Supplemental Digital Content is Available in the Text.
KEY WORDS: Endoscopic burr, Foraminal stenosis, Foraminotomy, Percutaneous endoscopic
Abstract
BACKGROUND:
Although several authors have reported the use of endoscopic techniques to treat lumbar foraminal stenosis, the practical application of these techniques has been limited to soft disc herniation.
OBJECTIVE:
To describe the details of the percutaneous endoscopic lumbar foraminotomy (ELF) technique for bony foraminal stenosis and to demonstrate the clinical outcomes.
METHODS:
Two years of prospective data were collected from 33 consecutive patients with lumbar foraminal stenosis who underwent ELF. The surgical outcomes were assessed using the visual analog scale, Oswestry Disability Index, and modified MacNab criteria. The procedure begins at the safer extraforaminal zone rather than the riskier intraforaminal zone. Then, a full-scale foraminal decompression can be performed using a burr and punches under endoscopic control.
RESULTS:
The mean age of the 18 female and 15 male patients was 64.2 years. The mean visual analog scale score for leg pain improved from 8.36 at baseline to 3.36 at 6 weeks, 2.03 at 1 year, and 1.97 at 2 years post-surgery (P < .001). The mean Oswestry Disability Index improved from 65.8 at baseline to 31.6 at 6 weeks, 19.7 at 1 year, and 19.3 at 2 years post-surgery (P < .001). Based on the modified MacNab criteria, excellent or good results were obtained in 81.8% of the patients, and symptomatic improvements were obtained in 93.9%.
CONCLUSION:
Percutaneous ELF under local anesthesia could be an efficacious surgical procedure for the treatment of foraminal stenosis. This procedure may offer safe and reproducible results, especially for elderly or medically compromised patients.
ABBREVIATIONS:
ELF,endoscopic lumbar foraminotomy
ODI, Oswestry Disability Index
VAS, visual analog scale
Conventional surgical methods for lumbar foraminal or far lateral stenosis can be categorized as total facetectomy with/without fusion and facet-preserving microforaminotomy. Total facetectomy offers sufficient decompression through the nerve root course. However, this often leads to segmental instability and back pain.1-4 A facet-preserving microdecompression technique using a paraspinal approach was reported by Reulen et al1 in 1987 and Wiltse and Spencer5 in 1988; this technique has since been modified by other surgeons. This technique enables direct access to a foraminal or far lateral lesion with minimal violation of the facet joint and less postoperative back pain.6 The success rates reported for open paraspinal foraminotomy are as high as 72% to 83%,4,7-14 and this technique has been considered the gold standard for the surgical treatment of lumbar foraminal or far lateral stenosis. However, some patients experience postoperative leg pain or dysesthesia, which are the primary causes of unfavorable outcomes.4,7,8,10,12 Excessive manipulation of the dorsal root ganglion is thought to cause dysesthesia.10,12 Moreover, a limited field of view in the paraspinal approach may lead to incomplete decompression.
To solve the above problems, various minimally invasive techniques have been developed. Some authors have reported the use of a technique known as percutaneous endoscopic foraminal discectomy and/or foraminoplasty.15-17 However, the application of these minimally invasive techniques has been limited to foraminal soft disc herniation or concurrent mild foraminal stenosis. We reported the technique of percutaneous endoscopic lumbar foraminotomy (ELF) for L5-S1 level.18 However, a safe approach to the stenotic foramen remains challenging, and a thorough decompression technique for hard bony stenosis has been difficult to achieve. Moreover, the practical application of this technique has been limited by its steep learning curve. We have developed a more practical form of this technique than that described in a previous study18 and applied it not only to the L5-S1 level but also other lumbar levels. The purpose of this study was to describe this advanced ELF technique and to demonstrate the clinical outcomes.
METHODS
This longitudinal cohort study was based on 35 patients with lumbar foraminal stenosis who underwent ELF between January 2009 and September 2011. During the 2-year follow-up period, 2 patients (5.7%) were lost to follow-up. Therefore, prospective data were collected from the remaining 33 patients.
Inclusion Criteria and Outcome Evaluation
The inclusion criteria were as follows: patients with symptomatic lumbar foraminal stenosis despite more than 6 weeks of conservative treatment, including selective nerve root blockade. Radiculopathy with foraminal or extraforaminal stenosis regardless of disc herniation was confirmed by both computed tomography and magnetic resonance imaging. The radiographic indications were moderate to severe foraminal stenosis with perineural fat obliteration or nerve root collapse based on the Wildermuth grading system.19 The exclusion criteria were intracanalicular stenosis, segmental instability, or coexisting pathological conditions such as acute inflammation, infection, and tumor.
Patient data were obtained from chart reviews and patient-based outcome questionnaires or telephone interviews. At each follow-up, the patients completed questionnaires that reflected their functional status and pain intensity. The patients' back pain and radicular leg pain levels were assessed using the visual analog scale (VAS) score. Functional status was assessed using the Oswestry Disability Index (ODI). The clinical outcomes were also assessed using the modified MacNab criteria.
Statistical Analysis
The comparison between pre- and postoperative clinical outcomes in pain and functional status was performed by using repeated-measures analysis of variance and a paired t test with Bonferroni method for adjustment of multiple comparisons. P values <.05 were considered significant.
Surgical Technique
The unique characteristic of the surgical technique in this study that distinguishes it from the standard transforaminal endoscopic discectomy technique20-22 is the use of epidural foraminal or extraforaminal procedures, which preserve the maternal disc, as opposed to intradiscal procedures. Discectomy is optional and performed when appropriate. The surgical process can be summarized into 3 steps: a safe extraforaminal landing, endoscopic foraminal unroofing, and sophisticated and full-scale foraminal decompression (see Video, Supplemental Digital Content, which demonstrates the 3 steps of the surgical process, http://youtu.be/aON-g_ZagqE).
Patient Preparation
As a premedication, midazolam (0.05 mg/kg) is injected intramuscularly 30 minutes before surgery. Fentanyl (0.8 μg/kg) is intravenously administered immediately before surgery, followed by additional doses as needed. The level of sedation should be titrated so that the patient is able to communicate with the surgeon during the procedure. The patient is placed in the prone position on a radiolucent table. The skin entry point is located 8 to 13 cm lateral to the midline, depending on the patient's waist size. To determine an appropriate entry point and approach angle, preoperative imaging studies and intraoperative fluoroscopy should be carefully considered.
Extraforaminal Landing
An 18-gauge spinal needle is inserted after the administration of local anesthesia (Figure 1A). Unlike the usual transforaminal endoscopic discectomy technique, the main target of this procedure is not the herniated disc fragment, but the foraminal nerve root entrapment by bony stenosis and thickened foraminal ligaments. Thus, the target point of the initial needling is the surface of the facet joint, not the intradiscal portion. The needle can be firmly engaged with the facet joint and then replaced by a guidewire. A tapered obturator is inserted over the guidewire, slid along the facet surface into the foramen using gentle manual pressure, and finally firmly engaged with the foramen. This maneuver prevents nerve root damage and enables a safe working space by pushing the exiting nerve root away from the surgical field. If the patient experiences pain during the procedure, the obturator can be placed on the surface of the superior facet. After the correct placement of the obturator in the foramen (not in the disc), a bevel-ended working cannula is introduced over the obturator and is placed on the undersurface of the facet joint. After the obturator is withdrawn, an ellipsoidal working channel endoscope is inserted. Initially, the surgeon can view the surface of the superior facet via endoscopic visualization. At this point, the direct intraforaminal placement of the working cannula or endoscope might be painful or dangerous due to nerve root injury in the stenotic foraminal space. Thus, the working cannula and endoscope should be placed outside the foramen and just contact the facet surface (floating-endoscopy technique).
FIGURE 1.
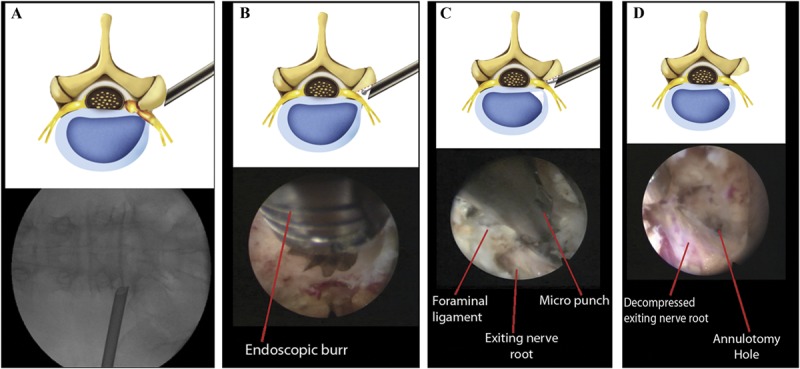
Schematic illustrations and corresponding intraoperative fluoroscopic and endoscopic views of the surgical procedure. A, extraforaminal placement of the working cannula for foraminal decompression. B, foraminal unroofing using an endoscopic burr. C, sophisticated foraminal decompression using various instruments, including punches, forceps, and a laser. Note the exiting nerve root decompression using a micropunch. D, final view of the full-scale foraminal decompression status. Note the decompressed exiting nerve root and annulotomy site.
Endoscopic Foraminal Unroofing
The hypertrophied part of the facet is removed using a specially designed endoscopic burr (Figure 1B). This foraminal unroofing process can be safely performed under both endoscopic and fluoroscopic guidance. In cases of osteoporosis or weak bones, a bone-removing trephine (bone reamer) can also be used to rapidly cut the facet. However, for the removal of normal bone, an endoscopic drill is safer and more effective than a bone reamer. The direction of the bone removal should be from the outside to the inside and from the inferior pedicle to the superior pedicle. The drilling should be performed until the ligamentum flavum and epidural fat begin to appear. After the hypertrophied superior facet is undercut, intraforaminal structures such as the foraminal ligament, ligamentum flavum, perineural fat covering the exiting nerve root, shoulder osteophyte, and disc surface should appear clearly. The working cannula and endoscope are now firmly engaged with the widened foraminal portion; a sophisticated exploration can now be performed. At this time, the sharp end of the bevel-ended working cannula should be positioned far from the exiting nerve root by rotating the cannula.
Full-Scale Foraminal Decompression
The hypertrophied foraminal ligament, including the ligamentum flavum and shoulder osteophyte, can be removed using various instruments (Figures 1C and 1D). Bone debris and tenacious ligaments can be removed using endoscopic graspers and punches. The redundant disc and soft tissues can be coagulated using bipolar radiofrequency. Supplementary use of a holmium yttrium-aluminum-garnet (Ho:YAG) laser may be helpful to ablate tissue debris. Any extruded disc fragments can also be removed as required. A flexible probe under both endoscopic and fluoroscopic guidance can be used to confirm the decompression. The surgeon can see and mobilize both the traversing nerve root and exiting nerve root under endoscopic visualization. The endpoint of the procedure is free mobilization of the exiting nerve root. After adequate hemostasis with a bipolar coagulator and hemostatic agent, the endoscope is withdrawn, and a sterile dressing is applied with a 1-point subcutaneous suture. The patient should be monitored for several hours if there are any postoperative problems. Patients usually undergo immediate postoperative magnetic resonance imaging and are permitted to be discharged (Figures 2 and 3).
FIGURE 2.
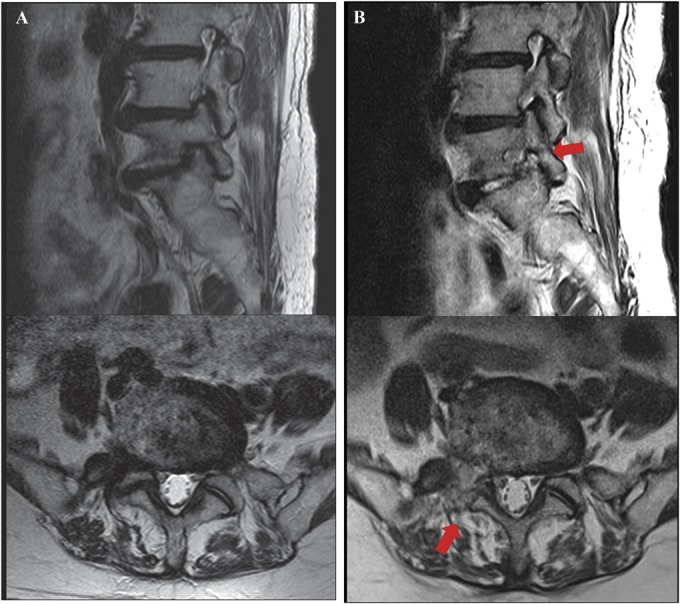
Illustrated case of a 78-year-old female patient. A, preoperative magnetic resonance (MR) images showing severe foraminal stenosis at the right L5-S1 level. B, postoperative MR images showing full-scale foraminal decompression (arrow) after endoscopic lumbar foraminotomy.
FIGURE 3.
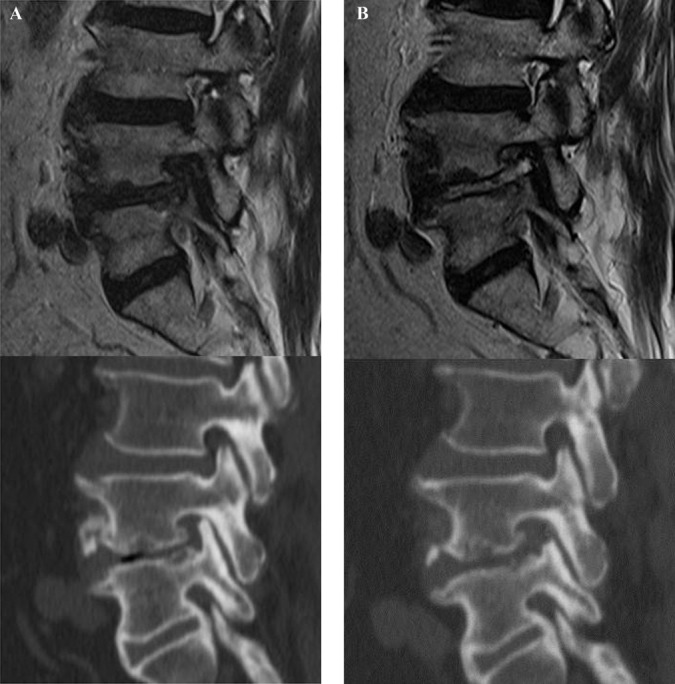
Illustrated case of a 67-year-old male patient. A, preoperative magnetic resonance and computed tomography (CT) images showing severe foraminal stenosis with disc herniation and facet impingement at the left L4-5 level. B, postoperative MR and CT images showing complete foraminal unroofing and visualization of exiting nerve root after endoscopic lumbar foraminotomy.
RESULTS
Demographics and Surgical Data
There were 18 women and 15 men (33 patients) with a mean age of 64.2 years (range, 27-81 years). The operated-on levels were L2-3 in 2 patients, L3-4 in 8, L4-5 in 15, and L5-S1 in 11 (36 levels). Eleven patients had a history of lumbar surgery; 5 of these patients underwent open discectomy at the same level, 2 underwent open discectomy at different levels, and 4 underwent fusion surgery at the adjacent levels. Eighteen of the 33 patients had neurological deficits in the form of muscle weakness or sensory deficits in the distribution of the affected nerve root. The mean operative time was 55.6 minutes per level (SD = 19.1 minutes; range, 35-120 minutes). The mean hospital stay after the procedure was 1.36 days (SD = 0.65 days; range, 1-4 days).
Clinical Outcomes
The mean preoperative VAS score for back pain was 5.36 (SD = 2.09). This score improved to 3.15, 2.61, 2.12, and, finally, 2.09 (SD = 1.77) at postoperative 6 weeks, 6 months, 1 year, and 2 years, respectively (P < .001) (Figure 4A). The mean preoperative VAS score for leg pain was 8.36 (SD = 1.32). This score changed to 3.36, 2.70, 2.03, and, finally, 1.97 (SD = 1.86) at postoperative 6 weeks, 6 months, 1 year, and 2 years, respectively (P < .001) (Figure 4B). The mean preoperative ODI score was 65.8% (SD = 17.4%). The mean postoperative ODI score was 31.6%, 28.0%, 19.7%, and, finally, 19.3% (SD = 16.7%) at postoperative 6 weeks, 6 months, 1 year, and 2 years, respectively (P < .001) (Figure 5). At the final follow-up review, the modified MacNab criteria were rated as follows: excellent in 13 patients (39.4%), good in 14 patients (42.4%), fair in 4 patients (12.1%), and poor in 2 patients (6.1%). Therefore, excellent or good results were obtained in 81.8% of the patients, and the rate of symptomatic improvement was 93.9% (Figure 6). One patient who underwent ELF at the L4-5 level required subsequent open facetectomy and fusion surgery because of incomplete decompression. A hidden disc extrusion missed during an earlier endoscopic foraminotomy was found during the second surgery. Two patients experience postoperative dysesthesia in the same dermatomal distribution. Both patients required selective nerve root block and oral gabapentin medication. Their symptoms were transient and improved over a 3-month period. There were no other major complications.
FIGURE 4.
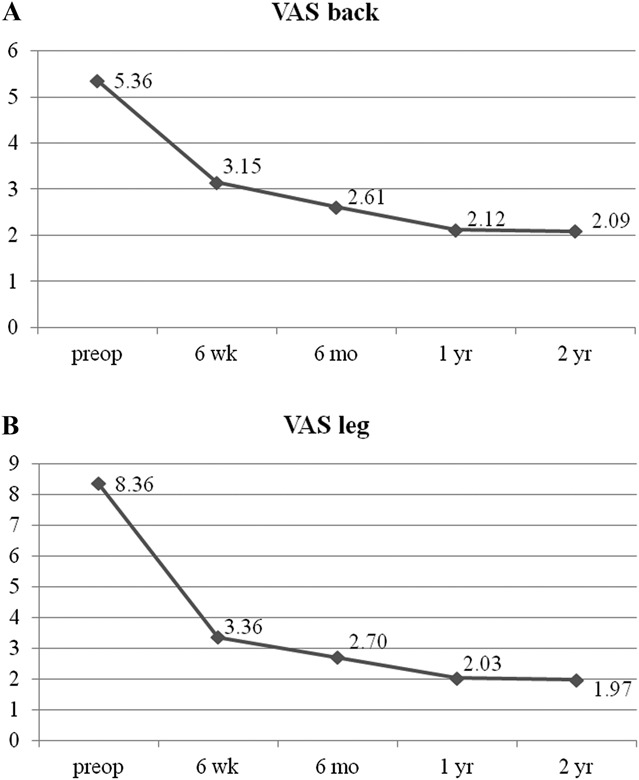
A, Visual analog scale (VAS) pain score for back pain preoperatively (preop) and at 6 weeks, 6 months, 1 year, and the final review (2 years) post-surgery. B, VAS pain score for radicular pain preoperatively and at 6 weeks, 6 months, 1 year, and the final review (2 years) post-surgery.
FIGURE 5.
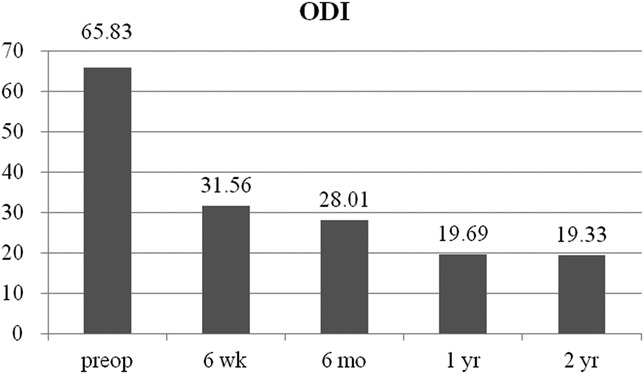
Oswestry Disability Index (ODI) scores preoperatively (preop) and at 6 weeks, 6 months, 1 year, and the final review (2 years) post-surgery.
FIGURE 6.
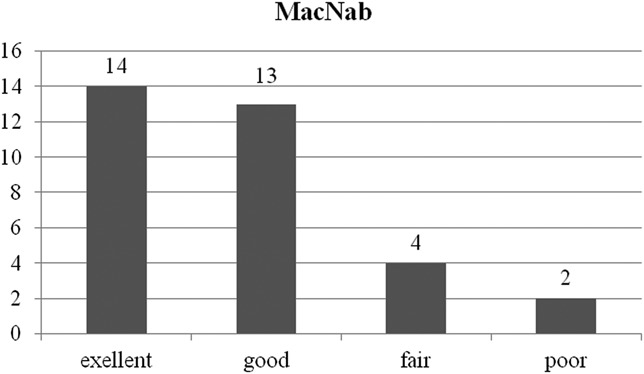
The global outcome according to the modified MacNab criteria. Twenty-seven of the 33 patients (81.8%) experienced excellent or good results, and 31 of the 33 patients (93.9%) experienced symptomatic improvement.
DISCUSSION
History of Percutaneous Endoscopic Foraminal Decompression
Since Hijikata,23 Onik et al,24 and Kambin and Sampson25 introduced the concept of posterolateral percutaneous lumbar disc decompression, several researchers have developed percutaneous endoscopic techniques for foraminal decompression. Knight et al15,16,26 introduced laser foraminoplasty for various foraminal nerve root entrapment syndromes. They used a side-firing laser to ablate soft tissues such as foraminal ligaments and osteophytes compressing the exiting nerve root. We described an ELF technique using a bone reamer and laser.18 Schubert and Hoogland17 also reported the use of a bone reamer for foraminoplasty in cases of migrated disc herniation. However, the previous techniques have limited applications in definite foraminal decompression. The use of a laser is effective only for neural entrapment caused by soft tissue or fragile osteophytes. Unfortunately, the laser may be less effective and more time-consuming for severe bony stenosis. Bone reamers can rapidly cut the hypertrophied bone. However, the use of bone reamers has an inherent disadvantage: as a blind technique, it does not allow direct visual control. Some authors have reported a percutaneous endoscopic technique for foraminal/extraforaminal disc herniation.27,28 However, their techniques can be applied only in cases of soft disc herniation, not bony stenosis of the foramen.
Characteristics of ELF
The main target of the ELF technique is lumbar foraminal stenosis that compresses the exiting nerve root by various pathologies such as hypertrophied bone and ligament, osteophytes, and/or redundant disc. Therefore, the surgeon should continually confirm the position of the exiting nerve root throughout the entire procedure. Full-scale foraminal decompression can then be performed around the exiting nerve root. A selective discectomy is optional, and the maternal disc can be preserved as required. The distinctive characteristics of this ELF technique can be summarized into 2 points: extraforaminal landing with a floating-endoscopy technique and full-scale foraminal decompression using various instruments such as a burr and punch under endoscopic vision control. First, an extraforaminal landing is one of the most important technical tips for safe foraminal decompression. The procedure begins at the safer extraforaminal zone rather than the riskier intraforaminal or intradiscal zone. In the usual transforaminal approach, the working cannula should be placed in the foraminal or intradiscal zone before the decompression process. However, the usual intraforaminal landing to the stenotic foramen may be difficult and challenging. The narrowness of the foraminal space impedes the proper placement of the working cannula and may irritate the exiting nerve root during the approach process. Therefore, severe approach-related pain or postoperative flares may occur due to nerve root irritation or injury. Instead, we place the working cannula at the extraforaminal zone (more precisely, on the surface of the superior facet) and then initiate the decompression process (floating-endoscopy technique). The working field is then gradually changed from outside to inside the foramen as the decompression process proceeds. This floating-endoscopy technique is useful for preventing neural damage or irritation. Second, the full-scale foraminal decompression process can be performed using various surgical instruments, such as an endoscopic burr, punch and flexible forceps, under clear endoscopic visualization, instead of using a blind bone reamer. As mentioned previously, a bone reamer can quickly cut the hypertrophied facet or osteophyte under fluoroscopic guidance. However, sculpting the bone tissue with a bone reamer is essentially a blind technique. This technique may cause unintended bleeding, inadequate bone removal, or even neural tissue damage. The use of a high-speed drill under clear endoscopic visualization enables safer and more efficacious bone removal. With the articulating bone burr (TipControl; Richard Wolf GmbH, Knittlingen, Germany), we were able to adjust the drilling direction and cover a wider working space. After the removal of the hypertrophied facet and part of the pedicle, the remaining bony shell and soft tissues can be removed via endoscopic punches, forceps, and a laser.
Technical Issues in Successful Decompression
We consider the most important aspect of this procedure to be identification of the location of the exiting nerve root before sophisticated decompression. The stenotic foramen is very narrow, and the exiting nerve root is subject to injury during the foraminal approach. When the working cannula is placed on the foramen, the sharp end of the bevel-ended cannula should be positioned far from the exiting nerve root by rotating the cannula. Once the working cannula is engaged around the foramen, the surgeon should locate the course of the exiting nerve root and confirm a safe working zone from the beginning of the endoscopic procedure. Another important point is that foraminal unroofing should be performed until the ligamentum flavum is exposed. It is not sufficient to remove only the lateral part of the superior facet, which exposes only a part of the exiting nerve root. The exiting nerve root should be fully decompressed from the proximal beginning point to the extraforaminal part.
Clinical Outcome and Surgical Data
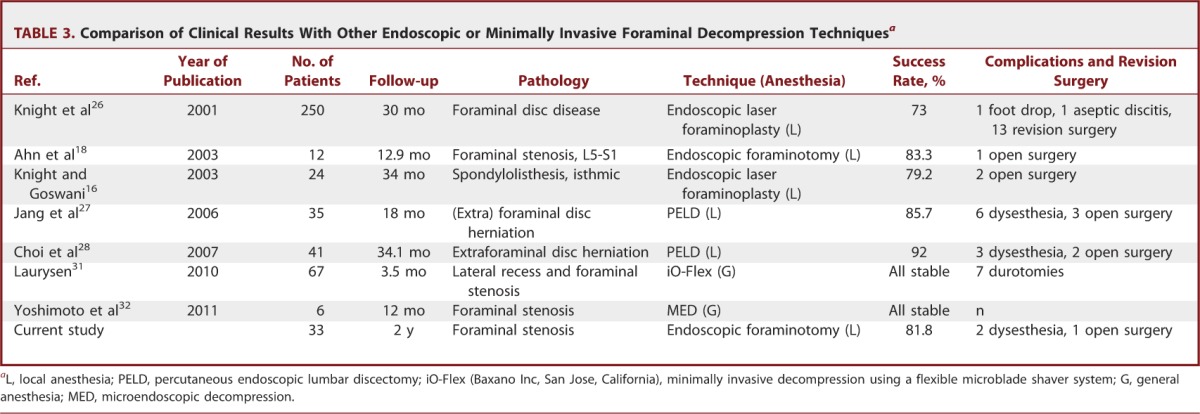
We found significant improvements in both pain score and functional status at the reviews performed at 6 weeks, 6 months, 1 year, and 2 years. The mean decrease in the VAS score at the final review was 6.36 (SD = 2.19) for leg pain and 3.24 (SD = 2.02) for back pain (P < .001). These data indicate that this technique is efficient for decompressing the exiting nerve root, and this effect continued over a 2-year follow-up period. The mean decrease in the ODI score at the final review was 46.5 (SD = 24.2) (P < .001). A reduction in the ODI score of more than 20% is considered clinically relevant.29,30 Therefore, our series exhibited clinically significant levels of functional improvement. According to the modified MacNab criteria, the global success rate in our series was 81.8%, and the symptomatic improvement was 93.9%. These outcomes are comparable to those of other published series of open decompression surgery patients (Table 1).4,7-14 Regarding surgical data, the mean operative times reported for open foraminal decompression surgery were 65.8 to 156 minutes, and the estimated blood losses were 56 to 90 mL.10,11,13 In the current work, the ELF procedures were performed for 55.6 minutes on average with patients under local anesthesia with negligible blood loss. Therefore, our surgical data demonstrate a relatively shorter operative time and less blood loss compared with open decompression surgery (Table 2). Our data are also comparable to those of other endoscopic or minimally invasive decompression techniques.16,18,26-28,31,32 The overall success rates of the case series ranged from 73% to 100%. We think that direct comparison among those studies is difficult because the type and degree of foraminal pathologies might be somewhat different. However, it would be helpful to shape the future of minimally invasive foraminal decompression techniques (Table 3).
TABLE 1.
Comparison of Clinical Results With Conventional Open Decompression Surgery for Lumbar Foraminal Stenosis
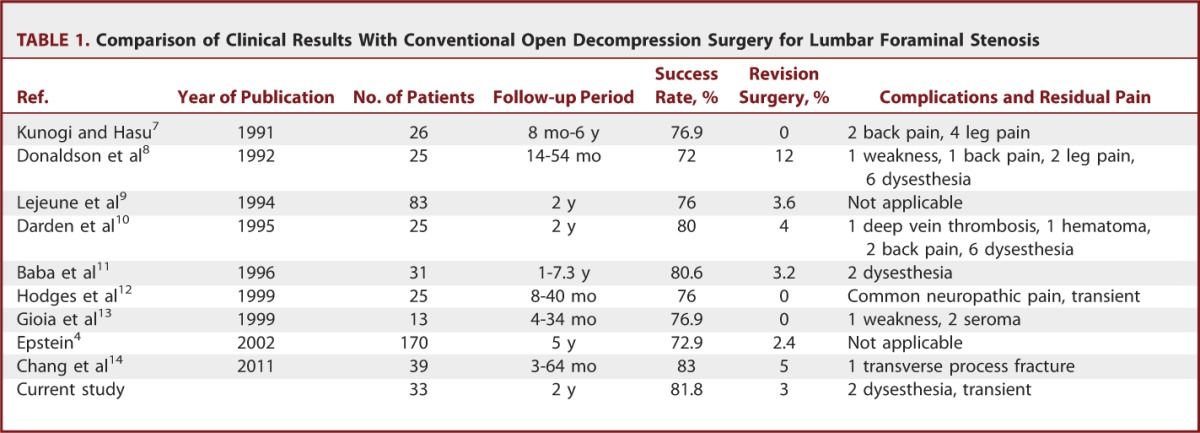
TABLE 2.
Comparison of Perioperative Data With Conventional Open Decompression Surgery for Lumbar Foraminal Stenosis
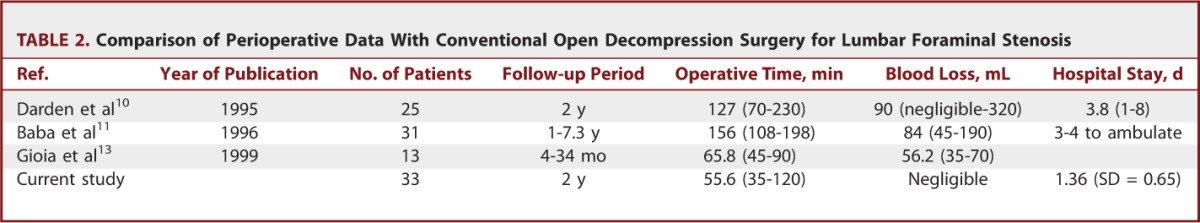
TABLE 3.
Comparison of Clinical Results With Other Endoscopic or Minimally Invasive Foraminal Decompression Techniquesa
Complications and Reoperation
Excessive manipulation or irritation of the dorsal root ganglion during foraminal decompression surgery may cause postoperative dysesthesia. The reported rates of postoperative dysesthesia or numbness after open decompression surgery are 6.5% to 24%.8,10,11 In our series, there were 2 patients with transient postoperative dysesthesia (6.1%); this symptom subsided within 3 months. There were no other major complications in our series. Regarding the reoperation rate, the rates reported for open decompression surgery are 2.4% to 12%.4,8-11,14 In our series, 1 (3%) of the 33 patients underwent open fusion surgery due to incomplete decompression. Therefore, our complication rate and reoperation rate are in agreement with published values for open decompression surgery (Table 1). In general, the primary advantages of endoscopic spine procedures are less traumatization and a lower complication rate.33-35 Our surgical data and clinical outcomes of endoscopic foraminotomy demonstrated considerable benefits in terms of tissue trauma.
Limitations and the Future of ELF
Although this technique has the benefits of full-scale foraminal decompression with less trauma, there are some limitations. First, there may be a learning curve for this novel technique. The use of a drill and punches under endoscopic control is unfamiliar to most spine surgeons. Second, in cases of extreme far lateral stenosis caused by hypertrophied sacral ala at the L5-S1 level, the ELF technique may still be challenging. A different approach angle and the rotation of the endoscope should be required for the removal of the sacral ala.
CONCLUSION
Endoscopic lumbar foraminotomy with patients under local anesthesia is a safe and effective technique for lumbar foraminal stenosis. This surgical technique has become more practical and standardized. Therefore, this technique may offer more reliable and reproducible results, especially for elderly or medically compromised patients.
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Acknowledgments
The authors express a special acknowledgment to Jae-Eun Park, BS, and Sol Lee, BS, for preparing the figures and manuscript.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.neurosurgery-online.com).
COMMENTS
In this study, the authors describe their version of percutaneous endoscopic lumbar foraminotomy (ELF) and evaluate the outcomes and complications in 33 patients. Standard measures including VAS pain score, ODI, and the modified MacNab criteria were assessed, and outcomes were comparable to the equivalent open procedure based on the reported literature. In general, complications were low, including postoperative dysesthesia, which was attributed to the use of the floating-endoscopy technique. As with all minimally invasive procedures, the primary benefit is decreased adjacent tissue injury, which typically translates into less bleeding and a shorter hospital stay. This benefit was seen in this study with negligible blood loss and a mean stay of 2 days, which, when compared with the literature on open decompression, was an improvement. The greatest issue is that most neurosurgeons are not trained in spinal endoscopy so the learning curve involved is likely steep. Given that the outcomes seem comparable to those with the open procedure and relative benefits of percutaneous ELF (negligible vs mean <100 mL blood loss in the open cases) are not great, it remains to be seen how well this procedure will be adopted. The authors should be congratulated for describing this technique and advancing the field of minimally invasive spine surgery.
Paul Park
Ann Arbor, Michigan
The authors are to be commended for their contribution in describing a novel minimally invasive technique for lumbar foraminal decompression. This is a well-structured, nonblinded prospective study, providing level II evidence, analyzing an endoscopic extraforaminal technique for lumbar nerve root decompression in 33 patients. The clinical outcomes are impressive, and, as such, the technique appears to have merit. The results appear to be favorable compared with those of other published studies of open and minimally invasive or endoscopic decompression.
Although the clinical outcomes speak for themselves, I believe that there may be some criticism regarding the degree of foraminal decompression that is possible with this technique. The authors do provide pre- and postoperative images demonstrating a good result; however, it would be more powerful to quantify the degree of decompression for the entire cohort given that pre- and postoperative imaging was done in each case. It is my understanding that such a study is under way by this group, and I look forward to reviewing the results.
Dean Karahalios
Evanston, Illinois
Endoscopic lumbar procedures have appeared in the neurosurgical literature for roughly 20 years, although the mainstay of treatment has been open procedures. A not insignificant reason is that most lumbar issues can be adequately addressed via open approaches with very good results. However, a particular area of difficulty remains with foraminal stenosis as the open approaches often require significant bony resection of the facet, larger incisions for lateral exposure, and the like. This study reviews 33 patients undergoing endoscopic lumbar foraminotomy in which the clinical outcomes (VAS score, ODI score, modified McNab) were excellent with minimal complications (2 patients with transient dysesthesia, 1 patient requiring reoperation). The authors offer a thorough explanation of the procedure, and the accompanying video demonstrates the procedure clearly. Although the study was not blinded, the results are nevertheless impressive.
The allures of percutaneous endoscopic techniques are the potential for less tissue trauma, faster recoveries, and shortened hospital stays. These endpoints will need to be compared in blinded studies to show the superiority of this application. Time will tell whether these techniques will eclipse the more standard approaches. As with all endoscopic procedures, surgeons wishing to pursue them will take on novel learning curves and therefore the benefit will need to be demonstrable to effect that change. However, for the application as described, this procedure is certainly exciting and may offer a competitive advantage to current techniques.
Nathan E. Simmons
Lebanon, New Hampshire
In this nicely illustrated report, the authors describe their experience in surgically treating 33 patients with foraminal stenosis with a minimally invasive, extraforaminal approach. Their technique places the incision 8-13 centimeters off of the midline and utilizes the bony facet as a docking point during fluoroscopic positioning of a percutaneous access cannula. Favorable results were seen in over 90 percent of patients with statistically significant improvement in the VAS leg pain scores at two-year follow up. One patient required reoperation for incomplete decompression and two patients complained of postoperative dysesthesia that regressed over the course of three months.
As a practical matter, it is not clear that the described technique provides a magnitude of benefit that would prompt wide adoption by neurosurgeons who, as a group, are generally more comfortable performing surgery using the operating microscope than the endoscope. Still, the report is of interest with regard to the “outside in” description of the operative technique that emphasizes the importance of not attempting to initially place tools within the stenotic foramen that might cause neurologic injury and the use of specialized tools, the specially designed endoscopic burr in particular.
John K. Houten
Bronx, New York
Figure.
No available caption
REFERENCES
- 1.Reulen HJ, Pfaundler S, Ebeling U. The lateral microsurgical approach to the “extracanalicular” lumbar disc herniation. I: a technical note. Acta Neurochir (Wien). 1987;84(1-2):64-67 [DOI] [PubMed] [Google Scholar]
- 2.Garrido E, Connaughton PN. Unilateral facetectomy approach for lateral lumbar disc herniation. J Neurosurg. 1991;74(5):754-756 [DOI] [PubMed] [Google Scholar]
- 3.Jenis LG, An HS. Spine update. Lumbar foraminal stenosis. Spine (Phila Pa 1976). 2000;25(3):389-394 [DOI] [PubMed] [Google Scholar]
- 4.Epstein NE. Foraminal and far lateral lumbar disc herniations: surgical alternatives and outcome measures. Spinal Cord. 2002;40(10):491-500 [DOI] [PubMed] [Google Scholar]
- 5.Wiltse LL, Spencer CW. New uses and refinements of the paraspinal approach to the lumbar spine. Spine (Phila Pa 1976). 1988;13(6):696-706 [PubMed] [Google Scholar]
- 6.Chang SB, Lee SH, Ahn Y, Kim JM. Risk factor for unsatisfactory outcome after lumbar foraminal and far lateral microdecompression. Spine (Phila Pa 1976). 2006;31(10):1163-1167 [DOI] [PubMed] [Google Scholar]
- 7.Kunogi J, Hasue M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine (Phila Pa 1976). 1991;16(11):1312-1320 [DOI] [PubMed] [Google Scholar]
- 8.Donaldson WF, III, Star MJ, Thorne RP. Surgical treatment for the far lateral herniated lumbar disc. Spine (Phila Pa 1976). 1993;18(10):1263-1267 [DOI] [PubMed] [Google Scholar]
- 9.Lejeune JP, Hladky JP, Cotten A, Vinchon M, Christiaens JL. Foraminal lumbar disc herniation. Experience with 83 patients. Spine (Phila Pa 1976). 1994;19(17):1905-1908 [DOI] [PubMed] [Google Scholar]
- 10.Darden BV, II, Wade JF, Alexander R, Wood KE, Rhyne AL, III, Hicks JR. Far lateral disc herniations treated by microscopic fragment excision. Techniques and results. Spine (Phila Pa 1976). 1995;20(13):1500-1505 [DOI] [PubMed] [Google Scholar]
- 11.Baba H, Uchida K, Maezawa Y, Furusawa N, Okumura Y, Imura S. Microsurgical nerve root canal widening without fusion for lumbosacral intervertebral foraminal stenosis: technical notes and early results. Spinal Cord. 1996;34(11):644-650 [DOI] [PubMed] [Google Scholar]
- 12.Hodges SD, Humphreys SC, Eck JC, Covington LA. The surgical treatment of far lateral L3-L4 and L4-L5 disc herniations. A modified technique and outcomes analysis of 25 patients. Spine (Phila Pa 1976). 1999;24(12):1243-1246 [DOI] [PubMed] [Google Scholar]
- 13.Gioia G, Mandelli D, Capaccioni B, Randelli F, Tessari L. Surgical treatment of far lateral lumbar disc herniation. Identification of compressed root and discectomy by lateral approach. Spine (Phila Pa 1976). 1999;24(18):1952-1957 [DOI] [PubMed] [Google Scholar]
- 14.Chang HS, Zidan I, Fujisawa N, Matsui T. Microsurgical posterolateral transmuscular approach for lumbar foraminal stenosis. J Spinal Disord Tech. 2011;24(5):302-307 [DOI] [PubMed] [Google Scholar]
- 15.Knight MT, Vajda A, Jakab GV, Awan S. Endoscopic laser foraminoplasty on the lumbar spine—early experience. Minim Invasive Neurosurg. 1998;41(1):5-9 [DOI] [PubMed] [Google Scholar]
- 16.Knight M, Goswami A. Management of isthmic spondylolisthesis with posterolateral endoscopic foraminal decompression. Spine (Phila Pa 1976). 2003;28(6):573-581 [DOI] [PubMed] [Google Scholar]
- 17.Schubert M, Hoogland T. Endoscopic transforaminal nucleotomy with foraminoplasty for lumbar disk herniation. Oper Orthop Traumatol. 2005;17(6):641-661 [DOI] [PubMed] [Google Scholar]
- 18.Ahn Y, Lee SH, Park WM, Lee HY. Posterolateral percutaneous endoscopic lumbar foraminotomy for L5-S1 foraminal or lateral exit zone stenosis. Technical note. J Neurosurg. 2003;99(suppl 3):320-323 [DOI] [PubMed] [Google Scholar]
- 19.Wildermuth S, Zanetti M, Duewell S, et al. Lumbar spine: quantitative and qualitative assessment of positional (upright flexion and extension) MR imaging and myelography. Radiology. 1998;207(2):391-398 [DOI] [PubMed] [Google Scholar]
- 20.Yeung AT, Tsou PM. Posterolateral endoscopic excision for lumbar disc herniation: surgical technique, outcome, and complications in 307 consecutive cases. Spine. 2002;27:722-731 [DOI] [PubMed] [Google Scholar]
- 21.Ahn Y, Lee SH, Park WM, Lee HY, Shin SW, Kang HY. Percutaneous endoscopic lumbar discectomy for recurrent disc herniation: surgical technique, outcome, and prognostic factors of 43 consecutive cases. Spine. 2004;29(16):E326. [DOI] [PubMed] [Google Scholar]
- 22.Ahn Y. Transforaminal percutaneous endoscopic lumbar discectomy: technical tips to prevent complications. Expert Rev Med Devices. 2012;9(4):361-366 [DOI] [PubMed] [Google Scholar]
- 23.Hijikata S. Percutaneous discectomy: a new treatment method for lumbar disc herniation. J Toden Hosp. 1975;5:5-13 [Google Scholar]
- 24.Onik G, Helms CA, Ginsburg L, Hoaglund FT, Morris J. Percutaneous lumbar diskectomy using a new aspiration probe. AJR Am J Roentgenol. 1985;144(6):1137-1140 [DOI] [PubMed] [Google Scholar]
- 25.Kambin P, Sampson S. Posterolateral percutaneous suction-excision of herniated lumbar intervertebral discs. Report of interim results. Clin Orthop Relat Res. 1986;207:37-43 [PubMed] [Google Scholar]
- 26.Knight MT, Goswami A, Patko JT, Buxton N. Endoscopic foraminoplasty: a prospective study on 250 consecutive patients with independent evaluation. J Clin Laser Med Surg. 2001;19(2):73-81 [DOI] [PubMed] [Google Scholar]
- 27.Jang JS, An SH, Lee SH. Transforaminal percutaneous endoscopic discectomy in the treatment of foraminal and extraforaminal lumbar disc herniations. J Spinal Disord Tech. 2006;19(5):338-343 [DOI] [PubMed] [Google Scholar]
- 28.Choi G, Lee SH, Bhanot A, Raiturker PP, Chae YS. Percutaneous endoscopic discectomy for extraforaminal lumbar disc herniations: extraforaminal targeted fragmentectomy technique using working channel endoscope. Spine (Phila Pa 1976). 2007;32(2):E93-E99 [DOI] [PubMed] [Google Scholar]
- 29.Ng LC, Tafazal S, Sell P. The effect of duration of symptoms on standard outcome measures in the surgical treatment of spinal stenosis. Eur Spine J. 2007;16(2):199-206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). 2008;33(1):90-94 [DOI] [PubMed] [Google Scholar]
- 31.Lauryssen C. Technical advances in minimally invasive surgery: direct decompression for lumbar spinal stenosis. Spine (Phila Pa 1976). 2010;35(suppl 26):S287-S293 [DOI] [PubMed] [Google Scholar]
- 32.Yoshimoto M, Takebayashi T, Kawaguchi S, et al. Minimally invasive technique for decompression of lumbar foraminal stenosis using a spinal microendoscope: technical note. Minim Invasive Neurosurg. 2011;54(3):142-146 [DOI] [PubMed] [Google Scholar]
- 33.Mayer HM, Brock M. Percutaneous endoscopic discectomy: surgical technique and preliminary results compared to microsurgical discectomy. J Neurosurg. 1993;78(2):216-225 [DOI] [PubMed] [Google Scholar]
- 34.Hermantin FU, Peters T, Quartararo L, Kambin P. A prospective, randomized study comparing the results of open discectomy with those of video-assisted arthroscopic microdiscectomy. J Bone Joint Surg Am. 1999;81(7):958-965 [DOI] [PubMed] [Google Scholar]
- 35.Ruetten S, Komp M, Merk H, Godolias G. Full-endoscopic interlaminar and transforaminal lumbar discectomy versus conventional microsurgical technique: a prospective, randomized, controlled study. Spine (Phila Pa 1976). 2008;33(9):931-939 [DOI] [PubMed] [Google Scholar]