Abstract
Most human physiologic set points like body temperature are tightly regulated and show little variation between healthy individuals. Red blood cell (RBC) characteristics such as hematocrit (HCT) and mean cell volume (MCV) are stable within individuals but can vary by 20% from one healthy person to the next. The mechanisms for the majority of this inter-individual variation are unknown and do not appear to involve common genetic variation. Here we show that environmental conditions present during development, namely in utero iron availability, can exert long-term influence on a set point related to the RBC life cycle. In a controlled study of rhesus monkeys and a retrospective study of humans, we use a mathematical model of in vivo RBC population dynamics to show that in utero iron deficiency is associated with a lowered threshold for RBC clearance and turnover. This in utero effect is plastic, persisting at least two years after birth and after the cessation of iron deficiency. Our study reports a rare instance of developmental plasticity in the human hematologic systems and also shows how mathematical modeling can be used to identify cellular mechanisms involved in the adaptive control of homeostatic set points.
Keywords: Anemias, Erythrocyte, Hematopoiesis- erythrocytes, Iron- anemia (experimental models), Red blood cell morphology
Introduction
Nutrition and other environmental factors acting during the fetal period are increasingly seen to influence metabolic and cardiovascular disease risk and outcomes in adulthood [1–3]. Epidemiologic evidence for this developmental plasticity in humans is significant, but it is difficult to infer mechanisms for persistent pathophysiologic changes from such retrospective studies. Also, the available controlled experimental evidence is largely limited to laboratory rodents and therefore does not explore the significant role of the prolonged fetal development of humans and other primates. An important fetal nutritional concern is third trimester iron deficiency, commonly seen in malnourished populations but also in populations with access to iron fortified foods and prenatal iron supplements. In 2007 the prevalence of third trimester anemia in the United States was 33% [4]. Anemia has long-term consequences for brain development but other effects are not clear [5, 6]. Effects of third-trimester iron deficiency on the infant’s postnatal hematologic status are largely unknown. During the fetal period in primates, important developmental transitions occur in erythropoietic processes including change of hematopoietic site (liver to bone marrow) [7], change in globin expression pattern (fetal to adult hemoglobin) [7, 8] and extensions in RBC half-life [9]. Most of the variation in hematologic characteristics in the healthy adult human population cannot be explained by common genetic variation [10–16]. It is possible that persistent changes in genes regulating cellular hematopoietic processes could occur in response to iron deficiency in the fetal period, perhaps via epigenetic mechanisms [17].
To investigate the effects of developmental iron status on longer-term hematologic function, we studied RBC population dynamics in rhesus monkeys and humans. We found steady state differences in RBC traits associated with developmental iron status: iron deprivation early in development was associated with lower mean RBC volume (MCV), mean RBC hemoglobin content (MCH), mean RBC hemoglobin concentration (MCHC), and higher RBC count. These in utero effects are plastic, persisting at least two years after birth and after the cessation of iron deficiency. We evaluated mechanisms for these steady state differences using a mathematical model of in vivo RBC population dynamics [18]. We find that that in utero iron deficiency is associated with a lowered threshold for RBC clearance and turnover. Our study reports a rare instance of developmental plasticity in the human hematologic system and also shows how mathematical modeling can be used to identify cellular mechanisms involved in the adaptive control of homeostatic set points.
Methods
Ethics Statement
All procedures followed the Guide for the Care and Use of Laboratory Animals of the National Research Council. CNPRC is accredited by the Association for Accreditation of Laboratory Animal Care. Protocols for this project were approved prior to implementation by the UC Davis Institutional Animal Care and Use Committee (IACUC). The human research protocol was approved by the Partners Healthcare Institutional Review Board.
Methods for the Rhesus Monkey Study
The rhesus monkey model for diet-induced third trimester anemia has been described previously [19]. Briefly, time-mated rhesus monkeys were assigned to iron sufficient (100 ppm iron, IS group) or iron deficient (10 ppm iron, ID group) purified diets at the time of identification of pregnancy at 39–42 days gestation. The study was conducted in two yearly cohorts of 10 dams each, 5 ID and 5 IS. Breeders were screened for prior reproductive history, and the two groups were balanced as much as possible for age, weight, and parity. There were no differences between iron deficient (ID) and iron sufficient (IS) dams contributing offspring to the study in background parameters (Supplementary Table S-I). All but two of the dams (one IS, one ID) were born at the California National Primate Research Center (CNPRC). Two sets of two infants (one ID, one IS) had the same sire, whereas the remaining infants had unique sires. For the present study, sex of the conceptus was also determined at this time [20], and only pregnancies with a male fetus were selected for the study. Ultrasound exams were conducted at gestation day (GD) 90 to confirm fetal viability. Feeding of the experimental diets continued until birth (spontaneous vaginal delivery). Pregnancies continuing beyond 175 days (expected gestation length 165 ±10 days) were considered post term and were terminated by c-section.
Details of diet composition and environmental control have been described [19]. The present study differed from previous experiments in the sex of the infants studied (male rather than mixed-sex). Males were chosen to increase power by decreasing intragroup variability due to sex differences. Erythropoiesis has been described as more active in male than female fetuses based on neonatal reticulocyte maturation index and serum transferrin [21], and RBC traits differ between male and female infants 4–9 month of age [22]. Other differences from previous work were in time of initiation of the experimental diet (GD45 vs. GD35 due to time required for determining fetal sex), and postnatal rearing (mother reared vs. nursery reared).
After birth, iron supplementation was provided to replete iron stores and terminate the period of iron deprivation. ID dams received iron dextran (10 mg/kg, i.m.), once per week for 4 weeks, beginning immediately following parturition. Three IS dams who were identified as anemic by veterinarians at birth also received the iron dextran treatment. ID infants were dosed orally with iron (Fer-in-Sol Iron Supplement Drops for Infants, 2 mg/kg) mixed with 5% dextrose (low iron infants) or dextrose only (control infants) beginning at 2 weeks of age. Dosing was done once per week until 5 weeks of age (4 times total). These iron supplementation regimens were adapted from veterinary recommendations for the dams and from human clinical recommendations for newborns. Infants remained with their dams through four months of age, after which weaning and social grouping were performed as described previously [23]. Beginning at 6 months of age infants participated in an extensive battery of behavioral tests, and noninvasive assessment including PET imaging, until 2 years of age when they were released from the study. Behavioral tests, along with growth measures and CNS assessments, have been reported separately [23].
Blood samples were obtained from infants at birth, 4, 6, 12 and 22.5 months for complete blood count (CBC), as well as measurement of ferritin and zinc protoporphyrin (ZPP). An additional serum sample was obtained at 6 and 12 months for measurement of serum transferrin, total iron binding capacity (TIBC), transferrin saturation (TfS), and serum iron. Methods for these assays have been described previously [19, 24]. When the persistent differences in RBC numbers seen in the first cohort were confirmed in the second cohort, a more detailed hematological assessment was conducted in the infants of the second cohort prior to release from the project at 2 years of age. CBCs were performed with the Siemens Advia 120 automated hematology analyzer to obtain distributions of single-RBC and single-reticulocyte volume and hemoglobin concentration.
Maternal endpoints were analyzed by ANOVA with diet group as the independent variable using general linear modeling (GLM) procedures in JMP9 (SAS, Cary NC). Infant CBC endpoints were analyzed by repeated measure analysis of variance (RMANOVA). Additionally, group differences were determined at each age by ANOVA for infant iron status endpoints. Multivariate analysis of this data was limited by the small sample size, but background variables (dam age, weight and parity, gestation length, birthweight) were screened for covariates and used in ANOVAs when relevant. Effect sizes were moderate (0.58–0.68) as analyzed by RMANOVA. Gestation length influenced MCV and MCH as noted previously in human studies [25] and consistent with increasing RBC lifespan with fetal age [9]. The statistical significance of diet group effects on these RBC parameters was increased by inclusion of gestation length as a covariate.
Methods for Mathematical Modeling of RBC Populations
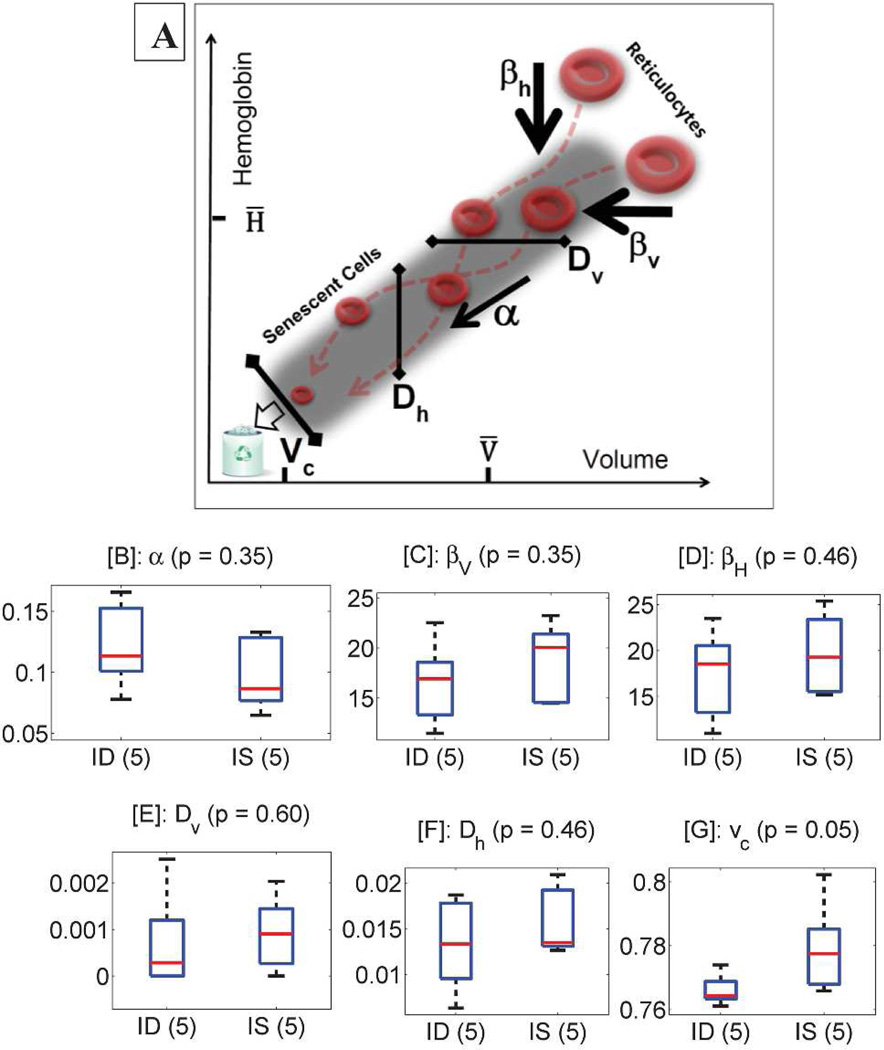
RBC population dynamics for monkeys and humans were modeled using single-cell measurements of RBC volume and hemoglobin concentration described above and a published mathematical model. See Figure 2A for a schematic. RBCs become smaller and lose hemoglobin as they age, with an initial fast phase of reduction following by slow phase [26, 27]. The rates in the initial fast phase are quantified for each individual by model parameters βv and βh. The initial phase of RBC maturation characterized by these fast rates of change is followed by a slower rate of change in both volume and hemoglobin, and the model quantifies this slower rate with the parameter α. The magnitude of variation in those rates across the RBC population (Dv and Dh) along with the RBC clearance threshold (vc) can also be inferred. Detailed methods have previously been published [18].
Figure 2. Dynamics of RBC maturation and clearance as modeled in rhesus monkeys.
Reticulocytes (top right of panel A) are released from the bone marrow into the circulation. The volume (x-axis) and hemoglobin content (y-axis) of individual reticulocytes decline rapidly at first and then more slowly, with rapid rates quantified by model parameters βv (panel C) and βh (D) and the slower rate by parameter α (panel D). The magnitude of variation in rates across the population of cells is quantified by model parameters Dv (panel E) and Dh (panel F), and the clearance threshold by vc (panel G). All parameters are dimensionless. See “Methods for Mathematical Modeling of RBC Populations” for detail. Boxplots show the extent and diversity for each estimated parameter. See the caption for Figure S2 for additional detail on boxplots.
Methods for the Human Study
We identified women with demonstrated iron deficiency or iron sufficiency during pregnancy at the study hospital, Massachusetts General Hospital. Iron deficiency corresponded to the presence of at least one low serum ferritin or iron measurement during the pregnancy, with a low measurement defined by the hospital reference range, which was typically 10 ng/ml for ferritin and 30 µg/ml for iron. Iron sufficiency was defined as the absence of documented anemia during the 300 days prior to delivery along with the absence of low iron or serum ferritin. Anemia corresponded to a hematocrit measurement below the hospital reference range. We then identified offspring resulting from these pregnancies who had had complete blood counts at the study hospital or an affiliated outpatient clinic between the ages of 1.6 years and 2.4 years. In order to focus our analysis on normal healthy hematologic function, we excluded any complete blood counts with low hematocrit, low MCV, low ferritin, or low iron, as well as any children with documented hemoglobinopathy, history of transfusion, or current iron deficiency. See Supplementary Table S-II for more detail on the study population size.
Results
We first investigated RBC dynamics in a nonhuman primate model, rhesus macaques, where third trimester iron deficiency is induced by a low iron diet [19, 24, 28]. In utero dietary iron deprivation (ID) was comparable to the 25th percentile of iron intake among pregnant US women [19], and thus represents a very mild perturbation which was not expected a priori to have an observable effect on infant hematology. Iron supply was normalized at birth, and hematologic characteristics were evaluated in offspring over the next 24 months, corresponding to about the first 8 years of life in humans. This experimental design parallels a common human scenario where mothers develop iron deficiency in the third trimester, but infants receive iron supplements or iron fortified formula and do not develop iron deficiency postnatally, thus limiting the iron deficiency to late fetal development in utero. There were no differences in dam CBC or iron status parameters at the initiation of the study or at gestational day 45 (GD45). See Supplementary Tables S-III and S-IV. As expected, group comparisons at the third trimester timepoint (GD150) showed significantly lower mean iron levels in the ID population as well as microcytic anemia: low RBC number density, hemoglobin concentration, MCV, and ferritin. Transferrin (TIBC) was elevated in the ID group. By one month after delivery, no group differences were seen in the dams.
There were no ID effects on gestation length, birthweight, or pregnancy outcome. Details of morphometric growth exams were reported separately [23]. Reports on normative CBC values for infant monkeys [29–33] indicate that human thresholds for anemia based on hemoglobin are applicable. Colony wide data on 3–4 month infants at CNPRC indicate an incidence of 34/695 (5%) infants with anemia based on this hemoglobin cutoff [34]. Based on this low incidence, there was insufficient power (21%) to detect 10% increased anemia incidence in the small experimental sample. However, none of the data analyzed here were from infants that met an anemia criterion, as shown in Figure 1.
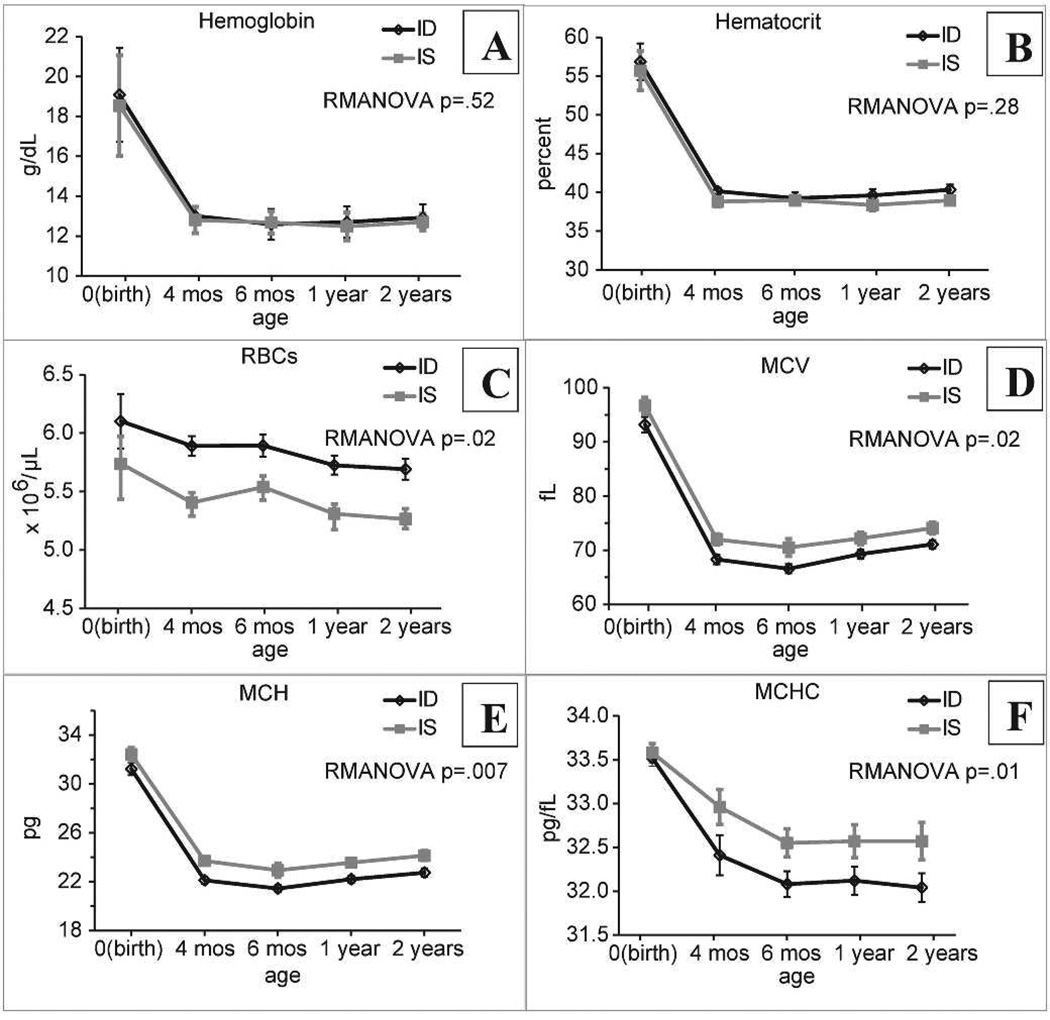
Figure 1. Red blood cell (RBC) characteristics.
Complete blood counts (CBCs) were performed between birth and two years of age for monkeys whose mothers received iron deprived (ID) or iron sufficient (IS) diets during pregnancy. Data are shown as mean ± SEM. N=10/group. Mean RBC volume (MCV); mean RBC hemoglobin content (MCH); mean RBC hemoglobin concentration (MCHC). P values for group comparisons from RMANOVA are shown in the figure. There was a significant effect of age for all endpoints (p=0.02–0.01), with no significant age*group interactions for any endpoint.
We note that while none of the ID infants was iron deficient at any age (see Supplementary Table S-V), there was less iron readily available to the ID animals in terms of iron stores at birth and transferrin saturation later in the postnatal period. Also, while maternal dietary iron deprivation was the independent variable in our study, the ID group also differed from the IS group in receiving iron supplementation after birth to terminate the period of iron deprivation. Thus it is possible that early iron supplementation, or iron deprivation followed by supplementation, were responsible for the diet group differences in infant RBC endpoints. Figure 1 shows that infant hemoglobin concentration (Fig. 1A) and hematocrit (Fig. 1B) were similar in both groups, declining in expected age-dependent fashion after birth to steady levels by six months of age. After the neonatal period, none of the infants was anemic at any time during the first two years of life, maintaining hemoglobin values > 11 g/dL.
Despite similar levels of overall blood oxygen carrying capacity (as measured by hemoglobin concentration and hematocrit), there were surprising and significant differences in characteristics of individual RBCs, and these single-RBC differences persisted for two years beyond the cessation of iron deprivation. The RBC concentration (Fig. 1C) was elevated in the ID group, while MCV, MCH, and MCHC were lower than in iron sufficient (IS) animals (Fig. 1D–F). There was no significant difference in the coefficient of variation (RDW) for volume (Supplementary Figure S1). The life cycle of the RBC can be divided into three phases: precursor proliferation in the bone marrow, maturation in the peripheral circulation, and clearance. Adaptive regulation likely occurs during all phases to provide RBCs of appropriate number, size, and hemoglobin content to support adequate oxygen delivery. Steady state characteristics of the circulating RBC population are determined both by the characteristics of the young RBCs (reticulocytes) entering from the bone marrow and by the dynamic processes of maturation and clearance taking place in the peripheral circulation [26, 27].
To investigate potential mechanisms for the persistent differences in hematologic set points seen in the ID animals, we measured single-RBC volumes and hemoglobin concentrations for populations of RBCs and reticulocytes from five ID and five IS animals at 24 months of age. We then applied a mathematical model of in vivo RBC population dynamics to quantify and compare the effect of iron deprivation on each part of the RBC life cycle [18]. See Figure 2A for a schematic of this mathematical model and “Methods for Mathematical Modeling of RBC Populations” for more detail. If changes in erythropoietic output were responsible for the set point differences, we would expect to find significant differences in reticulocyte population characteristics between the two groups. If changes in RBC maturation or clearance were responsible, then we would expect greater divergence between the steady state RBC populations as compared to that between the reticulocyte populations.
We found no significant difference in erythropoietic output (See Supplementary Figure S2), with similar values for mean reticulocyte volume (rMCV), variation in reticulocyte volume from one cell to the next (rRDW), mean hemoglobin content (rMCH), mean reticulocyte hemoglobin concentration (rMCHC), and reticulocyte percentage (retic). There was a trend toward lower single-RBC hemoglobin content in the ID animals, but this trend did not reach statistical significance.
Thus the observed differences in RBC characteristics likely reflect alterations in the dynamic processes of RBC maturation and clearance. RBCs become smaller and lose hemoglobin in part through vesicle formation as they mature [26, 27]. The molecular and biophysical mechanisms for these maturation processes are unclear and involve multiple types of effector cells operating at distributed anatomic sites in vivo and consequently present significant experimental challenges for direct characterization. A mathematical model of RBC population dynamics[18] enables us to estimate typical rates for these in vivo maturation processes and to compare them in different individuals who are healthy or have only mild disease. The model (Equation 1) describes the volume and hemoglobin dynamics of a typical RBC as a function (f) of its current volume and hemoglobin and accounting for fluctuations in these rates over time (ζ). The dynamics of the full RBC population include production of reticulocytes (b) and clearance of senescent cells (c). See Figure 2A for a schematic of RBC maturation and its parameterization by the model.
| (1) |
We found no significant differences in the average rates of volume and hemoglobin reduction for ID and IS animals. Both the initial rapid phase of maturation (quantified by model parameters βv and βh in Fig. 2C–D) and the subsequent slower phase (quantified by model parameter α in Fig. 2B) were comparable in the two groups. These single-cell rates of change vary for different RBCs in an individual, and Figures 2E and 2F show that there was no significant difference in the magnitude of variation for volume reduction (Dv) or for hemoglobin reduction (Dh). We did find a significant difference in the RBC clearance threshold (vc in Figure 2G). The detailed mechanism for RBC clearance in general is not well understood and is unlikely to be determined by volume or hemoglobin content alone, but because the clearance signal is highly correlated with these quantities, we can compare empirical clearance thresholds between the groups in terms of volume and hemoglobin content. We find that RBCs in ID animals continue to circulate at significantly lower volumes and hemoglobin contents than they do in IS animals, even after normalizing for the lower MCV and MCH in the ID cohort. In IS animals, most RBCs have been removed before their volume reaches 77% of the animal’s MCV, while in ID animals the clearance threshold is lower, and cells continue to mature and circulate beyond this point.
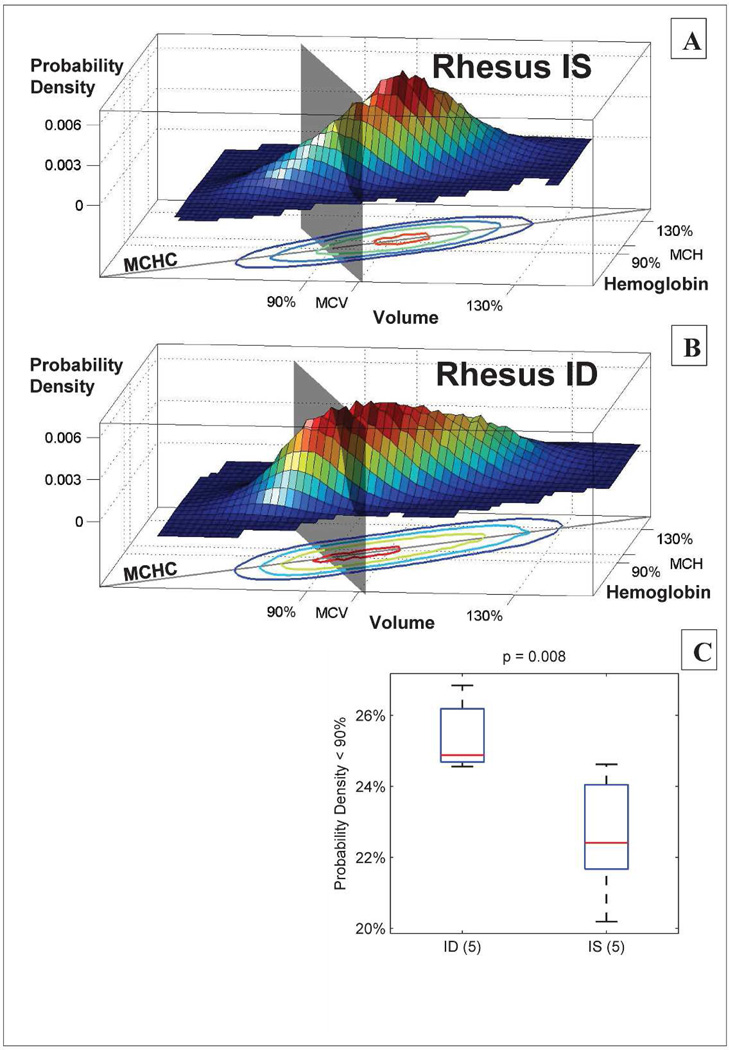
Cleared RBCs cannot be observed, but different clearance thresholds will lead to differences in the remaining circulating cell population. If the ID clearance threshold is reduced as predicted, then there should be more cells with low volume and hemoglobin in the ID animals. We tested this prediction and found it to be true with high significance (p = 0.008). Figure 3 shows that a greater fraction of the circulating cells in ID animals are smaller than 90% of the MCV and contain less than 90% of the MCH. A delayed clearance in the ID animals will increase the number of circulating cells with small volumes and low hemoglobin contents, thereby lowering the MCV and MCH, as is observed. This clearance delay will not have any effect on the rMCV and rMCH, and one would therefore expect the rMCV/MCV ratio to be larger in the ID animals, leading to an expansion in the number of cells with volumes that are large relative to the MCV, as is seen by comparing the very high-volume end of the contour plots in Figure 3. We thus find evidence independent of the model that a mild decrease in in utero iron availability is associated with a persistent delay in RBC clearance in rhesus macaques.
Figure 3. In utero iron deficiency is associated with persistent delay in RBC clearance in two-year-old rhesus monkeys.
Panels A and B show the single-RBC volume and hemoglobin distributions for an IS (panel A) and an ID (panel B) animal. A gray plane divides the populations at 90% of the MCV and MCH. In the IS RBC population, 21% of the circulating RBCs are located between the plane and origin at steady state, as compared to 27% in the ID animal. Panel C shows that this difference between the ID (5 animals) and IS (5 animals) groups is significant with a p-value of 0.008 (one-sided rank sum test), supporting the conclusion that in utero iron deprivation causes a long-lasting delay in RBC clearance with more low volume and hemoglobin cells in the ID animals. The “MCHC” lines in A and B show the mean single-RBC hemoglobin concentration. Contour lines in A and B enclose 90%, 75%, 50%, and 12.5% of cells.
The RBC clearance thresholds for two-year-old monkeys compared in Fig. 3C are presented as dimensionless values normalized by each animal’s MCV in order to control for inter-individual variation in MCV. Because a delay in RBC clearance will increase the fraction of cells with small volume thereby decreasing MCV, some of this inter-individual variation in MCV is a direct result of different clearance thresholds, and the dimensional clearance thresholds may be a more meaningful characterization of the RBC clearance processes. Supplementary Figure S3 compares clearance thresholds in units of absolute volume and shows a similar difference with even greater statistical significance. A lower clearance threshold may lead to an increased average RBC lifespan. However, it is also possible that the lowered clearance threshold is associated with an increased rate of maturation, which could offset the effect of a lowered clearance threshold on the average RBC lifespan. Even without an associated increase in maturation rate, the modest reduction in clearance threshold (~1% in Fig. 2G) would yield at most a similarly modest increase in average RBC lifespan.
Ferritin, serum iron, TIBC, TfS, ZPP, and serum iron in Supplementary Table S-V showed anticipated age-related changes over the first two years of life. ID effects were identified across all ages only for transferrin saturation (RMANOVA: diet F(1,18)=13.04, p=0.002) which was consistently lower in the ID group after birth. Also, ferritin was significantly lower in ID than IS infants at birth (p=0.007) indicating lower initial iron stores entering the postnatal period. Thus, although none of the ID infants had iron deficiency anemia at any age in terms of hemoglobin, less iron seemed to be available to the ID animals in terms of iron stores at birth and transferrin saturation later in the postnatal period. Notably, at two years of age, the ID group showed lower values than the IS group for three parameters (TIBC, transferrin saturation, and serum iron). These changes could be influential in producing the RBC characteristics observed and warrant further investigation.
We next looked for empirical evidence of this developmental plasticity in RBC homeostasis in humans. Because it is not ethical to perform a randomized clinical trial of in utero iron deficiency in humans, we analyzed historical RBC data from children whose mothers had documented iron status during pregnancy. We compared children at age 2, a developmental stage comparable to 6 months in the rhesus monkeys when the most dramatic age-related changes in erythroid physiology have occurred and the hematologic characteristics have become relatively stable, as seen in Figure 1. We considered only children with normal HCT and MCV to focus on the processes controlling healthy hematologic function. See “Methods for the Human Study” for more detail.
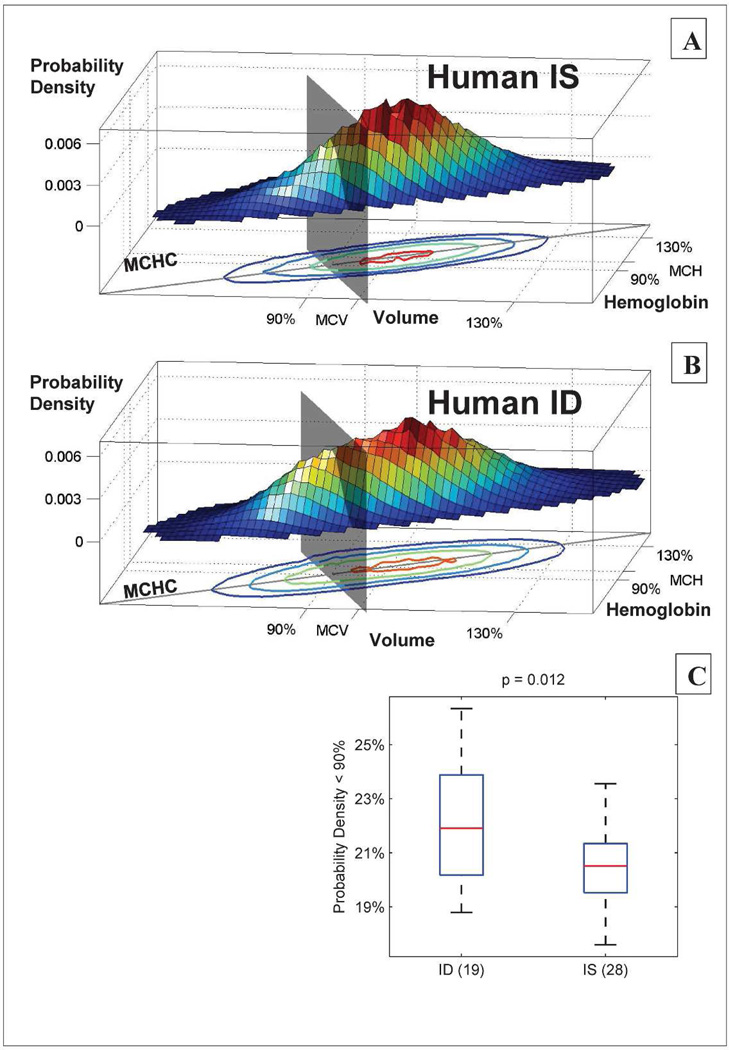
We found evidence for the same clearance delay in two-year-old healthy human children whose mothers were iron deficient during pregnancy. Figure 4 shows that there were more circulating RBCs with low volume and hemoglobin in children whose mothers were iron deficient during pregnancy. This significant difference is particularly surprising because pregnant women identified as iron deficient are typically treated immediately to reverse the deficit, reducing the duration of iron deficiency and also reducing the likelihood that we would see evidence of plasticity. This lowered clearance threshold in ID children was associated with an increase in the RDW. We also found a slight but significant reduction in HCT for the typical ID child compared to the typical IS child. See Supplementary Figure S4 for detail. No child had a high RDW, and all HCTs were within study hospital reference ranges by design. Overall, we find that in utero iron deficiency leads to a persistent lowering of the RBC clearance threshold, making a significant contribution to the healthy phenotypic variation in hematologic set points.
Figure 4. In utero iron deficiency is associated with persistent delay in RBC clearance in two-year-old humans.
Panels A and B show the single-RBC volume and hemoglobin distributions for a typical IS (panel A) and a typical ID (panel B) child. A gray plane divides the populations al 90% of the MCV and MCH. For the IS child, 19% of the circulating RBCs are located between the plane and origin at steady state, as compared to 25% for the ID child. Panel C shows that this difference between the 19 ID children and 28 IS children is significant with a p-value of 0.012 (one-sided rank sum test), supporting the model prediction that in utero iron deprivation causes a long-lasting delay in RBC clearance with more low volume and hemoglobin cells in the ID children. The “MCHC” lines in A and B show the mean single-RBC hemoglobin concentration. Contour lines in A and B enclose 90%. 75%, 50%, and 12.5% of cells.
Discussion
Developmental plasticity acts by definition over long time-scales, linking conditions present during early stages to phenotypes that may not arise for many years [1]. These phenotypes, such as hypertension or risk of diabetes, involve pathology at the organ system level or above, and mechanisms are thus challenging to elucidate even when inciting conditions are more proximal. Our study shows that mathematical modeling can decompose these complex phenotypes into different mechanistic components, enabling us to identify the specific cellular-level process responsible for composite phenotypic differences. Taken in isolation, the individual findings in the study would be modestly persuasive, but the integration of the modeled clearance threshold difference seen in the small cohort of monkeys typical for a non-human primate study (Fig. 2G), the signature of delayed clearance in their raw blood count data (Fig. 3), and the confirmation of this same specific finding in a two-year-old human population (Fig. 4) provides a compelling mechanistic argument for developmental plasticity of human RBC set points.
Despite altered RBC clearance thresholds, the overall hemoglobin levels in the ID monkeys and ID children were normal. It is possible that the shifted RBC clearance is an adaptive strategy to maintain total circulating red cell mass (and oxygen carrying capacity) despite initially reduced erythropoietic output, as has been suggested for the lowered RBC clearance threshold seen in mild iron deficiency in adult humans [18]. This altered homeostatic set point may thus represent a hematologic example of the developmental plasticity seen for metabolic traits and comprising the “thrifty phenotype” [3]. This pattern of delayed clearance with normal hemoglobin level has been previously reported in humans with thalassemia trait [18], the most common clinically significant human genetic variant worldwide [35, 36]. The developmental plasticity reported here may therefore also share some common features with the congenital response to thalassemic genetic variants. Previous studies have shown a systematic increase in RBC lifespan during late gestation, implicating developmental regulation of RBC maturation and clearance that could potentially result in different set points depending on the fetal environment. This possibility is further supported by the effects of gestation length on infant RBC endpoints at birth through the first two years of life in our study, previously reported only at birth in humans [7, 25].
The long-term behavioral effects of fetal iron deprivation justify further study to determine how much prenatal iron deprivation is needed to trigger persistent alterations in RBC traits and the role of these hematologic alterations in long-term brain development and function. This study shows how mechanistic mathematical modeling of human cellular physiology enables characterization and identification of the key in vivo cellular processes governing homeostasis and focuses future investigations of their molecular mechanisms. Our work also motivates analogous study of healthy variation in other homeostatic set points where long-lasting adaptation to environmental conditions present during development may explain some of the healthy human phenotypic heterogeneity that does not appear to have a basis in common genetic variation.
Supplementary Material
Acknowledgments
The authors wish to thank and acknowledge the contributions of the animal care and veterinary medicine staff of CNPRC; Abigail Spinner who conducted the CBCs; Joan Shewmaker who conducted the measurement of individual RBC population distributions; Rosy Jaromin who provided database support for medical record searches; and Maureen Hames, Ana Fernandes, Alexandra Barrasso, and Judith Oakley who assisted with medical record review. Medical record queries were facilitated by the Partners Healthcare Research Patient Data Repository. All mathematical modeling and simulation was done on the Harvard Medical School Orchestra computing cluster. MSG and CEH acknowledge the support of NIH grants HD39386 and P51OD011107. JMH acknowledges that this work was funded by the National Institutes of Health through the NIH Director’s New Innovator Award Program, 1-DP2-DK098087 and NIH DK083242.
Footnotes
Author Contributions
MSG designed monkey experiment, wrote manuscript; CEH scheduled and supervised monkey sample collection, prepared monkey database and summaries; RM analyzed and interpreted single-cell monkey and human reticulocyte and RBC data; JMH designed human experiment, analyzed and interpreted single-cell monkey and human reticulocyte and RBC data, and wrote manuscript.
Conflicts of Interest
The authors have no conflicts of interest.
References
- 1.Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gluckman PD, Hanson MA. Living with the past: Evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 3.Hales CN, Barker DJP. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- 4.Reinold C, Dalenius K, Smith B, et al. Prenancy Nutrition Surveillance 2007 Report. Atlanta: U. S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2009. [Google Scholar]
- 5.Allen LH. Anemia and iron deficiency: effects on pregnancy outcome. Am J Clin Nutr. 2000;71:1280S–1284S. doi: 10.1093/ajcn/71.5.1280s. [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Beard J, Connor J, et al. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi JW, Pai SH. Change in erythropoiesis with gestational age during pregnancy. Ann Hematol. 2001;80:26–31. doi: 10.1007/s002770000229. [DOI] [PubMed] [Google Scholar]
- 8.Stamatoyannopoulos G. Control of globin gene expression during development and erythroid differentiation. Exp Hematol. 2005;33:259–271. doi: 10.1016/j.exphem.2004.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brace RA, Langendorfer C, Song TB, et al. Red blood cell life span in the ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1196–R1204. doi: 10.1152/ajpregu.2000.279.4.R1196. [DOI] [PubMed] [Google Scholar]
- 10.Okada Y, Kamatani Y. Common genetic factors for hematological traits in humans. J Hum Genet. 2012;57:161–169. doi: 10.1038/jhg.2012.2. [DOI] [PubMed] [Google Scholar]
- 11.Ganesh SK, Zakai NA, van Rooij FJA, et al. Multiple loci influence erythrocyte phenotypes in the CHARGE Consortium. Nature Genet. 2009;41 doi: 10.1038/ng.466. 1191-U1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soranzo N, Spector TD, Mangino M, et al. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nature Genet. 2009;41 doi: 10.1038/ng.467. 1182-U1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira MAR, Hottenga JJ, Warrington NM, et al. Sequence Variants in Three Loci Influence Monocyte Counts and Erythrocyte Volume. Am J Hum Genet. 2009;85:745–749. doi: 10.1016/j.ajhg.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang Q, Kathiresan S, Lin JP, et al. Genome-wide association and linkage analyses of hemostatic factors and hematological phenotypes in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S12. doi: 10.1186/1471-2350-8-S1-S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kullo IJ, Ding K, Jouni H, et al. A genome-wide association study of red blood cell traits using the electronic medical record. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benjamin EJ, Dupuis J, Larson MG, et al. Genome-wide association with select biomarker traits in the Framingham Heart Study. BMC Med Genet. 2007;8(Suppl 1):S11. doi: 10.1186/1471-2350-8-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanhees K, Coort S, Ruijters EJ, et al. Epigenetics: prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J. 2011;25:797–807. doi: 10.1096/fj.10-172155. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JM, Mahadevan L. Physiological and pathological population dynamics of circulating human red blood cells. Proc Natl Acad Sci U S A. 2010;107:20587–20592. doi: 10.1073/pnas.1012747107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golub MS, Hogrefe CE, Tarantal AF, et al. Diet-induced iron deficiency anemia and pregnancy outcome in rhesus monkeys. Am J Clin Nutr. 2006;83:647–656. doi: 10.1093/ajcn.83.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jimenez DF, Tarantal AF. Fetal gender determination in early first trimester pregnancies of rhesus monkeys (Macaca mulatta) by fluorescent PCR analysis of maternal serum. J Med Primatol. 2003;32:315–319. doi: 10.1046/j.1600-0684.2003.00041.x. [DOI] [PubMed] [Google Scholar]
- 21.Choi JW, Kim CS, Pai SH. Erythropoietic activity and soluble transferrin receptor level in neonates and maternal blood. Acta Paediatr. 2000;89:675–679. doi: 10.1080/080352500750043981. [DOI] [PubMed] [Google Scholar]
- 22.Domellof M, Lonnerdal B, Dewey KG, et al. Sex differences in iron status during infancy. Pediatrics. 2002;110:545–552. doi: 10.1542/peds.110.3.545. [DOI] [PubMed] [Google Scholar]
- 23.Golub MS, Hogrefe CE, Unger EL. Influence of prenatal iron deficiency and MAOA genotype on response to social challenge in rhesus monkey infants. Genes, Brain and Behavior. 2012;11:278–290. doi: 10.1111/j.1601-183X.2012.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golub MS, Hogrefe CE, Germann SL, et al. Behavioral consequences of developmental iron deficiency in infant rhesus monkeys. Neurotoxicol Teratol. 2006;28:3–17. doi: 10.1016/j.ntt.2005.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alur P, Devapatla SS, Super DM, et al. Impact of race and gestational age on red blood cell indices in very low birth weight infants. Pediatrics. 2000;106:306–310. doi: 10.1542/peds.106.2.306. [DOI] [PubMed] [Google Scholar]
- 26.Waugh RE, Narla M, Jackson CW, et al. Rheologic properties of senescent erythrocytes: loss of surface area and volume with red blood cell age. Blood. 1992;79:1351–1358. [PubMed] [Google Scholar]
- 27.Willekens FL, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, et al. Hemoglobin loss from erythrocytes in vivo results from spleen-facilitated vesiculation. Blood. 2003;101:747–751. doi: 10.1182/blood-2002-02-0500. [DOI] [PubMed] [Google Scholar]
- 28.Golub MS, Hogrefe CE, Germann SL. Iron deprivation during fetal development changes the behavior of juvenile rhesus monkeys. J Nutr. 2007;137:979–984. doi: 10.1093/jn/137.4.979. [DOI] [PubMed] [Google Scholar]
- 29.Anderson J, Keen C, Lonnerdal B. Iron deficiency in outdoor corral housed juvenile rhesus monkeys. Lab Anim Sci. 1983;33:494. [Google Scholar]
- 30.Bicknese EM, George JW, Hird DW, et al. Prevalence and risk factors for iron deficiency anemia in weanling rhesus macaques. Lab Anim Sci. 1993;43:434–438. [PubMed] [Google Scholar]
- 31.Kriete MF, Champoux M, Suomi SJ. Development of iron deficiency anemia in infant rhesus macaques. Lab Anim Sci. 1995;45:15–21. [PubMed] [Google Scholar]
- 32.Lubach GR, Coe CL. Preconception maternal iron status is a risk factor for iron deficiency in infant rhesus monkeys (Macaca mulatta) J Nutr. 2006;136:2345–2349. doi: 10.1093/jn/136.9.2345. [DOI] [PubMed] [Google Scholar]
- 33.Munro N. A three year study of iron deficiency and behavior in rhesus monkeys. International Journal of Biosocial Research. 1987;9:35–62. [Google Scholar]
- 34.Golub MS, Hogrefe CE, Widaman KF, et al. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol. 2009;51:47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fucharoen S, Winichagoon P, Wisedpanichkij R, et al. Prenatal and postnatal diagnoses of thalassemias and hemoglobinopathies by HPLC. Clin Chem. 1998;44:740–748. [PubMed] [Google Scholar]
- 36.Bain B. Haemoglobinopathy diagnosis. Malden, MA: Blackwell Pulications; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.