Abstract
Spinal cord injury (SCI) results in loss of sensory and motor function below the level of injury and has limited available therapies. Multiple channel bridges have been investigated as a means to create a permissive environment for regeneration, with channels supporting axonal growth through the injury. Bridges support robust axon growth with myelination of the axons, and herein we investigated the cell types that are myelinating the axons and whether trophic factors can enhance myelination. Lentivirus encoding for neurotrophin-3 (NT3), sonic hedgehog (SHH) and the combination of these factors was delivered from bridges implanted into a lateral hemisection defect at T9/T10 in mice, and the response of endogenous progenitor cells within the spinal cord was investigated. Relative to control, the localized sustained expression of these factors significantly increased growth of regenerating axons into the bridge and enhanced axon myelination 8 weeks after injury. SHH decreased Sox2+ cells and increased Olig2+ cells, whereas NT3 alone or in combination with SHH enhanced GFAP+ and Olig2+ cells relative to control. For delivery of lentivirus encoding for either factor, we identified cells at various stages of differentiation along the oligodendrocyte lineage (e.g., O4+, GalC+). Expression of NT3 enhanced myelination primarily by infiltrating Schwann cells, whereas SHH over-expression substantially increased myelination by oligodendrocytes. Gene delivery represents a promising tool to direct activation and differentiation of endogenous progenitor cells for applications in regenerative medicine.
Introduction
Regeneration of spinal cord tissue can occur after injury when presented with inductive cues. Towards this goal, we have developed implantable bridges to support the long-distance growth of spared axons, defined as axons near the injury that did not undergo Wallerian-type degeneration, through the injury1–4, and the delivery of neurotrophic factors promotes the additional extension of axons into the injury5; yet, these regenerating axons must be myelinated in order to appropriately relay the electrical signals needed to restore motor and sensory function. Following injury, myelinating cells in the spinal cord consist of Schwann cells and oligodendrocytes. Schwann cells from the peripheral nervous system (PNS) rapidly invade the spinal cord after injury and can spontaneously remyelinate spared axons in the central nervous system (CNS); however, Schwann cells generally do not form myelin sheaths thick enough to restore axonal conductance6,7. In contrast, oligodendrocytes, which normally produce myelin in the CNS, can myelinate multiple spared axons to enhance and integrate their conductance8. However, remyelination by mature oligodendrocytes rarely occurs, as few mature, pre-existing oligodendrocytes survive after spinal cord injury (SCI)9–11 and are not capable of proliferation or extensive migration11. Instead, remyelination by CNS-derived cells is typically mediated by oligodendrocyte progenitors (Olig2+, NG2+), which reside in the adult spinal cord and are activated in response to injury12–17. However, myelination by oligodendrocyte progenitors is significantly hindered by the lack of pro-oligodendrogenic factors18 and the abundance of factors that inhibit differentiation18,19 in the tissue microenvironment following SCI.
Recent strategies to enhance the myelination of axons after SCI have targeted endogenous oligodendrocyte progenitors18–23. Oligodendrocyte myelination requires the migration, proliferation and differentiation of progenitors near to the injury12,13,16,17. Sonic hedgehog (SHH)22–26 and neurotrophin-3 (NT3)27–31 have been identified as factors that enhance the proliferation and differentiation of oligodendrocyte progenitors in vitro and in vivo. In addition, SHH promotes sparing of both neurons32,33 and white matter20 after CNS injury. SHH is also chemoattractant for neural-lineage progenitors during CNS development34 and after injury35. NT3 is known to promote neuronal survival, sprouting of spared axons and regeneration of injured axons after injury5,27,31. Furthermore, NT3 amplifies the proliferation of oligodendrocyte progenitors in vivo30,31. These reports reveal inductive factors can increase progenitor presence after SCI; however, the ability of these endogenous progenitors to differentiate into mature oligodendrocytes and subsequently myelinate large numbers of regenerating axons remains unknown.
In this report, we investigated NT3 and SHH individually and in combination for their ability to enhance the recruitment, proliferation and differentiation of oligodendrocyte progenitors and their ability to myelinate large numbers of regenerating axons that are growing through a biomaterial bridge implanted into the injury. Previously, we fabricated multiple channel bridges from biodegradable poly(lactide-co-glycolide) (PLG) containing a high density of linear channels that enabled the guidance of regenerating axons across the injury site when implanted into a lateral hemisection lesion in the mouse spinal cord2,36. In addition, the bridge contains a network of interconnected pores that permitted the rapid infiltration of multiple cell types from the adjacent tissue1–4. Functionalization of these bridges with lentivirus provided robust, sustained transgene expression after SCI5,36,37. Axons are completely severed in the lesion before bridge implantation; thus axons extending through the bridge can be attributed to regeneration of injured axons or sprouting of contralateral axons and are not spared axons originating from the injury site. The capacity of these axons regenerating through the injury to contribute to restoration of circuitry within the spinal cord will require remyelination to establish conduction; therefore, our analysis focused on the recruitment and differentiation of endogenous glial progenitor cells within the bridge implants, and on the extent and source of myelination within the bridge. Using a mouse model, lentiviral delivery was employed to obtain localized and persistent expression of SHH and/or NT3, and histology was employed to determine the characteristics of the regenerating tissue. Lentiviral delivery allowed for sustained expression of one or more factors for the duration of the study. Furthermore, expression is greatest within the implant, with decreasing concentrations in adjacent spinal cord segments that creates a concentration gradient that may direct cell migration towards the implant. The ability for the biomaterial and localized gene delivery to modulate the local environment represents a significant advance towards regenerating spinal circuitry through the injured spinal cord.
Results
Transgene expression in the spinal cord
Initial studies characterized the location of transgene expression following bridge-mediated lentivirus delivery. Bridges were implanted into a lateral hemisection model at T9-T10 (Fig. 1a, b), with extensive cell infiltration and integration with the host tissue1–4. Additionally, delivery of lentivirus from these bridges has promoted transgene expression that exceeded 60 days36. Lentiviral particles (~5 × 107 LP) encoding Firefly luciferase (FLuc) were delivered from PLG bridges and localization of transgene expression was assessed 4 weeks after injury. Upon extraction of the spinal cord, more than half of the transgene expression resided within the implantation site (Fig. 1c). Additionally, over 80% of transgene expression was within two vertebrae adjacent to the implantation site. This distribution of transgene expression demonstrates the ability to locally deliver gene therapy vectors. We confirmed that expression is sustained over at least 60 days (8 weeks) when two lentiviral vector encoding for different transgenes (FLuc and green fluorescent protein, GFP) were simultaneously delivered from a single bridge platform (Fig. 1d). Co-expression of both transgenes was confirmed by immunohistochemistry in spinal cords extracted 8 weeks after injury (Fig. 1e, f). Most cells expressing either GFP or FLuc expressed both transgenes.
Figure 1.
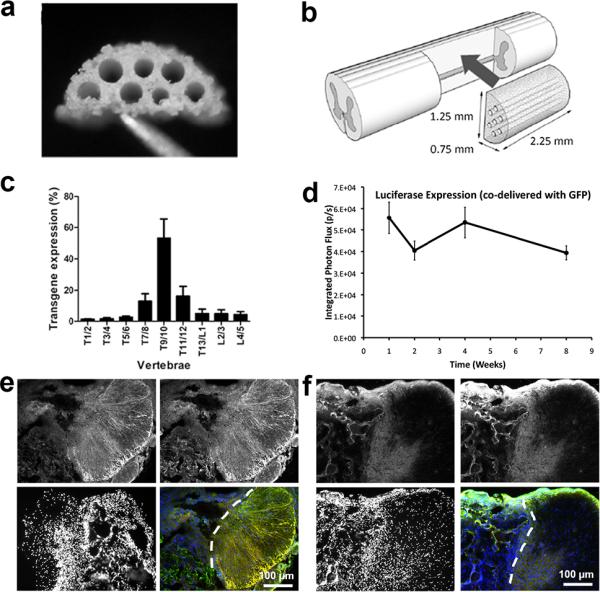
(a) PLG bridge. (b) Schematic of bridge implantation into lateral hemisection injury. (c) Distribution of transgene expression along the spinal cord 4 weeks after injury (mean +/− SD). Note the bridge is implanted at T9/10. (d) Sustained expression of FLuc in vivo over 8 weeks with simultaneous delivery of GFP and FLuc lentiviral vectors from the bridge. Background signal remained less than 1×103 photons/s during all measurements (e,f) Immunohistochemistry of spinal cord tissues extracted 8 weeks after injury with co-delivery of GFP and FLuc vectors (e) or bridge alone with no lentiviral delivery (f). Panels in (e) and (f) show staining for FLuc (upper left, red), GFP (upper right, green) and Hoescht (lower left, blue). are shown in the (green - GFP, upper right; red - FLuc, upper left; blue - Hoescht, lower right). Bottom right panels show overlaid images, where cell co-expressing GFP and FLuc appear yellow. Dashed lines denote the interface between bridge (left) implants and spinal cord tissue (right).
Recruitment and differentiation of endogenous progenitors
Lentivirus from the bridge was subsequently employed to express NT3, SHH or a combination of these factors in order to modulate the tissue microenvironment within and adjacent to the implantation site. An analysis of sparing of grey and white matter contra-lateral to the injury was performed. The contralateral tissue had damage to both the grey and white matter in all conditions, and expression of NT3, SHH, or their combination did not significantly impact the extent of damage to the grey and white matter in the contralateral tissue. Here, we define “damaged” tissue as tissue that no longer presents the normal morphology of healthy spinal cord tissue. In healthy spinal cord, the “butterfly” shape of the grey matter is visible and myelinated axon bundles are distributed throughout the white matter. In addition, the total cell number in the bridges was similar for all conditions (Supp. Fig. 1).
Staining of tissue sections for adult spinal cord, neural lineageprogenitors (Sox2+) were present throughout the bridge implants (Fig. 2a-e). In the case of SHH over-expression, a significantly decreased number of Sox2+ cells was observed relative to delivery of the control vector (FLuc) (Fig. 2f). NT3 over-expression exhibited a trend toward reducing the number of Sox2+ cells relative to control, though the decrease was not significant. The combination of SHH and NT3 over-expression produced levels of Sox2+ cells that were approximately similar to controls. Sox2+ progenitor cells in the process of differentiating along astrocyte or oligodendrocyte lineages may co-express glial fibrillary acidic protein (GFAP) and cytoplasmic Olig238–41 or nuclear Olig216,42, respectively. Thus, we assessed co-localization of Sox2 and these markers. Overlap of these markers within single cells, as visualized from widefield fluorescence imaging, was confirmed using confocal microscopy (Supp. Fig. 2). The percentage of Sox2+ cells that were also GFAP+ and/or Olig2+ was similar across experimental conditions (Fig. 2g), with uncommitted neural progenitor cells (Sox2+/GFAP-/Olig2-) trending toward the greatest percentage of Sox2+ cells with SHH or NT3/SHH expression. Likewise, there were no significant differences in the density of Sox2+ cells that were also GFAP+ and/or Olig2+ across experimental conditions (Fig. 2h). However, the data again indicate a trend towards increased presence of uncommitted neural progenitors. Finally, we note that a trend towards increased proliferation of Sox2+ cells with expression of NT3 or NT3/SHH in combination, though the levels were not significant (Supp. Fig. 3a).
Fig. 2.
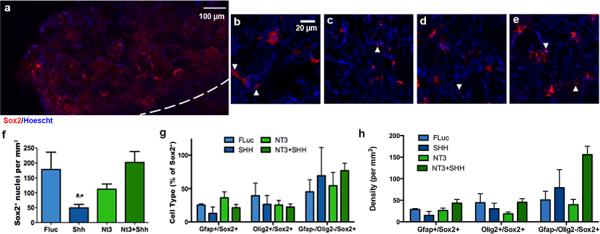
Sox2+(red)/Hoescht+(blue) nuclei 8 wks after injury. (a) Immunofluorescence of Sox2+ cells from bridge implants delivering lentivirus encoding SHH. Dashed line indicates border between bridge implant and host tissue. Sox2+ immunofluorescence at higher magnification from bridges delivering (b) FLuc, (c) SHH, (d) NT3, or (e) NT3 and SHH. Arrows indicate Sox2+ nuclei. (f) Quantification of Sox2+ nuclei in FLuc, NT3, SHH and NT3+SHH conditions (mean +/− SD). (g) Quantification of sub-populations of Sox2+/Hoescht+ nuclei (co-expression of GFAP or Olig2) as a percentage of total Sox2+/Hoescht+ cells (mean +/− SD). (h) Quantification of sub-population densities of Sox2+/Hoescht+ nuclei (co-expression of GFAP or Olig2) (mean +/− SD).
Presence of glial-restricted precursors was investigated within the bridge area by immunostaining for Olig2 (Fig. 3a-e). Over-expression of SHH, NT3, or their combination increased the number of cells expressing nuclear Olig2 by 5 to 9-fold relative to control (Fig. 3f). As Olig2 expression was confined to the nucleus in the majority of Olig2+ cells, the percentage of both GFAP+/Olig2+ and Sox2+/Olig2+ cells was relatively unaffected by expression of any factor. However, a trend was observed for increased percentage of GFAP-/Sox2−/Olig2+ cells with expression of SHH, NT3, or their combination (Fig. 3g). Similarly, no significant differences were observed in the densities of GFAP+/Olig2+, Sox2+/Olig2+ or GFAP-/Sox2-/Olig2+ cells across conditions (Fig. 3h). However, there were trends indicating the presence of fewer Sox2+/Olig2+ cells in NT3 only case and more GFAP−/Sox2−/Olig2+ cells in the SHH only case. GFAP−/Sox2−/Olig2+ likely represent oligodendroctye precursor cells or immature oligodendrocytes43. The number of Olig2+/Ki67+ cells (proliferative oligodendrocyte precursors) demonstrated a trend toward increased proliferation in the presence of SHH, NT3, or the combination (≈3 to 5 fold increase relative to FLuc) (Supp. Fig. 3b). Consistent with Olig2 staining, staining for NG2 was also observed within the bridges, which stains glia-restricted progenitors, yet may also stain other infiltrating cell types, such as meningeal fibroblasts, non-myelinating Schwann cells, and macrophages (Supp. Fig. 4ad)44,45. In addition, markers for oligodendrocyte progenitors at later stages of maturation were also observed, such as O4 (Supp. Fig. 4eh) and galactosylceramidase (GalC) (Supp. Fig. 4i-l), which stain immature oligodendrocytes and immature and mature oligodendrocytes respectively.
Fig. 3.
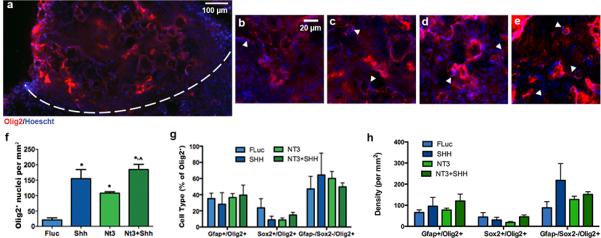
Olig2+(red)/Hoescht+(blue) nuclei 8 wks after injury. (a) Immunofluorescence of Olig2+ cells from bridge implants delivering lentivirus encoding SHH. Dashed line indicates border between bridge implant and host tissue. Olig2+ Immunofluorescence at higher magnification from bridges delivering (b) FLuc, (c) SHH, (d) NT3, or (d) NT3 and SHH. Arrows indicate Olig2+ nuclei. Brightness and contrast were adjusted for clarity. (e) Quantification of Olig2+ nuclei in FLuc, NT3, SHH and NT3+SHH conditions (mean +/−SD). (f) Quantification of sub-populations of Olig2+/Hoescht+ nuclei (co-expression of GFAP or Sox2) as a percentage of total Olig2+/Hoescht+ cells (mean +/− SD). (h) Quantification of sub-population densities of Olig2+/Hoescht+ nuclei (co-expression of GFAP or Sox2) (mean +/− SD).
Astrocytes were identified by staining for GFAP, which indicated these cells were present at the injury (Fig. 4a-e), primarily at the interface of the bridge and host tissue. The density of GFAP+ cells in the bridge significantly increased when NT3 was over-expressed, regardless of whether SHH was also over-expressed (Fig. 4f). The number of Ki67+/GFAP+ cells (proliferative astrocytes, astrocyte precursors or proliferative neural progenitors) also significantly increased when NT3 was overexpressed (Supp. Fig. 3c), again independent of SHH delivery. In the scar adjacent to the bridge, the presence of GFAP was unaffected by factor delivery. As numbers of GFAP+/Sox2+/Olig2− cells (astrocyte precursors) remained low and unvarying across conditions (Fig. 2g, 2h), the additional GFAP+ cells with NT3 expression were most likely mature, reactive astrocytes.
Fig. 4.
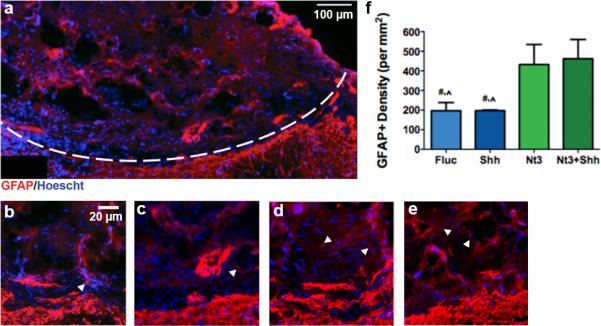
GFAP+ cells (red) 8 wks after injury. (a) Immunofluorescence of GFAP+ cells from bridge implants delivering lentivirus encoding SHH. Dashed line indicates border between bridge implant and host tissue. GFAP+ immunofluorescence at higher magnification from bridges delivering (a) FLuc, (b) SHH, (c) NT3, or (d) NT3 and SHH. Interface between bridge and contralateral tissue is visible in each image. Arrows indicate GFAP+ cells. Brightness and contrast were adjusted for clarity. (e) Quantification of GFAP+ cells in FLuc, NT3, SHH and NT3+SHH conditions (mean +/− SD).
Axon regeneration, myelination, and sparing
Both unmyelinated (neurofilament-160, NFM+) and myelinated (NFM+/myelin basic protein, MBP+) nerve fibers were present throughout the bridge in control animals that over-expressed Fluc (Fig. 5a-e). NFM+ staining was typically confined to individual, unbundled nerve fibers. Control animals had approximately 1500 neurites/mm2 (Fig. 5f), 17% of which were myelinated (Fig. 5g). This observation has previously been attributed to the bridge architecture, consisting of linear channels and interconnected pores2, which allows for the infiltration of host cells that create a growth inductive environment. Regenerating axons typically appeared bundled as pairs, triplicates or more, consistent with previous reports2,3,5 (Fig. 5b-e). Upon expression of SHH or NT3 alone, the total number of axons that extended into the middle of the bridge significantly increased by approximately 2-fold relative to control (Fig. 5f). Similar to axon number, the number of myelinated axons (NFM+/MBP+) also significantly increased in the presence of either SHH or NT3 by approximately 7-fold and 6-fold respectively, relative to controls. The tandem expression of SHH and NT3 did not further enhance the number of axons or myelinated axons in the bridges. The percentage of myelinated axons, which was determined from the ratio of the number of NFM+/MBP+ axons divided by the number of NFM+ axons, was similar for SHH, NT3, or the combination, and was significantly increased relative to control (Fig. 5g).
Fig. 5.
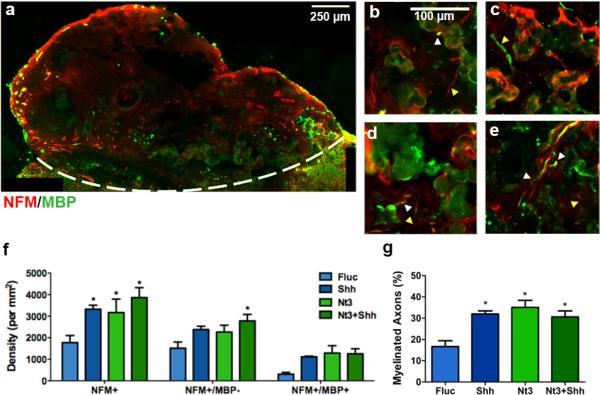
Myelinated axons 8 wks after injury. (a) Immunofluorescence of myelinated (NFM+/MBP+: red/green, respectively) and total (NFM+: red) axons from bridge implants delivering lentivirus encoding SHH. Dashed line indicates border between bridge implant and host tissue. NFM+/MBP+ Immunofluorescence at higher magnification from bridges delivering (b) FLuc, (c) SHH, (d) NT3, or (e) NT3+SHH. White arrows show unmyelinated neurofilaments and yellow arrows show myelinated neurofilaments. Brightness and contrast were adjusted for clarity. (f) Quantification of total axon numbers in FLuc, NT3, SHH and NT3+SHH conditions (mean +/−SD). (g) Percentage of axons that were myelinated (mean +/− SD).
The source of myelination in the bridge was investigated by immunostaining to identify whether remyelinated fibers were ensheathed by Schwann cell (NFM+/MBP+/P0+) (Fig. 6a-e) or oligodendrocyte-derived myelin (NFM+/MBP+/P0−) (Fig. 6f). In control animals, significantly fewer numbers of myelinated axons were observed relative to delivery of NT3 or SHH alone or their combination (Fig. 6f). In the controls, the percentage of these myelinated fibers ensheathed by oligodendrocytes-derived myelin was 31% (Fig. 6g), with the percentage determined from the ratio of the number of MBP+/PO+ axons divided by the number of MBP+ axons,. Over-expression of SHH resulted in significantly greater percentage of oligodendrocyte-myelinated fibers (72%) compared to control (Fig. 6g). Over-expression of NT3 resulted in an increase of both oligodendrocyte and Schwann cell myelin relative to control (Fig. 6f,g), with oligodendrocytes myelinating approximately 40% of myelinated fibers. Furthermore, NT3 expression significantly increased the percentage of Schwann cell-myelinated fibers compared to all other groups (Fig. 6g). NT3 has been shown in vitro to promote the migration of Schwann cells46, which may influence myelination. Additionally, the combination of SHH and NT3 over-expression also increased both Schwann cell and oligodendrocyte-derived myelin relative to control, with 45% of axonal fibers within the bridge implant ensheathed by oligodendrocyte-derived myelin (Fig. 6g). However, the combination of NT3 and SHH delivery resulted in a significantly lower percentage of oligodendrocytemyelinated fibers when compared to SHH alone.
Fig. 6.
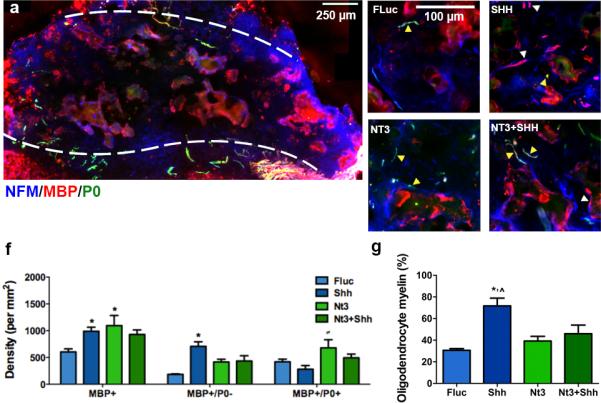
Source of myelination. (a) Widefield view of immunofluorescence of Schwann cell- (NFM+/MBP+/P0+: blue/red/green, respectively) and oligodendrocyte- (NFM+/MBP+/P0−) derived myelin fibers from bridge implants delivering lentivirus encoding SHH. Dashed line indicates border between bridge implant and host tissue. Immunofluorescence at higher magnification from bridges delivering (b) FLuc, (c) SHH, (d) NT3, or (e) NT3+SHH. White arrows show fibers wrapped by Schwann cell-derived myelin and yellow arrows show fibers myelinated by oligodendrocytes. Brightness and contrast were adjusted for clarity. (f) Quantification of total axon numbers in FLuc, NT3, SHH and NT3 and SHH conditions (mean +/− SD). (g) Percentage of axons that were myelinated by oligodendrocytes (mean +/− SD).
Discussion
We investigated myelination of regenerating axons by host cells recruited into multiple channel PLG bridges implanted into a mouse lateral hemisection spinal cord lesion. We have previously reported the ability of the bridge architecture to encourage axon regeneration after SCI due to the enhanced infiltration of multiple cell types and guidance of severed axons through linear channels1–4. Moreover, lentiviral delivery from the bridges resulted in sustained, localized transgene expression capable of altering the injury microenvironment2,5,36. In particular, delivery of lentivirus encoding for NT3 further increased the number of axons extending through the bridge implant5. Notably, lentiviral delivery from PLG bridges provides a platform for long-term, localized production of therapeutic proteins, which is difficult to achieve and often requires the use of osmotic pumps. Unlike other viral vectors, lentivirus does not influence the cellular behavior of progenitors47 nor invoke a significant inflammatory response48. Lentiviral vectors exhibit the same physical properties, independent of the encoding gene. Therefore, in contrast to protein delivery, the same delivery system can be applied to deliver multiple vectors encoding various regenerative factors without modification to the biomaterial platform. In these studies, lentiviral vectors encoding two distinct transgenes were delivered from PLG bridges and transgene expression altered the local environment for at least 8 wks after injury to promote axonal regeneration and remyelination. This combination of biomaterials and gene delivery technology have provided a means for local delivery of multiple regenerative factors. Furthermore, expression of these factors resulted in a gradient along the spinal cord, with the greatest concentration at the implantation site, which was sustained for at least 60 days. Approximately 50% of transgene expression was localized at the level of the implant (T9/10), with an additional 30% in adjacent segments (T7/8 and T11/12). This steep decrease in expression in either direction along the spinal cord resulted in a gradient of the expressed regenerative factors. Gradients of inductive factors, and specifically of NT-3 and SHH, are well-established for their role in directing cell movement during development34,35,49-52 and maintenance of this gradient may contribute to the recruitment and differentiation of progenitor cells within the spinal cord.
The bridge implants provide a defined space for analysis of progenitor cell recruitment to the injury site. The contribution of endogenous progenitor cells to repair after SCI has been challenging to characterize in many common injury models. However, in these studies the bridges are acellular at the time of implantation so that any cells present in the bridge at the time of extraction must have migrated from the host tissue. Likewise, as axons in the hemisection injury model are completely severed prior to bridge implantation, any axons observed entering the implant space can be attributed to either regeneration of injured axons or novel sprouting of axons from the contralateral tissue. The contribution of these axons to restoring circuitry within the spinal cord requires remyelination of the axons. These studies focused on histological investigation of endogenous progenitor cells and axonal processes that migrate or extend into the bridge from the adjacent spinal cord tissue. Spontaneous rewiring of corticospinal circuits is observed that results from sprouting of spared axons53-57. In contrast, the long-distance regeneration of axons through an injury and subsequent myelination remains a substantial challenge, yet promises to enhance recovery. The analysis of regeneration herein demonstrated the potential of axons to extend through a spinal cord lesion and the potential for influencing the differentiation of endogenous progenitor cells to facilitate tissue repair and restoration of function.
In bridges lacking lentivirus encoding for inductive factors, the abundance of progenitor cells (identified as Sox2+ and/or Olig2+) and proliferating progenitors (co-labeled as Ki67+) within the bridges was relatively low in the absence of inductive factors. Furthermore, the paucity of immature (O4+) and mature (GalC+) oligodendrocytes along with the lack of axonal myelination within the bridges indicates that Sox2+ and Olig2+ progenitor cells did not effectively undergo differentiation into functional, myelinating oligodendrocytes. Results reported herein with the bridge alone are consistent with previous reports that few oligodendrocyte precursors, which are required for remyelination of axons17, are present12–16. This lack of immature oligodendrocytes near the injury may have limited remyelination by glia of CNS origin. Thus, in our control bridges (no lentivirus), comparable levels of myelination by oligodendrocytes and infiltrating Schwann cells were observed.
Addition of lentivirus encoding for NT3 to the bridge sufficiently altered the injury environment to enhance recruitment and differentiation of oligodendrocyte precursors and enhanced myelination of regenerating nerve fibers, consistent with previous report in rat models5,58. NT3 over-expression has promoted robust regeneration of axons across the injury site and increased axonal myelination; however, the majority of these axons remain unmyelinated5,58. After injury, remyelination occurs when endogenous progenitor cells migrate towards the injury, proliferate and differentiate into mature oligodendrocytes12–17. The improved myelination with NT3 expression can be attributed to increases in both Schwann cell-and oligodendrocyte-derived myelin at the injury, with increases in Schwann cell-derived myelin greater than that of oligodendrocyte-derived myelin. NT3 likely enhanced recruitment of Schwann cells into the bridge46. An increase in Olig2+ staining was observed within the bridge for NT3 over-expression (Supp. Fig. 4), suggesting recruitment of oligodendrocyte progenitors; however, NT3 was apparently insufficient to promote their differentiation into mature, myelinating oligodendrocytes.
Delivery of SHH-encoding lentiviral vectors from the bridge significantly increased numbers of myelinated axons at the injury relative to NT3 over-expression and negative controls, and significantly amplified the percentage of axons myelinated by CNS-derived oligodendrocytes rather than PNS-derived Schwann cells. We hypothesized that SHH would enhance myelination after injury by improving recruitment of oligodendrocyte progenitors to the lesion and subsequently promoting their maturation into functional, myelinating oligodendrocytes. This hypothesis was supported by the results that over-expression of SHH increased the numbers of oligodendrocyte progenitors (Olig2+) recruited to the injury while simultaneously maintaining low numbers of astrocytic cells (GFAP+) at levels similar to controls and significantly reduced relative to NT3 over-expression. Expression of SHH-or NT3-encoding lentivirus alone resulted in a decreased presence of Sox2+ progenitor cells, while simultaneous delivery of both SHH- and NT3-encoding lentivirus increased the presence of these neural-lineage progenitors relative to either factor alone. These findings suggest that combinatorial delivery of multiple inductive factors can substantially perturb the recruitment of endogenous progenitor cells at various stages of differentiation.
Previous studies have reported that SHH delivery increases both the presence and proliferation of endogenous progenitors after SCI (NG2+ 23 and nestin+ 21 cells) or traumatic brain injury (Olig2+ cells)26. Although we observed a significant increase in the numbers of progenitor cells with SHH over-expression, we did not observe an effect of SHH on proliferation. However, these previous studies measured proliferation within 3 wks of injury20,21,23, while the data presented in this manuscript were acquired 8 wks after injury. Thus, it is possible that NT3 or SHH over-expression promoted progenitor proliferation at relatively soon after SCI, while by 8 wks progenitors may have exited the cell cycle and proceeded toward differentiation.
The combined delivery of NT3 and SHH resulted in axon extension and myelination similar to NT3 alone, despite the presence of SHH. This result suggests that NT3 expression may inhibit oligodendrocyte maturation, which is supported by reports that NT3 acts to maintain oligodendrocyte progenitors in a proliferative state30,59. Alternatively, NT3 may be more effective at promoting myelination by Schwann cells than by oligodendrocytes. It is also possible that over-expression of inductive factors may have influenced glial progenitors from the PNS to differentiate into CNS-like glia. PNS-derived glial progenitors can differentiate to myelinate CNS axons and express markers typically associated with myelinating oligodendrocytes (e.g., GalC)60. Likewise, these transdiffentiated cells do not express P0, a common marker for Schwann cell-derived myelin. Thus, SHH over-expression may promote myelination from CNS progenitors or trans-differentiation of PNS progenitors. In all experimental conditions, the majority of myelinated axons in the bridge appeared in the regions closest to the contralateral tissue (Supp. Fig. 1a), suggesting that oligodendrocyte progenitors and Schwann cells (or PNS progenitors) migrated into the injury from the adjacent host spinal cord and dorsal root ganglia, respectively. This pattern of myelination indicates that the ability of glial cells to migrate within close proximity of regenerating axons may have substantial effects on remyelination after injury and that additional recruitment of these cells may be beneficial.
Conclusions
We report the ability of two inductive factors (NT3 and SHH) to promote axon extension and remyelination by endogenous progenitor cells following SCI. In these studies both axon extension and myelination were increased to similar levels when NT3 and SHH were delivered alone or in combination, when compared to negative controls. However, SHH promoted greater myelination by oligodendrocytes, consistent with its ability to increase the presence of oligodendrocyte progenitor cells (Olig2+). In contrast, NT3 promoted myelination by both CNS-derived oligodendrocytes and PNS-derived Schwann cells. Overall, we have demonstrated that lentivirus delivered from implanted bridges provides a viable strategy to provide long-term, combinatorial delivery of regenerative factors that target multiple barriers to regeneration.
Experimental
Heparin-modified PLG bridges
Porous bridges, with a porosity of 90%, sized for a mouse spinal cord hemisection lesion were formed based on a previously established technique2. In brief, a 1:1 mixture of PLG (75:25 ratio of D,L-lactide to L-glycolide, inherent viscosity: 0.76 dL/g; Lakeshore Biomaterials, Birmingham, AL, USA) and 63-106 μm salt coated cylindrical sugar fibers drawn from 220°C caramelized sucrose, was packed into a mold, was equilibrated under 800 psi of carbon dioxide for 16 hours and was then released at 60 psi/min to foam into the final structure. The bridges were sectioned, leached 15 min in distilled water, dried and stored desiccated at room temperature until use.
Bridges were modified by first drying chitosan (8183 mol. wt., Sigma Aldrich, St. Louis, MO, USA) onto the surface and then immersing the bridge for 2 hours into 1 mL of 1 M 2-(N-morpholino)ethanesulfonic acid (MES, Sigma Aldrich) buffer in the presence of 9 mg 1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC, CreoSalus Inc., Louisville, KY, USA) and 6 mg N-hydroxysulfosuccinimide (NHS, Research Organics, Cleveland, OH, USA). Heparin (180 USP/mg, Sigma Aldrich) was attached onto the chitosan-modified PLG by drying heparin onto the chitosan-modified bridge and then immersing the bridge into the above EDC/NHS in MES solution overnight36. The modified bridges were washed with distilled water, dried and stored desiccated at room temperature for up to one week before use.
Lentivirus production
Human embryonic kidney cells (HEK 293T, ATCC CRL-11268) cells were cultured in Dulbecco's modified eagle medium containing 10% fetal bovine serum and 1% penicillin-streptomycin. All cell culture materials were purchased from Life Technologies (Carlsbad, CA, USA). Lentivirus was prepared using a previously established technique25,36,37. Cells were co-transfected with vectors encoding plenti-CMV-Firefly luciferase, plenti-CMV-human SHH or plenti-CMV-human NT3 and the following packaging vectors: pMDL-GagPol, pRSV-Rev, pIVSVSV-G using Lipofectamine 2000 (Life Technologies). Supernatant was collected after 48 hours, concentrated in PEG-it (SystemBiosciences, Mountain View, CA) for 24 hours, precipitated using ultracentrifugation and resuspended in PBS. Titer was determined using a qPCR lentivirus titer kit (Applied Biological Materials, Inc., Richmond, BC, Canada).
Gene expression in vivo
For in vivo delivery, lentivirus (~1.5 × 107 particles total) was pipetted directly onto a porous, 7-channel PLG bridge, allowed to adsorb onto the bridge surface and stored at –80 °C until use. Mice were treated according the Animal Care and use Committee guidelines at Northwestern University and all animal procedures were pre-approved by the Committee. SCI surgery and bridge implantation was performed as previously described (n = 4 per condition)2. C57Bl6 females (4-6 wks old, Charles River, Wilmington, MA, USA) were anesthetized using isoflurane (2%). A dorsal laminectomy was performed at T9-T10 for bridge implantation into a 2.25 mm long hemisection lesion. The injury site was covered by Gelfoam and stabilized by suturing the dorsal muscles and stapling the skin to close the wound. Baytril (enrofloxacin 2.5 mg/kg, once a day for 14 days), buprenorphine (0.01 mg/kg, twice a day for 3 days), and lactate ringer solution (5 mL/100 g, once a day for 5 days) were subcutaneously administered post-operatively. Bladders were expressed twice daily until urinary function recovered.
To quantify luciferase expression, spinal cords were extracted 4 weeks after implantation, sectioned every two vertebrae along the length of the spinal column, and frozen at −80 °C. Thawed sections were homogenized in 100 μL of 1× reporter lysis buffer (Promega, Madison, WI, USA), centrifuged at 14,000 rpm for 10 min at 4 °C, and supernatant collected. Luciferase activity in the supernatant was then assessed by measuring light production in the presence of the substrate D-luciferin (Promega) using a luminometer (10 s integration time).
Immunohistochemistry
Spinal cords containing bridge implants were extracted 8 weeks after SCI and flash frozen in isopentane. Spinal cords were then cyrosectioned transversally (18 μm sections). Sections in the middle of the bridge were used for all analyses. Immuno-stained tissue sections were imaged using both widefield (Leica DMRB, Wetzlar, Germany) and confocal (Leica SP5) fluorescence microscopes. Widefield images were acquired using standard fluorescence filter cube sets with a 16-bit, monochromatic CCD (CoolSNAP, Photometrics, Tuscon, AZ, USA). Hoescht+ nuclei were quantified by selecting positive area using a color threshold and summing the number of particles after applying the watershed feature in FIJI/ImageJ (NIH, Bethesda, MD, USA).
The following antibodies were used for primary detection: anti-Ki67 (1:300, ab16667, AbCam, Cambridge, MA, USA), anti-Ki67 (1:100, sc-7846, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-GFAP (1:1000, G3893, G4546, Sigma Aldrich), anti-GFAP (1:1000, ab53554, AbCam), anti-CS56 (1:200, C8035, Sigma Aldrich), anti-NG2 (1:200, ab5320, Millipore, Billerica, MA), anti-Sox2 (1:400, ab97959, AbCam), anti-Olig2 (1:200, MABN50, Millipore), anti-GalC (1:200, MAB342, Millipore), anti-O4 (1:200, MAB345, Millipore), anti-NFM (1:1000, MAB1621, Millipore), anti-MBP (1:1000, sc-13914, Santa Cruz Biotechnology), anti-P0 (1:500, MPZ, Aves Labs, Tigard, OR, USA). Species-specific antibodies were used for secondary detection (1:500-1:1000, Life Technologies, unless otherwise noted): AlexaFluor 488 goat anti-rabbit IgG (A-11034), AlexaFluor 555 goat anti-rabbit IgG (A-21429), AlexaFluor 488 goat anti-mouse IgG (A-11029), AlexaFluor 555 goat anti-mouse IgG (A-21424), AlexaFluor 488 goat anti-mouse IgM (A-21042), AlexaFluor 488 donkey anti-goat IgG (A-11055), AlexaFluor 555 donkey anti-goat IgG (A-21432), AlexaFluor 546 donkey anti-goat IgG (A-11056), AlexaFluor 633 donkey anti-goat IgG (A-21082), AlexaFluor 647 donkey anti-goat IgG (A-21447), AlexaFluor 555 donkey anti-mouse IgG (A-31570), AlexaFluor 647 donkey anti-mouse IgG (A-31571), fluorescein anti-chicken IgY (F-1005, Aves Labs).
Numbers of immuno-positive cells and axons were quantified manually by two researchers independently. Co-staining for multiple markers was always assessed by evaluating overlap of pixels above a set threshold in images acquired over identical sample areas. Co-localization of Sox2, GFAP and Olig2, was evaluated to determine the numbers of neural progenitors, astrocytes and oligodendrocyte progenitors, respectively. Overlap of markers within single cells was confirmed using confocal fluorescence imaging. Because the PLG material generally exhibits high background, cells were counted as Olig2, Sox2 or GFAP positive only when appearance of those markers spatially overlapped Hoescht+ nuclei (Supp. Fig. 5).
To assess the numbers of regenerated and myelinated axons, a double stain for NFM and MBP was performed. Only axons within the bridge were included and axon counts were normalized to bridge area (Supp. Fig. 6a-c). The fraction of myelinated axons was calculated as the ratio of MBP+/NFM+ axons divided by the total number of axons (NFM+). To assess the source of the myelin, a triple stain of NFM, MBP and P0 was performed. MBP+/P0−/NFM+ axons were considered to be myelinated by oligodendrocytes while MBP+/P0+/NFM+ axons were considered to be myelinated by Schwann cells (Supp. Fig. 6d-g). The fraction of axons ensheathed with CNS-derived myelin was calculated as MBP+/P0−/NFM+ axons divided by MBP+/NFM+ axons. Sparing of spinal cord tissue was quantified by measuring the area of abnormal or pathological tissue contralateral to the injury in transverse sections. Area was normalized to the length of the scar boundary dividing the lesion and the contralateral tissue.
Statistics
Multiple comparisons pairs were analyzed using a one-way or two-way ANOVA with a Bonferonni post-hoc test as appropriate. Significance was defined at a level of p<0.05 unless otherwise indicated.
Supplementary Material
Acknowledgements
This work was supported by the NIH (RO1 EB005678, R21 EB006520, RO1 EB003806, R01 CA173745, F32 NS081961 (SKS)). In vivo bioluminescence imaging work was performed at the Northwestern University Center for Advanced Molecular Imaging generously supported by NCI CCSG P30 CA060553 awarded to the Robert H Lurie Comprehensive Cancer Center. Confocal microscopy was performed at the Northwestern University Biological Imaging Facility. Technical support for animal studies from the Northwestern University Center for Comparative Medicine.
References
- 1.De Laporte L, Yang Y, Zelivyanskaya ML, et al. Plasmid releasing multiple channel bridges for transgene expression after spinal cord injury. Mol Ther. 2009;17(2):318–326. doi: 10.1038/mt.2008.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas AM, Kubilius MB, Holland SJ, et al. Channel density and porosity of degradable bridging scaffolds on axon growth after spinal injury. Biomaterials. 2013;34(9):2213–2220. doi: 10.1016/j.biomaterials.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuinstra HM, Margul DJ, Goodman AG, et al. Long-term characterization of axon regeneration and matrix changes using multiple channel bridges for spinal cord regeneration. Tissue Eng A. 2014;11:2013. doi: 10.1089/ten.tea.2013.0111. published online Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, De Laporte L, Zelivyanskaya ML, et al. Multiple channel bridges for spinal cord injury: cellular characterization of host response. Tissue Eng Part A. 2009;15(11):3283–3295. doi: 10.1089/ten.tea.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tuinstra HM, Aviles MO, Shin S, et al. Multifunctional, multichannel bridges that deliver neurotrophin encoding lentivirus for regeneration following spinal cord injury. Biomaterials. 2012;33(5):1618–1626. doi: 10.1016/j.biomaterials.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 2005;192(2):384–393. doi: 10.1016/j.expneurol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 7.Powers BE, Lasiene J, Plemel JR, et al. Axonal thinning and extensive remyelination without chronic demyelination in spinal injured rats. J Neurosci. 2012;32(15):5120–5125. doi: 10.1523/JNEUROSCI.0002-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamazaki Y, Hozumi Y, Kaneko K, et al. Oligodendrocytes: Facilitating axonal conduction by more than myelination. Neuroscientist. 2010;16(1):11–18. doi: 10.1177/1073858409334425. [DOI] [PubMed] [Google Scholar]
- 9.Li GL, Farooque M, Holtz A, Olsson Y. Apoptosis of oligodendrocytes occurs for long distances away from the primary injury after compression trauma to rat spinal cord. Acta Neuropathol. 1999;98(5):473–480. doi: 10.1007/s004010051112. [DOI] [PubMed] [Google Scholar]
- 10.Casha S, Yu WR, Fehlings MG. Oligodendroglial apoptosis occurs along degenerating axons and is associated with FAS and p75 expression following spinal cord injury in the rat. Neurosci. 2001;103(1):203–218. doi: 10.1016/s0306-4522(00)00538-8. [DOI] [PubMed] [Google Scholar]
- 11.Grossman SD, Rosenberg LJ, Wrathall JR. Temporal–spatial pattern of acute neuronal and glial loss after spinal cord contusion. Exp Neurol. 2001;168(2):273–282. doi: 10.1006/exnr.2001.7628. [DOI] [PubMed] [Google Scholar]
- 12.Gensert JM, Goldman JE. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron. 1997;19(1):197–203. doi: 10.1016/s0896-6273(00)80359-1. [DOI] [PubMed] [Google Scholar]
- 13.Yang H, Lu P, McKay HM, et al. Endogenous neurogenesis replaces oligodendrocytes and astrocytes after primate spinal cord injury. J Neurosci. 2006;26(8):2157–2166. doi: 10.1523/JNEUROSCI.4070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mi S, Miller RH, Tang W, et al. Promotion of central nervous system remyelination by induced differentiation of oligodendrocyte precursor cells. Ann Neurol. 2009;65(3):304–315. doi: 10.1002/ana.21581. [DOI] [PubMed] [Google Scholar]
- 15.Sellers DL, Maris DO, Horner PJ. Postinjury niches induce temporal shifts in progenitor fates to direct lesion repair after spinal cord injury. J Neurosci. 2009;29(20):6722–6733. doi: 10.1523/JNEUROSCI.4538-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnabé-Heider F, Goritz C, Sabelström H, et al. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7(4):470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 17.Yang Y, Lewis R, Miller RH. Interactions between oligodendrocyte precursors control the onset of CNS myelination. Develop Bio. 2011;350(1):127–138. doi: 10.1016/j.ydbio.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Cheng X, He Q, et al. Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci. 2011;31(16):6053–6058. doi: 10.1523/JNEUROSCI.5524-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J, Leong SY, Schachner M. Differential expression of cell fate determinants in neurons and glial cells of adult mouse spinal cord after compression injury. Eur J Neurosci. 2005;22(8):1895–1906. doi: 10.1111/j.1460-9568.2005.04348.x. [DOI] [PubMed] [Google Scholar]
- 20.Bambakidis NC, Miller RH. Transplantation of oligodendrocyte precursors and sonic hedgehog results in improved function and white matter sparing in the spinal cords of adult rats after contusion. Spine J. 2004;4(1):16–26. doi: 10.1016/j.spinee.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Bambakidis NC, Horn EM, Nakaji P, et al. Endogenous stem cell proliferation induced by intravenous hedgehog agonist administration after contusion in the adult rat spinal cord. J Neurosurg Spine. 2009;10(2):171–176. doi: 10.3171/2008.10.SPI08231. [DOI] [PubMed] [Google Scholar]
- 22.Lowry N, Goderie SK, Adamo M, et al. Multipotent embryonic spinal cord stem cells expanded by endothelial factors and Shh/RA promote functional recovery after spinal cord injury. Exp Neurol. 2008;209(2):510–522. doi: 10.1016/j.expneurol.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Lowry N, Goderie SK, Lederman P, et al. The effect of long-term release of Shh from implanted biodegradable microspheres on recovery from spinal cord injury in mice. Biomaterials. 2012;33(10):2892–2901. doi: 10.1016/j.biomaterials.2011.12.048. [DOI] [PubMed] [Google Scholar]
- 24.Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nature Neurosci. 2003;6(1):21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 25.Zhu, G. G, Mehler MF, Zhao J, Yu Yung S, Kessler JA. Sonic hedgehog and BMP2 exert opposing actions on proliferation and differentiation of embryonic neural progenitor cells. Develop Bio. 1999;215(1):118–129. doi: 10.1006/dbio.1999.9431. [DOI] [PubMed] [Google Scholar]
- 26.Amankulor NM, Hambardzumyan D, Pyonteck SM, et al. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J Neurosci. 2009;29(33):10299–10308. doi: 10.1523/JNEUROSCI.2500-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bamber NI, Li H, Lu X, et al. Neurotrophins BDNF and NT3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur J Neurosci. 2001;13(2):257–268. [PubMed] [Google Scholar]
- 28.Barres B, Raff M, Gaese F, et al. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature. 1994;367:371–375. doi: 10.1038/367371a0. [DOI] [PubMed] [Google Scholar]
- 29.Engel U, Wolswijk G. Oligodendrocyte-type-2A astrocyte (O-2A) progenitor cells derived from adult rat spinal cord: In vitro characteristics and response to PDGF, bFGF and NT3. Glia. 1996;16(1):16–26. doi: 10.1002/(SICI)1098-1136(199601)16:1<16::AID-GLIA3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Kahn MA, Dinh L, de Vellis J. NT3-mediated TrkC receptor activation promotes proliferation and cell survival of rodent progenitor oligodendrocyte cells in vitro and in vivo. J Neurosci Res. 1998;54(6):754–765. doi: 10.1002/(SICI)1097-4547(19981215)54:6<754::AID-JNR3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18(14):5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu CL, Chen SD, Hwang CS, Yang DI. Sonic hedgehog mediates BDNF-induced neuroprotection against mitochondrial inhibitor 3-nitropropionic acid. Biochem Biophys Res Commun. 2009;385(1):112–117. doi: 10.1016/j.bbrc.2009.04.145. [DOI] [PubMed] [Google Scholar]
- 33.Wu J, Zhang S, Sun X. Neuroprotective effect of upregulated sonic hedgehog in retinal ganglion cells following chronic ocular hypertension. Invest Ophthalmol Visual Sci. 2010;51(6):2986–2992. doi: 10.1167/iovs.09-4151. [DOI] [PubMed] [Google Scholar]
- 34.Merchan P, Bribian A, Sanchez-Camacho C, et al. Sonic hedgehog promotes the migration and proliferation of optic nerve oligodendrocyte precursors. Mol Cell Neurosci. 2007;36(3):355–368. doi: 10.1016/j.mcn.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Hor CHH, Tang BL. Sonic hedgehog as a chemoattractant for adult NPCs. Cell Adhesion & Migration. 2010;4(1):1–3. doi: 10.4161/cam.4.1.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas AM, Shea LD. Polysaccharide-modified scaffolds for controlled lentivirus delivery in vitro and after spinal cord injury. J Controlled Release. 2013;170(3):421–429. doi: 10.1016/j.jconrel.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shin S, Tuinstra HM, Salvay DM, Shea LD. Phosphatidylserine immobilization of lentivirus for localized gene transfer. Biomaterials. 2010;31(15):4353–4359. doi: 10.1016/j.biomaterials.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassiani-Ingoni R, Coksaygan T, Xue H, et al. Cytoplasmic translocation of Olig2 in adult glial progenitors marks the generation of reactive astrocytes following autoimmune inflammation. Exp Neurol. 2006;201(2):349–358. doi: 10.1016/j.expneurol.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 39.Magnus T, Coksaygan T, Korn T, et al. Evidence that nucleocytoplasmic Olig2 translocation mediates brain-injury-induced differentiation of glial precursors to astrocytes. J Neurosci Res. 2007;85(10):2126–2137. doi: 10.1002/jnr.21368. [DOI] [PubMed] [Google Scholar]
- 40.Foret A, Quertainmont R, Botman O, et al. Stem cells in the adult rat spinal cord: plasticity after injury and treadmill training exercise. J Neurochem. 2010;112(3):762–772. doi: 10.1111/j.1471-4159.2009.06500.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee HJ, Wu J, Chung J, Wrathall JR. SOX2 expression is upregulated in adult spinal cord after contusion injury in both oligodendrocyte lineage and ependymal cells. J Neurosci Res. 2013;91(2):196–210. doi: 10.1002/jnr.23151. [DOI] [PubMed] [Google Scholar]
- 42.Brazel CY, Limke TL, Osborne JK, et al. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell. 2005;4(4):197–207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- 43.Marshall CA, Novitch BG, Goldman JE. Olig2 directs astrocyte and oligodendrocyte formation in postnatal subventricular zone cells. J Neurosci. 2005;25(32):7289–7298. doi: 10.1523/JNEUROSCI.1924-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang X, Davies JE, Davies SJA. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res. 2003;71(3):427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- 45.McTigue DM, Tripathi R, Wei P. NG2 colocalizes with axons and is expressed by a mixed cell population in spinal cord lesions. J Neuropathol Exp Neurol. 2006;65(4):406–420. doi: 10.1097/01.jnen.0000218447.32320.52. [DOI] [PubMed] [Google Scholar]
- 46.Yamauchi J, Chan JR, Shooter EM. Neurotrophins regulate Schwann cell migration by activating divergent signaling pathways dependent on Rho GTPases. Proc Natl Acad Sci USA. 2004;101(23):8774–8779. doi: 10.1073/pnas.0402795101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hughes SM, Moussavi-Harami F, Sauter SL, Davidson BL. Viral-mediated gene transfer to mouse primary neural progenitor cells. Mol Ther. 2002;5(1):16–24. doi: 10.1006/mthe.2001.0512. [DOI] [PubMed] [Google Scholar]
- 48.Abdellatif AA, Pelt JL, Benton RL, et al. Gene delivery to the spinal cord: Comparison between lentiviral, adenoviral, and retroviral vector delivery systems. J Neurosci Res. 2006;84(3):553–567. doi: 10.1002/jnr.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stamataki D, Ulloa F, Tsoni SV, Mynett A, Briscoe J. A gradient of Gli activity mediates graded Sonic Hedgehog signaling in the neural tube. Genes & Development. 2005;19:626–641. doi: 10.1101/gad.325905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26(38):9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park JY, Kim SK, Woo DH, Lee EJ, Kim JH, Lee SH. Differentiation of neural progenitor cells in a microfluidic chip-generated cytokine gradient. Stem Cells. 2009;27(11):2645–2654. doi: 10.1002/stem.202. [DOI] [PubMed] [Google Scholar]
- 52.Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. 2010;88:1182–1192. doi: 10.1002/jnr.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ballerman M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibers. Eur J Neurosci. 2006;23(8):1988–1996. doi: 10.1111/j.1460-9568.2006.04726.x. [DOI] [PubMed] [Google Scholar]
- 54.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in rats. Nature Neurosci. 2004;7(3):269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 55.Beauparlant J, van den Brand R, Barraud Q, et al. Undirected compensatory plasticity contributes to neuronal dysfunctional after severe spinal cord injury. Brain. 2013;136(11):3347–3361. doi: 10.1093/brain/awt204. [DOI] [PubMed] [Google Scholar]
- 56.Blesch A, Tuszynski MH. Spinal cord injury: Plasticity, regeneration and the challenge of translational drug development. Trends Neurosci. 2009;32(1):41–47. doi: 10.1016/j.tins.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 57.Rossignol S. Plasticity of connections underlying locomotor recovery after central and/or peripheral lesions in the adult mammals. Phil Trans Royal Soc B: Biol Sci. 2006;361(1473):1647–1671. doi: 10.1098/rstb.2006.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26(38):9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Neri M, Maderna C, Ferrari D, et al. Robust generation of oligodendrocyte progenitors from human neural stem cells and engraftment in experimental demyelination models in mice. PloS One. 2010;5(4):e10145. doi: 10.1371/journal.pone.0010145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Binder E, Rukavina M, Hassani H, et al. Peripheral nervous system progenitors can be reprogrammed to produce myelinating oligodendrocytes and repair brain lesions. J Neurosci. 2011;31(17):6379–6391. doi: 10.1523/JNEUROSCI.0129-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


