Summary
Background
Gamma irradiation is currently the standard care to avoid transfusion-associated graft-versus-host disease. Guidelines on gamma irradiation of blood components state that platelets (PLTs) can be irradiated at any stage in their 5-day storage and can thereafter be stored up to their normal shelf life of 5 days after collection. In this study, we explored whether the timing of irradiation has an effect on transfusion efficacy of apheresis PLT concentrates (APCs).
Methods
Based on the 1-hour percent PLT recovery (PPR1h), transfusion efficacy of 1,000 eligible APCs transfused to 144 children were evaluated retrospectively. PPR1h was compared in transfused APCs irradiated at the day of transfusion and APCs irradiated in advance.
Results
In univariate analysis, transfusion efficacy of APCs irradiated in advance was significantly lower than that of APCs irradiated at the day of transfusion (mean PPR1h 27.7 vs. 35.0%; p = 0.007). This was confirmed in multivariate analysis (p = 0.030). Compared to non-irradiated APCs, transfusion efficacy of APCs irradiated at the day of transfusion was not significantly inferior (mean difference −2.8%; 95% CI −6.1 to 0.5%; p = 0.092), but APCs irradiated in advance were clearly less efficient (mean difference −8.1%; 95% CI −12.2 to −4.0%; p < 0.001).
Conclusion
Our data strongly support that APCs should not be irradiated in advance, 1.e., ≥24 h before transfusion.
Keywords: Platelet transfusion, Platelet concentrates, Platelet storage, Gamma irradiation
Introduction
Platelet (PLT) transfusion in pediatric oncology is used for prevention or treatment of bleeding in thrombocytopenic patients with malignancies who have bone marrow failure caused by their disease or its treatment.
Transfusion-associated graft-versus-host disease (TA-GVHD) is a rare but usually fatal complication of transfusion of cellular blood components that results from the engraftment and clonal expansion of contaminating allogeneic donor lymphocytes, eliciting an acute immune dysregulation in the host [1, 2]. The risk associated with an individual transfusion depends on the number and viability of contaminating lymphocytes, the susceptibility of the patient's immune system to their engraftment, and the degree of immunological (HLA) disparity between donor and recipient. Since at least one case of TA-GVHD has been reported after transfusion of only 8 × 104 lymphocytes per kg of body weight, the threshold of the quantity of transfused lymphocytes that can cause TA-GVHD remains unknown [3]. Quality guidelines for leukocyte-reduced apheresis PLT concentrates (APCs) define a maximal leukocyte count of <5 × 106/l [4]. Since this limited number of remaining leukocytes may cause TA-GVHD, even leukocyte-reduced PLT concentrates have to be gamma-irradiated [5, 6]. For PLTs and plasma, but not for red blood cell concentrates (RBCs), photochemical pathogen reduction technologies like riboflavin plus ultraviolet light irradiation may be an alternative [7, 8]. Nonetheless, gamma irradiation is currently the only recommended method to prevent TA-GVHD [3, 9, 10]. By damaging the DNA of lymphocytes, ionizing radiation abolishes their mitotic capacity [9]. According to earlier transfusion studies, gamma irradiation of PLTs did not influence post-transfusion PLT increments [11, 12, 13, 14], whereas more recent work showed decreased increments [15, 16].
In everyday transfusion practice, both pre-storage gamma irradiation as well as irradiation of blood components immediately before transfusion are common. Both approaches are consistent with international guidelines, stating that gamma irradiation of PLT concentrates can be applied at any stage in their five-day storage, and thereafter PLTs can be stored up to their normal shelf life of 5 days after collection [3, 9, 10]. The impact of gamma irradiation of PLT concentrates on their transfusion efficacy in vivo is poorly investigated, and the results are inconsistent [11, 12, 13, 15, 16]. Therefore, taking into account all significant covariates affecting transfusion efficacy from our previous work [16, 17], the aim of the present study was to investigate whether gamma irradiation of APCs adversely affects transfusion efficacy and whether the timing of irradiation has an effect on transfusion efficacy.
Patients and Methods
Study Design
Within the hemovigilance program of our hospital, efficacy and adverse events of PLT transfusions are registered prospectively in all patients. Adverse events, however, were not analyzed in this study. In the present study, the prospectively registered transfusion efficacy of APCs was analyzed retrospectively by reviewing the records of all patients having received at least one APC between January 2004 and December 2010 at the Division of Pediatric Hematology and Oncology, Department of Pediatrics, University of Bern, Switzerland. This data set included the data of our two former prospective trials evaluating the effects of high-yield thrombocytapheresis on the quality of APCs [16] and the influence of ABO blood group matching between PLT donor and recipient on posttransfusion PLT increment [17]. In addition to the 1-hour percent PLT recovery (PPR1h), side effects of PLT transfusions and data on the production process of APCs were recorded. The latter included information on the apheresis procedure, storage medium, storage time, APC volume, gamma irradiation, and timing of irradiation.
Patients
Between January 2004 and December 2010, all consecutive patients with hematological malignancies, solid tumors, or aplastic anemia requiring at least one PLT transfusion at the Division of Pediatric Hematology and Oncology, Department of Pediatrics, University of Bern, Switzerland, were assessed for their eligibility for this retrospective analysis. Exclusion criteria were fever ≥38.5 °C before transfusion, clinically enlarged spleen, thrombosis, adverse events requiring interruption of transfusion, immune thrombocytopenia (ITP), and hemorrhage ≥ WHO grade 3. During the observation period there was no patient with PLT transfusion refractoriness. This study was approved by the institutional review board with waiver of informed consent.
Preparation and Delivery of APCs
PLTs were obtained from healthy volunteer donors, either by single- or double-needle thrombocytapheresis using an Amicus Crescendo device (n = 117; software version 2.51; Baxter, Deerfield, IL, USA), or by single-needle procedures using a Trima Accel device (n = 865; software version 5.0 or 5.1; Gambro BCT, Lakewood, CO, USA) or a Cobe Spectra device (n = 18; software version 7.0; Gambro BCT). Apheresis time was limited to 100 min. Depending on the yield, the apheresis product was split into APCs containing at least 2.0 × 1011 PLTs and 2.4 × 1011 PLTs, corresponding to the minimum content required by the standards of the Swiss Red Cross until the end of 2006 and starting with 2007, respectively. APCs produced with the Amicus device were stored in 65% T-Sol® (PAS II, Baxter) and 35% autologous plasma according to the manufacturer's instructions, whereas PLTs derived from the Trima and Spectra devices were stored in 100% autologous plasma. APCs were stored at 22 ± 2 °C for a maximum of 5 days on a flat-bed shaker with constant agitation until delivery for transfusion. Older products were delivered first, in line with the policy of our Division of Transfusion Medicine to avoid expiration of APCs.
PLT Transfusions
Most patients received prophylactic PLT transfusions, with only a few receiving PLT transfusions for therapeutic purposes. In uncomplicated thrombocytopenia no threshold for prophylactic PLT transfusion was defined. However, as shown in table 2, depending on clinical circumstances various thresholds between 10 and 100 × 109/l were applied. For example, in children with a PLT count ≤ 10 × 109/l before RBC transfusion, an APC was given. This was done due to the observation that in patients with severe anemia there is significant peripheral vasoconstriction that will dissolve after transfusion of RBCs, probably increasing the bleeding risk. In children with severe neutropenia and uncomplicated thrombocytopenia, no threshold for prophylactic PLT transfusion was defined. However, due to the increased bleeding risk when fever appears, a PLT trigger of ≤ 10 × 109/l was applied.
Table 2.
Indications for transfusion and corresponding pre-transfusion PLT counts
| Indications | Pre-transfusion PLT count × 109/l |
Transfusions (%)a | |
|---|---|---|---|
| median | range | ||
| Therapeutic use | |||
| Minor bleeding (WHO grade <3) | 20 | 2–95 | 39 (4%) |
| Prophylactic use | |||
| Before red blood cell transfusion | 10 | 1–4 | 314 (31%) |
| Before invasive interventionb | 19 | 0–97 | 238 (24%) |
| Fever in severe neutropenia | 9 | 1–90 | 133 (13%) |
| Prevention of further bleeding after major hemorrhage | 18 | 2–66 | 90 (9%) |
| Before discharge from hospital | 9 | 1–32 | 63 (6%) |
| Autologous stem cell transplantation | 13 | 2–55 | 123 (12%) |
n = 1,000.
Intramuscular injection, surgery, insertion of a central venous catheter, lumbar puncture.
In case of circulating leukemic cells in the peripheral blood before lumbar puncture, PLTs were transfused when the PLT count was <100 × 109/l [27].
Bleeding episodes due to thrombocytopenia were managed with PLTs until controlled. In all other situations, infants received 10–15 ml/kg body weight of an APC. Children <2 years old received half an APC, and patients aged ≥2 years received a full APC. In line with our routine practice, we recorded pulse rate and blood pressure before starting the transfusion as well as every 3 min during transfusion. Body temperature was measured before and after transfusion. Patients were monitored for adverse events until 1 h after the end of transfusion.
Efficacy Measures: 1-Hour Percent PLT Recovery (PPR1h)
Because in infants and children, blood volume is proportional to body weight and is only sub-optimally related to body surface area, we measured transfusion efficacy by the PPR1h instead of the more widely used 1-hour corrected count increment (CCI1h) [18, 19]. This surrogate outcome measure is calculated as PPR1h (%) = (post-transfusion count (109/l) – pre-transfusion count (109/l)) × blood volume (l) / PLT dose (1011/l). Blood volume was determined according to Linderkamp et al. [20]. According to the British Committee for Standards in Haematology, an unsuccessful PLT transfusion was defined as one with a PPR1h of 30% or less [4].
Determination of PLT Count
PLT count in blood samples of patients was measured with a CELL DYN 3500 R analyzer (Abbott Laboratories, Abbott Park, IL, USA). In case of a PLT count < 30 × 109/l or an abnormal histogram pattern, manual counting with a modified Neubauer counting chamber was performed.
In each APC, PLT count was determined with an ADVIA 120 Hematology System (Bayer, Leverkusen, Germany) according to the pre-analytic recommendations of the apheresis device manufacturers. After the donation, the APCs were stored during at least 1 h without agitation and then put on a PLT agitator for an additional 1-hour period. Thereafter the PLT count was measured.
Gamma Irradiation
When clinically required, gamma irradiation of APCs was performed with a cesium irradiator Gammacell 3000 Elan (MDS Nordion, Ottawa, ON, Canada) at a dose of 25 Gy. Gamma-irradiated APCs were administrated to the following patients: children undergoing allogeneic or autologous stem cell transplantation, patients after treatment with anti-thymocyte globulin, and infants undergoing highly myelotoxic chemotherapy. The choice of APCs and the timing of the gamma irradiation were at the discretion of the staff of the Division of Transfusion Medicine. Since only the date, but not the exact time of gamma irradiation was documented, transfusions were categorized as irradiated at the same day as transfused (<24 h) in the case when irradiation and transfusions occurred on the same date. Being well aware that in some transfusions the time lag between irradiation and transfusion could be less than 24 h, all transfusions with different dates of irradiation and of transfusion were categorized to ≥24 h. According to these definitions, transfusions were divided into three groups: non-irradiated APCs, APCs gamma-irradiated at the day of transfusion (<24 h before transfusion), and APCs gamma-irradiated in advance (≥24 h).
Statistical Analysis
Means and their standard deviation (SD) were calculated. For analysis, univariate and multivariate linear mixed-effect (LME) regression was used. With a random intercept per patient, LME accounts for the correlation among multiple transfusions given to the same patient. For multivariate analysis, storage time, applied apheresis procedure, apheresis yield, ABO transfusion constellation, body weight of recipients, and PLT count before transfusion were used as covariates [16, 17]. Two-sided tests were used throughout, and p values < 0.050 were considered significant. The R 2.10.1. software (R Foundation for Statistical Computing, Vienna, Austria) was used.
Results
Patient Characteristics, PLT Transfusions, and Indications
During the study period, 152 children and adolescents received 1,272 APC transfusions of which 272 (23%) were excluded from analysis for the following reasons: fever ≥ 38.5 °C before transfusion (n = 125), missing pre- or post-transfusion PLT count (n = 48), clinically enlarged spleen (n = 16), thrombosis (n = 14), APC obtained from a foreign blood bank (n = 32), refusal of study participation (n = 22), adverse events requiring interruption of transfusion (n = 9), ITP (n = 4), and hemorrhage ≥ WHO grade 3 (n = 2). Adverse reactions were shivering (n = 6), dyspnea (n = 1), urticaria (n = 1), and transfusion-related acute lung injury (n = 1). The eligible study population consisted of 144 children (69 girls, 75 boys) with a median age of 6.1 years at first transfusion (range 0.1–20.0 years). They presented with thrombocytopenia due to the malignancy itself or the applied chemotherapy, or because of aplastic anemia (table 1). The median number of APCs transfused per patient was 5 (interquartile range 3–10). Of 1,000 PLT transfusions, 961 (96%) were given prophylactically and 39 (4%) therapeutically (table 2).
Table 1.
Patient's diagnoses, number of transfused APCs, and distribution of non-irradiated and gamma-irradiated APCs
| Diagnosis | Patients (%) | Transfused APCs (%) |
|||
|---|---|---|---|---|---|
| total | non-irradiated | irradiated at the day of transfusion | irradiated ≥24 h before transfusion | ||
| Acute lymphoblastic leukemia | 61 (42%) | 404 (40%) | 323 (80%) | 48 (12%) | 33 (8%) |
| Acute myeloid leukemia | 13 (9%) | 187 (19%) | 29 (16%) | 85 (45%) | 73 (39%) |
| Lymphoma | 5 (3%) | 19 (2%) | 6 (32%) | 10 (52%) | 3 (16%) |
| Tumor of the central nervous system | 23 (16%) | 141 (14%) | 31 (22%) | 78 (55%) | 32 (23%) |
| Extracranial solid tumor | 34 (24%) | 157 (16%) | 38 (24%) | 94 (60%) | 25 (16%) |
| Aplastic anemia | 8 (6%) | 92 (9%) | 34 (37%) | 50 (54%) | 8 (9%) |
| Total | 144 (100%) | 1,000 (100%) | 461 (46%) | 365 (37%) | 174 (17%) |
Distribution of Gamma-Irradiated and Non-Irradiated APCs
The 1,000 eligible PLT transfusions included 461 (46%) non-irradiated and 539 (54%) gamma-irradiated APCs. Among the 539 gamma-irradiated APCs, there were 365 (68%) irradiated at the day of transfusion and 174 (32%) irradiated in advance (≥24 h). In all groups of patients, except those with acute lymphoblastic leukemia (80% of APCs non-irradiated), the majority of APCs transfused were gamma-irradiated (table 1).
PLT Transfusion Efficacy
Table 3 shows the transfusion efficacy depending on gamma irradiation and timing of irradiation. In univariate analysis, the transfusion efficacy of gamma-irradiated APCs was inferior compared to non-irradiated APC (mean PPR1h 32.7 vs. 39.3%; p = 0.014). However, this was obviously due to APCs irradiated in advance (≥24 h) (mean PPR1h 27.7%; p < 0.001), while the efficacy of APCs irradiated at the day of transfusion was not significantly inferior than that of non-irradiated APCs (mean PPR1h 35.0%; p = 0.092). Correspondingly, the efficacy of APCs gamma-irradiated in advance (≥24 h) was clearly inferior compared to APCs irradiated at the day of transfusion (mean PPR1h 27.7 vs. 35.0%; p = 0.007) (table 4).
Table 3.
Linear mixed analysis of transfusion efficacy of non-irradiated and gamma-irradiated APCs
| APCs | n | Mean PPR1h |
PPR1h univariate |
PPR1h multivariate |
|||||
|---|---|---|---|---|---|---|---|---|---|
| % | SD | difference, % | 95% CI, % | p | difference, % | 95% CI, % | p | ||
| Total | 1,000 | 35.7 | 19.3 | ||||||
| Non-irradiated | 461 | 39.3 | 19.4 | reference | reference | ||||
| Gamma-irradiated | 539 | 32.7 | 18.7 | −3.8 | −6.8 to −0.8 | 0.014 | −3.8 | −6.6 to −1.1 | 0.006 |
| Day of transfusion | 365 | 35.0 | 18.5 | −2.8 | −6.1 to 0.5 | 0.092 | −3.3 | −6.3 to −0.3 | 0.030 |
| ≥24 h | 174 | 27.7 | 18.0 | −8.1 | −12.2 to −4.0 | <0.001 | −6.3 | −10.1 to −2.6 | <0.001 |
Table 4.
Linear mixed analysis of transfusion efficacy of APCs irradiated at the day of transfusion and in advance (≥24 h)
| Gamma-irradiated APCs | n | Mean PPR1h |
PPR1h univariate |
PPR1h multivariate |
|||||
|---|---|---|---|---|---|---|---|---|---|
| % | SD | difference, % | 95% CI, % | p | difference, % | 95% CI, % | p | ||
| Day of transfusion | 365 | 35.0% | 18.5 | reference | reference | ||||
| ≥24 h | 174 | 27.7% | 18.0 | −4.2 | −7.2 to −1.2 | 0.007 | −3.0 | −5.7 to −0.3 | 0.030 |
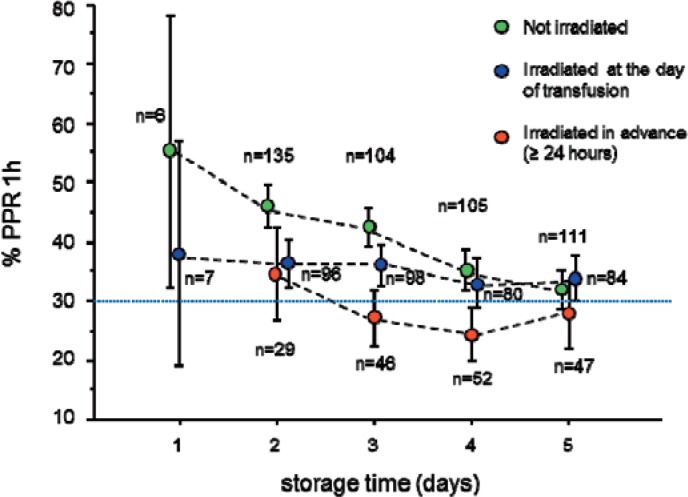
These results were confirmed by multivariate analysis, corrected for storage time of APCs, applied apheresis procedure, apheresis yield, ABO transfusion constellation between donor and recipient, body weight of recipients, and PLT count before transfusion (tables 3–5, fig. 1). Noteworthy, beyond this variety of parameters significantly influencing transfusion efficacy, the negative impact of gamma irradiation in advance (≥ 24 h) remained statistically significant.
Table 5.
Multivariate association of transfusion efficacy of APCs gamma-irradiated at the day of transfusion versus irradiated ≥24 h, n = 539
| Factors analyzed | Difference in PPR1h, results of LME analysis |
||
|---|---|---|---|
| estimate, % | 95% CI, % | p value | |
| Apheresis procedure with Amicus vs. others | −5.5 | −7.1 to −4.0 | <0.001 |
| Platelet yield × 1011 | −1.6 | −2.2 to −1.0 | <0.001 |
| ABO minor-mismatched vs. ABO identical | 2.2 | −2.6 to 6.9 | 0.37 |
| ABO major-mismatched vs. ABO identical | −5.8 | −9.1 to −2.5 | <0.001 |
| Storage time per day | −2.5 | −3.6 to −1.5 | <0.001 |
| Platelet count before transfusion per 10 × 109/l | −0.7 | −1.5 to 0.1 | 0.098 |
| Body weight of recipient per kg | 0.3 | 0.2 to 0.4 | <0.001 |
| Irradiation ≥24 h vs. at the day of transfusion | −3.0 | −5.7 to −0.3 | 0.030 |
Fig. 1.
Transfusion efficacy of 1000 APCs according to gamma irradiation, timing of irradiation, and storage time (error bars = 95% CI). Dotted blue line: According to the British Committee for Standards in Haematology an unsuccessful PLT transfusion is defined as one with a PPR1h of 30% or less [4].
Discussion
Although, since the introduction of allogeneic bone marrow transplantation to the medical armory, GVHD has been a known complication, it was not until the 1970s that TA-GVHD was recognized as a distinct entity [2]. It is a rare but usually fatal complication of transfusion of cellular blood components that results from the engraftment and clonal expansion of contaminating allogeneic lymphocytes. The pathophysiology of TA-GVHD, the patient population at risk, and the preventive measurements have been reviewed by Rühl et al. [2]. Although treatment of blood components by riboflavin plus ultraviolet light irradiation or other pathogen reduction techniques are considered to be equivalent alternatives [7, 8], gamma irradiation of cellular blood products is still the standard of care method for TA-GVHD prevention. Doses of 2,500–3,000 cGy completely inhibit T-cell proliferation and, thereby, initiation of TA-GVHD [3]. According to a more than 30-year-old study, there seems to be no evidence that blood components irradiated up to 5,000 cGy have undesired effects or are less effective than non-irradiated products. No demonstrable negative effect on the clinical efficacy of red blood cells, PLTs, and granulocytes was observed [14]. However, only recently proteomic technologies have been introduced to evaluate and optimize the quality of APCs [21]. By comparing photochemical pathogen reduction technologies (e.g., riboflavin plus ultraviolet light irradiation) with gamma irradiation at day 1 and day 5, it emerged that both methods induced proteome changes reflecting increasing PLT storage lesions at day 5, potentially influencing negatively transfusion efficacy [22, 23].
Various international guidelines state that gamma irradiation of PLT concentrates can be applied at any stage during storage, and thereafter PLTs can be stored up to their normal shelf life of 5 days after collection [3, 9, 10]. These guidelines are mainly based on studies published in the 1980s. Besides a multiplicity of in vitro studies, mainly three groups, Button et al. [14], Read et al. [11] and Duguid et al. [12], evaluated in vivo recovery and/or survival of gamma-irradiated PLTs. Different irradiation dosages, different timing of irradiation, and different storage times after gamma irradiation were evaluated. In conclusion, these studies showed that survival and recovery of gamma-irradiated PLTs were not different from non-irradiated PLTs. However, within the frame of a large, multi-institutional PLT transfusion trial (TRAP trial) on adult patients, Slichter and colleagues [15] demonstrated significantly decreased PLT responses after transfusion in both gamma-irradiated, filtered, pooled random donor PLT concentrates and ultraviolet B-irradiated pooled random donor PLT concentrates, but not in APCs. However, within our own pediatric study on the effect of high-yield thrombocytapheresis on the quality of PLT products, transfusion efficacy was significantly reduced in APCs by gamma irradiation [16]. In agreement with this observation, PLT storage lesions 5 days after gamma irradiation documented by proteome changes have been described [22, 23]. Additionally, gamma irradiation may suppress significantly the formation of immature PLTs in APCs and thereby potentially decrease the efficacy of PLT transfusions [24].
Although bleeding would be the only meaningful endpoint, such evaluations are very challenging, last but not least due to the difficulties to standardize their classification [25]. Further, in our study population the frequency of major or minor bleeding episodes was very low. Therefore, we based our analysis on a surrogate parameter. As previously mentioned, in children PPR1h is a more appropriate surrogate marker for transfusion efficacy than the more widely used CCI1h. The aim of the present study was to investigate whether gamma irradiation of APCs adversely affects transfusion efficacy and, particularly, whether the timing of irradiation has an effect on transfusion efficacy. Transfusion efficacy of gamma-irradiated APCs was significantly inferior as compared to non-irradiated APCs. However, this was particularly due to APCs irradiated ≥24 h before transfusion. Comparing non-irradiated APCs with APCs irradiated at the day of transfusion, no significant difference was observed. Correspondingly, transfusion efficacy of APCs irradiated ≥24 h before transfusion were significantly inferior to products irradiated at the day of transfusion (tables 3–5, fig. 1). In view of the fact that in some transfusions which were categorized as APCs irradiated in advance (≥24 h) (see methods), the time lag between gamma irradiation and transfusion could have been less than 24 h, the difference between the PPR1h obtained with APCs irradiated at the day of transfusion and APCs irradiated in advance (≥24 h) may have even been underestimated here.
One of the shortcomings of the present study is its retrospective nature, though data were collected prospectively, and also the fact that for the timing of gamma irradiation only two groups could be defined, irradiation ≥24 h before transfusion and irradiation at the day of transfusion, which were compared with non-irradiated PLT transfusions. Further, there is an unbalance between APCs gamma-irradiated at the day of transfusion and those irradiated in advance in favor of the former. It would be of interest to evaluate in more detail both the association between transfusion efficacy and the time frame between gamma irradiation of APCs and transfusion as well as the effect of treatment by riboflavin or amotosalen-HCI plus ultraviolet light also including the time frame between irradiation and transfusion, which in the later procedure could be up to 7 days [26].
In conclusion, in view of the present data, we suggest that at optimal conditions, PLTs should be gamma-irradiated as shortly as possible before transfusion, and in any other situation at least within 24 h before transfusion.
Disclosure Statement
No conflict of interest of any of the authors.
Acknowledgements
This work was supported by the Swiss Cancer League (Oncosuisse, Bern, Switzerland; grant OCS 01470-02-2004 and KLS-01828-02-2006), the Beatrice Borer-Foundation at Vinetum Foundation, Biel, Switzerland, and the Berner Stiftung für krebskranke Kinder und Jugendliche.
References
- 1.Brubaker DB. Immunopathogenic mechanisms of posttransfusion graft-vs-host disease. Proc Soc Exp Biol Med. 1993;202:122–147. doi: 10.3181/00379727-202-43519b. [DOI] [PubMed] [Google Scholar]
- 2.Ruhl H, Bein G, Sachs UJ. Transfusion-associated graft-versus-host disease. Transfus Med Rev. 2009;23:62–71. doi: 10.1016/j.tmrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Treleaven J, Gennery A, Marsh J, Norfolk D, Page L, Parker A, Saran F, Thurston J, Webb D. Guidelines on the use of irradiated blood components prepared by the British Committee for Standards in Haematology Blood Transfusion Task Force. Br J Haematol. 2011;152:35–51. doi: 10.1111/j.1365-2141.2010.08444.x. [DOI] [PubMed] [Google Scholar]
- 4.British Committee for Standards in Haematology, Blood Transfusion Task Force (Chairman P Kelsey) Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122:10–23. [Google Scholar]
- 5.Stroncek DF, Rebulla P. Platelet transfusions. Lancet. 2007;370:427–438. doi: 10.1016/S0140-6736(07)61198-2. [DOI] [PubMed] [Google Scholar]
- 6.Uchida S, Tadokoro K, Takahashi M, Yahagi H, Satake M, Juji T. Analysis of 66 patients definitive with transfusion-associated graft-versus-host disease and the effect of universal irradiation of blood. Transfus Med. 2013;23:416–422. doi: 10.1111/tme.12081. [DOI] [PubMed] [Google Scholar]
- 7.Fast LD, Nevola M, Tavares J, Reddy HL, Goodrich RP, Marschner S. Treatment of whole blood with riboflavin plus ultraviolet light, an alternative to gamma irradiation in the prevention of transfusion-associated graft-versus-host disease? Transfusion. 2013;53:373–381. doi: 10.1111/j.1537-2995.2012.03715.x. [DOI] [PubMed] [Google Scholar]
- 8.Fast LD. Developments in the prevention of transfusion-associated graft-versus-host disease. Br J Haematol. 2012;158:563–568. doi: 10.1111/j.1365-2141.2012.09197.x. [DOI] [PubMed] [Google Scholar]
- 9.Moroff G, Luban NL. The irradiation of blood and blood components to prevent graft-versus-host disease: Technical issues and guidelines. Transfus Med Rev. 1997;11:15–26. doi: 10.1016/s0887-7963(97)80006-5. [DOI] [PubMed] [Google Scholar]
- 10.Williamson LM. U.K. Guidelines for the irradiation of blood components. Transfus Sci. 1995;16:135–137. doi: 10.1016/0955-3886(95)97396-h. [DOI] [PubMed] [Google Scholar]
- 11.Read EJ, Kodis C, Carter CS, Leitman SF. Viability of platelets following storage in the irradiated state. A pair-controlled study. Transfusion. 1988;28:446–450. doi: 10.1046/j.1537-2995.1988.28588337334.x. [DOI] [PubMed] [Google Scholar]
- 12.Duguid JK, Carr R, Jenkins JA, Hutton JL, Lucas GF, Davies JM. Clinical evaluation of the effects of storage time and irradiation on transfused platelets. Vox Sang. 1991;60:151–154. doi: 10.1111/j.1423-0410.1991.tb00891.x. [DOI] [PubMed] [Google Scholar]
- 13.Sweeney JD, Holme S, Moroff G. Storage of apheresis platelets after gamma radiation. Transfusion. 1994;34:779–783. doi: 10.1046/j.1537-2995.1994.34994378279.x. [DOI] [PubMed] [Google Scholar]
- 14.Button LN, DeWolf WC, Newburger PE, Jacobson MS, Kevy SV. The effects of irradiation on blood components. Transfusion. 1981;21:419–426. doi: 10.1046/j.1537-2995.1981.21481275998.x. [DOI] [PubMed] [Google Scholar]
- 15.Slichter SJ, Davis K, Enright H, Braine H, Gernsheimer T, Kao KJ, Kickler T, Lee E, McFarland J, McCullough J, Rodey G, Schiffer CA, Woodson R. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105:4106–4114. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Julmy F, Ammann RA, Mansouri Taleghani B, Fontana S, Hirt A, Leibundgut K. Effects of high-yield thrombocytapheresis on the quality of platelet products. Transfusion. 2008;48:442–450. doi: 10.1111/j.1537-2995.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 17.Julmy F, Ammann RA, Taleghani BM, Fontana S, Hirt A, Leibundgut K. Transfusion efficacy of abo major-mismatched platelets (plts) in children is inferior to that of ABO-identical plts. Transfusion. 2009;49:21–33. doi: 10.1111/j.1537-2995.2008.01914.x. [DOI] [PubMed] [Google Scholar]
- 18.Hanson SR, Slichter SJ. Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood. 1985;66:1105–1109. [PubMed] [Google Scholar]
- 19.Davis KB, Slichter SJ, Corash L. Corrected count increment and percent platelet recovery as measures of posttransfusion platelet response: problems and a solution. Transfusion. 1999;39:586–592. doi: 10.1046/j.1537-2995.1999.39060586.x. [DOI] [PubMed] [Google Scholar]
- 20.Linderkamp O, Versmold HT, Riegel KP, Betke K. Estimation and prediction of blood volume in infants and children. Eur J Pediatr. 1977;125:227–234. doi: 10.1007/BF00493567. [DOI] [PubMed] [Google Scholar]
- 21.Schubert P, Culibrk B, Karwal S, Slichter SJ, Devine DV. Optimization of platelet concentrate quality: application of proteomic technologies to donor management. J Proteomics. 2012;76:329–336. doi: 10.1016/j.jprot.2012.06.023. [DOI] [PubMed] [Google Scholar]
- 22.Thiele T, Sablewski A, Iuga C, Bakchoul T, Bente A, Gorg S, Volker U, Greinacher A, Steil L. Profiling alterations in platelets induced by amotosalen/UVA pathogen reduction and gamma irradiation – a LC-ESI-MS/MS-based proteomics approach. Blood Transfus. 2012;10(suppl 2):s63–70. doi: 10.2450/2012.010S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrocco C, D'Alessandro A, Girelli G, Zolla L. Proteomic analysis of platelets treated with gamma irradiation versus a commercial photochemical pathogen reduction technology. Transfusion. 2013;53:1808–1820. doi: 10.1111/trf.12060. [DOI] [PubMed] [Google Scholar]
- 24.Hong H, Xiao W, Maitta RW. Steady increment of immature platelet fraction is suppressed by irradiation in single-donor platelet components during storage. PLoS One. 2014;9:e85465. doi: 10.1371/journal.pone.0085465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Estcourt LJ, Heddle N, Kaufman R, McCullough J, Murphy MF, Slichter S, Wood EM, Stanworth SJ. The challenges of measuring bleeding outcomes in clinical trials of platelet transfusions. Transfusion. 2013;53:1531–1543. doi: 10.1111/trf.12058. [DOI] [PubMed] [Google Scholar]
- 26.Picker SM, Oustianskaia L, Schneider V, Gathof BS. Functional characteristics of apheresis-derived platelets treated with ultraviolet light combined with either amotosalen-Hcl (S-59) or riboflavin (vitamin B2) for pathogen-reduction. Vox Sang. 2009;97:26–33. doi: 10.1111/j.1423-0410.2009.01176.x. [DOI] [PubMed] [Google Scholar]
- 27.Gajjar A, Harrison PL, Sandlund JT, Rivera GK, Ribeiro RC, Rubnitz JE, Razzouk B, Relling MV, Evans WE, Boyett JM, Pui CH. Traumatic lumbar puncture at diagnosis adversely affects outcome in childhood acute lymphoblastic leukemia. Blood. 2000;96:3381–3384. [PubMed] [Google Scholar]