Abstract
Objective
To evaluate prospectively the relationship between prepregnancy folate intake and risk of spontaneous abortion and stillbirth.
Methods
Women in the Nurses’ Health Study-II who self-reported a pregnancy between 1992 and 2009 were included in this analysis. Dietary folate and supplement use was assessed every 4 years, starting in 1991, by a food-frequency questionnaire. Pregnancies were self-reported, with case pregnancies lost spontaneously (spontaneous abortion <20 weeks of gestation and stillbirth 20+ weeks of gestation) and comparison pregnancies ending in ectopic pregnancy, induced abortion, or live birth.
Results
Among the 11,072 women, 15,950 pregnancies were reported of which 2,756(17.3%) ended in spontaneous abortion and 120(0.8%) ended in stillbirth. Compared to women in the lowest quintile of prepregnancy folate intake (<285μg/day), those in the highest quintile (>851μg/day) had a relative risk (RR) of spontaneous abortion of 0.91 (95% CI 0.82,1.02) after multivariable adjustment (P-trend=0.04). This association was primarily attributable to intake of folate from supplements. Compared to women without supplemental folate intake (0μg/day), those in the highest category (>730μg/day) had a RR of spontaneous abortion of 0.80 (95% CI 0.71,0.90) after multivariable adjustment (P-trend=<0.001). The association of prepregnancy supplemental folate with risk of spontaneous abortion was consistent across gestational period of loss. A similar inverse trend was observed with the risk of stillbirth, which fell short of conventional significance (P-trend=0.06).
Conclusions
Higher intake of folate from supplements was associated with reduced risk of spontaneous abortion. Women at risk of pregnancy should use supplemental folate for neural tube defect prevention and because it may decrease the risk of spontaneous abortion,.
Introduction
Approximately one-third of pregnancies are lost after implantation, many before clinical recognition (1), making pregnancy loss the most frequent adverse pregnancy outcome (2). While chromosomal abnormalities are implicated in about 50% of all spontaneous abortions, the remaining 50% may be preventable and related to environmental factors (3). Research on the role of dietary factors in human reproduction is limited but there is reason to believe that intake of certain nutrients, particularly folate, could positively influence reproductive success (4).
Folic acid prevents neural tube defects (5). The American College of Obstetrics and Gynecology (ACOG) recommends that all women planning or capable of pregnancy take 400 μg/day of folic acid (6). However, folic acid supplementation may have reproductive benefits beyond the prevention of neural tube defects. Folic acid supplementation in animals promotes embryo and fetal survival rates throughout gestation (7–9) yet the association between folate intake and fetal survival in humans is less clear.
The objective of this study was to evaluate the relationship between prepregnancy folate intake and risks of spontaneous abortion and stillbirth in a large, prospective cohort of women with a wide range of folate intake. We aimed to expand on previous studies by examining dose-response relationships comparing spontaneous abortion food with supplemental folate. We hypothesized that higher intake of folate, particularly supplemental folate (due to higher absorption and bioavailability and wider range of intake), is associated with reduced risk of pregnancy loss.
Materials and Methods
The Nurses’ Health Study-II is an ongoing prospective cohort of 116,480 female nurses, ages 24 to 44 years at the study’s inception in 1989. Questionnaires are distributed every 2 years to update lifestyle and medical characteristics and capture incident health outcomes. Response rates for each questionnaire cycle have exceeded 90%. Diet was first assessed in 1991 and updated every 4 years thereafter. Women were eligible for this analysis if they had no history of pregnancy loss in 1991 and reported at least one pregnancy during 1992–2009. Eligible participants contributed pregnancies until their first pregnancy loss or the end of follow-up. Women were censored after their first pregnancy loss to prevent reverse causation, that is, behavioral changes in response to an adverse outcome. Of the 19,451 eligible pregnancies, we excluded those with missing data on diet (n=2,475), implausible or missing gestational age (n=111), missing year of pregnancy (n=619), and diagnosis of type II diabetes (n=69), cardiovascular disease (n=86), or cancer (n=141) prior to the pregnancy. The final sample consisted of 15,950 pregnancies from 11,072 women. This study was approved by the institutional review board of Partners Health Care, Boston, Massachusetts.
Diet was evaluated using a validated 131-item food frequency questionnaire (FFQ)(10). Women reported how often, on average, they consumed specified amounts of each food during the previous year. Participants were also asked whether they used multivitamins and other nutrient supplements and if so, the brand, dose, and frequency of use. Nutrient intakes were estimated by summing nutrient contributions of each food item and supplement. Nutrient contents of each item were obtained from a nutrient database derived from the US Department of Agriculture and additional information from manufacturers (11). The database was updated in 1998 to reflect the universal folic acid supplementation of flour and cereals. Folate intake with this questionnaire has been validated against prospectively collected diet records (r = 0.71)(12), red blood cell folate (r = 0.51)(13), and plasma folate levels (r = 0.63)(14). Nutrient intakes were adjusted for total energy intake using the nutrient residual method (15). We used diet information from 1991 for pregnancies in 1992, 1993, 1994, 1995; the 1995 diet for pregnancies in 1996, 1997, 1998, 1999; and so forth. If a woman was missing diet (< 5% of women, the most recent dietary data was carried forward.
Outcome Assessment
Women reported their pregnancies in 1989 and in each biennial follow-up questionnaire. In the 2009 questionnaire, women also reported information on the year, length, complications, and outcomes of all previous pregnancies. Options for pregnancy outcomes were a singleton live birth, multiple birth, miscarriage or stillbirth, tubal or ectopic pregnancy, or induced abortion. Gestational lengths were reported in categories: < 8 weeks, 8–11 weeks, 12–19 weeks, 20–27 weeks, 28–31 weeks, 32–36 weeks, 37–39 weeks, 40–42 weeks, and 43+ weeks of gestation. Self-reported pregnancy outcome and gestation length have been shown to be validly reported (16). Spontaneous abortion was defined as a fetal loss under 20 completed weeks of gestation. Stillbirth was defined as a fetal loss at 20+ completed weeks of gestation. The validity of maternal recall of spontaneous abortion has not been assessed in this population; the sensitivity is estimated to be around 75% (17),(18). Non-cases were all pregnancies that did not end in fetal loss (live births, induced abortions, and tubal or ectopic pregnancies).
Covariate Assessment
Information on covariates was assessed in 1989 and during follow-up. For variables updated over follow-up, the most recent value prior to that pregnancy was used. Age was computed as the difference between year of birth and year of pregnancy. Physical activity was ascertained in 1991, 1997, 2001, and 2005. The questionnaire-based estimates correlated well with detailed activity diaries in a validation study (r=0.56)(19). Smoking status, multivitamin use, oral contraceptive use, and history of infertility were self-reported in 1989 and updated every two years. History of ovulation inducing medication use was self-reported starting in 1993 and updated every 2 years. Marital status was reported in 1989, 1993, and 1997. Weight was self-reported in 1989 and updated every two years thereafter. Race and height were reported in 1989. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. In a validation study, self- reported weight was highly correlated with weight measured by a technician (r=0.97)(20).
Statistical Analysis
Baseline characteristics were derived from the 1991 questionnaire for all women contributing eligible pregnancies. We divided women into groups according to quintiles of calorie-adjusted total folate intake and categories of supplemental folate intake. Differences in baseline characteristics by prepregnancy total and supplemental folate intake were compared using a chi-squared test for categorical variables and Kruskal-Wallis non-parametric tests for continuous variables.
The relative risk (RR) of spontaneous abortion and stillbirth in relation to prepregnancy folate intake was estimated using log-binomial regression. Generalized estimating equations with an exchangeable working correlation structure were used to account for the within-person correlation between pregnancies. Tests for linear trend were conducted by using the median values in each category as a continuous variable. In addition to age, calorie, and year adjusted models, multivariable models were further adjusted for a priori–selected prepregnancy covariables: BMI, smoking status, physical activity, history of infertility, marital status, and race. Categorical covariables included an indicator for missing data, if necessary.
We assessed whether the other B vitamins (thiamin, riboflavin, niacin, pantothenic acid, vitamin B6, or vitamin B12) were associated with spontaneous abortion, as these B vitamins come from similar dietary sources and are highly correlated with folate intake. In addition, we investigated whether the relation of folate with spontaneous abortion differed by gestational age at loss (<8 weeks, 8–11 weeks, and 12–19 weeks). Relative risks for specific gestational windows were estimated using log-binomial regressions. The reference group for fetal losses < 8 weeks was all initiated pregnancies, for fetal loses 8–11 weeks was all pregnancies lasting beyond 8 weeks, and for fetal losses 12–19 weeks was all pregnancies lasting beyond 12 weeks. P values for heterogeneity were derived from the cross-product interaction term added to the main-effects multivariable model.
To address the potential of residual confounding by factors strongly related to risk of pregnancy loss, we performed sensitivity analyses restricted to pregnancies from women 40 years or younger, pregnancies with no history of infertility, and first eligible pregnancies. To capture uncontrolled confounding by behaviors related to pregnancy planning and pregnancy recognition, we performed analyses restricted to married women not using oral contraception. To address the potential of misclassification of exposure due to the interval between diet assessments, we restricted analyses to pregnancies in the years closest to diet assessment (1992, 1996, 2000, and 2004). Effect modification by prepregnancy BMI (< 25 kg/m2 vs. ≥25 kg/m2), smoking status (current vs. never or former smokers), and maternal age (<35 yrs vs. ≥35 yrs) was tested using cross-product terms in the final multivariable model. All data were analyzed using SAS 9.1 (SAS Institute Inc, Cary, NC).
Results
Of the 15,950 eligible pregnancies, 2,756 (17.3%) ended in spontaneous abortion and 120 (0.8%) in stillbirth. Women in the cohort had a mean (SD) age of 31.6 (3.4) years and BMI of 23.3 (4.3) kg/m2 in 1991. The majority were Caucasian (93%), married (71%), never smokers (71%), and nulliparous (46%) in 1991. On average, women with higher folate intake were slightly heavier, reported more physical activity, were less likely to be current smokers and current users of oral contraceptives, and reported higher calorie intake and more frequent multivitamin consumption. These women were also more likely to be parous, Caucasian women who were married and had a history of infertility (Table 1).
Table 1.
Baseline Demographic Characteristics by Quintile of Baseline Total Folate Intake and Category of Supplemental Folate Intake in 1991 (n=11,072 Women)
| Total Folate Intake | Supplemental Folate Intake | |||
|---|---|---|---|---|
|
|
||||
| Quantile | Q1 | Q5 | Q1 | Q4 |
| Number of Women | 2215 | 2212 | 5478 | 1224 |
| Folate intake, μg/day, Median [Range] | 234 [88 – 274] | 1051 [788 – 2653] | 0 [0–0] | 1000 [800–2000] |
| Maternal age, yr | 31 [29, 34] | 31 [29, 34] | 31 [29, 34] | 31 [29, 34] |
| Prepregnancy BMI, kg/m2 | 22.1 [20.4, 25.1] | 22.9 [20.8, 25.8] | 22.1 [20.4, 24.9] | 23.6 [21.3, 26.6] |
| Physical Activity, MET-hrs/week | 10.9 [4.2, 24.8] | 16.5 [7.1, 33.8] | 14.7 [6.0, 30.9] | 15.2 [6.5, 30.2] |
| Smoking status, n % | ||||
| Never smoker | 1515 (68.4) | 1638 (74.1) | 3838 (70.1) | 928 (75.8) |
| Former smoker | 396 (17.9) | 464 (21.0) | 1053 (19.2) | 251 (20.5) |
| Current smoker | 299 (13.5) | 105 (4.8) | 577 (10.5) | 44 (3.6) |
| White, n% | 2022 (91.3) | 2075 (93.8) | 5089 (92.9) | 1146 (93.6) |
| Married, n% | 1511 (68.2) | 1735 (78.4) | 3713 (67.8) | 1043 (85.2) |
| Ever oral contraceptive use | ||||
| Never | 348 (15.7) | 346 (15.6) | 843 (15.4) | 196 (16.0) |
| Past | 1222 (55.2) | 1601 (72.4) | 3068 (56.0) | 961 (78.5) |
| Current | 645 (29.1) | 264 (11.9) | 1567 (28.6) | 67 (5.5) |
| History of infertility*, n% | 324 (14.6) | 344 (15.6) | 773 (14.1) | 190 (15.5) |
| History of infertility drug use†, n% | 209 (9.4) | 273 (12.3) | 546 (10.0) | 153 (12.5) |
| Parity, n% | ||||
| Nulliparous | 997 (45.0) | 878 (39.7) | 2496 (45.6) | 401 (32.8) |
| 1 | 593 (26.8) | 742 (33.5) | 1432 (26.1) | 476 (38.9) |
| 2 | 420 (19.0) | 363 (16.4) | 1046 (19.1) | 205 (16.8) |
| 3+ | 159 (7.2) | 135 (6.1) | 391 (7.1) | 75 (6.1) |
| Multivitamin use, n% | 149 (6.7) | 2176 (98.4) | 176 (3.2) | 1224 (100.0) |
| Energy Intake, kcal/day | 1659 [1293, 2071] | 1713 [1346, 2087] | 1709 [1360, 2100] | 1942 [1602, 2301] |
| % Energy from fat | 33.8 [30.0, 37.6] | 29.5 [26.1, 32.9] | 31.3 [27.6, 35.2] | 29.9 [26.7, 33.2] |
| % Energy from carbohydrates | 47.3 [42.5, 52.6] | 52.0 [47.8, 56.4] | 49.8 [45.1, 21.2] | 51.9 [47.8, 56.1] |
| % Energy from protein | 18.6 [16.3, 20.8] | 19.5 [17.5, 21.7] | 19.0 [16.9, 21.2] | 19.4 [17.4, 21.6] |
| Alcohol intake, g/day | 1.5 [0.0, 4.4] | 0.9 [0.0, 2.7] | 1.8 [0.0, 4.5] | 0.0 [0.0, 1.9] |
BMI, body mass indexMET-hr, metabolic equivalent task hours.
Sample sizes may vary due to missing data.
Assessed in 1989 and 1991.
Assessed in 1993 (first time questions was asked).
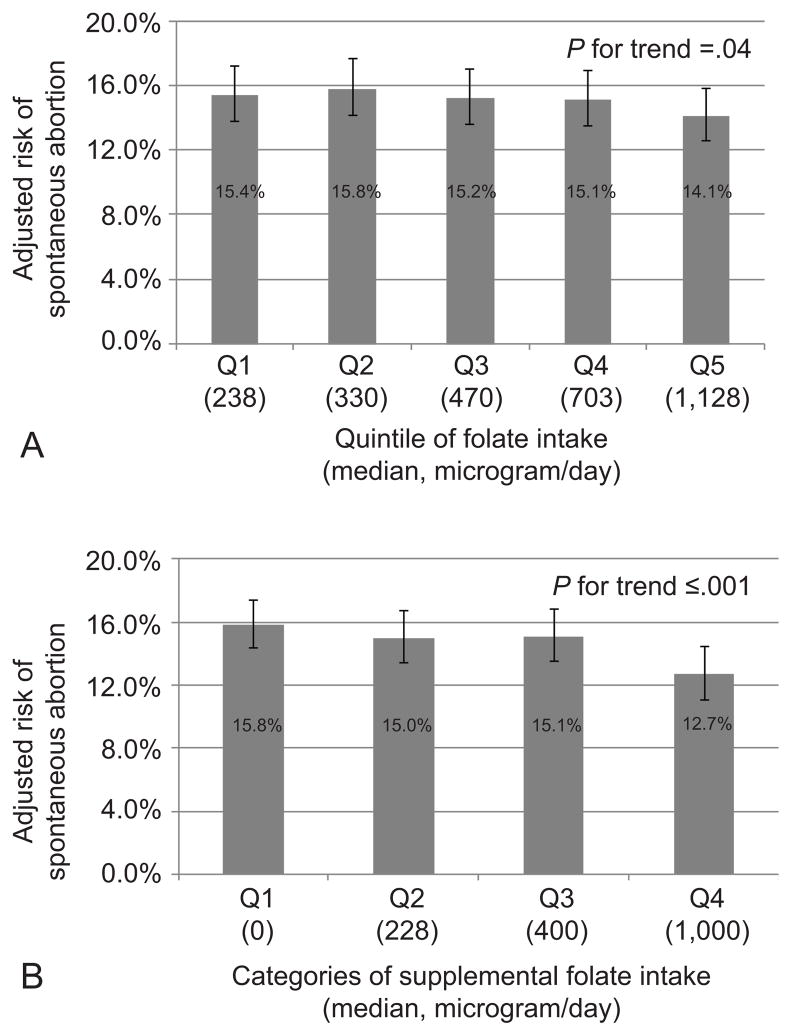
Higher intake of total folate prior to pregnancy was associated with reduced risk of spontaneous abortion (Table 2). Compared to women in the lowest quintile of prepregnancy folate intake (<285 μg/day), those in the highest quintile (>851 μg/day) had a RR of spontaneous abortion of 0.91 (95% confidence interval [CI] 0.82, 1.02) after adjusting for energy intake, maternal age, BMI, physical activity, year of pregnancy, history of infertility, marital status, and race(p-trend=0.04). This association was driven solely by folate from supplements. Specifically, after multivariable adjustment, women in the highest category of supplemental folate intake (>730 μg/day) had an RR of spontaneous abortion of 0.80 (95% CI 0.71, 0.90) compared to women who did not consume supplemental folate (p-trend= <0.001). The adjusted absolute risks of spontaneous abortion by quintile of folate intake and category of supplemental folate (Figure 1) suggest that 42 women would need to go from 400–729 μg/day of supplemental folate (Q3) to >730 μg/day (Q4) of supplemental folate to prevent one spontaneous abortion.
Table 2.
Prepregnancy Folate Intake and Relative Risks of Spontaneous Abortion
| Categories of Folate Intake (median, μg/day) | Cases/Total | % | Age, Year, & Energy- Adjusted RR (95% CI)* | Multivariate-Adjusted RR (95% CI)† |
|---|---|---|---|---|
| Total folate | ||||
| Q1 (238) | 537/3185 | 16.9 | 1.00 (Ref) | 1.00 (Ref) |
| Q2 (330) | 572/3192 | 17.9 | 1.03 (0.92, 1.14) | 1.02 (0.92, 1.14) |
| Q3 (470) | 559/3197 | 17.5 | 0.99 (0.89, 1.10) | 0.99 (0.89, 1.10) |
| Q4 (703) | 562/3186 | 17.6 | 0.97 (0.88, 1.08) | 0.98 (0.88, 1.09) |
| Q5 (1128) | 526/3190 | 16.5 | 0.91 (0.82, 1.01) | 0.91 (0.82, 1.02) |
| P for trend | 0.03 | 0.04 | ||
| Folate from foods | ||||
| Q1 (213) | 514/3177 | 16.2 | 1.00 (Ref) | 1.00 (Ref) |
| Q2 (267) | 497/3180 | 15.6 | 0.96 (0.86, 1.08) | 0.97 (0.86, 1.08) |
| Q3 (311) | 551/3204 | 17.2 | 1.03 (0.93, 1.15) | 1.03 (0.93, 1.15) |
| Q4 (364) | 576/3199 | 18.0 | 1.04 (0.94, 1.16) | 1.05 (0.94, 1.17) |
| Q5 (461) | 618/3190 | 19.4 | 1.06 (0.95, 1.18) | 1.07 (0.96, 1.19) |
| P for trend | 0.12 | 0.09 | ||
| Folate from supplements | ||||
| Q1 (0) | 1145/6638 | 17.3 | 1.00 (Ref) | 1.00 (Ref) |
| Q2 (228) | 701/3779 | 18.6 | 0.95 (0.87, 1.03) | 0.95 (0.87, 1.03) |
| Q3 (400) | 589/3351 | 17.6 | 0.95 (0.87, 1.04) | 0.95 (0.87, 1.04) |
| Q4 (1000) | 321/2182 | 14.7 | 0.80 (0.71, 0.89) | 0.80 (0.71, 0.90) |
| P for trend | <0.001 | <0.001 | ||
RR, relative risk; CI, confidence interval. Ranges for total folate quintiles in μg/day: Q1 (<284.9), Q2 (285–383.9), Q3 (384–580.9), Q4 (581–850.9), Q5 (>851). Ranges for food folate quintiles in μg/day: Q1 (<243.9), Q2 (244–288.9), Q3 (289–336.9), Q4 (337–398.9), Q5 (>399). Ranges for supplemental folate quartiles in μg/day: Q1 (0), Q2 (0.1–399.9), Q3 (400–729.9), Q4 (>730).
Adjusted for age (continuous), total energy intake (continuous), and year (continuous).
Age, year, and energy adjusted model further adjusted for body mass index (<18.5, 18.5–24.9, 25–29.9, ≥30, and missing), smoking status (never, former, current, and missing), physical activity (< 3 metabolic equivalent task [MET]-h/wk, 3–8.9 MET-h/wk, 9–17.9 MET-h/wk, 18–26.9 MET-h/wk, 27–41.9 MET-h/wk, >42 MET-h/wk, and missing), history of infertility (no, yes, and missing), marital status (married, not married), and race (white, other).
Figure 1.
Prepregnancy folate intake and adjusted absolute risks of spontaneous abortion.
(A) Total folate intake in quintiles. (B) Supplemental folate intake in categories. Adjusted risks are presented for the average age (35 years), total energy intake (1700 kcal/day), year (1992), body mass index (18.5–24.9 kg/m2), smoking status (never smoker), physical activity (9–17.9 MET-h/wk), history of infertility (none), marital status (married), and race (white) in our cohort.
After multivariable adjustment, higher intake of vitamin B12 was associated with lower risk of spontaneousspontaneous abortion (p-trend=0.04) (Table 3). With further adjustment for supplemental folate intake, however, intake of B12 was no longer related to spontaneous abortion (p-trend=0.93) whereas the inverse dose-response relation between supplemental folate intake and risk of spontaneous abortion remained statistically and clinically significant. In this model, the RR (95%CI) spontaneous abortionfor increasing quartiles of supplemental folate intake were 1.00 (REF), 0.94 (0.86, 1.04), 0.91 (0.81, 1.02), 0.78 (0.68, 0.90) (p-trend =< 0.001).
Table 3.
Prepregnancy B Vitamin Intake and Relative Risks of Spontaneous Abortion
| Quintile of Intake (median, μg/day) | Cases/Total | % | Multivariate-Adjusted RR (95% CI)* |
|---|---|---|---|
| Thiamin (B1) | |||
| Q1 (1.3) | 561/3172 | 17.7 | 1.00 (Ref) |
| Q2 (1.7) | 552/3179 | 17.4 | 1.00 (0.89, 1.11) |
| Q3 (2.4) | 549/3210 | 17.1 | 0.98 (0.88, 1.10) |
| Q4 (3.2) | 536/3204 | 16.7 | 0.95 (0.85, 1.06) |
| Q5 (5.1) | 558/3185 | 17.5 | 0.96 (0.86, 1.06) |
| P for Trend | 0.33 | ||
| Riboflavin (B2) | |||
| Q1 (1.6) | 588/3215 | 18.3 | 1.00 (Ref) |
| Q2 (2.2) | 531/3142 | 16.9 | 0.93 (0.84, 1.04) |
| Q3 (3.0) | 544/3231 | 16.8 | 0.94 (0.85, 1.05) |
| Q4 (4.0) | 549/3178 | 17.3 | 0.96 (0.86, 1.06) |
| Q5 (6.2) | 544/3184 | 17.1 | 0.90 (0.81, 1.00) |
| P for Trend | 0.13 | ||
| Niacin (B3) | |||
| Q1 (20.1) | 555/3175 | 17.5 | 1.00 (Ref) |
| Q2 (25.3) | 552/3187 | 17.3 | 1.01 (0.91, 1.12) |
| Q3 (33.3) | 533/3198 | 16.7 | 0.97 (0.87, 1.08) |
| Q4 (40.7) | 535/3205 | 16.7 | 0.96 (0.86, 1.07) |
| Q5 (53.0) | 581/3185 | 18.2 | 1.00 (0.90, 1.11) |
| P for Trend | 0.84 | ||
| Pantothenic Acid (B5) † | |||
| Q1 (4.0) | 548/3189 | 17.2 | 1.00 (Ref) |
| Q2 (5.0) | 571/3331 | 17.1 | 0.99 (0.89, 1.11) |
| Q3 (6.1) | 501/3041 | 16.5 | 0.96 (0.86, 1.08) |
| Q4 (11.5) | 528/3189 | 16.6 | 0.95 (0.85, 1.06) |
| Q5 (18.1) | 608/3200 | 19.0 | 1.04 (0.94, 1.15) |
| P for Trend | 0.39 | ||
| Vitamin B6† | |||
| Q1 (1.8) | 528/2956 | 17.9 | 1.00 (Ref) |
| Q2 (2.3) | 615/3562 | 17.3 | 1.00 (0.90, 1.11) |
| Q3 (3.4) | 537/3084 | 17.4 | 1.00 (0.90, 1.12) |
| Q4 (4.8) | 529/3170 | 16.7 | 0.94 (0.84, 1.05) |
| Q5 (13.7) | 547/3178 | 17.2 | 0.95 (0.85, 1.05) |
| P for Trend | 0.25 | ||
| Vitamin B12† | |||
| Q1 (4.0) | 746/4026 | 18.5 | 1.00 (Ref) |
| Q2 (6.0) | 293/1739 | 16.9 | 0.91 (0.81, 1.03) |
| Q3 (9.0) | 580/3622 | 16.0 | 0.88 (0.80, 0.97) |
| Q4 (12.0) | 588/3220 | 18.3 | 1.01 (0.91, 1.11) |
| Q5 (19.0) | 549/3343 | 16.4 | 0.87 (0.79, 0.96) |
| P for Trend | 0.04 | ||
RR, relative risk; CI, confidence interval.
Adjusted for age (continuous), energy intake (continuous), BMI (<18.5, 18.5–24.9, 25–29.9, ≥30, and missing), smoking status (never, former, current, and missing), physical activity (< 3, 3–8.9, 9–17.9, 18–26.9, 27–41.9, >42 MET-h/wk, and missing), year of pregnancy, history of infertility (no, yes, and missing), marital status (married, not married), and race (white, other).
1,500 pregnancies with missing values.
Overall, the magnitude of association between prepregnancy total and supplemental folate intake with risk of spontaneous abortion was fairly consistent across different gestational window (p-interaction, 0.92 and 0.71 respectively) (Table 4). Furthermore, total and supplemental folate intake had an inverse association with stillbirth which fell short of conventional significance (p-trend, 0.06 and 0.14, respectively). The adjusted absolute risks of stillbirth in Q1 and Q5 of total folate intake were 0.0072 and 0.0040 and in Q1 and Q4 of supplemental folate were 0.0064 and 0.0041.
Table 4.
Prepregnancy Folate Intake and Relative Risks of Spontaneous Abortion and Stillbirth Stratified by Gestational Length
| Categories of Intake (median, μg/day) | Fetal Loss < 8 Weeks | Fetal Loss 8–11 Weeks | Fetal Loss 12–19 Weeks | Fetal Loss 20+ Weeks | ||||
|---|---|---|---|---|---|---|---|---|
| Cases/Total | Multivariate-Adjusted RR (95% CI)* | Cases/Total | Multivariate-Adjusted RR (95% CI)* | Cases/Total | Multivariate-Adjusted RR (95% CI)* | Cases/Total | Multivariate-Adjusted RR (95% CI)* | |
| Total Folate | ||||||||
| Q1 (241) | 200/3185 | 1.00 (Ref) | 226/2886 | 1.00 (Ref) | 111/2596 | 1.00 (Ref) | 28/2467 | 1.00 (Ref) |
| Q2 (336) | 192/3192 | 0.92 (0.76, 1.11) | 250/2902 | 1.08 (0.91, 1.28) | 130/2594 | 1.10 (0.86, 1.41) | 25/2451 | 0.79 (0.46, 1.36) |
| Q3 (489) | 214/3197 | 1.00 (0.83, 1.21) | 224/2876 | 0.97 (0.81, 1.15) | 121/2603 | 1.00 (0.78, 1.29) | 26/2467 | 0.85 (0.50, 1.46) |
| Q4 (725) | 206/3186 | 0.95 (0.79, 1.15) | 245/2904 | 1.02 (0.86, 1.22) | 111/2621 | 0.90 (0.69, 1.16) | 23/2490 | 0.72 (0.41, 1.26) |
| Q5 (1161) | 189/3190 | 0.87 (0.72, 1.06) | 216/2941 | 0.88 (0.74, 1.06) | 121/2697 | 0.95 (0.74, 1.23) | 18/2568 | 0.55 (0.30, 1.00) |
| P for Trend | 0.22 | 0.08 | 0.31 | 0.06 | ||||
| Folate from Supplements | ||||||||
| Q1 (0) | 408/6638 | 1.00 (Ref) | 489/6030 | 1.00 (Ref) | 248/5416 | 1.00 (Ref) | 53/5134 | 1.00 (Ref) |
| Q2 (228) | 261/3779 | 0.96 (0.82, 1.12) | 292/3391 | 0.92 (0.80, 1.07) | 148/3048 | 0.96 (0.78, 1.18) | 31/2885 | 0.92 (0.58, 1.45) |
| Q3 (400) | 218/3351 | 0.97 (0.82, 1.14) | 245/3047 | 0.92 (0.79, 1.07) | 126/2756 | 0.93 (0.76, 1.15) | 23/2609 | 0.79 (0.48, 1.29) |
| Q4 (1000) | 114/2182 | 0.79 (0.64, 0.97) | 135/2041 | 0.76 (0.63, 0.92) | 72/1891 | 0.78 (0.60, 1.01) | 13/1815 | 0.66 (0.36, 1.21) |
| P for Trend | 0.03 | 0.005 | 0.05 | 0.14 | ||||
RR, relative risk; CI, confidence interval.
Adjusted for age (continuous), energy intake (continuous), body mass index (<18.5, 18.5–24.9, 25–29.9, ≥30, and missing), smoking status (never, former, current, and missing), physical activity (< 3 MET-h/wk, 3–8.9 MET-h/wk, 9–17.9 MET-h/wk, 18–26.9 MET-h/wk, 27–41.9 MET-h/wk, >42 MET-h/wk, and missing), year of pregnancy (continuous), history of infertility (no, yes, and missing), and marital status (married, not married), and race (white, other).
In sensitivity analyses (see the Appendix online at http://links.lww.com/xxx) limited to pregnancies in women less than 40 years of age, pregnancies without a history of infertility, and pregnancies among married women who were not using oral contraception, the results remained similar. Results became slightly stronger when analyses were restricted to the first eligible pregnancy and pregnancies closest to diet assessments. No substantial differences in effect estimates were seen when assessing consumption of folate and risk of spontaneous abortion in overweight vs. non-overweight women, in current vs. never or former smokers, and in younger vs. older women (< 35 yrs vs. ≥35 yrs).
Discussion
In this prospective cohort of 15,950 pregnancies, we found that the risk of spontaneous abortion was 20% lower among women in the highest category of supplemental folate intake (>730 μg/day) than in the lowest (0 μg /d) category.
Since 1992, the U.S. Preventive Services Task Force and Centers for Disease Control and Prevention recommend that all women planning or capable of pregnancy take 400 μg of folic acid daily to prevent neural tube defects (6). In the mid 1990’s the safety of folic acid supplementation was called into question on the basis of three papers (21–23), which suggested folic acid supplementation increased the risk of miscarriage. These findings were subsequently challenged due to methodological errors (24, 25) (e.g. using a one-tailed test) and incongruent conclusions. Specifically, the Hungarian trial found that folic acid increased fertility and multiple birth rates (in addition to miscarriage rates) which seemed implausible. Furthermore, the Medial Research Council Vitamin Study, which used a dose of folic acid approximately 5 times greater the Hungarian trial, found no detrimental effect of folic acid supplementation on miscarriage when the analysis was limited to only women receiving folic acid. Two follow-up studies from China (26) and Brazil (27) also provided evidence that periconceptional folic acid use did not increase miscarriage rates. Two recent cohort studies reported that the use of folic acid or multiple vitamins during pregnancy was associated with a 50–60% reduced risk of miscarriage (28, 29).Our study allowed us to improve on previous studies by contrasting the relationship between prepregnancy folate from food and supplements and spontaneous abortion and to examine a dose-response relationship across this broad range of intake.
Lower folate intake has been linked to reduced cell division, disrupted methylation reactions, and increased inflammatory cytokine production, oxidative stress levels, and apoptosis, all of which could subsequently affect the developing embryo (30). Folate deficiency has also been suggested as a risk factor for abruptio placentae and preeclampsia (31). Thus, the vascular effects related to folate deficiency might also increase the risk of spontaneous abortion and stillbirth. Another explanation is that low folate levels increase the incidence of neural tube defects, and fetuses affected with neural tube defects are more commonly aborted spontaneously (32). While plausible, neural tube defects are rare conditions and this could only explain a fraction of the association between low folate levels and spontaneous abortion.
Supplemental folate was more strongly related to spontaneous abortion than was folate from foods. This difference could be due partly to the greater absorption rates of synthetic folate (33). Relative to folic acid, natural food folate has a lower proportion of folate that is absorbed and available for metabolic reactions and storage. Several luminal factors also hinder the absorption of natural food folate (33). In addition, the range of supplemental folate intake was wider than the range of intakes from foods, increasing our ability to detect an association with supplemental folate intake.
We did not have information on diet during pregnancy and were thus unable to discern whether the association of prepregnancy folate intake with spontaneous abortion is independent of pregnancy folate intake. Nevertheless, prepregnancy folate intake is likely the more relevant time window since most spontaneous abortions occur early in pregnancy. Male partner data were also lacking. Previous studies have reported positive associations between folate and male fertility (34, 35); however, it is unlikely that supplemental folate consumption is highly correlated among partners, thus reducing the likelihood that male diet would be a strong confounder. Second, there is some concern about differential misclassification of fetal loss by gestational age and pregnancy intention. However, our sub-analyses addressing these issues confirmed a robust association. Third, it is possible that many spontaneous abortions were unrecognized and thus not reported. While plausible, underreporting of early losses is likely non-differential with respect to folate intake (due to the prospective design) and would be expected to attenuate the association. Fourth, misclassification of folate intake is likely, particularly because diet information was updated only every four years. As expected, when we limited our analyses to pregnancies in the years closest to diet assessment the results were stronger (Appendix, http://links.lww.com/xxx). Fifth, despite our adjustment and stratification for a variety of potential confounders, we cannot rule out the possibility that there may be residual or unmeasured confounding. However, differences between unadjusted and multivariate-adjusted effect estimates were small suggesting that any residual confounding is unlikely to have a large effect on our results. Our analyses of folate and risk of stillbirth were limited by low number of cases s which reduced statistical power. Finally, our study does not distinguish chromosomally normal from abnormal miscarriages
The strengths of this study are the large, prospective design, nearly-complete follow-up over 18 years, inclusion of early pregnancy losses, ability to examine a dose-response association across a broad range of folate intake, and the ability to contrast supplemental folate with food folate.
Our results have important public health and clinical implications. ACOG and the World Health Organization recommend that women of childbearing age in many countries, including the US, to take prenatal folic acid supplements. Despite this recommendation, the majority of US reproductive aged women consume far below the recommendation of 400 μg/day (36). In addition, food fortification with folic acid has been introduced in many countries and is being considered in others (37–39). Our results provide reassurance that higher intake of supplemental folate is not associated with increased risk of pregnancy loss; rather it may be an effective strategy to prevent spontaneous abortion. Given that the first prenatal visit is likely too late to initiate a discussion on the importance of folic acid supplements, annual OB/GYN visits might be the best opportunity to talk to women about the importance of folic acid.
Supplementary Material
Acknowledgments
Supported by NIH grant T32DK007703-16, T32HD060454, and UM1 CA176726
Footnotes
Presented at the annual meeting for the Society of Pediatric and Perinatal Epidemiology on June 17th, 2013 in Boston, MA.
References
- 1.Wilcox AJ, Weinberg CR, O’Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 2.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12:77–126. [PubMed] [Google Scholar]
- 3.Cramer DW, Wise LA. The epidemiology of recurrent pregnancy loss. Semin Reprod Med. 2000;18:331–9. doi: 10.1055/s-2000-13722. [DOI] [PubMed] [Google Scholar]
- 4.Homan GF, Davies M, Norman R. The impact of lifestyle factors on reproductive performance in the general population and those undergoing infertility treatment: a review. Hum Reprod Update. 2007;13:209–23. doi: 10.1093/humupd/dml056. [DOI] [PubMed] [Google Scholar]
- 5.De-Regil LM, Fernandez-Gaxiola AC, Dowswell T, Pena-Rosas JP. Folic acid supplements before conception and in early pregnancy (up to 12 weeks) for the prevention of birth defects. Cochrane Database Syst Rev. 2010 [Google Scholar]
- 6.Control CfD. Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR. 1992;41 [PubMed] [Google Scholar]
- 7.Matte JJ, Girard CL, Brisson GJ. Folic acid and reproductive performances of sows. J Anim Sci. 1984;59:1020–5. doi: 10.2527/jas1984.5941020x. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay GF, Matte JJ, Dufour JJ, Brisson GJ. Survival rate and development of fetuses during the first 30 days of gestation after folic acid addition to a swine diet. J Anim Sci. 1989;67:724–32. doi: 10.2527/jas1989.673724x. [DOI] [PubMed] [Google Scholar]
- 9.Habibzadeh N, Schorah CJ, Smithells RW. The effects of maternal folic acid and vitamin C nutrition in early pregnancy on reproductive performance in the guinea-pig. Br J Nutr. 1986;55:23–35. doi: 10.1079/bjn19860006. [DOI] [PubMed] [Google Scholar]
- 10.Willett WC, Lenart E. Chapter 6: Reproducibility and validity of Food Frequency Questionnaires. In: Willett WC, editor. Nutritional Epidemiology. 2. New York: Oxford University Press; 1998. [Google Scholar]
- 11.U.S. Department of Agriculture ARS. USDA National Nutrient Database for Standard Reference. Release 25. 2012; Available from: http://www.ars.usda.gov/ba/bhnrc/ndl.
- 12.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, et al. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med. 1998;129:517–24. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Jacques PF, Sulsky SI, Sadowski JA, Phillips JC, Rush D, Willett WC. Comparison of micronutrient intake measured by a dietary questionnaire and biochemical indicators of micronutrient status. Am J Clin Nutr. 1993;57:182–9. doi: 10.1093/ajcn/57.2.182. [DOI] [PubMed] [Google Scholar]
- 15.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 16.Olson JE, Shu XO, Ross JA, Pendergrass T, Robison LL. Medical record validation of maternally reported birth characteristics and pregnancy-related events: a report from the Children’s Cancer Group. Am J Epidemiol. 1997;145:58–67. doi: 10.1093/oxfordjournals.aje.a009032. [DOI] [PubMed] [Google Scholar]
- 17.Kristensen P, Irgens LM. Maternal reproductive history: a registry based comparison of previous pregnancy data derived from maternal recall and data obtained during the actual pregnancy. Acta Obstet Gynecol Scand. 2000;79:471–7. [PubMed] [Google Scholar]
- 18.Wilcox AJ, Horney LF. Accuracy of spontaneous abortion recall. Am J Epidemiol. 1984;120:727–33. doi: 10.1093/oxfordjournals.aje.a113940. [DOI] [PubMed] [Google Scholar]
- 19.Wolf AM, Hunter DJ, Colditz GA, Manson JE, Stampfer MJ, Corsano KA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Czeizel AE, Dudas I, Metneki J. Pregnancy outcomes in a randomised controlled trial of periconceptional multivitamin supplementation. Final report. Arch Gynecol Obstet. 1994;255:131–9. doi: 10.1007/BF02390940. [DOI] [PubMed] [Google Scholar]
- 22.Hook EB, Czeizel AE. Can terathanasia explain the protective effect of folic-acid supplementation on birth defects? Lancet. 1997;350:513–5. doi: 10.1016/S0140-6736(97)01342-1. [DOI] [PubMed] [Google Scholar]
- 23.Windham GC, Shaw GM, Todoroff K, Swan SH. Miscarriage and use of multi-vitamins or folic acid. Am J Med Genet. 2000;90:261–2. doi: 10.1002/(sici)1096-8628(20000131)90:3<261::aid-ajmg18>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Wald N, Hackshaw A. Folic acid and prevention of neural-tube defects. Lancet. 1997;350:665. doi: 10.1016/S0140-6736(05)63358-2. [DOI] [PubMed] [Google Scholar]
- 25.Wald NJ, Hackshaw AK. Folic acid and miscarriage: an unjustified link. Am J Med Genet. 2001;98:204. doi: 10.1002/1096-8628(20010115)98:2<204::aid-ajmg1032>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 26.Gindler J, Li Z, Berry RJ, Zheng J, Correa A, Sun X, et al. Folic acid supplements during pregnancy and risk of miscarriage. Lancet. 2001;358:796–800. doi: 10.1016/s0140-6736(01)05969-4. [DOI] [PubMed] [Google Scholar]
- 27.Vila-Nova C, Wehby GL, Queiros FC, Chakraborty H, Felix TM, Goco N, et al. Periconceptional use of folic acid and risk of miscarriage - findings of the Oral Cleft Prevention Program in Brazil. J Perinat Med. 2013:1–6. doi: 10.1515/jpm-2012-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan R, Olshan AF, Herring AH, Savitz DA, Siega-Riz AM, Hartmann KE. Self-reported vitamin supplementation in early pregnancy and risk of miscarriage. Am J Epidemiol. 2009;169:1312–8. doi: 10.1093/aje/kwp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrne J. Periconceptional folic acid prevents miscarriage in Irish families with neural tube defects. Ir J Med Sci. 2011;180:59–62. doi: 10.1007/s11845-010-0629-5. [DOI] [PubMed] [Google Scholar]
- 30.Forges T, Monnier-Barbarino P, Alberto JM, Gueant-Rodriguez RM, Daval JL, Gueant JL. Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update. 2007;13:225–38. doi: 10.1093/humupd/dml063. [DOI] [PubMed] [Google Scholar]
- 31.Ray JG, Laskin CA. Folic acid and homocyst(e)ine metabolic defects and the risk of placental abruption, pre-eclampsia and spontaneous pregnancy loss: A systematic review. Placenta. 1999;20:519–29. doi: 10.1053/plac.1999.0417. [DOI] [PubMed] [Google Scholar]
- 32.Byrne J, Warburton D. Neural tube defects in spontaneous abortions. Am J Med Genet. 1986;25:327–33. doi: 10.1002/ajmg.1320250219. [DOI] [PubMed] [Google Scholar]
- 33.McNulty H, Pentieva K. Folate bioavailability. Proc Nutr Soc. 2004;63:529–36. doi: 10.1079/pns2004383. [DOI] [PubMed] [Google Scholar]
- 34.Schmid TE, Eskenazi B, Marchetti F, Young S, Weldon RH, Baumgartner A, et al. Micronutrients intake is associated with improved sperm DNA quality in older men. Fertil Steril. 2012;98:1130–7. e1. doi: 10.1016/j.fertnstert.2012.07.1126. [DOI] [PubMed] [Google Scholar]
- 35.Young SS, Eskenazi B, Marchetti FM, Block G, Wyrobek AJ. The association of folate, zinc and antioxidant intake with sperm aneuploidy in healthy non-smoking men. Hum Reprod. 2008;23:1014–22. doi: 10.1093/humrep/den036. [DOI] [PubMed] [Google Scholar]
- 36.Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, McDowell MA, Yetley EA, et al. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am J Clin Nutr. 2010;91:231–7. doi: 10.3945/ajcn.2009.28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Food Standards Australia New Zealand. Final Assessment Report. Proposal P295: Consideration for Mandatory Fortification with Folic Acid. 2006 [Google Scholar]
- 38.Health Council of the Netherlands; Health Council of the Netherlands, editor. Towards an optimal use of folic acid. Hague: 2008. [Google Scholar]
- 39.Scientific Advisory Committee on Nutrition. Folic Acid and Colorectal Cancer Risk: Review of Recommendation for Mandatory Folic Acid Fortification. Norwich, UK: The Stationary Office; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.