Abstract
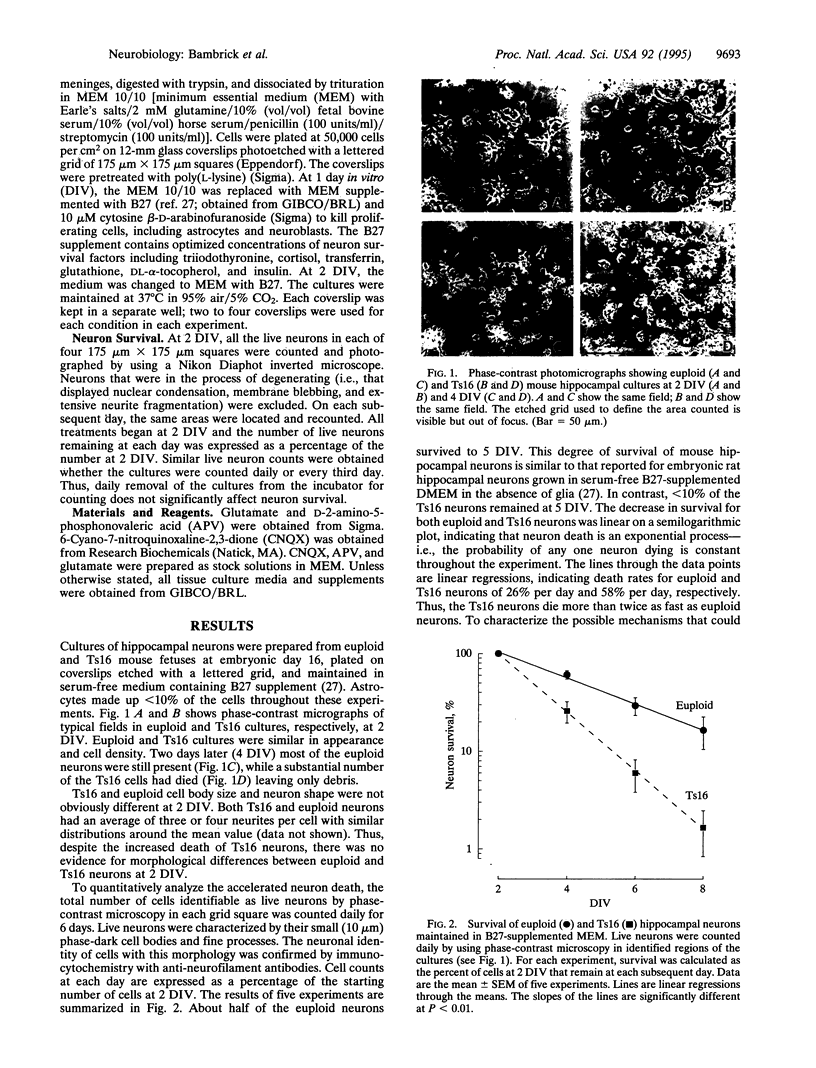
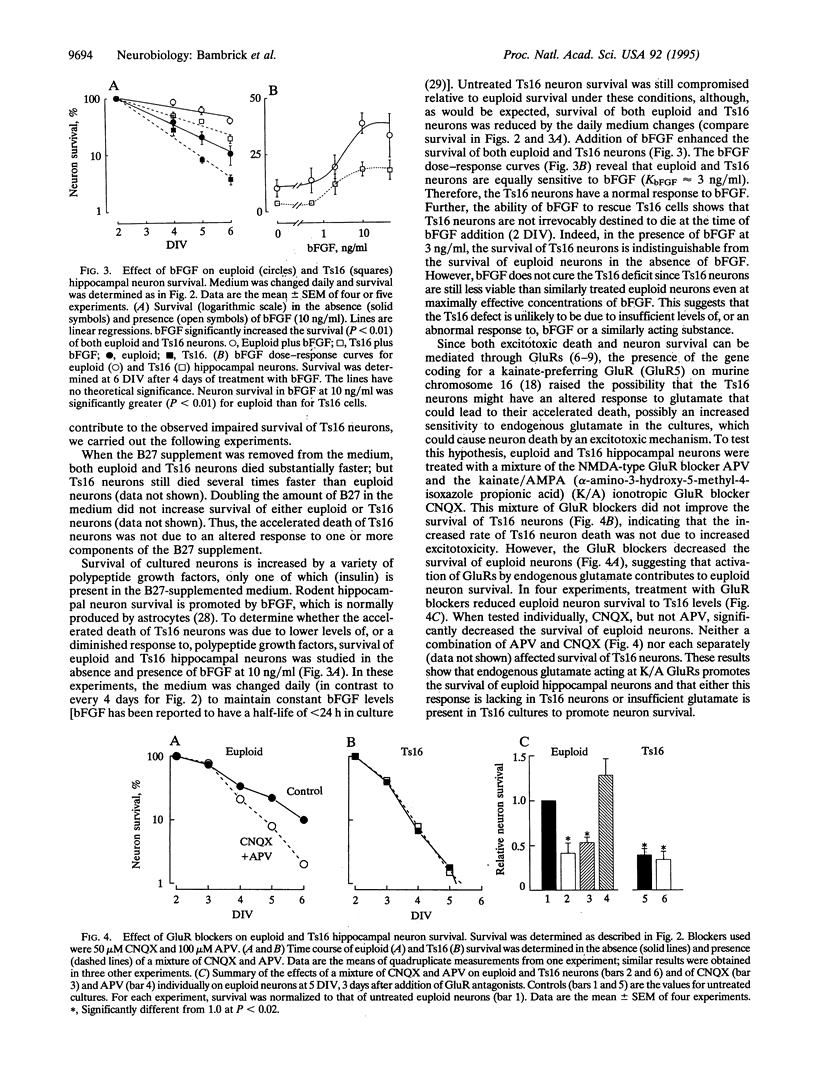
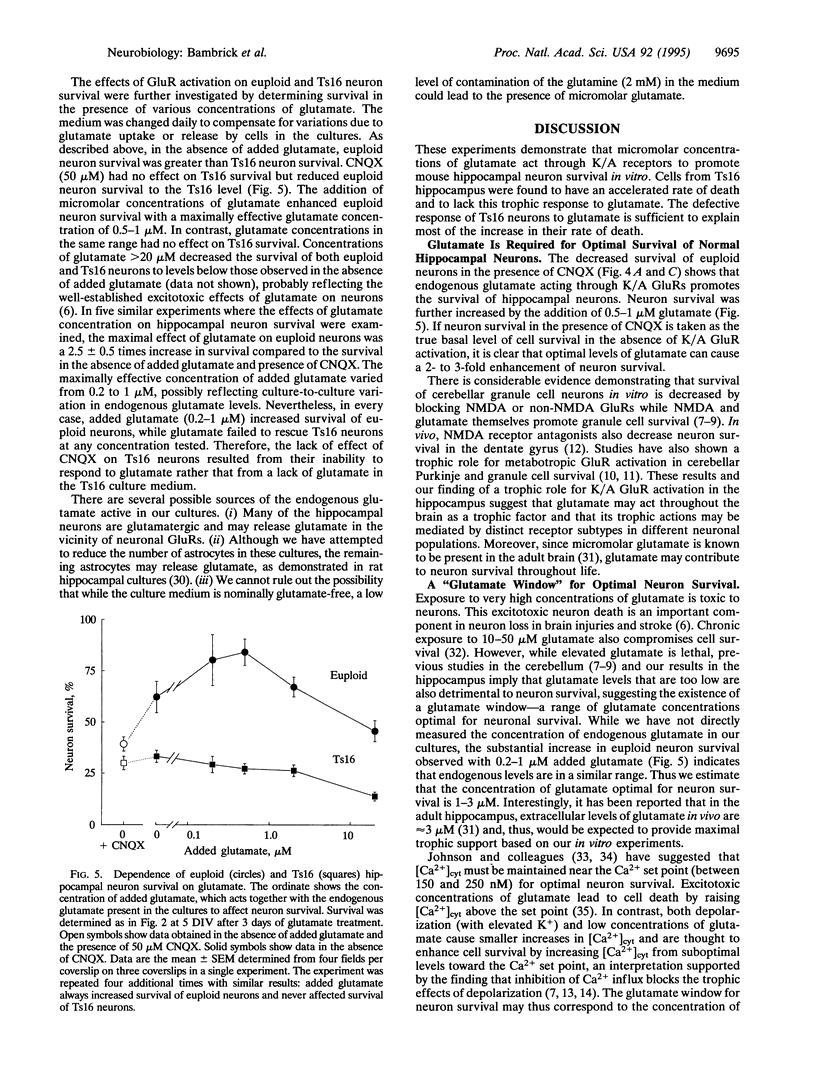
The survival of cultured mouse hippocampal neurons was found to be greatly enhanced by micromolar concentrations of the excitatory neurotransmitter glutamate. Blockade of kainate/AMPA (alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) glutamate receptors increased the rate of neuron death, suggesting that endogenous glutamate in the cultures promotes survival. Addition of glutamate (0.5-1 microM) further increased neuron survival, whereas glutamate in excess of 20 microM resulted in increased death. Thus, the survival vs. glutamate dose-response relation is bell-shaped with an optimal glutamate concentration near 1 microM. We found that hippocampal neurons from mice with the genetic defect trisomy 16 (Ts16) died 2-3 times faster than normal (euploid) neurons. Moreover, glutamate, at all concentrations tested, failed to increase survival of Ts16 neurons. In contrast, the neurotrophic polypeptide basic fibroblast growth factor did increase the survival of Ts16 and euploid neurons. Ts16 is a naturally occurring mouse genetic abnormality, the human analog of which (Down syndrome) leads to altered brain development and Alzheimer disease. These results demonstrate that the Ts16 genotype confers a defect in the glutamate-mediated survival response of hippocampal neurons and that this defect can contribute to their accelerated death.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balázs R., Jørgensen O. S., Hack N. N-methyl-D-aspartate promotes the survival of cerebellar granule cells in culture. Neuroscience. 1988 Nov;27(2):437–451. doi: 10.1016/0306-4522(88)90279-5. [DOI] [PubMed] [Google Scholar]
- Brewer G. J., Torricelli J. R., Evege E. K., Price P. J. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993 Aug 1;35(5):567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Graham M. E., Cambray-Deakin M. Neurotrophic effects of NMDA receptor activation on developing cerebellar granule cells. J Neurocytol. 1993 Sep;22(9):689–695. doi: 10.1007/BF01181314. [DOI] [PubMed] [Google Scholar]
- Choi D. W. Excitotoxic cell death. J Neurobiol. 1992 Nov;23(9):1261–1276. doi: 10.1002/neu.480230915. [DOI] [PubMed] [Google Scholar]
- Choi D. W., Maulucci-Gedde M., Kriegstein A. R. Glutamate neurotoxicity in cortical cell culture. J Neurosci. 1987 Feb;7(2):357–368. doi: 10.1523/JNEUROSCI.07-02-00357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F., Schmidt M. F., Guthrie P. B., Kater S. B. Sustained increase in intracellular calcium promotes neuronal survival. J Neurosci. 1991 Aug;11(8):2582–2587. doi: 10.1523/JNEUROSCI.11-08-02582.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copani A., Bruno V. M., Barresi V., Battaglia G., Condorelli D. F., Nicoletti F. Activation of metabotropic glutamate receptors prevents neuronal apoptosis in culture. J Neurochem. 1995 Jan;64(1):101–108. doi: 10.1046/j.1471-4159.1995.64010101.x. [DOI] [PubMed] [Google Scholar]
- Corsi P., Coyle J. T. Nerve growth factor corrects developmental impairments of basal forebrain cholinergic neurons in the trisomy 16 mouse. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1793–1797. doi: 10.1073/pnas.88.5.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle J. T., Oster-Granite M. L., Reeves R. H., Gearhart J. D. Down syndrome, Alzheimer's disease and the trisomy 16 mouse. Trends Neurosci. 1988 Sep;11(9):390–394. doi: 10.1016/0166-2236(88)90075-6. [DOI] [PubMed] [Google Scholar]
- Eide F. F., Lowenstein D. H., Reichardt L. F. Neurotrophins and their receptors--current concepts and implications for neurologic disease. Exp Neurol. 1993 Jun;121(2):200–214. doi: 10.1006/exnr.1993.1087. [DOI] [PubMed] [Google Scholar]
- Franklin J. L., Johnson E. M., Jr Suppression of programmed neuronal death by sustained elevation of cytoplasmic calcium. Trends Neurosci. 1992 Dec;15(12):501–508. doi: 10.1016/0166-2236(92)90103-f. [DOI] [PubMed] [Google Scholar]
- Gallo V., Kingsbury A., Balázs R., Jørgensen O. S. The role of depolarization in the survival and differentiation of cerebellar granule cells in culture. J Neurosci. 1987 Jul;7(7):2203–2213. doi: 10.1523/JNEUROSCI.07-07-02203.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart J. D., Oster-Granite M. L., Reeves R. H., Coyle J. T. Developmental consequences of autosomal aneuploidy in mammals. Dev Genet. 1987;8(4):249–265. doi: 10.1002/dvg.1020080408. [DOI] [PubMed] [Google Scholar]
- Gould E., Cameron H. A., McEwen B. S. Blockade of NMDA receptors increases cell death and birth in the developing rat dentate gyrus. J Comp Neurol. 1994 Feb 22;340(4):551–565. doi: 10.1002/cne.903400408. [DOI] [PubMed] [Google Scholar]
- Gregor P., Reeves R. H., Jabs E. W., Yang X., Dackowski W., Rochelle J. M., Brown R. H., Jr, Haines J. L., O'Hara B. F., Uhl G. R. Chromosomal localization of glutamate receptor genes: relationship to familial amyotrophic lateral sclerosis and other neurological disorders of mice and humans. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):3053–3057. doi: 10.1073/pnas.90.7.3053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman D. M., Li Y. W., DeArmond S. J., McKinley M. P., Gage F. H., Epstein C. J., Mobley W. C. Mouse model of neurodegeneration: atrophy of basal forebrain cholinergic neurons in trisomy 16 transplants. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1383–1387. doi: 10.1073/pnas.89.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humpel C., Hoffer B., Strömberg I., Bektesh S., Collins F., Olson L. Neurons of the hippocampal formation express glial cell line-derived neurotrophic factor messenger RNA in response to kainate-induced excitation. Neuroscience. 1994 Apr;59(4):791–795. doi: 10.1016/0306-4522(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Jr, Koike T., Franklin J. A "calcium set-point hypothesis" of neuronal dependence on neurotrophic factor. Exp Neurol. 1992 Jan;115(1):163–166. doi: 10.1016/0014-4886(92)90242-i. [DOI] [PubMed] [Google Scholar]
- Kiss J., Schlumpf M., Balázs R. Selective retardation of the development of the basal forebrain cholinergic and pontine catecholaminergic nuclei in the brain of trisomy 16 mouse, an animal model of Down's syndrome. Brain Res Dev Brain Res. 1989 Dec 1;50(2):251–264. doi: 10.1016/0165-3806(89)90201-0. [DOI] [PubMed] [Google Scholar]
- Lehmann A., Isacsson H., Hamberger A. Effects of in vivo administration of kainic acid on the extracellular amino acid pool in the rabbit hippocampus. J Neurochem. 1983 May;40(5):1314–1320. doi: 10.1111/j.1471-4159.1983.tb13572.x. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Wiegand S. J., Altar C. A., DiStefano P. S. Neurotrophic factors: from molecule to man. Trends Neurosci. 1994 May;17(5):182–190. doi: 10.1016/0166-2236(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Mattson M. P., Rychlik B. Glia protect hippocampal neurons against excitatory amino acid-induced degeneration: involvement of fibroblast growth factor. Int J Dev Neurosci. 1990;8(4):399–415. doi: 10.1016/0736-5748(90)90073-b. [DOI] [PubMed] [Google Scholar]
- McKinnon R. D., Matsui T., Dubois-Dalcq M., Aaronson S. A. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990 Nov;5(5):603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- Mount H. T., Dreyfus C. F., Black I. B. Purkinje cell survival is differentially regulated by metabotropic and ionotropic excitatory amino acid receptors. J Neurosci. 1993 Jul;13(7):3173–3179. doi: 10.1523/JNEUROSCI.13-07-03173.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V., Basarsky T. A., Liu F., Jeftinija K., Jeftinija S., Haydon P. G. Glutamate-mediated astrocyte-neuron signalling. Nature. 1994 Jun 30;369(6483):744–747. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- Potter H. Review and hypothesis: Alzheimer disease and Down syndrome--chromosome 21 nondisjunction may underlie both disorders. Am J Hum Genet. 1991 Jun;48(6):1192–1200. [PMC free article] [PubMed] [Google Scholar]
- Raff M. C., Barres B. A., Burne J. F., Coles H. S., Ishizaki Y., Jacobson M. D. Programmed cell death and the control of cell survival: lessons from the nervous system. Science. 1993 Oct 29;262(5134):695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Rothstein J. D., Jin L., Dykes-Hoberg M., Kuncl R. W. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer H. S., Tiemeyer M., Hedreen J. C., Gearhart J., Coyle J. T. Morphologic and neurochemical studies of embryonic brain development in murine trisomy 16. Brain Res. 1984 Aug;317(2):155–166. doi: 10.1016/0165-3806(84)90093-2. [DOI] [PubMed] [Google Scholar]
- Sugiyama K., Brunori A., Mayer M. L. Glial uptake of excitatory amino acids influences neuronal survival in cultures of mouse hippocampus. Neuroscience. 1989;32(3):779–791. doi: 10.1016/0306-4522(89)90298-4. [DOI] [PubMed] [Google Scholar]
- Sweeney J. E., Höhmann C. F., Oster-Granite M. L., Coyle J. T. Neurogenesis of the basal forebrain in euploid and trisomy 16 mice: an animal model for developmental disorders in Down syndrome. Neuroscience. 1989;31(2):413–425. doi: 10.1016/0306-4522(89)90384-9. [DOI] [PubMed] [Google Scholar]
- Unsicker K., Reichert-Preibsch H., Schmidt R., Pettmann B., Labourdette G., Sensenbrenner M. Astroglial and fibroblast growth factors have neurotrophic functions for cultured peripheral and central nervous system neurons. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5459–5463. doi: 10.1073/pnas.84.15.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan G. M., Ni B., Weller M., Wood K. A., Paul S. M. Depolarization or glutamate receptor activation blocks apoptotic cell death of cultured cerebellar granule neurons. Brain Res. 1994 Sep 5;656(1):43–51. doi: 10.1016/0006-8993(94)91364-1. [DOI] [PubMed] [Google Scholar]




