Summary
Introduction
Optimising the use of blood has become a core task of transfusion medicine. Because no general guidelines are available in Switzerland, we analysed the effects of the introduction of a guideline on red blood cell (RBC) transfusion for elective orthopaedic surgery.
Methods
Prospective, multicentre, before-and-after study comparing the use of RBCs in adult elective hip or knee replacement before and after the implementation of a guideline in 10 Swiss hospitals, developed together with all participants.
Results
We included 2,134 patients, 1,238 in 7 months before, 896 in 6 months after intervention. 57 (34 or 2.7% before, 23 or 2.6% after) were lost before follow-up visit. The mean number of transfused RBC units decreased from 0.5 to 0.4 per patient (0.1, 95% CI 0.08–0.2; p = 0.014), the proportion of transfused patients from 20.9% to 16.9% (4%, 95% C.I. 0.7–7.4%; p = 0.02), and the pre-transfusion haemoglobin from 82.6 to 78.2 g/l (4.4 g/l, 95% C. I. 2.15–6.62 g/l, p < 0.001). We did not observe any statistically significant changes in in-hospital mortality (0.4% vs. 0%) and morbidity (4.1% vs. 4.0%), median hospital length of stay (9 vs. 9 days), follow-up mortality (0.4% vs. 0.2%) and follow-up morbidity (6.9% vs. 6.0%).
Conclusions
The introduction of a simple transfusion guideline reduces and standardises the use of RBCs by decreasing the haemoglobin transfusion trigger, without negative effects on the patient outcome. Local support, training, and monitoring of the effects are requirements for programmes optimising the use of blood.
Keywords: Transfusion, Surgery, Red blood cells, Guideline
Introduction
Blood is a precious resource obtained from voluntary, not remunerated donors [1], and should be used to the greatest benefit of patients. In the last years, efforts have been undertaken in order to optimise the use of blood. Results of clinical trials published in recent years have shown that optimising the use of blood components, particularly red blood cell (RBC) concentrates, not only reduces the costs related to blood transfusion but also may influence positively the outcome of patients, or at least does not worsen it [2, 3, 4, 5, 6]. As a consequence, several guidelines for the use of RBCs have been developed by different societies and institutions [7, 8, 9, 10, 11, 12, 13]. Currently, further efforts to optimise patient care and use of blood components are ongoing under the concept of blood patient management [14, 15, 16, 17].
In Switzerland, national guidelines for the transfusion of RBCs do not yet exist. But every institution transfusing blood components should implement a quality system in accordance with the current state of the art [18]. Guidelines for the use of blood components, as well as the documentation and the evaluation of their use, should be part of this quality system. However, implementing and teaching a guideline as well as evaluating its effect is a major challenge for hospitals being more and more confronted with limited financial and personal resources. Furthermore, blood transfusion is in fact a complex but at the same time a very safe process that represents only a small proportion of the hospital costs and activities. Thus, hospitals usually depend on any kind of significant support/funding for their efforts to achieve the implementation of guidelines. With this purpose, 5 years ago our group applied for a grant from the Swiss Red Cross Humanitarian Foundation (SRC-HF), which amongst others supports research and development in the field of transfusion medicine. We planned to develop and introduce guidelines within Swiss hospitals interested to implement them and to evaluate their effect on the use of RBC concentrates. We targeted a multicentre approach including different kinds of hospitals for elective orthopaedic surgery and a single university hospital for cardiovascular surgery. In this article we describe principles, methods and results of the multicentre study in hip and knee prosthesis in the hope, that this will inspire others to take similar initiatives, possibly as a first step towards development of a complete patient blood management programme. Meanwhile, since the performance of the project, the number of delivered RBC concentrates has dropped by about 10% and even by about 15% on a per inhabitant basis in Switzerland [19], and this reduction is still ongoing. We are not able to state if the results are a consequence of this first and until now unique initiative in Switzerland trying to optimise the use of blood on a broad base, but during and after this project the use of blood components was repeatedly debated throughout the country, and several clinics implemented this or similar guidelines. Therefore, we hope to have given a valuable contribution to the current positive development.
Material and Methods
Study Design and Participating Hospitals
We designed the protocol as a prospective, multicentre, before-and-after cohort study in orthopaedic surgery, including all prospective patients planned for elective hip or knee replacement during the study period in the hospitals accepting to participate. During the first 6 months (August 2007 to January 2008), we monitored the RBC use and the patient outcomes (phase 1 of the protocol). At the end of phase 1, a RBC transfusion guideline, jointly developed with the participating hospitals, was introduced and implemented. Because the development process of the guideline took 1 month longer than planned, we prolonged phase 1 to 7 months (until February 2008). Transfusion data were continuously monitored until completion of a subsequent post-interventional period of 6 months (phase 2 of the protocol, March to August 2008). Because the aims of the project were part of the development of a mandatory quality system, in particular to implement measures for optimal blood use [18], the local Ethical Committee confirmed that no formal approval of the protocol was needed.
The primary outcome was the difference in the number of RBC concentrates per surgical intervention between phase 1 and 2. Secondary outcomes were the differences between the proportion of transfused patients, the pre-transfusion haemoglobin values and the patient outcomes at the end of their hospitalisation and at the first consultation at least 30 days after surgery. The patient outcomes comprised mortality and cumulative complication rate, including cardiovascular events, bleedings, and infections (CTCAE grade 3 or higher [20]).
Selection and Recruitment of Centres
We sent a letter to all Swiss hospitals known to include an orthopaedic unit, inviting them to participate in the study on a voluntary basis. Ten hospitals accepted to participate and completed the study. The 10 hospitals performed about 7% of the elective hip and knee replacements in Switzerland during the study period. They were distributed in the 3 Cantons of Bern (central-western part of Switzerland, 5 hospitals), St. Gallen (eastern part of Switzerland, 3 hospitals), and Ticino (southern part of Switzerland, 2 hospitals). They comprised 1 university hospital, 3 public cantonal hospitals, 4 public regional hospitals and 2 private regional hospitals.
Inclusion and Exclusion Criteria
All adult (at least 18 years of age) prospective patients undergoing elective hip or knee replacement during the study period were included, independently from comorbidities or risk factors. Excluded were non-elective interventions, patients with concomitant surgical procedures, patients younger than 18 years, patients refusing transfusions or patients for whom no adequate RBCs were available in the regional stock because of multiple RBC antibodies or rare blood groups.
Development of the Guideline
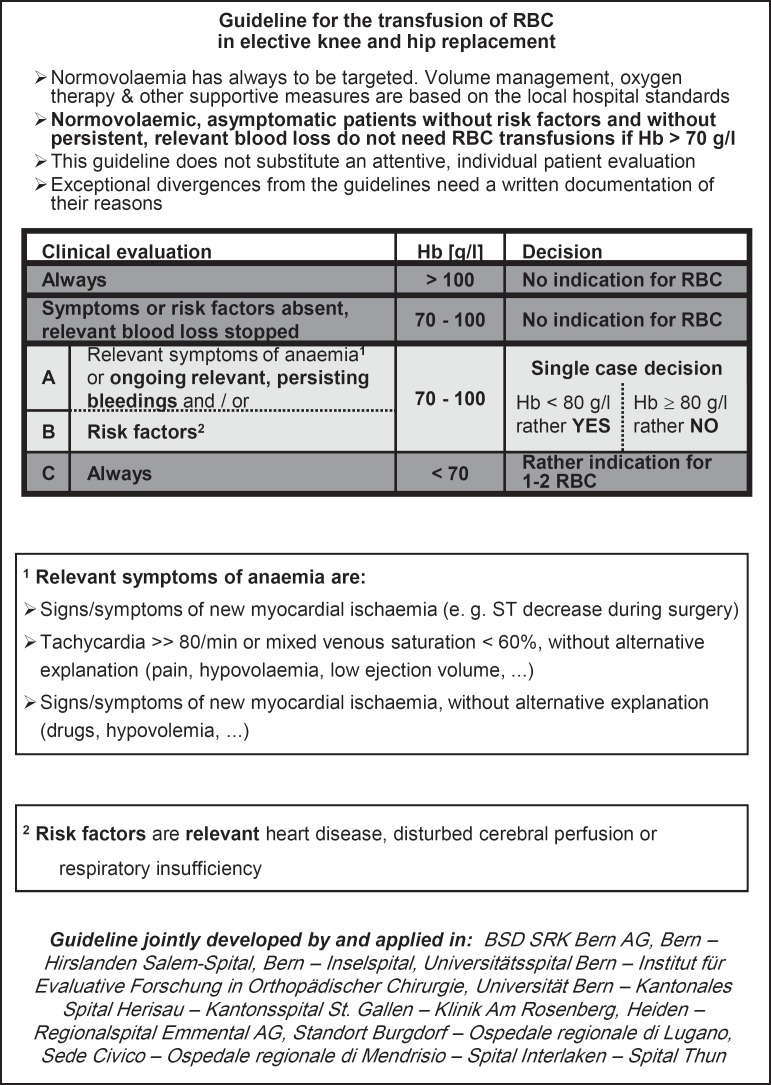
In order to improve the compliance, we developed a simple, evidence-based guideline together with the anaesthetists and/or orthopaedic surgeons responsible for the interventions in the participating hospitals (fig. 1). For this purpose, the authors of this paper reviewed the literature for available guidelines (considered guidelines are listed in table 1), checked their compatibility with the published controlled clinical trials at this time (considered studies see table 1) and distributed a draft to all responsible persons of the participating hospitals. To avoid a bias during phase 1, the draft guideline was discussed with all participants, finalised and trained locally in the hospitals to as many medical doctors as possible only at the end of the phase 1. The finalised guideline was subsequently implemented in the standard procedures of the participating hospitals. One copy was put in the medical records of every patient included in phase 2 of the study, and pocket leaflets were distributed to the medical doctors. In order to properly complete this process, we had to prolong phase 1 of the study by 1 month (i.e., 7 instead of 6 months).
Fig. 1.
The developed guideline for RBC transfusion.
Table 1.
A Practice guidelines and B clinical studies principally considered for the development of our guideline
| A | Practice guidelines |
| 1 | American Society of Anesthesiologists Task Force: Practice Guidelines for Perioperative Blood Transfusion and Adjuvant Therapies. |
| An Updated Report by the American Society of Anesthesiologists Task Force. Approved by the House of Delegates on October 22, 1995 and last amended on October 25, 2005. | |
| 2 | Expert Working Group. Guidelines for red blood cell and plasma transfusion for adults and children. Int J Risk Saf Med 1997;10:255-271 |
| 3 | Transfusion de globules rouges homologues: produits, indications, alternatives. Agence Française de Sécurité Sanitaire des Produits de Santé, 2002 |
| 4 | Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists. Perioperative blood transfusion and blood conservation in cardiac surgery: practice guideline. Ann Thorac Surg 2007:83(suppl 5):27–86 |
| 5 | British Committee for Standards in Haematology, Blood Transfusion Task Force: Guidelines for the clinical use of red cell transfusions. |
| Br J Haematol 2001;113:24-31 | |
| 6 | Vorstand und wissenschaftlicher Beirat der Bundesärztekammer: Leitlinien zur Therapie mit Blutkomponenten und Plasmaderivaten, |
| 3. Auflage. Deutscher Ärzteverlag, Köln 2003 | |
| 7 | Bundesärztekammer im Einvernehmen mit dem Paul-Ehrlich-Institut: Richtlinien zur Gewinnung von Blut und Blutbestandteilen und zur Anwendung von Blutprodukten (Hämotherapie). 2005, Änderungen und Ergänzungen 2007 |
| 8 | Blood Transfusion and the Anaesthetist: Blood Component Therapy. Association of Anaesthetists of Great Britain and Ireland, 2005 |
| 9 | Practice Guideline Development Task Force of the College of the American Pathologists_ Practice Parameter for the use of red blood cell transfusions. Arch Pathol Lab Med 1998;122:130–138 |
| B | Clinical studies |
| 1 | Hébert P, et al: A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med 1999;340:409–417 |
| 2 | Eindhoven GB, et al: Adjusted transfusion triggers improve transfusion practice in orthopedic surgery. Transfus Med 2005;15:13–18 |
| 3 | Wong CJ, et al: A cluster-randomized controlled trial of a blood conservation algorithm in patients undergoing total hip joint arthroplasty. Transfusion 2007;47:832–841 |
| 4 | Grover M, et al: Silent myocardial ischemia and hemoglobin concentration: a randomized controlled trial of transfusion strategy in lower limb arthroplasty. Vox Sang 2006;90:105–112 |
| 5 | Müller U, et al: Effect of a flow chart on use of blood transfusions in primary hip and knee replacement: prospective before and after study. |
| BMJ 2004;328:934–938 | |
| 6 | Carson JL, et al: Perioperative blood transfusion and postoperative mortality. JAMA 1998;279:199–205 |
| 7 | Weiskopf RB, et al: Human cardiovascular and metabolic response to acute, severe isovolemic anemia. JAMA 1998;279:217–221 |
| 8 | Heier HE, et al: Transfusion vs alternative treatment modalities in acute bleeding: a systematic review. Acta Anaesthesiol Scand 2006;50:920–931 |
Data Collection and Statistical Analysis
We collected data on patients’ characteristics, surgical interventions, haemoglobin values, transfusions and complications during hospitalisation and until the last follow-up. In order to control the patient groups for general condition and comorbidities, we registered the American Society of Anaesthesiology (ASA) status. Two study nurses, specifically trained for the project, prospectively collected all the data from the hospital clinical records and from the reports of the follow-up visits, and entered them in a web-based electronic database of the Institute for Evaluative Research in Orthopaedic Surgery of the University of Bern which also performed the statistical analyses using SAS 9.1 (SAS Institute Inc., Cary, NC, USA).
The primary endpoint, the comparison between phase 1 and phase 2 regarding the mean numbers of RBC concentrates transfused per patient was assessed by the Wilcoxon rank sum test. The pre-transfusion haemoglobin values were also compared by the Wilcoxon rank sum test and the remaining secondary endpoints by the chi square test. Because only one primary endpoint was defined, no correction for multiple comparisons was performed. In order to avoid bias related to differences in the populations between phase 1 and 2, the primary endpoint and the proportion of transfused patients were also re-analysed after adjustment for variables influencing the transfusion need detected by logistic regression analysis (see ‘Results’). Changes in the variability of outcomes between hospitals were evaluated by the Mood test. Other categorical variables were compared by the chi square test, and numeric variables by the Wilcoxon rank sum test.
Results
Out of 2,144 surgical interventions recorded during the study period, 2,134 could be evaluated for transfusion of RBC concentrates, haemoglobin trigger and in-hospital morbidity; data on surgery, transfusion or haemoglobin values were partially missing in 10 cases. 57 (2.7%) patients were lost during follow-up (34 in phase 1 (2.7%), 23 in phase 2 (2.6%)), in all cases because no medical reports of the follow-up visits were sent back to the study office. Consequently, 2,077 patients were evaluable at the last follow-up >30 days after surgery.
Basic characteristics of the patients and of the surgical procedures are reported in table 2. No differences were observed between phase 1 and 2, except for a higher number of prosthesis replacements and slightly higher preoperative haemoglobin values in phase 1. The distribution of surgical procedures between the hospitals is summarised in table 3; only minimal differences in the distribution between phase 1 and 2 were observed.
Table 2.
Patient characteristics and surgical procedures before (phase 1) and after (phase 2) introduction of the transfusion guideline
| Variable | Phase 1 | Phase 2 | p value |
|---|---|---|---|
| N | 1,238 | 896 | n.a. |
| Gender (m/f), % | 45.5/54.5 | 45.0/55.0 | n.s. |
| Mean age, years (standard deviation) | 68.1 (11.5) | 67.9 (11.8) | n.s. |
| Median ASA score (range) | 2 (1–4) | 2 (1–4) | n.s. |
| Mean preoperative haemoglobin value, g/l (standard deviation) | 138.0 (13.8) | 135.6 (15.0) | <0.001 |
| Hip/knee surgery, % | 57.7/42.3 | 58.8/41.2 | n.s. |
| Primary surgery / prosthesis replacement, %) | 91.9/8.1 | 95.5/4.5 | <0.001 |
| Mean duration of the surgical intervention, min (standard deviation) | 107 (35) | 107 (35) | n.s. |
| Regional/general anaesthesia, % | 69.8/30.2 | 68.2/31.8 | n.s. |
| Use of blood tourniquet, % | 36.1 | 34.5 | n.s. |
| Preoperative autologous blood donation, % | 4.9 | 3.8 | n.s. |
| Intraoperative blood saving (CellSaver), % | 3.6 | 4.0 | n.s. |
Table 3.
Percent distribution of the surgical interventions between the hospitals
| Hospital | Phase 1 | Phase 2 |
|---|---|---|
| 1 | 8.3% | 6.1% |
| 2 | 4.8% | 5.6% |
| 3 | 7.7% | 6.7% |
| 4 | 3.0% | 4.0% |
| 5 | 3.0% | 4.3% |
| 6 | 5.0% | 8.4% |
| 7 | 13.2% | 13.1% |
| 8 | 15.6% | 16.0% |
| 9 | 19.3% | 17.1% |
| 10 | 20.1% | 18.7% |
| Total | 100.0% | 100.0% |
After the introduction of the guideline, we observed a decrease of the mean number of RBC transfusions from 0.5 to 0.4 units per patient (p = 0.014), which corresponds to a 20% reduction of the transfused RBC concentrates. Less patients received any transfusion of RBCs, and the haemoglobin level prior to transfusion was lower in phase 2 (table 4). In order to test whether differences in baseline variables do have an influence on the results, the analyses for the primary outcome and for the proportion of transfused patients were repeated after adjustment for variables found to have an influence on the transfusion need by logistic regression. These comprise age (p < 0.001), preoperative haemoglobin (p < 0.001), % of prosthesis replacement (p < 0.001) and type of anaesthesia (p = 0.037). Additionally to the raw results in table 4, adjusted differences in RBC use or odds ratios for transfusion probability are reported in table 5. The adjusted data confirmed the findings of the unadjusted raw data.
Table 4.
Absolute number of RBC concentrates per patient (primary endpoint), proportion of transfused patients, and pre-transfusion haemoglobin (secondary endpoints), unadjusted.
| Outcome | Phase 1 | Phase 2 | Difference | 95% CI | p value |
|---|---|---|---|---|---|
| RBC per patient, units | 0.5 | 0.4 | 0.1 | 0.08–0.2 | 0.014 |
| Transfused patients, % | 20.9 | 16.9 | 4.0 | 0.70–7.40 | 0.020 |
| Pre-transfusion Hb, g/l | 82.6 | 78.2 | 4.4 | 2.15–6.62 | <0.001 |
Table 5.
Differences of transfused RBC units per patient and odds ratios of transfused patients, unadjusted and adjusted for variables influencing the transfusion need (see text)
| Outcome | Difference unadjusted | 95% CI | Difference adjusted | 95% CI |
|---|---|---|---|---|
| RBC per patient (units, difference) | 0.1 | 0.08–0.20 | 0.12 | 0.03–0.21 |
| Proportion of transfused patients (odds ratio) | 1.3 | 1.05–1.63 | 1.8 | 1.17–2.75 |
The variability between hospitals decreased in the phase 2 for both the number of transfused RBC units per patient and the proportion of transfused patients. The range of transfused RBC units per patient decreased from 0.11–1.16 in phase 1 to 0.14–0.69 in phase 2 (p < 0.001). The range of the proportion of transfused patients decreased from 4.6–43.7% in phase 1 to 6.9–28.3% in phase 2 (p < 0.001). The inter-hospital range of the pre-transfusion haemoglobin values in phase 1 was 76.9–88.9 and in phase 2 68.5–81.2 (not significant).
We did not observe any differences in length of hospital stay, mortality and cumulative complication rates between phase 1 and 2. The mean time point of the last follow-up was 3 days earlier in phase 2. The results are outlined in table 6.
Table 6.
Follow-up results
| Outcome | Phase 1 | Phase 2 | p value |
|---|---|---|---|
| Length of hospital stay, days | 9 | 9 | n.s. |
| Hospital morbidity, % | 4.1 | 4.0 | n.s. |
| Hospital mortality, % | 0.4 | 0.0 | n.s. |
| Time of follow up after intervention, days | 52 | 49 | 0.0014 |
| Morbidity on follow-up, % | 6.9 | 6.0 | n.s. |
| Mortality on follow-up, % | 0.4 | 0.2 | n.s. |
Discussion
In the last years, efforts to optimise the use of blood have been undertaken in several countries, transfusion guidelines for RBC concentrated have been published [7, 8, 9, 10, 11, 12, 13], and a further optimisation is ongoing under the concept of patient blood management [14, 15, 16, 17]. Projects aimed to achieve this goal are time-consuming, and under the current financial and performance pressure it is often difficult to find the resources needed. Our experience suggests that improvements can be achieved by a small group of persons leading and coordinating the activities of the major players in transfusion medicine. Key factors that may have contributed to the success of the guideline are in our opinion its simplicity, the lack of requirement for major changes, the concerted development in collaboration with the local opinion leaders, its wide distribution among the staffs and the development of a sense of ownership on the guideline and on the results achieved. Effectively, because the guideline was based on the current literature applicable for several patient categories and no negative effects after its introduction could be observed (see below), we could make it immediately available for all participating institutions after the study. The participating university hospital also applied the guideline for other non-orthopaedic wards like cardiovascular surgery. In this way we achieved our goal to implement guidelines as part of a quality management system making the first step towards the concept of patient blood management in the hospitals.
Our intervention also showed a significant effect in reducing the RBC use and the proportion of transfused patients, among others by reducing the mean haemoglobin level prior to transfusion. In this context, the project may have some limitations. First, confounding factors may have resulted in a lower transfusion need irrespective of the guideline. But this effect was still observed after adjustment for differences between the two phases, probably because in phase 2 the patients actually received fewer transfusions despite lower preoperative haemoglobin values. Furthermore, no hospital reported other changes in their surgical or other perioperative procedures, which could also have influenced the results. Second, the observed change may be related to a concomitant, general reduction of transfused RBC concentrates in Switzerland during the same time interval, independent of the initiative of our project. But this is not the case, as the RBC use in Switzerland actually increased by 1.6% between 2007 and 2008 [19]. Third, the effect of being under study may already have reduced the RBC need in phase 1. In order to avoid this effect, we restricted information about the project to the single responsible persons in the hospitals, and the staffs were informed only at the moment of the training and implementation of the guideline.
The observed effect was lower than that observed in a similar former study performed in a single centre in Switzerland [21]. The RBC use and its variability in Switzerland seem also to be relatively low when compared to other countries [22, 23]. This suggests that the potential for improvement in RBC use may be limited, at least for elective orthopaedic surgery in Switzerland. Alternatively, the institutions accepting to participate could have been those where physicians have been already aware of good transfusion practices and therefore beforehand have applied certain transfusion rules. Our result from a parallel survey at a university clinic showed a rate of transfused patients of 67.9% in elective patients undergoing aortic aneurysm, coronary artery bypass and hearth valve surgery [24], and the comparison with the literature suggests that further improvement may be achieved also in other indications than orthopaedic surgery [25]. Furthermore, the observed preoperative haemoglobin values were relatively close to the lower norm value for men [26, 27], who represented 45% of the patients in our collective. Thus, we suggest that developing towards a complete patient blood management programme by correcting preoperative anaemia should further reduce the transfusion need.
There is now evidence from randomised trials that a reduction of the haemoglobin transfusion trigger does not influence negatively the morbidity or mortality [2, 3, 4, 5, 6]. Consistently, we found no differences between phase 1 and 2, neither with regard to the in-hospital complications nor during the time until the follow-up performed >30 days after surgery. However, two elements have to be considered in the interpretation of the follow-up results. First, the mean time point of the last follow-up was 3 days earlier in phase 2 than in phase 1 (49 vs. 52 days, see table 6). This may cause an underestimation of the proportion of adverse events in phase 2. However, because of the moderate extent of the difference, the elapsed time interval since the intervention and the trend towards a lower number of events in phase 2, it seems at least unlikely that the introduction of our guideline may have caused a higher morbidity. Second, 57 patients were lost from follow-up. Their number was low, the reason for the missing data for all patients the same, and the patient number was nearly balanced between phase 1 and 2. Therefore, it is unlikely that an additional bias may have influenced the results. But obviously the validity of the results should be demonstrated in a randomised trial.
In conclusion, a simple transfusion guideline (fig. 1) could be introduced in 10 Swiss hospitals. It was broadly accepted and quickly implemented; it reduced and standardised the use of RBCs by decreasing the haemoglobin transfusion trigger without negative effects on the patient outcome. Furthermore, it complied successfully with the legal demand of introducing quality systems in the hospitals. Since the end of this project, the necessity to implement guidelines and other instruments optimising the use of blood has been repeatedly debated in Switzerland: the use of RBC concentrates per 1,000 inhabitants has dropped by about 15% so far (end of 2013) [19]. Furthermore, several hospitals are now evaluating blood patient management programmes, and additional efforts to optimise the use of blood are still ongoing. With the presented project we hope to have given a valuable contribution to the current positive development and to inspire others to take similar initiatives.
Disclosure Statement
The authors have no competing interests.
Acknowledgments
All responsible persons and staffs of following hospitals supported locally the project and contributed considerably to the development, training and implementation of the guidelines: Hirslanden Salem-Spital Bern, Inselspital Universitätsspital Bern, Kantonales Spital Herisau, Kantonsspital St. Gallen, Klinik Am Rosenberg Heiden, Regionalspital Emmental AG Standort Burgdorf, Ospedale regionale di Lugano Sede Civico, Ospedale regionale di Mendrisio, Spital Interlaken, Spital Thun. L Krummen and A Lädrach contributed essentially to the data collection.
The project was supported by the grant Nr. 76 of the Humanitarian Foundation of the Swiss Red Cross.
References
- 1.European Directorate for the Quality of Medicines and HealthCare. Guide to the Preparation, Use and Quality Assurance of blood Components. 17th ed. Strasbourg: Council of Europe; 2013. [Google Scholar]
- 2.Hébert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 3.Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, Fukushima J, Kalil Filho R, Sierra DB, Lopes NH, Mauad T, Roquim AC, Sundin MR, Leão WC, Almeida JP, Pomerantzeff PM, Dallan LO, Jatene FB, Stolf NA, Auler JO., Jr Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 4.Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, Nemo G, Dragert K, Beaupre L, Hildebrand K, Macaulay W, Lewis C, Cook DR, Dobbin G, Zakriya KJ, Apple FS, Horney RA, Magaziner J FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Villanueva C, Colomo A, Bosch A, Concepción M, Hernandez-Gea V, Aracil C, Graupera I, Poca M, Alvarez-Urturi C, Gordillo J, Guarner-Argente C, Santaló M, Muñiz E, Guarner C. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 6.So-Osman C, Nelissen R, Brand R, Faber F, Slaa RT, Stiggelbout A, Brand A. The impact of a restrictive transfusion trigger on post-operative complication rate and well-being following elective orthopaedic surgery: a post-hoc analysis of a randomized study. Blood Transf. 2013;11:289–295. doi: 10.2450/2013.0172-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manual of Optimal Blood Use (Optimal Blood Use Project) 2010 www.optimalblooduse.eu (last accessed May 13, 2014).
- 8.United Kingdom Blood Transfusion and Tissue Transplantation Services www.transfusionguidelines.org.uk (last accessed May 13, 2014).
- 9.Bundesärtztekammer (German Medical Association) Cross-Sectional Guidelines for Therapy with Blood Components and Plasma Derivatives 4th ed. 2008. www.bundesaerztekammer.de/downloads/Querschnittsleitlinie_Gesamtdokument-englisch_07032011.pdf (last accessed May 13, 2014).
- 10.Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 11.Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, Hebert PC, Anderson GL, Bard MR, Bromberg W, Chiu WC, Cipolle MD, Clancy KD, Diebel L, Hoff WS, Hughes KM, Munshi I, Nayduch D, Sandhu R, Yelon JA American College of Critical Care Medicine of the Society of Critical Care Medicine; Eastern Association for the Surgery of Trauma Practice Management Workgroup. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124–3157. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 12.Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Song HK, Clough ER, Shore-Lesserson LJ, Goodnough LT, Mazer CD, Shander A, Stafford-Smith M, Waters J, Baker RA, Dickinson TA, FitzGerald DJ, Likosky DS, Shann KG. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 13.Carson JL, Grossman BJ, Kleinman S, Tinmouth AT, Marques MB, Fung MK, Holcomb JB, Illoh O, Kaplan LJ, Katz LM, Rao SV, Roback JD, Shander A, Tobian AA, Weinstein R, Swinton McLaughlin LG, Djulbegovic B. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 14.Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet. 2013;381:1845–1854. doi: 10.1016/S0140-6736(13)60650-9. [DOI] [PubMed] [Google Scholar]
- 15.Spahn DR, Goodnough LT. Alternatives to blood transfusion. Lancet. 2013;381:1855–1865. doi: 10.1016/S0140-6736(13)60808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology. 2010;113:482–495. doi: 10.1097/ALN.0b013e3181e08e97. [DOI] [PubMed] [Google Scholar]
- 17.Gombotz H, Hofmann A. Patient blood management: three pillar strategy to improve outcome through avoidance of allogeneic blood products. Anaesthesist. 2013;62:519–527. doi: 10.1007/s00101-013-2199-1. [DOI] [PubMed] [Google Scholar]
- 18.Swissmedic (Swiss Agency for Therapeutic Products) Quality Systems for the Use of Blood Components (in German) https://www.swissmedic.ch/marktueberwachung/00138/00188/index.html?lang=de&download=NHzLpZeg7t,lnp6I0NTU042l2Z6ln1acy4Zn4Z2qZpnO2Yuq2Z6gpJCDdH12fWym162epY-bg2c_JjKbNoKSn6A– (last accessed May 13, 2014).
- 19.Blood Transfusion Services of the Swiss Red Cross Yearly Reports 2008-2013 (in German) Available from: www.weltblutspendetag.ch/medien-de/publikationen.php last accessed May 13, 2014).
- 20.DCTD, NCI, NIH, DHHS. Common Terminology Criteria for Adverse Events (CTCAE), Version 3.0. 2006.
- 21.Müller U, Exadaktylos A, Roeder C, Pisan M, Eggli S, Jüni P. Effect of a flow chart on use of blood transfusions in primary total hip and knee replacement: prospective before and after study. BMJ. 2004;328:934–938. doi: 10.1136/bmj.328.7445.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gombotz H, Rehak PH, Shander A, Hofmann A. Blood use in elective surgery: the Austrian benchmark study. Transfusion. 2007;47:1468–1480. doi: 10.1111/j.1537-2995.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 23.Chen AF, Klatt BA, Yazer MH, Waters JH. Blood utilization after primary total joint arthroplasty in a large hospital network. HSS J. 2013;9:123–128. doi: 10.1007/s11420-013-9327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fontana S, Müller U, Perler M, Staub L, Eberle B, Regli B, Carrel TP, Schmidli J, Schmid P, Mansouri Taleghani B. Red blood cell (RBC) use in elective cardiac and vascular surgery: a prospective assessment prior to the implementation of transfusion guidelines in a Swiss university hospital. Transfusion. 2009;49(suppl):166A. [Google Scholar]
- 25.Bennet-Guerrero E, Zhao Y, O'Brien SM, Ferguson Jr TB, Peterson ED, Gammie JS, Song HK. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304:1568–1575. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- 26.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–1750. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Worldwide Prevalence of Anaemia 1993-2005. World Geneva: Health Organization; 2008. [Google Scholar]