Dear Editor,
Growth of cells at clonal density, that is, <<1% confluence, is useful for studying cell migration and cell–cell interactions. Clonal growth from single cells is essential for isolating stem cells, separating cell types descended from a single stem cell precursor, and establishing clonal cell strains from humans, mutant mice, or genetically modified cells (Bennett et al., 1989; Miyazaki et al., 2008; Nishikawa-Torikai et al., 2011). Clonal survival distinguishes proliferative survival from cell cycle arrest, senescence, or delayed death, for which metabolic survival assays often lead to misleading conclusions about a drug's effectiveness. Clonality is the basis for detecting the creation of mutant cells, which occur so rarely that biochemical methods are inadequate. However, primary human melanocytes grow poorly, if at all, below ~ 10% confluence and cannot be established at clonal density on plastic. Feeder layers require a compromise between the growth media optimal for feeder cell and melanocyte. The lethal doses of inactivating carcinogens induce unknown stress responses, and the feeder cells must later be purified away before molecular assays can be carried out on the melanocytes.
HaCaT human keratinocytes deposit an extracellular matrix (ECM) that has been used to culture melanocytes at high density (Gilchrest et al., 1985). At high melanocyte density (≥25 000 cells/cm2), the laminin-332 in this ECM supports adhesion, spreading, and migration, as well as phosphorylation of focal adhesion kinase (Chung et al., 2011). ECM from the 804G rat bladder cell line supports these parameters and cell proliferation in epidermal carcinoma and pancreatic beta cells, with laminin-332 again the important component (Gonzales et al., 1999; Langhofer et al., 1993; Liu et al., 2009; Parnaud et al., 2009). Laminin-332 is major laminin in skin (Todorovic et al., 2010), and, unlike other laminins in the basement membrane, it supports the formation of hemi-desomsomes (Langhofer et al., 1993). Here, we report that cell-free ECM deposited by 804G cells, and to a lesser extent HaCaT, allows survival and proliferation of single primary human melanocytes and that laminin-332 is crucial.
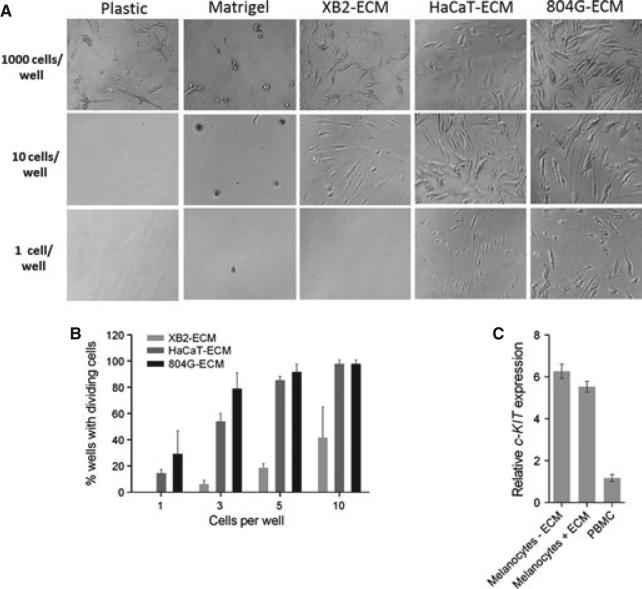
We used HaCaT, 804G, and the keratinocyte cell line XB2 to deposit human, rat, or mouse ECM, respectively, onto wells of a 96-well plate (Appendix S1) or used ECM containing laminin-111 (Matrigel). One, 10, or 1000 primary human melanocytes were flow-sorted and deposited into the ECM-coated wells. Seven or 14 days after sorting, primary human melanocytes were observed multiplying on ECM even at low density (Figure 1A). Clonal growth from a single cell occurred only on ECM from 804G or HaCaT cells. Cell attachment and proliferation were quantified by counting wells containing more than the initial number of cells after 14 days (Figure 1B). When three cells were deposited per well, melanocyte proliferation was observed in 79, 54, and 6% of wells coated with ECM from 804G, HaCaT, and XB2 cells, respectively. Precise plating efficiency was obtained by the P0 method from the percentage of wells containing no dividing cells after 14 days. For 804G ECM, plating efficiencies were 0.53 ± 0.15, 0.55 ± 0.20, and 0.36 ± 0.25 for 5, 3, or 1 cell per well, respectively, while HaCaT ECM gave half those of 804 ECM, 0.38 ± 0.06, 0.28 ± 0.02, and 0.16 ± 0.03. The –50% plating efficiency on 804G ECM is comparable to many immortal cell lines at high density. Growth kinetics and plating efficiency for several human donors are shown in Figure S1. Primary human melanocytes grown on ECM retained normal melanocytic characteristics such as dendrites (Figure 1A) and pigmentation (Figure 2). Another indication of normal differentiation was that mRNA expression of melanocyte marker c-KIT on 804G ECM was equal to that on plastic (Figure 1C).
Figure 1.
Clonal growth of primary human melanocytes on cell-deposited extracellular matrix (ECM). (A) Clonal growth occurs on ECM from cell lines secreting laminin-332, but not on ECM containing laminin-111 (Matrigel). Cell strain C6. Image at 1000 cells/well is from day 7; images for 10 and 1 cells/well from day 14. 200×. (B) Plating efficiency measured as percentage of wells containing dividing cells. Two repeats using donor C6 (mean ± SEM). (C) c-KIT mRNA expression is the same in primary human melanocytes cultured on plastic or 804G ECM. Expression is 8-fold lower in human peripheral blood monocytes. Cell strain C189 plated at 250 000 cells per 60-mm dish. Mean ± SEM.
Figure 2.
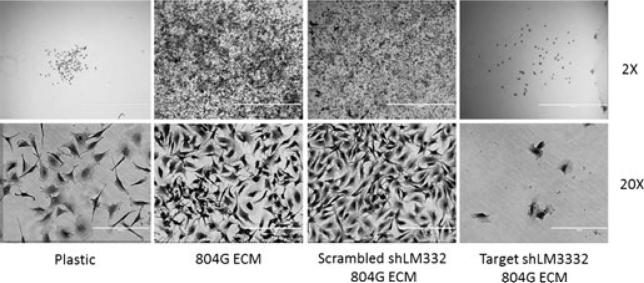
Requirement for laminin-332. Clonal growth on 804G ECM is suppressed when 804G cells have been stably transduced with retrovirus carrying shRNA against the γ2 subunit of laminin-332, but not by scrambled shRNA. Ten donor C189 melanocytes seeded per 35-mm dish by limiting dilution and assessed after 10 days. Scale bar, 400 μm.
We next examined whether the laminin-332 deposited by 804G and HaCaT cells was responsible for clonal melanocyte growth. Western blotting for the γ2 chain of laminin-332 showed that HaCaT and 804G cells contained higher levels of laminin-332 protein than the poorly supportive XB2 cells (Figure S2A). 804G cells, which gave the highest plating efficiency, contained only the fully processed form, cleaved from 150 kDa to 105 kDa (Kariya et al., 2012). This form supports the creation of hemidesmosomes (Goldfinger et al., 1998). We then tested whether clonal growth on HaCaT ECM was diminished by blocking antibody. P3H9-2 against human laminin-332 reduces proliferation of breast epithelial cells by 50% (Gonzales et al., 1999). Control mouse IgG did not affect growth on HaCaT human ECM (P > 0.05), but pre-incubation of ECM with the antilaminin-332 antibody at 10 and 30 μg/ml inhibited melanocyte growth by 57.4 and 46.7%, respectively (Figure S2B). To test laminin-332 in 804G cells, we obtained these cells stably transduced with retrovirus expressing shRNA for laminin-332 γ2 chain (shLM332) or a scrambled control shRNA (Scrambled shLM332) (Liu et al., 2009). Figure 2 shows occasional sparse miniclones on plastic, but 804G ECM fostered profuse growth of dendritic, pigmented cells. Scrambled shLM332 had little effect, but shRNA targeted to LM332 profoundly inhibited growth and health of primary melanocytes. The decrease in clone number was ~80% for clones greater than 100 cells (Figure S2C).
The clonal survival of primary human melanocytes could be measured using the ECM method (Figure S3). Seven days after exposure to ultraviolet radiation (UVB), 74% of the plated cells formed colonies after 1000 J/m2 UVB, compared with the plating efficiency of unirradiated cells, and 42% after 2000 J/m2.
The results imply that a major determinant of melanocyte clonal growth lies in the basement membrane. Using ECM for growth of single melanocytes should permit studies of cell migration, cell–cell communication, melanocyte stem cells and their lineages, and drivers of clonal expansion, enhancing our understanding of both therapeutics and basic biology.
Supplementary Material
Acknowledgements
We thank Drs. J. C. R. Jones, G. Merlino, and A. Weaver for the kind gifts of 804G, XB2, and shLM332-transfected 804G cells and Drs. D. Bennett, V. Muthusamy, and Z. Zhao for helpful conversations. Supported by Department of Defense CDMRP Grants CA093473P1 and CA093473 to DEB and RH. Normal human melanocytes were provided by the Specimen Resource Core of the Yale SPORE in Skin Cancer, NIH Grant CA121974 to RH; flow cytometry core facility supported by NCI Grant P30CA0 16359 to Yale Comprehensive Cancer Center.
References
- Bennett DC, Cooper PJ, Dexter TJ, Devlin LM, Heasman J, Nester B. Cloned mouse melanocyte lines carrying the germline mutations albino and brown: complementation in culture. Development. 1989;105:379–385. doi: 10.1242/dev.105.2.379. [DOI] [PubMed] [Google Scholar]
- Chung H, Suh EK, Han IO, Oh ES. Keratinocyte-derived laminin-332 promotes adhesion and migration in melanocytes and melanoma. J. Biol. Chem. 2011;286:13438–13447. doi: 10.1074/jbc.M110.166751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrest BA, Albert LS, Karassik RL, Yaar M. Substrate influences human epidermal melanocyte attachment and spreading in vitro. In Vitro Cell. Dev. Biol. 1985;21:114–120. doi: 10.1007/BF02620952. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JC. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J. Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M, Haan K, Baker SE, Fitchmun M, Todorov I, Weitzman S, Jones JC. A cell signal pathway involving laminin-5, alpha3beta1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol. Biol. Cell. 1999;10:259–270. doi: 10.1091/mbc.10.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya Y, Sato H, Katou N, Miyazaki K. Polymerized laminin-332 matrix supports rapid and tight adhesion of keratinocytes, suppressing cell migration. PLoS ONE. 2012;7:e35546. doi: 10.1371/journal.pone.0035546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhofer M, Hopkinson SB, Jones JC. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J. Cell Sci. 1993;105(Pt 3):753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Liu S, Yamashita H, Weidow B, Weaver AM, Quaranta V. Laminin-332-beta1 integrin interactions negatively regulate invadopodia. J. Cell. Physiol. 2009;223:134–142. doi: 10.1002/jcp.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Futaki S, Hasegawa K, Kawasaki M, Sanzen N, Hayashi M, Kawase E, Sekiguchi K, Nakatsuji N, Suemori H. Recombinant human laminin isoforms can support the undifferentiated growth of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2008;375:27–32. doi: 10.1016/j.bbrc.2008.07.111. [DOI] [PubMed] [Google Scholar]
- Nishikawa-Torikai S, Osawa M, Nishikawa S. Functional characterization of melanocyte stem cells in hair follicles. J. Invest. Dermatol. 2011;131:2358–2367. doi: 10.1038/jid.2011.195. [DOI] [PubMed] [Google Scholar]
- Parnaud G, Hammar E, Ribaux P, Donath MY, Berney T, Halban PA. Signaling pathways implicated in the stimulation of beta-cell proliferation by extracellular matrix. Mol. Endocrinol. 2009;23:1264–1271. doi: 10.1210/me.2009-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovic V, Desai BV, Eigenheer RA, Yin T, Amargo EV, Mrksich M, Green KJ, Patterson MJ. Detection of differentially expressed basal cell proteins by mass spectrometry. Molecular & cellular proteomics: MCP. 2010;9:351–361. doi: 10.1074/mcp.M900358-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.