.1 years) than those with nongerminomatous tumors (11.4 years), with a male:female ratio of greater than 2.5:1 for both groups. The most common symptoms at presentation were headache (54%), vision abnormalities (38%), nausea and vomiting (34%), and diabetes insipidus (31%). Pineal region tumors predominated, and disease was disseminated at diagnosis in 4 of 12 patients with germinoma and 3 of 14 children with nongerminomatous tumors. Four patients with germinoma had modest elevations of HCG in CSF (6.9– 21 mI/ml), three of these four with elevations in CSF but not in serum. Thirteen of 14 patients with nongerminomatous tumors had serum or CSF elevations of HCG (12 patients) and/or AFP (9 patients). Of nine nongerminomatous children with both serum and CSF evaluations, four had CSF values higher than serum and five had CSF values less than serum. Two patients had marked disparities in blood versus CSF values. Patient 10 had an AFP in blood of 5,190 ng/ml and in CSF obtained the previous day of 26 ng/ml, and Patient 27 had HCG in CSF of 2,392 mIU/ml with a normal serum value the same day. The only nongerminomatous tumor patient with normal serum and CSF values was a 10-year-old boy with malignant teratoma that had adenocarcinomatous features.
TABLE III.
Germinoma Patients' Tumor Characteristics and Treatment Outcome
| Patient number | Sex/age (yrs) | Site | Metastases | β-hcg blood/CSF | Surgery | Chemotherapy response | Status |
|---|---|---|---|---|---|---|---|
| 1 | M/17.7 | Pineal | Ventricle CSF | NL/NL | Biopsy | PR | PFS 63 mos |
| 3 | M/15.1 | Thalamic | NL/NL | 75% resection | PR | PFS 70 mos | |
| 4 | M/15.5 | Para-pineal | NL/NL | 80% resection | CR | PFS 73 mos | |
| 6 | M/15.6 | Pineal | NL/NL | Biopsy | CR | PFS 65 mos | |
| 7 | F/11.1 | Suprasellar | CSF spine | NL/NL | Biopsy | PR | PFS 66 mos |
| 8 | M/13.1 | Pineal | CPA | NL/NL | Partial removal | PR | PFS 71 mos |
| 11 | M/15.2 | Bilat basal ganglia | 11/21 mIU/ml | Biopsy | SD | PFS 64 mos | |
| 13 | M/9.5 | Pineal | NL/NL | GTR | Not Eval. | PFS 68 mos | |
| 17 | M/11.6 | Basal ganglia | NL/8.2 mIU/ml | Biopsy | CR | PFS 67 mos | |
| 18 | M/15.2 | Thalamic–pineal | NL/NL | Biopsy | PR | PFS 66 mos | |
| 19 | M/15.6 | Pineal-suprasellar | Spine | NL/6.9 mIU/ml | Biopsy | PR* | PFS 61 mos |
| 21 | F/10.1 | Suprasellar | NL/7.6 | Partial removal | CR** | PFS 10 mos | |
| OS ** 56 mos |
Yrs, years; HCG, beta subunit of human chorionic gonadotrophin; CSF, cerebrospinal fluid; M, male; F, female; CPA, cerebellar pontine angle; NL, normal; CR, complete response; PR, partial response; SD, stable disease; Eval, evaluable; PFS, progression-free survival; mos, months; OS, overall survival.
All patients received radiation therapy as per protocol except:
Patient 19 who was treated as a CR (central review ranked response as PR)
Patient 21 who did not receive radiation (parents' request), developed PD at 10 mos, then received neuraxis radiation and was progression-free at 56 mos.
TABLE IV.
Nongerminomatous Germ Cell Patients' Tumor Characteristics and Treatment Outcome
| Patient number |
Sex/age (yrs) |
Pathology | Site | Metastases | HCG mIU/ml blood/CSF |
AFP ng/ml blood/CSF |
Surg | Chemo resp |
Status |
|---|---|---|---|---|---|---|---|---|---|
| 2 | M/12.5 | Terato-chorio carcinoma | Pineal | 2,424/220 | 10.5/4.5 | GTR | Not eval | PFS 67 mos | |
| 5 | F/13.9 | Germinoma | Pituitary | 5,496/not done | NL/not done | PR | PR (96%) | PFS 71 mos | |
| 9 | M/16.3 | None | Pineal | NL/NL | 286/314 | Shunt only | PRa | PFS 61 mos | |
| 10 | M/16.9 | Yolk-sac teratoma | Pineal | 13/NL | 5,190/26 | 75% resect | Early death | DOD 2 mos | |
| 12 | M/18.11 | Mixed yolk-sac | Pineal | 13/not done | 274/not done | PR | PD | DOD 15 mos | |
| 15 | F/11.2 | Germinoma | Pineal | Frontal horn | 9,990/9,800 | 1,141/153 | Biopsy | PRb | PFS 64 mos |
| 22 | M/10.4 | Adenoca-teratoma | Pineal | NL/NL | NL/NL | GTR | Not eval | PFS 61 mos | |
| 24 | M/11.6 | Germ | Pineal | CSF periventricular | 74/59 | NL/NL | Biopsy | CRb | PFS 58 mos |
| 25 | M/7.11 | None | Pineal | 165/1,550 | 14/8 | None | SDb | PFS 43 mos | |
| 26 | M/10.7 | Germ-chorio | Pineal | NL/471 | NL/NL | GTR | Not eval b | PFS 52 mos | |
| 27 | F/6.5 | Germ-chorio | Supra-sellar | NL/2,392 | NL/NL | PR | SDb | Died of a sz 21 mos | |
| 28 | M/16.2 | None | Pineal | 200/74 | 80/12 | None | SD | PFS 51 mos | |
| 29 | F/14 | Mixed germ | Supra-sellar | 719/ | 3,247/ | PR | CR | PFS 50 mos | |
| 30 | M/10.4 | Mixed yolk-sac terat | Pineal | 4/ | 156/NL | GTR | Not eval | PFS 50 mos |
Yrs, years; HCG, beta subunit of human chorionic gonadotrophin; CSF, cerebrospinal fluid; AFP, α-fetoprotein; chemo, chemotherapy; Resp, response; M, male; GTR, gross total resection; Eval, evaluable; PFS, progression-free survival; F, female; NL, normal; PR, partial response; resect, resection; DOD, dead of disease; germ, germinoma; CR, complete response; SD, stable disease; sz, seizure; terat, teratoma.
Serum β-hcg + a-fp normal (CSF not done).
Serum and CSF β-hcg + α-fp normalized.
Surgery
Five of 12 children with germinoma underwent resection (four partial, one gross total resection), five children had stereotactic biopsies, and two had open biopsies. Pathology confirmed pure germinoma in all cases. Four of 14 children with a nongerminomatous tumor underwent gross total resections (pineal region), 5 had partial resections (2 pineal, 2 suprasellar, 1 pituitary), 2 children underwent biopsy, and 3 did not have surgical tissue obtained but were diagnosed by elevated markers. Seven of 12 low-risk germinoma patients and 13 of 14 high-risk patients were on dexamethasone at the start of chemotherapy.
Chemotherapy Toxicity
Three of the children with diabetes insipidus received continuous IV vasopressin during chemotherapy in order to maintain normal serum sodium; two of the other five children had transient asymptomatic hypo or hyper-natremia (Na 127; Na 160) while receiving chemotherapy and intranasal DDAVP. Patient 10 presented with depressed level of consciousness, received initial chemotherapy while intubated, and developed fungal sepsis with hypotension while on prolonged dexamethasone. He received only course 1 of chemotherapy, remained neurologically unstable on dexamethasone, and began radiation at week 10. Patient 12, while neutropenic after course 4, had probable aspiration pneumonia with pleural effusion, requiring mechanical ventilation with pressor support.
Response to Chemotherapy (Tables III and IV)
Low-risk
Ten of eleven patients evaluable for response had complete (four) or partial response (six) to chemotherapy. One patient (#11) with multifocal disease in bilateral basal ganglia remained stable through chemotherapy and was progression-free at 44 months.
High-risk
Five of nine patients evaluable for response had CR (two patients) or PR (three patients) to chemotherapy; four patients underwent gross total resection and were inevaluable for response. Although normalization of AFP and HCG were not required for response determinations, both patients with CR and two patients with PR had normalization of previously elevated values. All three patients classified as having SD after chemotherapy had some evidence of tumor response. Patient 27 had a 32% estimated decrease in tumor volume. Patients 25 and 28 had not undergone initial surgery but were eligible because of elevated HCG and AFP. Both patients had normalization of all markers during chemotherapy, with follow-up scans demonstrating stable tumor volume (both patients remain progression-free at 49 and 62 months). Patient 10 (see above) received only one course of chemotherapy and was inevaluable for response. This child had evidence of improvement on MRI, and serum AFP dropped from 5,190 to 30 mIU/ml, but he died of PD documented on MRI during radiation, which had been delayed for 10 weeks. Patient 12 developed PD with new leptomeningeal seeding just before RT after course 4. This patient had undergone gross total resection of a pathologically benign teratoma in the pineal region 5 years earlier, then underwent partial resection of a mixed yolk-sac tumor in the same area before chemotherapy. Although MRI before course 4 showed slight shrinkage of tumor mass, MRI at week 18 before RT (delayed secondary to toxicities) showed PD. Despite protocol RT, this patient died of disease 9 months after treatment.
Radiation Therapy
Low-risk
Three children with CR after chemotherapy and one child with gross total resection of primary disease received reduced RT 30.6 Gy to gross tumor volume as per protocol; the fourth child with CR received no RT at parents’ request. Patient 19 with pineal and suprasellar tumor was classified as CR by the local institution (PR by central review) and was treated with reduced RT: 30.6 Gy to gross tumor + 23.4 Gy neuraxis. The remaining six patients with germinomas received full-dose RT as per protocol.
High-risk
All patients with nongerminomatous tumors received RT as per protocol.
Outcome (Figures 1 and 2)
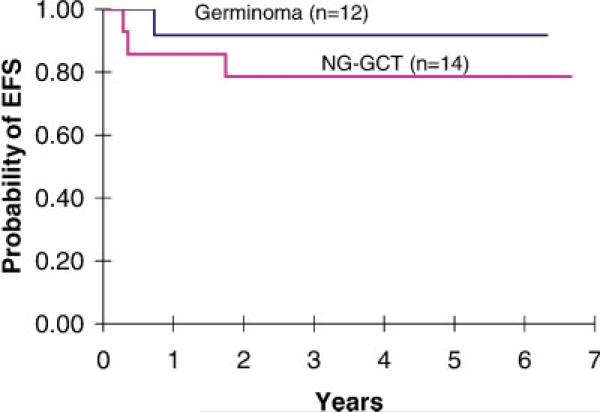
Fig. 1.
Event-free survival (EFS) for POG 9530 eligible patients. Three-year EFS was 92± 8% for low-risk germinoma patients, and ±79 11% for high-risk or nongerminomatous patients. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
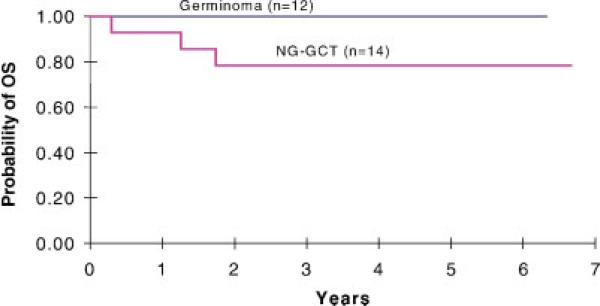
Fig. 2.
Overall survival (OS) for POG 9530 eligible patients. Three-year OS was 79± 11% for high-risk or nongerminomatous patients. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
For low-risk patients with pure germinoma, 11 of 12 children were progression-free at median 66 months (range, 61–73 months). One patient in CR after chemotherapy, whose parents refused RT, recurred at 10 months, then received craniospinal RT and was progression-free at 56 months. For high-risk patients with nongerminomatous tumors, 11 of 14 were progression-free at median 58 months (range, 43–71 months). One inevaluable patient received only one course of chemotherapy and died during RT; one patient (#27) with SD died at 21 months of a seizure at home. Of 24 evaluable patients treated on this study, only one patient with a mixed yolk-sac tumor developed PD before RT and died of disease 9 months later. Outcome data on quality of life and neuro-psychologic status was not obtained in this preliminary pilot study, which was designed to assess feasibility and accrual. Overall, 23 of 26 eligible patients were progression-free at median 61 months (range, 43– 73 months).
DISCUSSION
The responsiveness of CNS germ cell tumors to the same chemotherapeutic agents used effectively for gonadal germ cell tumors offers a potential opportunity to decrease traditional high-dose RT. In this first North American pilot trial, we documented four CRs and six PRs to pre-radiation chemotherapy for 11 evaluable children with germinoma, five of whom then received reduced RT. All germinoma patients were alive at a median 66 months after RT. Five of nine evaluable children with high-risk nongerminomatous tumors responded to high-dose chemotherapy, and 11 of 14 patients (79%) were progression-free at 27–80 months. Only three of 26 eligible patients developed PD, of whom two did not receive planned therapy.
CCDP, etoposide, and a VCR were used in this study based on reported response rates for germ cell tumors of the testes [27,28], and of the CNS [21,23,29]. CMP was added due to high response rates reported for single agent CNS treatment [30,31]. For nongerminomatous patients, the platinum dose was doubled based on data for systemic germ cell tumors, and for CNS nongerminomatous patients (86% survival after 400 mg/m2 vs. 56% following 200 mg/m2) [14,32]. The CMP dose was increased because of responses noted after single agent treatment in newly diagnosed and refractory disease [23].
Toxicities from chemotherapy were as expected: on the high-risk arm there were increased infections and in two patients significant hearing loss. More worrisome was the child who developed fungal disease while intubated postoperatively on prolonged steroids. Serious consideration should be given to postponing myelotoxic therapy in seriously ill or neurologically unstable patients, particularly in the setting of high-dose steroids.
We treated germinoma patients with HCG > 50 mIU/ml on the high-risk treatment arm in concordance with recent French and German trials [14,33]. While 5% of germinomas may contain syncytiotrophoblastic giant cells that secrete HCG [34], controversy exists regarding the prognostic significance of modest elevations of HCG [35]. Buckner et al. noted excellent response and survival in three patients with CSF HCG 79.6–147 mIU/ml [16], whereas Balmaceda et al. noted a trend toward increased PD (P 0.06) in 29 patients [23]. It remains unclear whether patients¼with pure germinoma and HCG 50–2,000 mIU/ml (e.g., Patient 24) should be classified as high-risk patients, but our intent was to follow the guidelines of previous European series [5,14,25,33,35–37]. If Patient 24 were to be excluded, the PFS for the high-risk group would be comparable at 76% (10 of 13 patients). Although for patients with nongerminomatous tumors very high tumor markers have been associated with a poor prognosis [38,39], elevated HCG in our nongerminomatous patients was not a prognostic factor with responses noted in three patients with blood and/or CSF HCG levels of 719– 9,990 mIU/ml.
Ten of 11 evaluable patients with low-risk germinoma achieved CR or PR on central review, which compares favorably to other series [3,12,13,15,16,19,35,38,40–42]. In the most recent series 10 of 11 patients with pure germinomas were disease-free, whereas 5 of 11 patients with elevated CSF HCG relapsed, including 2 with HCG 101–233 mIU/ml, after chemotherapy plus local RTof 24 Gy [15]. It is possible that patients with high HCG are more likely to relapse if local control is minimized. However, modest reductions in RT have not had a significant effect on event-free survival (EFS) [12–16,35,38]. Lowering radiation dose by 5 Gy (45 Gy + 30 Gy neuraxis) was reported in the German ′89 study to have a negligible effect (EFS 87% for 49 patients) [35]. Several other studies have reported decreased radiation dose or field after neoadjuvant chemotherapy [12–14,16,38]. The largest recent French trial noted response to pre-radiation chemotherapy in all 38 evaluable patients with a 3-year EFS of 96.4% for 57 patients [33,36]. In the larger Japanese studies cited above, local radiation was reduced further to 24–30 Gy with EFS of 80– 94% [15,39,43]. However, when RT is not given, most patients relapse despite excellent response rates to up-front chemotherapy [44].
For nongerminomatous tumors and high-risk germinomas, our response rate (5 of 9) and PFS were comparable to recent reports: 21 of 26 [23], 12 of 18 [24], 21 of 27 [39], 16 of 17 [45]. However, these studies do not clarify whether increased intensification of chemotherapy, with concomitant increased toxicity, improves survival. Although not specifically recommended in our study, second-look surgery after response evaluation is now encouraged, particularly for patients with nongerminomatous tumors where residual mass after normalization of markers may represent benign teratoma [46].
While patients with germ cell tumors may present with permanent hormone deficiencies, brain and ventricular RT may cause neurocognitive impairment [47–53], as well as additional hormonal dysfunction. Neuraxis RT also causes growth retardation. Although formal neuropsychology follow-up was not planned for this pilot study, future trials will include formal longitudinal testing to evaluate the late effects of combined therapy.
This first North American trial found significant tumor shrinkage in 10 of 11 children with germinoma, and in 5 of 9 with nongerminomatous tumors. Only one child with a nongerminomatous tumor failed protocol therapy and died of tumor progression. With excellent patient accrual documen ted in this POG pilot (28 eligible and evaluable patients in 2 years), future COG trials will evaluate in a Phase III study chemotherapy plus response-based radiation versus standard radiation for germinoma patients, and intensification with stem cell transplant for nonresponding nongerminoma patients.
REFERENCES
- 1.Jennings MT, Gelman R, Hochberg F. Intracranial germ-cell tumors: Natural history and pathogenesis. J Neurosurg. 1985;63:155–167. doi: 10.3171/jns.1985.63.2.0155. [DOI] [PubMed] [Google Scholar]
- 2.Dearnaley DP, A'Hern RP, Whittaker S, et al. Pineal and CNS germ cell tumors: Royal Marsden Hospital experience 1962-1987. Int J Radiat Oncol Biol Phys. 1990;18:773–781. doi: 10.1016/0360-3016(90)90396-2. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman HJ, Otsubo H, Hendrick EB, et al. Intracranial germ-cell tumors in children. J Neurosurg. 1991;74:545–552. doi: 10.3171/jns.1991.74.4.0545. [DOI] [PubMed] [Google Scholar]
- 4.Sawamura Y, Ikeda J, Shirato H, et al. Germ cell tumours of the central nervous system: Treatment considerations based on 111 cases and their long-term clinical outcomes. Eur J Cancer. 1998;34:104–110. doi: 10.1016/s0959-8049(97)10045-4. [DOI] [PubMed] [Google Scholar]
- 5.Bamberg M, Kortmann RD, Calaminus G, et al. Radiation therapy for intracranial germinoma: Results of the German Cooperative Prospective Trials MAKEI 83/86/89. J Clin Oncol. 1999;17:2585–2592. doi: 10.1200/JCO.1999.17.8.2585. [DOI] [PubMed] [Google Scholar]
- 6.Duffner PK, Cohen ME, Thomas PR, et al. The long-term effects of cranial irradiation on the central nervous system. Cancer. 1985;56:1841–1846. doi: 10.1002/1097-0142(19851001)56:7+<1841::aid-cncr2820561325>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Lannering B, Marky I, Lundberg A, et al. Long-term sequelae after pediatric brain tumors: Their effect on disability and quality of life. Med Pediatr Oncol. 1990;18:304–310. doi: 10.1002/mpo.2950180410. [DOI] [PubMed] [Google Scholar]
- 8.Radcliffe J, Packer RJ, Atkins TE, et al. Three- and four-year cognitive outcome in children with noncortical brain tumors treated with whole brain radiotherapy. Ann Neurol. 1992;32:551–554. doi: 10.1002/ana.410320411. [DOI] [PubMed] [Google Scholar]
- 9.Constine LS, Woolf PD, Cann D, et al. Hypothalamic-pituitary dysfunction after radiation for brain tumors. N Engl J Med. 1993;328:87–94. doi: 10.1056/NEJM199301143280203. [DOI] [PubMed] [Google Scholar]
- 10.Mulhern R, Kepner JL, Thomas PR, et al. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: A Pediatric Oncology Group Study. J Clin Oncol. 1998;16:1723–1728. doi: 10.1200/JCO.1998.16.5.1723. [DOI] [PubMed] [Google Scholar]
- 11.Sands SA, Kellie SJ, Davidow AL, et al. Long-term quality of life and neuropsychologic functioning for patients with CNS germ-cell tumors: From the First International CNS Germ-Cell Tumor Study. Neuro-Oncology. 2001;3:174–183. doi: 10.1093/neuonc/3.3.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen J, Kim JH, Packer RJ. Neoadjuvant chemotherapy for newly diagnosed germ-cell tumors of the central nervous system. J Neurosurg. 1987;67:65–70. doi: 10.3171/jns.1987.67.1.0065. [DOI] [PubMed] [Google Scholar]
- 13.Allen JC, DaRosso RC, Donahue B, et al. A Phase II trial of preirradiation carboplatin in newly diagnosed germinoma of the central nervous system. Cancer. 1994;74:940–944. doi: 10.1002/1097-0142(19940801)74:3<940::aid-cncr2820740323>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 14.Calaminus G, Bamberg M, Baranzelli MC, et al. Intracranial germ cell tumors: A comprehensive update of the European data. Neuropediatrics. 1994;25:26–32. doi: 10.1055/s-2008-1071577. [DOI] [PubMed] [Google Scholar]
- 15.Aoyama H, Shirato H, Ikeda J, et al. Induction chemotherapy followed by low-dose involved-field radiotherapy for intracranial germ cell tumors. J Clin Onc. 2002;20:857–865. doi: 10.1200/JCO.2002.20.3.857. [DOI] [PubMed] [Google Scholar]
- 16.Buckner JC, Peethambaram PP, Smithson WA, et al. Phase II trial of primary chemotherapy followed by reduced-dose radiation for CNS germ cell tumors. J Clin Onc. 1999;17:933–940. doi: 10.1200/JCO.1999.17.3.933. [DOI] [PubMed] [Google Scholar]
- 17.Kida Y, Kobayashi T, Yoshida J, et al. Combination chemotherapy with cisplatin and etoposide for malignant intracranial germ cell tumors. An experimental and clinical study. J Neurosurg. 1986;65:470–475. doi: 10.3171/jns.1989.70.5.0676. [DOI] [PubMed] [Google Scholar]
- 18.Itoyama Y, Kochi M, Yamamoto H, et al. Clinical study of intracranial nongerminomatous germ cell tumors producing alpha-fetoprotein. Neurosurgery. 1990;27:454–460. doi: 10.1097/00006123-199009000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Patel SR, Buckner JC, Smithson WA, et al. Cisplatin-based chemotherapy in primary central nervous system germ cell tumors. J Neurooncol. 1992;12:47–52. doi: 10.1007/BF00172456. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida J, Sugita K, Kobayashi T, et al. Prognosis of intracranial germ cell tumors: Effectiveness of chemotherapy with cisplatin and etoposide (CDDP and VP-16). Acta Neurochir (Wein) 1993;130:111–117. doi: 10.1007/BF02112027. [DOI] [PubMed] [Google Scholar]
- 21.Chang TK, Wong TT, Hwang B. Combination chemotherapy with vinblastine, bleomycin, cisplatin, and etoposide (VBPE) in children with primary intracranial germ cell tumors. Med Pediatr Oncol. 1995;24:368–372. doi: 10.1002/mpo.2950240606. [DOI] [PubMed] [Google Scholar]
- 22.Itoyama Y, Kochi M, Kuratsu J, et al. Treatment of intracranial nongerminomatous malignant germ cell tumors producing alpha fetoprotein. Neurosurg. 1995;36:459–464. doi: 10.1227/00006123-199503000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Balmaceda C, Heller G, Rosenblum M, et al. Chemotherapy without irradiation a novel approach for newly diagnosed CNS germ cell tumors: Results of an international cooperative trial. J Clin Onc. 1996;12:2908–2915. doi: 10.1200/JCO.1996.14.11.2908. [DOI] [PubMed] [Google Scholar]
- 24.Robertson PL, DaRosso RC, Allen JC. Improved prognosis of intracranial non-germinomatous germ cell tumors with multi-modality therapy. J Neurooncol. 1997;32:71–80. doi: 10.1023/a:1005732105727. [DOI] [PubMed] [Google Scholar]
- 25.Gobel U, Schneider DT, Calaminus G, et al. Germ-cell tumors in childhood and adolescence. Ann Oncol. 2000;11:263–271. doi: 10.1023/a:1008360523160. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi T, Yoshida J, Ishiyama J, et al. Combination chemo-therapy with cisplatin and etoposide for malignant intracranial germ cell tumors. An experimental and clinical study. J Neurosurg. 1989;70:676–681. doi: 10.3171/jns.1989.70.5.0676. [DOI] [PubMed] [Google Scholar]
- 27.Williams S, Birch R, Einhorn L. Treatment of disseminated germ cell tumors with cisplatin, bleomycin and either vinblastine or etoposide. N Engl J Med. 1987;316:1435–1440. doi: 10.1056/NEJM198706043162302. [DOI] [PubMed] [Google Scholar]
- 28.Einhorn LH. Clinical trials in testicular cancer. Cancer. 1993;71:3182–3184. doi: 10.1002/1097-0142(19930515)71:10<3182::aid-cncr2820711046>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 29.Matsutani M, Takakura K, Sano K. Primary intracranial germ cell tumors: Pathology and treatment. Prog Exp Tumor Res. 1987;30:307–312. doi: 10.1159/000413688. [DOI] [PubMed] [Google Scholar]
- 30.Allen JC, Bosl G, Walker R. Chemotheray trials in recurrent primary intracranial germ cell tumors. J Neurooncol. 1985;3:147–152. doi: 10.1007/BF02228891. [DOI] [PubMed] [Google Scholar]
- 31.Jereb B, Zupanic N, Petric J. Intracranial germinoma: Report of seven cases. Pediatr Hematol Oncol. 1990;7:183–188. doi: 10.3109/08880019009033389. [DOI] [PubMed] [Google Scholar]
- 32.Cushing B, Giller R, Cullen JW, et al. Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high-dose or standard-dose cisplatin in children and adolescents with high-risk malignant germ cell tumors: A Pediatric Intergroup Study—Pediatric Oncology Group 9049 and Children's Cancer Group 8882. J Clin Oncol. 2004;22:2691–2700. doi: 10.1200/JCO.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Baranzelli MC, Patte C, Bouffet E, et al. An attempt to treat pediatric intracranial aFP and b-HCG secreting germ cell tumors with chemotherapy alone—SFOP experience with 18 cases. J Neuro-Oncol. 1998;37:229–239. doi: 10.1023/a:1005863601481. [DOI] [PubMed] [Google Scholar]
- 34.Matsutani M, Sano K, Takakura K, et al. Primary intracranial germ cell tumors: A clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86:446–455. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 35.Bamberg M, Kortmann RD, Calaminus G, et al. Radiation therapy for intracranial germinoma: Results of the German Cooperative Prospective Trials MAKEI 83/86/89. J Clin Oncol. 1999;17:2585–2592. doi: 10.1200/JCO.1999.17.8.2585. [DOI] [PubMed] [Google Scholar]
- 36.Bouffet E, Baranzelli MC, Patte C, et al. Combined treatment modality for intracranial germinomas: Results of a multicentre SFOP experience—Societe Francaise d'Oncologie Pediatrique. Br J Cancer. 1999;79:1199–1204. doi: 10.1038/sj.bjc.6690192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Calaminus G, Andreussi L, Garrre M-L, et al. Secreting germ cell tumors of the central nervous system (CNS). First results of the cooperative German/Italian pilot study (CNS sGCT). Klin Padiatr. 1997;209:222–227. doi: 10.1055/s-2008-1043954. [DOI] [PubMed] [Google Scholar]
- 38.Shibamoto Y, Takahashi M, Sasai K. Prognosis of intracranial germinoma with syncytiotrophoblastic giant cells treated by radiation therapy. Int J Radiat Oncol Biol Phys. 1997;37:511–515. doi: 10.1016/s0360-3016(96)00611-6. [DOI] [PubMed] [Google Scholar]
- 39.Matsutani M. Combined chemotherapy and radiation therapy for CNS germ cell tumor—The Japanese experience. J Neurooncol. 2001;54:311–316. doi: 10.1023/a:1012743707883. [DOI] [PubMed] [Google Scholar]
- 40.Modak S, Gardner S, Dunkel IJ, et al. Thiotepa-based high-dose chemotherapy with autologous stem-cell rescue in patients with recurrent or progressive CNS germ cell tumors. J Clin Oncol. 2004;22:1934–1943. doi: 10.1200/JCO.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 41.Fuller BG, Kapp DS, Cox R. Radiation therapy of pineal region tumors: 25 new cases and a review of 208 previously reported cases. Int J Radiat Oncol Biol Phys. 1993;28:2229–2245. doi: 10.1016/0360-3016(94)90162-7. [DOI] [PubMed] [Google Scholar]
- 42.Takakura K. Clinical Neurosurgery, Proceedings of the Congress of Neurological Surgeons. Williams & Wilkins, Inc.; Baltimore: 1984. Intracranial germ cell tumors. pp. 429–444. [PubMed] [Google Scholar]
- 43.Sawamura Y, Shirato H, Ikeda J, et al. Induction chemotherapy followed by reduced-volume radiation therapy for newly diagnosed central nervous system germinoma. J Neurosurg. 1998;88:66–72. doi: 10.3171/jns.1998.88.1.0066. [DOI] [PubMed] [Google Scholar]
- 44.Kellie SJ, Boyce H, Dunkel IJ, et al. Intensive cisplatin and cyclophosphamide-based chemotherapy without radiotherapy for intracranial germinomas: Failure of a primary chemotherapy approach. Pediatr Blood Cancer. 2004;43:126–133. doi: 10.1002/pbc.20026. [DOI] [PubMed] [Google Scholar]
- 45.Kellie SJ, Boyce H, Dunkel IJ, et al. Primary chemotherapy for intracranial nongerminomatous germ cell tumors: Results of the Second International CNS Germ Cell Study Group Protocol. J Clin Oncol. 2004;22:846–853. doi: 10.1200/JCO.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 46.Weiner HL, Finlay JL. Surgery in the management of primary intracranial germ cell tumors. Childs Nerv Syst. 1999;15:770–773. doi: 10.1007/s003810050469. [DOI] [PubMed] [Google Scholar]
- 47.Packer R, Sutton LN, Atkins TE, et al. A prospective study of cognitive function in children receiving whole-brain radiotherapy and chemotherapy: 2-year results. J Neurosurg. 1989;70:707–713. doi: 10.3171/jns.1989.70.5.0707. [DOI] [PubMed] [Google Scholar]
- 48.Chapman CA, Waber DP, Bernstein JH, et al. Neurobehavioral and neurologic outcome in long-term survivors of posterior fossa brain tumors: Role of age and perioperative factors. J Child Neurol. 1995;10:209–212. doi: 10.1177/088307389501000308. [DOI] [PubMed] [Google Scholar]
- 49.Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children's Cancer Group Study. J Clin Oncol. 1999;17:2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- 50.Jenkin D, Berry M, Chan H, et al. Pineal region germinomas in childhood. Treatment considerations. Int J Radiat Oncol Biol Phys. 1990;18:541–545. doi: 10.1016/0360-3016(90)90058-r. [DOI] [PubMed] [Google Scholar]
- 51.Matsutani M, Sano K, Takakura K. Long-term follow-up of patients with primary intracranial germinomas. In: Packer RJ, Bleyer WA, Pochedly C, editors. Pediatric Neuro-oncology. Harwood Academic Pub; Paris: 1992. pp. 254–260. [Google Scholar]
- 52.Sutton LN, Radcliffe J, Goldwein JW, et al. Quality of life of adult survivors of germinomas treated with craniospinal irradiation. Neurosurgery. 1999;45:1292–1298. doi: 10.1097/00006123-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 53.Merchant TE, Sherwood SH, Mulhern RK, et al. CNS germinoma: Disease control and long-term functional outcome for 12 children treated with craniospinal irradiation. Int J Radiat Oncol Biol Phys. 2000;46:1171–1176. doi: 10.1016/s0360-3016(99)00375-2. [DOI] [PubMed] [Google Scholar]