Abstract
Serotonin (5-HT) receptors are neuromodulator neurotransmitter receptors which when activated generate a signal transduction pathway within cells resulting in cell-cell communication. 5-hydroxytryptamine (serotonin) receptor 2B (5-HT2B) is a subtype of the seven members of 5-hydroxytrytamine (5-HT) family of receptors which is the largest member of the super family of 7-transmembrane G-protein coupled receptors (GPCRs). Not only do 5-HT receptors play physiological roles in the cardiovascular system, gastrointestinal and endocrine function and the central nervous, but they also play a role in behavioral functions. In particular 5-HT2B receptor is wide spread with regards to its distribution throughout bodily tissues and is expressed at high levels in the lungs, peripheral tissues, liver, kidney and prostate just to name a few. Hence 5-HT2B participates in multiple biological functions including CNS regulation, regulation of gastrointestinal motality, cardiovascular regulation and 5-HT transport system regulation. While 5-HT2B is a viable drug target and has therapeutic indications for treating obesity, psychotherapy, Parkinson’s disease etc. there is a growing concern regarding adverse drug reactions, specifically valvulopathy associated with 5-HT2B agonists. Due to the sequence homology experienced by 5-HT2 subtypes there is also a concern regarding the off target effects of 5-HT2A and 5-HT2C agonists. The concept of subtype selectivity is of paramount importance and can be tackled with the aid of in silico studies, specifically cheminformatics, to develop models to predict valvulopathy associated toxicity of drug candidates prior to clinical trials. This review has highlighted three in silico approaches thus far that have been successful in either predicting 5-HT2B toxicity of molecules or identifying important interactions between 5-HT2B and drug molecules that bring about valvulopathy related toxicities.
Keywords: 5-hydroxytryptamine (serotonin) receptor 2B, valvular heart disease, G protein-coupled receptors, in silico, cheminformatics, quantitative structure–activity relationship, homology modeling, molecular docking, molecular dynamics, virtual screening
1. INTRODUCTION
5-hydroxytryptamine (serotonin) receptor 2B (5-HT2B), a subtype of the seven members of 5-hydroxytrytamine (5-HT) family of receptors with the exception of 5-HT3 receptor which is a part of the ligand-gated ion channel associated receptors, is the largest member of the super family of 7-transmembrane G-protein coupled receptors (GPCRs). GPCRs are one of the most exploited drug targets accounting for over 40% of the blockbuster drugs currently on the market. 1,2 The large diversity of 5-HT receptor is as a consequence of evolutionary growth over a long period of time, which adds to the pharmacological complexity of these receptors. To date seven 5-HT families, 5-HT1 through 5-HT7, have been identified with a total of nineteen known subfamilies (Table 1).3,4
Table 1.
List of human serotonin receptor family and subtypes.
| Receptor Family | 5-HT1 | 5-HT2 | 5-HT3 | 5-HT4 | 5-HT5 | 5-HT6 | 5-HT7 |
|---|---|---|---|---|---|---|---|
| 5-HT1A | 5-HT2A | 5-HT3A | 5-HT4A | 5-HT5A | |||
| 5-HT1B | 5-HT2B | 5-HT3B | 5-HT4B | ||||
| 5-HT1D | 5-HT2C | 5-HT4C | |||||
| 5-HT1E | 5-HT4D | ||||||
| Subtypes | |||||||
| 5-HT1F | 5-HT4E | ||||||
| 5-HT4F | |||||||
| 5-HT4G | |||||||
| 5-HT4H |
Seretonin (5-HT) receptors are neuromodulator neurotransmitter receptors which when activated generate a signal transduction pathway within cells resulting in cell-cell communication. In mammals, serotonin plays a variety of physiological roles in the cardiovascular system, gastrointestinal and endocrine function and the central nervous. Not only do 5-HT receptors have physiological functions they also regulate behavioral functions such as maintenance of aggression and mood, appetite control, control of sleep-wake cycles, sex and memory.5 These receptors are neither excitatory nor inhibitory rather they mediate the actions of serotonin neurotransmitters to which they bind.
2. 5-HT2BRECEPTOR
5-HT2B, a member of the 5-HT2 group of receptors, is widely distributed body tissues. The receptor is expressed at high levels in the liver, kidney, alimentary canal, fundus of the stomach and intestines, which partially makes up the gastrointestinal tract where 95% of total body serotonin is synthesized. To a lesser extent 5-HT2B is also expressed in the lungs, cardiovascular tissues and central nervous system (CNS) of mammals.6-9 However, more recent studies are reporting the expression of several 5-HT receptors in the human heart.10 5-HT2B receptors are known to participate in multiple biological functions, three of the more infamous roles being;
A. CNS regulation - The mechanism by which 5-HT2B functions in the brain is unknown but we do know that 5-HT2B is associated with behavioral disorders such as reduced grooming, non-rapid eye movement sleep regulation etc.11
B. Regulation of gastrointestinal motality – In mice, of the 5-HT receptors, 5-HT2B mRNA was the only found in the interstitial cells of cajal (ICC). In mice, 5-HT is found to regulate ICC proliferation through 5-HT2B .12 ICC regulation is necessary for the proper function of the gastrointestinal (GI) system as it generates slow wave electrical activity, that causes phasic contraction of the GI tract. The GI system is able to process and transport food via the control of the motor patterns of smooth muscles which is ultimately in part controlled by the ICC.2,13 Thus 5-HT2B is known to play key roles in intestinal disorders like irritable bowel syndrome (IBS). It has also been proposed the effects of 5-HT in the human colon are mediated by 5-HT2B receptor.14
C. Cardiovascular regulation – 5-HT2B plays a vital role in the regulation of cardiovascular functions and development both at the embryonic stage and adulthood. Thus irregular levels of 5-HT2B receptor concentration may lead to vascular pathologies which will be discussed further in the later section of this review.15
D. 5-HT transport system regulation – Plasma serotonin level are controlled by 5-HT2B receptors. Studies of serotonin plasma level in mice that had acute agonist and antagonist stimulation revealed serotonin levels increased with agonist stimulation and decreased with antagonist stimulation.16
There is a large phenogenetic similarity between members of the 5-HT2 family due to the presence of highly conserved motifs within the seven transmembrane regions.5 The human 5-HT2B receptor shows 58% homology to 5-HT2A and 51% homology to 5-HT2C in overall sequence identity, however in the transmembrane regions homology ranges between 68-79%.2,17 Although the homology among the subtypes are relatively close and the binding properties are similar, binding of the 5-HT2B is distinctly different. This was observed in a study by Chan and Eglen et.al where SB 204741, a 5-HT2B antagonist, had a 20 fold selectivity to human 5-HT2B compared to 5-HT2C. Similarly 5-HT2B receptor had a low affinity for ritanserin and higher affinity for yohimbine than 5-HT2C and 5-HT2A .6,18 This trend in affinity is observed by many 5-HT2 ligands and supports the conclusion above.19
Hence, subtype selectivity becomes an important consideration in the design of drugs to target 5-HT2 receptors. A lack of selectivity will result in residual affinities for undesirable targets causing off-target effects. Additionally, the metabolites of drugs should be devoid of any agonist effect, simply put, drug metabolites cannot bind to other targets causing or triggering a response. Such was the case with the drug flenfluramine (trade name Pondimin) and 3, 4-methylenedioxymethamphetamine (MDMA, “Ecstasy”).
2.1 Valvular Heart Disease
Valvular heart disease (VHD) , a cardiovascular disease (CVD), which refers to disorders or defects of the heart valves, is a major health concern in the United States and worldwide. According to the 2011 report from the American Heart Association, in 2007 alone 2200 Americans die from CVD per day resulting in 150,000 deaths that year. Of the 150,000 Americans who died from CVD, 23,313 died as a result of VHD.20 Valvulopathy is the main cause of human mortality worldwide with costly and invasive surgery as the treatment option. VHD is an iatrogenic induced cardiopathy associated with pharmaceutical agents like Ergot alkaloids, amphetamines/hallucinogens, anorexigens and dopamine agonists acting through a serotonergic pathways involving the activation of 5-HT2B. Evidence of this has emerged from a variety of studies including, experimental, clinical and observational studies. As a result, many drugs for treatment of obesity, depression, psychotherapy, pain etc. have been removed from the market.
Flenfluramine is a serotonin agonist approved by the U.S. Food and Drug Administration (FDA) in 1973 for the treatment of obesity. After reports of VHD and pulmonary hypertension the drug was withdrawn from the market in 1997.21-23 Rothman et al. in 2009 provided evidence supporting the hypothesis that VHD is as a result of the activation of various serotonin receptors, specifically and most importantly 5-HT2B receptor by amphetamine derivative, norfenfluramine, the metabolite of fenfluramine, eliciting a agonist activity.22,24 Receptorome screening by Setola et al. 2003 has also revealed the affinity for the popular amphetamine derivative, 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”), to preferentially bind to and activate 5-HT2B receptors inducing mitogenesis in human heart valve resulting in VHD.25 Reports of other VHD-associated drugs include ergot derivatives like peroglide, methysergide and ergotamine which are used in the treatment of Parkinson's disease, restless leg syndrome and migraines respectively. Due to the structural similarity of these ergot derivatives to serotonin (Figure 1), it is thought that iatrogenic induced VHD is associated with the accumulation of excess serotonin.10,26,27
Fig. 1.
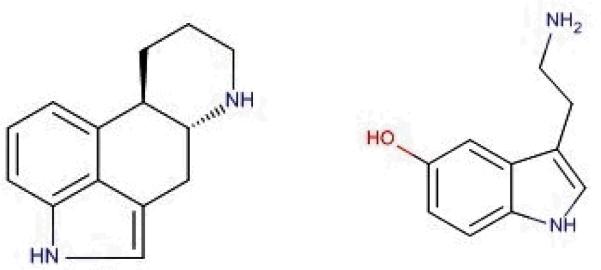
The chemical structure of Ergot (Left) and Serotonin (Right).
Subsequently other drugs of various classes that have a serotonergic mechanism that can activate 5-HT2B receptor may elicit VHD and should be avoided. Thus preclinical screening of the valvulopathogenic actions of drugs is a leading topic of research focus. This review focuses on human 5-HT2B receptor, its role in cardiovascular disease, explicitly valvular heart disease and recent efforts in in silico predictions of drug toxicity prior to clinical trials.
3. MECHANISM OF ACTION
In order to develop a method to predict cardiopathic toxicities, specifically induced-VHD, of drug candidates, we must clearly understand the mechanism of action of 5-HT2B activation by valvulopathic agents. Due to a 300 fold higher concentration of 5-HT2B receptor transcripts in the human heart valves and pulmonary arteries, 5-HT2B is the likely candidate contributing to VHD.28
The following classes of drugs, erogot alkaloids, amphetamines/hallucinogens, anorexigens and dopamine agonists, exert an agonist effect when bound to 5-HT2B receptors. Drugs that target transporter proteins such as serotonin receptor's/transporters (SERTs) are categorized into two types based on their mechanism of action; (1) reuptake inhibitors and (2) substrate-type releasers. Reuptake inhibitors inhibit the transporter-mediated reuptake of neurotransmitters from synapse thereby leading to an increase in the extracellular transmitter concentration. While substrate-type releasers bind to transporter proteins and are transported into the cell cytoplasm.29
There are three proposed mitogenic pathways by which 5-HT2B receptors are activated resulting in VHD 10,24,30;
1. The drug molecules first bind to and activate 5-HT2B receptor in the cell membrane which leads to the dissociation of the G-protein releasing Gαq subunits. Gαq subunit in turn activates phospholipase C-β (PLC-β) causing the activation of protein kinase C (PKC).
2. In addition to the activation of PLC-β, Gαq is also thought to activate Src-P which upregulates the activity of transforming growth factor-β1 (TFG-β1).31
3. Finally the mitogenesis involving the phosphorylation of retinoblastoma protein via activation of 5-HT2B recpetors causing excess cell division and proliferation resulting in valvulopathy.
4. SELECTIVE AGONISTS AND ANTAGONISTS OF 5-HT2BRECEPTOR
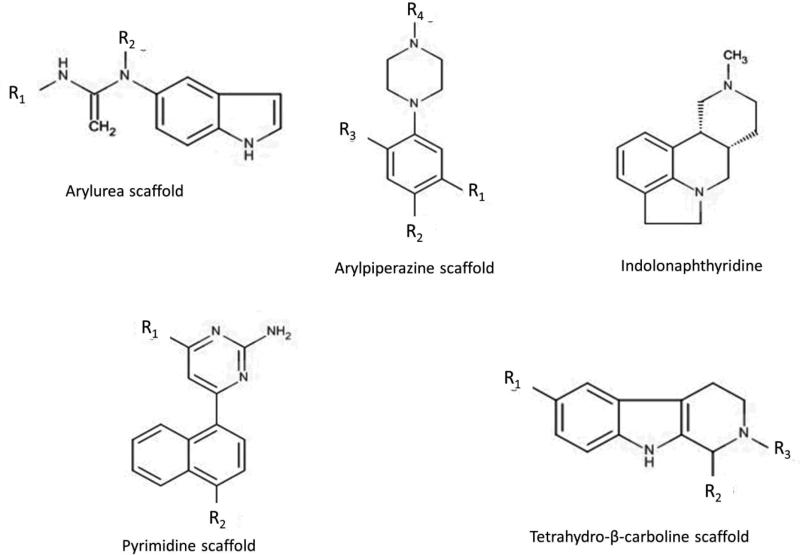
So far little information regarding selective binders of 5-HT2B receptor has been reported, while the number of non-selective agonist and antagonists appears to be large (Table 2). Recently there have been reports of a few molecular scaffolds considered as selective agonists of 5-HT2B receptor. These scaffolds include; (1) arylureas, (2) arylpiperazines, (3) indolonaphthyridines, (4) pyrimidines and tetrahydro-β-carbolines (Figure 3).32 Arylureas have a high affinity for 5-HT2B > 5-HT2C which seems to be caused by the conformational restrictions of the ring system that lowers the affinity for 5-HT2C. On the other hand pyrimidines and some tetrahydro-β-carbolines exhibit a 100 fold selectivity for 5-HT2B compared to 5-HT2A/C.
Table 2.
List of some human 5-HT2B receptor agonists and antagonists and their therapeutic indications.
| 5-HT2B Agents | Name | Structure | Mechanism of action | Therapeutic Indication |
|---|---|---|---|---|
| Fenfluramine22 |
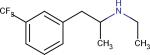
|
Increases Serotonin levels | Controls food intake or suppresses Obesity | |
| Norfenfluramine22 |
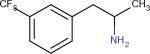
|
Metabolite of Fenfluramine. Increases Serotonin levels | Controls food intake or suppresses Obesity | |
| 3,4-methylenedioxymeth amphetamine (MDMA, “Ecstasy”)25 |
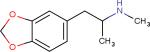
|
Increases Serotonin levels | Posttraumatic stress disorder, Psychotherapy | |
| Alpha-methyl-5 -HT33 |
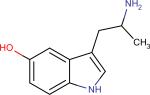
|
Increases Serotonin levels | Its prodrug alpha- methyltryptophan is used for treatment of disorders where serotonin is deficient | |
| AGONISTS | Lysergic acid diethylamide (LSD)3 |
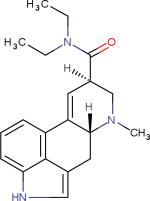
|
5-HT receptor agonist | Psychotherapy, Pain, Alcoholism etc. |
| Carbergoline42 |
|
Dopamine agonist | hyperprolactinemia, Parkinson's disease etc. | |
| Dihydroergotamine42 |
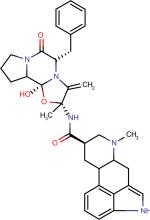
|
Serotonin receptor agonist | Migraine treatment | |
| Methylergonovine42 |
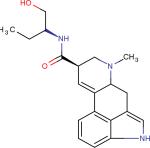
|
Partial agonist/antagoni st on serotonergic, dopaminergic and alpha- adrenergic receptors | Control of postpartum hemorrhage & Migraines | |
| Pergolide42 |

|
Dopamine agonist | Parkinson’s Disease and Hyperprolactinemic Disorders | |
| Agomelatine3 |
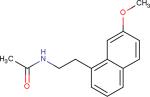
|
MTi and MT2 agonist as well as 5-HT2C antagonist | Antidepressant | |
| ANTAGONI STS | Asenapine3 |

|
Schizophrenia and mania associated with bipolar disorder | |
| Ketanserin3 |
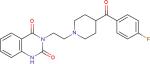
|
alpha-1 adrenergic receptor antagonist | Antihypertensive | |
| Methysergide3 |
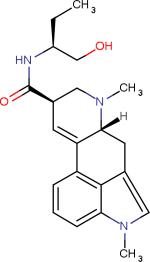
|
5-HT2B receptor agonist | Treatment of Migraines | |
| Ritanserin3 |
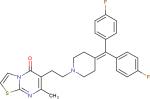
|
5HT2A/2C antagonist | Treatment of schizophrenia | |
| Tegaserod3 |
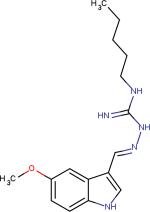
|
5-HT4 agonist | Management of irritable bowel syndrome and constipation | |
| Yohimbine3 |

|
Alpha-2 andrenergic receptor antagonist | Treatment of impotence or erectile dysfunction |
Fig. 3.
Amphetamine (phenyl-isopropylamine) scaffold and examples of amphetamine-derived hallucinogens (MDMA, LSD).
The ergot alkaloid derivatives can be considered privileged structures as their molecular scaffolds (Figure 1) bind to multiple targets thus displaying diverse biological activity.33 Ergot alkaloids such as, Carbergoline34, Methylergonovine and Pergolide35, exhibit interactions with serotonergic, dopaminergic, and adrenergic receptors (Table 2). Not only do these privileged structures act as agonists, they also can act as partial agonists and antagonists as seen with Methylergonovine and Methysergide. As mentioned in section 2.1, there is structural similarity between the ergot derivatives and serotonin. A close analysis of the ergot scaffold reveals a constrained serotonin structure embedded inside.
Hallucinogens are agents that produce hallucinogenic effect and bind to 5-HT2 receptors. There are various classes of classical hallucinogens such as: (1) ergoline, (2) indolealkylamine and (3) phenylalkyamine classes.36 MDMA is a drug of the amphetamine class which are a part of the phenylalkylamine class of hallucinogens, while LSD is a part of the ergoline class (Figure 3).
Nelson et.al and colleagues have studied the structure activity relationship of a large number of phenyl-isopropylamines (Figure 3, 4) and their binding affinities to all three subtypes of 5-HT2 class of receptors. Their findings revealed significant correlations among binding data targeting 5-HT2A, 5-HT2B and 5-HT2C. This information correlates with a study conducted by Porter et. al which ranked the potency of pheny-isoproplyamines at 5-HT2 receptor site to be 5-HT2A > 5-HT2B > 5-HT2C.37 Nelson et. al also reported substitutions of hydrophobic groups at R1 displayed selectivity for 5-HT2A/2C receptors but there was no significant difference in binding affinities for 5-HT2B.38 These findings indicate there are structural differences at the active sites of 5-HT2 receptors that should be taken into account in the development of selective agents for 5-HT2B receptors so as to eliminate the ADRs-like VHD.
Fig. 4.
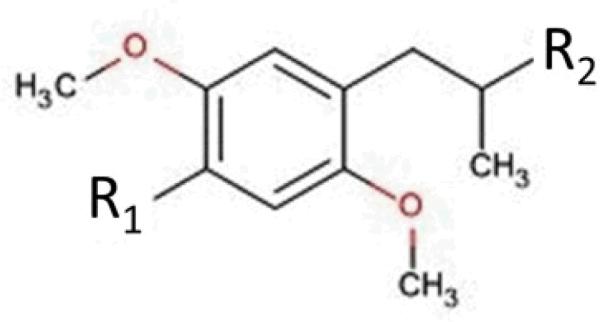
The chemical structure of Phenyl-isopropylamine derivatives.
Rothman et. al., Porter et. al. and Fitzgerald et. al. have all reported norfenfluramines display a significantly higher binding affinity to 5-HT2B receptor when compared to fenfluramines which exhibit a low affinity to all 5-HT2 receptors.28,37,39 The only structural difference between fenfluramines and norfenfluramines is the presence of a secondary amine as opposed to a primary amine respectively (Figure 5).
Fig. 5.
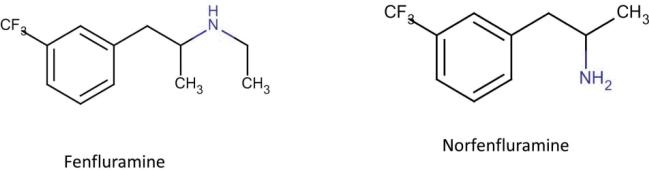
The chemical structure of Fenfluramine derivatives
From the studies mentioned in section 4, it is quite obvious that; (1) even though there is close structural homology between 5-HT2 receptor subtypes there is however structural differences at the active sites of each subtype, and (2) small changes of substituent groups can cause significant differences in selectivity to each subtype.
5. CURRENT IN SILICO STUDIES TO PREDICT DRUG TOXICITY
Increasing evidence suggests that activation of 5-HT2B is the primary cause of drug induced-VHD. It has led to increasing interest in developing analytical methods for determining whether a drug candidate or drug is 5-HT2B active. Stahura et.al proposed the use of high through put (HTS) screening of molecules against biological targets. The disadvantages of this method being: (1) it is expensive and (2) it is time consuming.40 Therefore, fast, simple and efficient new methods to predict adverse drug reactions (ADRs) prior to clinical trials have been under intense research. One option to explore is in silico research which is less expensive to conduct and will ultimately improve the safety and cost of drug development. Thus far and to the best of our knowledge, there have been two in silico studies reported in literature that have proposed methods of predicting 5-HT2B toxicity of investigational drugs as well as drugs currently on the market which we will discuss in detail below.41
5.1. Cheminformatics Studies on 5-HT2B Receptor Ligands
A promising cost effective and efficient method of preselecting 5-HT2B active molecules is with the aid of in silico techniques for conducting virtual screening (VS).
5.1.1. 2D Classification QSAR Studies and Screening
Tropsha et. al. has successfully employed cheminformatic models to accurately identify ( with a 90% hit rate) compounds with a high affinity toward 5-HT2B receptor.42 A dataset of 800 compounds obtained from the Psychoactive Drug Screening Program (PDSP), which consist of 2200 compounds, was the basis for the model generation. Upon completion of the data curation process the data was randomly split into a modeling set (used for model generation) and a validation set (used for validating the model accuracy). An additional independent external validation was conducted on the remaining 1400 compounds from PDSP. QSAR methods for model development included the use of k-Nearest Neighbor algorithm (k-NN), classification based on association (CBA) and distance weighted discrimination (DWD). To ensure the validity and robustness of the models generated Y-randomization tests were included. If y-randomized models resulted in a high correct correlation rate (CCR) it indicates the employed QSAR method is unable generate an acceptable classification model. The most statistically predictive and robust models based on the results of both internal and external datasets were employed for virtual screening of the world drug index (WDI) database, which led to the identification of 122 hit molecules targeting 5-HT2B actives. Of the 122 possible 5-HT2B hits, 10 structurally diverse compounds were chosen for experimental validation of which 9 were experimentally confirmed to be 5-HT2B active. This study suggests the use of cheminformatics as a possible tool to quickly and efficiently proactively screen drug candidates for potential valvulopathy toxicity.
5.1.2. 3D Classification QSAR Studies
Within the same realm of cheminformatics is the use of shape signatures for toxicology modeling.43,44 Welsh et. al. proposed a novel method which utilizes shape-dependent signatures to generate 3-D descriptors which are used to classify molecules with respect to their biological activity. The premise of shape signature is to generate a detailed 3-D representation of the shape of molecules, with the aid of smooth molecular surface triangular algorithm (SMART) and ray tracing algorithm to derive probability distributions that characterize the ray trace and then use the information as compact descriptors of the shape (1D signatures). This approach also includes the use of molecular electrostatic potential (MEP) to define the 2D signature. Thus the 3D molecular features are a composition of the overall molecular shape and distribution of charges of a molecule. Welsh has extended the use of shape signatures 3D molecular descriptors to generate 5-HT2B toxicity models utilizing classification techniques such as k-Nearest Neighbor algorithm (k-NN), Support Vector Machine (SVM) and Kohonen self-organizing maps (SOM). The results of this study exhibited an overall prediction accuracy of the test set ranging from 70-84%. There are advantages of using shape signature approach as opposed to the traditional descriptor-based QSAR methods (Cheminformatics) such as:
1. Shape signature will work for all types of compounds example, organics, inorganics, organometallics, ions etc. Whereas adequate descriptors have not been developed for inorganics and organometallics, thus these molecules are not included in any cheminformatic modelling.45
2. Models generated by shape signatures are much less sensitive to the chemical structures in the training set compared to cheminformatic models. Applicability domain becomes a major concern when accessing QSAR model validity and is one of the six basic principles implemented when using QSARs for regulatory purposes.46,47
3. Models generated from shape signatures once created do not have to be refitted to accommodate for new task.
4. Preprocessing/Data curation is necessary when generating the traditional QSAR model, and it is a time consuming process. No preprocessing of data is necessary for shape signature methods.
5.2. Structure-based Studies on 5-HT2B Receptor and Its Ligands
Some in silico studies are not designed to develop predictive toxicity models for 5-HT2B, instead they are designed to understand 5-HT2B selectivity. As a result of the high homology (~ 80%) experienced between 5-HT2 receptors, there is concern regarding similar affinities for receptor antagonists, specifically between 5-HT2B and 5-HT2C. A good understanding of the subtype selectivity of 5-HT2 receptor subtypes will aid in the design of more selective drug molecules for 5-HT2 subtypes thereby greatly reducing the risk of toxicity by reducing the risk of off-target effects. To attempt to do so without a crystal structure of 5-HT2B receptor is difficult. Kim et. al and colleagues were able to not only predict the structure of 5-HT2B receptor but also the structure of 5-HT2C receptor using MembEnsemb technique.48,49 They have also predicted the binding site, known agonists/antagonists and identified the key residues in the active site that determine the selectivity of 5-HT2B ligands using a GenMSCDock technique. The homology modeling approach employed by Kim et.al involved the use of MembEnsemb Program v.4. The first step was to predict the seven transmembrane (TM) regions of 5-HT2B receptor by analyzing sequence alignments and conducting a hydropathicity analysis. The next step was to then decipher the hydrophobic centers which are integral to maintaining the structural integrity of the TMs. Upon predicting the hydrophobic centers they were then able to attempt to generate a PDB template of 5-HT2B receptor from frog rhodopsin (fRho).50 Structures of known GPCRs exhibit α-helical extensions above the surface membranes which Kim et.al has predicted for the 5-HT2 receptors using a secondary standard structure prediction sever (PSIPRED), located at the N-terminus of TM6 which includes a (D/E)RY motif.51 Further studies were conducted to decipher the role of the ionic locks in the (D/E)RY motif of the TM6 extensions, followed by a helix scan to determine the best packing structure of 5-HT2B. MembEnsemb in combination with BiHelix methods were employed to attain this feat. The loops were generated by CCBB LOOP builder. Upon complete elucidation of the 5-HT2B receptor, 5-HT2C receptor was mutated from the predicted 5-HT2B receptor. From docking studies using GenMSDock, which utilized known agonists and antagonists of 5-HT2B receptor, Kim et.al and colleagues were able to identify the binding sites as well as key interaction that determined 5-HT2B/5-HT2C selectivity. It was concluded that, antagonist selectivity of 5-HT2B receptor involved key interactions with two valine (V103, V190) residues, two leucine (L132, L347) residues and an alanine (A225) residue in the active site. Finally, molecular dynamic simulation of the predicted agonist-5-HT2B and antagonist- 5-HT2B revealed that the state of the (D/E)RY motif was crucial for antagonist or agonist behavior of molecules. The salt bridges in the (D/E)RY motif was maintained for antagonists dynamics and destroyed in agonist dynamics.
More recently Wacker et. al. elucidated the complete crystal structures of 5-HT2B in complex ERG and 5-HT1B/ERG in an effort to investigate the differences in the protein structures that can account for the biased signaling and potential selectivity.52 Three major differences between 5-HT2B and 5-HT1B structures outlined by Wacker et. al. are:
1. At the cytoplasmic end of the receptor subtypes, 5-HT1B helix rotates clockwise towards the active-site while this effect is absent in 5-HT2B.
2. An important motif of 5-HT receptors is D(E)/RY which makes a salt bridge between D(E) and R. While the salt bridge is maintained by 5-HT2B receptor it broken in the 5-HT1B receptor structure.
3. Also structural differences in the extracellular loop region of both subtypes can explain their selectivity. An observed kink in the 5-HT2B loop that is stabilized by a water molecule via hydrogen bonding causes additional conformational changes within the protein.
6. CONCLUSIONS
There has been a great concern regarding ADRs, specifically occurrence of drug induced valvulopathy, associated with 5-HT2B agonists. This is a legitimate and important concern for pharmaceutical industry as drugs designed to target 5-HT receptors for the treatment pain, psychotherapy and obesity etc. are blockbuster drugs. With the relatively new discovery of the role that 5-HT2B receptors play in bringing about various cardiopathies, development of cost effective and efficacious methods for preclinical screening of the valvulopathogenic actions of drugs is a topic of research focus currently. An area of considerable interest in drug discovery research is in silico methods of modeling toxicity-related proteins like 5-HT2B to identify ADRs early in the drug discovery process. This review has highlighted three in silico approaches thus far that have been successful in either predicting 5-HT2B toxicity of molecules or identifying important interactions between 5-HT2B and drug molecules that bring about toxicities. We hope this review will bring awareness to the current issues of predicting drug induced cardiotoxicities and highlight the fact that limited research is being conducted in this area.
Fig. 2.
The chemical scaffolds of selective 5-HT2B binders.
ACKNOWLEDGEMENT
This research project was supported in part by the National Institutes of Health Administrative Supplements for U.S. - China Biomedical Collaborative Research (5P30A1087714-02); the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number G12MD007597; and the District of Columbia Developmental Center for AIDS Research (P30AI087714). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERNCES
- 1.Filmore D. It's a GPCR world. Modern Drug Discovery. 2004;7(11):24–28. [Google Scholar]
- 2.Brea J, Castro-Palomino J, Yeste S, Cubero E, Parraga A, Dominguez E, Loza MI. Emerging opportunities and concerns for drug discovery at serotonin 5-HT2B receptors. Curr. Top. Med. Chem. 2010;10(5):493–503. doi: 10.2174/156802610791111524. [DOI] [PubMed] [Google Scholar]
- 3.Pytliak M, Vargova V, Mechirova V, Felsoci M. Serotonin receptors - from molecular biology to clinical applications. Physiol Res. 2011;60(1):15–25. doi: 10.33549/physiolres.931903. [DOI] [PubMed] [Google Scholar]
- 4.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 1994;46(2):157–203. [PubMed] [Google Scholar]
- 5.Nichols DE, Nichols CD. Serotonin receptors. Chem. Rev. 2008;108(5):1614–1641. doi: 10.1021/cr078224o. [DOI] [PubMed] [Google Scholar]
- 6.Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, Chan HW, Eglen RM. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br. J. Pharmacol. 1995;115(4):622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi DS, Birraux G, Launay JM, Maroteaux L. The human serotonin 5-HT2B receptor: pharmacological link between 5-HT2 and 5-HT1D receptors. FEBS Lett. 1994;352(3):393–399. doi: 10.1016/0014-5793(94)00968-6. [DOI] [PubMed] [Google Scholar]
- 8.Choi DS, Maroteaux L. Immunohistochemical localisation of the serotonin 5-HT2B receptor in mouse gut, cardiovascular system, and brain. FEBS Lett. 1996;391(1-2):45–51. doi: 10.1016/0014-5793(96)00695-3. [DOI] [PubMed] [Google Scholar]
- 9.Duxon MS, Flanigan TP, Reavley AC, Baxter GS, Blackburn TP, Fone KC. Evidence for expression of the 5-hydroxytryptamine-2B receptor protein in the rat central nervous system. Neuroscience. 1997;76(2):323–329. doi: 10.1016/s0306-4522(96)00480-0. [DOI] [PubMed] [Google Scholar]
- 10.Smith SA, Waggoner AD, de las FL, Davila-Roman VG. Role of serotoninergic pathways in drug-induced valvular heart disease and diagnostic features by echocardiography. J. Am. Soc. Echocardiogr. 2009;22(8):883–889. doi: 10.1016/j.echo.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doly S, Valjent E, Setola V, Callebert J, Herve D, Launay JM, Maroteaux L. Serotonin 5-HT2B receptors are required for 3,4-methylenedioxymethamphetamine-induced hyperlocomotion and 5-HT release in vivo and in vitro. J. Neurosci. 2008;28(11):2933–2940. doi: 10.1523/JNEUROSCI.5723-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wouters MM, Gibbons SJ, Roeder JL, Distad M, Ou Y, Strege PR, Szurszewski JH, Farrugia G. Exogenous serotonin regulates proliferation of interstitial cells of Cajal in mouse jejunum through 5-HT2B receptors. Gastroenterology. 2007;133(3):897–906. doi: 10.1053/j.gastro.2007.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Sanders KM, Koh SD, Ward SM. Interstitial cells of cajal as pacemakers in the gastrointestinal tract. Annu. Rev. Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 14.Borman RA, Tilford NS, Harmer DW, Day N, Ellis ES, Sheldrick RL, Carey J, Coleman RA, Baxter GS. 5-HT(2B) receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br. J. Pharmacol. 2002;135(5):1144–1151. doi: 10.1038/sj.bjp.0704571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffre F, Bonnin P, Callebert J, Debbabi H, Setola V, Doly S, Monassier L, Mettauer B, Blaxall BC, Launay JM, Maroteaux L. Serotonin and angiotensin receptors in cardiac fibroblasts coregulate adrenergic-dependent cardiac hypertrophy. Circ. Res. 2009;104(1):113–123. doi: 10.1161/CIRCRESAHA.108.180976. [DOI] [PubMed] [Google Scholar]
- 16.Callebert J, Esteve JM, Herve P, Peoc'h K, Tournois C, Drouet L, Launay JM, Maroteaux L. Evidence for a control of plasma serotonin levels by 5-hydroxytryptamine(2B) receptors in mice. J. Pharmacol. Exp. Ther. 2006;317(2):724–731. doi: 10.1124/jpet.105.098269. [DOI] [PubMed] [Google Scholar]
- 17.Schmuck K, Ullmer C, Engels P, Lubbert H. Cloning and functional characterization of the human 5-HT2B serotonin receptor. FEBS Lett. 1994;342(1):85–90. doi: 10.1016/0014-5793(94)80590-3. [DOI] [PubMed] [Google Scholar]
- 18.Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 19.Baxter GS. Novel discriminatory ligands for 5-HT2B receptors. Behav. Brain Res. 1996;73(1-2):149–152. doi: 10.1016/0166-4328(96)00087-3. [DOI] [PubMed] [Google Scholar]
- 20.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de SG, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics--2011 update: a report from the American Heart Association. Circulation. 2011;123(4):e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Connolly HM, Crary JL, McGoon MD, Hensrud DD, Edwards BS, Edwards WD, Schaff HV. Valvular heart disease associated with fenfluramine-phentermine. N. Engl. J. Med. 1997;337(9):581–588. doi: 10.1056/NEJM199708283370901. [DOI] [PubMed] [Google Scholar]
- 22.Rothman RB, Baumann MH. Serotonergic drugs and valvular heart disease. Expert. Opin. Drug Saf. 2009;8(3):317–329. doi: 10.1517/14740330902931524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardiac Valvulopathy Associated with Exposure to Fenfluramine or Dexgenfluramine. Centers For Disease Control and Prevention; Nov 4, p. 97. [Google Scholar]
- 24.Roth BL. Drugs and valvular heart disease. N. Engl. J. Med. 2007;356(1):6–9. doi: 10.1056/NEJMp068265. [DOI] [PubMed] [Google Scholar]
- 25.Setola V, Hufeisen SJ, Grande-Allen KJ, Vesely I, Glennon RA, Blough B, Rothman RB, Roth BL. 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) induces fenfluramine-like proliferative actions on human cardiac valvular interstitial cells in vitro. Mol. Pharmacol. 2003;63(6):1223–1229. doi: 10.1124/mol.63.6.1223. [DOI] [PubMed] [Google Scholar]
- 26.Pritchett AM, Morrison JF, Edwards WD, Schaff HV, Connolly HM, Espinosa RE. Valvular heart disease in patients taking pergolide. Mayo Clin. Proc. 2002;77(12):1280–1286. doi: 10.4065/77.12.1280. [DOI] [PubMed] [Google Scholar]
- 27.Donnelly KB. Cardiac valvular pathology: comparative pathology and animal models of acquired cardiac valvular diseases. Toxicol. Pathol. 2008;36(2):204–217. doi: 10.1177/0192623307312707. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol. Pharmacol. 2000;57(1):75–81. [PubMed] [Google Scholar]
- 29.Rothman RB, Baumann MH. Therapeutic and adverse actions of serotonin transporter substrates. Pharmacol. Ther. 2002;95(1):73–88. doi: 10.1016/s0163-7258(02)00234-6. [DOI] [PubMed] [Google Scholar]
- 30.Elangbam CS. Drug-induced valvulopathy: an update. Toxicol. Pathol. 2010;38(6):837–848. doi: 10.1177/0192623310378027. [DOI] [PubMed] [Google Scholar]
- 31.Jian B, Xu J, Connolly J, Savani RC, Narula N, Liang B, Levy RJ. Serotonin mechanisms in heart valve disease I: serotonin-induced up-regulation of transforming growth factor-beta1 via G-protein signal transduction in aortic valve interstitial cells. Am. J. Pathol. 2002;161(6):2111–2121. doi: 10.1016/s0002-9440(10)64489-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poissonnet G, Parmentier JG, Boutin JA, Goldstein S. The emergence of selective 5-HT 2B antagonists structures, activities and potential therapeutic applications. Mini. Rev. Med. Chem. 2004;4(3):325–330. doi: 10.2174/1389557043487312. [DOI] [PubMed] [Google Scholar]
- 33.DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA. Privileged structures: applications in drug discovery. Comb. Chem. High Throughput. Screen. 2004;7(5):473–494. doi: 10.2174/1386207043328544. [DOI] [PubMed] [Google Scholar]
- 34.Sharif NA, McLaughlin MA, Kelly CR, Katoli P, Drace C, Husain S, Crosson C, Toris C, Zhan GL, Camras C. Cabergoline: Pharmacology, ocular hypotensive studies in multiple species, and aqueous humor dynamic modulation in the Cynomolgus monkey eyes. Exp. Eye Res. 2009;88(3):386–397. doi: 10.1016/j.exer.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E. Dopamine agonists and the risk of cardiac-valve regurgitation. N. Engl. J. Med. 2007;356(1):29–38. doi: 10.1056/NEJMoa062222. [DOI] [PubMed] [Google Scholar]
- 36.Glennon RA. Classical hallucinogens: an introductory overview. NIDA Res. Monogr. 1994;146:4–32. [PubMed] [Google Scholar]
- 37.Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br. J. Pharmacol. 1999;128(1):13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson DL, Lucaites VL, Wainscott DB, Glennon RA. Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, -HT(2B) and 5-HT2C receptors. Naunyn Schmiedebergs Arch. Pharmacol. 1999;359(1):1–6. doi: 10.1007/pl00005315. [DOI] [PubMed] [Google Scholar]
- 39.Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102(23):2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- 40.Stahura FL, Bajorath J. Virtual screening methods that complement HTS. Comb. Chem. High Throughput. Screen. 2004;7(4):259–269. doi: 10.2174/1386207043328706. [DOI] [PubMed] [Google Scholar]
- 41.Scheiber J, Jenkins JL, Sukuru SC, Bender A, Mikhailov D, Milik M, Azzaoui K, Whitebread S, Hamon J, Urban L, Glick M, Davies JW. Mapping adverse drug reactions in chemical space. J. Med. Chem. 2009;52(9):3103–3107. doi: 10.1021/jm801546k. [DOI] [PubMed] [Google Scholar]
- 42.Hajjo R, Grulke CM, Golbraikh A, Setola V, Huang XP, Roth BL, Tropsha A. Development, validation, and use of quantitative structure-activity relationship models of 5-hydroxytryptamine (2B) receptor ligands to identify novel receptor binders and putative valvulopathic compounds among common drugs. J. Med. Chem. 2010;53(21):7573–7586. doi: 10.1021/jm100600y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chekmarev DS, Kholodovych V, Balakin KV, Ivanenkov Y, Ekins S, Welsh WJ. Shape signatures: new descriptors for predicting cardiotoxicity in silico. Chem. Res. Toxicol. 2008;21(6):1304–1314. doi: 10.1021/tx800063r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zauhar RJ, Moyna G, Tian L, Li Z, Welsh WJ. Shape signatures: a new approach to computer-aided ligand- and receptor-based drug design. J. Med. Chem. 2003;46(26):5674–5690. doi: 10.1021/jm030242k. [DOI] [PubMed] [Google Scholar]
- 45.Fourches D, Muratov E, Tropsha A. Trust, but verify: on the importance of chemical structure curation in cheminformatics and QSAR modeling research. J. Chem. Inf. Model. 2010;50(7):1189–1204. doi: 10.1021/ci100176x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tunkel J, Mayo K, Austin C, Hickerson A, Howard P. Practical considerations on the use of predictive models for regulatory purposes. Environ. Sci. Technol. 2005;39(7):2188–2199. doi: 10.1021/es049220t. [DOI] [PubMed] [Google Scholar]
- 47.Jaworska J, Nikolova-Jeliazkova N, Aldenberg T. QSAR applicabilty domain estimation by projection of the training set descriptor space: a review. Altern. Lab Anim. 2005;33(5):445–459. doi: 10.1177/026119290503300508. [DOI] [PubMed] [Google Scholar]
- 48.Bray JK, Goddard WA., III The structure of human serotonin 2c G-protein-coupled receptor bound to agonists and antagonists. J. Mol. Graph. Model. 2008;27(1):66–81. doi: 10.1016/j.jmgm.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 49.Kim SK, Li Y, Abrol R, Heo J, Goddard WA., III Predicted structures and dynamics for agonists and antagonists bound to serotonin 5-HT2B and 5-HT2C receptors. J. Chem. Inf. Model. 2011;51(2):420–433. doi: 10.1021/ci100375b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martí-Renom MA, Stuart AC, Fiser A, Sanchez R, Melo F, Sali A. COMPARATIVE PROTEIN STRUCTURE MODELING OF GENES AND GENOMES. Annu. Rev. Biophys. Biomol. Struct. 2000;(29):291–325. doi: 10.1146/annurev.biophys.29.1.291. [DOI] [PubMed] [Google Scholar]
- 51.Bryson K, McGuffin LJ, Marsden RL, Ward JJ, Sodhi JS, Jones DT. Protein structure prediction servers at University College London. Nucleic Acids Res. 2005;33:W36–W38. doi: 10.1093/nar/gki410. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wacker D, Wang C, Katritch V, Han GW, Huang XP, Vardy E, McCorvy JD, Jiang Y, Chu M, Siu FY, Liu W, Xu HE, Cherezov V, Roth BL, Stevens RC. Structural Features for Functional Selectivity at Serotonin Receptors. Science. 2013 doi: 10.1126/science.1232808. [DOI] [PMC free article] [PubMed] [Google Scholar]