Summary
Background
The storage of red blood cells (RBC) is associated with impairment of their properties that can induce a circulatory risk to recipients. In a preceding study (2009), we reported that post-storage rejuvenation (RJ) of stored RBC (St-RBC) efficiently reduced the storage-induced RBC/endothelial cell interaction, while only partially reversing the level of intracellular Ca2+, reactive oxygen species, and surface phosphatidylserine. In the present study, we examined the RJ effectiveness in repairing St-RBC mechanical properties.
Methods
RBC, stored in CPDA-1 without pre-storage leukoreduction, were subjected to post-storage RJ, and the deformability, osmotic fragility (OF), and mechanical fragility (MF) of the rejuvenated St-RBC (St-RBCRj) were compared to those of untreated St-RBC and of freshly-collected RBC (F-RBC).
Results
5-week storage considerably increased OF and MF, and reduced the deformability of St-RBC. All alterations were only partially (40–70%) reversed by RJ, depending on the extent of the damage: the greater the damage, the lesser the relative effect of RJ.
Conclusion
The findings of the present and preceding studies suggest that different St-RBC properties are differentially reversed by RJ, implying that some of the changes occur during storage and are irreversible.
Keywords: Rejuvenation, RBC osmotic fragility, RBC mechanic fragility, RBC deformability
Introduction
Red blood cells (RBC) have specific mechanical properties, specifically deformability, osmotic fragility (OF) and mechanical fragility (MF), which are important determinants of their hemodynamic function and survival in the circulation. As shown previously, the mechanical behavior of RBC is predominantly determined by cell shape, surface area-to-volume ratio, membrane and skeleton condition, and surface charge [1, 2, 3], properties that are altered during cold storage of RBC [4, 5]. Cold storage of RBC in blood banks has been reported to be associated with changes in various RBC properties, including increased cell volume [6], oxidation of membrane protein and lipids [7], loss of cellular antioxidant capability [8], changes in K+ and Na+ concentration [9], changes in lipid in/out distribution on the RBC surface [8, 10], and decreased cell membrane area [11, 12]. Storage-induced damage to RBC, which can be noted as early as the second week of storage and progresses with storage duration [4], has been shown to impair the functionality of stored RBC (St-RBC) [5] and to facilitate their removal from circulation following transfusion [13, 14, 15, 16]. In order to minimize the adverse effect of ‘aged’ St-RBC, methods for attenuation of storage-induced damage, using pre-storage and post-storage treatments of St-RBC units, have been proposed and some tested [14].
Various protocols are used for RBC storage, such as pre-storage leukoreduction or use of different storage media (e.g. CPDA-1, AS, SAGM, PAGGSM, CP2D, AS3). These are likely to differentially affect St-RBC properties, as well as the extent of storage-induced damage and its kinetics [5, 17, 18, 19, 20, 21]. Pre-storage leukoreduction has been shown to attenuate storage-induced damage to RBC properties, including deformability [5]. Accordingly, pre-storage leukoreduction is becoming more accepted in blood banking, but it is not universally practiced as this procedure is laborious and costly, and some blood banks question its cost/benefit ratio.
Some of the storage-induced deficiencies, such as reduced adenosine triphosphate (ATP), are restored following transfusion of stored RBC [16, 22], but other changes, such as decreased surface area-to-volume ratio due to vesiculation [23] or decreased surface charge through loss of sialic acid [24, 25], are not expected to be reversible. Attempts to reverse storage-induced damage to RBC functionality have been made by post-storage rejuvenation treatment, i.e., supplementing RBC units with ‘rejuvenating’ materials, e.g. Rejuvesol® (enCyte Systems, Inc., Braintree, MA, USA), aimed at bringing St-RBC to the status of freshly-collected blood units. This has been found to reverse the storage-induced impairment of certain RBC properties to varying degrees.
In a previous study [17], we found that post-storage rejuvenation (with Rejuvesol) of un-leukoreduced St-RBC (stored in CPDA-1) only partially rectified storage-induced biochemical lesions, including elevated levels of reactive oxygen species (ROS) and intracellular Ca2+ as well as phosphatidylserine (PS) externalization [17]. On the other hand, the rejuvenation treatment exerted practically a complete reduction of the storage-induced elevation of RBC adhesion to vascular endothelial cells (EC) [17]. These finding suggest that rejuvenation might differentially affect storage-induced changes in RBC properties. On these grounds, the present research was undertaken to extend our previous studies and determine the capacity of rejuvenation treatment to reverse storage-induced impairment of St-RBC mechanical/rheological properties, specifically RBC deformability, OF, and MF.
Material and Methods
Each 50 ml of Rejuvesol contains sodium pyruvate (550 mg), inosine (1.34 g), adenine (0.034 g), dibasic sodium phosphate 0.50 g, and monobasic sodium phosphate (monohydrate, 0.20 g), in water for injection, pH 6.7–7.4.
RBC Storage Conditions
Similar to our previous [17, 18] as well as other studies [24, 26, 27], we used non-leukoreduced St-RBC. Blood was drawn from 9 healthy donors at the Hadassah Hospital Blood Bank, following informed consent according to the Helsinki Committee Regulations Permit (98290, Hadassah Hospital, Jerusalem, Israel), and collected (non-leukoreduced) into standard sterile bags (Fresenius Kabi AG, Homburg, Germany), containing citrate phosphate dextrose (CPD). Immediately following collection, RBC were isolated by centrifugation (Roto Silenta 630RS, Tuttlingen Germany) for 6 min (2,367 rounds per minute (RPM), 24 °C) followed by removal of the plasma. Units of concentrated RBC were stored in CPDA-1 under standard conditions (2–6 °C for 5 weeks) practiced at the Hadassah Hospital Blood Bank. RBC mechanical properties were determined immediately following donation, and the units were denoted fresh RBC (F-RBC) and RBC after 35 days of cold storage.
Preparation of St-RBC Suspension
A total of 5 ml of cells were drawn from St-RBC units and supplemented with either 1 ml of glucose phosphate-buffered saline (GPBS: 140 mmol/l sodium chloride (NaCl), 10 mmol/l Na+/K+ phosphate, 5.5 mmol/l glucose, pH 7.4) or with 1 ml of Rejuvesol (St-RBCRj). We used a buffer with a physiological level of glucose, needed for maintenance of RBC hemodynamics [28, 29]. Both samples were incubated for 60 min at 37 °C. The RBC were then washed once by centrifugation (500 × g for 10 min) in GPBS, and re-suspended at 10% hematocrit in the GPBS buffer.
Preparation of F-RBC Suspension
A blood sample (5 ml) was taken from each donor during donation and used as control. After separation by centrifugation (500 × g for 10 min) and washing twice by suspension and centrifugation in GPBS, RBC were re-suspended at 10% hematocrit in the GPBS buffer.
Determination of RBC Deformability
The present research employed the computerized Cell Flow Properties Analyzer (CFA) [18], conceived and developed in our laboratory for monitoring RBC flow properties (aggregability, deformability, and adhesiveness) by direct visualization of RBC in a narrow-gap flow chamber and their dynamic organization under controllable flow conditions resembling those in a micro-vessel. RBC deformability is determined in the CFA by monitoring the elongation of RBC adherent to the slide, under flow-induced shear stress [18]. In brief, 50 μl of RBC suspension (1% hematocrit, in GPBS) are inserted into the flow chamber (adjusted to 200 µm gap), containing an un-coated polystyrene slide (Electron Microscopy Science, Washington, PA, USA). The adherent RBC are then subjected to flow-induce shear stress (3.0 Pa), and their deformability is determined by the change in cell shape, expressed by the elongation ratio, ER = a/b, where a/b = the major/the minor cell axes. ER = 1 reflects round RBC, undeformed under the applied shear stress. The CFA image analysis program provides this measure for each cell (individually) and its distribution in a large RBC population (at least 2,500 ± 300 cells) [18]. The accuracy of axes measurement is about 10%. Accordingly, RBC with ER ≤ 1.1 are defined as ‘undeformable’ cells (UDC), namely cells that do not deform under high shear stress (3.0 Pa). As shown and discussed in our previous studies, when considering the potential of reduced RBC deformability to induce microvascular occlusion, the portion of UDC is more clinically relevant than the shift in average values [30, 31, 32].
Determination of RBC Hemolysis (% Hemolysis)
Following application of mechanical or osmotic stress on the RBC suspension, the supernatant was then transferred to a spectrophotometer cuvette (1.5 ml semimicro ultraviolet methacrylate cuvette, Fisher Scientific, Hampton, NH, USA), and the induced RBC hemolysis was determined by measuring hemoglobin optical density (OD) at 540 nm. Although this method does not measure methemoglobin and oxidized forms of hemoglobin (which comprise about 1–3% of the total hemoglobin), it is commonly used when measuring experimentally induced RBC lysis [21, 30, 31]. The hemolysis level (% hemolysis) was calculated according to the following formula:
where the subscript 0, S, and T correspond to the OD of supernatant obtained for control (no stress) RBC, RBC subjected to mechanical or osmotic stress, and for total hemolysis, respectively. Total hemolysis was applied by conventional incubation (10 min) of RBC in water [21].
Determination of RBC Mechanical Fragility
For assessment of MF, we used the procedure described by Kameneva's group [33, 34, 35] with slight changes. 3 ml of RBC suspension (control, stored, and stored-rejuvenated) were rocked in glass test tubes (13 × 100 mm, Fisher Scientific), containing 5 steel beads (1/8-inch stainless-steel beads, Nationskander California Corp., Anaheim, CA, USA). After 1 h of rocking (RM-500 Roller Mixer, Digisystem Laboratory Instruments, Inc., New Taipei City, Taiwan) at 40 cycles/min, the suspension was centrifuged at 500 × g for 10 min at room temperature, and the supernatant was collected and subjected to measurements of hemolysis level as above, providing the MF index (MFI), where MFI = % hemolysis for RBC subjected to mechanical stress.
Determination of RBC Osmotic Fragility
RBC OF was determined by subjecting the RBC to osmotic pressure, according to the method of Beutler [36]: 10 µl of RBC are mixed with 1 ml NaCl solution at varying concentrations (0, 3.0, 3.5, 4.0, 4.5, 5.0, 6.0, 7.0, 8.0, and 9.0 g/l), incubated at room temperature for 1 h, and centrifuged at 500 × g for 10 min. The hemolysis level is determined by hemoglobin determination in the supernatant as described above. The OFI was determined by using NaCl, exerting 50% hemolysis.
Extent of Storage-Induced Cell Damage
To assess the extent of storage-induced damage to RBC deformability (Def) and MF, as expressed by the change in UDC and MFI, we formulated the damage index (DI) as follows:
where St and F refer to St-RBC and F-RBC, respectively. Accordingly, DI = 0 when these parameters are not altered by cold storage (i.e., MFISt = MFIF or UDCSt = UDCF), and the DI value elevates with increasing alteration.
Rejuvenation Effectiveness
For assessment of rejuvenation (Rj) effectiveness, we formulated the rejuvenation effectiveness index (REI), describing the portion of storage-induced damage to an RBC property (P) that is repaired by the rejuvenation treatment, as follows:
where St, St-Rj, and F refer to St-RBC, St-RBC subjected to rejuvenation, and F-RBC, respectively. Accordingly, REI varies between 0 and 100% for no improvement (PSt = PSt-Rj) or complete recovery (PF = PSt-Rj), respectively.
Statistical Analysis
Differences between tested groups were analyzed for statistical significance using a repeated measures analysis of variance (one-way ANOVA; SigmaStat-3, Systat Software, Inc., San Jose, CA, USA) with Tukey HSD test. As described above, in our research we provided only paired measurements. Each RBC sample was subjected to determination of deformability, OF, and MF immediately after blood collection and following 35 days of storage (before and after rejuvenation treatment), and to analysis by ANOVA for the 3 groups and paired t-tests for fresh and stored groups (F-RBC/St-RBC). Statistical significance was set at p < 0.05.
Results
Hematological parameters of the tested blood units are given in table 1.
Table 1.
St-RBC properties prior to and after 35 days of cold storage
| Parameter | Before storage, mean ± SD | After storage, mean ± SD | p value (paired t-test) |
|---|---|---|---|
| Hemolysis, % | 0.16 ± 0.09 | 0.37 ± 0.08 | <0.005 |
| pHa | 7.00 ± 0.06 | 6.41 ± 0.08 | <0.005 |
| MCV, flb | 92.00 ± 3.03 | 95.15 ± 4.05 | <0.001 |
| MCHC, g/dlb | 33.23 ± 0.32 | 31.56 ± 0.21 | <0.05 |
| OFI, % | 0.46 ± 0.53 | 0.53 ± 0.02 | 0.0002 |
| MFI, % | 0.97 ± 0.29 | 1.80 ± 0.48 | <0.0001 |
| UDC, % | 1.91 ± 0.69 | 6.39 ± 1.55 | <0.0001 |
Measured in supernatant from units at room temperature.
Measured by standard blood bank procedure using the Model S Coulter Counter (Coulter Electronics, Hialeah, FL, USA).
SD = Standard deviation.
As noted in the introduction, previous studies have reported that cold storage induces elevation of RBC rigidity (reduced deformability), as well as OF and MF properties [5, 18, 34, 36, 37, 38, 39]. In accordance with that, we found in the present study that after 5 weeks of cold storage in CPDA-1, RBC were considerably more fragile and rigid (table 2). As shown in the respective figures, all 3 mechanical properties were improved to varying degrees by rejuvenation treatment of St-RBC.
Table 2.
Statistical analysis (one-way repeated ANOVA and Tukey HSD test) of the differences in mechanical properties of F-RBC, St-RBC, and St-RBCRj
| Parameter | F-RBC, mean ± SD | St-RBC, mean ± SD | St-RBCRj, mean ± SD | ANOVA |
p value for Tukey HSD test Vs St-RBCRj for |
||
|---|---|---|---|---|---|---|---|
| Fa | p | F-RBC | St-RBC | ||||
| OFI, % | 0.46 ± 0.53 | 0.53 ± 0.02 | 0.50 ± 0.05 | 38.9 | <0.001 | <0.001 | 0.002 |
| MFI, % | 0.97 ± 0.29 | 1.89 ± 0.48 | 1.34 ± 0.41 | 83.2 | <0.001 | <0.001 | <0.001 |
| UDC, % | 1.91 ± 0.69 | 6.39 ± 1.55 | 4.18 ± 1.02 | 116.3 | <0.001 | <0.001 | <0.001 |
F-ratio can be thought of as a measure of how different the means are relative to the variability within each measured parameter. The larger this value, the greater the likelihood that the differences between the means are due to something other than chance alone, namely real effects (characterized by p value).
SD = Standard deviation.
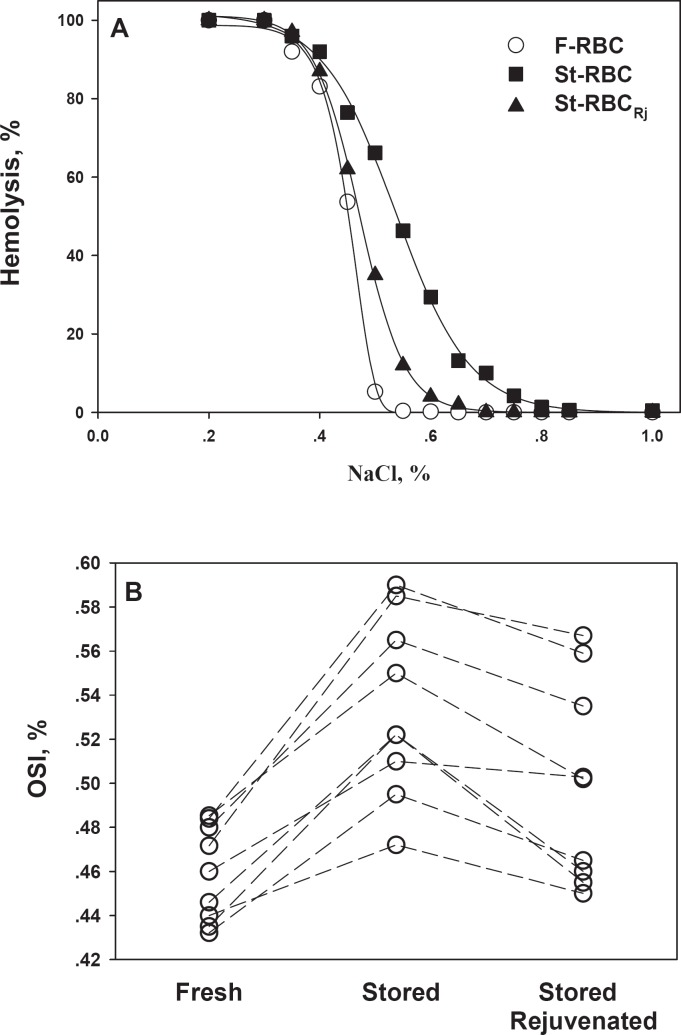
Figure 1A depicts a representative OF curve, namely the % hemolysis as function of extracellular osmolarity (NaCl in suspension medium). The figure shows a right shift of the OF curve for St-RBC, meaning that following storage the RBC are more susceptible to a decrease in osmotic pressure as they undergo lysis following smaller reduction in NaCl compared to F-RBC. Rejuvenation treatment partially improved OF of St-RBC. Figure 1B depicts the OFI, namely NaCl exerting 50% hemolysis, for the individual RBC units tested. The figure shows that in all cases the OF of RBC is increased during storage and only partially improved by rejuvenation treatment which did not reduce the OF to the level of F-RBC.
Fig. 1.
RBC osmotic fragility for fresh and stored cells. A Typical RBC OF curves in control (F-RBC), stored (St-RBC), and rejuvenated (St-RBCRj, following storage) cells. B The value of OFI (concentration of NaCl that will produce 50% hemolysis of RBC). Each line connects the data points (for F-RBC, St-RBC, and St-RBCEj) obtained from the same donor.
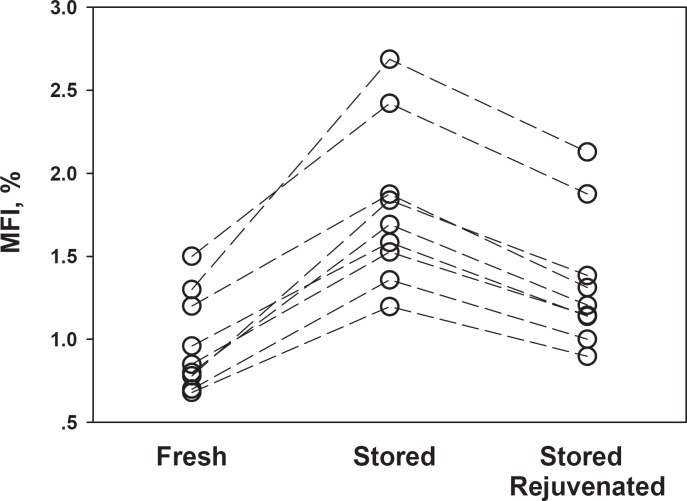
Figure 2 depicts the MF index for F-RBC, St-RBC, and St-RBC subjected to rejuvenation treatment (St-RBCRj) for the individual RBC units tested. As shown, the fragility is increased during storage and is rectified by rejuvenation for all RBC units. Similar to OF, the rejuvenation treatment only partially reversed the effects of storage on MF.
Fig. 2.
Partial reversal of St-RBC MF by rejuvenation treatment. Each line connects the data points obtained from the same donor.
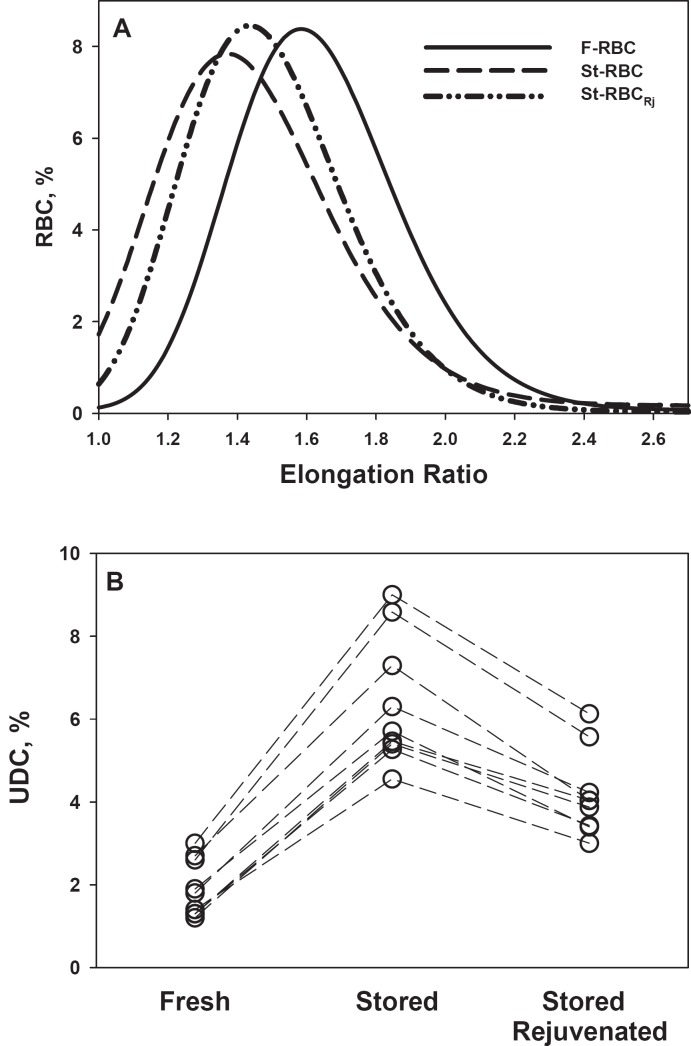
In our study, RBC deformability is described by the RBC elongation ratio (ER) distribution (fig. 3A) and by the percentage of UDC (fig. 3B) in the RBC population [5, 18, 31, 32]. Figure 3A, depicting a representative ER curve, shows that cold storage induced an overall reduction in RBC deformability as shown by the shift of the St-RBC curve to lower ER values (elevation of the presence of low-deformable cells in the population) compared to F-RBC, and this was improved by rejuvenation. Figure 3B, depicting the change in the percentage of UDC in the RBC population relative to that in F-RBC, shows that for each of the tested RBC units cold storage induced a considerable increase in the percentage of UDC (up to 4.5-fold), and this effect was attenuated by rejuvenation.
Fig. 3.
Partial reversal of St-RBC deformability by rejuvenation treatment. A Typical distribution of elongation ratio for control (F-RBC), stored (St-RBC), and rejuvenated (St-RBCEj, following storage) cells under shear stress of 3.0 Pa. B Concentration of undeformable cells (UDC) in the RBC population (under shear stress of 3.0 Pa). Each line connects the data points obtained from the same donor.
The results presented above clearly show that, in general, routine cold storage induces impairment of RBC properties to varying degrees, and that this is partially reversed by post-storage treatment with Rejuvesol. These findings are summarized in table 2, presenting the storage-induced damage to St-RBC mechanical properties, and their improvement by rejuvenation.
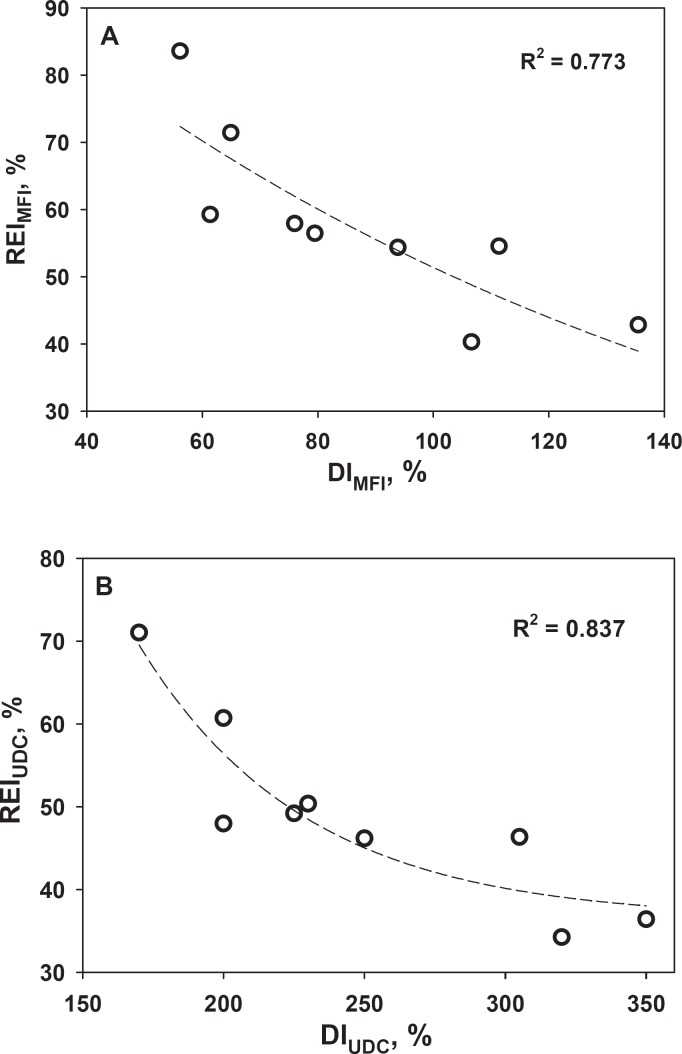
Table 3 summarizes the effectiveness of the rejuvenation treatment (described by REI) in improving storage-induced damage to RBC mechanical properties (determined in the present study), as well as other RBC properties determined in our previous study [17]. This table clearly shows that treatment of St-RBC with Rejuvesol rectified all tested properties of St-RBC, but the extent of improvement differed considerably between the different measures. Figure 4 shows that the effectiveness of rejuvenation (REI) in restoring St-RBC deformability and MF is inversely related to the extent of storage-induced damage (expressed as DI).
Table 3.
Rejuvenation treatment induces partial (REI < 100%) recovery of St-RBC properties
| Parameter | % recovery (REI), mean ± SD |
|---|---|
| OFI, % | 49.3 ± 26.8 |
| MFI, % | 57.9 ± 13.3 |
| UDC, % | 49.2 ± 11.3 |
| (Ca2+)i, μmol/la | 67.3 ± 12.1 |
| ROS, %a | 55.3 ± 16.1 |
| Annexin V-labeled RBC, %a | 69.1 ± 13.4 |
| Adhesion of RBC to ECb, RBC/mm2 | 93.2 ± 6.1 |
Fig. 4.
Effectiveness of rejuvenation (rejuvenation effectiveness index, REI) of St-RBC in reversing A cell MF and B deformability as functions of strength of cell damage (damage index, DI). Relation between REI and DI expressed by equation y = Y0 + ae-bx.
Discussion
In previous attempts to attenuate impairment of RBC functionality under cold storage, it was found that rejuvenation, i.e. treatment mainly by Rejuvesol, can completely reverse the storage-induced reduction of St-RBC levels of biochemical measures, specifically ATP and 2,3-DPG, and of mechanical properties, specifically OF, MF, and deformability [34, 40, 41, 42]. However, as shown in table 3, in the present and previous [17] studies we found that all these properties are only partially rectified by treatment with Rejuvesol. These discrepancies might be explained by differences in storage conditions, methods used to determine RBC characteristics, and/or their dependence on ATP levels, as discussed in the following.
While Gelderman and Vostal [41] reported complete reversal of St-RBC OF and MF, we found only partial improvement of these alterations (fig. 1 and 2, table 3). This can be explained by differences in the storage medium. A number of studies have demonstrated the effect of storage medium on the alteration of St-RBC during storage. Specifically, it has been reported [34] that with any storage duration the increase in fragility of St-RBC was significantly higher when stored in SAGM [34] or PAGGSM [43] compared to AS-5 medium. In the study of Gelderman and Vostal [41], RBC were stored in CP2D/AS3 medium [41], while in the present study RBC were stored in CPDA-1. In a study comparing RBC storage media, Heaton et al. [22] found that following 35 days of storage in CP2D/AS3, ATP levels were approximately 2-fold higher than after storage in CPDA-1 (3.22 ± 0.58 vs. 1.71 ± 0.45 μmol/g Hb). In a recent study, it was suggested that reduced ATP decreases rejuvenation capability [42]. It is thus likely that the rejuvenation effectiveness in restoring ATP-dependent properties of RBC stored in CPDA-1 would be lower than with CP2D/AS3.
As to the dependence on the methods used to determine RBC characteristics, with specific consideration for RBC deformability, d'Almera et al. [40] reported that treatment of St-RBC stored in CPDA-1 (for 29 days) with Rejuvesol completely reversed the storage-induced reduction of RBC deformability, while we found that storage in the same medium (for 35 days) only partially rectified the storage-induced increase in RBC rigidity (fig. 3, tables 2 and 3). This discrepancy can be explained by differences in the methods used to determine deformability. d'Almera et al. [40] measured deformability by membrane displacement applied by aspiration of single cells into a micropipette tip. In this method, applied to a maximum of less than 200 cells, the effects of surface area/volume ratios and of cytoplasmic viscosity on the overall RBC deformability are largely eliminated, causing the results to refer only to the membrane-specific changes that have occurred under cold storage and rejuvenation [40]. In our study, we measured the actual RBC deformability by direct visual monitoring of the changes in RBC shape under flow-induced shear stress, and the deformability parameters are derived from a large cell population (2,500 ± 300 cells), thereby enabling the derivation of parameters such as percentage of UDC, which better express St-RBC capacity to induced circulatory disorders [31, 32].
The rejuvenation procedure is aimed primarily at restoring St-RBC ATP levels, but this effect decreases with increasing storage duration [42]. It is well known that various RBC functions and properties are largely, but possibly not solely, determined by the cell ATP level [42]. It is plausible that rejuvenation would be more effective in restoring St-RBC properties that are strongly ATP-dependent. This is supported by the results presented in table 3, showing that the REI is higher for intracellular Ca2+ levels known to be determined by the ATP level [44], and subsequently for the surface PS level known to be dependent on both the Ca2+ and the ATP level [45], and increases with cell age [46] and storage duration [14]. Table 3 (which includes results from a previous study) also shows that rejuvenation treatment practically reversed St-RBC/EC adhesion to the level of F-RBC, although surface PS, which is the predominant determinant of St-RBC adherence, is not fully reversed. As observed and discussed in our previous study [17], the percentage of PS-positive RBC following rejuvenation is slightly higher than that of F-RBC, while this treatment suppressed RBC/EC adhesion to the level of F-RBC. This might indicate that the correlation between RBC/EC adherence and surface PS level is not equal. It is likely that achieving RBC adhesion under shear stress requires a threshold/permissive level of surface PS. The possible dependence of this threshold on the shear stress applied to the cells is yet to be explored [17]. In contrast, the rejuvenation efficacy in restoring St-RBC deformability, which is ATP-independent [47], is lower than 50% (table 3). Of particular interest are the findings presented in figure 4, which show that the efficacy of the rejuvenation treatment in rectifying the storage-induced impairment of RBC mechanical properties depends on the extent of the damage – the greater the damage (DI), the smaller the rejuvenation efficacy (REI). Changes in RBC mechanical properties are often associated with irreversible components of structural alterations, such as changes in the RBC surface-to-volume ratio due to vesiculation [23] or reduction in surface charge due to sialic acid degradation [24, 25], which are not expected to be affected by rejuvenation treatment aimed at restoring ATP levels in RBC. The above changes in RBC membrane structure have been reported to increase with storage duration [24, 25], thereby explaining the finding that rejuvenation efficacy is inversely related to the extent of storage-induced damage to St-RBC mechanical properties. This observation conforms with that of Pompeo et al. [39] and Girasole et al. [37] who used atomic force microscopy to examine storage-induced structural and metabolic changes in St-RBC. These authors have detected irreversible transformations in the RBC structure and metabolism, as early as after 8 days of storage. The authors concluded that some changes, located at the membrane surface, appear when the ATP synthesis capacity declines to apparently subviable levels and the membrane skeleton has been permanently damaged [39]. These changes were not reversed by rejuvenation treatment with IPP (inosine-pyruvate-phosphate) medium. In addition, Karon et al. [38] showed that St-RBC biochemical and morphological properties are changed in an irreversible manner in their own time scale; Band-3 aggregation, cholesterol loss, and defects in RBC morphology occur early during storage (in the first 7 days), while the loss of protein-rich vesicles occurs later [38]. This is supported by in-vivo studies of transfused St-RBC by Luten et al. [16] and Beutler [36], concluding that RBC storage is associated with irreversible damage which increases with storage duration.
Study Limitations
As noted above, the present and respective previous studies were confined to specific storage conditions, namely non-leukoreduced RBC and CPDA-1 storage (routinely practiced in our and other medical centers [18, 26, 27]). Since the practice of leukoreduction, and the use of new improved storage media, is becoming more common in blood banking, the extrapolation from the conditions tested in the present study to other storage conditions has to be further examined.
Conclusion
The findings and considerations discussed above suggest that different properties of St-RBC are differentially impaired during cold storage. Rejuvenation treatment with Rejuvesol restores storage-impaired RBC properties to varying degrees, with preference to ATP-dependent properties. The extent of reversal is inversely proportional to the extent of damage, and some of the changes cannot be fully reversed.
Disclosure Statement
All authors declared no conflict of interest.
Acknowledgement
This work was supported by grants from the National Blood Foundation (to S. Yedgar and G. Barshtein), and the Walter and Greta Stiel Chair for Heart Studies (to S. Yedgar). We are grateful to O. Fredman for her assistance.
References
- 1.Mohandas N, Chasis JA. Red blood cell deformability, membrane material properties and shape: regulation by transmembrane, skeletal and cytosolic proteins and lipids. Semin Hematol. 1993;30:171–192. [PubMed] [Google Scholar]
- 2.Mohandas N, Chasis JA, Shohet SB. The influence of membrane skeleton on red cell deformability, membrane material properties, and shape. Semin Hematol. 1983;20:225–242. [PubMed] [Google Scholar]
- 3.Mohandas N, Clark MR, Jacobs MS, Shohet SB. Analysis of factors regulating erythrocyte deformability. J Clin Invest. 1980;66:563–573. doi: 10.1172/JCI109888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess JR. Red cell storage. J Proteomics. 2010;73:368–373. doi: 10.1016/j.jprot.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Barshtein G, Manny N, Yedgar S. Circulatory risk in the transfusion of red blood cells with impaired flow properties induced by storage. Transfus Med Rev. 2011;25:24–35. doi: 10.1016/j.tmrv.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Arduini A, Minetti G, Ciana A, Seppi C, Brovelli A, Profumo A, Vercellati C, Zappa M, Zanella A, Dottori S, Bonomini M. Cellular properties of human erythrocytes preserved in saline-adenine-glucose-mannitol in the presence of L-carnitine. Am J Hematol. 2007;82:31–40. doi: 10.1002/ajh.20753. [DOI] [PubMed] [Google Scholar]
- 7.Willekens FL, Werre JM, Kruijt JK, Roerdinkholder-Stoelwinder B, Groenen-Dopp YA, van den Bos AG, Bosman GJ, van Berkel TJ. Liver Kupffer cells rapidly remove red blood cell-derived vesicles from the circulation by scavenger receptors. Blood. 2005;105:2141–2145. doi: 10.1182/blood-2004-04-1578. [DOI] [PubMed] [Google Scholar]
- 8.Dumaswala UJ, Wilson MJ, Wu YL, Wykle J, Zhuo L, Douglass LM, Daleke DL. Glutathione loading prevents free radical injury in red blood cells after storage. Free Radic Res. 2000;33:517–529. doi: 10.1080/10715760000301061. [DOI] [PubMed] [Google Scholar]
- 9.Cicha I, Suzuki Y, Tateishi N, Shiba M, Muraoka M, Tadokoro K, Maeda N. Gamma-ray-irradiated red blood cells stored in mannitol-adenine-phosphate medium: rheological evaluation and susceptibility to oxidative stress. Vox Sang. 2000;79:75–82. doi: 10.1159/000031216. [DOI] [PubMed] [Google Scholar]
- 10.Bratosin D, Leszczynski S, Sartiaux C, Fontaine O, Descamps J, Huart JJ, Poplineau J, Goudaliez F, Aminoff D, Montreuil J. Improved storage of erythrocytes by prior leukodepletion: flow cytometric evaluation of stored erythrocytes. Cytometry. 2001;46:351–356. doi: 10.1002/cyto.10005. [DOI] [PubMed] [Google Scholar]
- 11.Jank H, Salzer U. Vesicles generated during storage of red blood cells enhance the generation of radical oxygen species in activated neutrophils. ScientificWorldJournal. 2011;11:173–185. doi: 10.1100/tsw.2011.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kriebardis AG, Antonelou MH, Stamoulis KE, Economou-Petersen E, Margaritis LH, Papassideri IS. RBC-derived vesicles during storage: ultrastructure, protein composition, oxidation, and signaling components. Transfusion. 2008;48:1943–1953. doi: 10.1111/j.1537-2995.2008.01794.x. [DOI] [PubMed] [Google Scholar]
- 13.Bosman GJ, Werre JM, Willekens FL, Novotny VM. Erythrocyte ageing in vivo and in vitro: structural aspects and implications for transfusion. Transfus Med. 2008;18:335–347. doi: 10.1111/j.1365-3148.2008.00892.x. [DOI] [PubMed] [Google Scholar]
- 14.Bosman GJ, Cluitmans JC, Groenen YA, Werre JM, Willekens FL, Novotny VM. Susceptibility to hyperosmotic stress-induced phosphatidylserine exposure increases during red blood cell storage. Transfusion. 2011;51:1072–1078. doi: 10.1111/j.1537-2995.2010.02929.x. [DOI] [PubMed] [Google Scholar]
- 15.Bosman GJ, Lasonder E, Groenen-Dopp YA, Willekens FL, Werre JM, Novotny VM. Comparative proteomics of erythrocyte aging in vivo and in vitro. J Proteomics. 2010;73:396–402. doi: 10.1016/j.jprot.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Luten M, Roerdinkholder-Stoelwinder B, Schaap NP, de Grip WJ, Bos HJ, Bosman GJ. Survival of red blood cells after transfusion: a comparison between red cells concentrates of different storage periods. Transfusion. 2008;48:1478–1485. doi: 10.1111/j.1537-2995.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 17.Koshkaryev A, Zelig O, Manny N, Yedgar S, Barshtein G. Rejuvenation treatment of stored red blood cells reverses storage-induced adhesion to vascular endothelial cells. Transfusion. 2009;49:2136–2143. doi: 10.1111/j.1537-2995.2009.02251.x. [DOI] [PubMed] [Google Scholar]
- 18.Relevy H, Koshkaryev A, Manny N, Yedgar S, Barshtein G. Blood banking-induced alteration of red blood cell flow properties. Transfusion. 2008;48:136–146. doi: 10.1111/j.1537-2995.2007.01491.x. [DOI] [PubMed] [Google Scholar]
- 19.Uyuklu M, Meiselman HJ, Baskurt OK. Effect of hemoglobin oxygenation level on red blood cell deformability and aggregation parameters. Clin Hemorheol Microcirc. 2009;41:179–188. doi: 10.3233/CH-2009-1168. [DOI] [PubMed] [Google Scholar]
- 20.Barjas-Castro ML, Brandao MM, Fontes A, Costa FF, Cesar CL, Saad ST. Elastic properties of irradiated RBCs measured by optical tweezers. Transfusion. 2002;42:1196–1199. doi: 10.1046/j.1537-2995.2002.00201.x. [DOI] [PubMed] [Google Scholar]
- 21.Blasi B, D'Alessandro A, Ramundo N, Zolla L. Red blood cell storage and cell morphology. Transfus Med. 2012;22:90–96. doi: 10.1111/j.1365-3148.2012.01139.x. [DOI] [PubMed] [Google Scholar]
- 22.Heaton A, Keegan T, Holme S. In vivo regeneration of red cell 2,3-diphosphoglycerate following transfusion of DPG-depleted AS-1, AS-3 and CPDA-1 red cells. Br J Haematol. 1989;71:131–136. doi: 10.1111/j.1365-2141.1989.tb06286.x. [DOI] [PubMed] [Google Scholar]
- 23.Safeukui I, Buffet PA, Deplaine G, Perrot S, Brousse V, Ndour A, Nguyen M, Mercereau-Puijalon O, David PH, Milon G, Mohandas N. Quantitative assessment of sensing and sequestration of spherocytic erythrocytes by human spleen: implications for understanding clinical variability of membrane disorders. Blood. 2012;120:424–430. doi: 10.1182/blood-2012-01-404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godin C, Caprani A. Effect of blood storage on erythrocyte/wall interactions: implications for surface charge and rigidity. Eur Biophys J. 1997;26:175–182. doi: 10.1007/s002490050069. [DOI] [PubMed] [Google Scholar]
- 25.Huang YX, Wu ZJ, Mehrishi J, Huang BT, Chen XY, Zheng XJ, Liu WJ, Luo M. Human red blood cell aging: correlative changes in surface charge and cell properties. J Cell Mol Med. 2011;15:2634–2642. doi: 10.1111/j.1582-4934.2011.01310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayhan B, Yuruk K, Koene S, Sahin A, Ince C, Aypar U. The effects of non-leukoreduced red blood cell transfusions on microcirculation in mixed surgical patients. Transfus Apher Sci. 2013;49:212–222. doi: 10.1016/j.transci.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Sadaka F, Aggu-Sher R, Krause K, O'Brien J, Armbrecht ES, Taylor RW. The effect of red blood cell transfusion on tissue oxygenation and microcirculation in severe septic patients. Ann Intensive Care. 2011;1:46. doi: 10.1186/2110-5820-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abed M, Balasaheb S, Towhid ST, Daniel C, Amann K, Lang F. Adhesion of annexin 7 deficient erythrocytes to endothelial cells. PLoS One. 8:e56650. doi: 10.1371/journal.pone.0056650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livshits L, Srulevich A, Raz I, Cahn A, Barshtein G, Yedgar S, Eldor R. Effect of short-term hyperglycemia on protein kinase C alpha activation in human erythrocytes. Rev Diabet Stud. 2013;9:94–103. doi: 10.1900/RDS.2012.9.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barshtein G, Abramovitch R, Katz M, Pappo O, Zelig O, Corchia N, Yedgar S, Matot I. Aged versus fresh blood for the treatment of hemorrhagic shock; differential effect on liver outcome and possible mechanism. Vox Sang. 2010;99:68–69. [Google Scholar]
- 31.Kaul DK, Koshkaryev A, Artmann G, Barshtein G, Yedgar S. Additive effect of red blood cell rigidity and adherence to endothelial cells in inducing vascular resistance. Am J Physiol Heart Circ Physiol. 2008;295:H1788–1793. doi: 10.1152/ajpheart.253.2008. [DOI] [PubMed] [Google Scholar]
- 32.Matot I, Katz M, Pappo O, Zelig O, Corchia N, Yedgar S, Barshtein G, Guerrero EB, Abramovitch R. Resuscitation with aged blood exacerbates liver injury in a hemorrhagic rat model. Crit Care Med. 2013;41:842–849. doi: 10.1097/CCM.0b013e3182711b38. [DOI] [PubMed] [Google Scholar]
- 33.Kameneva MV, Marad PF, Brugger JM, Repko BM, Wang JH, Moran J, Borovetz HS. In vitro evaluation of hemolysis and sublethal blood trauma in a novel subcutaneous vascular access system for hemodialysis. Asaio J. 2002;48:34–38. doi: 10.1097/00002480-200201000-00008. [DOI] [PubMed] [Google Scholar]
- 34.Raval JS, Waters JH, Seltsam A, Scharberg EA, Richter E, Daly AR, Kameneva MV, Yazer MH. The use of the mechanical fragility test in evaluating sublethal RBC injury during storage. Vox Sang. 2010;99:325–331. doi: 10.1111/j.1423-0410.2010.01365.x. [DOI] [PubMed] [Google Scholar]
- 35.Yazer MH, Waters JH, Elkin KR, Rohrbaugh ME, Kameneva MV. A comparison of hemolysis and red cell mechanical fragility in blood collected with different cell salvage suction devices. Transfusion. 2008;48:1188–1191. doi: 10.1111/j.1537-2995.2008.01670.x. [DOI] [PubMed] [Google Scholar]
- 36.Beutler E, Kuhl W, West C. The osmotic fragility of erythrocytes after prolonged liquid storage and after reinfusion. Blood. 1982;59:1141–1147. [PubMed] [Google Scholar]
- 37.Girasole M, Pompeo G, Cricenti A, Longo G, Boumis G, Bellelli A, Amiconi S. The how, when, and why of the aging signals appearing on the human erythrocyte membrane: an atomic force microscopy study of surface roughness. Nanomedicine. 2012;6:760–768. doi: 10.1016/j.nano.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Karon BS, Van Buskirk CM, Jaben EA, Hoyer JD, Thomas DD. Temporal sequence of major biochemical events during blood bank storage of packed red blood cells. Blood Transfus. 2012. pp. 1–9. [DOI] [PMC free article] [PubMed]
- 39.Pompeo G, Girasole M, Cricenti A, Boumis G, Bellelli A, Amiconi S. Erythrocyte death in vitro induced by starvation in the absence of Ca(2+) Biochim Biophys Acta. 2012;1798:1047–1055. doi: 10.1016/j.bbamem.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 40.D'Almeida MS, Jagger J, Duggan M, White M, Ellis C, Chin-Yee IH. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA-1 for 29 days: implications for animal models of transfusion. Transfus Med. 2000;10:291–303. doi: 10.1046/j.1365-3148.2000.00267.x. [DOI] [PubMed] [Google Scholar]
- 41.Gelderman MP, Vostal JG. Rejuvenation improves roller pump-induced physical stress resistance of fresh and stored red blood cells. Transfusion. 2011;51:1096–1104. doi: 10.1111/j.1537-2995.2010.02972.x. [DOI] [PubMed] [Google Scholar]
- 42.Meyer EK, Dumont DF, Baker S, Dumont LJ. Rejuvenation capacity of red blood cells in additive solutions over long-term storage. Transfusion. 2012;51:1574–1579. doi: 10.1111/j.1537-2995.2010.03021.x. [DOI] [PubMed] [Google Scholar]
- 43.Zehnder L, Schulzki T, Goede JS, Hayes J, Reinhart WH. Erythrocyte storage in hypertonic (SAGM) or isotonic (PAGGSM) conservation medium: influence on cell properties. Vox Sang. 2008;95:280–287. doi: 10.1111/j.1423-0410.2008.01097.x. [DOI] [PubMed] [Google Scholar]
- 44.Maher AD, Kuchel PW. The Gardos channel: a review of the Ca2+-activated K+ channel in human erythrocytes. Int J Biochem Cell Biol. 2003;35:1182–1197. doi: 10.1016/s1357-2725(02)00310-2. [DOI] [PubMed] [Google Scholar]
- 45.Bevers EM, Comfurius P, Dekkers DW, Harmsma M, Zwaal RF. Regulatory mechanisms of transmembrane phospholipid distributions and pathophysiological implications of transbilayer lipid scrambling. Lupus. 1998;7(suppl 2):S126–131. doi: 10.1177/096120339800700228. [DOI] [PubMed] [Google Scholar]
- 46.Ghashghaeinia M, Cluitmans JC, Akel A, Dreischer P, Toulany M, Koberle M, Skabytska Y, Saki M, Biedermann T, Duszenko M, Lang F, Wieder T, Bosman GJ. The impact of erythrocyte age on eryptosis. Br J Haematol. 2012;157:606–614. doi: 10.1111/j.1365-2141.2012.09100.x. [DOI] [PubMed] [Google Scholar]
- 47.Karger R, Lukow C, Kretschmer V. Deformability of red blood cells and correlation with ATP content during storage as leukocyte-depleted whole blood. Transfus Med Hemother. 2012;39:277–282. doi: 10.1159/000339809. [DOI] [PMC free article] [PubMed] [Google Scholar]