Summary
Background
During the transfusion of blood components, the transfer of allogeneic donor white blood cells (WBCs) can mediate transfusion-associated graft-versus-host disease (TA-GVHD). To minimize the reaction, exposure of blood products to gamma irradiation is currently the standard of care. The aim of our study was to evaluate and compare hemostatic function, transfusion efficacy, and safety of gamma-irradiated single-donor apheresis platelet concentrates (PCs) and of conventional non-irradiated PCs in patients with chemotherapy-induced thrombocytopenia.
Methods
20 double-dose single-donor leukoreduced PCs were split in two identical units; one was gamma-irradiated with 25 Gy (study arm A) and the other remains non-irradiated (study arm B). Both units were stored under equal conditions. Hematologic patients were randomly assigned to receive gamma-irradiated or conventional non-irradiated PCs. Hemostatic function was evaluated by thrombelastography (TEG). TEG measurements were taken pre transfusion and 1 and 24 h post transfusion. TEG profiles were measured, noting the time to initiate clotting (R), the angle of clot formation (α), and the maximum amplitude (clot strength (MA)). Whole blood samples were collected from these thrombocytopenic patients at 1 and 24 h for PLT count increments (CIs) and corrected count increments (CCIs) with assessments of transfusion efficacy. Time to next PLT transfusion, transfusion requirement of RBCs, active bleeding, and adverse events (AEs), were analyzed.
Results
No differences could be found in hemostatic function parameters (MA, R, and α) between study arms A and B (all p values > 0.096) pre transfusion as well as 1 and 24 h post transfusion. No differences between study arms A and B were observed for mean (± standard deviation (SD)) 1-hour CCI (12.83 ± 6.33 vs. 11.59 ± 5.97) and 24-hour CCI (6.56 ± 4.10 vs. 5.76 ± 4.05). Mean 1-hour CI and 24-hour CI were not significantly different in both study arms (p = 0.254 and p = 0.242 respectively). Median time to the next PC transfusion after study PC was not significantly different between groups: (2.4 vs. 2.2 days, p = 0.767). No differences could be found in transfusion requirement of red blood cells (p = 0.744) between both study arms. There were also no regarding bleeding, adverse events, and acute transfusion reaction(s).
Conclusions
This study confirms safety of gamma-irradiated PCs for treatment thrombocytopenia. Hemostatic function, transfusion efficacy, bleeding, and safety of single-donor apheresis PCs treated with gamma irradiation versus untreated control PCs are comparable.
Keywords: Hemostatic function, Transfusion efficacy, Apheresis platelet concentrates, Gamma irradiation, Thrombocytopenic patients
Introduction
Allogeneic platelet (PLT) transfusions are fundamental to the supportive care of patients with severe thrombocytopenia, but risks are associated with the use of any blood component [1, 2]. Transfusion-associated graft-versus-host disease (TA-GVHD) occurs when functional white blood cells (WBCs) are transfused into a patient who is unable to mount an immune response to the allogeneic donor cells due to human leukocyte antigen (HLA) compatibility or to immunosuppression. TA-GVHD is a rare but highly lethal complication caused by engraftment of allogeneic T lymphocytes in the recipient after transfusion of a blood component [3]. Because there is currently no available treatment for TA-GVHD, the goal has been to reduce the likelihood of occurrence. Attempts to reduce these undesirable effects have included exposure of the blood products to gamma irradiation and the use of leukoreduction filters. To date the gold standard for the inactivation of all leukocyte subsets is gamma irradiation [4]. A dose of 25 Gy is commonly used to inactivate viable T cells to prevent TA-GVHD without damaging the platelets [5].
Evaluation of the function of PLTs is only possible by the use of laborious and complicated in vitro tests and markers [6]. Moreover, these in vitro tests do not provide information on the in vivo hemostatic potential of PLTs. In general, hemostatic status has been monitored with plasma-based coagulation tests such as PT/INR and APTT, which underestimate coagulopathy [7]. Moreover, these tests are insensitive for hypocoagulant conditions and thus may be of limited value for the detection of impaired coagulation activity upon dilution [8, 9, 10]. Recent studies have shown that hemostatic function after transfusion of apheresis platelet concentrates (PCs) as assessed by whole blood tests (thrombelastography (TEG)). TEG records the viscoelastic properties of clot formation and the quality and stability of the formed clot. TEG provides a rapid assessment of thrombus generation dynamics and fibrinolysis as a real-time graphical display, allowing identification of clinically relevant coagulopathies and guidance of transfusion therapy in various clinical settings [11]. Transfusion efficacy of study arm A and B was evaluated by 1- and 24-hour corrected count increments (CCIs). According to international guidelines, a successful transfusion was defined as a CCI of more than 7.5 for immediate clinical effect (PLT recovery) and a CCI of more than 4.5 for late clinical effect (PLT survival) [2, 3, 4, 12, 13, 14].
In our study we aim to evaluate and compare hemostatic function, transfusion efficacy, and safety in hematologic patients receiving gamma-irradiated PCs or conventional non-irradiated PCs transfusions because of severe chemotherapy-induced thrombocytopenia.
Material and Methods
Study Design and Study Population
A single-center, randomized design was used in this pilot study to evaluate hemostatic function, transfusion efficacy, and safety of PCs treated by gamma irradiation. The study targeted an enrollment of 40 patients with thrombocytopenia requiring platelet transfusion support. Patients were recruited in The First Affiliated Hospital of Anhui Medical University. The study protocol was approved by Anhui Medical University ethics committees, and all patients gave informed consent. Patients admitted for chemotherapy treatment of one of the following conditions, myelodysplastic syndrome, acute myelogenous leukemia, acute lymphoblastic leukemia, multiple myeloma, aplastic anemia and plasma cell leukemia, were enrolled in the study if they were thrombocytopenic or expected to develop thrombocytopenia requiring platelet transfusion.
Exclusion criteria included the following: history of refractoriness to PLT transfusions, presence of HLA antibodies, positive lymphocytotoxicity, or previously documented alloimmunization; disseminated intravascular coagulation; treatment with IL-11 or other investigational platelet growth factors; previous exposure to gamma-irradiated PLTs; active bleeding requiring one or more transfusions of red blood cells (RBCs); coagulation disorders, use of anticoagulants, use of acetyl salicylic acid within the preceding.
10 days, or use of non-steroidal anti-inflammatory drugs within the last 24 h; exposure to an investigational product within 30 days before randomization; splenomegaly or splenectomy; history or diagnosis of immune or idiopathic thrombocytopenic purpura, thrombotic thrombocytopenia purpura, or hemolytic uremic syndrome; pregnant or lactating females; any other medical condition that would compromise the participation of the patient in the study. Finally, patients who developed thrombosis, as demonstrated by ultrasonography, were also excluded. Patients were assigned to the two study arms based on allocation within the hematologic ward. The ward was divided into a ‘gamma irradiation side’ and an ‘untreated control side’ and only subjects admitted to the gamma irradiation side were asked to participate in the study to receive gamma-irradiated PCs. Patients were assigned to the untreated control side received conventional PCs and served as a control group.
PLT Collection, Preparation, and Study Procedures
PLTs were collected by standard apheresis procedures (COBE Spectra v.7.0 LRS Turbo and Trima Accel v.5.0 LRS, Gambro BCT, Lakewood, CO, USA) from 20 healthy donors. Acid-citrate-dextrose (Gambro BCT) was used as anticoagulant agent. Contaminating WBCs were removed by the leukoreduction system of the cell separators. To avoid reduction in PLT concentration and volume below the requirements and the recommendations of the Chinese guidelines, 20 double-dose single-donor PCs containing more than 250 × 109 PLTs (2.89 ± 0.20 vs. 2.90 ± 0.19, p = 0.989) on the day of donation (day 0) were divided into two study arms (A and B). PCs in study arm A were irradiated with 25 Gy on day 0 (Gamma cell 3000 Elan, MDS Nordion, Ottawa, ON, Canada), PCs assigned to study arm B were used as untreated controls. Gamma-irradiated and non-irradiated PCs were stored in bags being part of each collection kit for the cell separators on an agitator (Model LPR-3, Melco Engineering, Glendale, CA, USA; or Model PFS84, Helmer, Noblesville, IN, USA) in an incubator (Model PC2200, Helmer) at a stable temperature of 22 ± 2 °C under gentle agitation up to 3 days. Gamma-irradiated PCs and non-irradiated PCs were randomly administered to 40 patients.
Transfusion of Gamma-Irradiated PCs and Non-Irradiated PCs
ABO- and RhD-identical PLT transfusions were the preferred option and provided whenever possible. PLT transfusions were requested by the patient's physician in accordance with international guidelines [15, 16, 17, 18]. Prophylactic PLT transfusions were given to non-febrile and clinically stable patients with peripheral blood PLT counts below 10 × 109/l and to febrile patients when PLT counts were below 20 × 109/l. In patients with increased risk of bleeding (e.g., before invasive procedures or recent hemorrhage) or ongoing bleeding, PLT transfusions were administered when peripheral blood PLT counts were below 20 × 109 to 50 × 109/l.
Study Endpoints
TEG Measurements
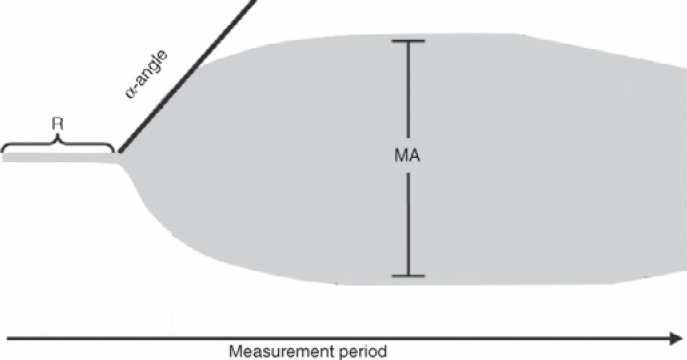
For comparative analysis of hemostatic function, transfusion efficacy and safety in study arm A and B, the primary endpoint was TEG measurements and 1-hour CCI. Secondary endpoints were 24-hour CCI, absolute count increment (CI) at 1 and 24 h, mean time to next PLT transfusion, time to next RBC transfusion, safety, and bleeding assessments. Blood samples were drawn for enumeration of PLT counts and measurement of TEG variables once before the transfusion (<60 min) and about 1 and 24 h after transfusion. Rotational TEG was performed using a thromboelastograph from Haemoscope (Model 5000, Software Version 4.1.73, Database Version 1.0.16;Haemoscope Corporation, Niles, IL, USA) according to the manufacturer's recommendations. Briefly, at the specified time points peripheral whole blood samples were drawn into tubes containing citrate at a ratio of 9 volumes of blood to 1 volume of 0.129 mol/l citrate. Samples were held at room temperature and were tested within 60 min of collection. Coagulation was initiated with the addition of kaolin and recalcification with calcium chloride. 1 ml of whole blood was added to a kaolin-loaded tube and mixed by inverting the tube five times. A 340-μl aliquot of the kaolin-activated sample was added to a warmed TEG sample cup preloaded with 20 μl of 0.2 mol/l CaCl2. The sample was incubated at 37 °C for the duration of testing. Aspects of the trace have been quantified to allow comparison of results and to assist clinical decision making. There are three main parameters of clot formation (fig. 1).
Fig. 1.
TEG trace, which gives a graphical representation of the sum of PLT function, coagulation factors, fibrin polymerization and the fibrinolytic system. The R time represents the length of time taken from the beginning of the test to initial fibrin formation. The α angle represents the rate of fibrin formation and cross-linking. MA is the greatest amplitude reached on the TEG trace and represents maximum clot strength, related directly to fibrin and platelet interaction. Modified according to Konig et al. [20].
PLT CI and CCI
For comparative analysis of transfusion efficacy between the group of patients receiving gamma-irradiated PCs and those receiving non-irradited PCs, 1-hour CI and CCI as well as 24-hour CI and CCI were calculated. PLT counts before transfusion and 1 h and 24 h after transfusion were obtained for each PLT transfusion to calculate 1- and 24-hour CCI, according to the following formula:
CCI = (post-transfusion PLT count (× 109/l) – pre-transfusion
PLT count (×109/l)) × body surface area (m2) / PLT dose (× 1011) (1).
Body surface area was estimated by the formula of DuBois and DuBois [21].
Safety Assessments
Patients that received gamma-irradiated PCs were monitored for 24 h after transfusion for adverse events (AEs) to evaluate safety. When an AE was reported, complementary information for assessment of causality and grade of severity was collected. If an AE was assessed as possibly related, probably related, or related to gamma-irradiated PCs, it was defined as an acute transfusion reaction (ATR). Bleeding assessments were performed prior to transfusion for each patient. In case bleeding was present prior to transfusion, post-transfusion bleeding status was also assessed. The respective clinical bleeding status was graded according to the WHO grading system (0, 1, 2, 3, 4; with grade 0 = no bleeding) [19].
Statistical Analysis
Statistical analysis was performed using chi-square test, Wilcoxon's rank sum or log rank test where appropriate. A p value of less than 0.05 was considered significant.
Results
Demographic, Clinical, Laboratory Characteristics and Baseline Characteristics of Study Population
Between January 1, 2011, and December 31, 2012, a total of 40 patients admitted to the gamma irradiation side (arm A) and untreated control side (arm B) of the hematology ward were asked to participate in the study. The distributions of age, sex, height, and weight were similar in both groups. The vast majority of the patients in both groups were treated for malignant hematologic diseases, with no significant difference in the distribution of single diagnoses. The proportions of patients undergoing autologous and allogeneic hematopoietic stem cell transplantation (HSCT) were not significantly different between groups. A total of 40 patients (19 males and 21 females) had been enrolled into the study. All enrolled patients (aged 18–70 years) had a prior history of successful PLT transfusion with no transfusion reactions. All transfusions were ABO- and RhD-identical, by means of recipient having no antibodies incompatible with the RBC type of donor. Analysis of the primary and secondary outcomes was based upon the per protocol (PP) cohort, while the intention to treat (ITT) cohort was followed for safety data. Baseline demographics for the pooled ITT cohorts are summarized in table 1.
Table 1.
Patients demographics*
| Characteristic | Gamma irradiation group (n = 20) | Control group (n = 20) | p value† (ITT) |
|---|---|---|---|
| Sex (male/female) | 10/10 | 11/9 | 0.752 |
| Age, years | 52.5 (18–70) | 54.5 (22–68) | 0.871 |
| Height, cm | 167.5 (152–182) | 169.0 (155–183) | 0.786 |
| Weight, kg | 67.6 (48.1–91.0) | 68.5 (54.1–92.1) | 0.981 |
| Body surface area, m2 | 1.74 (1.39–2.12) | 1.75 (1.49–2.14) | 0.927 |
| Blood group | |||
| O | 7 | 6 | |
| A | 7 | 8 | |
| B | 6 | 6 | |
| Rh factor | |||
| Negative | 1 | 0 | |
| Positive | 19 | 20 | |
| Diagnosis | |||
| MDS | 4 | 3 | |
| AML | 4 | 4 | |
| ALL | 6 | 7 | |
| MM | 1 | 1 | |
| AA | 3 | 3 | |
| PCL | 2 | 2 |
MDS = Myelodysplastic syndrome; AML = acute myelogenous leukemia; ALL = acute lymphoblastic leukemia; MM = multiple myeloma; AA = aplastic anemia; PCL = plasma cell leukemia.; NS = not significant (p ≥ 0.05).
Data are reported as median (range).
Control group (ITT) compared to gamma irradiation group (ITT).
PLT Product Characteristics
A total of 20 PLT transfusions each were administered in the control group and in the treatment group; the PLT doses were within the range of 2.52–3.48 × 1011 and 2.58–3.45 × 1011 platelets per unit for the gamma-irradiated PCs and the non-irradiated PCs, respectively. Swirl was assessed positive (score 2) in all PCs tested. The product pH was 7.21 ± 0.06 for gamma-irradiated and 7.24 ± 0.06 for non-irradiated PCs (p = 0.086), and the mean storage time was 2.15 ± 0.49 days for gamma-irradiated and 1.95 ± 0.51 days for non-irradiated PCs (p > 0.211). The RBC contamination was <3 × 106/ml and the WBC content below 1 × 106 per unit, meeting quality control requirements.
Primary Endpoint
Hemostatic Function Evaluated by TEG and 1-Hour CCI
PLT transfusion had an immediate but transient effect on initial clot formation that was reflected in the time to initiate clotting (R) and the angle of clot formation (α), whereas the effect on PLT clot strength and stability, the maximum amplitude (MA), persisted until the next day. No significant trends were observed for differences in pre-transfusion α (p = 0.096), pre-transfusion MA (p = 0.105), pre-transfusion R (p = 0.630) in both study groups.
At 1 h post transfusion, all efficacy variables were significantly different from pre-transfusion values for both the treatment (p < 0.05) and the control group (p < 0.17). At 24 h post transfusion, differences in R and α were no longer significantly different from pre-transfusion values in patients treated with gamma-irradiated PCs (R, p = 0.343; α, p = 0.204) as well as in the control group (R, p = 0.105; α, p = 0.064), whereas significant trends were observed in both groups for differences in MA (arm A, p = 0.004; arm B, p = 0.001). When comparing study arm A and B, none of the TEG measurement variables was significantly different at 1 h and at 24 h post transfusion. Hemostatic function variables, including the pre- and posttransfusion TEG variables, are summarized in table 2.
Table 2.
The TEG coagulation variables PP population
| Variable | Gamma irradiation group |
Control group |
p value (1 h/24 h)§ | ||||
|---|---|---|---|---|---|---|---|
| pre transfusion | 1 h post transfusion | 24 h post transfusion | pre transfusion | 1 h post transfusion | 24 h post transfusion | ||
| R, min | 9.85 ± 3.31† | 8.30 ± 2.31† | 9.31 ± 2.90† | 9.97 ± 2.84† | 8.41 ± 2.07† | 9.30 ± 2.50† | 0.930/0.988 |
| α, ° | 39.90 ± 9.24† | 49.55 ± 7.31† | 42.65 ± 8.63† | 42.28 ± 7.04† | 52.34 ± 6.34† | 44.96 ± 6.66† | 0.148/0.215 |
| MA, mm | 39.30 ± 9.23† | 47.81 ± 6.45† | 45.75 ± 7.24† | 40.99 ± 6.50† | 49.26 ± 3.93† | 47.16 ± 4.29† | 0.397/0.293 |
Values are expressed as means ± SD.
Gamma irradiation group (1 h post transfusion) compared to control group (1 h post transfusion); gamma irradiation group (24 h post transfusion) compared to control group (24 h post transfusion).
n = 19 due to missing TEG data for 1 patient.
NS = Not significant (p ≥ 0.05).
An additional primary endpoint of this study was whether gamma-irradiated PCs provided sufficient 1-hour CCI post transfusion compared to non-irradiated control PCs. Of the 40 patients of this study who received a PLT transfusion, 37 patients were eligible for the 1-hour CCI PP analyses (table 3).
Table 3.
1-hour CI and CCI – PP population
| Parameters of transfusion efficacy | Gamma irradiation group (n = 18)† | Control group (n = 19)§ | p value |
|---|---|---|---|
| Pre-transfusion platelet count (× 109/l) | |||
| Mean ± SD | 10.7 ± 4.15 | 11.0 ± 5.14 | 0.715 |
| Median (range) | 9.6 (5.0–25.1) | 10.0 (4.3–29.1) | |
| 1-hour post-transfusion PLT count (× 109/l) | |||
| Mean ± SD | 29.9 ± 13.62 | 32.7 ± 13.68 | 0.346 |
| Median (range) | 28.8 (8.5–62.3) | 33.8 (8.8–65.8) | |
| 1-hour CI | |||
| Mean ± SD | 19.5 ± 10.63 | 21.6 ± 10.74 | 0.254 |
| Median (range) | 19.0 (3.5–49.9) | 22.4 (2.9–48.7) | |
| 1-hour CCI | |||
| Mean ± SD§ | 11.6 ± 5.97 | 12.8 ± 6.33 | 0.171 |
| Median (range) | 11.1 (2.2–29.9) | 13.0 (2.0–30.9) |
Calculated for 18 patients: 2 patients had a single PLT transfusion with no post-transfusion PLT count.
Calculated for 19 patients: 1 patient had a single PLT transfusion with no post-transfusion PLT count.
NS = Not significant (p ≥ 0.05).
Secondary Endpoint
1, 24-Hour CIs and 24-Hour CCI
In the PP population analysis, the mean 1-hour CI did not significantly differ between patients assigned to treatment with non-irradiated PCs and those receiving gamma-irradiated PCs. The results of the comparative analysis are given in table 3. Similarly, the 24-hour CI and 24-hour CCI of patients treated with gamma-irradiated PCs were not significantly different from those of patients receiving non-irradiated PCs (table 4).
Table 4.
24-hour CI and CCI – PP population
| Parameters of transfusion efficacy | Gamma irradiation group (n = 19)† | Control group (n = 18)§ | p value |
|---|---|---|---|
| 24-hour post-transfusion PLT count (× 109/l) | |||
| Mean ± SD | 20.3 ± 10.15 | 22.1 ± 9.82 | 0.316 |
| Median (range) | 18.9 (8.0–46.1) | 20.8 (7.9–50.4) | |
| 24-hour CI | |||
| Mean ± SD | 9.8 ± 7.11 | 11.0 ± 6.61 | 0.242 |
| Median (range) | 8.7 (1.5–32.4) | 9.8 (2.0–33.3) | |
| 1-hour CCI | |||
| Mean ± SD§ | 5.8 ± 4.05 | 6.6 ± 4.10 | 0.167 |
| Median (range) | 5.0 (1.1–19.4) | 5.7 (1.4–21.1) |
Calculated for 19 patients: one patient had a single PLT transfusion with no post-transfusion PLT count.
Calculated for 18 patients: two patients had a single PLT transfusion with no post-transfusion PLT count.
NS = Not significant (p ≥ 0.05).
Additional Platelet Transfusions and Active Bleeding
The median time to the next PLT transfusion after the study transfusion was 2.2 days for the gamma-irradiated group and 2.4 days for the control group (p = 0.767, log-rank test). Within 15 days after the study transfusion, a median of 3 PLT transfusions was administered in both treatment groups.
Bleeding was assessed within 12 h before and after each study transfusion. During the pre-transfusion assessments, three bleeding events (2 petechiae, 1 gingivorrhagia) were recorded before the gamma-irradiated PC transfusions and two (1 petechiae, 1 purpura) before the non-irradiated PC transfusions. Post transfusion, there were three bleeding events (3 petechiae or purpura) after gamma-irradiated PC transfusion and three bleeding events (3 petechiae or purpura) after non-irradiated PC transfusion. None of the transfusion bleeding events did exceed WHO grade 1 severity, and no inferences could be made about the relationship to PLT CI or MA modification.
Additional RBCs Transfusions
The majority of patients in both study arms did not require RBC transfusions within 24 h after the study transfusion. Only 7 of 20 (35%) patients in the control group and 8 of 20 (40%) patients in the group receiving gamma-irradiated PCs must be transfused with RBCs (p = 0.744).
Safety Analysis
Patients were observed for any AEs for 24 h after the start of the study PLT transfusion, and all AEs, whether or not classified as related to the study transfusion, were recorded. About 15% of the patients underwent at least 1 ATR within 24 h after the study platelet transfusion (15% in study arm A vs. 15% in study arm B), with the majority being allergic manifestations. All AEs occurred within 24 h after a transfusion; these AEs comprised a spectrum of signs and symptoms including fever (5% in study arm A vs. 10% in study arm B), chills (5% in study arm A vs. 0% in study arm B), skin rash (5% in study arm A vs. 0% in study arm B) and nausea/vomiting (0% in study arm A vs. 5% in study arm B). There were no statistically significant differences between treatment groups in the frequency of any AE deemed by the investigator as related to the study transfusion within the first 24 h or during the 4 days following study transfusion.
All patients were followed up from randomization until 30 days after study transfusion for serious AEs. All serious AEs were considered unrelated to the study transfusion. Two fatal serious AEs were considered not related to gamma-irradiated PCs. No case of transfusion-transmitted infection, TA-GVHD, or transfusion-related acute lung injury was observed. Three study patients died; two deaths occurred in the control group, and one death occurred in the treatment group. All other deaths were considered related to the patient's underlying disease.
Discussion
The immunologic consequences of the transfusion of blood products can range from donor anti-recipient responses such as TA-GVHD to recipient anti-donor responses such as the production of alloantibodies, generation of regulatory responses, and responses that can eliminate the donor PLTs. While TA-GVHD only occurs in a limited number of transfusion recipients, the almost inevitable mortality associated with TA-GVHD led transfusion specialists to focus on preventing TA-GVHD [22]. Gamma irradiation of blood components is performed to reduce the risk for patients to develop this highly lethal complication which is caused by T lymphocytes in blood components [3]. As a result, gamma irradiation with 25 Gy was adopted as the standard treatment of blood products for the prevention of TA-GVHD.
To our knowledge, there are few studies reporting on the effect of gamma irradiation on apheresis PLTs [23, 24]. We found no difference in pH between gamma-irradiated and control PCs, which is in accordance with some previous reports. However, there are also studies that have reported differences in pH between irradiated and non-irradiated PCs [4]. Swirling was well maintained and at a similar level for irradiated and non-irradiated PCs. All PCs in our study complied with Chinese regulatory requirements (minimum PLT content of 2.52 × 1011/unit).
The utility of TEG to provide rapid assessments of global hemostatic function in the operating theater and the emergency room is well established, and clot strength has repeatedly been reported to have the best correlation with clinically relevant blood loss [8, 25, 26, 27]. Its use in hematology-oncology patients with thrombocytopenia is relatively novel, but interest is growing [8, 28]. We found that both gamma-irradiated and untreated PCs significantly improved all TEG variables within 1 h of transfusion, indicating that the transfused PLTs were hemostatically active and that TEG was sensitive to the effects of a single PLT transfusion. All TEG variables indicated improved hemostatic function after transfusion of both gamma-irradiated and untreated PCs, with increases in α and MA most indicative of the role of PLTs in coagulation. MA was lower at 1 h post transfusion in gamma-irradiated PCs when compared to untreated PCs. Such thresholds for TEG variables have not yet been defined to guide transfusion practices in patients with thrombocytopenia. Comparing the two posttransfusion time points, MA in the group receiving gamma-irradiated PCs has not significantly changed while it has significantly dropped in the group receiving non-irradiated PCs.
This study is limited by its small sample size, and results must be interpreted with caution. Despite the small sample size, it is clear that TEG measurements demonstrated a hemostatic effect due to PLT transfusion in hematology-oncology patients with thrombocytopenia, and MA results were consistent with PLT counts. However, a common criticism of TEG is that it has not been correlated with more clinically relevant outcome measures such as bleeding events [8]. Apelseth et al. [18] recently reported that MA measured by TEG correlates with PLT CIs and bleeding, thus reflecting both PLT viability and functionality in hematology-oncology patients and demonstrating TEG's clinical utility in this patient population.
CI as well as CCI are still regarded as parameters predictive for the response to PLT transfusions and thus the success of a transfusion [4, 29]. In evaluating CCI, particular consideration must be given to product- and patient-related factors. First, it is essential to consider the characteristics of the PCs transfused. PLT yield of apheresis is dependent on the cell separator used [30]. All PLT products in our study were collected by single-donor apheresis with one type of apheresis device at a single center, and all were resuspended in 100% plasma. After apheresis, all products either were gamma-irradiated with 25 Gy or did not receive any additional treatment. It is important to note that gamma-irradiated PCs are registered for WBC inactivation for prevention of TA-GVHD in China. All were PCs stored under the same conditions and transfused according to the same guidelines to a homogenous patient population.
In our analysis we did not detect any significant difference in 1-hour CCI between patients receiving gamma-irradiated PCs and those receiving non-irradiated PCs. The current study demonstrated that transfusion of gamma-irradiated PCs stored for 0–3 days resulted in a mean 1-hour CCI that was 9.6% lower than that of the group transfused with non-irradiated PCs; however, this difference did not reach statistical significance (p = 0.171). There was no effect on hemorrhagic AEs, and the use of RBC transfusions within 24 h after the start of the study transfusion was similar in both groups (study arm A: 40.0% vs. study arm B: 35.0%; p = 0.744). Even though the post-transfusion 24-hour CCI in the group receiving gamma-irradiated PCs was 12.2% lower than that of the control group, this difference did not reach statistical significance (p = 0.167). Moreover, the median time to the next PLT transfusion within 15 days of the study transfusion was similar in both groups (2.2 days in study arm A vs. 2.4 days in study arm B; p = 0.767). Although the CCI theoretically corrects for PLT dose, there is evidence that suggests that this correction is not complete and that lower PLT dose might yield lower CCIs [31]. Resuspending PCs in PAS is linked with lower CCI compared to PCs in 100% plasma [30, 32, 33]. Moreover, PAS allows for PCs to be transfused regardless of ABO barrier between donor and recipient, and ABO incompatibility may negatively affect CCI as well [34]. Theoretically, gamma irradiation of PCs is likely to add a further lesion to the PLTs, resulting in lower efficacy and CCI of PCs. On the other hand, non-irradiated PCs should therefore yield higher CCI. Based on our results, we conclude that gamma-irradiated single-donor apheresis PCs yielded comparable CCI to non-irradiated PCs resuspended in 100% plasma. As already mentioned, gamma irradiation is another factor known to negatively affect CCI [31].
We utilized other supporting endpoints directly indicative for hemostasis including active bleeding, AEs, and ATR. The safety profile was similar in both study groups for the first 24 h and for the 4 days following study transfusion. The AEs being classified as related to transfusion of gamma-irradiated PCs were infrequent, and all of them were of mild severity. The incidence of ATR was low too, and all reported ATRs were of mild severity. Our results are in line with our previous experience and with that of larger studies showing a generally low incidence of transfusion reactions associated with gamma-irradiated PCs. The ATRs observed were mild allergic reactions in the majority of cases. These events were all attributed to the progression of underlying illness and were considered unrelated to gamma irradiation of PCs. Our study showed that most bleedings were determined as bleeding score 0–1, with the majority involving the mucocutaneous organ system, and indicated that gamma irradiation of PCs was effective to support hemostasis and to prevent bleeding. Fewer patients had bleedings after transfusion than prior to transfusion. The study analyzed the safety and efficacy of gamma irradiation of PCs in a large cohort of hematologic patients. Gamma-irradiated PCs were found to be equivalent to non-irradiated PCs in terms of bleeding control. Most patients in this cohort had hematological diseases treated with high-dose chemotherapy, and about one third received autologous peripheral blood stem cell transplantations. Due to their very severe underlying diseases, the patients were at high risk for development of TA-GVHD. In the course of this study, no case of TA-GVHD was reported.
In conclusion, our study demonstrates similar hemostatic function and transfusion efficacy of gamma-irradiated PCs when compared to non-irradiated PCs in hematologic patients. Gamma irradiation treatment of PCs is safe in routine use even for such high-risk patients who are severely immuno-compromised and have undergone stem cell transplantation.
Disclosure Statement
The authors declare that they have no conflict of interest in the subject matter of the manuscript.
Acknowledgments
We thank Dr. B-L Wang for his excellent technical and financial assistance and valuable advice during the preparation of this manuscript.
References
- 1.Blajchman MA, Slichter SJ, Heddle NM, Murphy MF. New strategies for the optimal use of platelet transfusions. ASH Educ Program Book. 2008. pp. 198–204. [DOI] [PubMed]
- 2.Sensebe L, Giraudeau B, Bardiaux L, Deconinck E, Schmidt A, Bidet ML, Leniger C, Hardy E, Babault C, Senecal D. The efficiency of transfusing high doses of platelets in hematologic patients with thrombocytopenia: results of a prospective, randomized, open, blinded end point (PROBE) study. Blood. 2005;105:862–864. doi: 10.1182/blood-2004-05-1841. [DOI] [PubMed] [Google Scholar]
- 3.Tynngård N, Studer M, Lindahl TL, Trinks M, Berlin G. The effect of gamma irradiation on the quality of apheresis platelets during storage for 7 days. Transfusion. 2008;48:1669–1675. doi: 10.1111/j.1537-2995.2008.01746.x. [DOI] [PubMed] [Google Scholar]
- 4.Schlenke P, Hagenah W, Irsch J, Sundin D, Corash L, Lin L, Kirchner H, Wagner T. Safety and clinical efficacy of platelet components prepared with pathogen inactivation in routine use for thrombocytopenic patients. Ann Hematol. 2011;90:1457–1465. doi: 10.1007/s00277-011-1222-3. [DOI] [PubMed] [Google Scholar]
- 5.van der Meer PF, Pietersz RN. Gamma irradiation does not affect 7-day storage of platelet concentrates. Vox Sang. 2005;89:97–99. doi: 10.1111/j.1423-0410.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 6.Roeloffzen WW, Kluin-Nelemans HC, Veeger NJ, Bosman L, de Wolf JT. Transfused stored platelets have the same haemostatic function as circulating native platelets. Vox Sang. 2010;99:123–130. doi: 10.1111/j.1423-0410.2010.01337.x. [DOI] [PubMed] [Google Scholar]
- 7.Shah U, Ma AD. Tests of platelet function. Curr Opin Hematol. 2007;14:432–437. doi: 10.1097/MOH.0b013e3282b9747b. [DOI] [PubMed] [Google Scholar]
- 8.Johansson PI, Simonsen AC, Brown PN, Ostrowski SR, Deberdt L, Van Hoydonck P, Yonemura SS, Goodrich RP. A pilot study to assess the hemostatic function of pathogen-reduced platelets in patients with thrombocytopenia. Transfusion. 2013;53:2043–2052. doi: 10.1111/trf.12055. [DOI] [PubMed] [Google Scholar]
- 9.Schols SE, Feijge MA, Lancé MD, Hamulyák K, ten Cate H, Heemskerk JW, van Pampus EC. Effects of plasma dilution on tissue-factor-induced thrombin generation and thromboelastography: partly compensating role of platelets. Transfusion. 2008;48:2384–2394. doi: 10.1111/j.1537-2995.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 10.Mitra B, Mori A, Cameron PA, Fitzgerald M, Street A, Bailey M. Massive blood transfusion and trauma resuscitation. Injury. 2007;38:1023–1029. doi: 10.1016/j.injury.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 11.Kamal AH, Tefferi A, Pruthi RK. How to interpret and pursue an abnormal prothrombin time, activated partial thromboplastin time, and bleeding time in adults. Mayo Clin Proc. 2007;82:864–873. doi: 10.4065/82.7.864. [DOI] [PubMed] [Google Scholar]
- 12.Holland LL, Brooks JP. Toward rational fresh frozen plasma transfusion: the effect of plasma transfusion on coagulation test results. Am J Clin Pathol. 2006;126:133–139. doi: 10.1309/NQXH-UG7H-ND78-LFFK. [DOI] [PubMed] [Google Scholar]
- 13.Ostrowski SR1, Bochsen L, Windeløv NA, Salado-Jimena JA, Reynaerts I, Goodrich RP, Johansson PI. Hemostatic function of buffy coat platelets in additive solution treated with pathogen reduction technology. Transfusion. 2011;51:344–356. doi: 10.1111/j.1537-2995.2010.02821.x. [DOI] [PubMed] [Google Scholar]
- 14.McFarland JG. Platelet and granulocyte antigens and antibodies. In: Roback JD, editor. Technical Manual. Bethesda: American Association of Blood Banks; 2008. pp. 525–540. [Google Scholar]
- 15.Stanworth SJ, Hyde C, Heddle N, Rebulla P, Brunskill S, Murphy MF. Prophylactic platelet transfusion for haemorrhage after chemotherapy and stem cell transplantation. Cochrane Database Syst Rev. 2004;4:CD004269. doi: 10.1002/14651858.CD004269.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Schiffer CA, Anderson KC, Bennett CL, Bernstein S, Elting LS, Goldsmith M, Goldstein M, Hume H, McCullough JJ, McIntyre RE, Powell BL, Rainey JM, Rowley SD, Rebulla P, Troner MB, Wagnon AH. Platelet transfusion for patients with cancer: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19:1519–1538. doi: 10.1200/JCO.2001.19.5.1519. [DOI] [PubMed] [Google Scholar]
- 17.Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122:10–23. doi: 10.1046/j.1365-2141.2003.04468.x. [DOI] [PubMed] [Google Scholar]
- 18.Apelseth TO, Bruserud O, Wentzel-Larsen T, Hervig T. Therapeutic efficacy of platelet transfusion in patients with acute leukemia: an evaluation of methods. Transfusion. 2010;50:766–775. doi: 10.1111/j.1537-2995.2009.02540.x. [DOI] [PubMed] [Google Scholar]
- 19.McCullough J, Vesole D, Benjamin R, Slichter S, Pineda A, Snyder E, Stadtmauer EA, Lopez-Plaza I, Coutre S, Strauss RG, Goodnough LT, Fridey JL, Raife T, Cable R, Murphy S, Howard F, 4th, Davis K, Lin JS, Metzel P, Corash L, Koutsoukos A, Lin L, Buchholz DH, Conlan MG. Therapeutic efficacy and safety of platelets treated with a photochemical process for pathogen inactivation: the SPRINT trial. Blood. 2004;104:1534–1541. doi: 10.1182/blood-2003-12-4443. [DOI] [PubMed] [Google Scholar]
- 20.Konig G, Yazer MH, Waters JH. The effect of salvaged blood on coagulation function as measured by thromboelastography. Transfusion. 2013;53:1235–1239. doi: 10.1111/j.1537-2995.2012.03884.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 22.Fast LD, DiLeone G, Marschner S. Inactivation of human white blood cells in platelet products after pathogen reduction technology treatment in comparison to gamma irradiation. Transfusion. 2011;51:1397–1404. doi: 10.1111/j.1537-2995.2010.02984.x. [DOI] [PubMed] [Google Scholar]
- 23.Apelseth TO, Hervig TA, Wentzel-Larsen T, Bruserud O. Cytokine accumulation in photochemically treated and gamma-irradiated platelet concentrates during storage. Transfusion. 2006;46:800–810. doi: 10.1111/j.1537-2995.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 24.Apelseth TO, Bruserud O, Wentzel-Larsen T, Bakken AM, Bjorsvik S, Hervig T. In vitro evaluation of metabolic changes and residual platelet responsiveness in photochemical treated and gamma-irradiated single-donor platelet concentrates during long-term storage. Transfusion. 2007;47:653–665. doi: 10.1111/j.1537-2995.2007.01167.x. [DOI] [PubMed] [Google Scholar]
- 25.Johansson P, Stissing T, Bochsen L, Ostrowski SR. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand J Trauma Resusc Emerg Med. 2009;17:45. doi: 10.1186/1757-7241-17-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson PI, Stensballe J. Effect of haemostatic control resuscitation on mortality in massively bleeding patients: a before and after study. Vox Sang. 2009;96:111–118. doi: 10.1111/j.1423-0410.2008.01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nystrup KB, Windelov NA, Thomsen AB, Johansson PI. Reduced clot strength upon admission, evaluated by thrombelastography (TEG), in trauma patients is independently associated with increased 30-day mortality. Scand J Trauma Resusc Emerg Med. 2011;19:1–8. doi: 10.1186/1757-7241-19-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flisberg P, Rundgren M, Engstrom M. The effects of platelet transfusions evaluated using rotational thromboelastometry. Anesth Analg. 2009;108:1430–1432. doi: 10.1213/ane.0b013e31819bccb7. [DOI] [PubMed] [Google Scholar]
- 29.Slichter S, Davis K, Enright H, Braine H, Gemsheimer T, Kao K-J, Kickler T, Lee E, McFarland J, McCullough J, Rodey G, Schiffer CA, Woodson R. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105:4106–4114. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fontana S, Mordasini L, Keller P, Taleghani BM. Prospective, paired crossover comparison of multiple, single-needle plateletpheresis procedures with the Amicus and Trima Accel cell separators. Transfusion. 2006;46:2004–2010. doi: 10.1111/j.1537-2995.2006.01009.x. [DOI] [PubMed] [Google Scholar]
- 31.Sigle JP, Infanti L, Studt JD, Martinez M, Stern M, Gratwohl A, Passweg J, Tichelli A, Buser AS. Comparison of transfusion efficacy of amotosalen-based pathogen-reduced platelet components and gamma-irradiated platelet components. Transfusion. 2013;53:1788–1797. doi: 10.1111/j.1537-2995.2012.03959.x. [DOI] [PubMed] [Google Scholar]
- 32.Davis KB, Slichter SJ, Corash L. Corrected count increment and percent platelet recovery as measures of posttransfusion platelet response: problems and a solution. Transfusion. 1999;39:586–592. doi: 10.1046/j.1537-2995.1999.39060586.x. [DOI] [PubMed] [Google Scholar]
- 33.de Wildt-Eggen J, Nauta S, Schrijver JG, van Marwijk Kooy M, Bins M, van Prooijen HC. Reactions and platelet increments after transfusion of platelet concentrates in plasma or an additive solution: a prospective, randomized study. Transfusion. 2000;40:398–403. doi: 10.1046/j.1537-2995.2000.40040398.x. [DOI] [PubMed] [Google Scholar]
- 34.Kerkhoffs JL, Eikenboom JC, Schipperus MS, van Wordragen-Vlaswinkel RJ, Brand R, Harvey MS, de Vries RR, Barge R, van Rhenen DJ, Brand A. A multicenter randomized study of the efficacy of transfusions with platelets stored in platelet additive solution II versus plasma. Blood. 2006;108:3210–3215. doi: 10.1182/blood-2006-04-020131. [DOI] [PubMed] [Google Scholar]