Abstract
The identification and characterization of the claudin family of tight junction (TJ) proteins in the late 1990s ushered in a new era for research into the molecular and cellular biology of intercellular junctions. Since that time, TJs have been studied in the contexts of many diseases including deafness, male infertility, cancer, bacterial invasion and liver and kidney disorders. In this review, we consider the role of claudins in the nervous system focusing on the mechanisms by which TJs in glial cells are involved in neuronal function. Electrophysiological evidence suggests that claudins may operate in the central nervous system (CNS) in a manner similar to polarized epithelia. We also evaluate hypotheses that TJs are the gatekeepers of an immune-privileged myelin compartment and that TJs emerged during evolution to form major adhesive forces within the myelin sheath. Finally, we consider the implications of CNS myelin TJs in the contexts of behavioral disorders (schizophrenia) and demyelinating/hypomyelinating diseases (multiple sclerosis and the leukodystrophies), and explore evidence of a possible mechanism governing affective disorder symptoms in patients with white matter abnormalities.
Keywords: radial component, transverse bands, axoglial, proteolipid protein, oligodendrocyte specific protein, experimental allergic encephalomyelitis, saltatory conduction
Introduction
Tight junctions (TJs) are evolutionarily-conserved structural and physiological components of polarized epithelia that are essential for life [reviewed in 1, 2]. The function and molecular composition of TJs have been unravelled over the last few decades and found to be comprised of intramembranous strands of protein polymers that form a dynamic meshwork at the apical edges of polarized epithelial cells. Tight junctions play multiple roles in epithelia, including: the generation of paracellular permeability barriers across epithelial sheets to regulate the flux of macromolecules into or out of the organism; the generation of macromolecular diffusion barriers within the plane of membranes to maintain cell polarity between the apical and basolateral domains and; the formation of ion selective paracellular pores to maintain electrochemical gradients and to regulate the composition of the epithelial microenvironment.
Genetic and biochemical studies show that the claudin family are principal mediators of the diverse morphological and physiological properties of TJs in higher eukaryotes [reviewed in 3], and many of these proteins are expressed in various cell types throughout the nervous system. In addition to their importance in elaborating and maintaining blood-brain-barriers in the choroid plexus and vasculature, TJs also play crucial roles in motor and sensory systems. Tight junction proteins are rarely expressed by neurons, but one exception is olfactory neurons. The dendrites of these cells traverse the paracellular space of the nasal epithelium and localize claudins to intercalate with and maintain the epithelial barrier while carrying out their function to detecting odors [4]. The utilization of TJs in this manner is somewhat analogous to intestinal dendritic cells, which extend claudin-bearing projections between intestinal epithelial cells so as not to disrupt the TJ barrier while sampling intestinal flora [5].
Tight junctions have been involved in nervous system function in early metazoan evolution with demonstrated importance at the blood-nerve barrier in Drosophila [6]. In the mammalian CNS, TJs are key structural components of oligodendrocytes and support neuron function by increasing conduction velocity along small myelinated fibers [7, 8]. Tight junctions are also present in PNS myelin elaborated by Schwann cells but likely perform different functions to TJs in CNS myelin [7, 8]. Of major focus in the current review, the CNS phenotype in Claudin 11-null mice demonstrates the importance of claudin 11 in myelin to maximizing nerve conduction.
The necessity for reliable conduction to plasticity and temporal processing in neural circuits implicates claudin 11 TJs broadly in brain function, but also in behavioral disorders such as schizophrenia and other affective disorders associated with myelin abnormalities. A likely theme to emerge in the coming years will be the importance of TJs to neuron physiology because they form resistive barriers that maintain the composition of the extracellular microenvironment in the nervous system. This is consistent with the extensively characterized function of TJs in polarized epithelia in peripheral tissues.
Function of TJs in the invertebrate nervous system
The claudin family of TJ proteins stems from 20 – 30 genes in mammals [reviewed in 1], with most of the expansion of this family occurring relatively recently during the evolution of marine and terrestrial vertebrates [9]. The genome of the invertebrate model organism Drosophila harbors six claudin family members expressed in different polarized epithelia, but two of these, megatrachea and sinuous, are expressed by peripheral nerve glial cells. These cells form several concentric layers around the nervous system, and generate an extracellular diffusion barrier (the blood-nerve barrier) that isolates neurons from the potassium-rich hemolymph bathing all organs in Drosophila.
Pleated septate junctions (pSJs) mediate the Drosophila blood-nerve barrier and are comprised of several adhesion molecule complexes which include megatrachea and sinuous . The major adhesion molecules forming pSJs are neurexin IV, contactin and neuroglian, which are conserved in mammalian axoglial junctions (Fig. 1A) [reviewed in 10]. However, orthologs of megatrachea and sinuous are not present in axoglial junctions, suggesting that structural and functional segregation of junctional elements has occurred during evolution [11, 12].
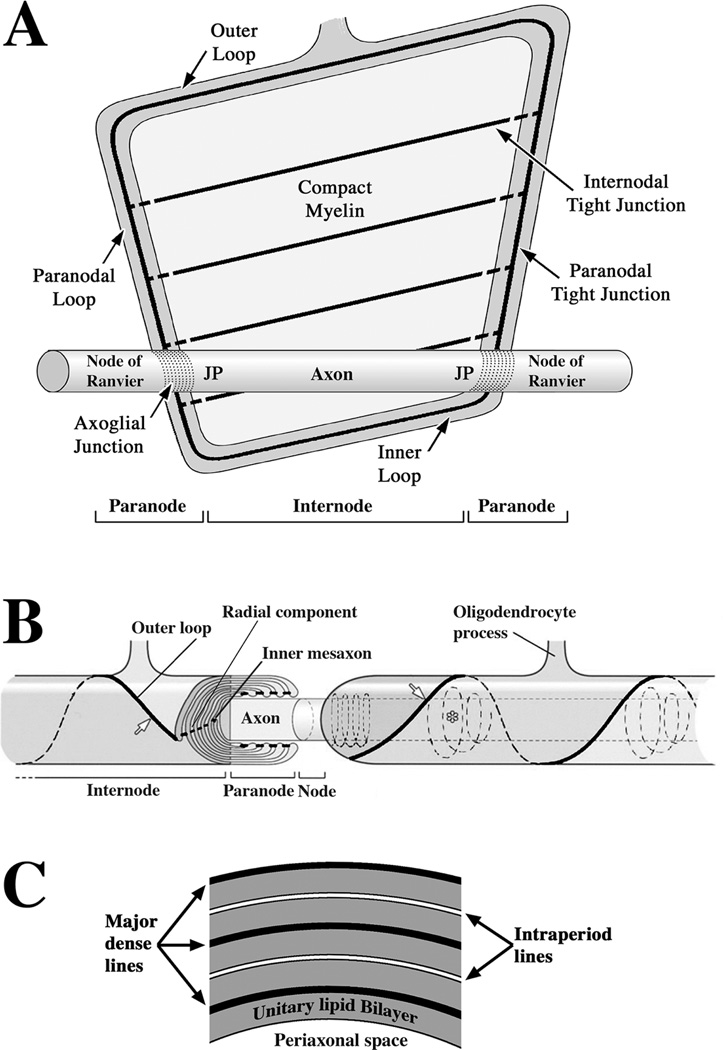
Figure 1. Organization of claudin 11 TJs in CNS myelinated fibers.
A: A CNS myelin sheath is unravelled to reveal the structure and organization of the multilayered myelin membrane that spirals around the axon. Myelin is a flat membrane envelope initially filled with cytoplasm on the inside which is extruded to the cell body as the sheath matures. Most of the membrane sheet compacts and the cytoplasmic membrane surface are closely apposed. A continuous channel of cytoplasm persists around the perimeter of the membrane. Autotypic TJs are also located around the perimeter and seal the edges of the membrane to block diffusion of macromolecules and increase the electrical resistance of the membrane. Veins of TJs run the length of the myelin internode through the compact region, which overlie each other on successive membrane layers in a radial alignment (the radial component). Membrane domains on the axon are also shown: nodes of Ranvier, which are short segments of bare axon between successive myelin sheaths in which sodium channels are clustered; axoglial junctions, which adheres the paranodal myelin to the axon in a continuous spiral; juxtaparanodal regions (JP) where potassium channels are clustered and the remaining axon internode (Axon).
B: Portions of two myelin sheaths are shown in their native conformation spirally wrapped around an axon and separated by a node of Ranvier (Node). To the left, the myelin sheath is cut to show the membrane spiral and the radial component. Claudin 11 TJs occlude the extracellular space at the inner mesaxon and outer loop (arrow), and between successive paranodal membrane layers (paranodal loops). In large myelinated fibers, Claudin 11 TJs also localize to spiral cytoplasmic channels (Schmidt-Lantermann incisures, not shown) within the compact region (asterisk). Reprinted from [77].
C: Schematic of a transverse section of compact myelin to show the major morphological features visible under the electron microscope. The major dense line and intraperiod line appear in myelin as alternating dark and light lines, although they actually spiral around the axon. The unitary lipid bilayer would comprise the envelope that is described in the legend for Fig. 1A.
Stork and colleagues [6] have demonstrated that loss-of-function alleles of either megatrachea or sinuous results in disorganization of pSJs and infiltration of large molecular weight fluorescent dextrans into Drosophila nerves. Thus, these claudins are clearly involved in blood-nerve barrier function which likely serves to sequester axons from hemolymph that would abolish action potentials and paralyze the animals. However, the loss of any of the adhesive proteins causes dissolution of the pSJs, so it remains unclear if the claudins or the neurexin IV/contactin/neuroglian adhesion complex underlie the blood-nerve diffusion barrier in Drosophila. In contrast, the mammalian orthologs of this invertebrate adhesion complex do form a diffusion barrier at paranodal axoglial junctions in absence of claudins.
Another important aspect of blood-nerve barrier biology in Drosophila remains unresolved and clouds the assignment of specific functions to megatrachea and sinuous. Canonical ultrastructural features of vertebrate TJs, including membrane kissing points and intramembranous TJ strands, have not been observed in pSJs using transmission or freeze-fracture electron microscopy. The absence of such direct evidence may stem from the difficulty of preserving sufficient ultrastructural detail in pSJs, which are dominated by dense arrays of septa between glial cells. Alternatively, drosophila claudins may only perform supporting roles for the adhesive molecules in septa, which would suggest that they do not form TJs and are not functionally equivalent to vertebrate claudins.
Function of TJs in CNS myelin
Myelin sheath development and structure
The mechanisms underlying myelin formation require complex sequential interactions between neurons and oligodendrocytes beginning with contact and recognition of axons by oligodendrocyte processes. Each process adheres to the axon and expands to form a large flat membrane sheet that wraps around the axon multiple times and elongates along the axon for several hundred micrometers to form a membrane tube (Fig. 1). Initially, the glial membrane is filled with cytoplasm, which is extruded as the membrane compacts around the axon and forms the characteristic stacked membrane spiral of the myelin sheath (Fig. 1B). Sodium channels are assembled in high density clusters in short segments of axonal membrane, called nodes of Ranvier, between adjacent myelin sheaths to enable rapid saltatory conduction.
Dominant ultrastructural features of mature myelin sheaths that are visible under the electron microscope include the alternating major dense line (MDL) and intraperiod line (IPL). The MDL arises from the close apposition of cytoplasmic surfaces of the membrane and deposition of structural proteins to stabilize and possibly fuse the membrane surfaces. The IPL arises from the close apposition of extracellular surfaces of the membrane. Unlike the MDL, the membrane surfaces at the IPL remain separated by a distance that is likely determined by the extracellular domains of transmembrane proteins acting as spacers [13, 14]. The distance between successive layers of the MDL or IPL, known as the myelin period, is constant at approximately 16 nm in the CNS [reviewed in 15].
Major structural proteins in CNS myelin
Three proteins - myelin basic protein (MBP), proteolipid protein 1 (PLP1) and claudin 11 - comprise as much as 90% of the total protein content of CNS myelin. Importantly, the distributions of these proteins in myelin are distinct. MBP is a highly-charged cytoplasmic extrinsic membrane protein that is localized to the MDL and stabilizes membrane apposition after compaction [16]. PLP1 is an intrinsic membrane protein with four transmembrane domains. Its amino- and carboxyl-termini are exposed to the membrane cytoplasmic surface and may interact with MBP [17, 18]. Two domains of PLP1 protrude from the extracellular membrane surface and may form homomeric interactions in trans with PLP1 from juxtaposed lamellae to set the spacing of the IPL. Claudin 11 is also an integral membrane protein with a topology likely resembling PLP1. Unlike MBP and PLP1, which are evenly distributed throughout myelin sheaths, claudin 11 is confined to narrow proteinaceous veins which may only be a few molecules wide and extend the length of the sheath. These veins are reminiscent of a structural feature of CNS myelin, the radial component, which occupies a small fraction of the cross-sectional area of myelin.
TJs form the radial component in CNS myelin
In pioneering observations, Peters [19, 20] identified the radial component in CNS myelin from ferricyanide- and permanganate-postfixed white matter tracts. The radial component appears as regularly spaced rod-like thickenings of the IPL in transmission electron micrographs and was hypothesized to comprise autotypic TJs (Fig. 2A). These morphological findings were confirmed by other groups [21, 22]. Early experiments indicated that the radial component conferred limited permeability of macromolecules and metal ions such as lanthanum into the myelin [22, 23], which is consistent with the role that TJs play in polarized epithelia. Further attesting to its importance in myelin, the radial component is evolutionarily conserved in terrestrial vertebrates, amphibians and fish [22, 24]. Final evidence of the correspondence between the radial component and TJs (Fig. 2B–F) was provided by immunofluorescence labeling of white matter with anti-claudin 11 antibodies and ablation of the Claudin 11 gene by homologous recombination in mice [25, 26]. Importantly, the absence of claudin 11 in myelin is apparently without consequence to myelin ultrastructure other than the loss of the radial component [8, 25], indicating that claudin 11 is not crucial for myelin compaction.
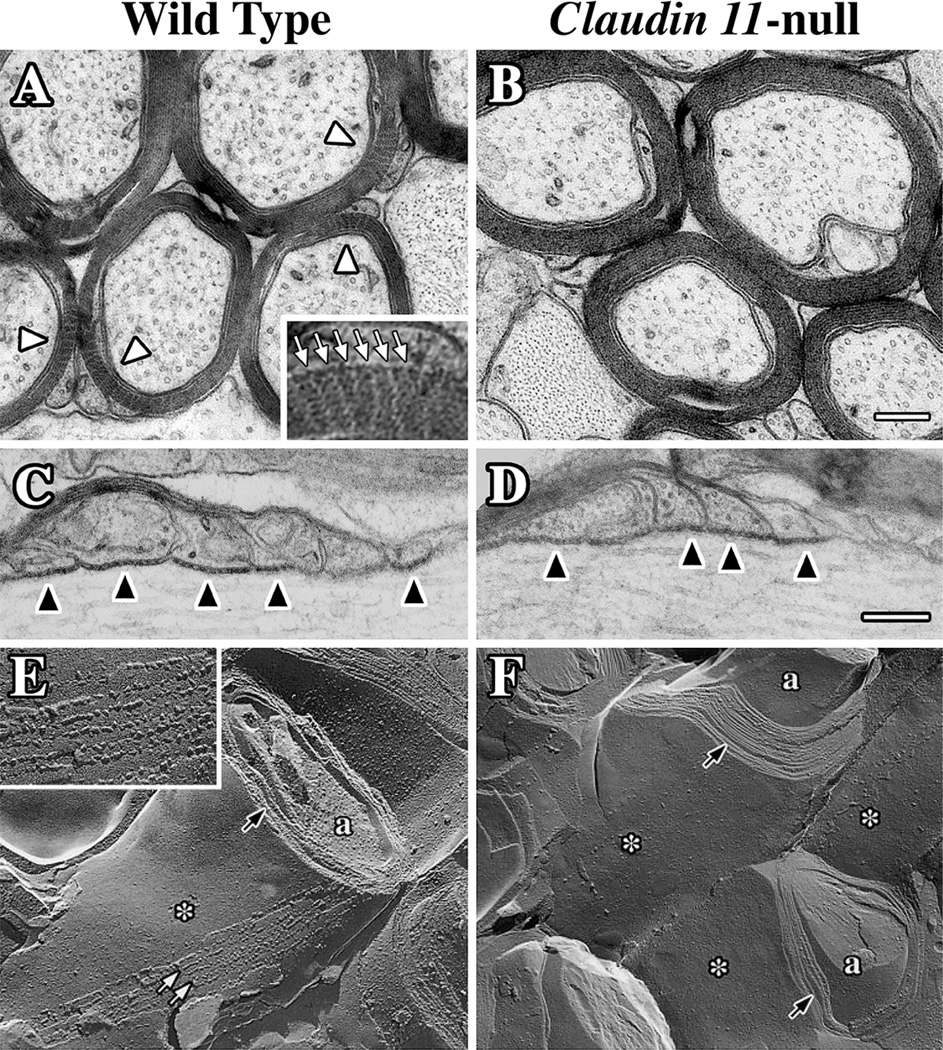
Figure 2. Tight junctions are absent in CNS myelin from Claudin 11-null mice.
A-D: Electron micrographs of transverse (A and B) and longitudinal (C and D) sections of optic nerve myelinated fibers from adult wild type (A and C) and Claudin 11-null (B and D) mice. The optic nerves were postfixed with ferricyanide [19] to reveal the radial components (white arrowheads) in the myelin. Radial components appear as electron-lucent radial lines through compact myelin in wild type mice (inset in A, white arrows), but are absent from Claudin 11-null mice. Overall myelin structure in the internode (A and B) and at paranodes (C and D) are unchanged by the absence of TJs, and axoglial junctions (also called transverse bands, black arrowheads in C and D) are morphologically normal. Scale bars = 0.2 µm. E-F: Freeze fracture replicas of CNS myelinated fibers from adult wild type (E) and Claudin 11-null (F) mice.
Intramembranous strands (white arrows in E) run the length of the internode (see Figure 1) and form the radial component within compact myelin (black arrow). The membranes are otherwise relatively smooth (asterisk). At high magnification (inset in E), the strands appear as discrete particles which represent monomers or multimeric complexes of claudin 11 in linear arrays. The strands are absent in Claudin 11-null mice (F) but the myelin membranes are compacted (black arrows in F) and otherwise morphologically normal. © Devaux and Gow, 2008. Originally published in [8, 25].
TJs contribute to myelin membrane resistance
As a starting point for analyzing the function of claudin 11 TJs in myelin, it is reasonable to draw parallels with TJs in polarized epithelia, even though myelin TJs are autotypic rather than homotypic or heterotypic, and even though many of the cytoplasmic plaque proteins associated with most TJs (such as occludin and the MAGUK family of zonula occludens proteins, ZO-1 to 3) are absent from myelin [25]. Thus, myelin TJs may play occluding and resistive roles by sealing the edges of the membrane along the length of the sheath and at paranodes (Fig. 1B). In addition, they may maintain asymmetrical distributions of membrane proteins and lipids in paranodal regions of myelin that contact the axon and control extracellular fluid composition at the IPL, particularly when other structural myelin proteins are altered.
The absence of claudin 11 confers several phenotypes in mice, including tremors, gait abnormalities and motor coordination deficits (Fig. 3). Examination of the electrophysiological properties of CNS myelinated tracts in optic nerve and spinal cord of Claudin 11-null mice [8] reveal significant reductions in conduction and the excitability threshold of axons. These changes are more pronounced for small axons in optic nerve (those below 1 µm in diameter), which are normally ensheathed by relatively thin myelin sheaths. In contrast, large axons such as those in the ventral spinal cord, with correspondingly thicker myelin sheaths, are unaffected or only mildly affected by the absence of TJs. These data suggest that TJs contribute to the insulation of axons by significantly increasing the membrane resistance of thin myelin segments.
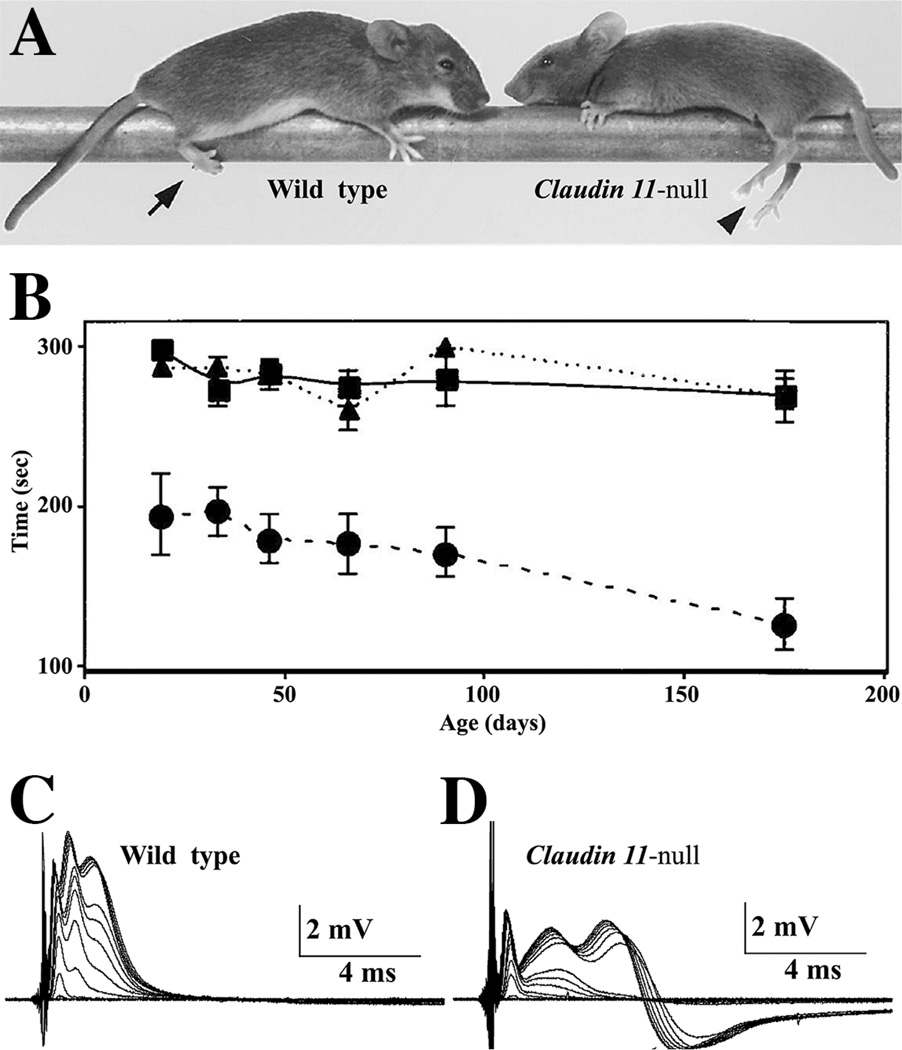
Fig. 3. Neurological and central conduction deficits in Claudin 11-null mice.
A: Hind limb weakness is a life-long phenotype of Claudin 11-null mice which does not enable these animals to grasp objects (arrowhead). Wild type mice are able to perform this task (arrow). B: The motor skills of wild type (squares) and heterozygous (triangles) mice from weaning to adulthood are constant and indistinguishable. However, Claudin 11-null mice (circles) perform this task poorly at all ages and are progressively impaired. Error bars, ± SEM. Currently, it is unclear if this performance stems from central or peripheral deficits, because Claudin 11 is expressed by oligodendrocytes and epaxial muscles during development [25]. C-D: Compound APs (CAPs) elicited in the optic nerves of adult wild type and Claudin 11-null mice by voltage pulses of increasing amplitude. Compound APs show three components in the optic nerve, reflecting fiber populations with different conduction velocities and diameters. The latencies of the second and third CAP components are substantially increased in Claudin 11-null mice, which indicates that conduction velocity is decreased. In addition, fibers contributing to the second and third components require higher stimulation intensities for recruitment, indicating that the threshold of nodal excitability in these fibers is abnormal. In addition, CAPs from Claudin 11-null mice are followed by hyperpolarizing afterpotentials (D) that are not observed in wild type nerves (C). Afterpotentials reflect the activation of internodal Kv1.1/Kv1.2 channels in the absence of myelin TJs; thus, internodal depolarization. © Devaux and Gow, 2008. Originally published in [8, 25].
Computer simulations of myelin TJs corroborate these conclusions (Fig. 4D) and reveal possible mechanisms by which myelin resistance and axon excitability threshold are altered [7]. Analogous to polarized epithelia, TJs diminish the paracellular current pathway between membrane layers in the myelin sheath, and thereby decrease its capacitive charge during action potential (AP) propagation. Thus, TJs diminish the time to charge the myelin capacitance, which favors the rapid regeneration and propagation of APs between nodes of Ranvier (Fig. 5A and B).
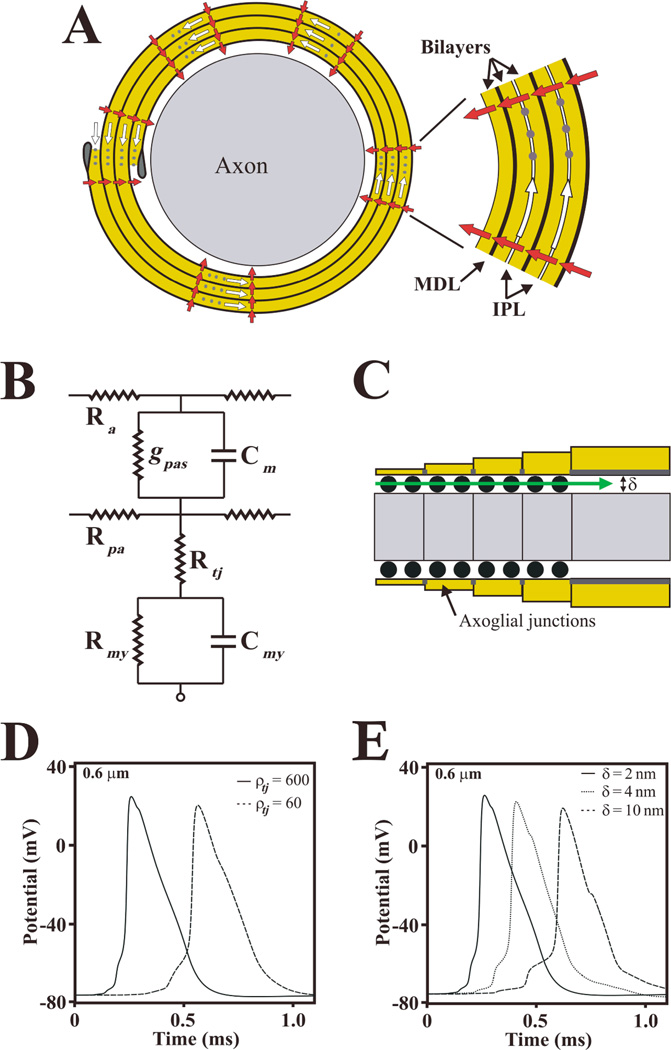
Fig. 4. Claudin 11 TJs may form a resistive pathway in the myelin.
A: Schematic representation of a cross section of CNS axon ensheathed with four myelin wraps. The inset shows the myelin membrane bilayers in greater detail, including intraperiod lines (IPL) and major dense lines (MDL). Myelin can be represented in an electrical circuit as a cable surrounding the axon, where current can flow through the IPL (open arrows) between myelin layers or across the myelin membrane (closed arrows). Myelin TJs (grey dots) occlude the extracellular space between the membrane layers and increase the resistance of this compartment, thereby limiting the current path at the IPL. This is analogous to a paracellular barrier formed by TJs in polarized epithelia that increases the transepithelial resistance. B: An equivalent electrical circuit of the myelin internode that is proposed to approximate the function of TJs. Thus, TJs may form a resistance (Rtj) in series with the myelin resistance (Rmy) and capacitance (Cmy). This resistive path decreases the capacitive charge of the myelin membrane and reduces the internodal delay during AP propagation. Ra and Rpa represent the resistances of the axoplasm and of the periaxonal space; gpas and Cm represent the conductance and capacitance of the axonal membrane. C: Schematic of a longitudinal section through the paranodal region, showing axoglial junctions (black dots) which occupy approximately 50% of the extracellular space at paranodes. Axoglial junctions maintain the width of the periaxonal space (δ) at paranodes and may form an axial resistance (Rpa; closed arrows) which prevents AP invasion of the juxtaparanodal and internodal regions. D: Computer simulations of APs generated by a 0.6 µm diameter myelinated axon in the presence (ρtj = 600 Ohm.cm2) and absence of claudin 11 TJs (ρtj = 60 Ohm.cm2). Lowering TJ resistivity (ρtj) in the myelin increases the latency (reduces the conduction velocity) of APs in small diameter myelinated axons. E: Computer simulations of APs generated in a 0.6 µm diameter myelinated axon with TJs and with functional axoglial junctions (δ = 2 nm; extracellular occupancy 50%) or without axoglial junctions where the paranodes are detached from the axon (δ = 4 nm or 10 nm). The absence of axoglial junctions also reduces conduction velocity. Surprisingly, simulations indicate that conduction block does not occur in larger fibers even when δ is > 100 nm. Together, the simulations indicate that TJs and AJs are both required for normal conduction, particularly in small fibers; however, they are assembled independently in CNS myelin and function differently from each other. Printed from [7] with permission from Cambridge University Press.
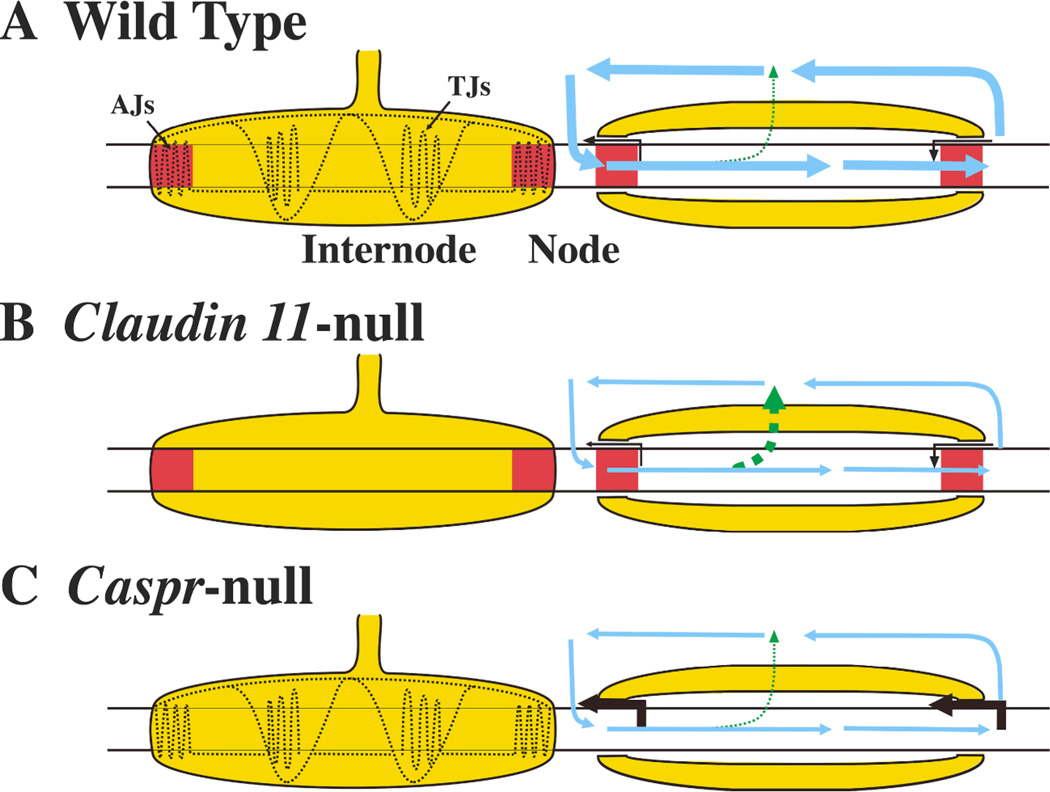
Fig. 5. Roles of TJs and axoglial junctions in axonal conduction.
The schematics depict myelinated axons from (A) wild type mice with TJs and axoglial junctions (AJs), (B) Claudin 11-null mice with AJs but no TJs and (C) Caspr-null mice with TJs but no AJs. Current flow (right) through a myelin internode is also shown for each genotype. In wild type animals, the majority of the axial current (looping arrows) flows to the next node because of the high resistance formed by the myelin around the axon. Small currents flow longitudinally through AJs at paranodes (black arrows) and radially through the myelin (dashed arrow). In Claudin 11-null mice, AJs are present; however, the loss of myelin TJs increases radial current flow through the IPL compartment of the myelin sheath (dashed arrows). This, in turn, decreases longitudinal current flow along the axon. In Caspr-null mice, the absence of AJs increases the distance between the axon and the myelin at paranodes, which in turn increases the current leak at paranodes and decreases longitudinal current flow along the axon. It is likely that similar changes occur in Contactin-null and Neurofascin 155-null mice. Thus, AJs and TJs play distinct but complementary functions in axonal conduction.
Axoglial junctions contribute to axonal conduction independently of TJs
In addition to TJs, CNS myelin sheaths also contain axoglial junctions (AJs), which are septate-like adhesive junctions localized to paranodal regions of the sheath (Fig. 2C and D). These junctions function to: attach the lateral cytoplasmic loops of myelin to the surface of the axon (Fig. 1), specify the distance between the axonal and myelin membranes at paranodes and define axonal membrane domains by lowering the lateral diffusion of axonal proteins such as ion channels. In addition, AJs are widely thought to form an impermeability barrier in the periaxonal space, although recent data suggest that AJs are more likely to act as space-filling struts that are relatively permeable even to small proteins [8].
Three major components constitute AJs in mammals– caspr, contactin and neurofascin 155 – and the targeted deletion of any one of these components in mice abolishes AJs in CNS myelin [27–30]. Consequences of the absence of AJs include conduction slowing, severe ataxia and early death, which probably stem from dysfunctional nodes of Ranvier and large increases in the width of the periaxonal space at paranodes. Computer simulations indicate that such increases can cause conduction slowing and conduction block, particularly in small diameter fibers (Fig. 4E). Interestingly, the absence of AJs does not cause the disassembly of TJs [30], indicating that these intercellular junctions form independently and serve complementary roles in promoting myelin insulation of the internode and saltatory conduction in small myelinated fibers (Fig. 5C).
Are TJs immune-protective or adhesive components of myelin?
Several hypotheses have been advanced to account for the presence of TJs in compact myelin. However, subsequent careful scrutiny has often brought into question the validity and veracity of the assumptions and conclusions leading to these hypotheses. For example, myelin TJs have been proposed as barriers to leukocyte migration which prevent them from coming into contact with myelin proteins that could be recognized as foreign antigens. In addition, myelin TJs have been suggested to be primary sites of adhesion that stabilize the multilamellar organization of compact myelin.
Early interest in myelin TJs revolved around the notion that these barriers might sequester myelin proteins away from immune cell surveillance, in similar fashion to the proposed function of TJs at the blood-testis barrier. The blood-testis barrier was originally thought to establish an immune-privileged compartment to preclude immune cell infiltration and interaction with novel antigens expressed in adolescence during spermiogenesis. Because myelination is also a late developmental event with respect to the perinatal establishment of immune tolerance, Mugnaini and colleagues [31] argued, by analogy, that myelin TJs were part of the blood-brain barrier and protected the CNS against demyelinating diseases such as multiple sclerosis. Contemporary opinions on immunomodulation challenge this view [32, 33], and an analysis of Claudin 11-null mice indicates that these mutants do not exhibit signs of immune cell infiltration, focal or disseminated demyelination or spontaneous encephalomyelitis [25].
In a variety of studies, TJs also have been proposed to be adhesive structures that confer myelin stability. For example, hexachlorophene toxicity in rodents induces splitting of CNS myelin lamellae except in the vicinity of the radial component [22]. Rosenbluth and colleagues have investigated the adhesiveness of myelin TJs in optic nerves from Plp1-null mice [34, 35]. Their ultrastructural analyses indicate, despite normal myelin compaction [also see 36], that the IPL in the mutants is disrupted by infiltration with hypotonic solutions, except at the radial component. Mobius and colleagues [36, 37] also have asserted that these data provide evidence for the adhesiveness of TJs in myelin.
However, such interpretations do not exclude other explanations like tissue processing artifacts. Prior to hypotonic treatment, Rosenbluth and colleagues [35] aldehyde-fixed the optic nerves, which undoubtedly crosslinked the claudin 11. Because 80 – 90% of the protein normally present at the IPL is missing in Plp1-null myelin, there is little wonder that the radial component remained intact in their study while swelling and splitting elsewhere at the surface of the virtually protein-free IPL was extensive.
Two studies have further explored the function of myelin TJs in the CNS of mice lacking both Claudin 11 and Plp1 genes (Claudin 11:Plp1-null). These double mutant mice exhibit significant clinical signs characterized by resting body tremors and ataxia with an onset around two weeks of age (A.G., unpublished data). At the ultrastructural level, Chow and colleagues [38] found dramatic disruption of myelin in cross-sections of optic nerve and spinal cord from double mutant mice with membrane whorls around many axons. This pathology was interpreted as indicating myelin instability and decompaction at the IPL in similar fashion to Plp1-null myelin.
However as reported here, Claudin 11:Plp1-null double mutants exhibit more subtle disruption of myelin architecture than reported previously [38], and is characterized by essentially compacted but loosely wrapped myelin around the vast majority of axons (Fig. 6) with some fiber loss, intra-axonal accumulations of vesicles, redundant myelin sheaths and an increased incidence of isolated cytoplasmic pockets within compact myelin. In general, the density of microtubules and neurofilaments is also increased in double mutant axons compared to controls (asterisks). The radial component, which is prevalent in control myelin (arrowheads), is absent in all Claudin 11-null genotypes and so too are TJs in non-compact regions of the myelin sheath such as the inner loop in the internode (Fig. 6C, D). Schmidt-Lantermann incisures are relatively rare in control rodent myelin because of the small size of axons [39] but are more frequent in the double mutants, suggesting that oligodendrocytes are compensating for deficiencies caused by structural changes in the mutant myelin (Fig. 6E, F).
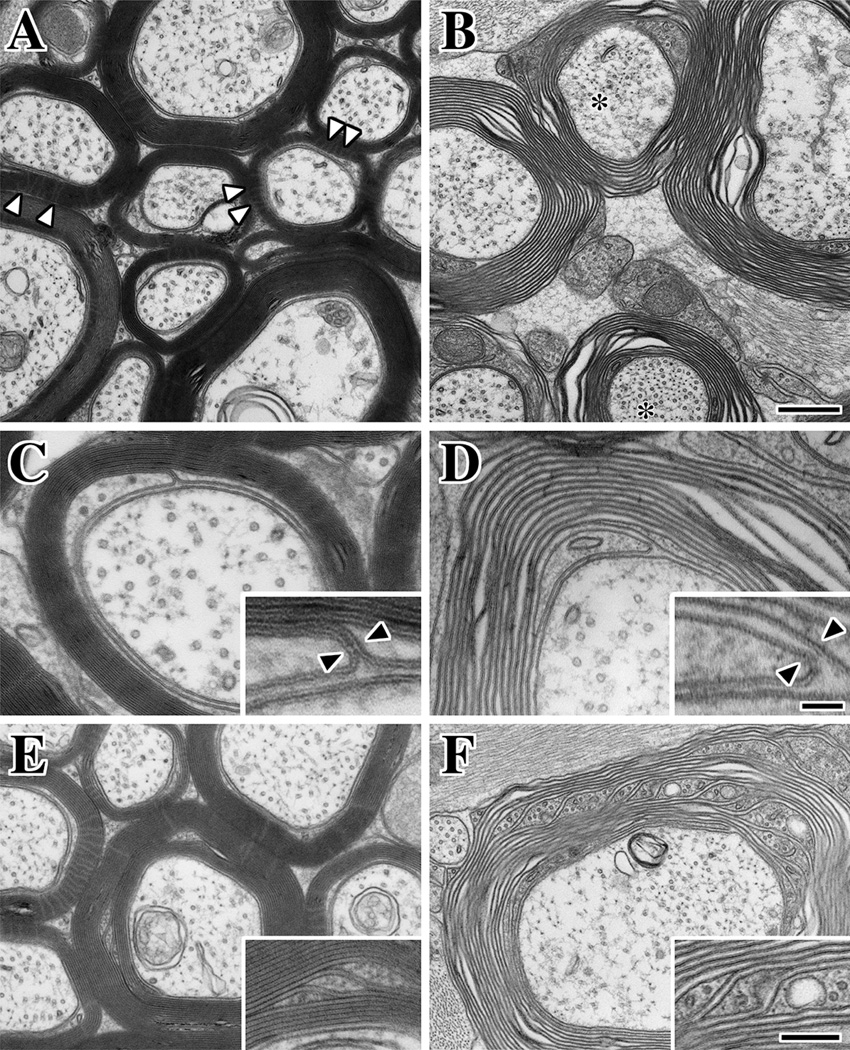
Fig. 6. ultrastructural abnormalities of CNS myelin in Claudin 11:Plp1-null mice.
A,B: Electron micrographs of optic nerve from adult Claudin 11-heterozygote control mice (A) and Claudin 11:Plp1-null double mutants (B). After glutaraldehyde fixation, the optic nerves were postfixed with osmium tetroxide and ferricyanide to reveal the radial component in almost all control myelin sheaths (arrowheads). Sheaths from double mutant optic nerves are multilamellar and largely compact, as indicated by the absence of cytoplasm in the internodal myelin. However, the membranes appear loosely wrapped around axons and sample preparation artifacts are present in most fibers. In general, the density of microtubules and neurofilaments is also increased in double mutant axons compared to controls (asterisk).
C,D: A characteristic feature of TJs at the inner loop in transverse sections of the myelin internode is the membrane kissing point at the junction between the periaxonal space and the intramyelinic compartment. The canonical pentalaminar appearance of the TJ is apparent in the inset (arrowheads) in Claudin 11-heterozygote mice (C). In Claudin 11:Plp1-null mice (D), the heptalaminar appearance of two closely apposed, but separate, membranes can be seen (arrowheads). The absence of a paracellular seal at the periaxonal-intramyelinic junction may provide access to the IPL for solutes from the periaxonal space and vice versa. Such access may disrupt electrostatic interactions that might keep the membrane surfaces of the IPL in close contact.
E,F: Schmidt-Lantermann incisures are less common in the rodent CNS than in larger mammals, likely because axons are much smaller [39]. However, incisures in control mice (E) have similar organization and architecture to other mammals and frequently contain one or more microtubules (inset in E). Incisures are several-fold more common in Claudin 11:Plp1-null mice, suggesting that oligodendrocytes are compensating for the structural abnormalities of the myelin in these mutants. Scale bars: A and B, 400 nm; C and D, 100 or 33 (insets) nm; E and F, 300 or 130 (insets) nm.
At high magnification (Fig. 7A), several abnormalities are apparent in the myelin from Claudin 11:Plp1-null double mutant mice. First, the myelin period is significantly greater and quite variable compared to controls (Fig. 7B). Second, the membrane surfaces at the IPL are much further apart than for the control, which probably accounts for the majority of the increased period. However, it is unclear if the lack of PLP1 at the IPL allows the membranes to move apart passively, or whether the absence of claudin 11 enables solutes to penetrate deep into the internode and cause generalized swelling. The latter explanation seems likely in light of previous investigations and the compressed appearance of internodal membranes in Plp1-null myelin, which contain TJs [13, 40, 41].
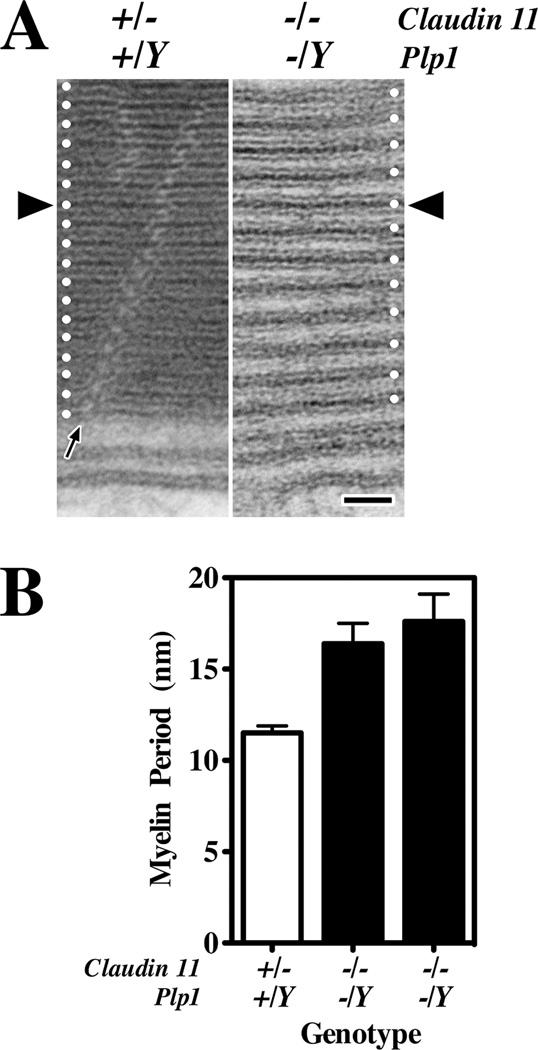
Fig. 7. abnormal myelin period, IPL and MDL in CNS myelin from Claudin 11:Plp1-null mice.
A: Electron micrographs of internodal myelin from optic nerves of adult Claudin 11-heterozygote and Claudin 11:Plp1-null mice at high magnification. The MDL from each mouse has been aligned at the black arrowheads and white dots on either edge show the spacing of the myelin lamellae for each animal. In the control myelin, the MDL is evenly spaced in each layer and the IPL appears as a narrow line at this magnification. The radial component, visualized by postfixing with ferricyanide, can be seen coursing diagonally through the myelin (black arrow). The myelin from the double mutant mouse is devoid of cytoplasm, but the lamellae do not appear completely compacted and several abnormalities are visible. Thus, the myelin period is abnormally large and variable; the IPL is too widely spaced; and the width of the MDL may be increased. B: Myelin period in adult optic nerve from one Claudin 11-heterozygote (control) and two Claudin 11:Plp1-null double mutant mice was determined by averaging three measurements for each of 30 myelinated fibers of different diameters (mean ± S.D.). These data reflect the micrographs in (A), that the myelin period is larger and more variable in the double mutants. Scale bar in A: 25 nm.
Finally and irrespective of the mechanism, the myelin from Claudin 11:Plp1-null mice appears as a spiral of pairs of lipid bilayers fused at the MDL, the width of which may be slightly greater than normal (compare MDLs in the vicinity of the black arrowheads). These data suggest that claudin 11 or, more likely PLP1, may contribute to stabilizing the cytoplasmic membrane apposition either independently of, or in conjunction with MBP. The PLP1-specific domain has a significant net positive charge to interact with negatively-charged lipid headgroups, which is similar to the proposed function of the cytoplasmic tail of the Po glycoprotein in PNS myelin [42].
From the perspective of myelin TJ function, the ultrastructural defects in Claudin 11:Plp1-null double mutant myelin reveal that oligodendrocytes can still assemble and largely compact a multilamellar membrane around axons even with as much as 60% of the total myelin protein missing and a near protein-free IPL. Such a remarkable feature of myelin lipid self-assembly into spiral membranes in the presence of MBP also has been demonstrated using purified components [43, 44]. Accordingly, these data imply that there may be little need for strongly adhesive proteins in myelin and cast substantial doubt on recent schemes that propose claudin 11 TJs as major adhesive structures.
Potential clinical relevance of CNS myelin TJs to neurological diseases
Schizophrenia
Schizophrenia is a complex mental disorder with genetic, neurological, and environmental causes. Schizophrenia symptoms are widely thought to arise from altered brain connectivity, particularly stemming from abnormal neurotransmitter metabolism [45, 46]. However, functional magnetic resonance imaging, tractography and post-mortem molecular analyses suggest white matter may be involved, with reduced myelin volume or integrity in some patients [47–49]. Consistent with this idea, schizophrenia symptoms are relatively common in several diseases with known myelin abnormalities including multiple sclerosis (MS), metachromatic leukodystrophy and X-linked adrenoleukodystrophy [49–54]. Because CNS axons require adequate myelination to maintain neurophysiological function, it is plausible that myelin dysfunction may negatively impact brain connectivity and executive functions in forebrain cortex.
Microarray analyses from several groups have revealed lower expression levels of several myelin-specific genes, including Claudin 11, in post-mortem tissue from affected regions of schizophrenia brain while expression of other myelin genes remains unaffected [55, 56]. These correlative data suggest that myelin function may be important for normal cognition and that white matter perturbations may play a role in mental disorders. Our observations in Claudin 11-null mice have lead us to postulate that reduced CLAUDIN 11 gene expression may slow the propagation of action potentials in small myelinated axons (< 1µm diameter) and thereby affect brain function in humans [8].
In vivo imaging implicates abnormal corpus callosum function in the development of schizophrenia [54] and this interhemispheric connective tract is largely (45 - 70%) comprised of axons below 1 µm diameter [57]. CLAUDIN 11 dysfunction may significantly increase transit times along this white matter tract and lead to callosal disconnectivity. If so, the disruption of myelin TJs may have broader implications and may impact more general brain processes. To date, mutations in the CLAUDIN 11 gene have not been implicated in familial forms of schizophrenia, although several single-nucleotide polymorphisms have been identified in the vicinity. Nevertheless, examining schizophrenia-related behavior and callosal connectivity in Claudin 11-null mice will shed light on the possibility that TJ function is linked to schizophrenia and possibly other affective disorders.
Multiple sclerosis
Multiple Sclerosis is the most common human demyelinating disease affecting CNS axons. MS is widely thought to stem from autoimmune attack, inflammatory demyelination and axonal damage; however, recent studies have challenged this notion and suggest that immune involvement is secondary to the primary defect [58]. Irrespective of the primary cause, many MS patients present circulating T-cells and antibodies reactive against myelin proteins [59, 60].
The abundance of claudin 11 in myelin has led to studies investigating whether claudin 11 is targeted by autoreactive T-cells or antibodies in MS patients. In one study, 50% of patients with relapsing-remitting MS harbored higher levels of anti-claudin 11 antibodies than controls [61], while a second study concluded that claudin 11-reactive T-cells were absent from MS patients [62]. These data suggest that a humoral response against myelin TJs may play a role in the development of relapsing-remitting MS. Nevertheless, claudin 11 induces experimental autoimmune encephalomyelitis (EAE) and optic neuritis in SJL/J mice with T-cell infiltration and demyelination [63–66] and EAE can be adoptively transferred to naïve mice by injecting claudin 11 reactive T-cells. Thus, the pathogenic actions of anti-claudin 11 antibodies in relapsing-remitting MS remain to be demonstrated and passive cotransfer of antibodies and T-cells reactive against claudin 11 will help determining whether anti-claudin 11 antibodies may destabilize myelin TJs and affect conduction.
Role of claudin 11 in CNS development
Other novel functions of claudin 11 in oligodendrocyte biology have recently emerged. Yeast-two-hybrid screens suggest that claudin 11 interacts with several transmembrane proteins, including: OAP-1, a member of the tetraspanin superfamily, Kv3.1, a voltage-rated potassium channel subunit, and integrin β1 [62, 67]. Overexpression of claudin 11 or OAP-1 in oligodendrocyte cell lines enhances their proliferation, while deletion of Claudin 11 or Kv3.1 gene reduces proliferation and migration of oligodendrocyte precursors in vitro. However, the relevance of these data to oligodendrocyte biology are controversial, because loss-of-function alleles of Kv3.1 and Claudin 11 do not confer overt defects in oligodendrocyte proliferation, migration or myelination in vivo. Nevertheless, it is intriguing that claudin 11 may be involved in oligodendrocyte differentiation during development, in contrast to the notion that TJs are only expressed by differentiated cells.
TJs in PNS myelin
Although there is strong correspondence between the distribution of TJs in myelin elaborated by oligodendrocytes and Schwann cells [68–70], it is unlikely that claudins function similarly in the CNS and PNS for several reasons. First and foremost, the absence of claudin 11 in CNS myelin causes conduction slowing in myelinated axons [7, 8, 25, 71] but there is little evidence that conduction in the PNS is perturbed in Claudin 19-null mice [72] or in patients with CLAUDIN 19 mutations [73, 74]. Second, TJs in CNS myelin are important for normal conduction in axons below 1µm diameter with thin myelin sheaths. In general, myelinated axons in the PNS are greater than 1µm in diameter [75] and the myelin is sufficiently thick so as not to benefit from the resistive properties of TJs. Finally, claudin 11 TJs in CNS myelin are continuous around the edges of the sheath and form a resistive barrier, but claudin 19 TJs are discontinuous in PNS myelin which would minimize the effectiveness of a barrier to current flow.
Irrespective of any differences in claudin function between CNS and PNS myelin, TJs may fulfill important roles in Schwann cells. Poliak and colleagues [76] suggest that TJs may coordinate the assembly and maintenance of microarchitectural features in myelinated fibers, such as gap junctions at Schmidt-Lantermann incisures. Gap junctions and TJ proteins co-localize in PNS myelin and incorporate cytoplasmic plaque anchoring proteins such as ZO-1. Perhaps, TJs reduce the width of the extracellular space between myelin lamellae at the IPL to mediate coupling of connexin hemi-channels at apposed membrane surfaces. Indeed, a role for claudin 11 TJs in maintaining gap junction microdomains has been established for basal cells in the stria vascularis of the cochlea [71].
Acknowledgements
This work has been supported by grants to J.D. from the Association Française contre les Myopathies and the National Multiple Sclerosis Society (RG3839A1/T) and to A.G. from NIDCD, NIH (DC006262), NINDS, NIH (NS43783), the National Multiple Sclerosis Society (RG2891) and the William and Marie Carls Foundation, Detroit, Michigan.
Contributor Information
Jérôme Devaux, Email: jerome.devaux@univmed.fr.
Bozena Fykkolodziej, Email: bfykkol@med.wayne.edu.
Alexander Gow, Email: agow@med.wayne.edu.
References
- 1.Southwood CM, Gow A. Functions of OSP/claudin 11-containing parallel tight junctions: implications from the knockout mouse. In: Anderson JM, Cereijido M, editors. Tight Junctions. New York: CRC Press; 2001. pp. 719–741. [Google Scholar]
- 2.Furuse M, Tsukita S. Claudins in occluding junctions of humans and flies. Trends in cell biology. 2006;16:181–188. doi: 10.1016/j.tcb.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Tsukita S, Yamazaki Y, Katsuno T, Tamura A, Tsukita S. Tight junction-based epithelial microenvironment and cell proliferation. Oncogene. 2008;27:6930–6938. doi: 10.1038/onc.2008.344. [DOI] [PubMed] [Google Scholar]
- 4.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. The Journal of cell biology. 1969;40:648–677. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nature immunology. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 6.Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C. Organization and function of the blood-brain barrier in Drosophila. J Neurosci. 2008;28:587–597. doi: 10.1523/JNEUROSCI.4367-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gow A, Devaux JJ. A Model of Tight Junction Function In CNS Myelinated Axons. Neuron Glia Biol. 2009;4:307–317. doi: 10.1017/S1740925X09990391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Devaux JJ, Gow A. Tight Junctions Potentiate The Insulative Properties Of Small CNS Myelinated Axons. The Journal of cell biology. 2008;183:909–921. doi: 10.1083/jcb.200808034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loh YH, Christoffels A, Brenner S, Hunziker W, Venkatesh B. Extensive expansion of the claudin gene family in the teleost fish, Fugu rubripes. Genome research. 2004;14:1248–1257. doi: 10.1101/gr.2400004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banerjee S, Sousa AD, Bhat MA. Organization and function of septate junctions: an evolutionary perspective. Cell Biochem Biophys. 2006;46:65–77. doi: 10.1385/CBB:46:1:65. [DOI] [PubMed] [Google Scholar]
- 11.Tepass U. Claudin complexities at the apical junctional complex. Nature cell biology. 2003;5:595–597. doi: 10.1038/ncb0703-595. [DOI] [PubMed] [Google Scholar]
- 12.Nunes FD, Lopez LN, Lin HW, Davies C, Azevedo RB, Gow A, Kachar B. Distinct subdomain organization and molecular composition of a tight junction with adherens junction features. Journal of cell science. 2006;119:4819–4827. doi: 10.1242/jcs.03233. [DOI] [PubMed] [Google Scholar]
- 13.Stecca B, Southwood CM, Gragerov A, Kelley KA, Friedrich VLJ, Gow A. The evolution of lipophilin genes from invertebrates to tetrapods: DM-20 cannot replace PLP in CNS myelin. J. Neurosci. 2000;20:4002–4010. doi: 10.1523/JNEUROSCI.20-11-04002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duncan ID. Dissection of the phenotype and genotype of the X-linked myelin mutants. In: Duncan ID, Skoff RP, Colman DR, editors. Myelination and Dysmyelination. Vol. 605. New York: New York Academy of Sciences; 1990. pp. 110–121. [DOI] [PubMed] [Google Scholar]
- 15.Kirschner DA, Ganser AL, Caspar DLD. Diffraction studies of molecular organization and membrane interactions in myelin. In: Morell P, editor. Myelin. New York: Plenum; 1984. pp. 51–95. [Google Scholar]
- 16.Omlin F, Webster Hd.F, Pulkovits CG, Cohen SR. Immunocytochemical localization of BP in major dense line regions of central and peripheral myelin. J. Cell Biol. 1982;95:242–248. doi: 10.1083/jcb.95.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Popot J-L, Pham-Dinh D, Dautigny A. Major myelin proteolipid: the 4-alpha-helix topology. J. Membr. Biol. 1991;120:233–246. doi: 10.1007/BF01868534. [DOI] [PubMed] [Google Scholar]
- 18.Gow A, Gragerov A, Gard A, Colman DR, Lazzarini RA. Conservation of topology, but not conformation, of the proteolipid proteins of the myelin sheath. J. Neurosci. 1997;17:181–189. doi: 10.1523/JNEUROSCI.17-01-00181.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters A. A radial component of central myelin sheaths. J Biophys Biochem Cytol. 1961;11:733–735. doi: 10.1083/jcb.11.3.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters A. Further observations on the structure of myelin sheaths in the central nervous system. Journal of Cell Biology. 1964;20:281–296. doi: 10.1083/jcb.20.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnapp B, Mugnaini E. Membrane architecture of myelinated fibers as seen by freeze-fracture. In: Waxman SG, editor. Physiology and Pathobiology of Axons. New York: Raven; 1978. pp. 83–123. [Google Scholar]
- 22.Tabira T, Cullen MJ, Reier PJ, Webster H. An experimental analysis of interlamellar tight junctions in amphibian and mammalian C.N.S. myelin. J. Neurocytol. 1978;7:489–503. doi: 10.1007/BF01173993. [DOI] [PubMed] [Google Scholar]
- 23.Hirano A, Becker NH, Zimmerman HM. Isolation of the periaxonal space of the central myelinated nerve fiber with regard to the diffusion of peroxidase. J. Histochem. Cytochem. 1969;17:512–516. doi: 10.1177/17.8.512. [DOI] [PubMed] [Google Scholar]
- 24.Shinowara NL, Beutel WB, Revel J-P. Comparative analysis of junctions in the myelin sheath of central and peripheral axons of fish, amphibians and mammals: a freeze-fracture study using complementary replicas. Journal of Neurocytology. 1980;9:15–38. doi: 10.1007/BF01205225. [DOI] [PubMed] [Google Scholar]
- 25.Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B, Lazzarini RA. CNS Myelin And Sertoli Cell Tight Junction Strands Are Absent In Osp/Claudin 11-Null Mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- 26.Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J. Cell Biol. 1999;145:579–588. doi: 10.1083/jcb.145.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pillai AM, Thaxton C, Pribisko AL, Cheng JG, Dupree JL, Bhat MA. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal domains. J Neurosci Res. 2009;87:1773–1793. doi: 10.1002/jnr.22015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 29.Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, Martin MS, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin iv/caspr/paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- 30.Zonta B, Tait S, Melrose S, Anderson H, Harroch S, Higginson J, Sherman DL, Brophy PJ. Glial and neuronal isoforms of Neurofascin have distinct roles in the assembly of nodes of Ranvier in the central nervous system. The Journal of cell biology. 2008;181:1169–1177. doi: 10.1083/jcb.200712154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mugnaini E, Schnapp B. Possible role of zonula occludens of the myelin sheath in demyelinating conditions. Nature. 1974;251:725–727. doi: 10.1038/251725a0. [DOI] [PubMed] [Google Scholar]
- 32.Yule TD, Mahi-Brown CA, Tung KS. Role of testicular autoantigens and influence of lymphokines in testicular autoimmune disease. J. Reprod. Immunol. 1990;18:89–103. doi: 10.1016/0165-0378(90)90026-3. [DOI] [PubMed] [Google Scholar]
- 33.Pelletier RM, Byers SW. The blood-testis barrier and Sertoli cell junctions: structural considerations. Microsc. Res. Tech. 1992;20:3–33. doi: 10.1002/jemt.1070200104. [DOI] [PubMed] [Google Scholar]
- 34.Rosenbluth J, Nave KA, Mierzwa A, Schiff R. Subtle myelin defects in PLP-null mice. Glia. 2006;54:172–182. doi: 10.1002/glia.20370. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbluth J, Schiff R, Lam P. Effects of osmolality on PLP-null myelin structure: implications re axon damage. Brain research. 2009;1253:191–197. doi: 10.1016/j.brainres.2008.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mobius W, Patzig J, Nave KA, Werner HB. Phylogeny of proteolipid proteins: divergence, constraints, and the evolution of novel functions in myelination and neuroprotection. Neuron Glia Biol. 2009:1–17. doi: 10.1017/S1740925X0900009X. [DOI] [PubMed] [Google Scholar]
- 37.Mobius W, Patzig J, Nave KA, Werner HB. Phylogeny of proteolipid proteins: divergence, constraints, and the evolution of novel functions in myelination and neuroprotection. Neuron Glia Biol. 2008;4:111–127. doi: 10.1017/S1740925X0900009X. [DOI] [PubMed] [Google Scholar]
- 38.Chow E, Mottahedeh J, Prins M, Ridder W, Nusinowitz S, Bronstein JM. Disrupted compaction of CNS myelin in an OSP/Claudin-11 and PLP/DM20 double knockout mouse. Molecular and cellular neurosciences. 2005;29:405–413. doi: 10.1016/j.mcn.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Blakemore WF. Schmidt-Lanterman incisures in the central nervous system. J. Ultrastruct. Res. 1969;29:496–498. doi: 10.1016/s0022-5320(69)90069-0. [DOI] [PubMed] [Google Scholar]
- 40.Rosenbluth J, Stoffel W, Schiff R. Myelin structure in proteolipid protein (PLP)-null mouse spinal cord. J. Comp. Neurol. 1996;371:336–344. doi: 10.1002/(SICI)1096-9861(19960722)371:2<336::AID-CNE12>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 41.Klugmann M, Schwab MH, Puhlhofer A, Schneider A, Zimmermann F, Griffiths IR, Nave K-A. Assembly of CNS myelin in the absence of proteolipid protein. Neuron. 1997;18:59–70. doi: 10.1016/s0896-6273(01)80046-5. [DOI] [PubMed] [Google Scholar]
- 42.Martini R, Mohajeri MH, Kasper S, Giese KP, Schachner M. Mice doubly deficient in the genes for P0 and myelin basic protein show that both proteins contribute to the formation of the major dense line in peripheral nerve myelin. J Neurosci. 1995;15:4488–4495. doi: 10.1523/JNEUROSCI.15-06-04488.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riccio P, Fasano A, Borenshtein N, Bleve-Zacheo T, Kirschner DA. Multilamellar packing of myelin modeled by lipid-bound MBP. J Neurosci Res. 2000;59:513–521. doi: 10.1002/(SICI)1097-4547(20000215)59:4<513::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 44.Mateu L, Luzzati V, London Y, Gould RM, Vosseberg FG. X-ray diffraction and electron microscope study of the interactions of myelin components. The structure of a lamellar phase with a 150 to 180 A repeat distance containing basic proteins and acidic lipids. Journal of molecular biology. 1973;75:697–709. doi: 10.1016/0022-2836(73)90302-1. [DOI] [PubMed] [Google Scholar]
- 45.Benes FM, Berretta S. Amygdalo-entorhinal inputs to the hippocampal formation in relation to schizophrenia. Annals of the New York Academy of Sciences. 2000;911:293–304. doi: 10.1111/j.1749-6632.2000.tb06733.x. [DOI] [PubMed] [Google Scholar]
- 46.Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- 47.Andreasen NC, Arndt S, Swayze V, 2nd, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WT. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science (New York, N.Y 266. 1994:294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 48.Flynn SW, Lang DJ, Mackay AL, Goghari V, Vavasour IM, Whittall KP, Smith GN, Arango V, Mann JJ, Dwork AJ, Falkai P, Honer WG. Abnormalities of myelination in schizophrenia detected in vivo with MRI, and post-mortem with analysis of oligodendrocyte proteins. Mol Psychiatry. 2003;8:811–820. doi: 10.1038/sj.mp.4001337. [DOI] [PubMed] [Google Scholar]
- 49.Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Archives of general psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 50.Denier C, Orgibet A, Roffi F, Jouvent E, Buhl C, Niel F, Boespflug-Tanguy O, Said G, Ducreux D. Adult-onset vanishing white matter leukoencephalopathy presenting as psychosis. Neurology. 2007;68:1538–1539. doi: 10.1212/01.wnl.0000260701.76868.44. [DOI] [PubMed] [Google Scholar]
- 51.Feinstein A, du Boulay G, Ron MA. Psychotic illness in multiple sclerosis. A clinical and magnetic resonance imaging study. Br J Psychiatry. 1992;161:680–685. doi: 10.1192/bjp.161.5.680. [DOI] [PubMed] [Google Scholar]
- 52.Hyde TM, Ziegler JC, Weinberger DR. Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Archives of neurology. 1992;49:401–406. doi: 10.1001/archneur.1992.00530280095028. [DOI] [PubMed] [Google Scholar]
- 53.Kopala LC, Tan S, Shea C, Orlik H, Vandorpe R, Honer WG. Adrenoleukodystrophy associated with psychosis. Schizophrenia research. 2000;45:263–265. [PubMed] [Google Scholar]
- 54.Walterfang M, Wood SJ, Velakoulis D, Copolov D, Pantelis C. Diseases of white matter and schizophrenia-like psychosis. The Australian and New Zealand journal of psychiatry. 2005;39:746–756. doi: 10.1080/j.1440-1614.2005.01678.x. [DOI] [PubMed] [Google Scholar]
- 55.McCullumsmith RE, Gupta D, Beneyto M, Kreger E, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophrenia research. 2007;90:15–27. doi: 10.1016/j.schres.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katsel P, Davis KL, Haroutunian V. Variations in myelin and oligodendrocyte-related gene expression across multiple brain regions in schizophrenia: a gene ontology study. Schizophrenia research. 2005;79:157–173. doi: 10.1016/j.schres.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 57.Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain research. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- 58.Trapp BD, Nave KA. Multiple sclerosis: an immune or neurodegenerative disorder? Annu Rev Neurosci. 2008;31:247–269. doi: 10.1146/annurev.neuro.30.051606.094313. [DOI] [PubMed] [Google Scholar]
- 59.Cross AH, Trotter JL, Lyons J. B cells and antibodies in CNS demyelinating disease. J Neuroimmunol. 2001;112:1–14. doi: 10.1016/s0165-5728(00)00409-4. [DOI] [PubMed] [Google Scholar]
- 60.O'Connor KC, Bar-Or A, Hafler DA. The neuroimmunology of multiple sclerosis: possible roles of T and B lymphocytes in immunopathogenesis. Journal of clinical immunology. 2001;21:81–92. doi: 10.1023/a:1011064007686. [DOI] [PubMed] [Google Scholar]
- 61.Bronstein JM, Lallone RL, Seitz RS, Ellison GW, Myers LW. A humoral response to oligodendrocyte-specific protein in MS: a potential molecular mimic. Neurology. 1999;53:154–161. doi: 10.1212/wnl.53.1.154. [DOI] [PubMed] [Google Scholar]
- 62.Tiwari-Woodruff SK, Buznikov AG, Vu TQ, Micevych PE, Chen K, Kornblum HI, Bronstein JM. OSP/claudin-11 forms a complex with a novel member of the tetraspanin super family and beta1 integrin and regulates proliferation and migration of oligodendrocytes. The Journal of cell biology. 2001;153:295–305. doi: 10.1083/jcb.153.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaushansky N, Eisenstein M, Oved JH, Ben-Nun A. Activation and control of pathogenic T cells in OSP/claudin-11-induced EAE in SJL/J mice are dominated by their focused recognition of a single epitopic residue (OSP58M) International immunology. 2008;20:1439–1449. doi: 10.1093/intimm/dxn099. [DOI] [PubMed] [Google Scholar]
- 64.Kaushansky N, Hemo R, Eisenstein M, BenNun A. OSP/claudin-11-induced EAE in mice is mediated by pathogenic T cells primarily governed by OSP192Y residue of major encephalitogenic region OSP179-207. Eur J Immunol. 2007;37:2018–2031. doi: 10.1002/eji.200636965. [DOI] [PubMed] [Google Scholar]
- 65.Kaushansky N, Zhong MC, Kerlero de Rosbo N, Hoeftberger R, Lassmann H, Ben-Nun A. Epitope specificity of autoreactive T and B cells associated with experimental autoimmune encephalomyelitis and optic neuritis induced by oligodendrocyte-specific protein in SJL/J mice. J Immunol. 2006;177:7364–7376. doi: 10.4049/jimmunol.177.10.7364. [DOI] [PubMed] [Google Scholar]
- 66.Stevens DB, Chen K, Seitz RS, Sercarz EE, Bronstein JM. Oligodendrocyte-specific protein peptides induce experimental autoimmune encephalomyelitis in SJL/J mice. J Immunol. 1999;162:7501–7509. [PubMed] [Google Scholar]
- 67.TiwariWoodruff S, BeltranParrazal L, Charles A, Keck T, Vu T, Bronstein J. K+ channel K(V)3.1 associates with OSP/claudin-11 and regulates oligodendrocyte development. American Journal of Physiology - Cell Physiology. 2006;291:C687–C698. doi: 10.1152/ajpcell.00510.2005. [DOI] [PubMed] [Google Scholar]
- 68.Hall SM, Williams PL. The distribution of electron-dense tracers in peripheral nerve fibres. J. Cell Sci. 1971;8:541–555. doi: 10.1242/jcs.8.2.541. [DOI] [PubMed] [Google Scholar]
- 69.Mugnaini E, Osen KK, Schnapp B, Friedrich VL., Jr. Distribution of Schwann cell cytoplasm and plasmalemmal vesicles (caveole) in peripheral myelin sheaths. An electron microscopic study with thin sections and freeze-fracturing. Journal of Neurocytology. 1977;6:647–668. doi: 10.1007/BF01176378. [DOI] [PubMed] [Google Scholar]
- 70.Revel J-P, Hamilton DW. The double nature of the intermediate dense line in peripheral nerve myelin. Anatomical Record. 1969;163:7–16. doi: 10.1002/ar.1091630102. [DOI] [PubMed] [Google Scholar]
- 71.Gow A, Davies C, Southwood CM, Frolenkov G, Chrustowski M, Ng L, Yamauchi D, Marcus DM, Kachar B. Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J Neurosci. 2004;24:7051–7062. doi: 10.1523/JNEUROSCI.1640-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Miyamoto T, Morita K, Takemoto D, Takeuchi K, Kitano Y, Miyakawa T, Nakayama K, Okamura Y, Sasaki H, Miyachi Y, Furuse M, Tsukita S. Tight junctions in Schwann cells of peripheral myelinated axons: a lesson from claudin-19-deficient mice. The Journal of cell biology. 2005;169:527–538. doi: 10.1083/jcb.200501154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee NP, Tong MK, Leung PP, Chan VW, Leung S, Tam PC, Chan KW, Lee KF, Yeung WS, Luk JM. Kidney claudin-19: localization in distal tubules and collecting ducts and dysregulation in polycystic renal disease. FEBS letters. 2006;580:923–931. doi: 10.1016/j.febslet.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 74.Konrad M, Schaller A, Seelow D, Pandey AV, Waldegger S, Lesslauer A, Vitzthum H, Suzuki Y, Luk JM, Becker C, Schlingmann KP, Schmid M, Rodriguez-Soriano J, Ariceta G, Cano F, Enriquez R, Juppner H, Bakkaloglu SA, Hediger MA, Gallati S, Neuhauss SC, Nurnberg P, Weber S. Mutations in the tight-junction gene claudin 19 (CLDN19) are associated with renal magnesium wasting, renal failure, and severe ocular involvement. Am J Hum Genet. 2006;79:949–957. doi: 10.1086/508617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Waxman SG, Bennett MV. Relative conduction velocities of small myelinated and non-myelinated fibres in the central nervous system. Nature: New biology. 1972;238:217–219. doi: 10.1038/newbio238217a0. [DOI] [PubMed] [Google Scholar]
- 76.Poliak S, Matlis S, Ullmer C, Scherer SS, Peles E. Distinct claudins and associated PDZ proteins form different autotypic tight junctions in myelinating Schwann cells. The Journal of cell biology. 2002;159:361–372. doi: 10.1083/jcb.200207050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gow A. Major components of myelin in the mammalian central and peripheral nervous systems. In: Kalman B, Brannagan THI, editors. Neuroimmunology in Clinical Practice. Malden: Blackwell Publishing; 2008. pp. 12–25. [Google Scholar]