Abstract
Background
Crystalline silica is among the environmental exposures associated with increased risk of autoimmune diseases, including rheumatoid arthritis, systemic sclerosis and systemic lupus erythematosus. Silica exposure has also been related to the development of ANCA-associated vasculitides (AAV), but past studies appear to conflict as to the presence and magnitude of the associated risks of disease. We aimed to conduct a systematic review of the existing studies and meta-analysis of their results.
Methods
We searched EMBASE, MEDLINE and international scientific conference abstract databases for studies examining the association of silica exposure with AAV. Studies in English, French, or Spanish were included and those examining the association of silica with ANCA-positivity alone were excluded. We assessed study quality using the Newcastle-Ottawa scale. We meta-analyzed the results using random effects models and tested for heterogeneity. We performed sensitivity and subgroup analyses, examining studies that adjusted for smoking and occupational risk factors as well as studies that analyzed by subtypes of AAV.
Results
We identified 158 potential manuscripts and 3 abstracts related to silica exposure and risk of AAV. 147 were excluded after abstract review and 14 underwent detailed evaluation of full manuscript/abstract. After further application of exclusion criteria, 6 studies (all case-controls) remained. The studies had moderate heterogeneity in selection of cases and controls, exposure assessment, disease definition and controlling for potential confounders. We found an overall significant summary effect estimate of silica “ever exposure” with development of AAV (summary OR 2.56, 95% CI 1.51- 4.36), with moderate heterogeneity (I2=48.40%). ORs were similar for studies examining only MPA (OR 3.95, CI 95% 1.89-8.24), compared to those only studying GPA (OR 3.56, CI 95% 1.85-6.82).
Conclusion
Despite moderate heterogeneity among studies, the totality of the evidence after meta-analysis points to an association between silica exposure and risk for developing AAV.
Keywords: Antineutrophil cytoplasmic antibodies, ANCA-associated vasculitides, Silica, Meta-analysis
1. Introduction
Vasculitides associated with serum positivity for antineutrophil cytoplasmic antibodies (ANCAs) that affect small to medium-sized vessels are commonly known as ANCA-associated vasculitides (AAV). The most common disease entities that fall in the AAV category include granulomatosis with polyangiitis (GPA) [formerly Wegener granulomatosis], microscopic polyangiitis (MPA), and Churg-Strauss syndrome (CSS), all of which affect the blood vessels systemically, often affecting the kidney and lung. Focal glomerular necrosis, crescent formation, and the absence or paucity of immunoglobulin deposits characterize glomerulonephritis in patients with AAV. Lung involvement ranges from fleeting focal infiltrates or interstitial disease to massive pulmonary hemorrhagic alveolar capillaritis, a life-threatening manifestation of small-vessel vasculitis [1].
Environmental factors are thought to trigger or induce several autoimmune disorders in genetically susceptible subjects [2],[3]. While there may be a substantial genetic component to development of AAV, there is also a body of evidence supporting exposure to environmental factors, either directly or indirectly, in the development of AAV [4],[5]. These include bacterial infections (i.e. Staphylococcus aureus) [6], viral infections (i.e. parvovirus B19) [7], asbestos [8] and silica exposure [2, 5]. Exposure to crystalline silica classically takes place in the so-called “dusty trades”, mining, sand-blasting, stone-cutting or stone quarry work. High levels of silica exposure also occur in construction work, pottery, agricultural and outdoor workers in areas with silica in the soil [9],[10],[11]. During the 1990s, an increased frequency of ANCAs (for instance, anti-myeloperoxidase antibodies) among individuals in mining and construction occupations [12],[13] was reported, suggesting environmental exposure was important for antibody development. In a more recent occupational study [14], 32 different occupations were examined and, although no statistically significant associations were found, a borderline increased risk of AAV was reported for bakers (OR=1.6, 95% CI 1.0-2.6), paper workers (OR=1.8, 95% CI, 0.9-3.5), miners (OR= 1.9, 95% CI, 1.0-3.5) and animal keepers (OR=1.8, 95 % CI 0.9-3.5).
Exposure to crystalline silica has been associated with the development of a number of respiratory diseases, including silicosis, progressive pulmonary fibrosis, chronic obstructive pulmonary disease and lung cancer [15]. Has also been reported to be associated with renal insufficiency and rapidly progressive glomerulonephritis [16]. Additionally, silica is known as one of the strongest environmental substances causing overall autoimmunity [17]. Silica is known as a strong T cell adjuvant [17]. Chronic exposure to silica particles activates T responder cells and Treg cells [18]. Silica exposure had been related to the development of several other autoimmune diseases, including rheumatoid arthritis (RA) [19], systemic sclerosis (SSc) [20] and systemic lupus erythematosus (SLE) [21].
Several case-control studies have shown that among patients with AAV, a high percentage (20 to 45%) were previously exposed to silica [22], [23], [24], [25], [26], [27], [28], [29]. However, these studies have been difficult to interpret; factors such as small numbers of cases and diverse forms of classification for silica exposure contribute to this difficulty, as do the varying strengths of association between AAV and silica that are reported. Our objective was to perform a meta-analysis of all past studies examining the association of silica exposure and AAV to determine the strength of the association between silica exposure and the development of AAV.
2. Methods
2.1 Data sources
We searched EMBASE and MEDLINE databases from January 1965 until April 2013 for studies examining the association of ANCA vasculitis with silica exposure. The search was performed using the following keywords and Medical Subject Headings: [“silica” or “silic*”] AND [“vasculitis” or “systemic vasculitis” or “primary systemic vasculitis” or “ANCA vasculitis” or “Wegener's” or “Churg Strauss” or “microscopic polyangiitis” or “rapidly progressive glomerulonephritis” or “granulomatosis with polyangiitis”]. We also searched abstracts available online from the following annual meetings: American College of Rheumatology (ACR), European League Against Rheumatism (EULAR), and the International Vasculitis Workshop.
2.2 Study eligibility, selection, and data abstraction
Only studies in English, French, or Spanish were included. We included case-control studies and cohort studies. No randomized controlled trials were found. Case reports, case series, reviews, and letters to the editors were excluded from the study. Attempts were made to contact authors to collect more information from potentially eligible abstracts [30], [22]. Eligible studies compared silica exposure among those with AAV to those without AAV, or compared the prevalence of AAV among silica exposed and non-silica exposed populations. Studies examining the association of silica and ANCA-positivity alone were not included in this study, unless the studies also examined the association of silica with the development of clinical vasculitis. Studies examining the association of particular occupations and development of vasculitis were not included unless silica or silicosis was specifically mentioned as an exposure. The two first authors independently reviewed all manuscripts and abstracts to determine study eligibility. Data were then abstracted independently by the two authors. When there was disagreement at either stage, it was resolved by consensus. To facilitate study comparison and meta-analysis, we categorized silica exposure from all studies as ever/never.
2.3 Study Quality Assessment
The quality of each included paper was reviewed by the first two authors independently using the Newcastle-Ottawa Scale [31] and any disagreement between the scores given by the two reviewers was resolved by consensus. The Newcastle-Ottawa scale is a commonly used method to assess the quality of non-randomized studies used in a meta-analysis. For case-control studies, the scale assigns points based on: selection of study groups (0-4 points), comparability between study groups (0-2 points), and exposure ascertainment (0-3 points). An a priori decision was made to allocate points to given studies that adjusted for potential confounders.
2.4 Statistical Analysis
We tested for heterogeneity between studies using the Q value and I2 statistic [32]. We used a random effects model to calculate the summary odds ratio for all included studies, given significant heterogeneity in the included studies. We also performed a jack knife sensitivity analysis, in which one study at a time is excluded to test the robustness of the analysis results. Subgroup meta-analyses were also performed, classifying studies by: 1) study quality, those studies using crude association or adjusted; and 2) those studies examining predominantly renal involvement and those using pulmonary or no distinction. We examined for publication bias by visually assessing for asymmetry of the funnel plot, as well as statistically using Begg's and Egger's tests [33], [34], [35]. Comprehensive Meta-Analysis software (www.meta-analysis.com; ©2006 Biostat, Inc.)[36] was employed for all statistics.
3.Results
3.1 Study Characteristics and Quality:
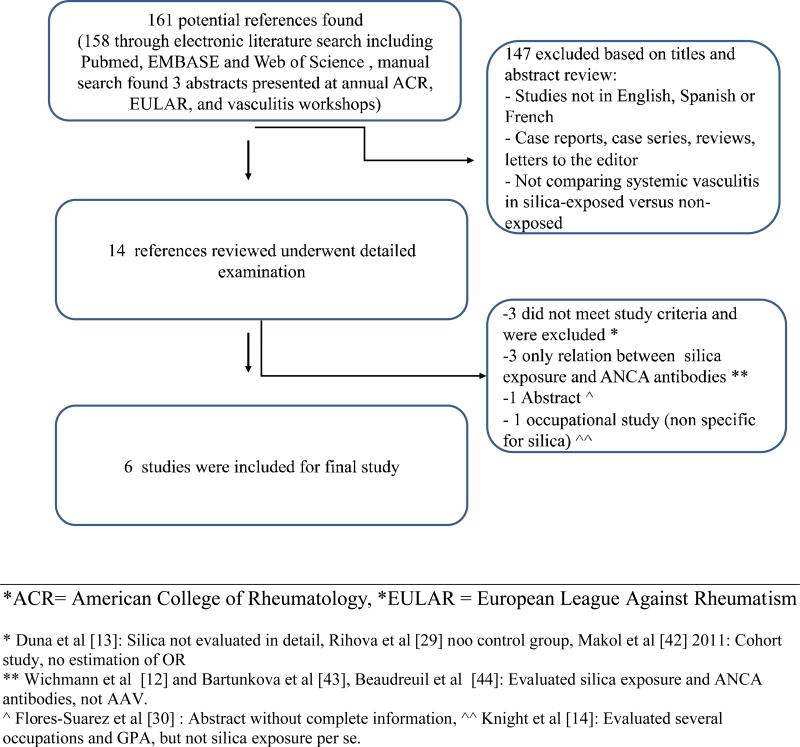
Figure 1 details our study selection process. The initial literature search identified a total of 161 potential references (158 through EMBASE and PUBMED and three abstracts presented at ACR, EULAR, and International Vasculitis Workshop annual scientific conferences). After application of the exclusion criteria described above, 14 references underwent detailed evaluation of the full text. After further application of exclusion criteria, six studies remained for inclusion in the analysis.
Figure 1.
Overview of Literature Search
3.2 Summary of Selected Studies
An overview of included studies is included in Table 1. All studies included in our analysis were designed as case-control studies.
Table 1.
Overview of Selected Papers Examining the Association of ANCA-associated Vasculitis with Silica Exposure
| First author, country, year [Ref] |
Assessment of silica exposure |
Case definition |
Number of cases |
Source of cases |
Overall time exposure in cases (yrs) |
Control definition |
Source of controls |
Number of controls |
Matched or Adjusted |
Odds ratio (95% CI) Ever silica exposure |
Quality Score* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gregorini, Italy, 1993 [23] | Personal interview of occupational exposure | ANCA-positive GN | 16 | All ANCA positive patients admitted | 26.3 | Nephropathy without vasculitis | Admitted patients | 32 | Matched: age, date of admission to hospital Adjusted: none | 14.0 (1.7 - 113.8) | S2/C1/E1 |
| Nuyts, Belgium, 1995 [24] | Occupational questionnaire | Renal GPA | 16 | All patients with GPA from six Belgian Renal Units (1991-1993) | 28.5 | General population | Belgian voter's list (>18 yrs) | 32 | Matched: age, sex, region Adjusted: none | 5.0 (1.4-11.6) | S3/C1/E2 |
| Hogan, USA, 2001 [25] | Occupational questionnaire | ANCA-positive GN | 65 | AAV from The Glomerular Disease Collaborative Network | Exposure > 2 yr in 84% | Nephropathy without vasculitis | Patients from 26 nephrology clinics | 65 | Matched: age, sex, race Adjusted: smoking and occupational exposure | 4.4 (1.36 -13.4) | S3/C2/E1 |
| Stratta, Italy, 2001 [26] | Occupational exposure survey | Renal vasculitis | 31 | Department of Nephrology Turin University | 14 | Nephropathy without vasculitis | Department of Nephrology of Turin University | 58 | Matched: age, sex, region Adjusted: none | 2.4 (1.02-6.5) | S2/C1/E1 |
| Lane, UK, 2003 [27] | Occupational exposure survey using the Standard Occupational Classification (SOC) 2000 | Primary systemic vasculitis | 75 | Patients having primary systemic vasculitis from NHUH | 23.7 | Non-inflammatory musculoskeletal disease | Inpatients and outpatients from Orthopedic and Rheumatology departments | 220 | Matched: age, sex Adjusted: smoking, occupational exposure, residence, allergy | 1.4 (0.7 - 2.7) | S2/C2/E2 |
| Hogan, USA, 2007 [28] | A structured telephone interview | ANCA-positive GN | 129 | Patients with biopsy proven renal vasculitis from 4 different states ** (1997-2003) | 13¥ | General population | List-assisted random-digit dialing | 109 | Matched: age, sex, state Adjusted: age, sex, state | 1.6 (0.9 - 2.8) | S3/C1/E1 |
The Newcastle-Ottawa scale [31] was used to assess quality of each study. Each study is scored on selection (S) of comparison groups (0-4 points), comparability (C) between the two groups (0-2 points), and exposure (E) ascertainment (0-3 points). NHUH: Norfolk and Norwich University Hospital.
North Carolina, South Carolina, Georgia and Virginia
Median exposure duration
Demographics data
Four out of six studies were conducted in Europe (two in Italy, one in Belgium and one in United Kingdom). The remaining two studies were done in U.S. A total of 332 cases (AAV) and 516 controls were included in the analysis. Five of out six studies selected AVV cases from nephrology clinics. Most of the cases were pauci-immune renal necrotizing vasculitis [n=171 (51%)], followed by GPA [n=88 (26.5%)], MPA [n=57 (17.1%)] and CSS [n=16 (4.8%)]. One study examined only renal GPA [24], and four examined renal vasculitis only [23], [25] ,[26], [28]. Three of the studies included used as controls patients with nephropathy but without evidence of vasculitis, two studies included healthy controls and one study included patients with non-inflammatory musculoskeletal diseases as controls. Sixty-nine percent of cases were male: all studies included both men and women except for one which was male only [23]. The mean age of cases was 57 ± 3.2 years, mean age of controls was 56.1 ± 3.1 years.
Most studies matched or adjusted for age and sex, and two further adjusted for smoking and other occupational exposures [25, 27]. More specific data regarding each of the studies can be found in Table 1.
3.3 Exposure measures
Exposure Assessment
Exposure to silica was assessed by a variety of different means. Silica exposure assessment was done by personal interview of occupational exposure by industrial hygienist in three studies [23], [24],[26], one study used self-reported occupational questionnaire [25], one study used a validated occupational exposure survey and the Standard Occupational Classification (SOC) 2000 [27], and one study used a structured telephone interview evaluated by one epidemiologist and one industrial hygienist [28].
Latency period
The latency period between the beginning of exposure and the diagnosis of AAV was only described in three studies [23],[26],[28]. Two studies found mean latency periods of 25 years (range 6-39) [23], and 32 years (range 6-39) [26], while another study found median latency period of 13 years (range 2-28) [28].
Degree of Exposure
The mean duration of silica exposure for cases from all included studies was 21 ± 7.1 years. The definition of the degree of exposure (i.e., type of exposure, amount of exposure over a period of time) in each study varied significantly from study to study. In one study, degree of exposure was not measured (just ever or never exposure) [23] . Two studies stratified exposure into three categories by the frequency of the exposure during a particular period of time [25], [28]. Two studies stratified in four categories by quantity of exposure (absence, low, moderate and high) [24], [27], and another study classified the exposure according five categories from none to high-intensity exposure [26]. One study also stratified exposure by type of exposure, i.e. whether due to agricultural or non-agricultural exposure [27].
Dose effect
Only two studies examined whether there was a “dose effect”, ie whether higher degree of exposure was associated with higher risk of development of disease [27], [28].
3.4 Publication Bias
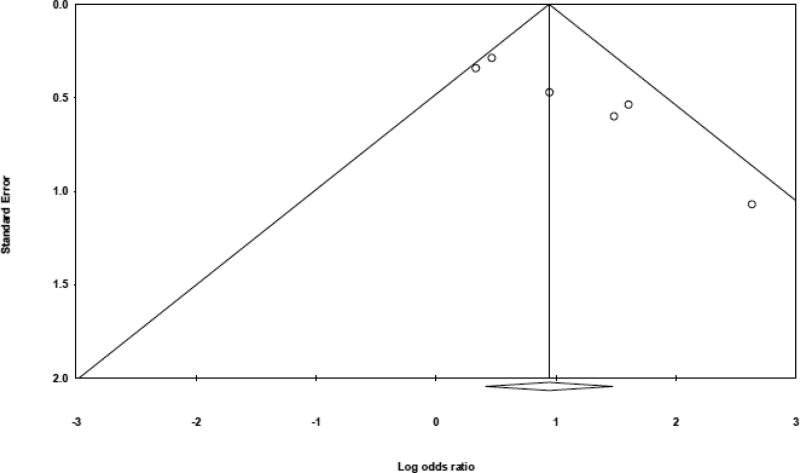
While there was no evidence of publication bias using the Begg's tests (two-tailed p-value = 0.06), the Egger's test with a two-tailed p-value = 0.01 and the funnel plot demonstrated some asymmetry (Figure 4). This suggests there may be potential publication bias on this topic, as small null studies with large standard errors were not identified in the published literature.
Figure 4.
Begg's funnel plot for detection of publication bias. The y-axis (standard error) measures precision of the results of the studies. The x-axis (log-odds ratio) measures the odds ratio of each study included in the meta-analysis. Therefore, smaller, less precise studies will likely have higher standard error and likely be more skewed from the center where larger, more precise studies lie. Therefore, asymmetry of the funnel plot suggests publication bias [33], [34],[35].
3.5 Summary Estimates of Association between Silica Exposure and AAV
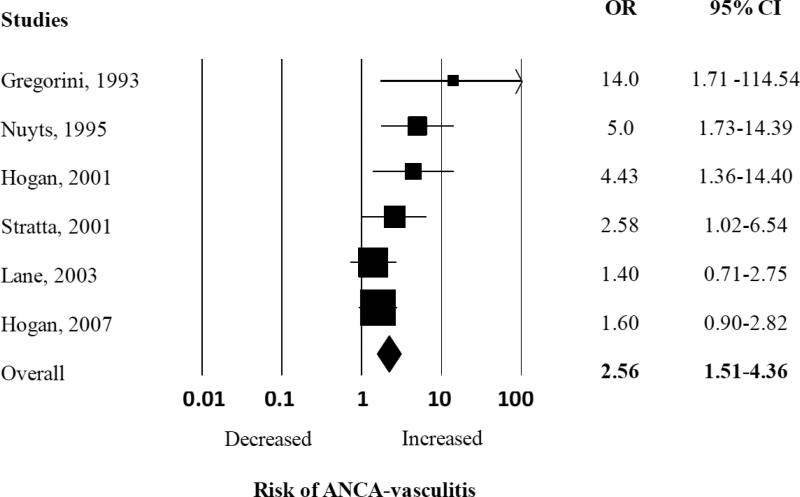
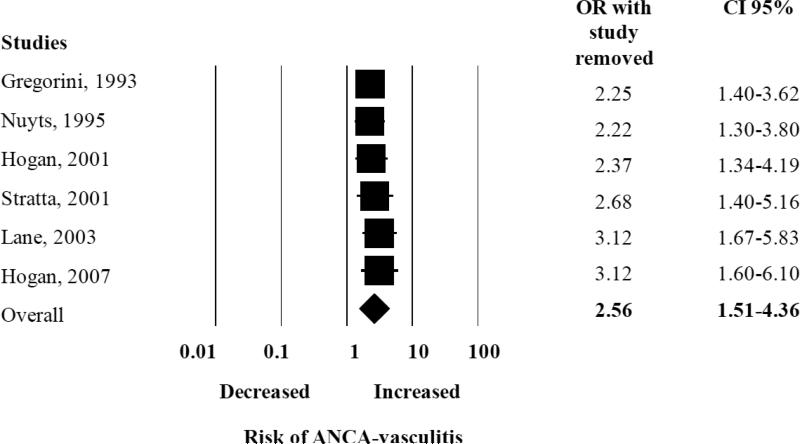
We found an elevated summary risk estimate for silica “ever” exposure with the development of AAV (pooled OR 2.57, 95% CI 1.51-4.36) (Figure 2). There was moderate heterogeneity among all the studies included in this analysis, with Q-value of 9.69 and I2 calculated at 48.40%. We performed a sub-analysis examining the effect of each study on the pooled OR, and did not find one study influencing the overall pooled OR (Appendix Figure 1).
Figure 2.
Random effects analysis of all studies for the association between ANCA-associated vasculitis with prior exposure to silica. Point (square) and overall (diamond) estimates are given as odds ratios with 95% confidence interval (CI) (horizontal bar).
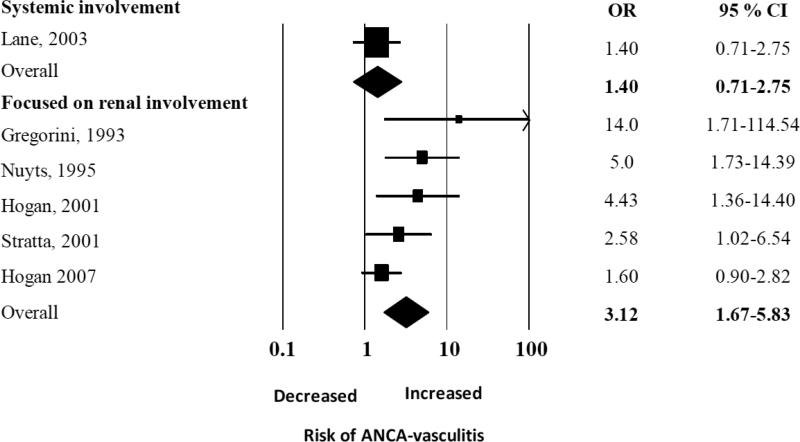
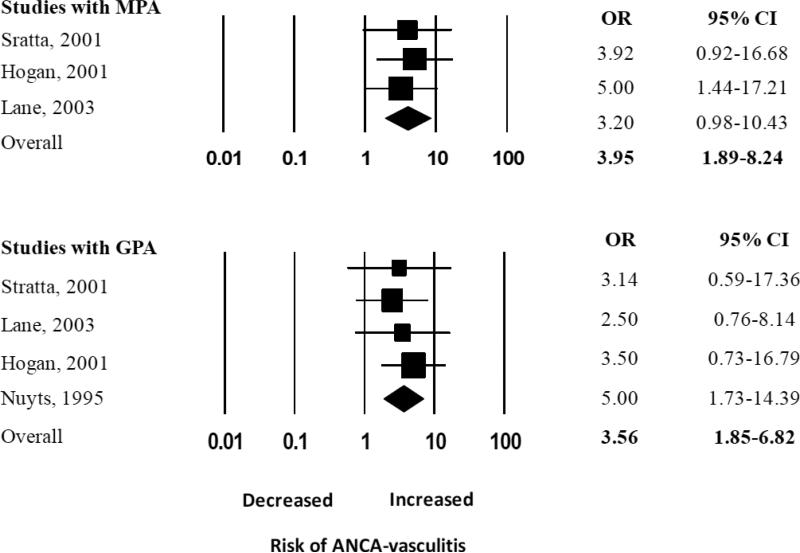
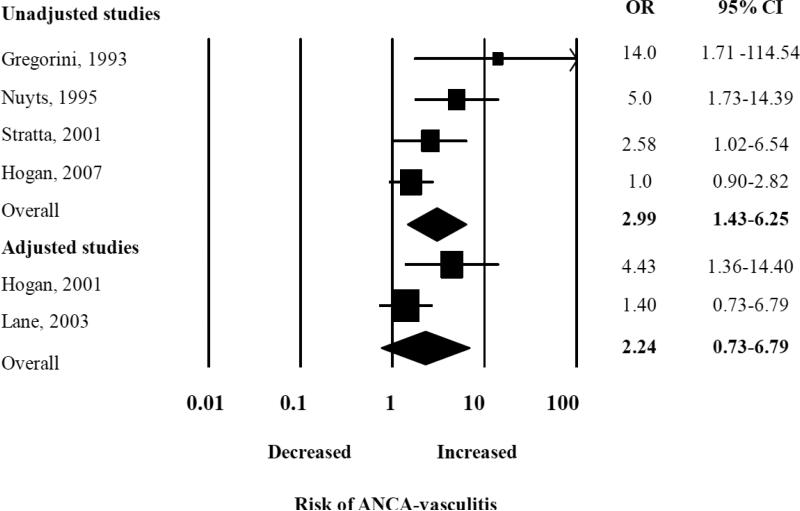
There was an elevated summary odds ratios for the five studies examining renal vasculitis alone (OR 3.13, 95% CI 1.68-5.84), but in a single study that examined both renal and pulmonary vasculitis the OR was not significantly elevated (OR 1.40, 95% CI 0.71 – 2.75) (Figure 3A). We performed a sub-analysis of studies that reported the odds of developing each type of vasculitis, and found the pooled ORs were similar for both GPA and MPA: the summary OR of history of ever being exposed to silica for GPA was 3.56 (95% CI 1.85 – 6.82) and for MPA, it was 3.95 (CI 95% 1.89 - 8.24) (Figure 3B). Interestingly, the summary OR for the association between silica exposure and AAV was not significant [2.24 (95% CI 0.74 -6.80)] for the only two studies that adjusted for smoking and occupational risk factors, compared to 2.99 (95% CI 1.43 – 6.25) for the four studies only reporting unadjusted estimates of association (Figure 3C). However, these two studies alone likely lacked power to detect a small effect given the large summary confidence interval.
Figure 3A.
Random effects analysis of all studies for the association between ANCA-associated vasculitis with prior exposure to silica grouped according to studies focused on renal involvement vs studies without differentiation between renal and other organ involvement (label as systemic). Point (square) and overall (diamond) estimates are given as odds ratios with 95% confidence interval (CI) (horizontal bar).
Figure 3B.
Random effects analysis of all studies for the association between ANCA-associated vasculitis with prior exposure to silica grouped according to studies focused in MPA and GPA. Point (square) and overall (diamond) estimates are given as odds ratios with 95% confidence interval (CI) (horizontal bar).
Figure 3C.
Random effects analysis of all studies for the association between ANCA-associated vasculitis with prior exposure to silica grouped according to studies that are adjusted and unadjusted. Point (square) and overall (diamond) estimates are given as odds ratios with 95% confidence interval (CI) (horizontal bar).
4. Discussion
While the exact pathogenesis of primary systemic vasculitis is unknown, there is concern that specific environmental factors may trigger AAV in patients with a genetic predisposition. Crystalline silica exposure has been associated with other autoimmune diseases and is a leading candidate among putative environmental triggers. Past studies investigating the potential association between silica exposure and the development of AAV have varied with regard to the presence and strength of the observed associations however. Our study therefore aimed to systemically review and meta-analyze the current literature, and to quantify and describe the potential association between silica and the development of AAV. In this meta-analysis of six studies, silica ever-exposure was associated with development of AAV, with a summary OR of 2.56, 95% CI 1.51-4.36.
Most of the studies included in our meta-analysis did not adjust for potential confounders, such as other occupational exposures and tobacco use, which may have led to a potential overestimation of risk attributed to silica. The studies may not have adjusted for these exposures; unlike in other autoimmune diseases such as RA, there is conflicting literature on the roles these exposures may play in the development of AAV. For example, there has been literature suggesting both decreased [27], [37] and increased [38] association of smoking with AAV development. Similarly, there is even scantier data concerning other occupational exposures, mainly asbestos exposure, although there is one study suggesting an association of asbestos with development of AAV [8]. Therefore, while the literature on environmental exposures and AAV is still growing, it may be important to consider these potential confounders in future studies.
There are limitations to this meta-analysis. After applying our exclusion criteria, a small number of studies were ultimately included. The studies that were included were at least of moderate quality as assessed by the Newcastle-Ottawa scale. In addition, moderate heterogeneity was found among the studies included. This heterogeneity likely resulted from differences in factors such as differing definitions of cases and controls. For example, some studies only examined particular AAV affecting primarily particular organ systems, such as renal involvement only, while other studies used a broader definition of AAV. We addressed this significant heterogeneity using random effects models, and by performing sub-analyses to determine whether significant differences between subgroups could be found. As with all meta-analyses including observational studies, we also are limited by confounding and bias inherent in each of the observational studies. In this case, studies may have been particularly prone to silica exposure misclassification and recall bias for AAV cases, considering that self-reporting was often used to collect information about exposure and that there were long mean latency periods between exposure and disease diagnosis. Lastly, we found the suggestion of publication bias, in which small null studies were not published.
Despite these limitations, our finding of increased risk of AAV with silica exposure is very interesting, particularly with regard to the pathogenesis of AAV. Crystalline silica may cause autoimmunity via at least two different proposed pathways [39]. First, silica has been shown to cause cell death by necrosis and apoptosis. When neutrophils undergo apoptosis, primary granule constituents translocate to the cell surface, therefore leading to the generation of self-antigens and potentially ANCA [40]. Secondly, individuals exposed to crystalline silica may have silica particles trapped in their alveoli permanently [15],[41]. Silica is known as a strong T cell adjuvant and a dysregulated immune response triggered in genetically predisposed individuals could induce an uncontrolled systemic inflammatory response, leading to AAV and tissue damage [17].
Our summary estimates lend support to the hypothesis that silica may act as an environmental “trigger” for the development of AAV, as well as other autoimmune diseases, and bring us closer to an understanding of the pathogenesis of AAV. However, further studies are warranted as the included studies may have inherent issues with confounding and bias, and had varied methods of defining exposures and performing analyses. While study of this topic is difficult considering the rarity of AAV and the long latency period needed for the appearance of disease after silica exposure (possibly several decades), further studies should be prospective studies and examine not only different types and durations of silica exposure, but also dose-effects of silica exposure and potential confounders and effect modification.
Take home messages.
Silica exposure has been related with the development of several autoimmune diseases, including rheumatoid arthritis, systemic sclerosis and systemic lupus erythematosus.
Despite moderate heterogeneity among all the studies, silica exposure was associated with more than 2 times higher risk for developing ANCA associated vasculitides.
Only two of the past studies adjusted for other potential confounders, including smoking and occupation.
Our subgroup analyses showed significantly elevated summary odds ratios for studies only examining renal vasculitis alone (OR 3.13; 95% CI 1.68-5.84) and for studies examining patients with GPA (OR 3.56; 95% CI 1.85 – 6.82) and MPA (OR 3.95; CI 95% 1.89 - 8.24).
Appendix Figure 1.
Random effects analysis of all studies for the association between ANCA-associated vasculitis with prior exposure to silica with jack knife sensitivity analysis. Point (square) and overall (diamond) estimates are given as odds ratios with 95% confidence interval (CI) (horizontal bar).
Acknowledgements
We are grateful to Michael Soto, PhD for his productive and valuable comments.
JA Gomez-Puerta, MD, PhD was founded by Fundación Alfonso Martin Escudero, Spain.
L. Gedmintas received support from the NIH grant no. 5T32AR055885-04.
References
- 1.Gomez-Puerta JA, Bosch X. Anti-neutrophil cytoplasmic antibody pathogenesis in small-vessel vasculitis: an update. Am J Pathol. 2009;175:1790–8. doi: 10.2353/ajpath.2009.090533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller FW, Alfredsson L, Costenbader KH, Kamen DL, Nelson LM, Norris JM, et al. Epidemiology of environmental exposures and human autoimmune diseases: findings from a National Institute of Environmental Health Sciences Expert Panel Workshop. J Autoimmun. 2012;39:259–71. doi: 10.1016/j.jaut.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoenfeld Y, Agmon-Levin N. ASIA’ - autoimmune/inflammatory syndrome induced by adjuvants. J Autoimmun. 2011;36:4–8. doi: 10.1016/j.jaut.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Chen M, Kallenberg CG. The environment, geoepidemiology and ANCA-associated vasculitides. Autoimmun Rev. 2010;9:A293–8. doi: 10.1016/j.autrev.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Costenbader KH, Gay S, Alarcon-Riquelme ME, Iaccarino L, Doria A. Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev. 2012;11:604–9. doi: 10.1016/j.autrev.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Popa ER, Stegeman CA, Kallenberg CG, Tervaert JW. Staphylococcus aureus and Wegener's granulomatosis. Arthritis Res. 2002;4:77–9. doi: 10.1186/ar392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkel TH, Torok TJ, Ferguson PJ, Durigon EL, Zaki SR, Leung DY, et al. Chronic parvovirus B19 infection and systemic necrotising vasculitis: opportunistic infection or aetiological agent? Lancet. 1994;343:1255–8. doi: 10.1016/s0140-6736(94)92152-0. [DOI] [PubMed] [Google Scholar]
- 8.Pelclova D, Bartunkova J, Fenclova Z, Lebedova J, Hladikova M, Benakova H. Asbestos exposure and antineutrophil cytoplasmic Antibody (ANCA) positivity. Arch Environ Health. 2003;58:662–8. doi: 10.3200/AEOH.58.10.662-668. [DOI] [PubMed] [Google Scholar]
- 9.Leung CC, Yu IT, Chen W. Silicosis. Lancet. 2012;379:2008–18. doi: 10.1016/S0140-6736(12)60235-9. [DOI] [PubMed] [Google Scholar]
- 10.Beaudry C, Lavoue J, Sauve JF, Begin D, Senhaji Rhazi M, Perrault G, et al. Occupational exposure to silica in construction workers: a literature-based exposure database. J Occup Environ Hyg. 2013;10:71–7. doi: 10.1080/15459624.2012.747399. [DOI] [PubMed] [Google Scholar]
- 11.Sauve JF, Beaudry C, Begin D, Dion C, Gerin M, Lavoue J. Silica exposure during construction activities: statistical modeling of task-based measurements from the literature. Ann Occup Hyg. 2013;57:432–43. doi: 10.1093/annhyg/mes089. [DOI] [PubMed] [Google Scholar]
- 12.Wichmann I, Sanchez-Roman J, Morales J, Castillo MJ, Ocana C, Nunez-Roldan A. Antimyeloperoxidase antibodies in individuals with occupational exposure to silica. Ann Rheum Dis. 1996;55:205–7. doi: 10.1136/ard.55.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duna GF, Cotch MF, Galperin C, Hoffman DB, Hoffman GS. Wegener's granulomatosis: role of environmental exposures. Clin Exp Rheumatol. 1998;16:669–74. [PubMed] [Google Scholar]
- 14.Knight A, Sandin S, Askling J. Occupational risk factors for Wegener's granulomatosis: a case-control study. Ann Rheum Dis. 2010;69:737–40. doi: 10.1136/ard.2009.107953. [DOI] [PubMed] [Google Scholar]
- 15.Greenberg MI, Waksman J, Curtis J. Silicosis: a review. Dis Mon. 2007;53:394–416. doi: 10.1016/j.disamonth.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 16.Osorio AM, Thun MJ, Novak RF, Van Cura EJ, Avner ED. Silica and glomerulonephritis: case report and review of the literature. Am J Kidney Dis. 1987;9:224–30. doi: 10.1016/s0272-6386(87)80059-8. [DOI] [PubMed] [Google Scholar]
- 17.Maeda M, Nishimura Y, Kumagai N, Hayashi H, Hatayama T, Katoh M, et al. Dysregulation of the immune system caused by silica and asbestos. J Immunotoxicol. 2010;7:268–78. doi: 10.3109/1547691X.2010.512579. [DOI] [PubMed] [Google Scholar]
- 18.Lee S, Hayashi H, Maeda M, Chen Y, Matsuzaki H, Takei-Kumagai N, et al. Environmental factors producing autoimmune dysregulation--chronic activation of T cells caused by silica exposure. Immunobiology. 2012;217:743–8. doi: 10.1016/j.imbio.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Khuder SA, Peshimam AZ, Agraharam S. Environmental risk factors for rheumatoid arthritis. Rev Environ Health. 2002;17:307–15. doi: 10.1515/reveh.2002.17.4.307. [DOI] [PubMed] [Google Scholar]
- 20.Mccormic ZD, Khuder SS, Aryal BK, Ames AL, Khuder SA. Occupational silica exposure as a risk factor for scleroderma: a meta-analysis. Int Arch Occup Environ Health. 2010;83:763–9. doi: 10.1007/s00420-009-0505-7. [DOI] [PubMed] [Google Scholar]
- 21.Finckh A, Cooper GS, Chibnik LB, Costenbader KH, Watts J, Pankey H, et al. Occupational silica and solvent exposures and risk of systemic lupus erythematosus in urban women. Arthritis Rheum. 2006;54:3648–54. doi: 10.1002/art.22210. [DOI] [PubMed] [Google Scholar]
- 22.Flores-Suarez L, Contreras I, Villa Ar. Primary systemic PSV) are associated with silica, solvents and livestock contact. Ann Rheum Dis. 2005;64:81. [Google Scholar]
- 23.Gregorini G, Ferioli A, Donato F, Tira P, Morassi L, Tardanico R, et al. Association between silica exposure and necrotizing crescentic glomerulonephritis with p-ANCA and anti-MPO antibodies: a hospital-based case-control study. Adv Exp Med Biol. 1993;336:435–40. doi: 10.1007/978-1-4757-9182-2_77. [DOI] [PubMed] [Google Scholar]
- 24.Nuyts GD, Van Vlem E, De Vos A, Daelemans RA, Rorive G, Elseviers MM, et al. Wegener granulomatosis is associated to exposure to silicon compounds: a case-control study. Nephrol Dial Transplant. 1995;10:1162–5. [PubMed] [Google Scholar]
- 25.Hogan SL, Satterly KK, Dooley MA, Nachman PH, Jennette JC, Falk RJ. Silica exposure in anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and lupus nephritis. J Am Soc Nephrol. 2001;12:134–42. doi: 10.1681/ASN.V121134. [DOI] [PubMed] [Google Scholar]
- 26.Stratta P, Messuerotti A, Canavese C, Coen M, Luccoli L, Bussolati B, et al. The role of metals in autoimmune vasculitis: epidemiological and pathogenic study. Sci Total Environ. 2001;270:179–90. doi: 10.1016/s0048-9697(00)00800-7. [DOI] [PubMed] [Google Scholar]
- 27.Lane SE, Watts RA, Bentham G, Innes NJ, Scott DG. Are environmental factors important in primary systemic vasculitis? A case-control study. Arthritis Rheum. 2003;48:814–23. doi: 10.1002/art.10830. [DOI] [PubMed] [Google Scholar]
- 28.Hogan SL, Cooper GS, Savitz DA, Nylander-French LA, Parks CG, Chin H, et al. Association of silica exposure with anti-neutrophil cytoplasmic autoantibody small-vessel vasculitis: a population-based, case-control study. Clin J Am Soc Nephrol. 2007;2:290–9. doi: 10.2215/CJN.03501006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rihova Z, Maixnerova D, Jancova E, Pelclova D, Bartunkova J, Fenclova Z, et al. Silica and asbestos exposure in ANCA-associated vasculitis with pulmonary involvement. Ren Fail. 2005;27:605–8. doi: 10.1080/08860220500200395. [DOI] [PubMed] [Google Scholar]
- 30.Flores-Suarez L, Contreras I, Brise Ca, Tilde N, Villa Ar. Environmental risk factors in primary vasculitides (PSV). Kidney Blood Press Res. 2003;26:249–302. [Google Scholar]
- 31.Wells Gs OCB, Peterson D, Welch J, Losos V, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011 Available from: www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 32.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–55. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 34.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 35.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis, Biostat. 2 ed. Englewood, NJ: 2005. [Google Scholar]
- 37.Haubitz M, Woywodt A, De Groot K, Haller H, Goebel U. Smoking habits in patients diagnosed with ANCA associated small vessel vasculitis. Ann Rheum Dis. 2005;64:1500–2. doi: 10.1136/ard.2004.033191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sessa A, Meroni M, Battini G, Vaccari M, Giordano F, Torri Tarelli L. Cigarette smoking and pauci-immune extracapillary glomerulonephritis with ANCA-associated idiopathic systemic vasculitis. A retrospective study. Contrib Nephrol. 2000;130:103–8. doi: 10.1159/000060054. [DOI] [PubMed] [Google Scholar]
- 39.Otsuki T, Maeda M, Murakami S, Hayashi H, Miura Y, Kusaka M, et al. Immunological effects of silica and asbestos. Cell Mol Immunol. 2007;4:261–8. [PubMed] [Google Scholar]
- 40.Pfau JC, Brown JM, Holian A. Silica-exposed mice generate autoantibodies to apoptotic cells. Toxicology. 2004;195:167–76. doi: 10.1016/j.tox.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Langley RJ, Kalra R, Mishra NC, Hahn FF, Razani-Boroujerdi S, Singh SP, et al. A biphasic response to silica: I. Immunostimulation is restricted to the early stage of silicosis in lewis rats. Am J Respir Cell Mol Biol. 2004;30:823–9. doi: 10.1165/rcmb.2003-0284OC. [DOI] [PubMed] [Google Scholar]
- 42.Makol A, Reilly MJ, Rosenman KD. Prevalence of connective tissue disease in silicosis (1985-2006)-a report from the state of Michigan surveillance system for silicosis. Am J Ind Med. 2011;54:255–62. doi: 10.1002/ajim.20917. [DOI] [PubMed] [Google Scholar]
- 43.Bartunkova J, Pelclova D, Fenclova Z, Sediva A, Lebedova J, Tesar V, et al. Exposure to silica and risk of ANCA-associated vasculitis. Am J Ind Med. 2006;49:569–76. doi: 10.1002/ajim.20327. [DOI] [PubMed] [Google Scholar]
- 44.Beaudreuil S, Lasfargues G, Laueriere L, El Ghoul Z, Fourquet F, Longuet C, et al. Occupational exposure in ANCA-positive patients: a case-control study. Kidney Int. 2005;67:1961–6. doi: 10.1111/j.1523-1755.2005.00295.x. [DOI] [PubMed] [Google Scholar]