Abstract
In mammalian testes, haploid spermatozoa are formed from diploid spermatogonia during spermatogenesis, which is a complicated cellular process. While these cellular events were reported in the 1960s and 1970s, the underlying molecular mechanism(s) that regulates these events remained unexplored until the past ~10 years. For instance, adhesion proteins were shown to be integrated components at the Sertoli cell–cell interface and/or the Sertoli–spermatid interface in the late 1980s. But only until recently, studies have demonstrated that some of the adhesion proteins serve as the platform for signal transduction that regulates cell adhesion. In this chapter, a brief summary and critical discussion are provided on the latest findings regarding these cell-adhesion proteins in the testis and their relationship to spermatogenesis. Moreover, antagonistic effects of two mammalian target of rapamycin (mTOR) complexes, known as mTORC1 and mTORC2, on cell-adhesion function in the testis are discussed. Finally, a hypothetic model is presented to depict how these two mTOR-signaling complexes having the “yin” and “yang” antagonistic effects on the Sertoli cell tight junction (TJ)-permeability barrier can maintain the blood–testis barrier (BTB) integrity during the epithelial cycle while preleptotene spermatocytes are crossing the BTB.
1. INTRODUCTION
Spermatogenesis takes place in the seminiferous epithelium, which is composed of germ and Sertoli cells with the Sertoli cell serving as the “mother” or the “nursery” cell that supports and nourishes germs cells at different stages of their development (Cheng and Mruk, 2010a; Griswold, 1998; Mruk and Cheng, 2004). Spermatogenesis is a complex and precisely regulated process that produces spermatozoa (haploid, 1n) from spermatogonia (diploid, 2n). Spermatogenesis is also tightly controlled by the hypothalamic–pituitary–testicular hormonal axis. This axis involves the production of gonadotropin- releasing hormone (GnRH) from the hypothalamus that induces the secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) from the pituitary gland. LH then stimulates the release of testosterone from Leydig cells via steroidogenesis to support Sertoli and germ cell function, which together with FSH that exerts its effects exclusively on Sertoli cells. These hormones together with locally produced hormones (e.g. inhibin, activin), steroids (e.g. estradiol-17β), and paracrine and autocrine factors (e.g. cytokines, fragments of laminins and collagens), thereby maintaining spermatogenesis in a unique microenvironment in the seminiferous epithelium (Carreau and Hess, 2010; Cheng and Mruk, 2012; O’Donnell et al., 2001; Sharpe, 1994; Walker, 2011; Winters and Moore, 2007). During spermatogenesis, a single type A spermatogonium undergoes 10 successive rounds of mitosis to give rise to 1024 primary spermatocytes, which then enter meiosis to produce 4096 spermatids theoretically (Cheng and Mruk, 2012; Ehmcke et al., 2006). Spermatids then undergo maturation via spermiogenesis to form spermatozoa which are to be released into the tubule lumen at spermiation (O’Donnell et al., 2011). However, it is estimated that the efficiency of spermatogenesis is only ~25%, and the majority of germ cells undergo apoptosis, which is regulated by estrogen produced by Leydig cells, Sertoli cells and germ cells (Barratt, 1995; Shaha, 2008; Tegelenbosch and de Rooij, 1993). This is to prevent overwhelming the capacity of Sertoli cells since each Sertoli cell can support ~30–50 developing germ cells (Billig et al., 1995; Weber et al., 1983). During spermatogenesis, the seminiferous epithelium can be organized into 14 stages in rats (stage I–XIV); 12 stages (stage I–XII) in mice and six stages (I–VI) in humans according to the different developmental stages of germ cells, in particular, the association of developing spermatids with Sertoli cells (de Kretser and Kerr, 1988; Hess and de Franca, 2008; Mruk et al., 2008; Parvinen, 1982). Throughout the seminiferous epithelial cycle, germ cells have to traverse the seminiferous epithelium, from the basal to the adluminal (apical) compartment, and finally reach the luminal edge of the seminiferous tubule at spermiation. This timely translocation of germ cells is synchronized with a series of cyclic junctional restructuring events at the Sertoli–Sertoli and Sertoli–germ cell interface (Cheng and Mruk, 2010b, 2012). These events are tightly regulated and precisely coordinated, their disruption can perturb spermatogenesis, leading to infertility.
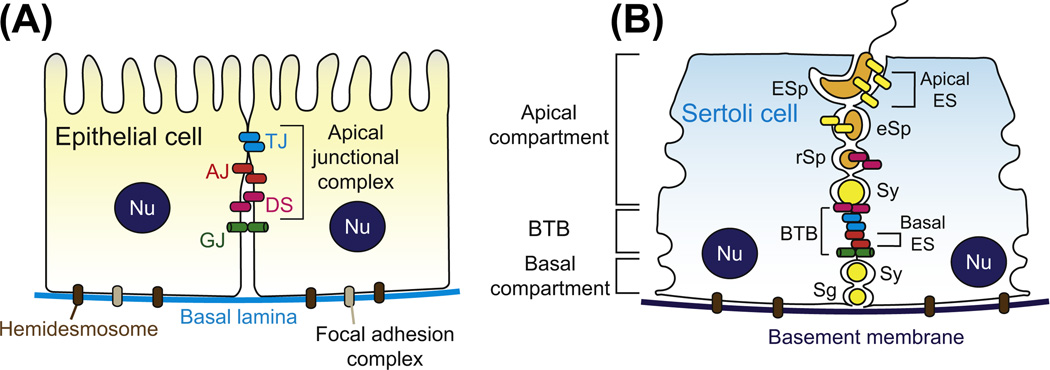
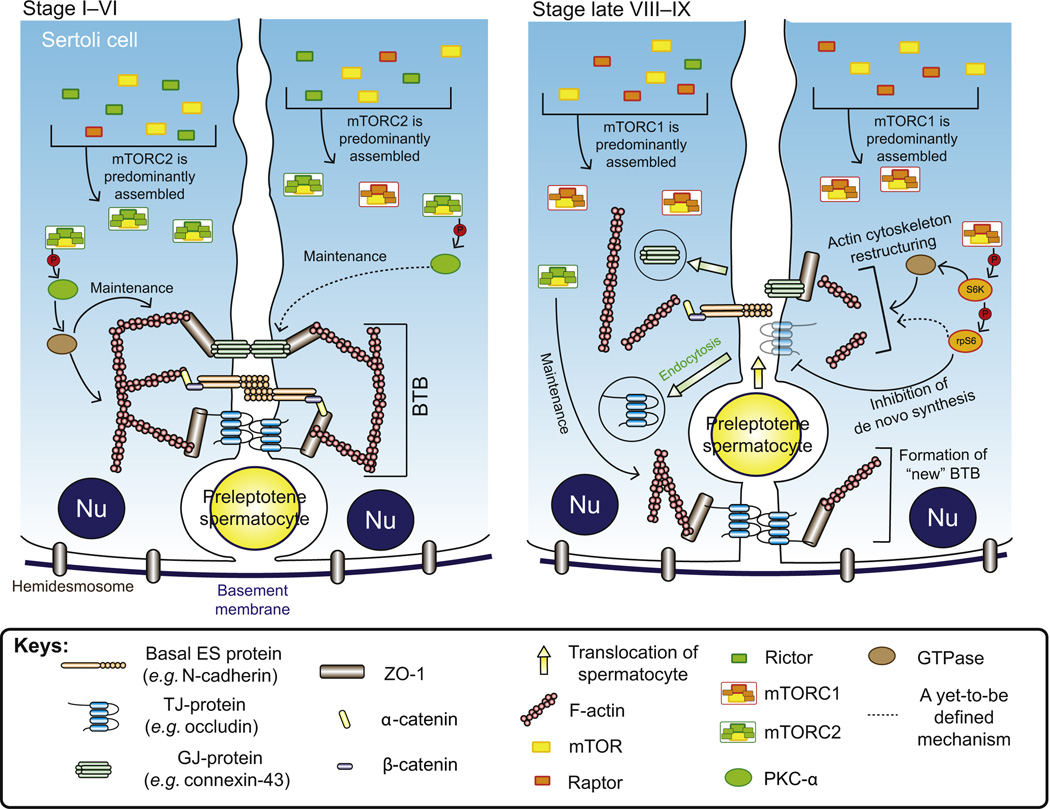
During the transit of preleptotene spermatocytes conneced in “clones” via intercellular bridges from the basal to the apical compartment, spermatocytes have first to travel across a blood–tissue junctional barrier, which physically separates the two compartments (Fig. 6.1). This junctional barrier, which located near the basement membrane, is formed by adjacent Sertoli cells known as the blood–testis barrier (BTB). The BTB is one of the tightest blood–tissue barriers, possibly because it is constituted by coexisting tight junction (TJ), basal ectoplasmic specialization [basal ES, a testis-specific adherens junction (AJ)], gap junction (GJ), and desmosome (DS) (Cheng and Mruk, 2012; Wong and Cheng, 2005). Except for DS which utilizes vimentin-based intermediate filaments as the attachment site, the above adhesion junctions are all connected to the actin cytoskeleton, especially the basal ES which possesses tightly packed actin filament bundles that lie perpendicular to the Sertoli cell plasma membrane and are sandwiched between cisternae of endoplasmic reticulum and the opposing Sertoli cell plasma membranes. This is also the hallmark ultrastructure of the BTB, which contributes to the unusual adhesive strength of the barrier (Cheng and Mruk, 2010b, 2011; Mruk et al., 2008). Despite the unusual tightness of the BTB, it undergoes cyclic restructuring during stage VIII–XI of the epithelial cycle to facilitate the transit of preleptotene spermatocytes at the BTB by assembling “new” BTB behind the transiting spermatocytes while the “old” BTB above the spermatocytes is being disassembled, so that the immunological barrier can remain intact (Cheng and Mruk, 2011; Cheng et al., 2010). Thus, the BTB serves as an immunological barrier to “seal” developing spermatocytes and spermatids from the systemic circulation, preventing the development of immune responses against germ cells residing at the apical compartment which arise at puberty (Fijak et al., 2011; Meinhardt and Hedger, 2011). This hypothesis about the coexistence of an “old” and a “new” BTB that enclose the spermatocytes in transit at the BTB was designated the intermediate compartment (Russell, 1977), and was shown in a lanthanum study using electron microscopy from our laboratory (Yan et al., 2008c) (Fig. 6.2). As different types of junctions at the BTB are connected to the actin cytoskeleton, BTB restructuring can be effectively regulated via cyclic reorganization of F-actin network utilizing different actin-regulating proteins. These actin-regulating proteins include epidermal growth factor pathway substrate 8 (Eps8) (Lie et al., 2009), which is an actin barbed-end capping and bundling protein (Hertzog et al., 2010), and actin-related protein 3 (Arp3) (Lie et al., 2010), which together with Arp2 forms the Arp2/3 complex that induces branched actin polymerization (Goley and Welch, 2006). Besides, accumulating evidence suggests that mammalian target of rapamycin (mTOR), a signaling molecule and a nonreceptor protein Ser/Thr kinase that is known to modulate an array of cellular events (Weichhart, 2012; Zoncu et al., 2011), is also responsible for the extensive reorganization of F-actin network to assist BTB restructuring during the epithelial cycle of spermatogenesis (Mok et al., 2012a; Mok et al., 2012c).
Figure 6.1. Differences in the morphological layouts of junction types between a typical epithelium/endothelium and the seminiferous epithelium.
(A) For the junctional complex in typical epithelia/endothelia, TJs, which are responsible for sealing the intercellular space to create the barrier function by regulating paracellular and transcellular transport, are located at the apical region of the lateral membrane between adjacent epithelial/endothelial cells. Underneath TJs, there are AJs that contribute to most of the adhesive force of the apical junctional complex by connecting to a dense F-actin network, creating the zonula adherens plaque, to be followed by desmosomes. Both TJ and AJ are actin-based cell–cell anchoring junctions, whereas DS is intermediate filament-based cell–cell anchoring junction. Other junctional molecules such as GJs, which are not part of the junctional complex, are localized basal to the junctional complex (constituted by TJ, AJ and DS). (B) Unlike the junctional complex in typical epithelia which are furthest away from the basal lamina, the BTB in seminiferous epithelium is located near the basement membrane (a modified form of extracellular matrix in the testis). Instead of being arranged as discrete structure as in other epithelia/ endothelia, TJs, basal ES (a testis-specific actin-rich AJ) and GJs are coexisting at the BTB, which together with DS are all involved in creating the BTB. The BTB physically separates the seminiferous epithelium into the basal and apical (adluminal) compartments. Spermatogonia and preleptotene spermatocytes reside at the basal compartment, and preleptotene spermatocytes that arise at stage VII-VIII of the epithelial cycle in the rat testis are the only germ cells that can traverse the BTB. After traversing the BTB, spermatocytes undergo meiosis and eventually differentiate into elongating/elongated spermatids, and spermatids (step 8–19 spermatids in the rat testis) anchored to the Sertoli cells by apical ES. Furthermore, hemidesmosomes (intermediate filament-based cell–matrix anchoring junction) and focal adhesion complexes (FAC, or known as focal contacts, an actin-based cell–matrix anchoring junction) are also found in most epithelia, but FAC is absent in the seminiferous epithelium. Abbreviations used: Sg, spermatogonium; Sy, spermatocyte; rSp, round spermatid; eSp, elongating spermatid; ESp, elongated spermatid; Nu, Sertoli cell nucleus; DS, desmosome; AJ, adherens junction; GJ, gap junction; TJ, tight junction; ES, ectoplasmic specialization. For color version of this figure, the reader is referred to the online version of this book.
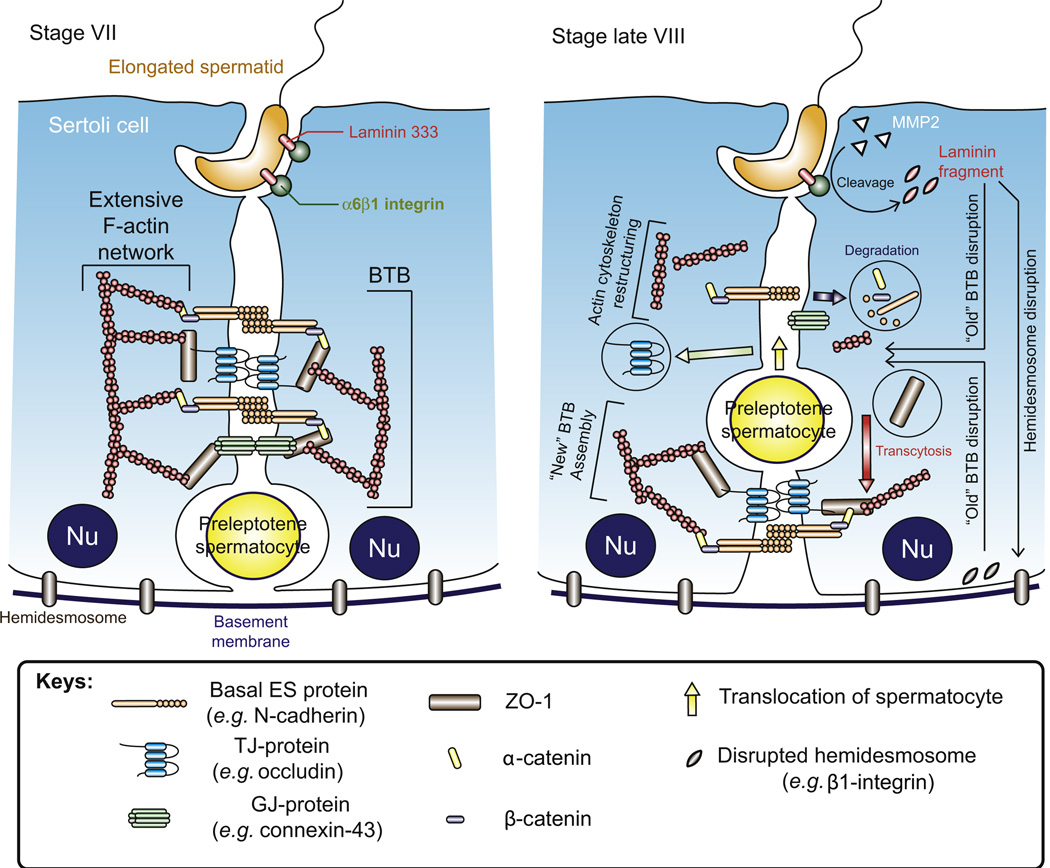
Figure 6.2. Restructuring of the BTB to facilitate the transit of preleptotene spermatocytes at stage VIII of the epithelial cycle.
Before BTB restructuring takes place, its integrity is maintained by coexisting TJs, basal ES and GJs which interact with each other and linked to actin cytoskeleton for structural support via adaptor proteins such as ZO-1. Besides, desmosome is also present at the Sertoli cell–cell interface at the BTB. On the other hand, elongated spermatids are also anchored to the Sertoli cell via a testis-specific apical ES protein complex in which laminin-333 residing at the elongating spermatid is linked to α6β1-integrin restricted to the Sertoli cell. At stage VIII of the epithelial cycle, when preleptotene spermatocytes are in transit at the BTB to enter the apical compartment for further development, the “old” BTB above the spermatocyte disassembles to “open” the BTB. This process is mediated by the apical ES–BTB–hemidesmosome functional axis, in which laminin 333 at the apical ES is cleaved by MMP2 to generate bioactive laminin fragments. The laminin fragments induce disruption of the “old” BTB and cause the loss of hemidesmosome function which also contributes to the “opening” of the “old” BTB. Besides, BTB restructuring is also facilitated by mTORC1 as well as by the reorganization of actin cytoskeleton mediated by actin-regulating proteins, such as the Arp2/3–N-WASP complex and Eps8 which induce a “branched/debundled” and “bundled” configuration of the actin filaments at the basal ES, respectively. Without the support from the dense F-actin network, BTB proteins are internalized through endocytosis and the internalized BTB proteins can either undergo degradation or being recycled for the assembly of “new” BTB via transcytosis at the base of the preleptotene spermatocytes. It is likely that molecules, such as testosterone, that promote BTB integrity may be working in concert with mTORC2 underneath the spermatocyte in transit to assemble a “new” BTB before the “old” BTB above the transiting spermatocyte is disassembled, so that the barrier function can remain intact during germ cell movement at the site. For color version of this figure, the reader is referred to the online version of this book.
In this review, we focus on the biology and regulation of the BTB, in particular, the involvement of the two mTOR signal complexes, namely mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), in regulating the intriguing dynamics of the BTB during the epithelial cycle. Studies on mTOR have largely been focused on the role of mTOR as a key modulator of cell survival, particularly in cancer biology (Khokhar et al., 2011; Wander et al., 2011), since mTOR plays a central role in regulating protein synthesis for cell growth, cell proliferation and survival (Howell and Manning, 2011; Senqupta et al., 2010). However, recent studies have shown that mTOR also takes part in a variety of cellular events including actin cytoskeleton reorganization, aging, autophagy, immune responses and barrier function (Inoki et al., 2011; Mok et al., 2012a; Mok et al., 2012c; Oh and Jacinto, 2011; Vassiliadis et al., 2011; Weichhart, 2012). Studies have shown that in podocytes, which are the cells that establish the blood–urine barrier in the kidney, a disruption of the mTOR signaling perturbs the barrier function as a result of internalization of the TJ-adaptor protein ZO-1 (Shorning et al., 2011) and reduced expression of slit diaphragm proteins (proteins which are essential for cell–cell contact and hence barrier function in podocytes) (Vollenbroker et al., 2009). More important, the involvement of mTOR in the BTB modulation via reorganization of actin cytoskeleton has been demonstrated in studies using RNAi to silence rpS6, a downstream signaling molecule of mTORC1 (Meyuhas, 2008), since its knockdown was found to promote TJ-barrier function (Mok et al., 2012c). On the other hand, the knockdown of rictor, a binding partner of mTORC2 (Sarbassov et al., 2004), was shown to disrupt BTB function (Mok et al., 2012a), illustrating the antagonistic effects of these two mTOR complexes on BTB dynamics. In order to have a better understanding of how the BTB is regulated by mTOR, we first provide an update on the latest status of research on the different junction types and the constituent adhesion proteins at the BTB, and how they interact with each other to maintain the barrier homeostasis. We then provide a brief background on mTOR such as the components of the two mTOR signaling complexes and their functions. Finally, we will examine some recent findings regarding the “yin” and “yang” of mTORs on BTB dynamics via the differential actions of mTORC1 and mTORC2 on BTB function.
2. ACTIN-BASED CELL JUNCTIONS AT BTB
Among all the blood–tissue barriers, such as the blood–brain barrier and the blood–urine barrier which are created between neighboring endothelial cells, cell junctions are typically arranged in which TJs are localized at the apical region, to be followed by discrete AJs and DS, which constitute the junctional complex (Fig. 6.1). In addition, GJs are located basal to the junctional complex (Hartsock and Nelson, 2008; Miyoshi and Takai, 2008) (Fig. 6.1). In these blood–tissue barriers, the permeability barrier is created almost exclusively by TJs which seal the intercellular space between adjacent membranes and confer cell polarity to restrict paracellular and transcellular transport of substances (Steed et al., 2010; Tsukita et al., 2001), whereas AJs which connect to a dense actin filament network confer the adhesion property (Harris and Tepass, 2010). Thus, the coexisting TJs, basal ES and GJs which contribute to the barrier and adhesion function of the BTB as an entity is in fact a unique feature amongst all the blood–tissue barriers (Fig. 6.1). Since TJs, basal ES and GJs are all linked to underlying actin cytoskeleton via corresponding adaptors, changes in the organization of actin filaments at the BTB during the epithelial cycle play a significant role in its restructuring. In this section, we briefly discuss each junction type at the BTB and how these junctions associate with the underlying F-actin cytoskeleton, interacting with each other.
2.1. Tight Junction
TJs appear as “kisses” between adjacent epithelial or endothelial cells under electron microscope where two plasma membranes fuse together as illustrated in the Sertoli cell BTB (Cheng and Mruk, 2010b; Steed et al., 2010; Tsukita et al., 2001). In other blood–tissue barriers, TJs are located apically in an epithelium or endothelium and act as “fences” that divide the membranes into apical and basolateral domains. Since integral membrane proteins are freely diffusible in plasma membrane, this “fence” function of the TJ restricts proteins to their respective apical or basal location (Steed et al., 2010; Tsukita et al., 2001), generating apicobasal polarity in an epithelium and to prevent transcellular transport of substances across the barrier. Although the intercellular space is sealed by TJs in which the TJ strands from two neighboring plasma membranes associate laterally with each other to form a “gate,” selected ions and/or solutes can pass through these “gates” via paracellular transport, which is dependent on their charge and size (Steed et al., 2010; Tsukita et al., 2001). This “gate” function of TJs varies among cell types due to the differences in the relative proportions of different TJ proteins (Steed et al., 2010; Tsukita et al., 2001). In addition, differences in TJ-strand density also affect permeability of the TJ (Steed et al., 2010; Tsukita et al., 2001). While the “fence” and “gate” functions imply TJs are considerably rigid in nature, TJs are actually dynamic ultrastructures by adjusting their permeability barrier function in response to changes in environment and/or physiological needs, such as development, cell migration and cell/tissue homeostasis (Steed et al., 2010; Tsukita et al., 2001). This flexibility of TJ is particularly important for the BTB, which undergoes cyclic restructuring to facilitate the transit of preleptotene spermatocytes while its integrity must be maintained to ensure proper development of spermatids via spermiogenesis behind the barrier. Furthermore, TJs are connected to actin cytoskeleton via adaptor proteins, which include zonula occludens-1, -2 and -3 (ZO-1, -2, -3). To date, many TJ proteins have been identified at the BTB, which include claudins, occludin, junctional adhesion molecules (JAMs), tricellulin and coxsackievirus and adenovirus receptor (CAR) (Cheng and Mruk, 2010b; Steed et al., 2010; Tsukita et al., 2001). Among these, claudins, occludin and JAMs are the best-studied TJ proteins at the BTB, which are briefly reviewed herein.
2.1.1. Claudins
Claudins are a family of TJ proteins, each has four transmembrane domains, two extracellular loops and a short cytoplasmic tail (Elkouby-Naor and Ben-Yosef, 2010). To date, 24 members of claudins have been identified (Elkouby-Naor and Ben-Yosef, 2010). Among these, claudin-1 through -8 and -11 have been identified by northerns in rodent testes (Furuse et al., 1998; Morita et al., 1999a, 1999b), whereas claudin 10, 12, and 23 were detected by microarray analysis using mRNAs from rodent testes (Singh et al., 2009). It is generally accepted that claudins are the backbone of TJ strands and are responsible for recruiting other TJ proteins, such as occludin to TJs. Forced expression of exogenous claudins in fibroblasts was able to induce cell adhesion activity by forming networks of TJ-strand-like ultrastructure at cell–cell contacts (Furuse et al., 1998; Kubota et al., 1999). Besides, the importance of claudins as the core structural component of TJs is demonstrated by the inability of forming an intact barrier in mice with specific claudin knockout. For example, mice lacking claudin 1 died shortly after birth due to dehydration as a result of failure in epidermal barrier function (Furuse et al., 2002). Deletion of claudin 5 in mice led to neonatal death, within 10 h after birth because of the absence of the blood–brain barrier (Nitta et al., 2003). Furthermore, knockout of claudin 18 in mice led to disruption of permeability barrier of gastric epithelia, causing paracellular H+ leakage that results in atrophic gastric epithelia (Hayashi et al., 2012). Knockout of claudin-11, which is expressed specifically in oligodendrocytes and Sertoli cells, led to infertility in mice due to the lack of BTB without TJ strands formed between Sertoli cells (Gow et al., 1999). Besides being the essential building block of TJs, claudins also determine the properties of TJ barriers by assembling TJs with different claudin members. For example, TJ strands formed by claudin-1 are highly branched network while claudin-11-based TJ strands, as those found in Sertoli cells, are mostly parallel strands with little branching (Gow et al., 1999; Morita et al., 1999b). Furthermore, the selectivity of ions and solutes of a permeability barrier is also dependent on the composition of claudins as illustrated by gain-or-loss function studies in animals, humans or cell lines involving specific claudins. For instance, overexpression of claudin-2, but not claudin-3, in MDCK I cells which express only claudin-1 and -4, leads to a “leaky” TJ barrier, as shown by a decrease in transepithelial electrical resistance (TER) across the cell epithelium. This thus reflects the differential ability among different claudins in conferring the TJ-barrier function (Furuse et al., 2001). Furthermore, in claudin-15 knockout mice, the small intestine displayed malabsorption of glucose due to a disruption of paracellular transport of Na+ ions across the TJ barrier (Tamura et al., 2011). Claudin-16, however, was shown to be important to paracellular transport of Mg2+ across the TJ barrier (Simon et al., 1999).
Claudins also play an important role in maintaining the BTB function during spermatogenesis. In fact, TJ strands at the BTB is contributed significantly by claudin-11 since deletion of claudin-11 leads to a loss of the BTB ultrastructure, resulting in the lack of TJ strands between Sertoli cells (Gow et al., 1999). Interestingly, Sertoli cells, which normally cease to divide after postnatal day 15, are found to be proliferating in adult claudin-11 knockout mice (Gow et al., 1999). This is probably due to the loss of contact inhibition after the disappearance of TJs. This thus suggests that the permeability barrier imposed by claudin-11 also has a role in regulating cell cycle function in Sertoli cells. Furthermore, a recent report has shown that claudin-3 may be a crucial protein involving in the intermediate compartment during translocation of spermatocytes across the BTB (Komljenovic et al., 2009). Immunofluorescence staining illustrated that during the transit of preleptotene spermatocytes across the BTB at stage VII–IX in mice, localization of claudin-3 at the BTB was found apically to preleptotene spermatocytes (“old” BTB) at stage VII; however, at stage VIII–early IX, claudin-3 was detected at both apically (“old” BTB) and basally (“new” BTB) of the translocating spermatocytes; and finally claudin-3 was detected only at the basal side (“new” BTB) of leptotene spermatocytes transformed from preleptotene spermatocytes (Komljenovic et al., 2009). Despite this stage-specific localization of claudin-3 coinciding with the intermediate compartment, this observation requires further verification by functional studies, such as if its knockdown would indeed impede the migration of spermatocytes at the BTB. Additionally, the role of claudin-3 may be species-specific since claudin-3 is not found at the BTB in the rat testis (Kaitu’u-Lino et al., 2007). Thus, much work is needed to define the role(s) of different claudin(s) in the cyclic restructuring events of the BTB during spermatogenesis.
2.1.2. Occludin
Occludin is the first integral membrane protein identified at the TJ (Furuse et al., 1993). Although occludin shares a similar topography with claudins by having four transmembrane domains, two extracellular loops and a cytoplasmic tail, there is no sequence homology between the two TJ proteins (Cummins, 2012; Furuse et al., 1998). Unlike claudins, which are composed of multiple members in the claudin gene family, no occludin-related gene has been identified thus far, but two occludin isoforms are produced by alternative splicing. Also, unlike claudins, occludin has a relative long cytoplasmic tail. Ser and Thr residues of its cytoplasmic tail are heavily phosphorylated; and studies have shown that phosphorylations at these sites via protein kinases are essential for regulating occludin localization and distribution in epithelia/endothelia. For instance, a study using primary Sertoli cell cultures in vitro has demonstrated that focal adhesion kinase (FAK) is structurally associated with occludin and it also regulates the structural interaction between occludin and ZO-1 (Siu et al., 2009a, 2009b). Furthermore, a knockdown of FAK in Sertoli cells led to a decrease in phosphorylation of Ser and Tyr, but not Thr in occludin, which, in turn, probably resulted in an increase in the internalization of occludin, thereby perturbing the TJ barrier (Siu et al., 2009a). Besides FAK, c-Yes is another nonreceptor protein tyrosine kinase known to be structurally associated with occluding at the Sertoli cell BTB (Xiao et al., 2011). When the intrinsic activity of c-Yes in Sertoli cells with an established functional TJ-permeability barrier that mimicked the BTB in vivo was inhibited by SU6656, a selective c-Yes inhibitor, redistribution of occludin from cell–cell interface to cell cytosol was found, contributing to the disruption of the Sertoli cell TJ barrier (Xiao et al., 2011). Besides FAK and c-Yes, protein kinase C (PKC) also plays a role in modulating the localization of occludin at TJs via its effects to confer the phosphorylation status in occludin. Study reported that upon stimulation of PKC by phorbol 12-myristate 13-acetate (PMA) and 1,2 dioctanoylglycerol (DiC8), phosphorylation of occludin was induced, leading to an increase in occludin localization at the cell–cell interface (Andreeva et al., 2001). The importance of occludin in spermatogenesis was also addressed by studies using synthetic occludin peptide. It was demonstrated that when occludin–occludin interaction between adjacent Sertoli cells was disrupted via intratesticular injection of peptide corresponding to a segment of the second extracellular loop of occludin, the BTB was compromised, leading to germ cell loss from the epithelium (Chung et al., 2001). Interestingly, when occludin was deleted, occludin knockout mice remained fertile by age 6 weeks at the time the first wave of spermatogenesis occurred (Saitou et al., 2000). However, these occludin knockout mice were found to be infertile by ~40–60 weeks of age, with their seminiferous tubules displayed atrophy and devoid of germ cells (Saitou et al., 2000). Subsequent studies by generating another genetic model of occludin knockdown confirmed that fertility was retained in these mice only from ~6–10 weeks of age (Takehashi et al., 2007), but all occludin knockout mice were infertile by 36–60 weeks of age with the tubules devoid of spermatocytes and spermatids (Saitou et al., 2000; Takehashi et al., 2007). Collectively, these findings illustrate that while other TJ proteins, such as claudins and JAMs, may be able to supersede the loss of occludin at the BTB to maintain spermatogenesis; however, occluding is absolutely essential to maintain the BTB function and spermatogenesis beyond 10 weeks of age in rodents during adulthood, illustrating the functional relationship between BTB and maintenance of spermatogenesis.
Interestingly, the necessity of occludin to spermatogenesis does not apply to humans as occludin was not found in human Sertoli cells in an earlier study (Moroi et al., 1998). However, a recent study by RT-PCR has identified occludin in human Sertoli cells (Xiao and Cheng, unpublished observations), illustrating further study on the function of occludin in huamn BTB is warranted. The lack of occludin in human seminiferous epithelium also illustrates that the BTB is a complex ultrastructure and its constituency is species-specific. Other studies have also shown that the role of occludin in blood–tissue barriers is organ- and/or tissue-specific. For instance, occludin is not essential for the formation of TJ strands; and in some cell types, it is not even needed for the maintenance of TJs. It was reported that occludin was not found in the TJ strands between porcine aortic endothelial cells (Hirase et al., 1997), revealing that in some tissues, occludin is not a constituent protein of the TJ barrier. Moreover, in occludin knockout mice, the TJ barrier formed between intestinal epithelial cells was indistinguishable from those of the wild type ultrastructurally (Saitou et al., 2000), demonstrating that in some epithelia that normally express occludin, a missing of occludin does not necessarily affect the formation and/or maintenance of the TJ barrier. Furthermore, although studies have shown that treatment of synthetic occludin peptide disrupted TJ barrier between Sertoli cells (Chung et al., 2001) as well as that between intestinal epithelial cells (Nusrat et al., 2005), a study in human intestinal T84 epithelial (T84) cell cultures has shown that the occludin peptide-induced TJ-barrier disruption was mediated by redistribution of other TJ proteins (e.g. claudin-1) and TJ adaptor (e.g. ZO-1) (Nusrat et al., 2005), illustrating occludin may act as a “signaling” regulatory TJ protein. More important, the use of monoclonal antibody against the second extracellular loop of occludin in T84 cells was found to disrupt epithelial cell polarity but not the TJ barrier (Tokunaga et al., 2007). Collectively, these findings illustrate the complex functional role of occludin at the TJ barrier, supporting the notion of its species- and/or tissue-specific function regarding its involvement in TJ-barrier formation and maintenance. Nonetheless, these findings illustrate that occludin, unlike claudins, may have other role(s) and serving as a signaling molecule in controlling the permeability in TJs, such as fine-tuning the barrier function, besides serving as the building block of TJs in some epithelia. This notion is also supported by studies illustrating that overexpression of exogenous occludin in fibroblasts was able to induce the formation of TJ strands, but these TJ strands were shorter and lesser in quantity when compared to those claudin-based TJ strands; and when fibroblasts were co-transfected with occludin and claudins, occludin was recruited to the TJ strands formed by claudins, and together they formed continuous belt-like ultrastructures at the cell–cell interface, which was in contrast to the punctuate pattern when occludin was overexpressed alone (Furuse et al., 1998). More important, while young adult occludin knockout mice at 6–10 weeks of age were fertile but when these mice reached adulthood by >30 weeks, besides being infertile with seminiferous tubules were found to be devoid of spermatocytes and spermatids, calcification in the brain, and chronic gastritis in the gastric epithelium were detected (Saitou et al., 2000), illustrating occludin, and perhaps TJs, may be playing more important cellular roles besides serving as an indispensable protein at the TJ barrier. In this context, it is of interest to note that studies have reported internalization of occludin by caveolae and/or clathrin-mediated endocytosis (Murakami et al., 2009; Schwarz et al., 2007; Shen and Turner, 2005), including the Sertoli cell TJ barrier (Wong et al., 2009; Yan et al., 2008c), illustrating occludin can be rapidly mobilized to other cellular domains to exert its function besides the TJ barrier.
2.1.3. Junctional Adhesion Molecules
JAMs are members of the immunoglobulin superfamily (IgSF) proteins; the extracellular region of these TJ-integral membrane proteins possess two Ig-like domains. Based on sequence homology, JAM family is composed of two subfamilies with one of them comprises three closely related members namely JAM-A (JAM-1), JAM-B (JAM-2) and JAM-C (JAM-3). Another subfamily, in which the members have a lower polypeptide sequence similarity, includes CAR, JAM-D (JAM-4) and JAM-like (JAM-L). Herein, we focus on the former subfamily since its members have been better characterized and studied in the testis. JAMs differ from claudins and occludin topologically since each JAM molecule has only one extracellular domain, a single transmembrane region and a cytoplasmic tail that varies in length among different isoforms (Mandell and Parkos, 2005; Severson and Parkos, 2009). Unlike claudins and occludin, JAMs alone is insufficient to from TJ strands as no TJs were detected in many primary cultures of fibroblasts and established fibroblast cell lines that expressed either JAM-A or JAM-C. However, JAMs are concentrated to the TJs when examined by immunofluorescence microscopy (Morris et al., 2006). JAMs are also distributed in and around TJ strands under electron microscopy, indicating their intimate association with the TJ barrier (Itoh et al., 2001). The involvement of JAM proteins in TJ-barrier function has been revealed in several studies. For instance, a study in T84 human intestinal epithelial cells using anti-JAM-A antibody has shown that JAM-A is necessary for recovery of Ca2+ depletion-induced TJ-barrier disruption as re-establishment of TJ barrier was disrupted due to the loss of JAM-A and occludin function following antibody treatment (Liu et al., 2000). JAMs are also required for the resealing of a disrupted TJ barrier induced by treatment of epithelial cells with synthetic peptides corresponding to the extracellular domain of JAMs (Liang et al., 2000). Moreover, a leaky TJ-permeability barrier was found in the intestinal epithelial cells of JAM-A knockout mice, indicating the significance of JAM proteins in barrier function (Laukoetter et al., 2007). Interestingly, such leaky TJ barrier might be the result of an induction of claudin-10 and -15 detected in the intestinal epithelial cells obtained from JAM-A knockout mice versus the wild-type. It was shown that an induction of certain claudins would lead to an increase in permeability of certain ions across the TJ barrier (Laukoetter et al., 2007). An induction of claudins after knockout of JAM-A and a down-regulation of occludin after JAM-A antibody treatment thus illustrate that JAMs may regulate the TJ barrier by altering the localization and/or expression of other TJ proteins (Severson and Parkos, 2009). Regardless of the importance of JAMs in modulating the barrier function in cell lines or intestinal epithelia, the significance of JAMs to the BTB remains unknown. Although JAM-A and JAM-B are found in the BTB (Morrow et al., 2010), deletion of JAM-A or homozygous mutation of JAM-B had no impact on the BTB integrity (Sakaguchi et al., 2006; Shao et al., 2008). It is known that mice with JAM-A deleted or JAM-B mutated remained fertile and their seminiferous epithelium was histologically normal (Sakaguchi et al., 2006; Shao et al., 2008). Even though deletion of JAM-A in mice led to reduced litter size, this is probably resulted from impaired motility of spermatozoa as JAM-A was also shown to be involved in sperm tail formation (Shao et al., 2008).
Unlike claudins and occludin whose functions are mostly related to the TJ-permeability barrier as these are structural components of the blood-tissue barriers, JAMs are involved in numerous cellular functions and pathological conditions, such as leukocyte migration, angiogenesis, hypertension and tumorigenesis (Bazzoni, 2011). Among them, the participation of JAMs in the transmigration of leukocyte across the endothelial TJ barrier during inflammation is of great interest since preleptotene spermatocytes may be utilizing JAMs to traverse the BTB with similar mechanism (Wang and Cheng, 2007). It is noted that besides Sertoli cells, germ cells also expressed JAM proteins including JAM-A and JAM-C (Wang and Cheng, 2007), thus it was proposed that other than playing the role for anchoring germ cells to Sertoli cells, JAMs may also be responsible for the spermatocyte transit at the BTB. In fact, the loss of JAM-C, an integrated component of the apical ES at the Sertoli–spermatid interface, led to failure of spermiogenesis and infertility (Gliki et al., 2004). In short, much work is needed to define the role of JAMs during spermatogenesis, in particular, its function at the BTB.
2.1.4. ZO Adaptor Proteins
Underneath the TJs, cytoplasmic plaques are formed via the cytoplasmic tails of TJ proteins directly associated with adaptor proteins, such as ZO proteins, at a 1:1 stoichiometric ratio (e.g. occludin-ZO-1, claudin-ZO-1, JAM-ZO-1), which in turn bind to the underlying actin filaments. As such, TJ proteins are linked to actin cytoskeleton for the support of barrier integrity. Three ZO proteins have been identified thus far and they are ZO-1, ZO-2 and ZO-3, which share sequence homology with each other and among them, ZO-1 is the predominant adaptor protein (Gonzalez-Mariscal et al., 2000; Tsukita et al., 2009). ZO proteins belong to the membrane-associated guanylate kinase (MAGUK) family, and beginning from their N-terminal region, they all have three PDZ domains, to be followed by an SH3 domain, a GUK domain and a cytoplasmic tail. The first PDZ domain was shown to bind to claudins (Itoh et al., 1999a) while the second one is necessary for homo- or heterodimerization between ZO proteins (Utepbergenov et al., 2006; Wittchen et al., 1999), and the third PDZ domain is needed for interacting with JAMs (Bazzoni et al., 2000; Ebnet et al., 2000). ZO proteins associate with occludin using the GUK domain (Furuse et al., 1994; Haskins et al., 1998; Itoh et al., 1999b), with actin filaments link to the ZO proteins via their cytoplasmic tails (Fanning et al., 1998; Itoh et al., 1997). The knockout of ZO-1 or ZO-2 in mice results in embryonic lethality (Katsuno et al., 2008; Xu et al., 2008). This demonstrates these two ZO proteins are essential for development, but little information can be deduced for their physiological function from these knockout mice. The importance of ZO proteins in recruitment of TJ proteins, especially claudins for the formation of TJs, was revealed by cultured epithelial cell line without endogenous ZO-3, whereas ZO-1 was knockout by homologous recombination, and ZO-2 was knockdown by RNAi (Umeda et al., 2006). Interestingly, when ZO truncated proteins containing only the N-terminus which has the three PDZ domains were forcibly localized to lateral membrane and dimerized, TJs formed by claudins were found to be distributed throughout the lateral membrane (Umeda et al., 2006). This is in sharp contrast to the TJs formed by overexpressing full length ZO-1 and ZO-2 as these TJs are precisely localized to the apical junctional complex. These observations thus illustrate that the interaction of SH3 domain and GUK domain in ZO proteins with AJs is necessary for directing TJ proteins to their correct cellular location (Umeda et al., 2006). The importance of ZO proteins in spermatogenesis was demonstrated in a study by injecting ZO-2−/− embryonic stem cells into wild-type blastocysts to generate viable ZO-2-deficient mice, these mice were found to have reduced fertility, resulting from impaired spermatogenesis, because the BTB was disrupted due to mislocalization of integral membrane proteins claudin 11 and Cx43 at the site (Xu et al., 2009).
These studies thus illustrate the significance of ZO-adaptor proteins in maintaining the TJ-barrier integrity by proper localization of TJ (and also GJ) integral membrane proteins, and this is mediated by their connection to the underlying actin filaments. This is particularly important for the BTB where an extensive F-actin network is present, and is under cyclic restructuring during the epithelial cycle of spermatogenesis. The maintenance and modulation of barrier function by actin reorganization is demonstrated in numerous studies. For instance, when actin depolymerization was induced by latrunculin A (Lat A, a toxin produced by red sea sponge) in MDCK cells, a disruption of the TJ barrier was detected, which was attributed to the internalization of occludin that caused by the loss of the apical peri-junctional F-actin ring underneath the TJs, illustrating the importance of actin cytoskeleton for proper TJ-protein localization (Shen and Turner, 2005). In a study using rat alveolar epithelial cells, strengthening of cortical actin filaments induced by treatment of keratinocyte growth factor (KGF) led to a tightening of the TJ barrier (LaFemina et al., 2010). The necessity of an actin cytoskeleton for the maintenance of the BTB integrity is best illustrated in studies using actin regulating proteins Eps8 and Arp3 (Lie et al., 2010, 2009). It was reported that after in vitro knockdown of Eps8 in Sertoli cells with an established functional TJ-permeability barrier by RNAi, actin disorganization was detected, leading to the redistribution of occludin and ZO-1 from the cell–cell interface into the cell cytosol (Lie et al., 2009). Moreover, in vivo knockdown of Eps8 in testis also led to truncation and mislocalization of F-actin and occludin, respectively, contributing to the disruption of the BTB integrity when assessed by an in vivo BTB functional assay (Lie et al., 2009). Furthermore, in a study using wiskostatin to block Arp3 activation in cultured Sertoli cells, the inhibition of branched actin polymerization that resulted in deposition of actin filament bundles at the cell–cell interface, led to a promotion of the Sertoli cell TJ-permeability barrier function (Lie et al., 2010). Indeed, one of the most important findings from the above studies was that it illustrated the two actin regulating proteins Eps8 and Arp3 that exhibited stage-specific and restrictive spatiotemporal expression at the BTB during the seminiferous epithelial cycle provided the means for cyclic reorganization of the actin cytoskeleton at the Sertoli cell BTB (Lie et al., 2010, 2009). In fact, besides binding to AJs, TJs and actin, adaptor proteins ZO-1/2/3 also bind to GJs, polarity proteins (e.g. PATJ), actin-binding proteins (e.g. cortactin, AF-6) and a variety of signaling molecules, such as kinases (e.g. c-Src, PKC), transcription factors (e.g. ZONAB, c-Jun) and G proteins (e.g. G protein α subunit) (Gonzalez-Mariscal et al., 2000; Tsukita et al., 2009). Thus, these adaptor proteins also act as scaffolding proteins at the TJ barrier by recruiting other regulatory proteins to the site and to provide cross talks among coexisting junctions at the BTB including TJs, basal ES and GJs.
2.2. Ectoplasmic Specialization (ES)
In epithelia and endothelia, AJ is localized below TJ in the basolateral region of two adjacent cells. It is a discrete structure physically segregated from TJ and is primarily responsible for cell–cell adhesion by connecting to a dense actin cytoskeleton that create a plaque-like ultrastructure known as zonula adherens (Hartsock and Nelson, 2008; Miyoshi and Takai, 2008). In the testis, however, AJ is distinctly different from those found in other epithelia/endothelia, instead a testis-specific ultrastructure known as ES is found. There are two ESs in the seminiferous epithelium dependent on its location. The ES that is found near the basement membrane between adjacent Sertoli cells, and is localized at the BTB is the basal ES, it coexists with TJ and GJ, and is responsible for Sertoli cell–cell adhesion (Cheng and Mruk, 2010b). The ES that is localized to the apical compartment and is the only anchoring device between Sertoli cells and spermatids (steps 8–19 in the rat testis) is the apical ES. ES is associated with an extensive actin filaments arranged in hexagonal bundles with unipolar orientation that lie perpendicular to the Sertoli cell plasma membrane (Mruk et al., 2008; Yan et al., 2007). Interestingly, these actin filaments are noncontractile in nature, thus they are not likely to be involved in germ cell movement as developing germ cells are immobile cells per se, lacking all the cell movement apparatus (e.g. lamellipodia) and Sertoli cells inside the seminiferous epithelium are also not actively motile cells per se (Mruk et al., 2008; Yan et al., 2007). As the actin filament bundles at the ES are restricted only to the Sertoli cell, but not in elongating/elongated spermatids, the ultrastructural features of the apical ES and basal ES are essentially identical except that actin filament bundles are found on both sides of Sertoli cells at the basal ES, but restricted only to the Sertoli cell at the apical ES (Cheng and Mruk, 2010b). Interestingly, the protein composition in both apical and basal ESs is quite different (Cheng and Mruk, 2010b). For instance, JAM-C, nectin-3, β1-integrin, laminin-α3,-β3,-γ3 are restricted to the apical ES, and JAM-A and -B are limited to the basal ES, whereas other proteins, such as CAR, are found in both apical and basal ES (Cheng and Mruk, 2010b). At the apical ES, other than AJ proteins that are usually found in epithelia/endothelia (e.g. N-cadherin, β-catenin, nectin-2), TJ proteins, GJ proteins, and focal adhesion complex (FAC, which is an anchoring junction at the cell–matrix interface) proteins are also found, making this a hybrid junction (Mruk et al., 2008; Wong et al., 2008; Yan et al., 2007).
2.2.1. Basal ES
The basal ES is restricted to adjacent Sertoli cells near the basement membrane at the site of the BTB, which is typified by the bundles of actin filaments sandwiched in-between cisternae of endoplasmic reticulum and the two opposing plasma membranes of Sertoli cells (Cheng and Mruk, 2010b). While the ultrastructural features of basal ES are indifferent from the apical ES, their constituent proteins are quite different as the basal ES shares some similarity with conventional AJ. For instance, constituent adhesion molecules at the basal ES are members of the cadherins and nectins family.
2.2.1.1. Cadherins
Being one of the major constituent proteins of AJs, the importance of cadherins is well demonstrated by the embryonic lethality of mice lacking classical cadherins, such as E-cadherin and N-cadherin (Radice et al., 1997). In rodent testis, the above two classical cadherins are found at the basal ES (Mruk et al., 2008; Yan et al., 2007). They are single span membrane protein having a divergent extracellular domain containing five repeats called ectodomain modules (ECs) and a conserved cytoplasmic tail (Harris and Tepass, 2010; Yonemura, 2011). Binding of Ca2+ ions is necessary for correct protein confirmation of the ECs, which participate in forming homotypic cis-dimers of cadherins on the same side of two neighboring cells. Two cis-dimers of cadherins from each adjacent cells then form homotypic trans-oligomers that create an AJ (Harris and Tepass, 2010; Yonemura, 2011). Although the binding between cadherin extracellular domains is weak, cell–cell adhesion is strengthened via lateral clustering of cadherins, which is a process mediated by nectins (Sakisaka et al., 2007; Takai et al., 2008). Cadherin clustering also required binding of p120-catenin and β-catenin to cadherin juxtamembrane region and cytoplasmic tail, respectively. p120-catenin is essential for the retention of cadherins at the plasma membrane. Studies using siRNA to knockdown p120-catenin or by overexpressing exogenous cadherins have shown that p-120 catenin–cadherin association is able to stabilize the cadherins by preventing cadherins at the cell surface from being internalized and degraded (Davis et al., 2003; Iyer et al., 2004; Maeda et al., 2006). On the other hand, β-catenin–cadherin association promotes cadherin clustering by connecting cadherins to actin cytoskeleton through the adaptor α-catenin, which can bind β-catenin and also actin filaments (Harris and Tepass, 2010; Yonemura, 2011). Studies have shown that during formation of AJs which is initiated by nectins, clustering of cadherins is aided by remodeling of actin cytoskeleton via actin regulating proteins such as the Arp2/3 complex which induces branched actin polymerization for capturing clusters of cadherins (Kametani and Takeichi, 2007; Le Clainche et al., 2007; Sato et al., 2006). However, a disruption of cortical actin filaments can lead to dissolution of cadherins at the cell–cell interface (Quinlan and Hyatt, 1999), illustrating the importance of actin filament network in recruiting cadherin-based AJs to cell–cell interface. It was long believed that AJs were maintained through the association of cadherin–β-catenin–α-catenin complex to actin filaments. However, it is now known that α-catenin cannot simultaneously bind to β-catenin and actin, implying a cadherin-β–catenin–α-catenin–actin association does not exist (Drees et al., 2005). Instead, α-catenin exists as monomers and dimers, which bind to β-catenin and actin, respectively. Clustering of cadherin-β–catenin–α-catenin complex during AJ formation induces a localized concentrated pool of α-catenin that favors its dimerization. Thus, α-catenin dissociates from β-catenin and forms dimers, which in turn associate with actin filaments. Association of α-catenin to actin filament inhibits the activity of the Arp2/3 complex and hence, reorganizing F-actin network from a “branched” to a “bundled” conformation (Drees et al., 2005), thereby stabilizing cell–cell adhesions with bundles of cortical actin filaments. In this context, it is of interest to note that while AJs may connect to the actin cytoskeleton via the nectin–afadin complex, the strong adhesion provided by AJs in an epithelium is difficult to achieve without the cadherin–β-catenin–α-catenin–actin association (Harris and Tepass, 2010). Moreover,when the actin-binding domain of α-catenin is deleted, the directional movement of cadherin–α-catenin fusion proteins to the apical junctional complex is abolished, illustrating binding of α-catenin to actin filaments is essential for actin cytoskeleton-mediated lateral flow of cadherins (Kametani and Takeichi, 2007). It seems that there are missing links regarding how α-catenin connects the cadherin–β-catenin complex to actin cytoskeleton, and additional research is needed in this area.
2.2.1.2. Nectins
Nectins are a family of immunoglobulin-like cell adhesion molecules with four members known to date, namely nectin-1 to -4. In general, each nectin has an extracellular domain which contains three Ig-like loops, a transmembrane region and a cytoplasmic tail (Sakisaka et al., 2007; Takai et al., 2008). Each nectin member first forms homotypic cis-dimers, which in turn form homotypic or heterotypic trans-dimers in a Ca2+-independent manner. Interestingly, the adhesive force between heterotypic trans-dimers is stronger than that between homotypic trans-dimers (Sakisaka et al., 2007; Takai et al., 2008). Nectins are connected to actin cytoskeleton via a cytoplasmic adaptor afadin (Sakisaka et al., 2007; Takai et al., 2008). Besides binding to nectins via PDZ domain and actin filaments via its C-terminal tail, afadin indeed has multiple domains, enabling it to associate with different proteins, such as c-Src, Rap1 (a small G protein), ZO-1, α-catenin (Sakisaka et al., 2007; Takai et al., 2008). This thus mediates signal transduction and provides cross talk between cadherin- and nectin-based junctions. Studies have demonstrated that by coupling with actin reorganization, nectins are responsible for initiating AJ formation and for recruiting cadherins to complete the process. As epithelial cells initiate cell–cell contact, trans-interacting nectins from adjacent cells were found to activate Cdc42 (a small GTPase of the Rho-subfamily), Rac (also a signaling GTPase) via c-Src in an afadin-independent manner (Fukuyama et al., 2005; Kawakatsu et al., 2005, 2002). Activated Cdc42 and Rac, in turn, trigger reorganization of actin cytoskeleton through the actin-binding protein IQGAP1, which induce branched actin polymerization via the Arp2/3 complex (Le Clainche et al., 2007; Sato et al., 2006) to recruit cadherins to the site. It is noted that at this step, the recruited cadherins are non-trans-interacting since they have yet to associate with cadherins from neighboring cells. Clustering of these non-trans-interacting cadherins is then assisted by afadin-associated trans-interacting nectins. This is achieved by activation of Rap1 by trans-interacting nectins, activated Rap1 then associates with afadin to form a complex, which in turn binds to p120-catenin to retain cadherins at plasma membrane (Hoshino et al., 2005; Sato et al., 2006). Hence, localized clustering of cadherins takes place which favors the trans-interaction of cadherins to establish AJs.
Nectin-2 is expressed in rodent Sertoli cells (Bouchard et al., 2000; Ozaki-Kuroda et al., 2002). Mice lacking nectin-2 are infertile illustrating nectin-2 is indispensable for spermatogenesis (Bouchard et al., 2000; Ozaki-Kuroda et al., 2002). Although studies of mice lacking nectin-2 were focused on apical ES (Kawakatsu et al., 2002) or spermatids (Bouchard et al., 2000), it was noted that the actin filament bundles at the apical ES in these mice were absent, suggesting that their BTB might have been disrupted due to a disorganized actin cytoskeleton.
2.2.1.3. Interplay between AJs and TJs Via Adaptor Proteins
As noted above, cell adhesion molecules cross talk with each other via their peripheral adaptors to maintain epithelial homeostasis. For instance, AJs are crucial for TJ assembly, and ZO-1 is a crucial player in this process (Hartsock and Nelson, 2008; Sakisaka et al., 2007). Studies have shown that nectin–afadin complex is able to recruit ZO-1, which was then used to recruit JAMs, claudins and occludin to the apical junctional complex to form TJs (Ooshio et al., 2010; Yokoyama et al., 2001). The necessity of trans-interacting nectins in the establishment of TJs was demonstrated when such interaction was blocked via the use of a chimeric protein that bound to the extracellular region of nectins, the recruitment of JAMs (Fukuhara et al., 2002a), claudins and occludin (Fukuhara et al., 2002b) for TJ assembly was impaired. Moreover, the importance of trans-interacting nectin–afadin association in initiating TJ assembly was shown by expressing nectins with a truncated C-terminus, rendering nectins incapable of binding to afadin, leading to an impairment to recruit ZO-1 to establish TJs (Yokoyama et al., 2001). Furthermore, interaction between afadin and ZO-1 is important for TJ assembly since a knockdown of either afadin or ZO-1, or over-expression of a truncated form of afadin that failed to bind to ZO-1 after the knockdown of endogenous afadin, impeded TJ formation (Ooshio et al., 2010). Besides playing a crucial role in TJ assembly, AJs are also essential for TJ maintenance, as a disruption of AJs often leads to TJ disassembly. For instance, when E-cadherin-mediated cell–cell adhesion was inhibited by treatment of an anti-E-cadherin antibody (Man et al., 2000), or when E-cadherin was downregulated after depletion of cellular polyamines (Guo et al., 2003), a disruption of the TJpermeability barrier was detected, illustrating a primary loss of AJ function leads to a secondary dysfunction of TJs. More important, cross talk between AJs and TJs is not unidirectional since AJ integrity is also dependent on the integrity of TJs. For instance, downregulation of occludin induced by transfecting PA4 (polyaxonal amacrine 4 cells of retina) epithelial cells with Raf-1, mislocalization of E-cadherin was observed, suggesting AJ disruption (Li and Mrsny, 2000). Collectively, these findings illustrate that while TJs and AJs are found in discrete locations in epithelia/endothelia, they are still functionally connected via their peripheral adaptor proteins. At the BTB, TJ and basal ES coexist in the same location, and such intimate relationship is especially important to elicit transient “opening” and “closing” of the barrier during the transit of preleptotene spermatocytes at stage VIII–IX of the epithelial cycle. It was noted that treatment of adult rats with adjudin at 50 mg/kg b.w. that was effective to induce germ cell loss from the epithelium except spermatogonia (Mok et al., 2012b; Yan and Cheng, 2005) did not impede the BTB integrity. During the process of adjudin-induced germ cell loss, the adaptor proteins α-catenin and ZO-1 at the basal ES and TJ, respectively, which were originally tightly associated (“engaged”) for linking basal ES and TJ together to reinforce the BTB integrity, became dissociated (“disengaged”). Thus, a primary disruption of the apical ES at the Sertoli–spermatid interface that facilitates germ cell loss do not perturb the TJ-barrier function at the BTB since the adaptors that link basal ES (e.g. catenins) and TJ (e.g. ZO-1) together are “disengaged” during adjudin-induced germ cell loss (Yan and Cheng, 2005). This thus illustrates that a novel mechanism is in place in the testis to safeguard the BTB integrity in response to changes in environment, such as following exposure to a toxicant, or during the epithelial cycle of spermatogenesis, when spermatids are in transit across the seminiferous epithelium involving localized apical ES restructuring, so that the BTB integrity can be maintained via “disengagement” of basal ES and TJ proteins.
2.2.2. Apical ES
In rodents, the apical ES, once it appears, is the only anchoring device between Sertoli cells and elongating spermatids (step 8–19 in rats). Besides conferring adhesion and structural support to developing spermatids, the apical ES also confers spermatid polarity during spermiogenesis so that the heads of developing spermatids are pointing toward the basement membrane, thus, the maximal number of spermatids can be packed in the seminiferous epithelium of a tubule (Wong and Cheng, 2009). Although the actin filament bundles, the hallmark ultrastructure of the ES, are only visible on the Sertoli cell, not the spermatid, at the apical ES (Cheng and Mruk, 2010b; Mruk et al., 2008), but the stage-specific expression of cadherins (Johnson and Boekelheide, 2002; Lee et al., 2003), nectin-3 (Ozaki-Kuroda et al., 2002) and laminin-α3, -β3, and γ-3 chains (Koch et al., 1999; Siu and Cheng, 2004; Yan and Cheng, 2006) by the spermatids during the epithelial cycle suggest that spermatids also play a role in establishing the apical ES. Apical ES is the strongest anchoring devices between Sertoli cells and spermatids (steps 8–19), significantly stronger than DSs between Sertoli cells and spermatids (steps 1–7) (Wolski et al., 2005). This unusual adhesive force is contributed by a number of factors. For instance, nectin-3 is exclusively expressed by elongating/elongated spermatids in the testis and this enables the formation of heterotypic trans-interaction between nectin-3 from germ cells and nectin-2 from Sertoli cells to yield a strong cell–cell adhesion. Furthermore, the hybrid nature of the apical ES also supports its adhesive strength. Among the different junction proteins present at the apical ES, it is believed that the interaction between laminin-333 (composed of laminin α3, β3, γ3 chains) from elongating/elongated spermatids and the α6β1-integrin from Sertoli cells contribute significantly to its adhesive force (Palombi et al., 1992; Salanova et al., 1995; Yan and Cheng, 2006). Interestingly, besides performing the anchoring function at apical ES, the laminin-333–α6β1-integrin protein complex also participates in regulating BTB integrity at the apical ES–BTB–hemidesmosome axis (Fig. 6.2). It was proposed that during spermiation, laminin chains at the apical ES was cleaved by matrix metalloproteinases, such as MMP-2, which was highly expressed at the apical ES at stage VIII of the epithelial cycle (Siu and Cheng, 2004), to facilitate the release of mature spermatids at spermiation (Yan et al., 2008a). Some of these fragments of laminin chains, which were shown to regulate cell-adhesion function in other epithelia (Yan et al., 2008b) were shown to perturb the Sertoli cell TJ-permeability barrier function (Yan et al., 2008a). This functional axis between the apical ES and the BTB was confirmed by adding purified recombinant laminin fragments into Sertoli cell cultures with an established TJ barrier, which was shown to disrupt the TJ barrier in vitro via down-regulation of integral membrane proteins occludin and JAM-A at the BTB, and similar observations were obtained by overexpressing these laminin fragments in Sertoli cells (Yan et al., 2008a). Surprisingly, laminin fragments were also found to reduce the level of β1-integrin at the hemidesmosome (an intermediate filament based cell–matrix anchoring junction present at the Sertoli cell–basement membrane interface) (Yan et al., 2008a). A knockdown of β1-integrin at the hemidesmosome in Sertoli cell epithelium in vitro also led to a disruption of the TJ barrier via redistribution of occludin and N-cadherin, with these proteins moved from the cell–cell interface into the cell cytosol (Yan et al., 2008a), illustrating there is a functional link between the hemidesmosome and the BTB. These findings thus illustrate that while spermiation and BTB restructuring that take place at the opposite ends of the epithelium at stage VIII of the epithelial cycle, they are functionally connected via the apical ES–BTB–hemidesmosome axis. The presence of this axis was recently confirmed by using a Sertoli cell injury model using phthalates, in which phthalate-induced apical ES disruption that led to spermatid lose and accompanied by a reducing level of laminins also induced a MMP-mediated BTB disruption (Yao et al., 2009, 2010).
2.3. Gap Junctions
The building blocks of GJs are integral membrane proteins known as connexins (Cx) such as Cx26, 33, 43. Six connexins form a hemichannel called connexon, and a connexon from one cell that docks with another connexon of an apposing or adjacent cell forms a functional GJ (Enders, 1993; Li et al., in press; Pointis et al., 2010). The primary function of GJs is to act as communicating channels between neighboring cells for mediating cell–cell communication for signal transduction (Bosco et al., 2011; Giepmans, 2004). In general, these channels allow diffusional exchange of ions and small molecules that are <1 kD in size, however, GJs assembled by different connexins indeed have variations among their pore size (Bosco et al., 2011; Giepmans, 2004). More than 20 connexins have been identified in rodent and human genomes. GJ can be composed of homotypic or heterotypic connexons, as such, a variety of GJs can be produced. Additionally, control of passage of molecules across GJs can be further modulated in a connexinspecific manner (Bosco et al., 2011; Giepmans, 2004). GJs can also interact with AJs and TJs through the shared adaptor ZO-1. Thus, ZO-1 also link GJs to actin cytoskeleton, which is important for proper localization of GJs (Giepmans and Moolenaar, 1998; Laing et al., 2001; Toyofuku et al., 1998). Besides mediating signaling between neighboring cells, GJs are also involved in modulating the function of AJs and TJs (Derangeon et al., 2009; Kojima et al., 2007) including TJ-barrier function at the BTB (Li et al., 2009). Studies have shown that in cultured Sertoli cells, a transient induction of Cx33 coincides with a surge in the expression of N-cadherin (Chung et al., 1999), and blocking the trans-interaction of connexons with synthetic peptides leads to mislocalization of N-cadherin (Lee et al., 2006), illustrating the involvement of GJs in the assembly and maintenance of AJs in the testis. Furthermore, the requirement of GJs in inducing TJ assembly and its maintenance was revealed in studies via overexpression of exogenous Cx32 in hepatocytes isolated from Cx32-deficient mice that led to an induction of TJs in these cells (Kojima et al., 2002). Furthermore, a disruption of GJ-communication in Caco-2 cells (human colonic epithelial cell line) resulted in TJ-barrier disruption (Morita et al., 2004). These studies illustrate GJ proteins themselves and/or GJ-mediated cell–cell communication is essential to the assembly and/or maintenance of AJs and TJs. Thus, GJs are expected to be crucial for BTB maintenance during spermatogenesis. In fact, spermatogenesis was disrupted in mice with Sertoli cell-specific deletion of Cx43 (Brehm et al., 2007; Carette et al., 2010). In these Cx43 SC only KO mice, spermatogenesis was arrested in which spermatogonia failed to differentiate beyond type A (Carette et al., 2010). Furthermore, a knockdown of Cx43 in cultured Sertoli cells with an established functional TJ-permeability barrier by RNAi perturbed the “resealing” of a disrupted TJ barrier induced by either Ca2+ depletion or treatment with bisphenol A (Li et al., 2010). Such a loss of the ability of the Sertoli cell to “reseal” the disrupted TJ barrier following Cx43 knockdown was shown to be mediated, at least in part, by changes in the localization of AJ and TJ proteins at the BTB, rendering their BTB proteins incapable of redistributing to their proper sites to “reseal” the disrupted BTB (Li et al., 2010). Moreover, in cultured Sertoli cells, the simultaneous knockdown of both Cx43 and plakophilin-2 (PKP-2 a desmosomal adaptor protein) was found to induce mislocalization of TJ proteins occludin and ZO-1, as well as an increase in endocytosis of N-cadherin, thereby destabilizing the TJ barrier (Li et al., 2009). Thus, these findings are consistent with studies in other epithelia that GJs are required for proper functioning of basal ES and TJs at the BTB in the rat testis, possibly mediated by transmitting signals among different junction types to coordinate their functions to maintain the BTB homeostasis during the epithelial cycle of spermatogenesis.
3. MAMMALIAN TARGET OF RAPAMYCIN (mTOR)
3.1. Introduction
The discovery of TOR, a Ser/Thr protein kinase, in yeasts was aided by using an antibiotic called rapamycin, which was found to specifically inhibit the activity of TOR and was thus designated “target of rapamycin (TOR).” Subsequent studies have identified its homolog in mammalian cells designated mammalian target of rapamycin (mTOR) (Brown et al., 1994; Chiu et al., 1994; Sabatini et al., 1994). Much attention was drawn to mTOR for its essential role in cell growth and proliferation as mTOR is the key regulator for sensing and integrating diverse environmental clues including growth factors, mitogens and nutrients so that appropriate cellular responses can occur in response to these changes (Laplante and Sabatini, 2012). Subsequent studies have shown that mTOR, besides protein synthesis that affects cell growth and proliferation, is virtually involved in almost all aspects of cellular function such as actin cytoskeleton reorganization, cell survival, and autophagy (Appenzeller-Herzog and Hall, 2012; Chi, 2012; Laplante and Sabatini, 2012; Nair and Ren, 2012), as well as pathogenesis such as carcinogenesis (Ekman et al., 2012; Fasolo and Sessa, 2012; Lieberthal and Levine, 2012; Posadas and Figlin, 2012; Sheppard et al., 2012). Dysregulation of mTOR signaling is observed in different pathological conditions, such as diabetes, cancer and obesity (Weichhart, 2012; Zoncu et al., 2011). mTOR belongs to PIKK (PI3K-related kinase) superfamily as its C-terminus shares strong homology to the catalytic domain of PI3K. However, instead of being a lipid kinase, mTOR is a Ser/Thr protein kinase. In order to execute its cellular functions, mTOR forms one of the two complexes, namely mTORC1 and mTORC2, by associating with different binding partners (Dazert and Hall, 2011; Laplante and Sabatini, 2012). mTORC1 is composed of mTOR, regulatory associated protein of mTOR (raptor), PRAS40, mLST8 and deptor. mTORC1 is responsible for the well-known roles of mTOR that regulates cell growth and proliferation by modulating protein synthesis. Moreover, mTORC1 is sensitive to rapamycin, which acts as an allosteric inhibitor for mTORC1 by associating with FKBP12 to form a complex. This complex binds to mTOR in a short stretch of sequence near its C-terminus known as the FKBP12–rapamycin-binding domain, causing dissociation of raptor from mTORC1 (Senqupta et al., 2010; Zhou and Huang, 2010). And for another mTOR complex, the mTORC2 was first described as rapamycin insensitive as FKBP12–rapamycin complex does not bind to mTORC2 (Oh and Jacinto, 2011; Zhou and Huang, 2010). The key binding partner of mTORC2 is rictor (rapamycin-insensitive companion of mTOR). Unlike mTORC1, mTORC2 regulates actin cytoskeleton and cell survival. Besides rictor, other subunits of mTORC2 include Sin1, mLST8, deptor, Hsp70 and protor-1/2. Interestingly, subsequent studies have shown that while mTORC2 is insensitive to rapamycin, but this is limited to short-term exposure since prolonged rapamycin challenge at up to 24 h leads to the dissociation of rictor from mTOR, disabling the mTORC2 signaling (Sarbassov et al., 2006). Although FKBP12–rapamycin complex does not bind to mTORC2, it was proposed that after long-term treatment, the availability of mTOR decreased as newly synthesized mTOR was occupied by FKBP12–rapamycin complex, preventing the formation of mTORC2. Different binding partners among mTORC1 and mTORC2 allow these kinases responding to different stimulating signals so that they can phosphorylate unique sets of substrates to induce distinctive physiological responses.
3.2. Mammalian Target of Rapamycin Complex 1 (mTORC1)
mTORC1 is composed of mTOR, raptor, proline-rich Akt/PKB substrate 40 kDa (PRAS40), mTOR associated protein LST8 homolog (mLST8) and DEP domain-containing mTOR-interacting protein (deptor) (Fig. 6.3). Among them, raptor is the key binding partner which acts as a critical scaffolding protein that controls mTORC1 assembly and the selection of substrates (Kim et al., 2002; Nojima et al., 2003; Schalm et al., 2003). In the absence of nutrients, raptor associates with mTOR stably to repress mTORC1 catalytic activity while under nutrient-rich conditions, the binding of raptor to mTOR is unstable but this unstable mTOR–raptor association is necessary for mTORC1 to carry out its kinase activity (Kim et al., 2002). Raptor can be phosphorylated at multiple sites for either up- or down-regulating mTORC1 activity (Zhou and Huang, 2010). For instance, under energy stress conditions, AMP-activated protein kinase (AMPK) phosphorylates raptor on S722 and S792 to induce binding of 14-3-3 protein to mTORC1 to elicit its inhibition, leading to cell cycle arrest (Gwinn et al., 2008). Activation of mTORC1 by mitogens, however, is mediated via phosphorylation of raptor on S719, S721 and S722 by p90 ribosomal S6 kinases (RSKs) (Carriere et al., 2008). Deptor (an inhibitor of mTOR) and mLST8 are common subunits among mTORC1 and mTORC2. Deptor binds to both mTOR complexes and functions as a negative regulator (Peterson et al., 2009). For mLST8, it is required for mTORC2 to maintain its activity (Guertin et al., 2006). However, the necessity for mLST8 in activating mTORC1 signaling remains unclear. The binding of mLST8 to mTORC1 was shown to stimulate mTORC1’s kinase activity toward S6K1 and 4E-BP1 (Kim et al., 2003). However, in mLST8-deficient fibroblasts, the association between mTOR and raptor, as well as the phosphorylation of substrates of mTORC1 are not impaired, indicating mLST8 has limited function for mTORC1 in fibroblasts (Guertin et al., 2006). Thus, it is of interest to determine whether there are mLST8-like protein(s) to rescue the function of mTORC1 in mLST8-deficient fibroblasts (Guertin et al., 2006). PRAS40 is another negative regulator of mTORC1 (Oshiro et al., 2007; Wang et al., 2007). PRAS40 inhibits mTORC1 activity by binding to mTORC1 via raptor, and phosphorylation of PRAS40 by PKB leads to its detachment from mTORC1, activating the complex (Wang et al., 2008). When mTORC1 is activated by appropriate signals, mTORC1 induces cell growth and proliferation via up-regulation of protein synthesis by phosphorylating S6 protein kinase (S6K) and eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) (Dazert and Hall, 2011; Laplante and Sabatini, 2012).
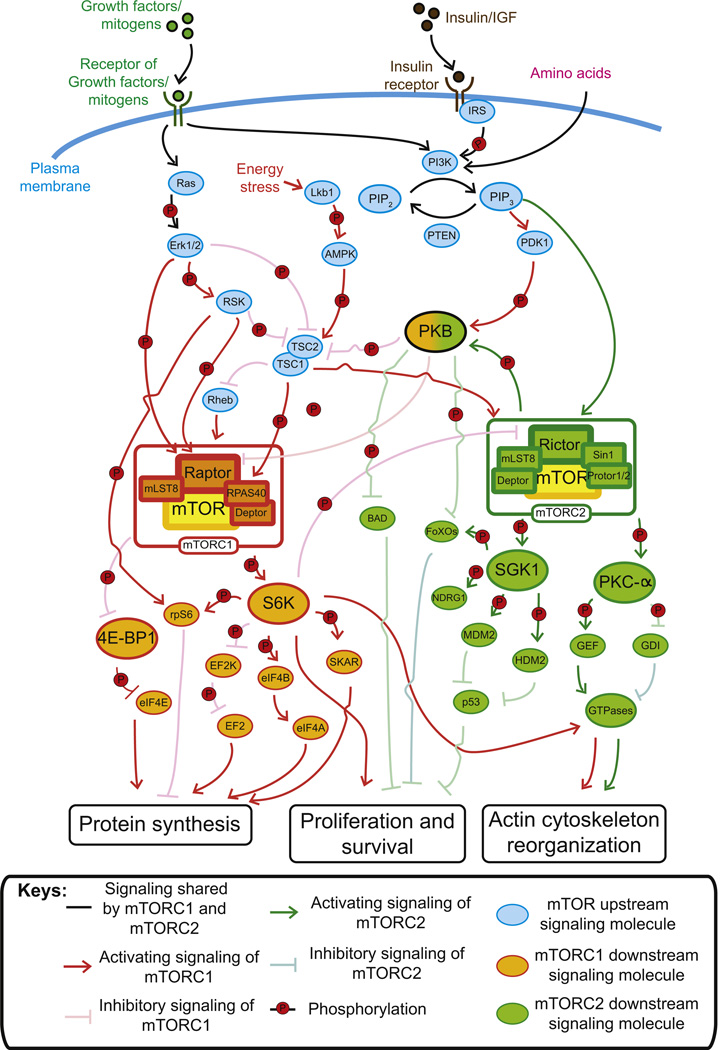
Figure 6.3. The likely mTOR signaling pathways involving mTORC1 and mTORC2 and the corresponding interacting/regulatory proteins that regulate different cellular events including BTB function in the testis via the effects on F-actin organization.
By assembling with different subunits, two mTOR complexes can be formed, namely, mTORC1 and mTORC2. Besides mTORC1 that is specifically regulated by the energy status of a cell, both mTOR complexes are activated by growth factors (e.g. insulin), mitogens and amino acids. Upon activation, except that upregulation of protein synthesis for cell growth is specifically mediated by mTORC1, the control of cell proliferation and survival as well as actin cytoskeleton organization is modulated by both complexes, despite the fact that they have their unique substrates and downstream signaling molecules. Moreover, mTORC1 and mTORC2 share several upstream signaling molecules. For example, PIP3 can activate both complexes while TSC1/2 complex inhibits mTORC1 but activates mTORC2. Furthermore, the signaling pathways of the two mTOR complexes are interconnected in which S6K1, the substrate of mTORC1, is able to phosphorylate rictor and thus inhibits mTORC2. As such, phosphorylation of PKB, which is the substrate of mTORC2, can be reduced. Since PKB phosphorylation is required for activating mTORC1, this leads to suppression of mTORC1 signaling and therefore, a negative feedback loop is established. For color version of this figure, the reader is referred to the online version of this book.
3.2.1. Upstream Signaling Molecules of mTORC1
As noted above, the activity of mTORC1 is modulated by stimuli such as growth factors, mitogens, amino acids and energy status (Fig. 6.3). For the growth factors that trigger mTORC1 signaling, insulin is among the best studied (Magnuson et al., 2012; Zoncu et al., 2011). Upon binding of insulin or insulin-like growth factor (IGF) to its receptors, autophosphorylation of these receptors takes place, which then phosphorylates the insulin receptor substrates (IRS). Activated IRS in turn phosphorylates PI3K, which catalyzes the conversion of phosphatidylinositol (4, 5)-bisphosphate (PIP2) to phosphatidylinositol-3, 4, 5-triphosphate (PIP3). This conversion can be reversed by phosphatases and tensin homolog on chromosome 10 (PTEN), which is an important negative regulator of mTORC1 pathway by converting PIP3 to PIP2, thus dysregulation of PTEN is detected in numerous kinds of cancer (Song et al., 2012). PIP3 recruits 3-phosphoinositide-dependent kinase 1 (PDK1) to phosphorylate PKB on T308 and for full activation, PKB is then phosphorylated by another kinase on S473 (Alessi et al., 1997; Andjelkovic et al., 1997) (Fig. 6.3). Activated PKB phosphorylates and inhibits tuberous sclerosis complex 2 (TSC2), which associates with TSC1 to form a complex that inhibits mTORC1 (Manning et al., 2002). As GTP-bound Ras-homolog enrich in brain (Rheb) is required for the activation of mTORC1, the inhibitory effect of TSC1/2 complex is mediated via its GTPase activity that acts on Rheb to maintain Rheb in a GDP-bound status. After the phosphorylation of TSC2, TSC1/2 complex is inhibited and hence, Rheb-GTP is accumulated for the activation of mTORC1.
In addition to TSC1/2 complex, PKB also promotes mTORC1 signaling by phosphorylating PRAS40. As such, PRAS40 is dissociated with raptor and hence, its inhibitory effect is removed (Wang et al., 2008). Moreover, besides the above PKB-mediated pathways, binding of growth factors or mitogens to their receptors can also activate mTORC1 via the Ras-Raf-MEK-ERK signaling. Upon the above activation, the small GTPase Ras eventually leads to phosphorylation of ERK1 and ERK2, which in turn inhibits the TSC1/2 complex by directly phosphorylating TSC2 or via activation of RSK1 that also phosphorylates and inhibits TSC2 (Ma et al., 2005; Roux et al., 2004). Furthermore, ERK1/2 and RSK1 also phosphorylate raptor to promote mTORC1 functions (Carriere et al., 2008, 2011) (Fig. 6.3). While mTORC1 signaling has to be “on” to upregulate protein synthesis in response to growth factors and mitogens, there are conditions that mTORC1 pathway has to be “off,” for example, when cells are in energy stress. When cellular ATP decreases, the rise of AMP/ATP ratio activates Lkb1 (liver kinase B1, also known as Ser/Thr kinase 11, STK11) to phosphorylate AMPK (Shackelford and Shaw, 2009), which in turn phosphorylates raptor, inhibiting mTORC1 as mentioned above (Gwinn et al., 2008). Besides, AMPK phosphorylates and activates TSC2, thus mTORC1 is suppressed by the TSC1/2 activity which catalyzes the conversion of Rheb-GTP to Rheb-GDP (Inoki et al., 2003).
3.2.2. Downstream Signaling Molecules of mTORC1
3.2.2.1. S6 Protein Kinases
Upon activation, mTORC1 up-regulates protein synthesis mainly through its two substrates S6K and 4E-BP1. In mammals, S6K is a family of protein kinases that has two members, namely S6K1 and S6K2, which are encoded by two different genes and share high homology (Lee-Fruman et al., 1999). The necessity of S6K in regulating cell and body size is well illustrated in genetic models deficient in either S6K1 or S6K2, or both. The loss of function of S6K in Drosophila led to a serve decline in body size due to reduction in cell size rather than cell number (Montagne et al., 1999). In mice lacking S6K1, body size was significantly smaller versus the wild type at birth and during postnatal growth, attributing by the reduced size in all organs (Shima et al., 1998). The reduction in size of organs was probably caused by decreased cell size, such as pancreatic β-cells (Pende et al., 2000) and myoblasts (Ohanna et al., 2005). In contrast, in mice lacking S6K2, body size was insignificantly different from the wild type at birth and during postnatal development, suggesting S6K2 is not required for regulating cell size in rodents. Furthermore, in mice deficient in both S6K1 and S6K2, body size of the surviving animals during embryonic and postnatal growth was not further reduced compared to that of S6K1-deficient mice (Pende et al., 2004) and the size of myoblasts from S6K1- and S6K2-deficient mice was similar to that of mice lacking S6K1 (Pende et al., 2004). These findings thus suggest that control of cell size seems to be mostly regulated by S6K1 in rodents. Moreover, it has been reported that mice lacking both forms of S6K are prone to suffer perinatal death, unlike mice lacking either form of S6K, which were viable and fertile (Pende et al., 2004). This indicates that although S6K2 may not contribute as much as S6K1 in regulating cell size, these two isoforms do have overlapping roles and therefore, loss of one isoform can be superseded, at least in part, by the other.
Ribosomal protein S6 (rpS6) was the first identified substrate of S6K1 for modulating protein synthesis (Gressner and Wool, 1974). Subsequent studies have identified other substrates of S6K1, which include elongation factor 2 (EF2) kinase, eukaryotic initiation factor 4B (eIF4B), programmed cell death 4 (PDCD4) and S6K Aly/REF-like substrate (SKAR) that promote protein synthesis via up-regulating translational activity. It is known that S6K1 phosphorylates and inactivates EF2 kinase (EF2K), leading to dephosphorylation and activation of EF2, which in turn promotes translation elongation (Wang et al., 2001). S6K1 also phosphorylates eIF4B on S422, resulting in enhanced translation initiation by stimulating the RNA helicase eIF4A to unwind mRNA for translation (Raught et al., 2004). The above process is further enhanced by phosphorylating the eIF4A inhibitor, PDCD4 (note: each PDCD4 molecule can bind two molecules of eIF4A) by S6K1 on S67 as such phosphorylation promotes PDCD4 degradation (Dorrello et al., 2006; Shahbazian et al., 2006). Furthermore, studies revealed that S6K1 also promoted protein translation by phosphorylating SKAR on S383 and S385 (Richardson et al., 2004). It is of interest to point out that SKAR was shown to be specifically phosphorylated by S6K1, but not S6K2, in regulating cell size (Richardson et al., 2004).
Besides regulating cell growth, S6K is also involved in stimulating cell proliferation. Rapamycin treatment has been shown to arrest cell cycle in mammalian lymphocytes at G1 phase; however, rapamycin treatment only delays cell cycle progression in other mammalian cell types (Abraham and Wiederrecht, 1996). This indicates the significance of mTORC1 signaling in cell cycle progression and S6K is one of the mediators since G1 phase progression was shown to be accelerated by overexpression of constitutively active S6K1 (Fingar et al., 2004). On the other hand, the importance of S6K2 in cell proliferation is illustrated in study demonstrating S6K2 was responsible for the interleukin-3 (IL-3)-driven cell proliferation since S6K2 was activated in lymphocytes and primary mouse bone marrow-derived mast cells upon IL-3 induced proliferation; and cell cycle progression was accelerated by overexpression of constitutively active S6K2 in lymphocytes (Cruz et al., 2005). Furthermore, the association of heterogeneous ribonucleoprotein (hnRNP) F with mTOR and S6K2, but not S6K1, is essential for driving cell proliferation (Goh et al., 2010). Taking collectively, both S6K1 and S6K2 are involved in mTORC1-mediated cell cycle progression. Interestingly, S6K1 is predominantly found in the cell cytosol versus S6K2 in the cell nucleus (Lee-Fruman et al., 1999).
3.2.2.2. Ribosomal Protein S6 (rpS6)
rpS6 was the first S6K substrate identified, and was thought to be its effector to upregulated protein synthesis (Magnuson et al., 2012). rpS6 is one of the ribosomal proteins of the 40S subunit of eukaryotic ribosomes (Wool, 1996). Much attention was given to rpS6 in the past since it was shown to undergo inducible phosphorylation upon a wide range of stimuli that upregulated protein synthesis (Gressner and Wool, 1974; Thomas et al., 1982; Wettenhall and Howlett, 1979; Wool, 1979). rpS6 can be phosphorylated in five residues located at the C-terminus: S235, S236, S240, S244 and S247 (Bandi et al., 1993; Krieq et al., 1988). It was suggested that phosphorylation progressed in an orderly manner that S236 is the primary phosphorylation site (Flotow and Thomas, 1992; Wettenhall et al., 1992). Full phosphorylation of rpS6 requires the presence of both S6K isoforms with S6K2 being the predominant kinase. However, studies reported in cells lacking both S6K or after rapamycin treatment wherein S6K activation was completely abolished, yet rpS6 was still being phosphorylated on S235 and S236. This thus illustrates S6K is not the only kinase for rpS6 (Pende et al., 2004). Indeed, rpS6 can be phosphorylated by RSK (p90 ribosomal S6 kinase), via the Ras-Raf-MEK-ERK signaling (Roux et al., 2007) (Fig. 6.3). Being the substrate of both S6K and RSK, which are kinases that are known to upregulate protein synthesis, it was once believed that rpS6 promoted protein translation. It is because upon stimulation of cells by growth factors, mitogens and/or nutrients, rpS6 phosphorylation was positively correlated to translational activation of a class of mRNAs having characteristic 5′ terminal oligopyrimidine (TOP) tract, as both events took place simultaneously. These mRNAs, known as TOP mRNAs, are responsible for encoding numerous translational apparatus. Hence, based on the fact that rpS6 is a subunit of ribosome that undergoes phosphorylation during protein synthesis upregulation, rpS6 was thought to be responsible for stimulating the translation of TOP mRNAs (Meyuhas, 2000). Furthermore, translational activation of TOP mRNAs upon stimulation by mitogens was abolished by rapamycin treatment in some cell lines seemingly reinforced the above hypothesis (Hornstein et al., 2001). This concept, however, has been challenged by subsequent studies. First, in several cell lines, only a minor or no suppression of TOP mRNAs translation was found after rapamycin treatment, regardless of a complete activation blockage of S6K or its substrate rpS6 by rapamycin (Tang et al., 2001). Moreover, in amino acid starved cells, neither phosphorylation of rpS6 nor activation of S6K1 was sufficient to stimulate the translation of TOP mRNAs, whereas overexpression of dominant negative S6K1 which inhibited the activity of S6K1 and rpS6 phosphorylation failed to cause translational repression of TOP mRNAs in amino acid refed cells (Tang et al., 2001). Besides, even in dividing lymphoblastoids that S6K1 was active and rpS6 was phosphorylated, translation of TOP mRNAs was constitutively repressed (Stolovich et al., 2005). Furthermore, in some cell lines, the relief of translation repression of TOP mRNAs by LiCl was found to be independent of S6K and rpS6 (Stolovich et al., 2005). Collectively, these studies indicate that rpS6 phosphorylation is not indispensable for translational activation of TOP mRNAs and this possibility was validated by a study demonstrating that in mice expressing knockin nonphosphorylatable rpS6 (rpS6p−/−), normal TOP mRNAs translation was detected (Ruvinsky et al., 2005). In short, it is increasingly clear that translational activation of TOP mRNAs is not mediated by rpS6 phosphorylation, and there is growing evidence to show that cell growth and even protein synthesis are not upregulated by phosphorylated rpS6, at least not in all mammalian cells. This notion is supported by studies using conditional rpS6 knockout mice or rpS6p−/− mice. It has been reported that after fasting that caused loses in weight and protein content in liver, the liver mass and total protein content of both wild-type and rpS6 conditional knockout mice recovered to the same extent and at the same rate, clearly demonstrating rpS6 is dispensable for cell growth and protein synthesis (Volarevic et al., 2000). Furthermore, in liver, relative proportion of ribosomes associated with polysomes was similar between rpS6p−/− and wild-type mice (Ruvinsky et al., 2005). More importantly, in mouse embryonic fibroblasts (MEFs) that derived from rpS6p−/− mice, instead of protein synthesis retardation, a significant increase in rate of protein synthesis was observed (Ruvinsky et al., 2005). The studies using rpS6p−/− mice revealed that phosphorylation of rpS6 was not necessary for the efficient polysome recruitment for translation, and in fact protein synthesis was negatively regulated by phosphorylated rpS6. Therefore, it is now generally accepted that upon stimulations, such as by growth factors, mitogens and nutrients, that induce cell growth, mTORC1 upregulates protein synthesis via its substrates, S6K and 4E-BP1. The role of rpS6 is likely to fine tune the above process by playing a role as a negative regulator (Ruvinsky and Meyuhas, 2006). Similar to the kinase S6K, rpS6 may also be involved in the regulation of cell proliferation, such as proliferation of liver cells (Volarevic et al., 2000). Also, mouse embryonic fibroblasts derived from rpS6p−/− displayed an accelerated cell division, indicating rpS6 phosphorylation regulates cell proliferation negatively in these fibroblasts (Ruvinsky et al., 2005).
3.2.2.3. 4E-Binding Protein 1
Besides S6K, another well-characterized substrate of mTORC1 for mediating protein synthesis is 4E-BP1, which is a repressor of the translation initiation factor eIF4E (Pause et al., 1994). When mTORC1 signaling is not activated, eIF4E is sequestered by hypophosphorylated 4E-BP1. However, upon stimulation such as growth factors and mitogens, activated mTORC1 phosphorylates 4E-BP1 at six sites: T37, T46, T70, S65, S83 and S112, leading to dissociation of 4E-BP1 from eIF4E. eIF4E is thus free to bind to eIF4G, which is a scaffolding protein that recruits eIF4A and coordinates the binding of small ribosomal subunits to the mRNA. Association of eIF4E with eIF4G and eIF4A forms a complex called eIF4F which binds to the 5′-end of mRNA (Marcitrigiano et al., 1999) for the recruitment of 40S ribosome and eventually results in the formation of 48S translation preinitiation complex (Gingras et al., 1999).
Other than regulating cell growth and proliferation, mTORC1 signaling plays a wide variety of physiological roles including autophagy, aging, memory and even actin reorganization (Weichhart, 2012; Zoncu et al., 2011). While mTORC1 and mTORC2 are two distinct signaling complexes having unique roles, they may work together in regulating many cellular events.
3.3. Mammalian Target of Rapamycin Complex 2 (mTORC2)
mTORC2 was discovered years after mTORC1, as such, less information is available for this signaling complex. Also, in contrast to the board downstream signaling molecules in the mTORC1 pathway, only a few substrates of mTORC2 have been identified, which include PKB, PKC-α and serum- and glucocorticoid-induced protein kinase 1 (SGK1) (Oh and Jacinto, 2011) (Fig. 6.3). mTORC2 signaling pathway is required for regulating cellular functions such as actin cytoskeleton organization and cell survival. Thus, malfunction of mTORC2 signaling is often found in different cancers with dysregulated actin organization and cell survival signals (Fang et al., 2012; Guo et al., 2012; Uesuqi et al., 2011). Besides the emerging necessities of mTORC2 for normal cell physiology, accumulating evidence has shown that these two mTOR complexes are interconnected, forming a complicated network of signaling molecules in mammalian cells in response to a wide range of stimuli.
Subunits of the mTORC2 include mTOR, rictor, Sin1 (stress-activated protein kinase (SAPK)-interacting protein 1), mLST8, deptor, Hsp70 and Protor-1/2. Among these, deptor and mLST8 are binding partners also found in mTORC1 and deptor serves as a negative regulator in both mTORC1 and mTORC2 (Peterson et al., 2009). While the function of mLST8 in mTORC1 is unclear, mLST8 is essential for the integrity of mTORC2 (Guertin et al., 2006). The importance of mLST8 to mTORC2 but not mTORC1 was revealed in a study in which raptor, rictor or mLST8 was deleted in mice. It was found that raptor-deficient mice died in early development; however, mice lacking mLST8 was able to survive until around embryonic day 10.5, similar to those lacking rictor, demonstrating the necessity of mLST8 to mTORC2 but not mTORC1 (Guertin et al., 2006). Also, upon knockout of mLST8, interaction between mTOR and raptor appeared to be normal and phosphorylation of S6K1 was not affected, whereas the association between mTOR and rictor, as well as the phosphorylation of PKB, were abolished (Guertin et al., 2006). Among the mTORC2 unique binding partners, rictor is the one that defines the function of mTORC2 by serving as a scaffolding protein for the assembly of the signaling complex (Powell et al., 2012). Mice lacking rictor led to a loss of PKB phosphorylation and embryonic lethality, demonstrating the significance of rictor for the assembly of mTORC2 to regulate development (Guertin et al., 2006). Additionally, rictor has up to 37 phosphorylation sites with most of them are located at its C-terminus (Dibble et al., 2009). Activity of the mTORC2 can be regulated via these phosphorylation sites in response to different stimuli. For example, phosphorylation of T1135, which is sensitive to amino acid and growth factors, leads to reduced phosphorylation of PKB (Dibble et al., 2009; Julien et al., 2010). Another mTORC2 exclusive subunit that is essential for the stability of whole complex is Sin1 since a knockdown of Sin1 was found to disrupt the interaction between mTOR and rictor, reducing PKB phosphorylation (Yang et al., 2006). Additionally, Sin1 may be able to modulate the activity of mTORC2 through the phosphorylation status of rictor since following a knockdown of Sin1, phosphorylation of rictor was reduced (Yang et al., 2006). Moreover, it is of interest to know that five Sin1 isoforms are generated through alternative splicing, and at least three distinctive mTORC2 complexes can be formed by three of the Sin1 isoforms, each of which may have unique but yet-to-be identified functions (Frias et al., 2006). Hsp70 is another mTORC2-specific subunit that is required for the association between mTOR and rictor. It was shown that after knockdown of Hsp70, binding between mTOR and rictor, but not raptor, was disrupted (Martin et al., 2008). Besides, only the phosphorylation of PKB, but not S6K1, was reduced after knockdown of Hsp70, indicating Hsp70 is indispensable for mTORC2, but not mTORC1 function (Martin et al., 2008). For Protor-1/2, although it is a subunit of mTORC2, the loss of Protor-1, but not Protor-2, led to reduced phosphorylation of SGK1, but not PKB and PKC-α, revealing Protor-1 may be needed by mTORC2 to activate SGK1 efficiently (Pearce et al., 2011).
3.3.1. Upstream Signaling Molecules of mTORC2
mTORC2 was first identified as a protein Ser/Thr kinase that phosphorylated PKB specifically on S473, which was stimulated by growth factors after serum depletion (Sarbassov et al., 2005). Subsequent study has demonstrated that besides PKB, SGK1 is another substrate of mTORC2 (Garcia-Martinez and Alessi, 2008). Since growth factors are known to (i) activate SGK1 and mTORC2 and (ii) generate PIP3 by PI3K, it was believed that PIP3 was able to stimulate mTORC2 for activating SGK1 via an unknown mechanism(s). It is now known that PIP3 can directly bind to and activate mTORC2 that leads to the phosphorylation of PKB and PKC-α by mTORC2 (Gan et al., 2011). Besides growth factors, mTORC2 can also be regulated by amino acids. After serum starvation, addition of Leu to HeLa cells is able to induce mTORC2-dependent cell migration via actin-reorganization mediated by Rac (Hernandez-Negrete et al., 2007). Amino acids also induced phosphorylation of PKB on S473 by mTORC2 and such phosphorylation was perturbed by a knockdown of rictor (Tato et al., 2011). Other than growth factors and amino acids, mTORC1 and mTORC2 also share other common upstream stimuli/signaling molecules. It has been demonstrated that TSC1/2 complex, an inhibitor of mTORC1, can be physically associated with mTOR2. However, in contrast to its inhibitory effect on mTORC1, TSC1/2 complex binds and activates mTORC2 in a manner independent of its GTPase-activating protein activity (Huang et al., 2008) (Fig. 6.3). It was shown that a loss of TSC1/2 complex led to impaired phosphorylation of all the known downstream effectors of mTORC2 including PKB, PKC-α and SGK1, thus confirming the necessity of TSC1/2 complex in mTORC2 activation (Huang et al., 2009). In addition to TSC1/2 complex, PTEN, which is also a suppressor of mTORC1, seems to exert its effect upstream of mTORC2 (Fig. 6.3). Among the upstream signaling molecules of mTORC2, perhaps the most interesting is S6K1, which is also a substrate of mTORC1. Growth factors are known to induce phosphorylation of rictor at T1135, which is directly mediated by S6K1 in a rapamycin-sensitive manner (Dibble et al., 2009; Julien et al., 2010; Treins et al., 2010). Although such phosphorylation does not affect mTORC2 integrity, intrinsic kinase activity or cellular localization, it triggers the binding of rictor to 14-3-3 proteins, which is a family of regulatory molecules that modulate numerous cellular events including spermatogenesis in the testis (Hermeking, 2003; Sun et al., 2009). In addition, overexpression of rictor with nonphosphorylatable T1135 in wild-type or rictor-null cells led to an increase of PKB phosphorylation on S473 while the phosphorylation status of PKC-α and SGK1 remained unchanged, indicating phosphorylation of rictor by S6K1 may indeed negatively regulate the activation of PKB by mTORC2. The findings summarized herein illustrate mTORC1 and mTORC2 form a connected signaling network that the two signaling complexes interact with each other functionally (Fig. 6.3). For instance, as PKB is needed for stimulating mTORC1, the suppression of mTORC2 on PKB activation by the mTORC1 substrate S6K1 may act as a negative feedback system to prevent overactivation of mTORC1.
3.3.2. Downstream Signaling Molecules of mTORC2
PKB, PKC-α and SGK1 are the three known downstream effectors of mTORC2 and they are members of the AGC kinase (PKA, PKG, PKC) family (Fig. 6.3). AGC kinases have highly conserved primary sequence within their kinase domains, and shared common structural features. For example, there is an activation loop in the catalytic domain of these molecules, and its phosphorylation leads to conformational changes which are essential to elicit the intrinsic catalytic activity of the enzyme (Parker and Parkinson, 2001; Pearce et al., 2010). Many AGC kinases also contain a hydrophobic motif located behind the kinase domain, and phosphorylation of this motif is required for stabilizing their active conformation. In addition, several AGC kinases have a turn motif (Parker and Parkinson, 2001; Pearce et al., 2010), which is an important phosphorylation site that promotes the integrity of the enzyme as well as maintaining its conformation for full kinase activity (Parker and Parkinson, 2001; Pearce et al., 2010).
3.3.2.1. Protein Kinase B
Among the substrates of mTORC2, PKB is the best characterized, which is known to be involved in regulating numerous cellular aspects including proliferation, survival, protein synthesis and metabolism. As mentioned previously, PIP3 produced upon growth factor stimulation is responsible for recruiting PKB to the plasma membrane, where it is phosphorylated by PDK1 at its activation loop on T308 (Alessi et al., 1997; Andjelkovic et al., 1997). In order for PKB to perform its kinase activity, it has to be further phosphorylated on S473 at the hydrophobic motif by mTORC2, and this phosphorylation is essential for PKB activation (Sarbassov et al., 2005). Furthermore, mTORC2 is also responsible for phosphorylating PKB on T450 at the turn motif (Oh et al., 2010). In short, mTORC2 phosphorylates PKB on S473 and T450 to elicit its full activation, and hence, PKB can effectively stimulate its substrates to regulate numerous cellular functions. For instance, FoxOs (transcription factors of the Forkhead box O class) are a family of transcription factors which promote the transcription of cell cycle inhibitors, and factors that induce apoptosis (Dijkers et al., 2000a, 2000b). Upon their phosphorylation by PKB, FoxOs are inhibited and hence, cell proliferation and survival are enhanced (Kloet and Burgering, 2011). Moreover, PKB also promotes cell survival with the aid of 14-3-3 protein. When exposed to survival factors, PKB phosphorylates BAD, a proapoptotic Bcl-2 family protein, on S136 and this phosphorylation leads to the association of 14-3-3 protein with BAD. As such, the accessibility of kinases, like PKB, to phosphorylate BAD on S155 is greatly enhanced and such phosphorylation inhibits BAD from interacting with prosurvival Bcl-2 family members to induce apoptosis (Datta et al., 1997, 2000). PKB also upregulates protein synthesis by phosphorylating and inhibiting TSC2 and PRAS40, leading to the activation of mTORC1 signaling that enhances protein synthesis via S6K1 and 4E-BP1. Furthermore, PKB also modulates the activity of enzymes involved in metabolism. For example, PKB has been shown to induce the localization of hexokinases to mitochondria, a process that can directly couple glucose metabolism to oxidative phosphorylation via yet-to-be defined mediator(s) (Gottlob et al., 2001). As a wide range of cellular physiology is mediated by PKB, it is not unexpected that dysregulation of PKB as well as its kinase mTORC2 are found to be involved in a variety of pathological conditions including cancers and diabetes (Hers et al., 2011; Oh and Jacinto, 2011). PKB has been localized to the BTB and apical ES in the seminiferous epithelium of rat testes, and its expression at these sites was found to be stage-specific, being highest at stage VI–VII but considerably diminished by early stage VIII and further diminished by late stage VIII of the epithelial cycle when BTB restructuring and apical ES degeneration take place to facilitate preleptotene spermatocyte migration and spermiation at the corresponding site (Siu et al., 2005). It is noted that this pattern of stage-specific expression of PKB at the apical ES is somewhat similar to the stage-specific expression of p-rpS6 at the apical ES (Mok et al., 2012c), illustrating PKB and rpS6 can be the downstream signaling molecules and substrates of mTORC2 and mTORC1, respectively, that mediate cross talk between the two mTOR signaling complexes.
3.3.2.2. Protein Kinase C-α
Unlike the other two mTORC2 effectors PKB and SGK1, which are substrates of mTORC2, it remains unclear whether PKC-α is directly phosphorylated by mTORC2 or through other mediator(s) (Sarbassov et al., 2004). However, after the knockdown of rictor by RNAi, phosphorylation of PKC-α on S657 was shown to be reduced, resulting in the change of cell shape due to actin reorganization in which actin filaments at the cortical sides became less prominent and stress fibers were formed in the cytosol. Similar morphology of actin cytoskeleton was observed after PKC-α knockdown, validating actin organization is indeed regulated by mTORC2 and is mediated through PKC-α (Sarbassov et al., 2004). In addition to that, a recent study showed that RNAi-mediated knockdown of rictor in cultured Sertoli cells also led to a reduced PKC-α phosphorylation, which in turn resulted in actin reorganization (Mok et al., 2012a). Furthermore, addition of serum to serum-starved fibroblasts induced rapid and robust stress-fiber formation, which was ablated by a knockdown of mTORC2 subunits mTOR, mLST8 and rictor (Jacinto et al., 2004). Furthermore, during the actin cytoskeleton restructuring due to the knockdown of mTORC2 subunits, a decline in GTP-bound Rac1 was observed. Whereas cells overexpressing constitutively active form of Rac1 and Rho were able to resist actin reorganization due to reduced mTORC2, this thus suggests that small GTPase Rac1 and Rho are possible mediators of mTORC2 in controlling actin organization (Jacinto et al., 2004). In fact, these small GTPases are likely downstream molecules of PKC-α (Fig. 6.3). This notion was supported by studies revealing that the Rho-induced actin rearrangement, such as stress-fibers formation, in endothelial cells was PKC-α-dependent. It was shown that upon treatment of TNF-α or thrombin, actin reorganization was induced and such actin reorganization was found to be mediated by activation of Rho GTPase activator p115RhoGEF (a guanine nucleotide exchange factor for Rho GTPase) by PKC-α (Holinstat et al., 2003; Peng et al., 2011). Moreover, it is known that PKC-α is also able to activate Rho by negatively regulating the Rho inhibitor, Rho-GDP guanine nucleotide dissociation inhibitor (GDI), via phosphorylation (Mehta et al., 2001). In short, the above studies suggest that small GTPases, such as Rho and Rac1, are downstream molecules of PKC-α in the mTORC2 signaling pathway that regulates actin cytoskeleton.
3.3.2.3. Serum- and Glucocorticoid-induced Protein Kinase 1 (SGK1)
SGK1 is an important substrate of mTORC2. Similar to PKB, following insulin and growth factor stimulation, SGK1 is phosphorylated by PDK1 at its activation loop on T256 (Biondi, 2004; Mora et al., 2004). In order to fully activate SGK1, mTORC2 is responsible for phosphorylating its S422 residue at the hydrophobic motif (Garcia-Martinez and Alessi, 2008). More important, it has been shown that in fibroblasts lacking mTORC2 subunits rictor, Sin1 or mLST8, the phosphorylation of SGK1 as well as its substrate N-myc downstream regulated gene 1 (NDRG1) is abrogated, illustrating the necessity of the functional mTORC2 in activating SGK1. SGK1 is also a Ser/Thr kinase that regulates a variety of cellular functions including ion transport, cell proliferation and survival (Garcia-Martinez and Alessi, 2008). Studies have shown that FoxOs are transcription factors that promote gene transcription involving in induction of apoptosis and inhibition of cell cycle progression (Dijkers et al., 2000a, 2000b), SGK1 was found to stimulate cell survival and proliferation by directly phosphorylating FoxO3a to inhibit its nuclear localization and transcription activity (Brunet et al., 2001; Dehner et al., 2008). Moreover, given the fact that p53 is able to trigger apoptosis (Soengas et al., 1999), SGK1 also enhances cell survival by phosphorylating an E3 ubiquitin ligase Mdm2 (murine double minute, an oncogene) on S166 to promote its binding to p53 for ubiquitination (Amato et al., 2009). Furthermore, mTORC2/SGK1 signaling is required for the expression of another p53 E3 ubiquitin ligase called human double minute 2 (HDM2). A knockdown of rictor or SGK1 by RNAi led to downregulation of HDM2, illustrating the significant of mTORC2/SGK1 pathway for inhibiting p53 to modulate cell survival (Lyo et al., 2010). Taken collectively, these studies show that besides PKB, mTORC2 can modulate cell proliferation as well as survival through SGK1. SGK1 is likely to play a crucial role in carcinogenesis since it is overexpressed in a variety of tumors (Fagerli et al., 2011; Simon et al., 2007; Zhang et al., 2005) and its suppression is able to reduce tumor development. For instance, mice lacking SGK1 were found to be more resistant to chemically induced colon cancer (Nasir et al., 2009). Whereas in adenoma polyposis coli (APC)-deficient mice, which developed gastrointestinal tumors spontaneously, the lack of SGK1 led to reduced intestinal tumor development (Wang et al., 2010). However, the role of SGK1 in spermatogenesis and other testicular function remain unexplored. Nontheless, these findings illustrate that SGK1 may be involved in regulating germ cell apoptosis during spermatogenesis.
3.4. The Interplay between mTORC1 and mTORC2 in Regulating Cellular Events
As described above, mTORC1 and mTORC2 have their distinctive downstream substrates and signaling molecules so that they regulate distinctive cellular functions. However, these two pathways are also interconnected and can interact with each other to affect phenotypes. For example, both signaling complexes are activated upon stimulation by growth factors and amino acids. Besides, they also share the same upstream regulator, TSC1/2 complex, which promotes the activity of mTORC1 but suppresses mTORC2 (Fig. 6.3). More important, S6K1, which is the substrate of mTORC1, can phosphorylate rictor, the critical binding partner of mTORC2, and inhibit the catalytic activity of mTORC2 on PKB, which is also the upstream regulator of mTORC1, thereby creating as a negative feedback loop (Fig. 6.3). Besides sharing common activating stimuli and regulators, recent studies have suggested that some of the cellular functions modulated by these signaling complexes are indeed overlapping, despite the fact that they have their specific substrates. For instance, mTORC1 regulates cell proliferation via S6K1 and rpS6, whereas mTORC2 modulates the same cellular process with PKB and SGK1. Furthermore, regulation of actin cytoskeleton was once regarded as a specific role of mTORC2, but several recent studies indicate that mTORC1 may be involved in this event. First, a study performed in yeasts revealed that rapamycin treatment which inhibited TORC1 signaling was found to perturb actin polarization within 10 min, and this treatment also delayed actin repolarization after glucose starvation (Aronova et al., 2007). Since significant actin depolarization was determined in such a short interval (within 10 min) after adding rapamycin, the actin reorganization should be attributed to a loss of TOR1 function only since mTORC2 remained unaffected during this short period of time (Aronova et al., 2007). Second, in Rh30 and dU-373 mammalian cancer cell lines, treatment of these cells with rapamycin for 2 h was found to inhibit the type I insulin-like growth factor (IGF-I)-stimulated F-actin reorganization, confirming the involvement of mTORC1 signaling in actin dynamics (Liu et al., 2008). Also, in ovarian cancer cells transfected with constitutively active S6K1, actin reorganization to facilitate the formation of actin-based lamellipodia, actin microspikes and filopodia were induced in these cells, and such actin cytoskeleton restructuring was mediated via Rac1 and Cdc42 (Ip et al., 2011). Furthermore, phosphorylated S6K1 was found to bind to F-actin, cross-linking actin filaments, thereby stabilizing F-actin as it significantly reduced the rate and extent of actin filament depolymerization induced by cofilin (Ip et al., 2011). In short, these recent findings illustrate that although mTORC1 and mTORC2 possess distinctive substrates and different downstream signaling molecules, they both regulate cell proliferation and F-actin organization in cells.
3.5. Regulation of Blood–Tissue Barrier Function by mTOR
3.5.1. Regulation of Barrier Function in The Kidney by mTOR
Among the numerous cellular processes mediated by mTOR, its effects on immune response in mammals are well characterized. Rapamycin, a potent inhibitor of mTOR, is an immunosuppressant drug widely used by kidney and heart transplant patients (Diekmann and Campistol, 2006; Kahan, 2001). However, after prolonged exposure to rapamycin, proteinuria (a pathological condition with excessive serum proteins found in urine) and even nephritic syndrome were observed in some patients (Aliabadi et al., 2008; Dittrich et al., 2004; Izzedine et al., 2005; van den Akker et al., 2006). Such pathological condition was later found to be the result of damages in podocytes, which are the cells responsible for maintaining the blood–urine filtration barrier of the renal glomerulus in the kidney. This selective barrier is created via a unique cell–cell contact called the slit diaphragm established by primary and secondary foot processes from podocytes (Paventadt et al., 2003). In cultured human immortal podocytes, prolonged treatment of rapamycin downregulated mTOR and rictor and thus reduced the formation of mTORC2, leading to reduced phosphorylation of PKB on S473 (Vollenbroker et al., 2009). The suppression of mTORC2 signaling disrupted the podocyte-based filtration barrier, which was the result of reduced cell adhesion. Such reduction of cell adhesion was mediated, at least in part, by a loss of slit diaphragm proteins, such as nephrin, and a reorganization of actin cytoskeleton. It was observed that formation of dot-like actin-rich structures were enhanced by rapamycin, and this actin reorganization was caused by a loss of Nck (non-catalytic region of tyrosine kinase adaptor protein 1), which is an actin regulating protein and a cytoskeleton adaptor that links nephrin to actin cytoskeleton (Vollenbroker et al., 2009). Besides long-term rapamycin treatment, diabetes also leads to malfunction of blood–urine filtration barrier, resulting in proteinuria. It was demonstrated that diabetes led to overactivation of mTOR signaling in damaged podocytes in diabetic mice, leading to mislocalization of slit diaphragm protein nephrin and also TJ adaptor ZO-1, moving from plasma membrane to cytosol (Inoki et al., 2011). The fact that the phenotypes of podocyte damages found in diabetic animals mimicked podocyte-specific TSC1 knockout mice (note: TSC1 is the mTORC1 upstream negative regulator, see Fig. 6.3), illustrating the involvement of mTORC1 signaling in the podocyte-based filtration barrier. The role of mTORC1 and mTORC2 in regulating the blood–urine filtration barrier was also illustrated in a study using podocyte-specific raptor or rictor knockout mice (Godel et al., 2011). Mice lacking mTORC1 in podocytes as the result of podocyte-specific raptor knockout developed significant albuminuria, a form of proteinuria. In contrast, loss of mTORC1 in podocytes of adult mice triggered by conditional knockout of raptor only had a mild effect and the level of protein excreted in urine in these mice was insignificantly higher than that of the wild-type (Godel et al., 2011). Additionally, it was shown that when conditional knockout of raptor was performed in mice with genetic background that was known to be more sensitive toward podocyte damage, significant proteinuria was induced (Godel et al., 2011). Taken together, these findings illustrate that mTORC1 signaling is required for proper development of podocytes to form the blood–urine filtration barrier; whereas in adult mice after podocytes are developed and the blood–urine filtration barrier is fully functional, mTORC1 is necessary for maintenance of podocyte functions, and mTORC1 is more important in animals with specific genetic background. It is noted that while podocytes are needed mTORC1 to maintain the filtration barrier function, overactivation of mTORC1 signaling in podocytes also leads to a disruption of the barrier. This indicates that a precise control on the availability of mTORC1 is needed to maintain the homeostasis of the barrier function. Regarding the role of mTORC2 in podocyte-mediated barrier function, it was shown that in podocyte-specific rictor knockout mice, only transient albuminuria was found when these mice were challenged by a BSA overload (Godel et al., 2011). However, when raptor and rictor were simultaneously knockout in podocytes, massive proteinuria was observed, suggesting mTORC2 signaling is necessary for podocytes to cope with stress conditions and both mTOR complexes work synergistically together to maintain the integrity of the filtration barrier in the kidney.
It was known that induction of mTORC1 activity by simultaneous deletion of PTEN and Lkb1, two negative upstream regulators of mTORC1 (Fig. 6.3), in mouse bladder epithelial cells led to a loss of AJ protein E-cadherin and TJ adaptor ZO-1, leading to tumor progression (Shorning et al., 2011). Moreover, it was reported that a knockdown of rictor by RNAi in glioma cells led to induction of matrix metalloproteinase-9 (MMP-9) mediated by activation of Raf-1-MEK-ERK pathway, and such activation was caused by the removal of the inhibitory effect from PKB due to a loss of mTORC2 function. Since MMP-9 is responsible for breaking down extracellular matrix via its action on collagen IV, its induction thus contributes to an increase in invasiveness of glioma tumor cells (Das et al., 2011). In addition, it was shown that in cultured Sertoli cells, an induction of MMP-9, such as by TNFα, that led to a disruption of the TJ barrier was mediated via a downregulation of TJ protein occluding (Siu et al., 2003). Collectively, these findings suggest that in Sertoli cells, suppression of mTORC2 activity may result in an MMP-9-mediated disruption of the BTB. In fact, a recent study has shown that a reduced mTORC2 activity perturbs the Sertoli BTB function (Mok et al., 2012a), whereas a reduced mTORC1 signaling function promotes the Sertoli TJ-permeability barrier (Mok et al., 2012c). These findings thus suggest that these two mTOR complexes work antagonistically to modulate BTB dynamics in the testis.
4. REGULATION OF BTB DYNAMICS BY mTOR
4.1. Background
The involvement of mTOR in BTB dynamics during spermatogenesis has not been explored until recently (Mok et al., 2012a; Mok et al., 2012c). As shown in Fig. 6.4, both mTOR and the crucial subunits that create mTORC1 (e.g. raptor) and mTORC2 (e.g. rictor) were localized in the seminiferous epithelium near the basement membrane, consistent with their localization at the BTB. However, it is noted that the stage-specific expression of raptor and rictor during the epithelial cycle is different, with raptor being the highest, but rictor at its lowest, at stage IX of the epithelial cycle (Fig. 6.4), implicating the mTORC1 and mTORC2 may have differential effects on the BTB. These recent findings (Mok et al., 2012a; Mok et al., 2012c) (Fig. 6.4) coupled with results of other studies in the field thus support a novel concept depicted in Fig. 6.5 regarding the “yin” and “yang” effects of the mTORC1 and mTORC2 signaling complexes on the BTB dynamics that regulate BTB restructuring during the seminiferous epithelial cycle of spermatogenesis, which is being critically evaluated in the following sections.
Figure 6.4. Stage-specific expression of raptor and rictor versus mTOR in the seminiferous epithelium of adult rat testes.
Relative expression level and localization of mTOR, raptor and rictor (red) in the seminiferous epithelium from stage V–IX tubules were examined by immunofluorescence microscopy. Cell nuclei were stained with DAPI (blue) to show the stages of the tubules. This figure shows that from stage V to IX, mTOR was expressed at relatively similar level at the basal compartment where BTB was located. On the other hand, the expression of raptor, which is the key binding partner of mTORC1, was transiently induced at stage IX (indicated by white arrow head) that begin in late stage VIII, most notably at the BTB, whereas the expression of rictor, which is the key subunit of mTORC2, was found to decline gradually from stage VII and became barely detectable at late stage VIII through stage IX (indicated by open arrow head), even though it remained weakly expressed in these stages. Bar, 50 μm, which applies to all micrograph. For interpretation of the references to color in this figure legend, the reader is referred to the online version of this book.
Figure 6.5. mTORC1 and mTORC2 display antagonistic effects on the BTB and their combined effects can protect the immunological barrier integrity during the transit of preleptotene spermatocytes at the BTB.
It is noted that rictor and raptor compete for mTOR for the formation of mTORC2 and mTORC1, respectively, to promote BTB and disrupt BTB integrity. At stages I–VI in which prior to BTB restructuring, the relative high expression of rictor favors the assembly of mTORC2, which is necessary for keeping the integrity of BTB by maintaining the dense F-actin network (namely the actin filament bundles at the basal ES), expression level of GJ proteins and GJ communication. On the other hand, in stage late VIII to stage IX that the BTB is transiently “open” to facilitate the transit of spermatocyte, the expression level of raptor is induced, whereas that of rictor is reduced. Thus, formation of mTORC2 is reduced and mTORC1 is favored. mTORC1 activates rpS6, which in turn disrupts the “old” BTB above the preleptotene spermatocytes in transit at the BTB by inhibiting de novo synthesis of BTB proteins. In addition, actin cytoskeleton reorganization during BTB restructuring is induced by (i) mTORC1 signaling via S6K1 and rpS6 and (ii) the decrease in phosphorylated PKC-α due to reduced mTORC2. The disorganized F-actin network leads to internalization of BTB proteins, which perturbs the “old” BTB. Furthermore, the decrease in GJ proteins and GJ communication caused by reduced mTORC2 also facilitates the disruption of the “old” BTB for the translocation of spermatocytes across the BTB. However, while mTORC2 expression is reduced, it remains to be robust enough to sustain the maintenance of the “new” BTB that is being assembled behind the preleptotene spermatocytes in transit. In short, utilizing the antagonistic effects of the mTORC1 and mTORC2 on the TJ-permeability barrier, the immunological barrier function can be maintained during the passage of preleptotene spermatocytes, which are connected in “clones” via intercellular bridges, at the BTB. For color version of this figure, the reader is referred to the online version of this book.
4.2. Regulation of BTB Dynamics by mTORC1
In the seminiferous epithelium of adult rat testes, rpS6, a crucial downstream signaling molecule of mTORC1 (Section 3.2.2.) was found to be highly expressed in the basal compartment of the seminiferous epithelium in all stages of the epithelial cycle, consistent with its localization at the BTB, implicating the likely involvement of mTORC1 signaling complex in BTB dynamics (Mok et al., 2012c). Interestingly, p-rpS6, the activated form of rpS6, was highly expressed at the BTB and colocalized with putative BTB proteins ZO-1, N-cadherin and Arp3, but restrictive to late stage VIII–IX, coinciding with the time of BTB restructuring to facilitate the transit of preleptotene spermatocytes at the site (Mok et al., 2012c). This timely upregulation in the phosphorylated and activated form of rpS6 at the BTB suggests that rpS6 may take part in the “opening” of the BTB for the transit of spermatocytes from the basal to the apical compartment. To confirm this postulate, rpS6 phosphorylation was abolished by inactivating mTORC1 signaling in cultured Sertoli cells with an established TJ-permeability barrier by either treatment of cells with rapamycin or a knockdown of rpS6 by RNAi, both approaches was shown to promote the Sertoli cell TJ barrier by making the BTB “tighter” following a blockade rpS6 activation or its knockdown (Mok et al., 2012c). In addition, the expression of TJ proteins, such as claudin-11, were upregulated with claudin-11 being redistributed and localized more intensely to the Sertoli cell–cell interface (Mok et al., 2012c), possibly being used to “strengthen” the TJ barrier. Furthermore, changes in the F-actin organization was detected with more actin filaments were found at the Sertoli cell–cell interface (Mok et al., 2012c), possibly being used to strengthen the Sertoli cell TJ barrier. In short, these findings illustrate that rpS6 was specifically activated and highly expressed at the site of the BTB in the seminiferous epithelium during its restructuring at stage VIII–IX of the epithelial cycle, whereas a suppression of rpS6 or its knockdown in Sertoli cells led to a “tightening” of the TJ barrier. These findings thus support the notion that the rpS6 activation is crucial to elicit BTB restructuring, such as at stage VIII–IX of the epithelial cycle. An earlier study has shown that mouse embryonic fibroblasts (MEFs, also known as feeder cells) from rpS6p−/− mice displayed a higher rate of global protein synthesis (Ruvinsky and Meyuhas, 2006), suggesting that a decline in phosphorylated rpS6 might trigger de novo synthesis of TJ proteins, which is consistent with our findings that a knockdown of rpS6 in Sertoli cell epithelium induced claudin-11 expression (Mok et al., 2012c). Furthermore, rpS6 might take part in regulating actin cytoskeleton similar to its upstream activator S6K1 since actin filament rearrangement was shown to be stimulated following a knockdown of rpS6; and to further support the role of rpS6 in actin dynamics, phosphorylated rpS6 was found to structurally interact with actin as demonstrated by coimmunoprecipitation (Mok et al., 2012c). Taking these findings collectively, it is clear that the promotion of the Sertoli cell TJ-barrier function after a suppression of rpS6 likely leads to an increase in the synthesis of TJ proteins (e.g. claudin-11), which coupled with redistribution and/or relocalization of BTB proteins to the Sertoli cell–cell interface, supported by an increase in F-actin bundles at the cortical region of the Sertoli cells in the epithelium, thereby strengthening the BTB integrity. In short, during the epithelial cycle of spermatogenesis, the timely activation of mTORC1 at stage VIII–IX that leads to phosphorylation of rpS6 during BTB restructuring may facilitate this process by transiently downregulating TJ proteins, and perturbing the supportive F-actin network underneath cell adhesion complexes that facilitates their endocytosis. In short, BTB is transiently “opened” above the preleptotene spermatocytes in transit at the BTB induced by an upregulation of p-rpS6, which facilitates the migration of these spermatocytes across the BTB to enter the adluminal compartment to prepare for meiosis I/II.
4.3. Regulation of BTB Dynamics by mTORC2
For mTORC2, its key binding partner rictor was shown to be highly expressed at the BTB from stages I–VI of the seminiferous epithelial cycle, however, it was downregulated from late stage VII and it was considerably diminished and barely detectable at stage IX (Mok et al., 2012a) (Fig. 6.4). This suggests that mTORC2 signaling may be involved in maintaining the BTB integrity during all the stages of the epithelial cycle of spermatogenesis except at stage VIII–IX when it is downregulated when the BTB is under restructuring (Mok et al., 2012a). To confirm this postulate, studies were performed in which a knockdown of rictor by RNAi in cultured Sertoli cells with an established TJ-permeability barrier was found to disrupt the TJ barrier, and this event was also associated with a reduced phosphorylation of PKC-α, but not PKB (Mok et al., 2012a). Thus, the Raf-1-MEK-ERK pathway, which is inhibited by PKB, was not activated and the level of MMP-9 remained unchanged (Mok et al., 2012a). As discussed in Section 3.2.1, mTORC2 signaling complex regulates actin cytoskeleton via PKC-α in multiple epithelia; thus, the knockdown of rictor by RNAi triggered actin reorganization, and actin filaments were rearranged in Sertoli cells with reduced F-actin to support the TJ-barrier function at the Sertoli cell–cell interface (Mok et al., 2012a). Interestingly, following the rictor knockdown in Sertoli cells by RNAi that led to a reduction in phosphorylated PKC-α, the expression of Cx26 and Cx43 in these Sertoli cells was also downregulated (Mok et al., 2012a). Furthermore, TJ proteins occluding and ZO-1 were also redistributed from the cell–cell interface and moved into the cell cytosol (Mok et al., 2012a), thereby destabilizing cell adhesion, leading to the Sertoli cell TJ-barrier disruption. These findings thus illustrate that a knockdown of rictor in Sertoli cells leads to restructuring of actin cytoskeleton, reducing cortical F-actin, this thus facilitates internalization of TJ proteins and hence weakening the TJ barrier. More important, it was demonstrated that a knockdown of rictor led to a disruption of GJ communication between adjacent Sertoli cells based on a functional GJchannel assay (Mok et al., 2012a). Collectively, these findings thus support the notion that during the seminiferous epithelial cycle of spermatogenesis, rictor and, hence, mTORC2 signaling is essential for maintaining BTB integrity. When rictor is downregulated during the epithelial cycle, such as at stage VIII at the time of BTB restructuring, this leads to PKC-α-mediated actin cytoskeleton reorganization that promotes endocytosis of TJ proteins to destabilize the BTB above the preleptotene spermatocytes in transient at the BTB. This process is also assisted by a downregulation of GJ proteins, which coordinates with the timely “disassembly” of TJ and basal ES at the site to facilitate the transit of spermatocytes.
4.4. A Hypothetic Model Based on The Antagonistic Effects of mTORC1 and mTORC2 on BTB Function to Regulate its Integrity during The Epithelial Cycle of Spermatogenesis
Based on recent findings as discussed above, it is clear that the action of mTORC1 is to promote the “disassembly” of the BTB while mTORC2 supports BTB integrity. It is very likely that the simultaneous presence of these two signaling complexes in the seminiferous epithelium that exert their antagonistic effects on the underlying actin cytoskeleton at the BTB that leads to changes in the localization of TJ proteins play a critical role in maintaining the BTB integrity during the transit of preleptotene spermatocytes, which are connected in “clones,” at the BTB. Figure 6.5 depicts a hypothetical model regarding the involvement of mTORC1 and mTORC2 in regulating BTB integrity during the epithelial cycle of spermatogenesis. It is hypothesized that during the epithelial cycle, upregulation of rictor at stages I–VII that favors the formation of mTORC2 is being used to maintain the BTB integrity, but not at stages VIII–IX when its expression is downregulated at the time of BTB restructuring. On the other hand, during stage late VIII–IX, the transient-induced expression of raptor favors the formation of mTORC1 for the disruption of the “old” BTB at the apical region of the transiting preleptotene spermatocytes at the site. This process is further facilitated by the reduction in mTORC2 due to a downregulation of rictor (Figs 6.4 and 6.5). Furthermore, the low level of rictor expressed during the BTB restructuring may be necessary for the “assembly” and “maintenance” of the “new” BTB that is being created at the basal region of the transiting preleptotene spermatocytes (Fig. 6.5). In fact, the dependence of relative abundance of raptor and rictor for the activation of mTORC1 or mTORC2 signaling has been demonstrated in other studies. For example, it was reported that the knockdown of raptor by RNAi in HEK-293T and HeLa cells led to an increase in PKB phosphorylation on S473, indicating mTORC2 signaling was enhanced. On the other hand, in the same cell types, knockdown of rictor caused increased phosphorylation of S6K1 with increased association between raptor and mTOR, revealing mTORC1 signaling was stimulated (Sarbassov et al., 2004). More important, it was revealed that in mTOR-mediated mitochondrial metabolism, a knockdown of raptor reduced oxygen consumption while a knockdown of rictor increased oxygen consumption and oxidative capacity (Schieke et al., 2006). These studies thus illustrate how a cellular function can be modulated based on the “yin-and-yang” effects of the two mTOC complexes mediated by the relative availability of raptor and rictor in a cellular microenvironment. In short, the combined antagonistic effects of the mTORC1 or mTORC2 signaling complexes can fine-tune a cellular event, such as the migration of preleptotene spermatocytes across the BTB as depicted in Fig. 6.5.
5. CONCLUDING REMARKS AND FUTURE
PERSPECTIVES
In this chapter, we have provided a critical update on the biology of adhesion junctions as well as the role of constituent proteins in regulating BTB dynamics in the testis. We have also reviewed the functional relationship between these proteins and the underlying actin cytoskeleton. While some of the discussions are based on findings in other epithelia/endothelia, this information will be helpful to design functional experiments in future studies to unravel the regulation of the BTB. We also provide an update on the latest development regarding the involvement of the two mTOR signaling complexes, namely mTORC1 and mTORC2, in regulating BTB dynamics during the seminiferous epithelial cycle of spermatogenesis. Although recent studies have shown that the mTORC1 and mTORC2 signaling complexes likely modulate BTB dynamics their antagonistic effects on the TJ-permeability barrier function via actin cytoskeleton, however, the actin regulatory proteins involved in these events remain to be identified and examined. Much work is needed to explore if mTOR complexes exert their effects on the F-actin via drebrin E, paladin, formins, filamins, Eps8, the Arp2/3 complex and others. Other small GTPases such as Rac and Rho and polarity proteins (e.g. PAR3, PAR6, 14-3-3, Scribble/Dlg/Lgl) may also be involved. Moreover, the molecular mechanism(s) by which rictor regulates the expression of GJ proteins and GJ communication, which in turn modulates BTB dynamics, remains to be identified. Additionally, we hypothesize that mTORC1 and mTORC2 regulate BTB dynamics via their antagonistic effects on BTB assembly and maintenance, and the activity of these two signal complexes are mediated by the relative expression of their key binding partners raptor and rictor and downstream signaling molecules, such as rpS6, in the seminiferous epithelium. While much work is needed, however, the model depicted in Fig. 6.5 provides a framework upon which functional studies can be designed to understand the interplay between mTOR complexes and other regulatory proteins that modulate the BTB function during spermatogenesis.
ACKNOWLEDGMENTS
Studies conducted in the authors’ laboratory were supported by grants from the National Institutes of Health (NICHD, U54 HD029990 Project 5 to CYC, R01 HD056034 to CYC).
ABBREVIATIONS
- AJ
adherens junction, also known as zonula adherens
- AMPK
AMP-activated protein kinase
- Arp2/3 complex
actin-related protein 2/3 complex
- BTB
blood–testis barrier
- Caco-2 cells
human colonic epithelial-2 cells
- CAR
coxsackievirus and adenovirus receptor
- Cx
connexin
- Deptor
DEP domain-containing mTOR-interacting protein, an inhibitor of mTORC1 and mTORC2
- Dlg
discs large
- DS
desmosome
- 4E-BP1
4E-binding protein 1
- EC
ectodomain module
- Eps8
epidermal growth factor pathway substrate 8
- ERK1/2
extracellular regulated kinase ½
- ES
ectoplasmic specialization
- FSH
follicle stimulating hormone
- GJ
gap junction
- HnRNP
heterogeneous ribonucleoprotein
- IGF
insulin-like growth factor
- JAM
junctional adhesion molecule
- KGF
keratinocyte growth factor
- Lgl
lethal giant larvae
- LH
luteinizing hormone
- mLST8
target of rapamycin complex subunit LST8, also known as mTOR associated protein LST8 homolog
- MAGUK
membrane-associated guanylate kinase
- MDCK cells
Madin-Darby canine kidney cells
- MMP
matrix metalloproteinase
- MTOR
mammalian target of rapamycin complex
- mTORC1
mTOR complex 1
- mTORC2
mTOR complex 2
- N-WASP
neuronal Wiskott-Aldrich syndrome protein
- PAR
partitioning defective protein
- PDK1
3-phosphoinositide-dependent kinase 1
- PI3K
phosphoinositide 3-kinase
- PIKK
PI3K-related kinase
- PIP2
phosphatidylinositol (4, 5)-bisphosphate
- PIP3
phosphatidylinositol-3, 4, 5-triphosphate
- PKB
protein kinase B also known as Akt
- PKC
protein kinase C
- PKP-2
plakophilin-2
- PRAS40
proline-rich Akt-PKB substrate 40 kDa
- PTEN
phosphatase and tensin homolog on chromosome 10
- Raptor
regulatory associated protein of mTOR
- Rheb
Ras-homolog enrich in brain
- Rictor
rapamycin-insensitive companion of mTOR
- rpS6
ribosomal protein S6
- RSK
p90 ribosomal S6 kinase
- S6K
S6 protein kinase also known as mTOR/p70, mTOR p70 ribosomal S6 kinase
- SGK1
serum- and glucocorticoid-inducible kinase 1
- SKAR
S6K Aly/REF-like substrate
- TJ
tight junction, also known as zonula occludens
- TOP
5′-terminal oligopyrimidine
- TSC1/2
tuberous sclerosis complex ½ containing TSC1 (hamartin) and TSC2 (tuberin)
- ZO-1
zonula occludens-1
REFERENCES
- Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu. Rev. Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- Aliabadi AZ, Pohanka E, Seebacher G, Dunkler D, Kammerstatter D, Wolner E, Grimm M, Zuckermann AO. Development of proteinuria after switch to sirolimus- based immunosuppression in long-term cardiac transplant patients. Am J. Transplant. 2008;8:854–861. doi: 10.1111/j.1600-6143.2007.02142.x. [DOI] [PubMed] [Google Scholar]
- Amato R, D’Antona L, Porciatti G, Agosti V, Menniti M, Rinaldo C, Costa N, Bellacchio E, Mattarocci S, Fuiano G, et al. Sgk1 activates MDM2-dependent p53 degradation and affects cell proliferation, survival, and differentiation. J. Mol. Med. (Berl) 2009;87:1221–1239. doi: 10.1007/s00109-009-0525-5. [DOI] [PubMed] [Google Scholar]
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucoq JM, Hemmings BA. Role of translocation in the activation and function of protein kinase. B.J. Biol. Chem. 1997;272:31515–31524. doi: 10.1074/jbc.272.50.31515. [DOI] [PubMed] [Google Scholar]
- Andreeva AY, Krause E, Muller EC, Blasig IE, Utepbergenov DI. Protein kinase C regulates the phosphorylation and cellular localization of occludin. J. Biol. Chem. 2001;276:38480–38486. doi: 10.1074/jbc.M104923200. [DOI] [PubMed] [Google Scholar]
- Appenzeller-Herzog C, Hall MN. Bidirectional crosstalk between endoplasmic reticulum stress and mTOR signaling. Trends Cell. Biol. 2012;22:274–282. doi: 10.1016/j.tcb.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Aronova S, Wedaman K, Anderson S, Yates JI, Powers T. Probing the membrane environment of the TOR kinases reveals functional interactions between TORC1, actin, and membrane trafficking in Saccharomyces cerevisiae. Mol. Cell. Biol. 2007;18:2779–2794. doi: 10.1091/mbc.E07-03-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandi HR, Ferrari S, Krieq J, Meyer HE, Thomas G. Identification of 40 S ribosomal protein S6 phosphorylation sites in Swiss mouse 3T3 fibroblasts stimulated with serum. J. Biol. Chem. 1993;268:4530–4533. [PubMed] [Google Scholar]
- Barratt CLR. Spermatogenesis. In: Grudzinskas JG, Yovich JL, editors. Gametes: The Spermatozoon. New York: Cambridge University Press; 1995. pp. 250–267. [Google Scholar]
- Bazzoni G. Pathobiology of junctional adhesion molecules. Antioxid. Redox Signal. 2011;15:1221–1234. doi: 10.1089/ars.2010.3867. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenons M, Citi S, Dejana E. Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J. Biol. Chem. 2000;275:20520–20526. doi: 10.1074/jbc.M905251199. [DOI] [PubMed] [Google Scholar]
- Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh AJ. Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology. 1995;136:5–12. doi: 10.1210/endo.136.1.7828558. [DOI] [PubMed] [Google Scholar]
- Biondi RM. Phosphoinositide-dependent protein kinase 1, a sensor of protein conformation. Trends Biochem. Sci. 2004;29:136–142. doi: 10.1016/j.tibs.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Bosco D, Haefliger JA, Meda P. Connexins: key mediators of endocrine function. Physiol. Rev. 2011;91:1393–1445. doi: 10.1152/physrev.00027.2010. [DOI] [PubMed] [Google Scholar]
- Bouchard MJ, Dong Y, McDermott BMJ, Lam DH, Brown KR, Shelanski M, Bellve AR, Racaniello VR. Defects in nuclear and cytoskeletal morphology and mitochondrial localization in spermatozoa of mice lacking nectin-2, a component of cell-cell adherens junctions. Mol. Cell. Biol. 2000;20:2865–2873. doi: 10.1128/mcb.20.8.2865-2873.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm R, Zeiler M, Ruttinger C, Herde K, Kibschull M, Winterhager E, Willecke K, Guillou F, Lecureuil C, Steger K, et al. A Sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am. J. Pathol. 2007;171:19–31. doi: 10.2353/ajpath.2007.061171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol. Cell. Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette D, Weider K, Gilleron J, Giese S, Dompierre J, Gergmann M, Brehm R, Denizot JP, Segretain D, Pointis G. Major involvement of connexin 43 in seminiferous epithelial junction dynamics and male fertility. Dev. Biol. 2010;346:54–67. doi: 10.1016/j.ydbio.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos. Trans. R Soc. Lond B Biol. Sci. 2010;365:1517–1535. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carriere A, Cargnello M, Julien LA, Gao H, Bonneil E, Thibault P, Roux PP. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr. Biol. 2008;18:1269–1277. doi: 10.1016/j.cub.2008.07.078. [DOI] [PubMed] [Google Scholar]
- Carriere A, Romeo Y, Acosta-Jaquez HA, Moreau J, Bonneil E, Thibault P, Fingar DC, Roux PP. ERK1/2 phosphorylate Raptor to promote Ras-dependent activation of mTOR complex 1 (mTORC1) J. Biol. Chem. 2011;286:567–577. doi: 10.1074/jbc.M110.159046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. The biology of spermatogenesis: the past, present and future. Philos. Trans. R Soc. Lond B Biol. Sci. 2010a;365:1459–1463. doi: 10.1098/rstb.2010.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. A local autocrine axis in the testes that regulates spermatogenesis. Nat. Rev. Endocrinol. 2010b;6:380–395. doi: 10.1038/nrendo.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. Regulation of spermiogenesis, spermiation and blood-testis barrier dynamics: novel insights from studies on Eps8 and Arp3. Biochem. J. 2011;435:553–562. doi: 10.1042/BJ20102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Mruk DD. The blood-testis barrier and its implication in male contraception. Pharmacol. Rev. 2012;64:16–64. doi: 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CY, Wong EW, Yan HH, Mruk DD. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: new insights and advances. Mol. Cell. Endocrinol. 2010;315:49–56. doi: 10.1016/j.mce.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi H. Regulation and function of mTOR signalling in T cell fate decisions. Nat. Rev. Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu MI, Katz H, Berlin V. RAPT1, a mammalian homolog of yeast, Tor, interacts with the FKBP12/rapamycin complex. Proc. Natl. Acad. Sci. U.SA. 1994;91:12574–12578. doi: 10.1073/pnas.91.26.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung NP, Mruk DD, Mo MY, Lee WM, Cheng CY. A 22-amino acid synthetic peptide corresponding to the second extracellular loop of rat occludin perturbs the blood-testis barrier and disrupts spermatogenesis reversibly in vivo. Biol. Reprod. 2001;65:1340–1351. doi: 10.1095/biolreprod65.5.1340. [DOI] [PubMed] [Google Scholar]
- Chung SS, Lee WM, Cheng CY. Study on the formation of specialized inter- Sertoli cell junctions in vitro. J. Cell. Physiol. 1999;181:258–272. doi: 10.1002/(SICI)1097-4652(199911)181:2<258::AID-JCP8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Cruz R, Hedden L, Boyer D, Kharas MG, Fruman DA, Lee-Fruman KK. S6 kinase 2 potentiates interleukin-3-driven cell proliferation. J. Leukoc. Biol. 2005;78:1378–1385. doi: 10.1189/jlb.0405225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins PM. Occludin: one protein, many forms. Mol. Cell. Biol. 2012;32:242–250. doi: 10.1128/MCB.06029-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G, Shiras A, Shanmuganandam K, Shastry P. Rictor regulates MMP-9 activity and invasion through Raf-1-MEK-ERK signaling pathway in glioma cells. Mol. Carcinog. 2011;50:412–423. doi: 10.1002/mc.20723. [DOI] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol. Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J. Cell. Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazert E, Hall MN. mTOR signaling in disease. Curr. Opin. Cell. Biol. 2011;23:744–755. doi: 10.1016/j.ceb.2011.09.003. [DOI] [PubMed] [Google Scholar]
- de Kretser D, Kerr J. The cytology of the testis. In: Knobil E, Neill J, Ewing L, Greenwald C, Markert C, Pfaff D, editors. The physiology of reproduction. New York: Raven Press; 1988. pp. 837–932. [Google Scholar]
- Dehner M, Hadjihannas M, Weiske J, Huber O, Hehrens J. Wnt signaling inhibits Forkhead box O3a-induced transcription and apoptosis through up-regulation of serum- and glucocorticoid-inducible kinase 1. J. Biol. Chem. 2008;283:19201–19210. doi: 10.1074/jbc.M710366200. [DOI] [PubMed] [Google Scholar]
- Derangeon M, Spray DC, Bourmeyster N, Sarrouihe D, Herve JC. Reciprocal influence of connexins and apical junction proteins on their expressions and functions. Biochem. Biophys. Acta. 2009;1788:768–778. doi: 10.1016/j.bbamem.2008.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol. Cell. Biol. 2009;29:5657–5670. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann F, Campistol JM. Conversion from calcineurin inhibitors to sirolimus in chronic allograft nephropathy: benefits and risks. Nephrol. Dial. Transplant. 2006;21:562–568. doi: 10.1093/ndt/gfi336. [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Lammers JW, Koenderman L, Coffer PJ. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 2000a;10:1201–1204. doi: 10.1016/s0960-9822(00)00728-4. [DOI] [PubMed] [Google Scholar]
- Dijkers PF, Medema RH, Pals C, Banerji L, Thomas NS, Lam EW, Burgering BM, Raaijmakers JA, Lammers JW, Koenderman L, et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1) Mol. Cell. Biol. 2000b;20:9138–9148. doi: 10.1128/mcb.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich E, Schmaldienst S, Soleiman A, Horl WH, Pohanka E. Rapamycin-associated post-transplantation glomerulonephritis and its remission after reintroduction of calcineurin-inhibitor therapy. Transpl. Int. 2004;17:215–220. doi: 10.1007/s00147-004-0700-0. [DOI] [PubMed] [Google Scholar]
- Dorrello NV, Peschiaroli A, Guardavaccaro D, Colburn NH, Sherman NE, Pagano M. S6K1- and betaTRCP-mediated degradation of PDCD4 promotes protein translation and cell growth. Science. 2006;314:467–471. doi: 10.1126/science.1130276. [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K, Schulz CU, Meyer Zu Brickwedde MK, Pendl GG, Vestweber D. Junctional adhesion molecule interacts with the PDZ domain-containing proteins AF-6 and ZO-1. J. Biol. Chem. 2000;275:27979–27988. doi: 10.1074/jbc.M002363200. [DOI] [PubMed] [Google Scholar]
- Ehmcke J, Wistuba J, Schlatt S. Spermatogonial stem cells: questions, models and perspectives. Hum. Reprod. Update. 2006;12:275–282. doi: 10.1093/humupd/dmk001. [DOI] [PubMed] [Google Scholar]
- Ekman S, Wynes MW, Hirsch FR. The mTOR pathway in lung cancer and implications for therpay and biomarker analysis. J. Thorac. Oncol. 2012;7:947–953. doi: 10.1097/JTO.0b013e31825581bd. [DOI] [PubMed] [Google Scholar]
- Elkouby-Naor L, Ben-Yosef T. Functions of claudin tight junction proteins and their complex interactions in various physiological systems. Int Rev. Cell. Mol. Biol. 2010;279:1–32. doi: 10.1016/S1937-6448(10)79001-8. [DOI] [PubMed] [Google Scholar]
- Enders G. Sertoli-Sertoli and Sertoli-germ cell communications. In: Russell L, Griswold M, editors. The Sertoli cell. Clearwater: Cache River Press; 1993. pp. 447–460. [Google Scholar]
- Fagerli UM, Ullrich K, Stuhmer T, Holien T, Kochert K, Holt RU, Bruland O, Chatterjee M, Nogai H, Lenz G, et al. Serum/glucocorticoid-regulated kinase 1 (SGK1) is a prominent target gene of the transcriptional response to cytokines in multiple myeloma and supports the growth of myeloma cells. Oncogene. 2011;30:3198–3206. doi: 10.1038/onc.2011.79. [DOI] [PubMed] [Google Scholar]
- Fang Z, Zhang T, Dizeyi N, Chen S, Wang H, Swanson KD, Cai C, Balk SP, Yuan X. Androgen receptor enhances p27 degradation in prostate cancer cells through rapid and selective TORC2 activation. J. Biol. Chem. 2012;287:2090–2098. doi: 10.1074/jbc.M111.323303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- Fasolo A, Sessa C. Targeting mTOR pathways in human malignancies. Curr. Pharm. Des. 2012;18:2766–2777. doi: 10.2174/138161212800626210. [DOI] [PubMed] [Google Scholar]
- Fijak M, Bhushan S, Meinhardt A. Immunoprivileged sites: the testis. Methods Mol. Biol. 2011;677:459–470. doi: 10.1007/978-1-60761-869-0_29. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/ eukaryotic translation initiation factor 4E. Mol. Biol. Cell. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotow H, Thomas G. Substrate recognition determinants of the mitogen-activated 70K S6 kinase from rat liver. J. Biol. Chem. 1992;267:3074–3078. [PubMed] [Google Scholar]
- Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, Sabatini DM. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 2006;16:1865–1870. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Fukuhara A, Irie K, Nakanish H, Takekuni K, Kawakatsu T, Ikeda W, Yamada A, Katata T, Honda T, Sato T, et al. Involvement of nectin in the localization of junctional adhesion molecule at tight junctions. Oncogene. 2002a;21:7642–7655. doi: 10.1038/sj.onc.1205875. [DOI] [PubMed] [Google Scholar]
- Fukuhara A, Irie K, Yamada A, Katata T, Honda T, Shimizu K, Nakanish H, Takai Y. Role of nectin in organization of tight junctions in epithelial cells. Genes Cells. 2002b;7:1059–1072. doi: 10.1046/j.1365-2443.2002.00578.x. [DOI] [PubMed] [Google Scholar]
- Fukuyama T, Oqita H, Kawakatsu T, Fukuhara T, Yamada T, Sato T, Shimizu K, Nakamura T, Matsuda M, Takai Y. Involvement of the c-Src-Crk-C3G-Rap1 signaling in the nectin-induced activation of Cdc42 and formation of adherens junctions. J. Biol. Chem. 2005;280:815–825. doi: 10.1074/jbc.M411099200. [DOI] [PubMed] [Google Scholar]
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and-2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell. Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J. Cell. Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hata M, Furuse K, Yoshida Y, Haratake A, Sugitani Y, Noda T, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J. Cell. Biol. 2002;56:1099–1111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J. Cell. Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions. J. Cell. Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan X, Wang J, Su B, Wu D. Evidence for direct activation of mTORC2 kinase activity by phosphatidylinositol 3,4,5-trisphosphate. J. Biol. Chem. 2011;286:10998–11002. doi: 10.1074/jbc.M110.195016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem. J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- Giepmans BN. Gap junctions and connexin-interacting proteins. Cardiovasc. Res. 2004;62:233–245. doi: 10.1016/j.cardiores.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Giepmans BN, Moolenaar WH. The gap junction protein connexin43 interacts with the second PDZ domain of the zona occludens-1 protein. Curr. Biol. 1998;8:931–934. doi: 10.1016/s0960-9822(07)00375-2. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- Gliki G, Ebnet K, Aurrand-Lions M, Imhof BA, Adams RH. Spermatid differentiation requires the assembly of a cell polarity complex downstream of junctional adhesion molecule-C. Nature. 2004;431:320–324. doi: 10.1038/nature02877. [DOI] [PubMed] [Google Scholar]
- Godel M, Hartleben B, Herbach N, Liu S, Zschiedrich S, Lu S, Debreczeni-Mor A, Lindenmeyer MT, Rastaldi MP, Hartleben G, et al. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. J. Clin. Invest. 2011;121:2197–2209. doi: 10.1172/JCI44774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh ET, Pardo OE, Michael N, Niewiarowski A, Totty N, Volkova D, Tsaneva IR, Seckl MJ, Gout I. Involvement of heterogeneous ribonucleoprotein F in the regulation of cell proliferation via the mammalian target of rapamycin/S6 kinase 2 pathway. J. Biol. Chem. 2010;285:17065–17076. doi: 10.1074/jbc.M109.078782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goley ED, Welch MD. The ARP2/3 complex: an actin nucleator comes of age. Nat. Rev. Mol. Cell. Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin. Cell. Dev. Biol. 2000;11:315–324. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- Gottlob K, Majewski N, Kennedy S, Kandel E, Robey B, Hay N. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 2001;15:1406–1418. doi: 10.1101/gad.889901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow A, Southwood CM, Li JS, Pariali M, Riordan GP, Brodie SE, Danias J, Bronstein JM, Kachar B, Lazzarini RA. CNS myelin and Sertoli cell tight junction strands are absent in Osp/claudin-11 null mice. Cell. 1999;99:649–659. doi: 10.1016/s0092-8674(00)81553-6. [DOI] [PubMed] [Google Scholar]
- Gressner AM, Wool G. The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit protein (S6) is phosphorylated. J. Biol. Chem. 1974;249:6917–6925. [PubMed] [Google Scholar]
- Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin. Cell. Dev. Biol. 1998;9:411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzqerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev. Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Guo X, Rao JN, Liu L, Zou TT, Turner DJ, Bass BL, Wang JY. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. AmJPhysiol. Cell. Physiol. 2003;285:C1174–C1187. doi: 10.1152/ajpcell.00015.2003. [DOI] [PubMed] [Google Scholar]
- Guo Z, Zhou Y, Evers BM, Wang Q. Rictor regulates FBXW7-dependent c-Myc and cyclin E degradation in colorectal cancer cells. BioChem Biophys. Res. Commun. 2012;418:426–432. doi: 10.1016/j.bbrc.2012.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Tepass U. Adherens junctions: from molecules to morphogenesis. Nat. Rev. Mol. Cell. Biol. 2010;11:502–514. doi: 10.1038/nrm2927. [DOI] [PubMed] [Google Scholar]
- Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J. Cell. Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi D, Tamura A, Tanaka H, Yamazaki Y, Watanabe S, Suzuki K, Suzuki K, Sentani K, Yasui W, Rakugi H, et al. Deficiency of claudin-18 causes paracellular H+ leakage, up-regulation of interleukin-1β, and atrophic gastritis in mice. Gastroenterology. 2012;142:292–304. doi: 10.1053/j.gastro.2011.10.040. [DOI] [PubMed] [Google Scholar]
- Hermeking H. The 14-3-3 cancer connection. Nat. Rev. Cancer. 2003;3:931–943. doi: 10.1038/nrc1230. [DOI] [PubMed] [Google Scholar]
- Hernandez-Negrete I, Carretero-Ortega J, Rosenfeldt H, Hernandez-Garcia R, Calderon- Salinas JV, Reyes-Cruz G, Gutkind JS, Vazquez-Prado J. P-Rex1 links mammalian target of rapamycin signaling to Rac activation and cell migration. J. Biol. Chem. 2007;282:23708–23715. doi: 10.1074/jbc.M703771200. [DOI] [PubMed] [Google Scholar]
- Hers I, Vincent EE, Tavare JM. Akt signalling in health and disease. Cell. Signal. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- Hertzog M, Milanesi F, Hazelwood L, Disanza A, Liu H, Perlade E, Malabarba MG, Pasqualato S, Maiolica A, Confalonieri S, et al. Molecular basis for the dual function of Eps8 on actin dynamics: bundling and capping. PLoS Biol. 2010;8:e1000387. doi: 10.1371/journal.pbio.1000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, de Franca LR. Spermatogenesis and cycle of the seminiferous epithelium. In: Cheng CY, editor. Molecular mechanisms in spermatogenesis. Austin, TX: Landes Bioscience/ Springer Science+Business Media; 2008. pp. 1–15. [Google Scholar]
- Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J. Cell. Sci. 1997;110:1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- Holinstat M, Mehta D, Kozasa T, Minshall RD, Malik AB. Protein kinase Calpha- induced p115RhoGEF phosphorylation signals endothelial cytoskeletal rearrangement. J. Biol. Chem. 2003;278:28793–28798. doi: 10.1074/jbc.M303900200. [DOI] [PubMed] [Google Scholar]
- Hornstein E, Tang H, Meyuhas O. Mitogenic and nutritional signals are transduced into translational efficiency of TOP mRNAs. Cold Spring Harb. Symp. Quant. Biol. 2001;66:477–484. doi: 10.1101/sqb.2001.66.477. [DOI] [PubMed] [Google Scholar]
- Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J. Biol. Chem. 2005;280:24095–24103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- Howell JJ, Manning BD. mTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol. Metab. 2011;22:94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Dibble CC, Matsuzaki M, Manning BD. The TSC1-TSC2 complex is required for proper activation of mTOR complex 2. Mol. Cell. Biol. 2008;28:4104–4115. doi: 10.1128/MCB.00289-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wu S, Wu CL, Manning BD. Signaling events downstream of mammalian target of rapamycin complex 2 are attenuated in cells and tumors deficient for the tuberous sclerosis complex tumor suppressors. Cancer Res. 2009;69:6107–6114. doi: 10.1158/0008-5472.CAN-09-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Mori H, Wang J, Suzuki T, Holng S, Yoshida S, Blattner SM, Ikenoue T, Ruegg MA, Hall MN, et al. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. J. Clin. Invest. 2011;121:2181–2196. doi: 10.1172/JCI44771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Ip CK, Cheung AN, Ngan HY, Wong AS. p70 S6 kinase in the control of actin cytoskeleton dynamics and directed migration of ovarian cancer cells. Oncogene. 2011;30:2420–2432. doi: 10.1038/onc.2010.615. [DOI] [PubMed] [Google Scholar]
- Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J. Cell. Biol. 1999a;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Morita K, Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J. Biol. Chem. 1999b;274:5981–5986. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J. Cell. Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J. Cell. Biol. 2001;154:491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA. VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004;286:L1143–L1153. doi: 10.1152/ajplung.00305.2003. [DOI] [PubMed] [Google Scholar]
- Izzedine H, Brocheriou I, Frances C. Post-transplantation proteinuria and sirolimus. N. Engl. J. Med. 2005;353:2088–2089. doi: 10.1056/NEJM200511103531922. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell. Biol. 2004;6 doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Johnson K, Boekelheide K. Dynamic testicular adhesion junctions are immunologically unique. II. Localization of classic cadherins in rat testis. Biol. Reprod. 2002;66:992–1000. doi: 10.1095/biolreprod66.4.992. [DOI] [PubMed] [Google Scholar]
- Julien LA, Carriere A, Moreau J, Roux PP. mTORC1-activated S6K1 phosphorylates Rictor on threonine: 1135 and regulates mTORC2 signaling. Mol. Cell. Biol. 2010;30:908–921. doi: 10.1128/MCB.00601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahan BD. Sirolimus: a comprehensive review. Expert Opin. Pharmacother. 2001;2:1903–1917. doi: 10.1517/14656566.2.11.1903. [DOI] [PubMed] [Google Scholar]
- Kaitu’u-Lino TJ, Sluka P, Foo CF, Stanton PG. Claudin-11 expression and localisation is regulated by androgens in rat Sertoli cells in vitro. Reproduction. 2007;133:1169–1179. doi: 10.1530/REP-06-0385. [DOI] [PubMed] [Google Scholar]
- Kametani Y, Takeichi M. Basal-to-apical cadherin flow at cell junctions. Nat. Cell. Biol. 2007;9:92–98. doi: 10.1038/ncb1520. [DOI] [PubMed] [Google Scholar]
- Katsuno T, Umeda K, Matsui T, Hata M, Tamura A, Itoh M, Takeuchi K, Fujimoto T, Nabeshima Y, Noda T, et al. Deficiency of zonula occludens-1 causes embryonic lethal phenotype associated with defected yolk sac angiogenesis and apoptosis of embryonic cells. Mol. Biol. Cell. 2008;19:2465–2475. doi: 10.1091/mbc.E07-12-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakatsu T, Oqita H, Fukuhara T, Fukuhara T, Minami Y, Shimizu K, Takai Y. Vav2 as a Rac-GDP/GTP exchange factor responsible for the nectin-induced, c-Src- and Cdc42-mediated activation of Rac. J. Biol. Chem. 2005;280:4940–4947. doi: 10.1074/jbc.M408710200. [DOI] [PubMed] [Google Scholar]
- Kawakatsu T, Shimizu K, Honda T, Fukuhara T, Hoshino T, Takai Y. Trans-interactions of nectins induce formation of filopodia and Lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J. Biol. Chem. 2002;277:50749–50755. doi: 10.1074/jbc.M209846200. [DOI] [PubMed] [Google Scholar]
- Khokhar NZ, Altman JK, Platanias LC. Emerging roles for mammalian target of rapamycin inhibitors in the treatment of solid tumors and hematological malignancies. Curr. Opin. Oncol. 2011;23:578–586. doi: 10.1097/CCO.0b013e32834b892d. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, Tempst P, Sabatini DM. GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol. Cell. 2003;11:895–904. doi: 10.1016/s1097-2765(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Kloet DE, Burgering BM. The PKB/FOXO switch in aging and cancer. Biochem. Biophys. Acta. 2011;1813:1926–1937. doi: 10.1016/j.bbamcr.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Koch M, Olson P, Albus A, Jin W, Hunter D, Brunken W, Burgeson R, Champliaud M. Characterization and expression of the laminin γ3 chain: a novel, non-basement membrane-associated, laminin chain. J. Cell. Biol. 1999;145:605–618. doi: 10.1083/jcb.145.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima T, Murata M, Go M, Spray DC, Sawada N. Connexins induce and maintain tight junctions in epithelial cellsJMembr. Biol. 2007;217:13–19. doi: 10.1007/s00232-007-9021-4. [DOI] [PubMed] [Google Scholar]
- Kojima T, Spray DC, Kokai Y, Chiba H, Mochizuki Y, Sawada N. Cx32 formation and/or Cx32-mediated intercellular communication induces expression and function of tight junctions in hepatocytic cell line. Exp. Cell. Res. 2002;276:40–51. doi: 10.1006/excr.2002.5511. [DOI] [PubMed] [Google Scholar]
- Komljenovic D, Sandhoff R, Teigler A, Heid H, Just WW, Gorgas K. Disruption of blood-testis barrier dynamics in ether-lipid-deficient mice. Cell. Tissue Res. 2009;337:281–299. doi: 10.1007/s00441-009-0809-7. [DOI] [PubMed] [Google Scholar]
- Krieq J, Hofsteenge J, Thomas G. Identification of the 40 S ribosomal protein S6 phosphorylation sites induced by cycloheximide. J. Biol. Chem. 1988;263:11473–11477. [PubMed] [Google Scholar]
- Kubota K, Furuse M, Sasaki H, Sonoda N, Fujita K, Nagafuchi A, Tsukita S. Ca2+-independent cell-adhesion activity of claudins, a family of integral membrane proteins localized at tight junctions. Curr. Biol. 1999;9:1035–1038. doi: 10.1016/s0960-9822(99)80452-7. [DOI] [PubMed] [Google Scholar]
- LaFemina MJ, Rokkam D, Chandrasena A, Pan J, Bajaj A, Johnson M, Frank JA. Keratinocyte growth factor enhances barrier function without altering claudin expression in primary alveolar epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010;299:L724–L734. doi: 10.1152/ajplung.00233.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing JG, Manley-Markowski RN, Koval M, Civitelli R, Steinberg TH. Connexin45 interacts with zonula occludens-1 and connexin43 in osteoblastic cells. J. Biol. Chem. 2001;276:23051–23055. doi: 10.1074/jbc.M100303200. [DOI] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;49:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukoetter MG, Nava P, Lee WY, Severson EA, Capaldo CT, Babbin BA, Williams IR, Koval M, Peatman E, Campbell JA, et al. JAM-A regulates permeability and inflammation in the intestine in vivoJExp. Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Clainche C, Schlaepfer D, Ferrari A, Klinqauf M, Grohmanova K, Veliqodskiy A, Didry D, Le D, Eqile C, Carlier MF, et al. IQGAP1 stimulates actin assembly through the N-WASP-Arp2/3 pathway. J. Biol. Chem. 2007;282:426–435. doi: 10.1074/jbc.M607711200. [DOI] [PubMed] [Google Scholar]
- Lee-Fruman KK, Kuo CJ, Lippincott J, Terada N, Blenis J. Characterization of S6K2, a novel kinase homologous to S6K1. Oncogene. 1999;18:5108–5114. doi: 10.1038/sj.onc.1202894. [DOI] [PubMed] [Google Scholar]
- Lee NP, Leung KW, Wo JY, Tam PC, Yeung WS, Luk JM. Blockage of testicular connexins induced apoptosis in rat seminiferous epithelium. Apoptosis. 2006;11:1215–1229. doi: 10.1007/s10495-006-6981-2. [DOI] [PubMed] [Google Scholar]
- Lee NPY, Mruk DD, Lee WM, Cheng CY. Is the cadherin/catenin complex a functional unit of cell-cell-actin-based adherens junctions (AJ) in the rat testis? Biol. Reprod. 2003;68:489–508. doi: 10.1095/biolreprod.102.005793. [DOI] [PubMed] [Google Scholar]
- Li D, Mrsny RJ. Oncogenic Raf-1 disrupts epithelial tight junctions via downregulation of occludin. J. Cell. Biol. 2000;148:791–800. doi: 10.1083/jcb.148.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MW, Mruk DD, Lee WM, Cheng CY. Connexin 43 is critical to maintain the homeostasis of the blood-testis barrier via its effects on tight junction reassembly. Proc. Natl. Acad. SciU.SA. 2010;107:17998–18003. doi: 10.1073/pnas.1007047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MWM, Mruk DD, Cheng CY. Gap junctions and blood-tissue barriers. Adv. Exp. Med. Biol. 2012;763:260–280. doi: 10.1007/978-1-4614-4711-5_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MWM, Mruk DD, Lee WM, Cheng CY. Connexin 43 and plakophilin-2 as a protein complex that regulates blood-testis barrier dynamics. Proc. Natl. Acad. Sci. U.SA. 2009;106:10213–10218. doi: 10.1073/pnas.0901700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang TW, DeMarco RA, Mrsny RJ, Gurney A, Gray A, Hooley J, Aaron HL, Huang A, Klassen T, Tumas DB, et al. Characterization of huJAM: evidence for involvement in cell-cell contact and tight junction regulation. Am. J. Physiol. Cell. Physiol. 2000;279:1733–1743. doi: 10.1152/ajpcell.2000.279.6.C1733. [DOI] [PubMed] [Google Scholar]
- Lie PPY, Chan AYN, Mruk DD, Lee WM, Cheng CY. Restricted Arp3 expression in the testis prevents blood-testis barrier disruption during junction restructuring at spermatogenesis. Proc. Natl. Acad. Sci. U.SA. 2010;107:11411–11416. doi: 10.1073/pnas.1001823107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie PPY, Mruk DD, Lee WM, Cheng CY. Epidermal growth factor receptor pathway substrate 8 (Eps8) is a novel regulator of cell adhesion and the blood-testis barrier integrity in the seminiferous epithelium. FASEB J. 2009;23:2555–2567. doi: 10.1096/fj.06-070573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberthal W, Levine JS. Mammalian target of rapamycin and the kidney. II. Pathophysiology and therapeutic implications. Am. J. Physiol. Ren. Physiol. 2012;303:F180–F191. doi: 10.1152/ajprenal.00015.2012. [DOI] [PubMed] [Google Scholar]
- Liu L, Chen L, Chung J, Huang S. Rapamycin inhibits F-actin reorganization and phosphorylation of focal adhesion proteins. Oncogene. 2008;27:4998–5010. doi: 10.1038/onc.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, Pochet M, Parkos CA. Human junction adhesion molecule regulates tight junction resealing in epithelia. J. Cell. Sci. 2000;113:2363–2374. doi: 10.1242/jcs.113.13.2363. [DOI] [PubMed] [Google Scholar]
- Lyo D, Xu L, Foster DA. Phospholipase D stabilizes HDM2 through an mTORC2/ SGK1 pathway. BioChem Biophys. Res. Commun. 2010;396:562–565. doi: 10.1016/j.bbrc.2010.04.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Chen Z, Erdjument-Bromage H, Tempst P, Pandolfi PP. Phosphorylation and functional inactivation of TSC2 by Erk implications for tuberous sclerosis and cancer pathogenesis. Cell. 2005;121:179–193. doi: 10.1016/j.cell.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Maeda M, Johnson E, Mandal SH, Lawson KR, Keim SA, Svoboda RA, Caplan S, Wahl JK, 3rd, Wheelock MJ, Johnson KR. Expression of inappropriate cadherins by epithelial tumor cells promotes endocytosis and degradation of E-cadherin via competition for p120(ctn) Oncogene. 2006;25:4595–4604. doi: 10.1038/sj.onc.1209396. [DOI] [PubMed] [Google Scholar]
- Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem. J. 2012;441:1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- Man Y, Hart VJ, Ring CJ, Sanjar S, West MR. Loss of epithelial integrity resulting from E-cadherin dysfunction predisposes airway epithelial cells to adenoviral infection. AmJRespir. Cell. Mol. Biol. 2000;23:610–617. doi: 10.1165/ajrcmb.23.5.4046. [DOI] [PubMed] [Google Scholar]
- Mandell KJ, Parkos CA. The JAM family of proteins. Adv. Drug Deliv. Rev. 2005;57:857–867. doi: 10.1016/j.addr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol. Cell. 2002;10:151–162. doi: 10.1016/s1097-2765(02)00568-3. [DOI] [PubMed] [Google Scholar]
- Marcitrigiano J, Gingras AC, Sonenberg N, Burley SK. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol. Cell. 1999;3:707–716. doi: 10.1016/s1097-2765(01)80003-4. [DOI] [PubMed] [Google Scholar]
- Martin J, Maris J, Bernath A, Nishimura RN, Gera J. Hsp70 associates with Rictor and is required for mTORC2 formation and activity. BioChem Biophys. Res. Commun. 2008;372:578–583. doi: 10.1016/j.bbrc.2008.05.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Rahman A, Malik AB. Protein kinase C-alpha signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J. Biol. Chem. 2001;276:22614–22620. doi: 10.1074/jbc.M101927200. [DOI] [PubMed] [Google Scholar]
- Meinhardt A, Hedger MP. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol. Cell. Endocrinol. 2011;335:60–68. doi: 10.1016/j.mce.2010.03.022. [DOI] [PubMed] [Google Scholar]
- Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. EurJBiochem. 2000;267:6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- Meyuhas O. Physiological roles of ribosomal protein S6: one of its kind. Int Rev. Cell. Mol. Biol. 2008;268:1–37. doi: 10.1016/S1937-6448(08)00801-0. [DOI] [PubMed] [Google Scholar]
- Miyoshi J, Takai Y. Structural and functional associations of apical junctions with cytoskeleton. Biochim. Biophys. Acta. 2008;1778:670–691. doi: 10.1016/j.bbamem.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Lee WM, Cheng CY. Rictor/mTORC2 regulates bloodtestis barrier dynamics via its effects on gap junction communications and actin filament network. FASEB J. 2012a doi: 10.1096/fj.12-212977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Lee WM, Cheng CY. Spermatogonial stem cells alone are not sufficient to re-initiate spermatogenesis in the rat testis following adjudin-induced infertility. Int. J. Androl. 2012b;35:86–101. doi: 10.1111/j.1365-2605.2011.01183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok KW, Mruk DD, Silvestrini B, Cheng CY. CYrpS6 regulates blood-testis barrier dynamics by affecting F-actin organization and protein recruitment. Endocrinology. 153:5036–5048. doi: 10.1210/en.2012-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne J, Stewart MJ, Stocker H, Hafen E, Kozma SC, Thomas G. Drosophila S6 kinase: a regulator of cell size. Science. 1999;285:2126–2129. doi: 10.1126/science.285.5436.2126. [DOI] [PubMed] [Google Scholar]
- Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell. Dev. Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Morita H, Katsuno T, Hoshimoto A, Hirano N, Saito Y, Suzuki Y. Connexin 26-mediated gap junctional intercellular communication suppresses paracellular permeability of human intestinal epithelial cell monolayers. Exp. Cell. Res. 2004;298:1–8. doi: 10.1016/j.yexcr.2004.03.046. [DOI] [PubMed] [Google Scholar]
- Morita K, Furuse M, Fujimoto K, Tsukita S. Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc. Natl. Acad. Sci. U.S.A. 1999a;96:511–516. doi: 10.1073/pnas.96.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S. Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J. Cell. Biol. 1999b;45:579–588. doi: 10.1083/jcb.145.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi S, Saitou M, Fujimoto K, Sakakibara A, Furuse M, Yosshida O, Tsukita S. Occludin is concentrated at tight junctions of mouse/rat but not human/guinea pig Sertoli cells in testes. AmJPhysiol. 1998;274:1708–1717. doi: 10.1152/ajpcell.1998.274.6.C1708. [DOI] [PubMed] [Google Scholar]
- Morris AP, Tawil A, Berkova Z, Wible L, Smith CW, Cunningham SA. Junctional Adhesion Molecules (JAMs) are differentially expressed in fibroblasts and co-localize with ZO-1 to adherens-like junctions. Cell. Commun. Adhes. 2006;13:233–247. doi: 10.1080/15419060600877978. [DOI] [PubMed] [Google Scholar]
- Morrow CM, Mruk DD, Cheng CY, Hess RA. Claudin and occludin expression and function in the seminiferous epithelium. Philos. Trans. R Soc. Lond B Biol. Sci. 2010;365:1679–1696. doi: 10.1098/rstb.2010.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mruk DD, Cheng CY. Sertoli-Sertoli and Sertoli-germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 2004;25:747–806. doi: 10.1210/er.2003-0022. [DOI] [PubMed] [Google Scholar]
- Mruk DD, Silvestrini B, Cheng CY. Anchoring junctions as drug targets: role in contraceptive development. Pharmacol. Rev. 2008;60:146–180. doi: 10.1124/pr.107.07105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami T, Felinski EA, Antonetti DA. Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factorinduced permeability. J. Biol. Chem. 2009;284:21036–21046. doi: 10.1074/jbc.M109.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair S, Ren J. Autophagy and cardiovascular aging: lesson learned from rapamycin. Cell Cycle. 2012;11 doi: 10.4161/cc.20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir O, Wang K, Foller M, Gu S, Bhandaru M, Ackermann TF, Bioni KM, Mack A, LKlingel K, Amato R, et al. Relative resistance of SGK1 knockout mice against chemical carcinogenesis. IUBMB Life. 2009;61:768–776. doi: 10.1002/iub.209. [DOI] [PubMed] [Google Scholar]
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J. Cell. Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojima H, Tokunaga C, Eguchi S, Oshiro N, Hidayat S, Yoshino K, Hara K, Tanaka N, Avruch J, Yonezawa K. The mammalian target of rapamycin (mTOR) partner, raptor, binds the mTOR substrates p70 S6 kinase and 4E-BP1 through their TOR signaling (TOS) motif. J. Biol. Chem. 2003;278:15461–15464. doi: 10.1074/jbc.C200665200. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Brown GT, Tom J, Drake A, Bui TT, Quan C, Mrsny RJ. Multiple protein interactions involving proposed extracellular loop domains of the tight junction protein occludin. Mol. Biol. Cell. 2005;16:1725–1734. doi: 10.1091/mbc.E04-06-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell L, Nicholls PK, O’Bryan MK, McLachlan RI, Stanton PG. Spermiation: the process of sperm release. Spermatogenesis. 2011;1:14–35. doi: 10.4161/spmg.1.1.14525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr. Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- Oh WJ, Jacinto E. mTOR complex 2 signaling and functions. Cell Cycle. 2011;10:2305–2316. doi: 10.4161/cc.10.14.16586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh WJ, Wu CC, Kim SJ, Facchinetti V, Julien LA, Finlan M, Roux PP, Su B, Jacinto E. mTORC2 can associate with ribosomes to promote cotranslational phosphorylation and stability of nascent Akt polypeptide. EMBO J. 2010;29:3939–3951. doi: 10.1038/emboj.2010.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohanna M, Sobering AK, Lapointe T, Lorenzo L, Praud C, Petroulakis E, Sonenberg N, Kelly PA, Sotiropoulos A, Pende M. Atrophy of S6K1(−/−) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat. Cell. Biol. 2005;7:286–294. doi: 10.1038/ncb1231. [DOI] [PubMed] [Google Scholar]
- Ooshio T, Kobayashi R, Ikeda W, Miyata M, Fukumoto Y, Matsuzawa N, Oqita H, Takai Y. Involvement of the interaction of afadin with ZO-1 in the formation of tight junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 2010;285:5003–5012. doi: 10.1074/jbc.M109.043760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro N, Takahashi R, Yoshino K, Tanimura K, Nakashima A, Eguchi S, Miyamoto T, Hara K, Takehana K, Avruch J, et al. The proline-rich Akt substrate of 40 kDa (PRAS40) is a physiological substrate of mammalian target of rapamycin complex 1. J. Biol. Chem. 2007;282:20329–20339. doi: 10.1074/jbc.M702636200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki-Kuroda K, Nakanish H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, et al. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr. Biol. 2002;12:1145–1150. doi: 10.1016/s0960-9822(02)00922-3. [DOI] [PubMed] [Google Scholar]
- Palombi F, Salanova M, Tarone G, Farini D, Stefanini M. Distribution of β1 integrin subunit in rat seminiferous epithelium. Biol. Reprod. 1992;47:1173–1182. doi: 10.1095/biolreprod47.6.1173. [DOI] [PubMed] [Google Scholar]
- Parker PJ, Parkinson SJ. AGC protein kinase phosphorylation and protein kinase C. Biochem. Soc. Trans. 2001;29:860–863. doi: 10.1042/0300-5127:0290860. [DOI] [PubMed] [Google Scholar]
- Parvinen M. Regulation of the seminiferous epithelium. Endocr. Rev. 1982;3:404–417. doi: 10.1210/edrv-3-4-404. [DOI] [PubMed] [Google Scholar]
- Pause A, Belsham GJ, Gingras AC, Donze O, Lin TA, Lawrence JCJ, Sonenberg N. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5’-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- Paventadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol. Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat. Rev. Mol. Cell. Biol. 2010;11:9–22. doi: 10.1038/nrm2822. [DOI] [PubMed] [Google Scholar]
- Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, Alessi DR. Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem. J. 2011;436:169–179. doi: 10.1042/BJ20102103. [DOI] [PubMed] [Google Scholar]
- Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, Klumperman J, Thorens B, Thomas G. Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature. 2000;408:994–997. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, Mueller M, Fumagalli S, Kozma SC, Thomas G. S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5’-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell. Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J, He F, Zhang C, Deng X, Yin F. Protein kinase C-α signals P115RhoGEF phosphorylation and RhoA activation in TNF-α-induced mouse brain microvascular endothelial cell barrier dysfunction. J. Neuroinflammation. 2011;8:28–37. doi: 10.1186/1742-2094-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, Gray NS, Sabatini DM. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointis G, Gilleron J, Carette D, Segretain D. Physiological and physiopathological aspects of connexins and communicating gap junctions in spermatogenesis. Philos. Trans. R Soc. Lond B Biol. Sci. 2010;365:1607–1620. doi: 10.1098/rstb.2009.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadas EM, Figlin RA. Systemic therapy in renal cell carcinoma: advancing paradigms. Oncology. 2012;26:290–301. [PubMed] [Google Scholar]
- Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan MP, Hyatt JL. Establishment of the circumferential actin filament network is a prerequisite for localization of the cadherin-catenin complex in epithelial cells. Cell. Growth Differ. 1999;10:839–854. [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev. Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, Polakiewicz RD, Sonenberg N, Hershey JW. Phosphorylation of eukaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J. 2004;23:1761–1769. doi: 10.1038/sj.emboj.7600193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson CJ, Broenstrup M, Fingar DC, Julich K, Ballif BA, Gygi S, Blenis J. SKAR is a specific target of S6 kinase 1 in cell growth control. Curr. Biol. 2004;14:1540–1549. doi: 10.1016/j.cub.2004.08.061. [DOI] [PubMed] [Google Scholar]
- Roux PP, Ballif BA, Anjum R, Gygi SP, Blenis J. Tumor-promoting phorbol esters and activated Ras inactivate the tuberous sclerosis tumor suppressor complex via p90 ribosomal S6 kinase. Proc. Natl. Acad. Sci. U S A. 2004;101:13489–13494. doi: 10.1073/pnas.0405659101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J. Biol. Chem. 2007;282:14056–14064. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. Movement of spermatocytes from the basal to the adluminal compartment of the rat testis. AM J. Anat. 1977;48:313–328. doi: 10.1002/aja.1001480303. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem. Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–2211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DM, Erdjument-Bromage H, Lui M, Tempst P, Snyder SH. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994;78:35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Saitou M, Furuse M, Sasaki H, Schulzke J, Fromm M, Takano H, Noda T, Tsukita S. Complex phenotype of mice lacking occludin, a component of tight junction strands. Mol. Biol. Cell. 2000;11:4131–4142. doi: 10.1091/mbc.11.12.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi T, Nishimoto M, Miyagi S, Iwama A, Morita Y, Iwamori N, Nakauchi H, Kiyonari H, Muramatsu M, Okuda A. Putative “stemness” gene jam-B is not required for maintenance of stem cell state in embryonic, neural, or hematopoietic stem cells. Mol. Cell. Biol. 2006;26:6557–6570. doi: 10.1128/MCB.00729-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakisaka T, Ikeda W, Oqita H, Fujita N, Takai Y. The roles of nectins in cell adhesions: cooperation with other cell adhesion molecules and growth factor receptors. Curr. Opin. Cell. Biol. 2007;19:593–602. doi: 10.1016/j.ceb.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Salanova M, Stefanini M, De Curtis I, Palombi F. Integrin receptor α6β1 is localized at specific sites of cell-to-cell contact in rat seminiferous epithelium. Biol. Reprod. 1995;52:79–87. doi: 10.1095/biolreprod52.1.79. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Senqupta S, Sheen JH, Hsu PP, Bagley AF, Markhard AL, Sabatini DM. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell. 2006;22:159–168. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sato T, Fujita N, Yamada A, Ooshio T, Okamoto R, Irie K, Takai Y. Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J. Biol. Chem. 2006;281:5288–5299. doi: 10.1074/jbc.M510070200. [DOI] [PubMed] [Google Scholar]
- Schalm SS, Fingar DC, Sabatini DM, Blenis J. TOS motif-mediated raptor binding regulates 4E-BP1 multisite phosphorylation and function. Curr. Biol. 2003;2003:797–806. doi: 10.1016/s0960-9822(03)00329-4. [DOI] [PubMed] [Google Scholar]
- Schieke SM, Philips D, McCoy JPJ, Aponte AM, Shen RF, Balaban RS, Finkel T. The mammalian target of rapamycin (mTOR) pathway regulates mitochondrial oxygen consumption and oxidative capacity. J. Biol. Chem. 2006;281:27643–27652. doi: 10.1074/jbc.M603536200. [DOI] [PubMed] [Google Scholar]
- Schwarz BT, Wang F, Shen L, Clayburgh DR, Su L, Wang Y, Fu YX, Turner JR. LIGHT signals directly to intestinal epithelia to cause barrier dysfunction via cytoskeletal and endocytic mechanisms. Gastroenterology. 2007;132:2383–2394. doi: 10.1053/j.gastro.2007.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senqupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson EA, Parkos CA. Structural determinants of Junctional Adhesion Molecule A (JAM-A) function and mechanisms of intracellular signaling. Curr. Opin. Cell. Biol. 2009;21:701–707. doi: 10.1016/j.ceb.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat. Rev. Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaha C. Estrogens and spermatogenesis. In: Cheng CY, editor. Molecular mechanisms in spermatogenesis. Austin, TX: Landes Bioscience and Springer Science+Business Media; 2008. pp. 42–64. [Google Scholar]
- Shahbazian D, Roux PP, Mieulet V, Cohen MS, Raught B, Taunton J, Hershey JW, Blenis J, Pende M, Sonenberg N. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006;25:2781–2791. doi: 10.1038/sj.emboj.7601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M, Ghosh A, Cooke VG, Naik UP, Martin-DeLeon PA. JAM-A is present in mammalian spermatozoa where it is essential for normal motility. Dev. Biol. 2008;313:246–255. doi: 10.1016/j.ydbio.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM. Regulation of spermatogenesis. In: Knobil E, Neill JD, editors. The physiology of reproduction. New York: Raven Press; 1994. pp. 1363–1434. [Google Scholar]
- Shen L, Turner JR. Actin depolymerization disrupts tight junctions via caveolaemediated endocytosis. Mol. Biol. Cell. 2005;16:3919–3936. doi: 10.1091/mbc.E04-12-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA. Targeting PI3 kinase/AKT/mTOR signalling in cancer. Crit. Rev. Oncog. 2012;17:69–95. doi: 10.1615/critrevoncog.v17.i1.60. [DOI] [PubMed] [Google Scholar]
- Shima H, Pende M, Chen Y, Fumagalli S, Thomas G, Kozma SC. Disruption of the p70(s6k)/p85(s6k) gene reveals a small mouse phenotype and a new functional S6 kinase. EMBO J. 1998;17:6649–6659. doi: 10.1093/emboj/17.22.6649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorning BY, Griffiths D, Clarke AR. Lkb1 and Pten synergise to suppress mTOR-mediated tumorigenesis and epithelial-mesenchymal transition in the mouse bladder. PLoS One. 2011;6:e16209. doi: 10.1371/journal.pone.0016209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DB, Lu Y, Choate KA, Velazquez H, AI-Sabban E, Praga M, Casari G, Bettinelli A, Colussi G, Rodriquez-Soriano J, et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. doi: 10.1126/science.285.5424.103. [DOI] [PubMed] [Google Scholar]
- Simon P, Schneck M, Hochstetter T, Koutsouki E, Mittelbronn M, Merseburger A, Weigert C, Niess A, Lang F. Differential regulation of serum- and glucocorticoid- inducible kinase 1 (SGK1) splice variants based on alternative initiation of transcription. Cell. Physiol. Biochem. 2007;20:715–728. doi: 10.1159/000110432. [DOI] [PubMed] [Google Scholar]
- Singh AP, Harada S, Mishina Y. Downstream genes of Sox8 that would affect adult male fertility. Sex. Dev. 2009;3:16–25. doi: 10.1159/000200078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ER, Wong EW, Mruk DD, Porto CS, Cheng CY. Focal adhesion kinase is a blood-testis barrier regulator. Proc. Natl. Acad. Sci. U S A. 2009a;106:9298–9303. doi: 10.1073/pnas.0813113106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu ER, Wong EWP, Mruk DD, Sze KL, Porto CS, Cheng CY. An occludin- focal adhesion kinase protein complex at the blood-testis barrier: a study using the cadmium model. Endocrinology. 2009b;150:3336–3344. doi: 10.1210/en.2008-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siu MK, Lee WM, Cheng CY. The interplay of collagen, IV, tumor necrosis factoralpha, gelatinase B (matrix metalloprotease-9), and tissue inhibitor of metalloproteases-1 in the basal lamina regulates Sertoli cell-tight junction dynamics in the rat testis. Endocrinology. 2003;144:371–387. doi: 10.1210/en.2002-220786. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Cheng CY. Interactions of proteases, protease inhibitors, and the β1 integrin/laminin γ3 protein complex in the regulation of ectoplasmic specialization dynamics in the rat testis. Biol. Reprod. 2004;70:945–964. doi: 10.1095/biolreprod.103.023606. [DOI] [PubMed] [Google Scholar]
- Siu MKY, Wong CH, Lee WM, Cheng CY. Sertoli-germ cell anchoring junction dynamics in the testis are regulated by an interplay of lipid and protein kinases. J. Biol. Chem. 2005;280:25029–25047. doi: 10.1074/jbc.M501049200. [DOI] [PubMed] [Google Scholar]
- Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell. Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell. Biol. 2010;20:142–149. doi: 10.1016/j.tcb.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Stolovich M, Lerer T, Bolkier Y, Cohen H, Meyuhas O. Lithium can relieve translational repression of TOP mRNAs elicited by various blocks along the cell cycle in a glycogen synthase kinase-3- and S6-kinase-independent manner. J. Biol. Chem. 2005;280:5336–5342. doi: 10.1074/jbc.M412434200. [DOI] [PubMed] [Google Scholar]
- Sun S, Wong EWP, Li MWM, Lee WM, Cheng CY. 14-3-3 and its binding partners are regulators of protein-protein interactions during spermatogenesis. J. Endocrinol. 2009;202:327–336. doi: 10.1677/JOE-09-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Ikeda W, Oqita H, Rikitake Y. The immunoglobulin-like cell adhesion molecule nectin and its associated protein afadin. Annu. Rev. Cell. Dev. Biol. 2008;24:309–342. doi: 10.1146/annurev.cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- Takehashi M, Kanatsu-Shinohara M, Miki H, Lee J, Kazuki Y, Inoue K, Ogonuki N, Toyokuni S, Oshimura M, Ogura A, et al. Production of knockout mice by gene targeting in multipotent germline stem cells. Dev. Biol. 2007;312:344–352. doi: 10.1016/j.ydbio.2007.09.029. [DOI] [PubMed] [Google Scholar]
- Tamura A, Hayashi H, Imasato M, Yamazaki Y, Haqiwara A, Wada M, Noda T, Watanabe M, Suzuki Y, Tsukita S. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterology. 2011;140:913–923. doi: 10.1053/j.gastro.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Tang H, Hornstein E, Stolovich M, Levy G, Livingstone M, Templeton D, Avruch J, Meyuhas O. Amino acid-induced translation of TOP mRNAs is fully dependent on phosphatidylinositol 3-kinase-mediated signaling, is partially inhibited by rapamycin, and is independent of S6K1 and rpS6 phosphorylation. Mol. Cell. Biol. 2001;21:8671–8683. doi: 10.1128/MCB.21.24.8671-8683.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tato I, Bartrons R, Ventura F, Rosa JL. Amino acids activate mammalian target of rapamycin complex 2 (mTORC2) via PI3K/Akt signaling. J. Biol. Chem. 2011;286:6128–6142. doi: 10.1074/jbc.M110.166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat. Res. 1993;290:193–200. doi: 10.1016/0027-5107(93)90159-d. [DOI] [PubMed] [Google Scholar]
- Thomas G, Martin-Perez J, Siegmann M, Otto AM. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982;30:235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Tokunaga Y, Kojima T, Osanai M, Murata M, Chiba H, Tobioka H, Sawada N. A novel monoclonal antibody against the second extracellular loop of occludin disrupts epithelial cell polarityJHistochem. Cytochem. 2007;55:735–744. doi: 10.1369/jhc.6A7165.2007. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Otsu K, Kuzuya T, Hori M, Tada M. Direct association of the gap junction protein connexin-43 with ZO-1 in cardiac myocytes. J. Biol. Chem. 1998;273:12725–12731. doi: 10.1074/jbc.273.21.12725. [DOI] [PubMed] [Google Scholar]
- Treins C, Warne PH, Magnuson MA, Pende M, Downward J. Rictor is a novel target of p70 S6 kinase-1. Oncogene. 2010;29:1003–1016. doi: 10.1038/onc.2009.401. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell. Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Katsuno T, Yamazaki Y, Umeda K, Tamura A, Tsukita S. Roles of ZO-1 and ZO-2 in establishment of the belt-like adherens and tight junctions with paracellular permselective barrier function. Ann. N Y Acad. Sci. 2009;1165:44–52. doi: 10.1111/j.1749-6632.2009.04056.x. [DOI] [PubMed] [Google Scholar]
- Uesuqi A, Kozaki K, Tsuruta T, Furuta M, Morita K, Imoto I, Omurs K, Inazawa J. The tumor suppressive microRNA miR-218 targets the mTOR component Rictor and inhibits AKT phosphorylation in oral cancer. Cancer Res. 2011;71:5765–5778. doi: 10.1158/0008-5472.CAN-11-0368. [DOI] [PubMed] [Google Scholar]
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, Matsui T, Tsukita S, Furuse M, Tsukita S. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Utepbergenov DI, Fanning AS, Anderson JM. Dimerization of the scaffolding protein ZO-1 through the second PDZ domain. J. Biol. Chem. 2006;281:24671–24677. doi: 10.1074/jbc.M512820200. [DOI] [PubMed] [Google Scholar]
- van den Akker JM, Wetzels JF, Hoitsma AJ. Proteinuria following conversion from azathioprine to sirolimus in renal transplant recipients. Kidney Int. 2006;70:1355–1357. doi: 10.1038/sj.ki.5001792. [DOI] [PubMed] [Google Scholar]
- Vassiliadis J, Bracken C, Matthews D, O’Brien S, Schiavi S, Wawersik S. Calcium mediates glomerular filtration through calcineurin and mTORC2/Akt signaling. J. Am.Soc. Nephrol. 2011;22:1453–1461. doi: 10.1681/ASN.2010080878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volarevic S, Stewart MJ, Ledermann B, Zilberman F, Terracciano L, Montini E, Grompe M, Kozma SC, Thomas G. Proliferation, but not growth, blocked by conditional deletion of 40S ribosomal protein S6. Science. 2000;288:2045–2047. doi: 10.1126/science.288.5473.2045. [DOI] [PubMed] [Google Scholar]
- Vollenbroker B, George B, Wolfgart M, Saleem MA, Paventadt H, Weide T. mTOR regulates expression of slit diaphragm proteins and cytoskeleton structure in podocytes. AmJPhysiol. Ren. Physiol. 2009;296:F418–F426. doi: 10.1152/ajprenal.90319.2008. [DOI] [PubMed] [Google Scholar]
- Walker WH. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis. 2011;1:116–120. doi: 10.4161/spmg.1.2.16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wander SA, Hennessy BT, Slingerland JM. Next-generation mTOR inhibitors in clinical oncology: how pathway complexity informs therapeutic strategy. J. Clin. Invest. 2011;121:1231–1241. doi: 10.1172/JCI44145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CQ, Cheng CY. A seamless trespass: germ cell migration across the seminiferous epithelium during spermatogenesis. J. Cell. Biol. 2007;178:549–556. doi: 10.1083/jcb.200704061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Gu S, Foller M, Ackermann TF, Klingel K, Kandolf R, Kuhl D, Stournaras C, Lang F. SGK1-dependent intestinal tumor growth in APC-deficient mice. Cell. Physiol. Biochem. 2010;25:271–278. doi: 10.1159/000276561. [DOI] [PubMed] [Google Scholar]
- Wang L, Harris TE, Lawrence JCJ. Regulation of proline-rich Akt substrate of 40 kDa (PRAS40) function by mammalian target of rapamycin complex 1 (mTORC1)- mediated phosphorylation. J. Biol. Chem. 2008;283:15619–15627. doi: 10.1074/jbc.M800723200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Harris TE, Roth RA, Lawrence JCJ. PRAS40 regulates mTORC1 kinase activity by functioning as a direct inhibitor of substrate binding. J. Biol. Chem. 2007;282:20036–20044. doi: 10.1074/jbc.M702376200. [DOI] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG. Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J. 2001;16:4370–4379. doi: 10.1093/emboj/20.16.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber JE, Russell LD, Wong V, Peterson RN. Three dimensional reconstruction of a rat stage V Sertoli cell: II. Morphometry of Sertoli-Sertoli and Sertoli-germ cell relationships. Am. J. Anat. 1983;167:163–179. doi: 10.1002/aja.1001670203. [DOI] [PubMed] [Google Scholar]
- Weichhart T. Mammalian target of rapamycin: a signaling kinase for every aspect of cellular life. Methods Mol. Biol. 2012;821:1–14. doi: 10.1007/978-1-61779-430-8_1. [DOI] [PubMed] [Google Scholar]
- Wettenhall RE, Erikson E, Maller JL. Ordered multisite phosphorylation of Xenopus ribosomal protein S6 by S6 kinase II. J. Biol. Chem. 1992;267:9021–9027. [PubMed] [Google Scholar]
- Wettenhall RE, Howlett GJ. Phosphorylation of a specific ribosomal protein during stimulation of thymocytes by concanavalin A and prostaglandin E1. J. Biol. Chem. 1979;254:9317–9323. [PubMed] [Google Scholar]
- Winters SJ, Moore JP. Paracrine control of gonadotrophs. Semin. Reprod. Med. 2007;25:379–387. doi: 10.1055/s-2007-984744. [DOI] [PubMed] [Google Scholar]
- Wittchen ES, Haskins J, Stevenson BR. Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3. J. Biol. Chem. 1999;274:35179–35185. doi: 10.1074/jbc.274.49.35179. [DOI] [PubMed] [Google Scholar]
- Wolski KM, Perrault C, Tran-Son-Tay R, Cameron DF. Strength measurement of the Sertoli-spermatid junctional complex. J. Androl. 2005;26:354–359. doi: 10.2164/jandrol.04142. [DOI] [PubMed] [Google Scholar]
- Wong CH, Cheng CY. The blood-testis barrier: its biology, regulation, and physiological role in spermatogenesis. Curr. Top. Dev. Biol. 2005;71:263–296. doi: 10.1016/S0070-2153(05)71008-5. [DOI] [PubMed] [Google Scholar]
- Wong EWP, Cheng CY. Polarity proteins and cell-cell interactions in the testis. Int. Rev. Cell. Mol. Biol. 2009;278:309–353. doi: 10.1016/S1937-6448(09)78007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Mruk DD, Cheng CY. Biology and regulation of ectoplasmic specialization, an atypical adherens junction type, in the testis. Biochem. Biophys. Acta. 2008;1778:692–708. doi: 10.1016/j.bbamem.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EWP, Sun S, Li MWM, Lee WM, Cheng CY. 14-3-3 protein regulates cell adhesion in the seminiferous epithelium of rat testes. Endocrinology. 2009;150:4713–4723. doi: 10.1210/en.2009-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool IG. The structure and function of eukaryotic ribosomes. Annu. Rev. Biochem. 1979;48:719–754. doi: 10.1146/annurev.bi.48.070179.003443. [DOI] [PubMed] [Google Scholar]
- Wool IG. Extraribosomal functions of ribosomal proteins. Trends Biochem. Sci. 1996;21:164–165. [PubMed] [Google Scholar]
- Xiao X, Mruk DD, Lee WM, Cheng CY. c-Yes regulates cell adhesion at the blood-testis barrier and the apical ectoplasmic specialization in the seminiferous epithelium of rat testes. Int. J. Biochem. Cell. Biol. 2011;43:651–665. doi: 10.1016/j.biocel.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Anuar F, Ali SM, Ng MY, Phua DC, Hunziker W. Zona occludens-2 is critical for blood-testis barrier integrity and male fertility. Mol. Biol. Cell. 2009;20:4268–4277. doi: 10.1091/mbc.E08-12-1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Kausalya PJ, Phua DC, Ali SM, Hossain Z, Hunziker W. Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol. Biol. Cell. 2008;28:1669–1678. doi: 10.1128/MCB.00891-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Cheng CY. Blood-testis barrier dynamics are regulated by an engagement/ disengagement mechanism between tight and adherens junctions via peripheral adaptors. Proc. Natl. Acad. Sci. U.SA. 2005;2005:11722–11727. doi: 10.1073/pnas.0503855102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Cheng CY. Laminin α3 forms a complex with β3 and γ3 chains that serves as the ligand for α6β1-integrin at the apical ectoplasmic specialization in adult rat testes. J. Biol. Chem. 2006;281:17286–17303. doi: 10.1074/jbc.M513218200. [DOI] [PubMed] [Google Scholar]
- Yan HH, Mruk DD, Lee WM, Cheng CY. Ectoplasmic specialization: a friend or a foe of spermatogenesis? Bioessays. 2007;29:36–48. doi: 10.1002/bies.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HH, Wong EWP, Lee WM, Cheng CY. An autocrine axis in the testis that coordinates spermiation and blood-testis barrier restructuring during spermatogenesis. Proc. Natl. Acad. Sci. U.SA. 2008a;105:8950–8955. doi: 10.1073/pnas.0711264105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Cheng CY. Junction restructuring and spermatogenesis: the biology, regulation, and implication in male contraceptive development. Curr. Top. Dev. Biol. 2008b;80:57–92. doi: 10.1016/S0070-2153(07)80002-0. [DOI] [PubMed] [Google Scholar]
- Yan HHN, Mruk DD, Lee WM, Cheng CY. Blood-testis barrier dynamics are regulated by testosterone and cytokines via their differential effects on the kinetics of protein endocytosis and recycling in Sertoli cells. FASEB J. 2008c;22:1945–1959. doi: 10.1096/fj.06-070342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Q, Ionki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–2832. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Richburg JH. TNFα-mediated disruption of spermatogenesis in response to Sertoli cell injury in rodents is partially regulated by MMP2. Biol. Reprod. 2009;80:581–589. doi: 10.1095/biolreprod.108.073122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PL, Lin YC, Richburg JH. Mono-(2-ethylhexyl) phthalate-induced disruption of junctional complexes in the seminiferous epithelium of the rodent testis is mediated by MMP2. Biol. Reprod. 2010;82:516–527. doi: 10.1095/biolreprod.109.080374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama S, Tachibana K, Nakanish H, Yamamoto Y, Irie K, Mandai K, Naqafuchi A, Monden M, Takai Y. alpha-catenin-independent recruitment of ZO-1 to nectin-based cell-cell adhesion sites through afadin. Mol. Biol. Cell. 2001;12:1595–1609. doi: 10.1091/mbc.12.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S. Cadherin-actin interactions at adherens junctions. Curr. Opin. Cell. Biol. 2011;23:515–522. doi: 10.1016/j.ceb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Zhang L, Cui R, Cheng X, Du J. Antiapoptotic effect of serum and glucocorticoid- inducible protein kinase is mediated by novel mechanism activating I{kappa}B kinase. Cancer Res. 2005;65:457–464. [PubMed] [Google Scholar]
- Zhou H, Huang S. The complexes of mammalian target of rapamycin. Curr. Protein Pept. Sci. 2010;11:409–424. doi: 10.2174/138920310791824093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell. Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]