Introduction
Breast cancer remains the most commonly diagnosed cancer in women, a diagnosis in which breast imaging has played a crucial role. Since the introduction of screening mammography, physicians and researchers have sought to improve breast imaging and extend its benefits to all patients. Yet, conventional breast imaging, in the form of mammography has not proved as beneficial in certain demographics of women as it has in others, namely women with dense breast tissue. Dense breasts have been found to be an independent risk factor for developing breast cancer[1] in addition to the decreased sensitivity of mammography in dense breast tissue[2, 3].
Positron Emission Mammography, or PEM, was introduced to meet the growing demand of breast specific metabolic imaging. PEM is similar to Positron Emission Tomography, or PET, in that it utilizes the localization of injectable radioisotopes in metabolically active cells and tissue to image disease processes. Unlike PET/CT, PEM has two adjustable plate detector heads that compress and stabilize the breast in a configuration not unlike a conventional mammogram. Although the use of whole body PET and whole body gamma imaging to image the breast have been discussed in the literature for years [4], the limited spatial resolution of Whole Body PET/CT has resulted in poor sensitivity and specificity of breast PET for lesions approximately 10 mm and smaller[5]. PEM has sought to improve its sensitivity in comparison to PET/CT by improving its special resolution by placing the heads of its detectors immediately adjacent to the breast. In comparison to Whole Body PET/CT, PEM has a spatial resolution of 2mm [6] and an improved count sensitivity. These imaging characteristics of PEM have translated into superior sensitivity and specificity in small lesions in clinical trials of patients with breast malignancies. Such advances have led to the logical question; if PEM can better image small lesions in the breast can it improve imaging of small lesions in other anatomical areas? To our knowledge there have been no studies to date on the feasibility of utilizing a breast dedicated high resolution positron emission scanner such as the PEMFlex Solo II to image lesions outside the breast.
Our institutional review board approved two separate protocols. A total of 14 patients with suspected or known FDG avid findings in the neck and extremities were imaged first with PET/CT and then with PEM. The objective of this imaging was to demonstrate that image acquisition with PEM was not only feasible but also capable of producing images of similar or better quality than PET. If imaging of small anatomical lesions were possible with a portable, high resolution positron emission scanner such as a PEM device then the potential uses of positron emission scanning could be greatly expanded. WB PET/CT is already being used to image osteomyelitis and there have been several studies evaluating the use of PET/CT in joint inflammation. If a high resolution scanner such as PEM could adequately image the extremities, in theory it could improve the accuracy and sensitivity of inflammation and infection detection.
Methods and Materials
This study is a retrospective chart review of 14 total patients initially imaged in two separate IRB approved protocols. All patients identified as a having a mass previously seen as FDG avid on WB PET/CT imaging located in the neck or in the extremities. Patients were consented for having PEM imaging of their masses immediately after PET/CT, thereby eliminating the need for an additional administration of FDG.
Imaging: F-18 FDG was administered to the patients based on weight, with the administered activity in this patient group ranging from 325.6 – 495.8 MBq (8.8 – 13.4 mCi), with average administered activity of 418.1 MBq (11.3 mCi). Imaging was performed on an integrated PET/CT system (DSTe, RX, VCT, GE Medical Systems, Milwaukee, WI) approximately 60–80 minutes after injection. Noncontrast CT scanning was performed for attenuation correction and image registration. The extent of scanning was tailored to the indication, with inclusion of the extremities as needed. Immediately after PET/CT patients were sent to PEM to have secondary imaging.
Like the technology upon which it is based, PEM utilizes F-18 FDG metabolism as a surrogate for evaluating metabolic activity of masses within the breast. PEM functions on the principal of improving spatial resolution and count sensitivity, relative to Whole Body PET/CT, by placing its two plate detectors immediately adjacent to the object of interest, such as the breast. Unlike PET/CT the detectors are not arranged in a 360 degree array around the object but instead can only image in the plane of positioning. Due to mammography convention images of the breast are typically acquired in the Medial Lateral Oblique orientation and Cranial Caudal orientations, in the case of our study images were acquired in the anterior and lateral orientations.
The PEM device utilized was a PEMFlex Solo II High Resolution PET (Naviscan, San Diego, CA). PEM imaging was performed between 1.5 and 2 hrs after tracer administration. Since PEM images were obtained immediately after PET/CT imaging no additional radiotracer was administered.
The positioning of the PEM adjustable detectors was performed by a trained Nuclear Medicine Technologist and images were obtained in both orientations for all lesions when possible. Due to restrictions of positioning, 3 of 14 patients were imaged in only one orientation.
Image reconstruction was performed with a limited angle image reconstruction algorithm that employs 3D MLEM. The implementation of this algorithm on the PEM solo system results in a fixed number of image slices (12 in this case) representing the volume between the two detector plates. Because of the limited number of projections due to scanner design, the resultant reconstructed image slices suffer from spatial anistrophy, or the distortion of object geometry, particularly in the axial direction (parallel to the two plate detectors); thus the exact geometry of the lesions was not consistent from slice to slice.
PET/CT images were sent to an Advantage Windows AW reading station (GE, Milwaukee) where they were reviewed by a board certified Imager. PEM images were sent to a work station with Naviscan’s proprietary image analysis software. A board certified imager reviewed the 12 images for each view and selected the image with the best qualitative lesion to background ratio.
Results
The lesion of interest first seen on PET/CT was easily identified by PEM in all 14 patients. The images of 8 representative patients are displayed below.
Discussion
The use of positron emission scanning in the form of whole body PET/CT to image and stage malignancy has revolutionized the practice of oncology. The strength of WB PET/CT is its ability to image large anatomical areas and produce an overall portrait of disease activity in a patient; its weakness is its limited spatial resolution [5]. Positron Emission Mammography in breast tissue is able to use metabolic imaging to detect lesions as small as 2mm [6] in the breast. Our study attempted to utilize PEM to image other small anatomical areas such as the hands, feet, arms and calves in the hope that PEM could impart similar improvements in resolution in these structures.
To our knowledge there is no published literature that supports the use of PEM to image the extremities. As a feasibility study, we observed several unique and interesting findings regarding PEM imaging in the extremities. Since PEM has thus far only imaged the breast, a structure devoid of muscle or bone, previously published PEM images have had a relatively uniform background which served to further emphasize the lesion of interest. Based on our observations of the imaging of the extremities, the background is composed of bone, which appears photopenic; muscle, which has a similar appearance to the background of breast; and skin which has minimally increased activity when compared to muscle. In the absence of a CT component as seen with PET/CT, the characteristic photopenia associated with bone is beneficial in localizing lesions to the bone versus soft tissue. Although our study is small, the appearance of bone as a photopenic structure with mildly increased counts at the periphery appears uniform throughout our images and we feel confident that further studies of the appearance of bone on PEM will demonstrate similar findings.
In addition to the general observations about the appearance of the extremities on PEM we were also able to demonstrate a few of PEM’s strengths. As demonstrated in the patients above, PEM was able to depict synovial activity, better resolve activity in the small bones of the feet, and clearly define melanoma’s hypermetabolic activity in the skin.
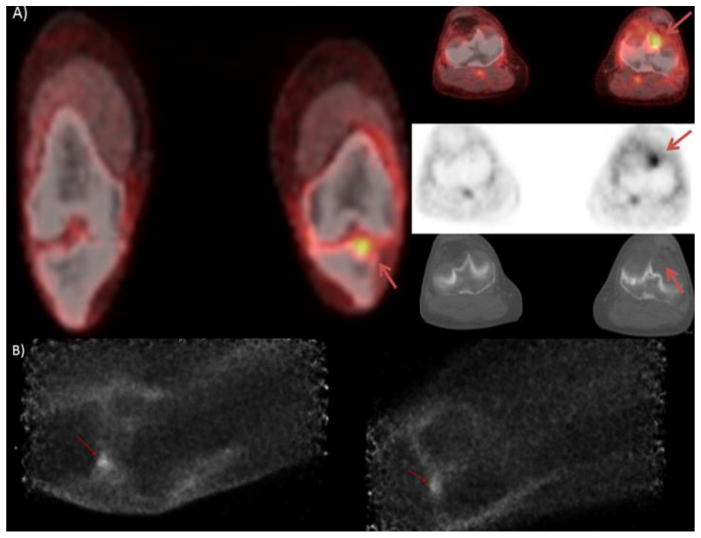
PEM’s ability to demonstrate the activity seen within the synovium in the knee of patient 6 illustrates its potential use in the realm of arthritis imaging. In patient 6, PEM’s ability to resolve the activity is clearly superior to PET/CT (which was unable to definitely localize the activity to bone or joint) and consistent with the patient’s biopsy proven diagnosis of PVNS, a synovial process. The experimental use of FDG PET/CT in the evaluation of rheumatoid arthritis has been extensively documented in the literature [7, 8]. In a study from Turkey, PET/CT was able to accurately assess response to therapy in knees affected with rheumatoid arthritis but found difficulty in accurately assessing disease in the hands due to low resolution of WB PET/CT [9]. A study from Leeds employed a nano PET (developed for small animal imaging) to study the distal interphalangeal joints of patients, demonstrating the potential use of a high resolution positron scanner. That study compared patients with psoriatic arthritis versus normal controls, finding marked difference in metabolism between the two groups [10]. The potential for a high resolution positron emission scanner to help manage rheumatoid arthritis exists, given PEM’s superior spatial resolution it may help play a role in the imaging of small joints, such as those of the hands.
Although, MRI is the current gold standard for osteomyelitis imaging in the diabetic foot, many diabetics are unable to obtain MR due to the contraindication of gadolinium in diabetic nephropathy. In these patients, bone scan and indium scanning has been used, but there is new interest in PET/CT. Studies have shown improved sensitivity and specificity of PET/CT over bone scan and indium scanning[11] and has been found to have excellent performance in the identification of osteomyelitis within the complicated diabetic foot [12]. The PEM images of patient 1 clearly show the activity within the cuboidal bone and improve upon the PET/CT images by showing more moderate activity in the adjacent distal calcaneus. High resolution imaging with PEM could theoretically help identify osteomyelitis in the smaller osseous structures and warrants further investigation.
There are two arenas in which PEM may be put into more immediate use. The first is imaging melanoma of the extremities, the superior spatial resolution of the PEM images of patient 5 demonstrate the potential utility of using PEM for surgical planning of resection or evaluation of response to therapy in melanoma. In these patients WB PET/CT often suffers from poor resolution coupled with misregistration of the PET to the CT which can make localization of the activity difficult. Although PEM does not have a CT component, its superior resolution with the potential help of radioactive markers could potentially help further define the spread of disease.
The second area in which PEM may be utilized almost immediately is in the imaging of the extremities after limb salvage surgery. The paper by Fritz [13] describes the difficulties of imaging these patients for local recurrence with CT and MR because of the artifacts created by their metallic hardware. On CT, beam hardening artifact and on MR blooming artifact, [14] limit the close evaluation of adjacent tissue. As demonstrated by the images we obtained, PEM has excellent resolution and should be able to demonstrate recurrence in the form of increased activity. Unlike CT and MR, PEM should not be affected by artifact from adjacent metallic structures. On PET there are often low levels of activity seen adjacent to orthopedic hardware in weight bearing structures. Although this activity may also be an issue on PEM there is real potential for an organ specific positron emission scanner such as PEM to play an increasing role in post-surgical surveillance in patients with metallic hardware.
To our knowledge this is the first study to utilize PEM as a potential high resolution PET imaging device for the extremities. Given the potential for obtaining functional imaging with improved spatial resolution, further research into such utilization appears warranted. Our preliminary results show that such imaging with PEM is not only feasible but in many instances adds value to the previously obtained PET/CT images.
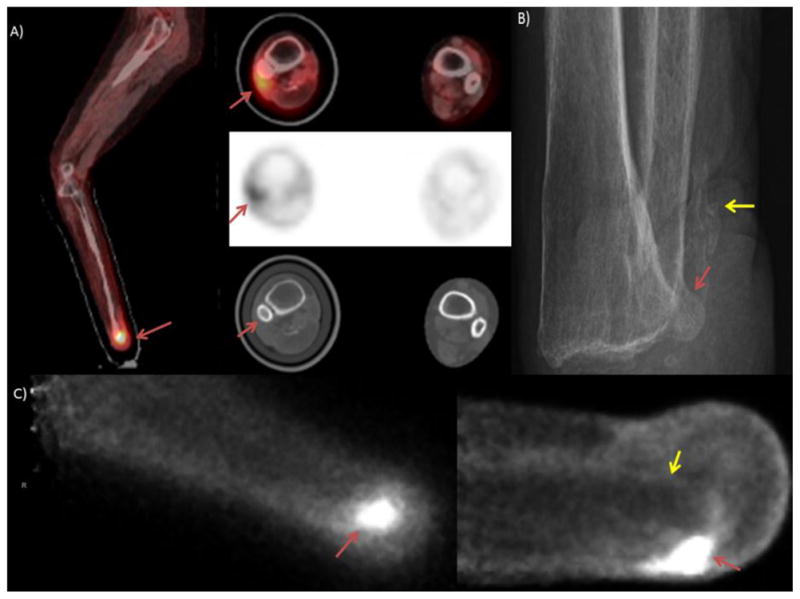
Figure 1.
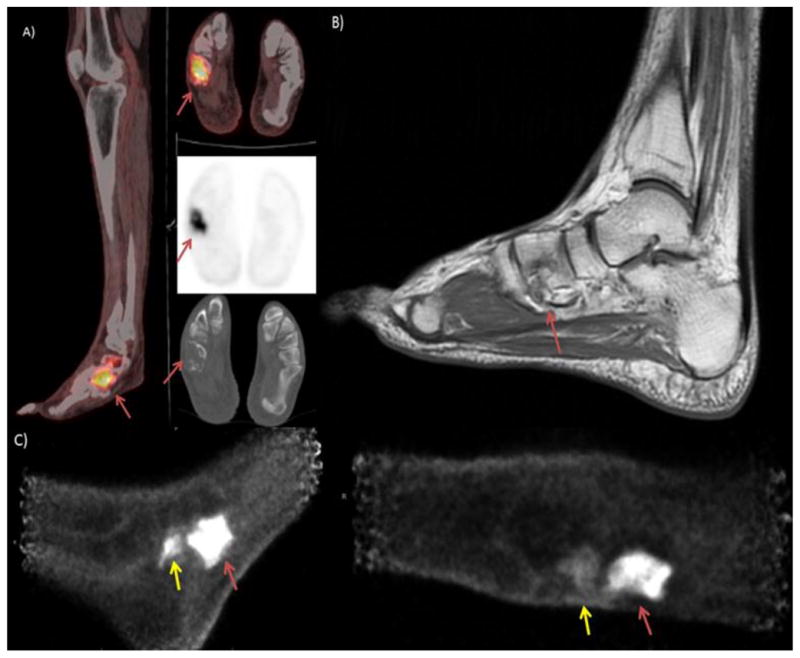
The patient is a 48 year old man who presented with right foot pain and was found to have a localized cuboidal spindle cell sarcoma which was treated with adjuvant chemotherapy. Disease was moderately refractory to treatment, the PET/CT image shown in image A) demonstrates persistent, intense uptake in a destructive lesion in the right cuboidal bone. Image B) Sagittal MRI with T2 images above and T1 images below show complete marrow replacement in the cuboid on the T2 images with heterogeneous signal in the calcaneus. This was initially read as indeterminate activity in the calcaneus but retrospectively appears suspicious for calcaneal involvement. C) PEM images show viable tumor in the cuboidal bone and increased uptake adjacent to that lesion in the distal calcaneus (yellow arrow) indicating presence of disease extending outside the cuboidal bone.
Figure 2.
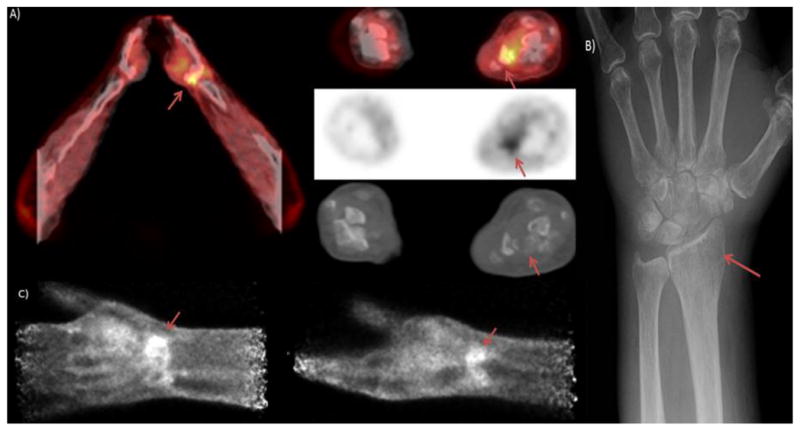
Patient is a 72 year old with metastatic non-small cell lung cancer. The PET/CT image A) depicts hypermetabolism in the region of the distal radius. The X-ray shown in B) demonstrates a mass like area of heterogeneous lucency in the distal radius. The PEM images in C) show a higher resolution depiction of hypermetabolism in the distal radius
Figure 3.
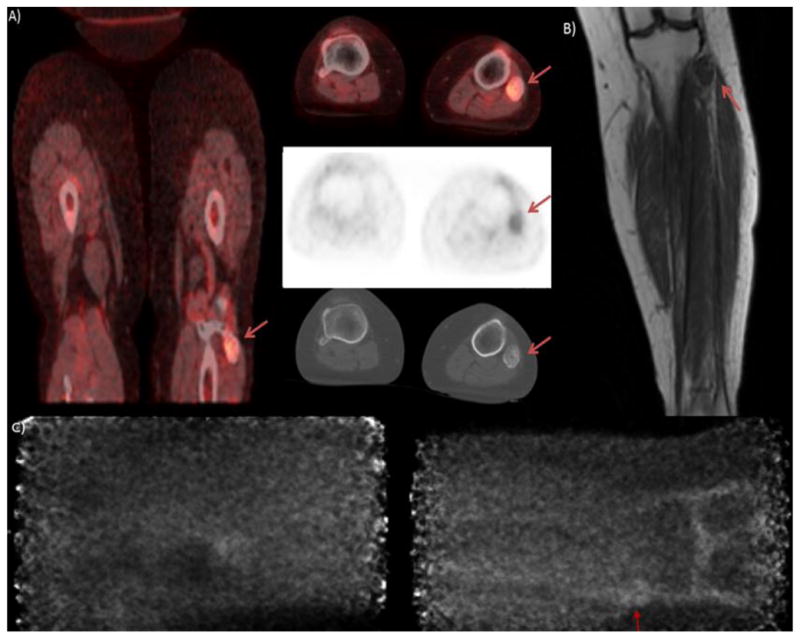
Patient is a 52 year old female with invasive ductal carcinoma suspected to be metastatic to the fibula. PET/CT A) shows mild hypermetabolism in the region of the fibula. The coronal T1 image of the patient’s MRI B) shows an isointense lesion occupying the entire width of the proximal fibula, the lesion was heterogeneously hyperintense on T2 images and was read as indeterminate. The lateral PEM image C) is non-specific but the anterior PEM image d) shows uptake in the head of the fibula (red arrow).
Figure 4.
Patient is a 53 year old female with invasive ductal carcinoma metastatic to the tibia. PET/CT showed a small, hypermetabolic blastic appearing lesion in the lateral aspect of the proximal tibia. The lesion measured 1.8 cm on PET/CT. PEM images show the focal, well-circumscribed and moderately hypermetabolic appearance of the lesion.
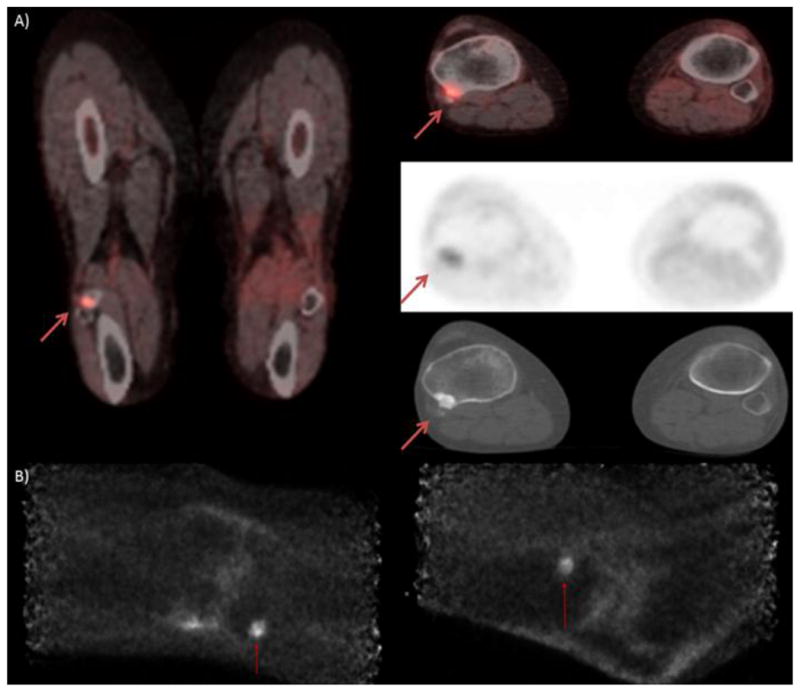
Figure 5.
Patient is a 60 year old female diagnosed with melanoma of the left heel. Patient had surgical excision of a lentiginous melanoma of the left heel five years prior. Patient presented with a left inguinal mass and on PET/CT images A) show mild, diffusely increased uptake of the left heel. PEM images B) show moderate to markedly increased activity of the superficial soft tissues of the left heel with demonstrably superior spatial resolution.
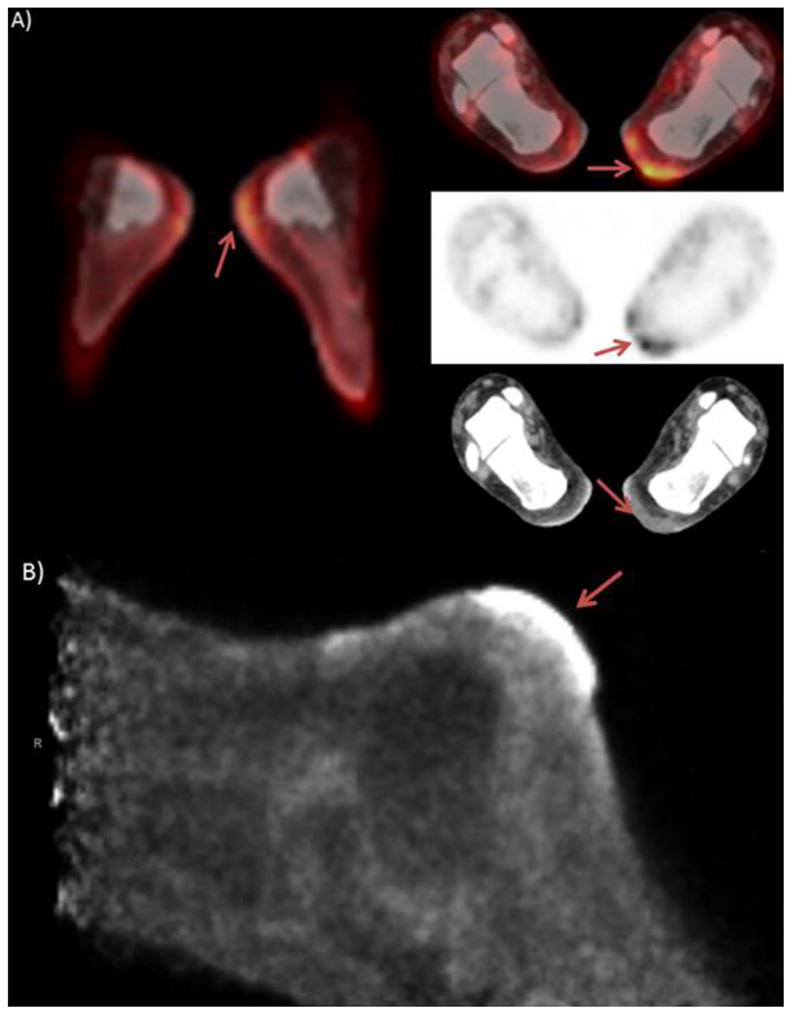
Figure 6.
Patient is an 80 year old male with a history of left knee melanoma, diagnosed and treated with wide surgical excision 13 year prior, patient was recurrence free until 2005 at which time he had recurrent disease in the left inguinal nodes, patient underwent local excision and had no evidence of disease recurrence to date. Serial PET/CT scanning revealed focal lesion in the joint space of the left knee, which was biopsy proven to be a PVNS. PET/CT images A) show mild focal activity at the tibial plateau, but slight misregistration makes exact localization difficult. Anterior PEM images B) depict the hypermetabolic activity within the joint space.
Figure 7.
Patient is a 57 year old female with metastatic adenocarcinoma of the lung with widespread metastasis to the bone and brain. Patient had a remote history of right foot amputation, on PET/CT A) moderate hypermetabolic activity was seen in the soft tissues adjacent to the distal fibula. There is irregularity of the fibula on the CT images but poor resolution of the PET images and mild misregistration appear to localize the hyperactivity to the soft tissue rather than the fibula. The patient’s x-ray B) shows myositis ossificans (yellow arrow) in reaction to the amputation and subtle erosions in the fibula (red arrow) which may be consistent with osteomyelitis. The PEM images C) show marked hypermetabolism in the location of the fibula. The yellow arrow demonstrates the photopenia in the location of the tibia associated with bone on these images.
Figure 8.
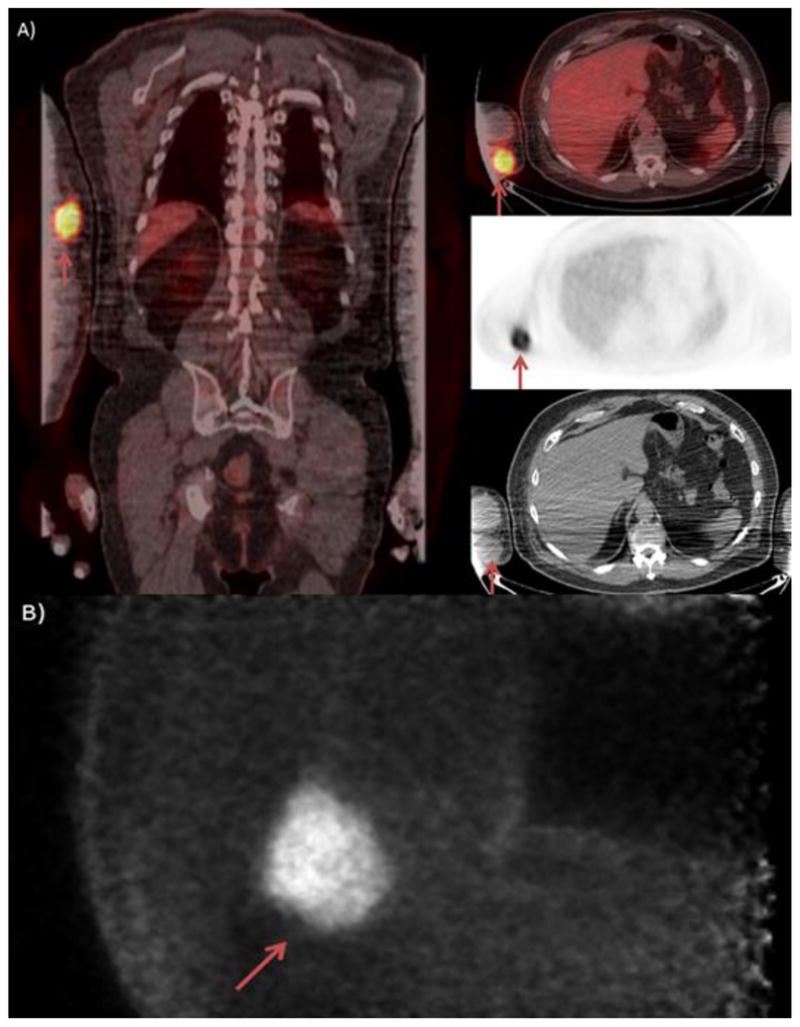
The patient is a 72 year old man with a metastatic merkel cell carcinoma. The patient initially presented with right arm swelling and was found to have axillary masses on imaging. On serial PET/CT imaging A) a Right hypermetabolic antecubital mass was seen in the soft tissues. PEM imaging B) identified the large soft tissue mass without evidence of the photopenia associated with bone, indicating that this lesion was indeed in the soft tissues.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Checka CM, et al. The relationship of mammographic density and age: implications for breast cancer screening. AJR Am J Roentgenol. 2012;198(3):W292–5. doi: 10.2214/AJR.10.6049. [DOI] [PubMed] [Google Scholar]
- 2.Jackson VP, et al. Imaging of the radiographically dense breast. Radiology. 1993;188(2):297–301. doi: 10.1148/radiology.188.2.8327668. [DOI] [PubMed] [Google Scholar]
- 3.Berg WA, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004;233(3):830–49. doi: 10.1148/radiol.2333031484. [DOI] [PubMed] [Google Scholar]
- 4.Kalles V, et al. The current status of positron emission mammography in breast cancer diagnosis. Breast Cancer. 2012 doi: 10.1007/s12282-012-0433-3. [DOI] [PubMed] [Google Scholar]
- 5.Avril N, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18(20):3495–502. doi: 10.1200/JCO.2000.18.20.3495. [DOI] [PubMed] [Google Scholar]
- 6.Schilling K, et al. Positron emission mammography in breast cancer presurgical planning: comparisons with magnetic resonance imaging. Eur J Nucl Med Mol Imaging. 2011;38(1):23–36. doi: 10.1007/s00259-010-1588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijayant V, et al. Potential of (18)F-FDG-PET as a valuable adjunct to clinical and response assessment in rheumatoid arthritis and seronegative spondyloarthropathies. World J Radiol. 2012;4(12):462–8. doi: 10.4329/wjr.v4.i12.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarma M, Vijayant V, Basu S. (18)F-FDG-PET assessment of early treatment response of articular and extra-articular foci in newly diagnosed rheumatoid arthritis. Hell J Nucl Med. 2012;15(1):70–1. [PubMed] [Google Scholar]
- 9.Roivainen A, et al. Correlation of (18)F-FDG PET/CT assessments with disease activity and markers of inflammation in patients with early rheumatoid arthritis following the initiation of combination therapy with triple oral antirheumatic drugs. Eur J Nucl Med Mol Imaging. 2013;40(3):403–10. doi: 10.1007/s00259-012-2282-x. [DOI] [PubMed] [Google Scholar]
- 10.Tan AL, et al. High-resolution [18F]fluoride positron emission tomography of the distal interphalangeal joint in psoriatic arthritis--a bone-enthesis-nail complex. Rheumatology (Oxford) 2013 doi: 10.1093/rheumatology/kes384. [DOI] [PubMed] [Google Scholar]
- 11.Kagna O, et al. FDG PET/CT imaging in the diagnosis of osteomyelitis in the diabetic foot. Eur J Nucl Med Mol Imaging. 2012;39(10):1545–50. doi: 10.1007/s00259-012-2183-z. [DOI] [PubMed] [Google Scholar]
- 12.Gnanasegaran G, Vijayanathan S, Fogelman I. Diagnosis of infection in the diabetic foot using (18)F-FDG PET/CT: a sweet alternative? Eur J Nucl Med Mol Imaging. 2012;39(10):1525–7. doi: 10.1007/s00259-012-2234-5. [DOI] [PubMed] [Google Scholar]
- 13.Fritz J, et al. Imaging of Limb Salvage Surgery. AJR. 2012;198:647–660. doi: 10.2214/AJR.11.7286. [DOI] [PubMed] [Google Scholar]
- 14.White L, Buckwalter K. Technical Considerations:CT and MR Imaging in the Postoperative Orthopedic Patient. Seminars in Muskuloskeletal Radiology. 2002;6(1):5–17. doi: 10.1055/s-2002-23160. [DOI] [PubMed] [Google Scholar]