Abstract
Endosomal signaling is emerging as one of the most important cellular events that regulate signaling function in mammalian cells or an epithelium in response to changes in environment such as the presence of stimuli mediated by cytokines, toxicants, heat, ions during growth and development, and other cellular processes such as cytokinesis and spermatogenesis. Recent studies have shown that protein endocytosis—the initial step of endosomal signaling—involves the participation of polarity proteins, such as partitioning defective protein 6 (Par6), Cdc42 and 14-3-3 (also known as Par5), which in turn is regulated by cytokines (e.g., TGF-β2, TGF-β3) and testosterone at the Sertoli cell blood–testis barrier (BTB) in the mammalian testis. In this short method paper, we provide a detailed protocol of assessing protein endocytosis, the initial and also the most critical step of endosomal signaling at the Sertoli cell BTB. This biochemical endocytosis assay summarizes our experience for the last decade, which should likely be performed in conjunction with the dual-labeled immunofluorescence analysis to assess protein endocytosis. While we are using a Sertoli cell in vitro system that mimics the BTB in vivo, this approach should be applicable to virtually all mammalian cells.
1. INTRODUCTION
Endosomal signaling is a rapidly evolving field. It refers to the transmission of incoming signals outside a mammalian cell via endosome-mediated trafficking, so that outside signals can be appropriately responded physiologically which are mediated by changes in specific microdomain(s) (e.g., basolateral or apical region of an epithelial cell) through re-arrangement of proteins through transcytosis, recycling or degradation within the cell, such that cells in an epithelium can make appropriate responses to incoming signals (Gonnord, Blouin, & Lamaze, 2012; Le Roy & Wrana, 2005a; Miaczynska & Bar-Sagi, 2010; Palfy, Remenyi, & Korcsmaros, 2012) during growth, development, and/or in response to stimuli from the environment, such as the presence of growth factors and toxicants. Endosomal signaling is known to affect multiple cellular events including cell migration, metabolism, survival, cell division, and proliferation (Le Roy & Wrana, 2005b; Leto & Saltiel, 2012; Neto, Collins, & Gould, 2011; Palfy et al., 2012; Polo & Di Fiorce, 2006; Schiefermeier, Teis, & Huber, 2011), which may also involve protein ubiquitination (Haglund & Dikic, 2012; Marchese & Trejo, 2013). Once inside the cell, endosomes can also serve as signaling platforms to mediate crosstalk between signaling pathways, so that appropriate responses can be made in response to changes in environment, during growth and development, or pathogenesis (Palfy et al., 2012). Furthermore, endosomes are also being used to deliver other intracellular proteins, such as Src family of non-receptor protein tyrosine kinases (Sandilands & Frame, 2008), Rho GTPase (Falkenberg & Loew, 2013) to specific microdomain of the plasma membrane to regulate cellular functions in response to changes in environment (Sandilands & Frame, 2008). The initial response of endosomal signaling is mediated by endosome-mediated internalization of cell surface proteins via endocytosis. While these changes can be correctly captured via dual-labeled immunofluorescence using confocal or regular fluorescence microscopy, such analysis can only be considered an initial step of assessment of endosomal signaling since these data are qualitative in nature. In order to provide a better semiquantitative data analysis, biochemical-based endocytosis assay provides better analysis in particular when the kinetics of endocytosis is being assessed. Biochemical analysis of cellular events pertinent to endocytosis, which include pinocytosis and phagocytosis, was first reported in the 1970s and 1980s (Bode, Baumann, & Kinne, 1975; Bode, Pockrandt-Hemstedt, Baumann, & Kinne, 1974; Daukas & Zigmond, 1985; Henning, Kaulen, & Stoffel, 1970; Loose, Megirian, & Turinsky, 1984; Quie, 1977). Since then, biochemical assay to assess protein endocytosis has been better developed with the initial use of radiolabeled proteins involving tedious biochemical steps such as ultracentrifugation (Wiley et al., 1991) to the use of protein biotinylation involving simple steps of lysate preparation and protein extraction with avidin-conjugated beads, to be following by immunoblot analysis using different specific antibodies (Le, Yap, & Stow, 1999; Morimoto et al., 2005) to track the events or kinetics of endocytosis.
In this short review, we provide a detailed step-by-step protocol based on our earlier experience using Sertoli cells in the rat testis as a study model to study protein endocytosis (Lie, Cheng, & Mruk, 2011; Wong, Mruk, Lee, & Cheng, 2010; Wong, Sun, Li, Lee, & Cheng, 2009; Yan, Mruk, Lee, & Cheng, 2008). While our experience is limited to the Sertoli cells in the testis, this approach, however, is applicable to other mammalian cells. Nonetheless, using this assay, we have shown that cellular events that occur in the seminiferous epithelium during spermatogenesis are coordinated by endocytic vesicle-mediated protein trafficking mediated by polarity proteins and under the influence of both cytokines and/or testosterone. For instance, testosterone and cytokines (e.g., TGF-β2) that have antagonistic effects on the blood–testis barrier (BTB) permeability function were found to have differential effects on endosome-based intracellular signaling in which testosterone facilitates endosome-mediated protein transcytosis/recycling, whereas TGF-β2 promotes endosome-mediated protein degradation based on biochemical assays (Yan et al., 2008). These findings have been subsequently confirmed using a fluorescence-based approach by staining Sertoli cells with specific markers of endocytosis, transcytosis, and recycling (Su, Mruk, Lee, & Cheng, 2010). Protein endocytosis was also shown to be crucial to TGF-β3-mediated disruptive effects on BTB function via the use of a Cdc42-dominant negative mutant for studies (Wong et al., 2010) in whichCdc42 is a critical component of the Par-based polarity protein complex (Wong & Cheng, 2009). For instance, it was shown that TGF-β3-mediated acceleration of protein endocytosis at the BTB is mediated by active Cdc42 since the deletion of Cdc42 functionality in the Sertoli cell epithelium via an overexpression of a dominant negative mutant of Cdc42 would insensitize these cells to TGF-β3 treatment (Wong et al., 2010), illustrating the Par6-Cdc42 complex is crucial in regulating protein endocytosis. This conclusion is also supported by findings in which a knockdown of Par3 or Par6 by RNAi in Sertoli cell epithelium was shown to induce mislocalization of integral membrane proteins at the Sertoli cell BTB (e.g., N-cadherin, JAM-A, nectin-2), with these proteins being redistributed, moved from the cell–cell interface and into the cell cytosol (Wong, Mruk, Lee, & Cheng, 2008), possibly via an increase in protein endocytosis. The notion that polarity proteins are crucial regulators of protein endocytosis is further supported by a study in which a knockdown of Par5 (14-3-3) was found to accelerate the kinetics of endocytosis of BTB integral membrane proteins JAM-A (junctional adhesion molecule-A) and N-cadherin (Wong et al., 2009). Taken collectively, these findings suggest that the initial event of endosomal signaling, namely, protein endocytosis, that occurs at the microenvironment of the BTB in the seminiferous epithelium of mammalian testis is primarily regulated by cytokines and testosterone, and with the involvement of polarity proteins such as Par and Cdc42, so that internalized proteins can be targeted to specific cellular domain(s) via transcytosis and recycling to mediate signaling function in response to changes in the environment, such as during the epithelial cycle of spermatogenesis or following exposure to toxicants and/or drugs. Results of these findings helped us to provide a biochemical-based model to study the processes of transport of preleptotene spermatocytes across the BTB during spermatogenesis (Cheng & Mruk, 2010; Cheng et al., 2011; Su, Mruk, & Cheng, 2013).
The assay protocol detailed below summarized our findings in assessing the effects of IL-1α, a cytokine in the testis known to accelerate protein endocytosis at the Sertoli cell BTB (Lie et al., 2011), on the kinetics of protein endocytosis using the in vitro Sertoli cell culture system which closely mimics the BTB in vivo. This assay, however, can be easily modified to assess changes in endocytosis following overexpression of any target proteins (e.g., Cdc42, a Par-based polarity protein complex component) using a mammalian cell expression vector (e.g., pCI-neo) (Wong et al., 2010), treatment of cells with toxicants (e.g., bisphenol A, cadmium) (Li, Mruk, Lee, & Cheng, 2009; Siu et al., 2009) as described in recently published reports. Thus, besides cytokines, steroids, and polarity proteins, other proteins that may regulate cellular functions via endosomal signaling can be rapidly assessed using this protocol.
2. ENDOCYTOSIS ASSAY
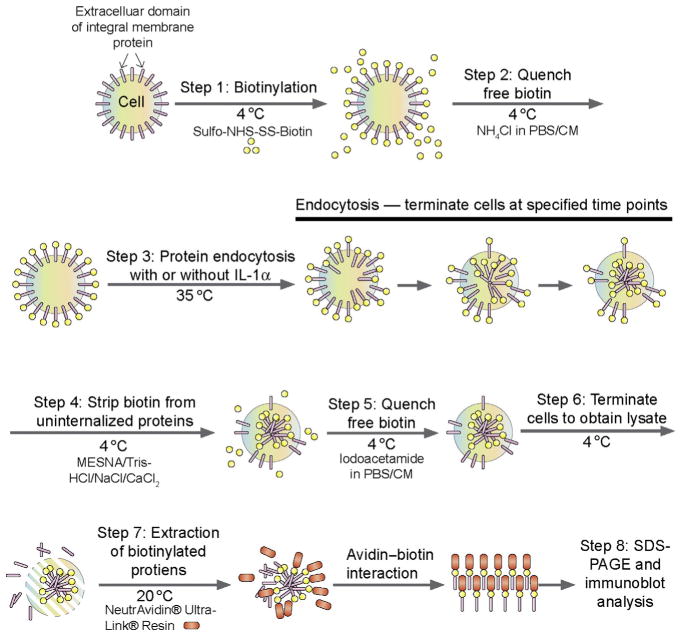
This assay is based on the use of biotin (also known as vitamin H) via biotinylation of cell surface proteins in which sulfo-NHS-biotin can be covalently and spontaneously conjugated to primary amines (such as lysine side chain ε-amines and N-terminal α-amines) in the amino acid residues of BTB integral membrane proteins such as CAR (coxsackievirus and adenovirus receptor) and JAM-A. Since sulfo-NHS-SS-biotin is water soluble and carries a charge, rendering it impermeable to plasma membranes, it only biotinylates the extracellular domains of BTB integral membrane proteins. Furthermore, a disulfide bond (S—S) is present in Sulfo-NHS-SS-biotin, which can be cleaved under reducing conditions in the presence of 2-mercaptoethanol to release the target protein in SDS-sample buffer using SDS-PAGE to be followed by immunoblotting using a specific antibody (see Fig. 10.1). Following biotinylation at 4 °C during which endocytosis fails to occur, non bound biotins will be removed by quenching, and endocytosis will be allowed to take place by placing cells in dishesina CO2 incubator (5%CO2/95%air, v/v) at 35 °C (which is the optimal temperature for testicular cells such as Sertoli cells—for other mammalian cells, 37 °C should be used instead) and cells will be terminated at specified time points as shown in Fig. 10.1 (see also Fig. 10.2). At termination, biotins on the uninternalized cell surface proteins will be stripped with 50 mM sodium 2-mercaptoethane sulfonate (MESNA) in 100 mM Tris–HCl, 100 mM NaCl, and2.5 mMCaCl2, pH 8.6 at 4 °C and quenched with5 mg/ml iodoacetamide in PBS/CM buffer at 4 °C for 15 min, so that only endocytosed biotinylated proteins will be subsequently analyzed (Fig. 10.1). Cell lysates will be obtained and biotinylated proteins will be pulled down with UltraLink® Immobilized NeutrAvidin® beads since avidin has an extraordinary affinity for biotin (KD = ~ 10−14 M) and the interaction between biotin and avidin is one of the strongest noncovalent molecular interactions in nature. Thereafter, the target proteins (e.g., CAR, JAM-A) are harvested with RIPA buffer and can be analyzed by SDS-PAGE following cleavage of the biotin in SDS sample buffer [0.125 M Tris, pH 6.8 at 22 °C containing 1% SDS (w/v), 1.6% 2-mercaptoethanol (v/v), 10% glycerol (v/v)], to be followed by immunoblot analysis.
Figure 10.1.
A schematic drawing illustrating the concept of endocytosis assay based on the use of biotinylation of cell surface proteins. The basic concept and detailed information of the various buffers and reagents can be found in the text.
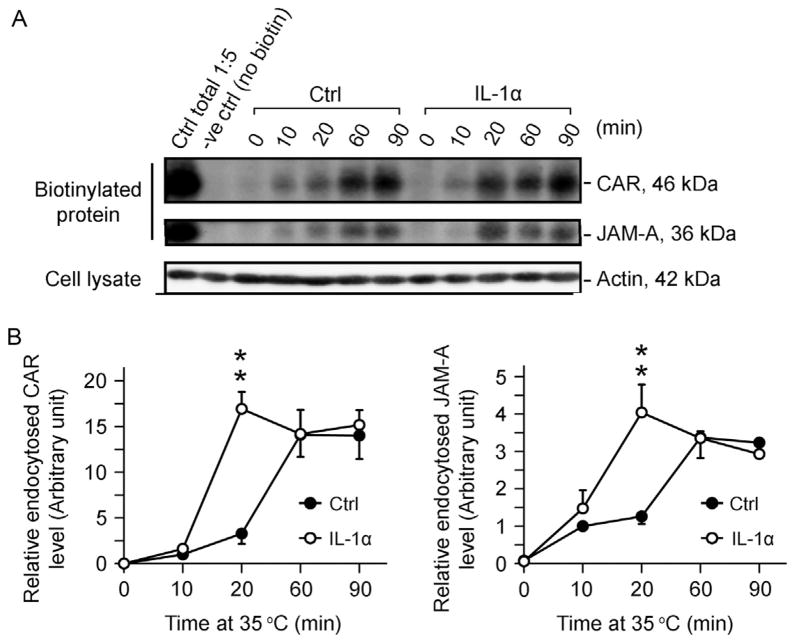
Figure 10.2.
Effects of IL-1α on the kinetics of endocytosis of CAR and JAM-A in Sertoli cells cultured in vitro with a functional tight junction-permeability barrier. Sertoli cells were cultured at 0.5 × 106 cells/cm2 for 4.5-day on Matrigel-coated dishes to allow the establishment of a functional TJ-permeability barrier. Thereafter, cells were subjected to biotinylation at 4 °C as described in text, and protein endocytosis was monitored at specified time points at 10, 20, 60, and 90 min versus time 0 in the absence (control, Ctrl) or presence of IL-1α (100 pg/ml) at 35 °C. (A) Endocytosed proteins at specified time points were monitored by extracting biotinylated proteins in cell lysates by using avidin-based resin for SDS-PAGE and immunoblot analysis after stripping of biotins from uninternalized biotinylated proteins on cell surface and quenching of the stripped free biotins in media. Following treatment of Sertoli cells with IL-1α which is known to perturb the Sertoli cell TJ-barrier function (Lie et al., 2011), an enhancement in endocytosis of CAR and JAM-A was noted. (B) Data were plotted against time to illustrate an increase in the kinetics of protein endocytosis following IL-1α treatment. Each data point is a mean ± SD of 3 replicates of a typical experiment, and this experiment was repeated three times using different batches of Sertoli cells. **P <0.01.
3. MATERIALS
Ten 20-day-old male Sprague–Dawley rats (Charles River Laboratories)
6-Well Culture Plate (Corning, 3516) coated with BD Matrigel™ Basement Membrane Matrix (BD Biosciences, 354234) diluted at 1:7 with DME/F-12
10 ml Stripette Serological Pipets (Corning, 4488)
EZ-Link™ Sulfo-NHS-SS-Biotin (Thermo Scientific, 21331)
NeutrAvidin® UltraLink® Resin (Thermo Scientific, 53151)
Recombinant Rat IL-1α (R&D Systems, 500-RL-005), stored in 5 μg/ml aliquots in sterile PBS containing 0.1% BSA at −20 °C
Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham (DME/F-12) (Sigma-Aldrich, D2906) supplemented with 10 μg/ml insulin, 5 μg/ml human transferrin, 2.5 ng/ml EGF and 5 μg/ml bacitracin
Antibodies for immunoblotting analysis: rabbit anti-CAR (Santa Cruz Biotechnology, sc-15405, 1:200 dilution); rabbit anti-JAM-A (Life Technologies Corporation, 36-1700, 1:250 dilution)
4. BUFFERS
All chemicals listed below were obtained from Sigma-Aldrich unless otherwise noted.
Buffers should be made fresh each time and stored at 4 °C prior to use.
PBS: 10 mM NaH2PO4, 0.15 M NaCl, pH 7.4 at 22 °C
PBS/CM: 10 mM NaH2PO4, 0.15 M NaCl, 0.9 mM CaCl2, 0.33 mM MgCl2, pH 7.4 at 22 °C
Labeling buffer: 0.5 mg/ml EZ-Link™ Sulfo-NHS-SS-Biotin in PBS/CM
Quenching buffer 1: 50 mM NH4Cl in PBS/CM
Stripping buffer: 50 mM MESNA, 100 mM Tris–HCl, 100 mM NaCl, 2.5 mM CaCl2, pH 8.6 at 22 °C
Quenching buffer 2: 5 mg/ml iodoacetamide in PBS/CM
RIPA buffer: 50 mM Tris–HCl, 150 mM NaCl, 5 mM EGTA, 0.2% SDS (i.e., 0.2 g/100 ml), 1% Triton X-100 (v/v), 1% Na deoxycholate (i.e., 1 g/100 ml), 2 mM N-ethylmaleimide, pH 8.0 at 22 °C, cleared by filtration through a 0.2-μm filtering unit. Freshly add protease inhibitors (2 mM PMSF, 1 μg/ml aprotinin and leupeptin) as well as Phosphatase Inhibitor Cocktail 2 (P5726) and 3 (P0044) at 1:100 dilution prior to use.
5. METHODS
-
Isolate Sertoli cells from ten 20-day-old male Sprague–Dawley rats and plate them in six 6-well culture plates at a high cell density (0.5×106 cells/cm2) as earlier described (Mruk & Cheng, 2011). Considering the 9.4-cm2 growth area in each well of the plate, one can anticipate isolating ~144×106 cells (which is the routine yield of Sertoli cells from 10 male pups) to seed in at least 24 wells, with four in each of the six plates. Culture the cells in DME/F-12 for 4.5 days to allow the establishment of a functional permeability barrier as detailed earlier (Mruk & Cheng, 2011).
Note: Each time point of the endocytosis assay requires a separate 6-well culture plate so that cells to be terminated later will not be disturbed.
Before experiment starts, warm 50 ml DME/F-12 containing IL-1α at 100 pg/ml (Note: IL-1α is dissolved in 0.1% BSA-PBS at 5 μg/ml as a stock) and another 50 ml DME/F-12 without cytokine to serve as control to 37 °C, respectively.
Immediately before use, weigh 6 mg Sulfo-NHS-SS-Biotin and dissolve it in 1 ml Milli-Q water (Model Advantage A10, Millipore). Use 1:12 dilution in PBS/CM to get 0.5 mg/ml Sulfo-NHS-SS-Biotin as the labeling buffer.
-
Take cells out of the incubator and put on ice, wash the cells with ice-cold PBS/CM twice.
Note: From now on, all steps should be done at 4 °C or on ice (so that endocytosis ceases to take place) unless otherwise noted.
Add labeling buffer from step 3 to each well (1 ml/well) except the negative-control well (−ve Ctrl) in which cells are incubated with plain PBS/CM. Incubate cells at 4 °C for 30 min with gentle agitation.
After the incubation, wash cells with PBS/CM once, and remove the free/excess Sulfo-NHS-SS-Biotin with quenching buffer 1 at 4 °C for 15 min with gentle agitation.
Wash with PBS/CM once. Lyse one well of cells as “total” biotinylated surface proteins with 1 ml RIPA buffer, and also harvest the −ve Ctrl cells.
Put cells in 35 °C incubator (to initiate endocytosis) with 5 ml/well DME/F-12 [with IL-1α and without (control)]. Take cells out (one plate at a time) at specified time points (with 0, 10, 20, 60, 90 min respective duration in 35 °C incubator, and each time point should have at least duplicate culture well), and put back on ice to proceed with step 9.
Wash with PBS/CM twice. Incubate in stripping buffer at 4 °C for 30 min with gentle agitation to remove biotin from uninternalized biotinylated cell surface proteins.
Wash with PBS/CM once. Incubate in quenching buffer 2 at 4 °C for 15 min with gentle agitation to quench free biotins.
Wash with PBS/CM once. Lyse cells with RIPA buffer.
-
Sonicate cells, obtain cell lysates in clear supernate by centrifugation at 14,000×g for 10 min at 4 °C, and determine the protein concentration (routinely around 1 mg/ml).
Note: Cell lysates can be stored at −20 °C with or without sonication/centrifugation until use.
-
Add ~20 μl NeutrAvidin® UltraLink® Resin to ~300 μg protein of cell lysate (~300 μl) for each reaction in a 0.6-ml microcentrifuge tube. Mix on a rocking platform for 4–6 h at room temperature.
Note: For optimization, wash the resin with RIPA buffer three times (washing is done by resuspending resin in RIPA buffer, gently mix the sample in a microcentrifuge tube and collect resin by centrifugation at 3000 g, 1 min each - do not vortex resin to avoid damaging its physicochemical properties).
Centrifuge the tube for 1 min at 3000–5000×g to obtain the resin-bound complex (i.e., avidin-biotinylated protein complexes) and discard supernate.
Wash the resin-bound complexes with RIPA buffer four times. In between spin down for 1 min at 3000–5000×g and discard supernate.
Heat the resin-bound complex in SDS sample buffer [0.125 M Tris, pH 6.8 at 22 °C containing 1% SDS (w/v), 1.6% 2-mercaptoethanol (v/v), 20% glycerol (v/v)] so that biotins can be cleaved from the proteins. Following SDS-PAGE, proteins will be analyzed by immunoblotting using specific antibodies.
6. CELL STAINING TO ASSESS ENDOCYTOSIS
Isolate Sertoli cells from 20-day-old male Sprague–Dawley rats and plate them onto glass coverslips (Thomas Scientific, 6662F43) inserted in 12-Well Culture Plate (Corning, 3513) coated with BD Matrigel™ Basement Membrane Matrix at a low cell density (0.05×106 cells/cm2). Culture the cells in DME/F-12 for 4.5 days to allow the establishment of a functional permeability barrier.
Fix cells with methanol at −20 °C for 5 min.
Wash with PBS three times. Incubate with blocking solution (1% BSA in PBS) for 30 min at room temperature.
Remove blocking solution by vacuum aspiration. Add ~50 μl of rabbit anti-CAR or anti-JAM-A using 1:100 dilution in PBS. Incubate for 4–6 h at room temperature.
Wash with PBS three times. Add ~50 μl of Alexa Fluor® 555 goat anti-rabbit IgG (H+L) (Life Technologies Corporation, A-21429) using 1:200 dilution in PBS. Incubate for 1 h at room temperature.
Wash with PBS three times. Dry coverslips in dark. Mount onto microscope slides (Thomas Scientific, 6686S50) with ProLong® Gold antifade reagent with DAPI (Life Technologies Corporation, P36935).
7. RESULTS
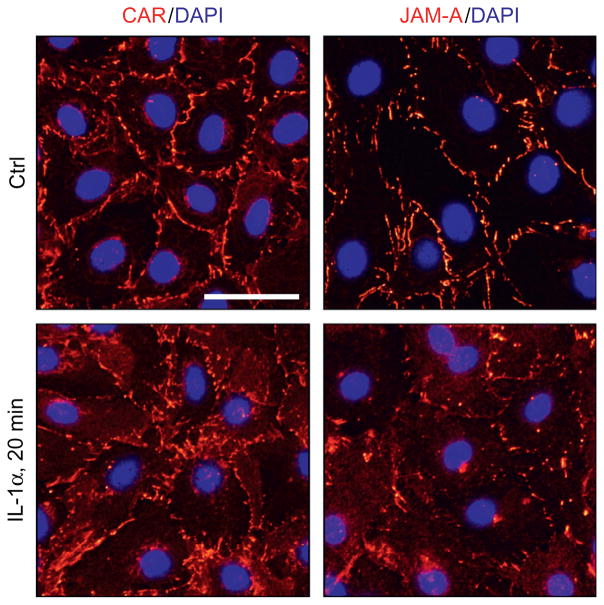
Figure 10.2A illustrates the results of a typical endocytosis assay in which exposure of Sertoli cells to IL-1α was found to enhance the kinetics of endocytosis of CAR (Fig. 10.2B, left panel) and JAM-A (Fig. 10.2B, right panel), which are integral membrane proteins at the Sertoli cell BTB, consistent with a recent report from our laboratory (Lie et al., 2011). These findings were confirmed by immunofluorescence microscopy as shown in Fig. 10.3, in which CAR and JAM-A were also found to become mis-localized following exposure of these cells to IL-1α wherein these proteins redistributed from the cell surface and moved into the cell cytosol, destabilizing the Sertoli cell tight junction barrier. Thus, these findings shown in Figs. 10.2 and 10.3 have unequivocally demonstrated that IL-1α perturbs Sertoli cell TJ-permeability barrier function via an increase in protein endocytosis, thereby disrupting the BTB function. Thus, a combination of the endocytosis assay and the fluorescence microscopy is a powerful technique to study endosomal signaling regulation in mammalian cells.
Figure 10.3.
A study by immunofluorescence microscopy to assess changes in protein distribution at the Sertoli cell cell–cell interface following treatment with IL-1α. Sertoli cells were cultured at 0.05 × 106 cells/cm2 for 4.5-day on Matrigel-coated coverslips which were placed in 12-well dishes with 2-ml DME/F-12 medium per well (supplemented with growth factors) to allow the establishment of a functional TJ-permeability that mimicked the Sertoli cell BTB in vivo as described (Xiao, Cheng, & Mruk, 2013; Xiao, Mruk, Lee, & Cheng, 2011). Thereafter, cells were treated without (control, Ctrl) or with IL-1α (100 pg/ml) for 20 min at 35 °C in a CO2 incubator. Sertoli cells were then harvested by fixing cells in methanol at −20 °C for 5 min and stained for either CAR or JAM-A (red fluorescence) as described in the text. Sertoli cell nuclei were visualized by DAPI (4′,6-diamidino-2-phenylindole) staining. It is noted that treatment of Sertoli cells accelerated the internalization of CAR and JAM-A, with these proteins redistributed from the cell surface into the cell cytosol, confirming data shown in Fig. 10.2. Scale bar, 60 μm, which applies to all other micrographs.
8. SUMMARY
This brief chapter has provided a detailed step-by-step protocol of studying protein endocytosis—an initial step in endosomal signaling function—in mammalian cells using Sertoli cells culture in vitro as a model. These studies are efficient, cost effective, and highly reproducible, and they can be performed in virtually any modern biochemistry and cell biology laboratory without expensive equipment and/or setup, applicable to all mammalian cells.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NICHD R01 HD056034 to C.Y.C.; U54 HD029990, Project 5 to C.Y.C.), National Science Foundation of China (NSFC 31371176 to X.X.), and The Hong Kong General Research Fund (GRF HKBU261812 to C.K.C.W.)
Footnotes
Disclosure: The authors have nothing to declare
References
- Bode F, Baumann K, Kinne R. Biochemical aspects of pinocytosis in kidney. Contributions to Nephrology. 1975;1:21–27. doi: 10.1159/000398225. [DOI] [PubMed] [Google Scholar]
- Bode F, Pockrandt-Hemstedt H, Baumann K, Kinne R. Analysis of the pinocytic process in rat kidney. I. Isolation of pinocytic vesicles from rat kidney cortex. Journal of Cell Biology. 1974;63:998–1008. doi: 10.1083/jcb.63.3.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nature Reviews Endocrinology. 2010;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Wong EWP, Lie PPY, Li MWM, Mruk DD, Yan HHN, et al. Regulation of blood-testis barrier dynamics by desmosome, gap junction, hemidesmosome and polarity proteins: An unexpected turn of events. Spermatogenesis. 2011;1:105–115. doi: 10.4161/spmg.1.2.15745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daukas G, Zigmond SH. Inhibtion of receptor-mediated but not fluid-phase endocytosis in polymorphonuclear keukocytes. Journal of Cell Biology. 1985;101:1673–1679. doi: 10.1083/jcb.101.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenberg CV, Loew LM. Computational analysis of Rho GTPase cycling. PLoS Computational Biology. 2013;9:e1002831. doi: 10.1371/journal.pcbi.1002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnord P, Blouin CM, Lamaze C. Membrane trafficking and signaling: Two sies of the same coin. Seminars in Cell & Developmental Biology. 2012;23:154–164. doi: 10.1016/j.semcdb.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Haglund K, Dikic I. The role of ubiquitylation in receptor endocytosis and endosomal sorting. Journal of Cell Science. 2012;125:265–275. doi: 10.1242/jcs.091280. [DOI] [PubMed] [Google Scholar]
- Henning R, Kaulen HD, Stoffel W. Biochemical analysis of the pinocytotic process. I. Isolation and chemical composition of the lysosomal and the plasma membrane of the rat liver cell. Hoppe-Seyler’s Zeitschrift für Physiologische Chemie. 1970;351:1191–1199. doi: 10.1515/bchm2.1970.351.2.1191. [DOI] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Clathrin- and non-clathrin-mediated endocytic regulation of cell signalling. Nature Reviews Molecular Cell Biology. 2005a;6:112–126. doi: 10.1038/nrm1571. [DOI] [PubMed] [Google Scholar]
- Le Roy C, Wrana JL. Signaling and endocytosis: A team effort for cell migration. Developmental Cell. 2005b;9:167–168. doi: 10.1016/j.devcel.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Le TL, Yap AS, Stow JL. Recycling of E-cadherin: A potential mechanism for regulating cadherin dynamics. Journal of Cell Biology. 1999;146:219–232. [PMC free article] [PubMed] [Google Scholar]
- Leto D, Saltiel AR. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nature Reviews Molecular Cell Biology. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- Li MWM, Mruk DD, Lee WM, Cheng CY. Disruption of the blood-testis barrier integrity by bisphenol A in vitro: Is this a suitable model for studying blood-testis barrier dynamics? International Journal of Biochemistry & Cell Biology. 2009;41:2302–2314. doi: 10.1016/j.biocel.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Cheng CY, Mruk DD. Interleukin-1α is a regulator of the blood-testis barrier. FASEB Journal. 2011;25:1244–1253. doi: 10.1096/fj.10-169995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loose LD, Megirian R, Turinsky J. Biochemical and functional alterations in macrophages after thermal injury. Infection and Immunity. 1984;44:554–558. doi: 10.1128/iai.44.3.554-558.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A, Trejo J. Ubiquitin-dependent regulation of G protein-coupled receptor trafficking and signaling. Cellular Signalling. 2013;25:707–716. doi: 10.1016/j.cellsig.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaczynska M, Bar-Sagi D. Signaling endosomes: Seeing is believing. Current Opinion in Cell Biology. 2010;22:535–540. doi: 10.1016/j.ceb.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, et al. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. Journal of Biological Chemistry. 2005;280:2220–2228. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. An in vitro system to study Sertoli cell blood-testis barrier dynamics. Methods in Molecular Biology. 2011;763:237–252. doi: 10.1007/978-1-61779-191-8_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto H, Collins LL, Gould GW. Vesicle trafficking and membrane remodelling in cytokinesis. Biochemical Journal. 2011;437:13–24. doi: 10.1042/BJ20110153. [DOI] [PubMed] [Google Scholar]
- Palfy M, Remenyi A, Korcsmaros T. Endosomal crosstalks: Meeting points for signaling pathways. Trends in Cell Biology. 2012;22:447–456. doi: 10.1016/j.tcb.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo S, Di Fiorce PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Quie PG. Disorders of phagocyte function: Biochemical aspects. Progress in Clinical and Biological Research. 1977;13:157–169. [PubMed] [Google Scholar]
- Sandilands E, Frame MC. Endosomal trafficking of Src tyrosine kinase. Trends in Cell Biology. 2008;18:322–329. doi: 10.1016/j.tcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Schiefermeier N, Teis D, Huber LA. Endosomal signaling and cell migration. Current Opinion in Cell Biology. 2011;23:615–620. doi: 10.1016/j.ceb.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ER, Wong EWP, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin-focal adhesion kinase protein complex at the blood-testis barrier: A study using the cadmium model. Endocrinology. 2009;150:3336–3344. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su WH, Mruk DD, Cheng CY. Regulation of actin dynamics and protein trafficking during spermatogenesis—insights into a complex process. Critical Reviews in Biochemistry and Molecular Biology. 2013;48:153–172. doi: 10.3109/10409238.2012.758084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L, Mruk DD, Lee WM, Cheng CY. Differential effects of testosterone and TGF-β3 on endocytic vesicle-mediated protein trafficking events at the blood-testis barrier. Experimental Cell Research. 2010;316:2945–2960. doi: 10.1016/j.yexcr.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley HS, Herbst JJ, Walsh BJ, Lauffenburger DA, Rosenfeld MG, Gill GN. The role of tyrosin kinase activity in endocytosis, compartmentation, and down-regulation of the epidermal growth factor receptor. Journal of Biological Chemistry. 1991;266:11083–11094. [PubMed] [Google Scholar]
- Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. International Review of Cell and Molecular Biology. 2009;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Lee WM, Cheng CY. Par3/Par6 polarity complex coordinates apical ectoplasmic specialization and blood-testis barrier restructuring during spermatogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:9657–9662. doi: 10.1073/pnas.0801527105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Lee WM, Cheng CY. Regulation of blood-testis barrier dynamics by TGF-β3 is a Cdc42-dependent protein trafficking event. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11399–11404. doi: 10.1073/pnas.1001077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–4723. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Cheng CY, Mruk DD. Intercellular adhesion molecule (ICAM)-1 is a regulator of blood-testis barrier function. Journal of Cell Science. 2013;125:5677–5689. doi: 10.1242/jcs.107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. International Journal of Biochemistry & Cell Biology. 2011;43:651–665. doi: 10.1016/j.biocel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB Journal. 2008;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]