Summary
Background
Drug treatments for patients with high-risk myelodysplastic syndromes provide no survival advantage. In this trial, we aimed to assess the effect of azacitidine on overall survival compared with the three commonest conventional care regimens.
Methods
In a phase III, international, multicentre, controlled, parallel-group, open-label trial, patients with higher-risk myelodysplastic syndromes were randomly assigned one-to-one to receive azacitidine (75 mg/m² per day for 7 days every 28 days) or conventional care (best supportive care, low-dose cytarabine, or intensive chemotherapy as selected by investigators before randomisation). Patients were stratified by French–American–British and international prognostic scoring system classifications; randomisation was done with a block size of four. The primary endpoint was overall survival. Efficacy analyses were by intention to treat for all patients assigned to receive treatment. This study is registered with ClinicalTrials.gov, number NCT00071799.
Findings
Between Feb 13, 2004, and Aug 7, 2006, 358 patients were randomly assigned to receive azacitidine (n=179) or conventional care regimens (n=179). Four patients in the azacitidine and 14 in the conventional care groups received no study drugs but were included in the intention-to-treat efficacy analysis. After a median follow-up of 21·1 months (IQR 15·1–26·9), median overall survival was 24·5 months (9·9–not reached) for the azacitidine group versus 15·0 months (5·6–24·1) for the conventional care group (hazard ratio 0·58; 95% CI 0·43–0·77; stratified log-rank p=0·0001). At last follow-up, 82 patients in the azacitidine group had died compared with 113 in the conventional care group. At 2 years, on the basis of Kaplan-Meier estimates, 50·8% (95% CI 42·1–58·8) of patients in the azacitidine group were alive compared with 26·2% (18·7–34·3) in the conventional care group (p<0·0001). Peripheral cytopenias were the most common grade 3–4 adverse events for all treatments.
Interpretation
Treatment with azacitidine increases overall survival in patients with higher-risk myelodysplastic syndromes relative to conventional care.
Introduction
Myelodysplastic syndromes are malignant diseases of bone-marrow stem-cells, characterised by ineffective haemopoiesis leading to peripheral-blood cytopenias and, in many patients, progression to acute myeloid leukaemia.1,2 Myelodysplastic syndromes are categorised morphologically with the French–American–British (FAB) and, more recently, WHO3,4 classifications. Individual prognosis is determined using the international prognostic scoring system.5
Patients with myelodysplastic syndromes who had intermediate-2 or high-risk scores on the international prognosis scoring system (known as higher-risk myelo-dysplastic syndromes) have a median survival of 1·2 years or 0·4 years, respectively,5 and a high-risk for progression to acute myeloid leukaemia.5 Although increasing survival and suppression of leukaemic transformation are the primary goals of treatment,6 no treatment strategies other than allogeneic stem-cell transplantation offer meaningful potential to change the natural history of the disease.7–15 Results of a Cancer and Leukemia Group B (CALGB) trial comparing treatment with azacitidine, a DNA methyl-transferase inhibitor, with best supportive care suggested improved overall survival with azacitidine, but the study was inconclusive because of its crossover design and absence of an active comparator.16
This large, prospective, randomised, phase III, clinical trial was done to assess the effect of treat ment on overall survival with azacitidine. The control arm included the three most commonly used treatments in higher-risk myelodysplastic syndromes (best supportive care, low-dose cytarabine, or intensive chemotherapy).6–15,17
Methods
Patients
Patients were eligible for enrolment if they were aged 18 years or older, with higher-risk myelodysplastic syndromes (an international prognosis scoring system rating of intermediate-2 or high risk) and FAB-defined refractory anaemia with excess blasts, refractory anaemia with excess blasts in transformation, or chronic myelomonocytic leukaemia3 with at least 10% bone-marrow blasts and a white-blood-cell count lower than 13×109 cells per L. Patients needed an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 and an estimated life expectancy of at least 3 months. Patients with therapy-related myelodysplastic syndrome, previous azacitidine treatment, or planned allogeneic stem-cell transplantation were excluded.
This phase III, international, multicentre, randomised, controlled, parallel-group, open-label trial was done in accordance with the Declaration of Helsinki. All patients provided written informed consent and the study was approved by the institutional review boards at all participating study sites. Enrolment to the trial and monitoring were done by site investigators and central pathology reviewers with standardised central review of all cytogenetic data.
Study design
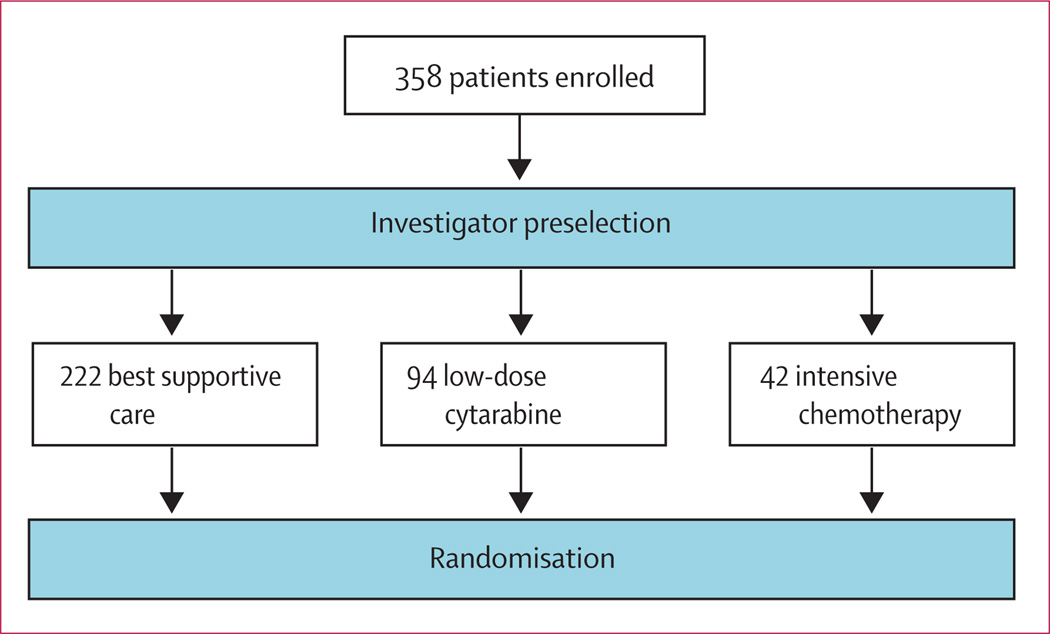
Before randomisation, investigators determined which of the three conventional care treatments (best supportive care, low-dose cytarabine, or intensive chemotherapy) was most appropriate for each patient, with clinical judgment on the basis of age, ECOG performance status, and comorbidities (figure 1). Patients were then randomly assigned one-to-one to receive azacitidine or conventional care regimens (figure 2). No crossover was allowed, and use of erythropoietin or darbepoetin was prohibited.
Figure 1. Investigator preselection.
Before randomisation, investigators preselected the most appropriate of the three conventional care regimens for all patients on the basis of age, general condition, comorbidities, and patient preference. Patients randomised to conventional care were to receive the investigator preselected treatment option.
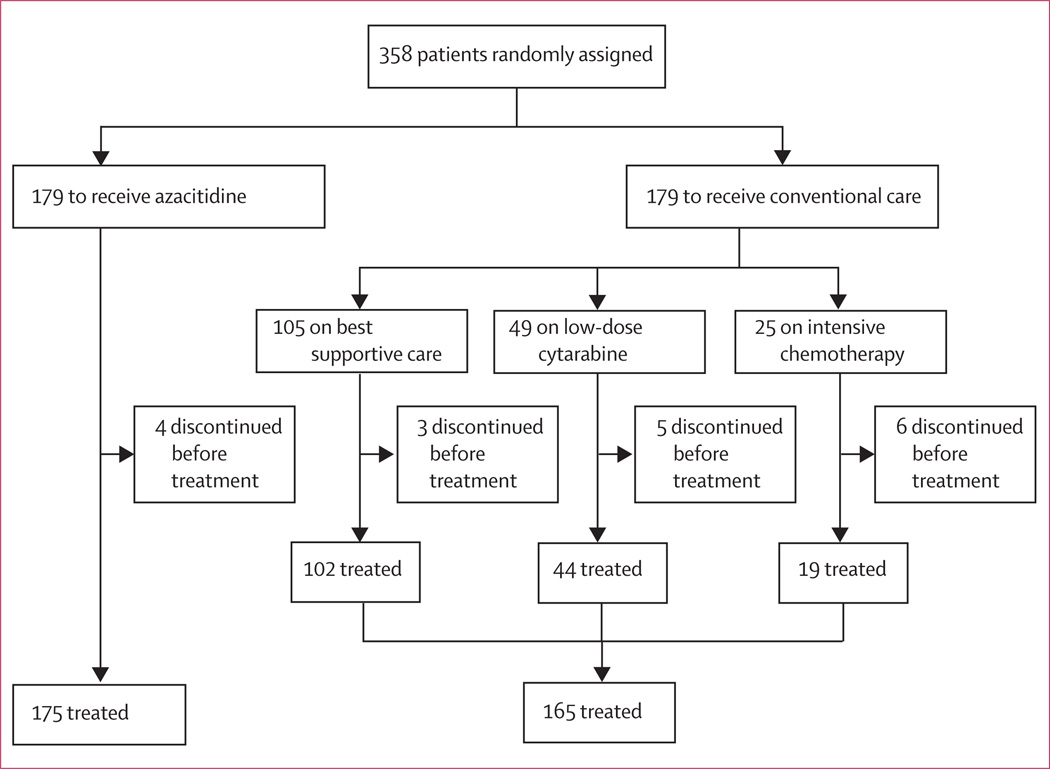
Figure 2.
Trial profile
Patients were stratified by investigators according to FAB and international prognostic scoring system classifications.3,5 Randomisation was done centrally, with allocation by telephone; patients were assigned to treatment in blocks of four within each stratum. The randomisation sequence was computer generated independently by Pharmaceutical Product Development (Wilmington, NC, USA). An independent data-safety monitoring board reviewed safety data and did an unblinded review of a scheduled interim efficacy analysis.
During the treatment phase of the trial, all treatments were continued until study completion (12 months after the last patient was assigned) or discontinuation due to relapse, unacceptable toxicity, or disease progression defined by the International Working Group (IWG 2000) criteria for myelodysplastic syndromes.18 Azacitidine was given subcutaneously at 75 mg/m² per day for 7 days every 28 days (delayed as needed until blood-count recovery), for at least six cycles. Conventional care regimens were given as follows: best supportive care only (including blood product transfusions and antibiotics with granulocyte-colony-stimulating factor for neutro penic infection); low-dose cytarabine, 20 mg/m² per day subcutaneously for 14 days, every 28 days (delayed as needed until blood-count recovery) for at least four cycles; or intensive chemotherapy (induction with cytarabine 100–200 mg/m² per day by continuous intravenous infusion for 7 days, plus 3 days of either intravenous daunorubicin [45–60 mg/m² per day], idarubicin [9–12 mg/m² per day], or mitoxantrone [8–12 mg/m² per day]). Patients who achieved complete or partial remission after induction (defined by the International Working Group criteria for acute myeloid leukaemia19) received one or two consolidation courses with reduced doses of the cytotoxic drugs used for induction followed by best supportive care. All patients could receive best supportive care as needed. After treatment discontinuation, all patients were followed up until death or study completion.
Assessment of efficacy and safety
Efficacy analyses were by intention to treat. Safety analyses included all patients who received at least one dose of study drug and one or more safety assessments thereafter. The primary endpoint was overall survival, analysed by comparison of the azacitidine and combined conventional care groups. A supportive analysis of overall survival assessed the potential effects of predefined subgroups on the basis of age; FAB subtype, risk group (intermediate-2 or high), and cytopenias (grades 0 or 1 and 2 or 3); cytogenetics (good, intermediate, and poor); –7/del(7q) cytogenetic abnormality, WHO classification; and serum lactate dehydrogenase. Another supportive analysis of overall survival, on the basis of investigator preselection, compared the azacitidine subgroups with the individual treatments of conventional care.
Secondary efficacy endpoints were time to trans-formation to acute myeloid leukaemia, haematological response, and improvement assessed with IWG 2000 criteria for myelodysplastic syndromes,18 independence from red-blood-cell transfusions for 56 consecutive days or more, number of infections requiring intravenous antimicrobials, and occurrence of adverse events. Bone-marrow samples were collected every 16 weeks during active treatment and as clinically indicated during follow-up. Infections requiring intravenous antimicrobials were counted from randomisation to last study visit. Adverse events were assessed with the National Cancer Institute’s Common Toxicity Criteria, version 2.0.
Statistical methods
This study was designed with 90% power—on the basis of a log-rank analysis—to detect an HR of 0·60 for overall survival in the azacitidine group compared with that in the conventional care group, with a two-sided α of 0·05. The protocol specified that about 354 patients were to be randomly assigned over 18 months and then monitored for at least 12 months of treatment and follow-up, resulting in at least 167 deaths over the 30-month trial period. Recruitment and a minimum follow-up of at least 12 months for all patients, however, necessitated a longer study period. With a study period of 42 months and 195 deaths, the study had 95% power under the assumptions of the design. One interim analysis was done with an O’Brien-Fleming monitoring boundary with a Lan-DeMets α spending function to control the overall α at 0·05 (data not shown).20
Overall survival was defined as the time from randomisation to death from any cause. Patients who remained alive were censored at the time of last follow-up. Time to transformation to acute myeloid leukaemia was measured from randomisation to development of 30% or greater bone-marrow blasts. Patients free from acute myeloid leukaemia transformation were censored for this analysis at the time of last adequate bone-marrow sample. Randomisation and analyses were stratified on FAB subtype and international prognostic scoring system group. Time-to-event curves were estimated with the Kaplan-Meier method21 and compared with stratified log-rank tests (primary analysis). Stratified Cox proportional hazards regression models22 were used to estimate hazard ratios (HRs) and associated 95% CIs. The primary analysis of overall survival between the azacitidine and combined conventional care groups used the stratified Cox proportional hazards model without any covariate adjustments to estimate the HR. Cox proportional hazards regression with stepwise selection was used to assess the baseline variables of sex, age, time since original diagnosis of myelodysplastic syndrome, ECOG performance status, number of previous red-blood-cell transfusions, number of previous platelet transfusions, measurements of haemoglobin, platelets, absolute neutrophil count, and lactate dehydrogenase, bone-marrow blast percentage, and presence or absence of cytogenetic –7/del(7q) abnormality. The final model included ECOG performance status, lactate dehydrogenase, haemoglobin, number of previous red-blood-cell transfusions, and presence or absence of the cytogenetic –7/del(7q) abnormality. Supportive overall survival analyses used the final Cox proportional hazards model. The consistency of treatment effect across subgroups was assessed with the difference in likelihood ratio between the full model with treatment subgroup and treatment-by-subgroup interaction, and the reduced model without the interaction. Additional supportive efficacy analyses by investigator preselection compared the azacitidine subgroups with the individual treatments that comprised conventional care (figure 1).
Haematological response, transfusion independence, and haematological improvement in the azacitidine and conventional care groups were compared with Fisher’s exact test. The rate of infection requiring intravenous antimicrobials was calculated with the number of recorded infections treated with intravenous antimicrobials divided by the total number of patient-years of follow-up. The relative risk of infection was calculated as the rate of infection in patients taking azacitidine compared with the rate in those receiving conventional care. The Mantel-Haenszel estimate of the common relative risk, the associated 95% CI, and the test that it equals unity were calculated.23 Analyses were done with SAS (version 9.13).
This study is registered with ClinicalTrials.gov, number NCT00071799.
Role of the funding source
The principal investigator and leading coinvestigators designed and did the study, provided oversight for the analysis of the data by Celgene, and wrote the article in consultation with Celgene. Additionally, Celgene elicited independent review of the statistical analysis plan by Kenneth J Kopecky (Fred Hutchinson Cancer Research Center, SW Oncology Group Statistical Center, Seattle, WA, USA) and Gary G Koch (University of North Carolina at Chapel Hill, Director, Biometric Consulting Lab, Chapel Hill, NC, USA) and independent review of the analyses, interpretation of the results, and review of this paper by Gary G Koch. The corresponding author and coauthors had access to all the trial data and had final responsibility for the decision to submit for publication.
Results
Between Feb 13, 2004, and Aug 7, 2006, 358 patients (intention-to-treat population) at 79 sites from 15 countries were randomly assigned to receive either azacitidine (n=179) or conventional care regimens (n=179). Of those assigned to conventional care, 105 were to receive best supportive care, 49 low dose cytarabine, and 25 intensive chemotherapy (figure 2). Median age was 69 years (range 38–88 years) with 258 (72%) of 358 patients age 65 years or older. Baseline characteristics were well balanced between the azacitidine and conventional care groups (table 1). The investigator preselection subgroups showed some imbalances as expected: namely, patients selected to receive intensive chemotherapy were younger and had better ECOG performance status and higher-risk disease (table 1). According to the WHO classification, 113 patients (32%) fulfilled criteria for acute myeloid leukaemia (marrow-blast percentage 20% or greater). The following protocol deviations were reported: 18 patients with international prognosis scoring system score of intermediate-1 were enrolled after central review (five in the azacitidine group; 13 in the conventional care group) and investigators decided to give eight patients (four in the azacitidine group and four in the conventional care group [two best supportive care; one low-dose cytarabine, and one intensive chemotherapy]) allogeneic transplantation during follow-up. Four patients in the azacitidine group and 14 in the conventional care group never received study drug but were followed for overall survival and are included in the intention to treat analysis (figure 2). All patients with protocol deviations are included in the intention to treat analysis.
Table 1.
Baseline demographics and disease characteristics by treatment group and investigator preselection
| Total ITT |
BSC only (n=222) |
Low-dose cytarabine (n=94) |
Intensive chemotherapy (n=42) |
|||||
|---|---|---|---|---|---|---|---|---|
| Azacitidine (n=179) |
CCR (n=179) |
Azacitidine (n=117) |
BSC (n=105) |
Azacitidine (n=45) |
Low-dose cytarabine (n=49) |
Azacitidine (n=17) |
Intensive chemotherapy (n=25) |
|
| Age (years) | 69 (42–83) | 70 (38–88) | 69 (52–83) | 70 (50–88) | 69 (42–82) | 71 (56–85) | 63 (45–78) | 65 (38–76) |
| ≤64 | 57 (32%) | 43 (24%) | 33 (28%) | 24 (23%) | 14 (31%) | 7 (14%) | 10 (59%) | 12 (48%) |
| ≥65 | 122 (68%) | 136 (76%) | 84 (72%) | 81 (77%) | 31 (69%) | 42 (86%) | 7 (41%) | 13 (52%) |
| Sex | ||||||||
| Men | 132 (74%) | 119 (67%) | 81 (69%) | 67 (64%) | 39 (87%) | 35 (71%) | 12 (71%) | 17 (68%) |
| Women | 47 (26%) | 60 (34%) | 36 (31%) | 38 (36%) | 6 (13%) | 14 (29%) | 5 (29%) | 8 (32%) |
| FAB classification | ||||||||
| RAEB | 104 (58%) | 103 (58%) | 69 (59%) | 68 (65%) | 27 (60%) | 25 (51%) | 8 (47%) | 10 (40%) |
| RAEB-T | 61 (34%) | 62 (35%) | 38 (33%) | 30 (29%) | 15 (33%) | 19 (39%) | 8 (47%) | 13 (52%) |
| CMMoL | 6 (3%) | 5 (3%) | 5 (4%) | 4 (4%) | 1 (2%) | 1 (2%) | 0 | 0 |
| AML | 1 (1%) | 1 (1%) | 0 | 0 | 1 (2%) | 0 | 0 | 1 (4%) |
| IPSS classification | ||||||||
| Intermediate-1 | 5 (3%) | 13 (7%) | 4 (3%) | 9 (9%) | 1 (2%) | 2 (4%) | 0 | 2 (8%) |
| Intermediate-2 | 76 (43%) | 70 (39%) | 48 (41%) | 46 (44%) | 22 (49%) | 21 (43%) | 6 (35%) | 3 (12%) |
| High | 82 (46%) | 85 (48%) | 57 (49%) | 46 (44%) | 19 (42%) | 21 (43%) | 6 (35%) | 18 (72%) |
| Karyotype risk | ||||||||
| Good | 83 (46%) | 84 (47%) | 53 (45%) | 47 (45%) | 24 (53%) | 28 (57%) | 6 (35%) | 9 (36%) |
| Intermediate | 37 (21%) | 39 (22%) | 25 (21%) | 23 (22%) | 7 (16%) | 12 (25%) | 5 (29%) | 4 (16%) |
| Poor | 50 (28%) | 50 (28%) | 33 (28%) | 31 (30%) | 13 (29%) | 8 (16%) | 4 (24%) | 11 (44%) |
| Missing | 9 (5%) | 6 (3%) | 6 (5%) | 4 (4%) | 1 (2%) | 1 (2%) | 2 (12%) | 1 (4%) |
| WHO classification | ||||||||
| RAEB-1 | 14 (8%) | 17 (10%) | 8 (7%) | 13 (12%) | 3 (7%) | 3 (6%) | 3 (18%) | 1 (4%) |
| RAEB-2 | 98 (55%) | 95 (53%) | 63 (54%) | 60 (57%) | 27 (60%) | 24 (49%) | 8 (47%) | 11 (44%) |
| CMMoL-1 | 1 (1%) | 0 | 1 (1%) | 0 | 0 | 0 | 0 | 0 |
| CMMoL-2 | 10 (6%) | 5 (3%) | 8 (7%) | 3 (3%) | 1 (2%) | 0 | 1 (6%) | 2 (8%) |
| AML | 55 (31%) | 58 (32%) | 36 (31%) | 27 (26%) | 14 (31%) | 20 (41%) | 5 (29%) | 11 (44%) |
| Indeterminate | 1 (1%) | 4 (2%) | 1 (1%) | 2 (2%) | 0 | 2 (4%) | 0 | 0 |
| ECOG performance status | ||||||||
| 0 | 78 (44%) | 80 (45%) | 47 (40%) | 36 (34%) | 21 (47%) | 29 (59%) | 10 (59%) | 15 (60%) |
| 1 | 86 (48%) | 86 (48%) | 59 (50%) | 59 (56%) | 21 (47%) | 17 (35%) | 6 (35%) | 10 (40%) |
| 2 | 13 (7%) | 10 (6%) | 11 (9%) | 8 (8%) | 1 (2%) | 2 (4%) | 1 (6%) | 0 |
| Missing | 2 (1%) | 3 (2%) | 0 | 2 (2%) | 2 (4%) | 1 (2%) | 0 | 0 |
| Time since original diagnosis (years) | ||||||||
| <1 | 92 (51%) | 95 (53%) | 53 (45%) | 53 (51%) | 29 (64%) | 28 (57%) | 10 (59%) | 14 (56%) |
| 1–2 | 37 (21%) | 45 (25%) | 29 (25%) | 27 (26%) | 7 (16%) | 12 (25%) | 1 (6%) | 6 (24%) |
| 2–3 | 20 (11%) | 10 (6%) | 14 (12%) | 6 (6%) | 4 (9%) | 3 (6%) | 2 (12%) | 1 (4%) |
| >3 | 30 (17%) | 29 (16%) | 21 (18%) | 19 (18%) | 5 (11%) | 6 (12%) | 4 (24%) | 4 (16%) |
Data are median (range) or number (%). CCR=conventional care regimen. ITT=intention to treat. BSC=best supportive care. FAB=French–American–British. RAEB=refractory anaemia with excess blasts. RAEB-T=RAEB in transformation. CMMoL=chronic myelomonocytic leukaemia. AML=acute myeloid leukaemia. IPSS=International prognostic scoring system. ECOG=Eastern Cooperative Oncology Group.
Azacitidine was given for a median of nine cycles (IQR four to 15), and 151 (86%) of 175 of patients who received azacitidine remained on 75 mg/m² per day throughout the study with no dose adjustments. The median azacitidine cycle-length was 28 days (IQR 28–35); 862 (54%) of the 1611 cycle-lengths were 28 days, 413 (26%) 29–35 days, and 336 (21%) longer than 35 days. Low-dose cytarabine was given for a median of four and a half cycles (IQR two to eight); 59 (29%) of 201 cycle-lengths were 28 days, 82 (41%) 29–35 days, and 60 (30%) longer than 35 days, the overall median was 35 days (IQR 28–36). Intensive chemotherapy was given for a median of one cycle (IQR one to three), and best supportive care for a median 6·2 months (IQR 3·6–10·3).
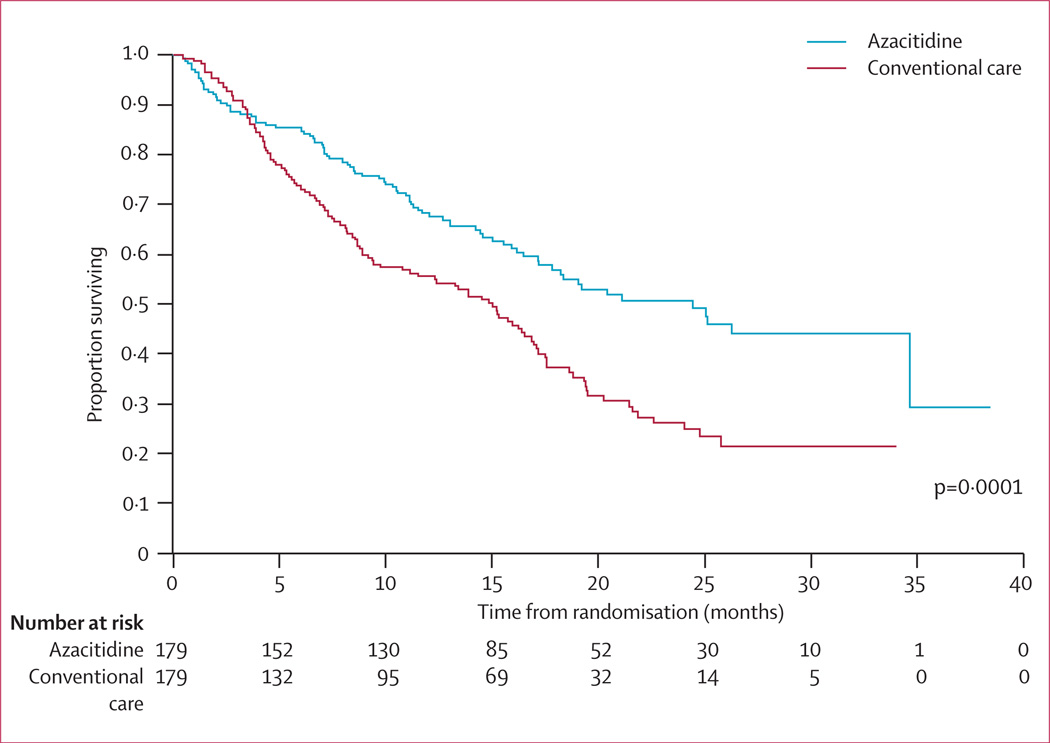
At the time of last follow-up, 82 patients in the azacitidine group had died compared with 113 in the conventional care group. After a median follow-up of 21·1 months (IQR 15·1–26·9), median Kaplan-Meier overall survival was 24·5 months (9·9–not reached) in the azacitidine group compared with 15 months (5·6–24·1) in the conventional care group, a difference of 9·4 months (stratified log-rank p=0·0001; figure 3). The HR for overall survival was 0·58 (95% CI 0·43–0·77). Kaplan-Meier survival curves for the azacitidine and conventional care groups separated permanently after about 3 months, at which time 140 (78%) of 179 patients receiving azacitidine had completed three cycles of treatment (figure 3). At 2 years, on the basis of Kaplan-Meier estimates, 50·8% (95% CI 42·1–58·8) of patients in the azacitidine group were alive compared with 26·2% (18·7–34·3) in the conventional care group (p<0·0001).
Figure 3.
Overall survival
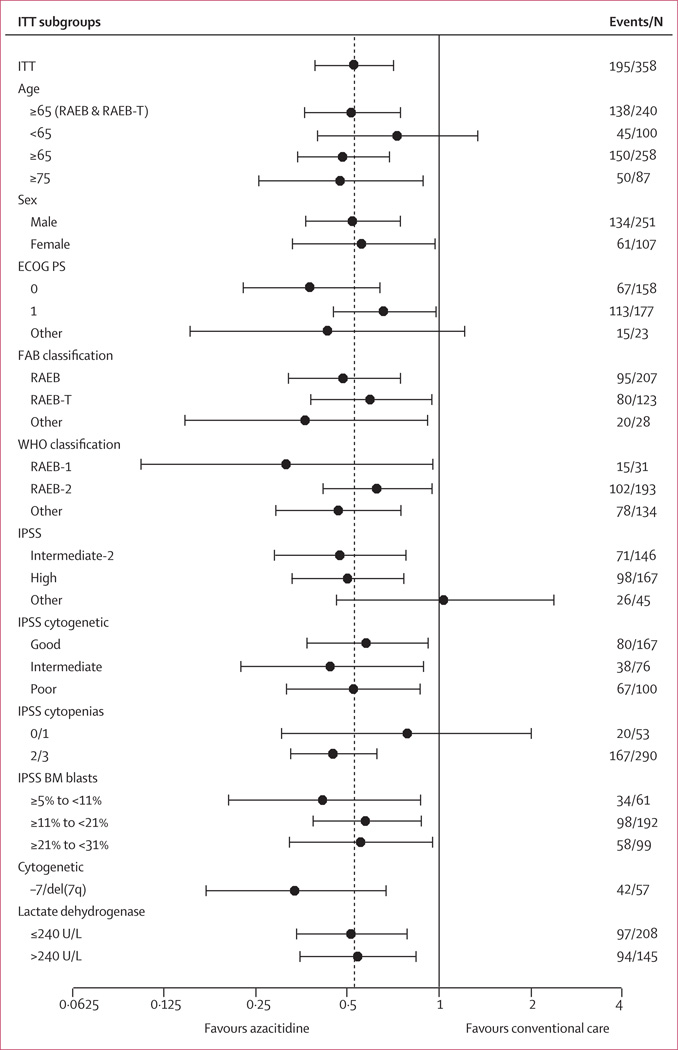
The supportive analysis of all predefined sub groups of patients showed consistency of the azacitidine effect on overall survival compared with conventional care (figure 4). In particular, overall survival was better for azacitidine than conventional care in all the cyto genetic subgroups on the international prognosis scoring system (poor prognosis HR 0·53, 95% CI 0·32–0·87, p=0·012; intermediate prognosis 0·44, 0·22–0·88, p=0·021; and good prognosis 0·59, 0·37–0·92, p=0·021). In patients with −7/del(7q), median Kaplan-Meier overall survival was 13·1 months (IQR 3·9–24·5; 95% CI 9·9–24·5) in the azacitidine group (n=30) compared with 4·6 months (2·9–9·3; 3·5–6·7) in the conventional care group (n=27) giving an HR of 0·34 (95% CI 0·17–0·67, p=0·0017; figure 4). Additionally sensitivity analyses exploring the effect of the eight patients who received allogeneic stem-cell transplantation included in the intention-to-treat analyses, determined they did not affect the significance of the overall survival results (data not shown).
Figure 4. Hazard ratio and 95% CI for overall survival in the intention-to-treat analysis.
Hazard ratios and CIs determined with stratified Cox proportional hazards model adjusted for treatment, subgroup, Eastern Cooperative Oncology Group performance status (ECOG PS), lactate dehydrogenase, haemoglobin, number of previous red-blood-cell transfusions, and presence or absence of the cytogenetic –7/del(7q) abnormality. No subgroup-by-treatment interactions were significant (p>0·20). The horizontal axis uses a logarithmic scale. The dotted line is the hazard ratio in the primary intention to treat (ITT) analysis; the hazard ratio and CI are from the stratified Cox regression model with treatment as the only term. FAB=French–American–British. RAEB=refractory anaemia with excess blasts. RAEB-T=RAEB in transformation. IPSS=international prognostic scoring system. BM=bone marrow.
Similar to the primary overall survival comparison (azacitidine vs conventional care), results from the investigator preselection subgroup analysis of overall survival showed significant differences favouring the study drug between azacitidine and best supportive care (9·6 months, p=0·0045) and azacitidine and cytarabine (9·2 months, p=0·0006). The difference in the comparison between azacitidine (n=17) and intensive chemotherapy (n=25), however, was not significant (9·3 months, p=0·51, table 2).
Table 2.
Overall survival and time to progression to acute myeloid leukaemia comparison for groups according to investigator preselection
| BSC only (n=222) |
Low-dose cytarabine (n=94) |
Intensive chemotherapy (n=42) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azacitidine (n=117) |
BSC (n=105) |
HR (95%CI) | p value | Azacitidine (n=45) |
Low-dose cytarabine (n=49) |
HR (95%CI) | p value | Azacitidine (n=17) |
Intensive chemotherapy (n=25) |
HR (95%CI) | p value | |
| Overall survival (months) |
21·1 (10·5-NR) |
11·5 (5·7-NR) |
0·58 (0·40–0·85) |
0·0045 | 24·5 (8·4–347) |
15·3 (4·9–25·8) |
0·36 (0·20–0·65) |
0·0006 | 25·1 (10·0-NR) |
15·7 (8·2–24·1) |
0·76 (0·33–1·74) |
0·51 |
| Time to transformation to AML (months) |
15·0 (8·8–27·6) |
10·1 (3·9–19·8) |
0·41 (0·27–0·63) |
<0·0001 | 15·0 (7·3–25·5) |
14.5 (4·9–192) |
0·55 (0·28–1·11) |
0·097 | 23·1 (6·4–25·4) |
10·7 (4·6–15·4) |
0·48 (0·16–1·45) |
0·19 |
Data are median (IQR). Hazard ratios calculated with stratified Cox proportional hazards model adjusted for treatment, subgroup, Eastern Cooperative Oncology Group performance status, lactate dehydrogenase, haemoglobin, number of previous red-blood-cell transfusions, and presence or absence of cytogenetic –7/del(7q) abnormality. No subgroup-by-treatment interactions were significant (p>0·20). BSC=best supportive care. NR=not reached. HR=hazard ratio. AML=acute myeloid leukaemia.
Median time to acute myeloid leukaemia transformation was 17·8 months (IQR 8·6–36·8; 95% CI 13·6–23·6) in the azacitidine group compared with 11·5 months (4·9-not reached; 8·3–14·5) in the conventional care group (HR 0·50, 95% CI 0·35–0·70; p<0·0001). Results from the investigator preselection subgroup showed a significant difference in time to acute myeloid leukaemia trans-formation for azacitidine versus best supportive care. Time to progression to acute myeloid leukaemia did not differ significantly in the comparisons of azacitidine with either low-dose cytarabine or intensive chemotherapy (table 2).
The proportion of patients with complete and partial remission was significantly higher in the azacitidine group than in the conventional care group (table 3). In the investigator preselection analysis, the proportion of patients with complete remission on azacitidine was significantly higher than with either best supportive care or low-dose cytarabine but not higher than with intensive chemotherapy (table 3). The proportion of patients with partial remission with azacitidine was higher than that with best supportive care, but no higher than with the other two treatments. Time to disease progression, relapse after complete or partial remission, and death were significantly longer in the azacitidine group (median 14·1 months, IQR 4·2–27·6) than in the conventional care group (8·8 months, 3·8–not reached; log-rank p=0·047). The proportions of erythroid and platelet improvements were higher in the azacitidine group than in the conventional care group (table 3), but there was no significant difference in the frequency of major neutrophil improvement between the two treatment groups. Duration of haematological response (complete and partial remission and any haematological improvement) was significantly longer in the azacitidine group (median 13·6 months, IQR 5·9–26·4; 95% CI 10·1–16·3) than in the CCR group (5·2 months; 2·9–12·2; 4·1–9·7; log-rank p=0·0002). Median duration of complete plus partial remission in the azacitidine group was 3·2 months (IQR 2·2–4·4; 95% CI 2·4–4·2) versus 3·0 months (2·1–4·0; 2·1–4·0; log-rank p=0·48) in the conventional care group. 50 (45%) of 111 patients (95% CI 35·6–54·8%) who were dependent on red-blood-cell transfusions at baseline in the azacitidine group became transfusion independent compared with 13 (11·4%) of 114 (6·2–18·7) in the conventional care group (p<0·0001).
Table 3.
Haematological response and improvement by treatment group and investigator preselection
| Total ITT (n=358) |
BSC only (n=222) |
Low-dose cytarabine (n=94) |
Intensive chemotherapy (n=42) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azacitidine (n=179) |
CCR (n=179) |
p value* | Azacitidine (n=117) |
BSC (n=105) |
p value* | Azacitidine (n=45) |
Low-dose cytarabine (n=49) |
p value* | Azacitidine (n=17) |
Intensive chemotherapy (n=25) |
p value* | |
| Haematological response | ||||||||||||
| Any remission | 51 (29%) | 21 (12%) | 0–0001 | 32 (27%) | 5 (5%) | <0–0001 | 14 (31%) | 6 (12%) | 0–042 | 5 (29%) | 10 (40%) | 0–53 |
| Complete remission | 30 (17%) | 14 (8%) | 0–015 | 14 (12%) | 1 (1%) | 0–0008 | 11 (24%) | 4 (8%) | 0–047 | 5 (29%) | 9 (36%) | 0–75 |
| Partial remission | 21 (12%) | 7 (4%) | 0–0094 | 18 (15%) | 4 (4%) | 0–0058 | 3 (7%) | 2 (4%) | 0–67 | 0 | 1 (4%) | 1–00 |
| Stable disease | 75 (42%) | 65 (36%) | 0–33 | 52 (44%) | 41 (39%) | 0–50 | 15 (33%) | 18 (37%) | 0–83 | 8 (47%) | 6 (24%) | 0–18 |
| Haematological imarovement† | ||||||||||||
| Any improvement | 87/177 (49%) | 51/178 (29%) | <0–0001 | 57/115 (50%) | 32/105 (31%) | 0–0058 | 24/45 (53%) | 12/48 (25%) | 0–0061 | 6/17 (35%) | 7/25 (28%) | 0–74 |
| Major erythroid improvement |
62/157 (40%) | 17/160 (11%) | <0–0001 | 39/100 (39%) | 8/96 (8%) | <0–0001 | 19/43 (44%) | 4/41 (10%) | 0–0005 | 4/14 (29%) | 5/23 (22%) | 0–70 |
| Major platelet improvement |
46/141 (33%) | 18/129 (14%) | 0–0003 | 27/89 (30%) | 8/78 (10%) | 0–0020 | 14/37 (38%) | 6/31 (19%) | 0–12 | 5/15 (33%) | 4/20 (20%) | 0–45 |
| Major neutrophil improvement |
25/131 (19%) | 20/111 (18%) | 0–87 | 13/85 (15%) | 13/66 (20%) | 0–52 | 9/33 (27%) | 3/28 (11%) | 0–12 | 3/13 (23%) | 4/17 (24%) | 1–00 |
Data are number (%) or number with improvement/number with data (%). Haematological response and improvement based on International Working Group 2000 criteria for myelodysplastic syndromes.19 CCR=conventional care regimen. BSC=best supportive care.
p value from Fisher’s exact test for comparing patients with response between the azacitidine group and the combined group of CCR, or within investigator preselection, between azacitidine and the individual CCR.
Haemotological improvement can include complete and partial remission.
The rate of infections treated with intravenous antimicrobials per patient year in the azacitidine group was 0·60 (95% CI 0·49–0·73) compared with 0·92 (0·74–1·13) in the conventional care group (relative risk 0·66, 95% CI 0·49–0·87; p=0·0032). There was a significant interaction of treatment by investigator preselection for the rate of infection (p=0·0004). In the investigator preselection analysis, per-patient-year rates were similar when comparing azacitidine (0·66) and best supportive care (0·61; relative risk 1·09, 95% CI 0·74–1·65; p=0·69), but significantly lower with azacitidine (0·44) compared with low-dose cytarabine (1·0; 0·44, 0·25–0·86; p=0·017) and with azacitidine (0·64) versus intensive chemotherapy (2·3; 0·28, 0·13–0·60; p=0·0059).
The most common grade 3–4 events were peripheral blood cytopenias for all treatments (table 4). The most common treatment-related non-haematological adverse events included injection site reactions with azacitidine, and nausea, vomiting, fatigue, and diarrhoea with azacitidine, low-dose cytarabine, and intensive chemotherapy. Treatment discontinuations before study completion in the azacitidine group compared with the conventional care group were mostly related to haematological adverse events (table 4). Table 4 lists the discontinuations because of haematological adverse events by investigator preselection.
Table 4.
Deaths, discontinuations, and grade 3 or 4 haematological toxicity by treatment group and investigator preselection
| Total ITT (n=358) |
BSC only (N=222) |
Low-dose cytarabine (N=94) |
Intensive chemotherapy (N=42) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azacitidine (n=179) |
Conventional care (n=179) |
Azacitidine (n=117) |
BSC (n=105) |
Azacitidine (n=45) |
Low-dose cytarabine (n=49) |
Azacitidine (n=17) |
Intensive chemotherapy (n=25) |
|||||
| Deaths | 82 (46%) | 113 (63%) | 53 (45%) | 66 (63%) | 20 (44%) | 31 (63%) | 9 (53%) | 16 (64%) | ||||
| Deaths during first 3 months* of treatment | 20 (11%) | 16 (9%) | 13 (11%) | 9 (9%) | 5 (11%) | 7 (14%) | 2 (12%) | 0 | ||||
| Safety population | 175 | 165 | 114 | 102 | 45 | 44 | 16 | 19 | ||||
| Discontinuation before study completion due to haematological adverse events† |
8 (5%) | 4 (2%) | 3 (3%) | 2 (2%) | 4 (9%) | 2 (5%) | 1 (6%) | 0 | ||||
| Grade 3 or 4 toxicity‡ | ||||||||||||
| Neutropenia | 159 (91%) | 126 (76%) | 104 (91%) | 70 (69%) | 40 (89%) | 39 (89%) | 15 (94%) | 17 (90%) | ||||
| Thrombocytopenia | 149 (85%) | 132 (80%) | 93 (82%) | 72 (71%) | 42 (93%) | 42 (96%) | 14 (88%) | 18 (95%) | ||||
| Anaemia | 100 (57%) | 112 (68%) | 62 (54%) | 67 (66%) | 29 (64%) | 34 (77%) | 9 (56%) | 11 (58%) | ||||
| Baseline grade 0–2 progressed to grade 3 or 4 during treatment† | ||||||||||||
| Neutropenia | 67/80 (84%) | 46/76 (61%) | 45/53 (85%) | 22/46 (48%) | 14/18 (78%) | 19/24 (79%) | 8/9 (89%) | 5/6 (83%) | ||||
| Thrombocytopenia | 72/97 (74%) | 68/94 (72%) | 49/69 (71%) | 29/54 (54%) | 17/20 (85%) | 29/30 (97%) | 6/8 (75%) | 10/10 (100%) | ||||
| Anaemia | 84/156 (54%) | 83/130 (64%) | 52/103 (51%) | 48/79 (61%) | 25/40 (63%) | 28/37 (76%) | 7/13 (54%) | 7/14 (50%) | ||||
Data are number (%) or number/number with data (%).
3 months = 91 days.
Study completion defined as 12 months after the last patient was randomised.
National Cancer Institute’s Common Toxicity Criteria toxicities based on laboratory data.
During the first 3 months of treatment, deaths occurred in 20 (11%) of 179 patients in the azacitidine group and 16 (9%) of 179 in the conventional care group (table 4). These deaths were primarily attributed to underlying disease (sepsis or bleeding) although four in the azacitidine group (two from sepsis and two from bleeding), and one in the conventional care group (receiving low-dose cytarabine) from cerebral ischaemia were probably related to treatment. Table 4 shows deaths in the first 3 months of treatment by investigator preselection.
Discussion
Treatment with azacitidine prolongs overall survival and lowers the risk of progression to acute myeloid leukaemia in patients with higher-risk myelodysplastic syndrome com pared with treatment with conventional care regimens.
The previous CALGB trial16 included a heterogeneous population of patients, best-supportive care as the only comparator, and a crossover design, and 53% of patients who received best-supportive care subsequently received azacitidine. Our study aimed to include only patients with higher-risk disease (87% were intermediate-2 or high on the international prognosis scoring system), and did not allow crossover. Furthermore, in the absence of a standard of care for the control regimen in higher-risk myelodysplastic syndromes, and with differing national, regional, institutional, or consensus guidelines,24,25 this trial compared azacitidine treatment with a control arm including the three most common treatments for higher-risk myelodysplastic syndromes over the past two decades (best supportive care, low-dose cytarabine, and intensive chemotherapy).6–15,17 Randomisation allowed valid comparisons within investigator preselected subgroups. Patients enrolled in the study were representative of those with higher-risk myelodysplastic syndrome in demographic characteristics, presenting signs and symptoms, and subtypes on the FAB classification. The proportions of patients selected to the best supportive care, low-dose cytarabine, and intensive chemotherapy groups were consistent with treatment practices (Germing U, Heinrich-Heine University, Dusseldorf, Germany, personal communication).26
Median overall survival in the azacitidine group exceeded that in the conventional care group by 9·4 months with a 2-year survival rate that was nearly doubled. The survival benefit with azacitidine was seen across all prognostic subgroups analysed, including those patients with poor, intermediate, and good cytogenetics according to the international prognosis scoring system. Patients with numerical or structural abnormalities of chromosome 7, who have a particularly poor outcome with traditional management strategies,17,27–29 had overall survival improvement with azacitidine.
The survival advantage in the azacitidine group was observed early in the treatment course compared with the conventional care group, with separation of the Kaplan-Meier survival curves occurring after about 3 months of treatment, corresponding to completion of about three cycles of azacitidine treatment by most patients. The median number of azacitidine treatment cycles was nine, suggesting that long-term treatment might give the best survival benefit.
Comparisons with the supportive investigator preselection analysis showed that treatment with azacitidine was associated with a significant improvement in overall survival compared with low-dose cytarabine or best supportive therapy. Previous studies of low-dose cytarabine in high-risk myelodysplastic syndrome or acute myeloid leukaemia have reported low proportions of patients with response and poor survival,30 particularly in patients with unfavourable cytogenetics.17,31,32 A previous trial that compared treatment with one cycle of low-dose cytarabine with best supportive care showed no survival differences.33 Although our trial was designed to maximise the potential of each treatment strategy by continuing treatment until evidence for disease progression, the median number of cycles with low-dose cytarabine was four and a half because of a combination of poor response, disease progression, and unacceptable toxicity.
The difference in median overall survival between the azacitidine and intensive chemotherapy groups was not statistically significant, possibly because of the small number of patients in this analysis. The proportion of patients with complete remission with intensive chemotherapy (40%) was in the range of published reports of myelodysplastic syndrome9–15 and higher than that observed with azacitidine in this and the CALGB studies.16 In patients who are candidates for allogeneic stem-cell transplantation and have a clear excess of marrow blasts (especially those with refractory anaemia with excess blasts in transformation, now classified as acute myeloid leukaemia in the WHO classification), intensive chemotherapy might be preferred to azacitidine before transplantation to provide better and more rapid reduction in marrow blast percentage. However, the value of reducing the blast percentage before transplantation in myelodysplastic syndromes is still disputed.12 Furthermore, intensive chemotherapy is associated with high proportions of complete remission in myelodysplastic syndrome only in the absence of an unfavourable karyotype,13,14 and patients transplanted after failure of intensive chemotherapy have a very poor outcome after transplantation.12 On the basis of results achieved with azacitidine in patients with unfavourable karyotype in the present study, this drug is being investigated before transplantation in patients with myelodysplastic syndrome with an excess of marrow blasts and unfavourable karyotype.34
In the investigator preselection analysis, grade 3 and 4 neutropenia was more common in patients receiving azacitidine, low-dose cytarabine, and intensive chemotherapy than in those receiving best supportive care. Thrombocytopenia was also more common with azacitidine than with best supportive care, but less common than with low-dose cytarabine and intensive chemotherapy. Despite the higher frequency of thrombocytopenia and neutropenia observed with azacitidine compared with best supportive care, the frequency of haemorrhagic complications and infection was similar for both treatments. Risk of infection requiring intravenous antimicrobials was a third lower in the azacitidine group than in the conventional care group.
Finally, mechanisms of the activity of azacitidine in myelodysplastic syndromes are not fully known. Aberrant DNA hypermethylation has been implicated in the progression of myelodysplastic syndromes, and DNA-methyltransferase inhibitors, such as azacitidine, undo hypermethylation and restore normal transcription of tumour suppressor genes.35,36 Additional mechanisms of action of azacitidine in myelodysplastic syndromes are, however, probable, including a certain degree of tumour-cell apoptosis. Research to identify these mechanisms is underway.35,37,38
This trial was international and multicentre in design with 79 investigative sites in 15 countries. In the comparison of azacitidine with the three most common treatments in higher-risk myelodysplastic syndromes, including two active treatments, treatment decisions were made in light of different treatment practices influenced by regional, national, and local guidelines and consensus criteria. For these reasons, the results are applicable to the improvement of the treatment of myelodysplastic syndromes internationally. Ultimately, intensive chemotherapy might remain the appropriate treatment in some situations in higher-risk myelodysplastic syndromes, especially before allogeneic stem-cell transplantation in candidates for this procedure who have an excess of marrow blasts without an unfavourable karyotype.
Increased survival time is the primary goal of treatment for patients with higher-risk myelodysplastic syndromes. However, with the exception of allogeneic haemopoietic stem-cell transplantation, which is suitable for only a few patients with myelodysplastic syndrome,27 no previous treatment strategies have shown a significant overall survival benefit. The results of this study indicate that azacitidine significantly lengthens overall survival and changes the natural history of myelodysplastic syndrome in patients with higher-risk disease.
Acknowledgments
The research was sponsored by Celgene Corporation. Neil Malone, an employee of Celgene, provided independent consultation for the writing of the paper. James W Vardiman provided adjudication of central pathology review and Anne Hagemeijer did the central cytogenetics review. Kenneth J Kopecky and Gary G Koch provided independent review of the statistical analysis plan describing the statistical approaches; and Gary G Koch provided independent review of the analyses, interpretation of the results, and the written paper.
Funding Celgene Corporation.
Footnotes
Contributors
PF, GJM, EH-L, AL, SDG, JFS, JMB, JBy, JBa, LZ, DM, CLB, LRS designed the trial. PF, GJM, EH-L, VS, CF AG, RS, NG, GS, and JFS did the research. DM of Celgene analysed the data. PF wrote the paper. All authors provided review and editing of the manuscript.
Study investigators
Australia—S Durrant, Royal Brisbane Hospital, Herston; A Enno, Newcastle Mater Misericordiae Hospital, Waratah; R Herrmann, The Royal Perth Hospital, Perth; N Horvath, Royal Adelaide Hospital, Adelaide; A Mills, Princess of Alexandra Hospital, Woolloongabba; A Spencer, The Alfred Hospital, Melbourne; J Szer, Royal Melbourne Hospital, Parkville; J Gallo, Liverpool Hospital; L Dunlop, Liverpool Hospital; C Arthur, Royal North Shore Hospital, St Leonards. Bulgaria— S Goranov, UMHAT St George, Clinic of Hematology, Plovdiv; D Peytchev, National Centre of Hematology and Transfusiology, Sofia; L Gercheva, MHAT St Marina, Clinic of Hematology, Varna. Czech Republic—J Cermak, Ustav Hematologie A. Krevni Transfuze, Praha; J Voglova, Fakultni Nemocnice Hradec Kralove. France—N Vey, Institut Paoli Calmettes, Marseille; F Dreyfus, Hôpital Cochin, Paris; G Laurent, CHU Purpan, Toulouse; B Quesnel, CHU de Lille; H Dombret, Hôpital Saint Louis, Paris, A Stamatoullas, Centre Henri Becquerel, Rouen; E Wattel, Hôpital Edouard Herriot, Lyon; M Hunault-Berger, CHU D’Angers. Germany—C Aul, St Johannes Hospital, Duisburg; A Giagounidis, St Johannes Hospital, Duisburg; U Duhrsen, University Essen; N Gattermann, Heinrich-Heine University Dusseldorf; U Platzbecker, Medizinische Klinik Und Poliklinik, Dresden; M Schmid, Universitatsklinikum Ulm; M Hanel, Klinikum Chemnitz Ggmbh; D Haase, Gerorg-August-Universitat Gottingen; W Fiedler, Universitatsklinikum Hamburg-Eppendorf, Hamburg; N Schmitz, Allgemeines Krankenhaus St Georg, Hamburg; W Hofmann, Universitatsklinikum Benjamin Franklin, Berlin; H Horst, Universitatsklinikum Kiel. Greece—N Anagnostopoulos, District General Hospital of Athens; V Pappa, University Hospital–Attikon, Athens; E Papadaki, University General Hospital of Heraklio, Crete; N Zoumbos, University General Hospital of Patra. Hungary—Z Borbenyi, University of Szeged; T Masszi, Orszagos Gyogyintezeti Kozpont, Budapest. Italy—M Baccarani, Policlinico S Orsola-Malpighi, Bologna; A Bacigalupo, Ospedale, San Martino, Genova; P Corradini, Instituto Nazionale Dei Tumori, Milano; G Leone, Policlinico Gemelli, Roma; S Sacchi, Centro Oncologico Modenese, Modena; A Bosi, Azienda Ospedaliera Careggi, Firenze; P Musto, Ospedale Casa Sollievo Della Sofferenza, San Giovanni Rotondo. Netherlands—P Muus, UMCN St. Radboud, Nijmegen. Poland—A Dmoszynska, Samodzielny Publiczny Szpital Kliniczny, Lublin; T Robak, Wojewodzki Szpital Specjalistyczny, Lodz; K Sulek, Wojskowy Instytut Medyczny, Warszawa; K Kuliczkowski, Samodzielny Publiczny Szpital Kliniczny, Wroclaw; W Jedrzejczak, Samodzielny Publiczny Centralny Szpital, Warszawa. Russia—A Zaritsky, Pavlov State Medical University, St Petersburg; K Abdulkadyrov, Institute of Haematology & Blood Transfusion, St Petersburg; E Podoltseva, City Hospital #31, St Petersburg; B Afanasiev, Pavlov State Medical University, St Petersburg. Spain—J Bargay, Hospital San Llatzer, Palma de Mallorca; S Brunet, Hospital Santa Creu I Sant Pau, Barcelona; C Del Canizo, Hospital Universitario De Salamanca; J Ribera, Hospital Universitario Germans Trias I Pujol, Barcelona; A Figuera Alvarez, Hospital Universitario de la Princesa, Madrid; J Diaz-Mediavilla, Hospital Clinico San Carlos, Madrid; M Canales, Hospital Universitario la Paz, Madrid; F Ramos y Ortega, Residencia Virgen Blanca, Leon. Sweden—L Nilsson, Lund University Hospital; A Olsson, Sahlgrenska University Hospital, Goteborg. UK—J Cavenagh, St. Bartholomew’s Hospital, London; J Parker, Norfolk and Norwich University Hospital, Norwich; S Killick, Royal Bournemouth General Hospital; A Kruger, Royal Cornwall Hospital, Truro, Cornwall; P Vyas, John Radcliffe Hospital, Oxford; M Dennis, Christie Hospital, Manchester. USA—L Cripe, Indiana University Cancer Center, Indianapolis, IN; J DiPersio, Washington University School of Medicine, St Louis, MO; P Emanuel, University of Alabama School of Medicine, Birmingham, AL.
Conflicts of interest
PF has participated in advisory board meetings for Celgene, Roche, Amgen, GlaxoSmithKlein, Merck, Novartis, Johnson and Johnson, and Cephalon. GJM has served as an advisory board member and consultant for Celgene, Amgen, and Genzyme, and as an advisory board member for Pharmion (now Celgene) and Johnson and Johnson. EH-L has participated in advisory board meetings for Celgene and Amgen and has given paid testimony for Celgene. VS has received honoraria from Celgene, Novartis, and Johnson and Johnson for lecturing. CF has no conflicts of interest. AG is a consultant to Celgene and participates on their speakers’ bureau. RS has nothing to disclose. NG has received honoraria for lecturing for Novartis, Roche, Celgene, and Janssen-Cilag and research support from Novartis, and Celgene. GS is a consultant for Celgene. AL has received honoraria from Celgene. SDG is a consultant for and owns stock in Celgene. JFS has participated on an advisory board for and has received honoraria from Celgene; he was a member of an advisory board and speaker’s bureau for Pharmion (now Celgene) and he received honoraria from them. JMB is a consultant for Celgene, Novartis, and Johnson and Johnson and is a member of a speakers’ bureau for Celgene. JBy is a consultant for Celgene. JBa, LZ, DM, and CLB are employees of Celgene and own stock in the company. LRS has received research funding and honoraria from Celgene.
References
- 1.Sloand EM. Myelodysplastic syndromes: introduction. Semin Hematol. 2008;45:1–2. doi: 10.1053/j.seminhematol.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Valent P, Horny HP, Bennett JM, et al. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: consensus statements and report from a working conference. Leuk Res. 2007;31:72–36. doi: 10.1016/j.leukres.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 3.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. [PubMed] [Google Scholar]
- 4.Harris NL, Jaffe ES, Diebold J, et al. World Health Organization classification of neoplastic diseases of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting-Airlie House, Virginia, November 1997. J Clin Oncol. 1999;17:3835–3849. doi: 10.1200/JCO.1999.17.12.3835. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2098. [PubMed] [Google Scholar]
- 6.List AF, Vardiman J, Issa JP, DeWitte TM. Myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program. 2004:297–317. doi: 10.1182/asheducation-2004.1.297. [DOI] [PubMed] [Google Scholar]
- 7.Cheson BD, Simon R. Low-dose ara-C in acute nonlymphocytic leukemia and myelodysplastic syndromes: a review of 20 years’ experience. Semin Oncol. 1987;14(2 suppl 1):126–133. [PubMed] [Google Scholar]
- 8.Gajewski JL, Ho WG, Nimer SD, et al. Efficacy of intensive chemotherapy for acute myelogenous leukemia associated with a preleukemic syndrome. J Clin Oncol. 1989;7:1637–1645. doi: 10.1200/JCO.1989.7.11.1637. [DOI] [PubMed] [Google Scholar]
- 9.Verbeek W, Wörmann B, Koch P, et al. S-HAM induction chemotherapy with or without GM-CSF in patients with high-risk myelodysplastic syndromes. Ann Hematol. 1997;74:205–208. doi: 10.1007/s002770050285. [DOI] [PubMed] [Google Scholar]
- 10.Estey EH, Thall PF, Cortes JE, et al. Comparison of idarubicin + ara-C-, fludarabine + ara-C-, and topotecan + ara-C-based regimens in treatment of newly diagnosed acute myeloid leukemia, refractory anemia with excess blasts in transformation, or refractory anemia with excess blasts. Blood. 2001;98:3575–3583. doi: 10.1182/blood.v98.13.3575. [DOI] [PubMed] [Google Scholar]
- 11.Prébet T, Ducastelle S, Debotton S, et al. A phase II study of intensive chemotherapy with fludarabine, cytarabine, and mitoxantrone in P glycoprotein-negative high-risk myelodysplastic syndromes. Hematol J. 2004;5:209–215. doi: 10.1038/sj.thj.6200363. [DOI] [PubMed] [Google Scholar]
- 12.de Witte T, Suciu S, Verhoef G, et al. Intensive chemotherapy followed by allogeneic or autologous stem cell transplantation for patients with myelodysplastic syndromes (MDSs) and acute myeloid leukemia following MDS. Blood. 2001;98:2326–2331. doi: 10.1182/blood.v98.8.2326. [DOI] [PubMed] [Google Scholar]
- 13.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 14.Knipp S, Hildebrand B, Kündgen A, et al. Intensive chemotherapy is not recommended for patients aged >60 years who have myelodysplastic syndromes or acute myeloid leukemia with high-risk karyotypes. Cancer. 2007;110:345–352. doi: 10.1002/cncr.22779. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann W, Heil G, Zander C, et al. Intensive chemotherapy with idarubicin, cytarabine, etoposide, and G-CSF priming in patients with advanced myelodysplastic syndrome and high-risk acute myeloid leukemia. Ann Hematol. 2004;83:498–503. doi: 10.1007/s00277-004-0889-0. [DOI] [PubMed] [Google Scholar]
- 16.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 17.Hellström-Lindberg E, Robert KH, Gahrton G, Lindberg G. A predictive model for the clinical response to low dose ara-C: a study of 102 patients with myelodysplastic syndromes or acute leukaemia. Br J Haematol. 1992;81:503–511. doi: 10.1111/j.1365-2141.1992.tb02982.x. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Kantarjian H, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000;96:3671–3674. [PubMed] [Google Scholar]
- 19.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Lan G, DeMets D. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–663. [Google Scholar]
- 21.Kaplan EL, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 22.Cox DR. Regression models and life-tables. J R Stat Soc Ser B Stat Meth. 1972;34:184–192. [Google Scholar]
- 23.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 24.Bowen D, Culligan D, Jowitt S, et al. Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol. 2003;120:187–200. doi: 10.1046/j.1365-2141.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 25.Alessandrino EP, Amadori S, Barosi G, et al. Evidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes: a statement from the Italian Society of Hematology. Haematologica. 2002;87:1286–1306. [PubMed] [Google Scholar]
- 26.Germing U. Düsseldorf MDS registry data—summary of classification systems and current treatment options for MDS patients with RAEB, RAEB-T, CMMol who are IPSS Int-2 or High. Personal Communication. Düsseldorf: Universitätsklinikum Düsseldorf; Oct 22, 2007. [Google Scholar]
- 27.Barrett AJ, Savani BN. Allogeneic stem cell transplantation for myelodysplastic syndrome. Semin Hematol. 2008;45:49–59. doi: 10.1053/j.seminhematol.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Kantarjian H, Beran M, Cortes J, et al. Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodysplastic syndrome. Cancer. 2006;106:1099–1109. doi: 10.1002/cncr.21699. [DOI] [PubMed] [Google Scholar]
- 29.van der Straaten HM, van Biezen A, Brand R, et al. Allogeneic stem cell transplantation for patients with acute myeloid leukemia or myelodysplastic syndrome who have chromosome 5 and/or 7 abnormalities. Haematologica. 2005;90:1339–1345. [PubMed] [Google Scholar]
- 30.Cheson BD. Standard and low-dose chemotherapy for the treatment of myelodysplastic syndromes. Leuk Res. 1998;22(suppl 1):S17–S21. doi: 10.1016/s0145-2126(98)00039-3. [DOI] [PubMed] [Google Scholar]
- 31.Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. doi: 10.1002/cncr.22496. [DOI] [PubMed] [Google Scholar]
- 32.Detourmignies L, Wattel E, Laï JL, Bauters F, Fenaux P. Is there still a role for low-dose cytosine arabinoside in de novo acute myeloid leukemia in the elderly? A report of 77 cases. Ann Hematol. 1993;66:235–240. doi: 10.1007/BF01738471. [DOI] [PubMed] [Google Scholar]
- 33.Miller KB, Kim K, Morrison FS, et al. The evaluation of low-dose cytarabine in the treatment of myelodysplastic syndromes: a phase-III intergroup study. Ann Hematol. 1992;65:162–168. doi: 10.1007/BF01703109. [DOI] [PubMed] [Google Scholar]
- 34.Field T, Perkins J, Alsina M, et al. Pre-transplant 5-azacitidine (Vidaza®) may improve outcome of allogeneic hematopoietic cell transplantation (HCT) in patients with myelodysplastic syndrome (MDS) Blood. 2006;108:1047a. [Google Scholar]
- 35.Raj K, John A, Ho A, et al. CDKN2B methylation status and isolated chromosome 7 abnormalities predict responses to treatment with 5-azacytidine. Leukemia. 2007;21:1937–1944. doi: 10.1038/sj.leu.2404796. [DOI] [PubMed] [Google Scholar]
- 36.Gore SD, Baylin S, Sugar E, et al. Combined DNA methyltransferase and histone deacetylase inhibition in the treatment of myeloid neoplasms. Cancer Res. 2006;66:6361–6369. doi: 10.1158/0008-5472.CAN-06-0080. [DOI] [PubMed] [Google Scholar]
- 37.Khan R, Schmidt-Mende J, Karimi M, et al. Hypomethylation and apoptosis in 5-azacytidine-treated myeloid cells. Exp Hematol. 2008;36:149–157. doi: 10.1016/j.exphem.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Stresemann C, Lyko F. Modes of action of the DNA methyltransferase inhibitors azacitidine and decitabine. Int J Cancer. 2008;123:8–13. doi: 10.1002/ijc.23607. [DOI] [PubMed] [Google Scholar]