Summary
Eukaryotic cell division is often regulated by extracellular signals. In budding yeast, signaling from mating pheromones arrests the cell cycle in G1 phase [1]. This arrest requires the protein Far1 [2], which is thought to antagonize the G1/S transition by acting as a Cdk inhibitor (CKI) [3, 4], although the mechanisms remain unresolved [5]. Recent studies found that G1/S cyclins (Cln1 and Cln2) recognize Cdk substrates via specific docking motifs, which promote substrate phosphorylation in vivo [6, 7]. Here, we show that these docking interactions are inhibited by pheromone signaling, and that this inhibition requires Far1. Moreover, Far1 mutants that cannot inhibit docking are defective at cell cycle arrest. Consistent with this arrest function, Far1 outcompetes substrates for association with G1/S cyclins in vivo, and it is present in large excess over G1/S cyclins during the pre-commitment period where pheromone can impose G1 arrest. Finally, a comparison of substrates that do and do not require docking suggests that Far1 acts as a multi-mode inhibitor that antagonizes both kinase activity and substrate recognition by Cln1/2-Cdk complexes. Our findings uncover a novel mechanism of Cdk regulation by external signals, and shed new light on Far1 function to provide a revised view of cell cycle arrest in this model system.
Results
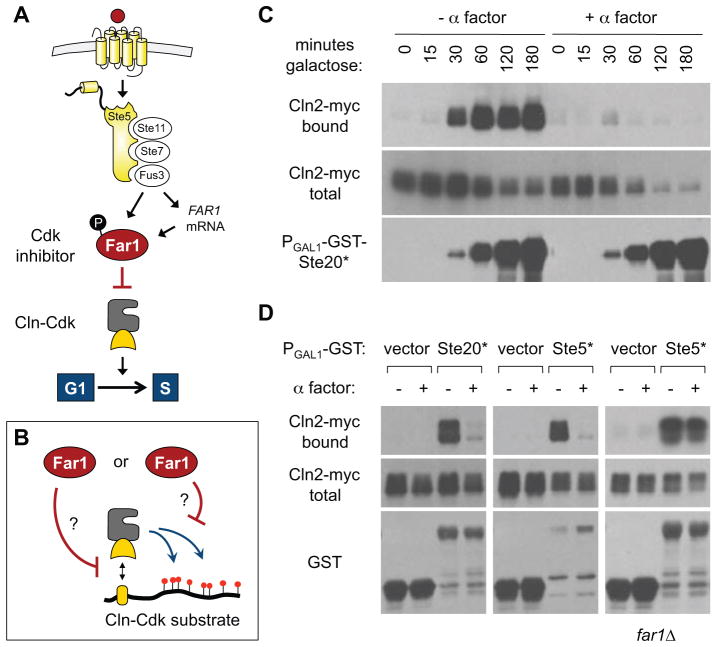
During cell cycle arrest by pheromone, Far1 is thought to act as a Cdk inhibitor (CKI) that antagonizes cyclin-Cdk complexes containing early cyclins (Cln1, Cln2, Cln3), which function in G1 to drive cell cycle entry (Figure 1A). Far1 binds these Cdk complexes in vivo [8] and appeared to inhibit Cln2-Cdk activity in vitro [4], but later studies failed to detect this inhibitory effect [5] and others suggested that Far1 might inhibit Cln3-Cdk or regulate Cln2 protein levels [9, 10]. Consequently, the precise effects of pheromone and Far1 on Cdk function in vivo have remained unresolved. Recent studies revealed that some Cln-Cdk phosphorylation events require docking interactions between Cln1/Cln2 and specific motifs in substrate proteins, including components of the mating pathway (Ste5, Ste20) and regulators of the G1/S transition (Sic1, Whi5) [6, 7]. Therefore, we asked if pheromone signaling and/or Far1 might disrupt these docking interactions, either in addition to or as an alternative to direct inhibition of Cdk activity per se (Figure 1B).
Figure 1. Pheromone signaling disrupts Cln2-substrate interactions.
(A) Mating pheromones signal through a MAP kinase cascade, leading to phosphorylation and increased expression of Far1, which is thought to induce G1 arrest by inhibiting Cln-Cdk complexes.
(B) Far1 could inhibit cyclin-substrate docking (left) or Cln-Cdk kinase activity (right).
(C) Cells harboring Cln2-myc (expressed from the CYC1 promoter; see Figure S1) and a galactose-inducible GST fusion to the Ste20 N-terminus (Ste20*) were induced with galactose with or without pheromone (α factor) for varying times. Complexes were captured on glutathione-sepharose. Bound and input samples were analyzed by anti-myc and anti-GST blots.
(D) Binding of Cln2-myc to galactose-inducible GST-Ste20* or GST-Ste5*, induced with or without pheromone. Note that pheromone disruption of Cln2-substrate binding was lost in far1Δ cells.
To monitor docking, we used an assay in which a GST-substrate fusion and an epitope-tagged cyclin (Cln2) were co-expressed and co-precipitated [6]. (Here, we took steps to prevent CLN2 expression and pheromone signaling from interfering with each other; see Supplemental Experimental Procedures and Figure S1.) First, we tested a GST fusion to a Cln2-binding fragment of Ste20 (residues 72–333, designated Ste20*)[6], expressed from an inducible promoter (PGAL1). Without pheromone, we observed Cln2-myc binding as soon as GST-Ste20* expression was detected, but binding was strongly inhibited when pheromone was included (Figure 1C). (Note, total Cln2 levels were often reduced by prolonged pheromone treatments, as in earlier studies [10], so we used short treatment times where possible to minimize this effect.) Pheromone also inhibited Cln2-myc binding to another, similar GST fusion (GST-Ste5*), in which the Cln2 docking site from Ste20 was replaced with one from Ste5 (Figure 1D). Remarkably, this inhibition was not observed in far1Δ cells (Figure 1D). Therefore, pheromone signaling can disrupt Cln2-substrate binding interactions in a manner that depends on Far1.
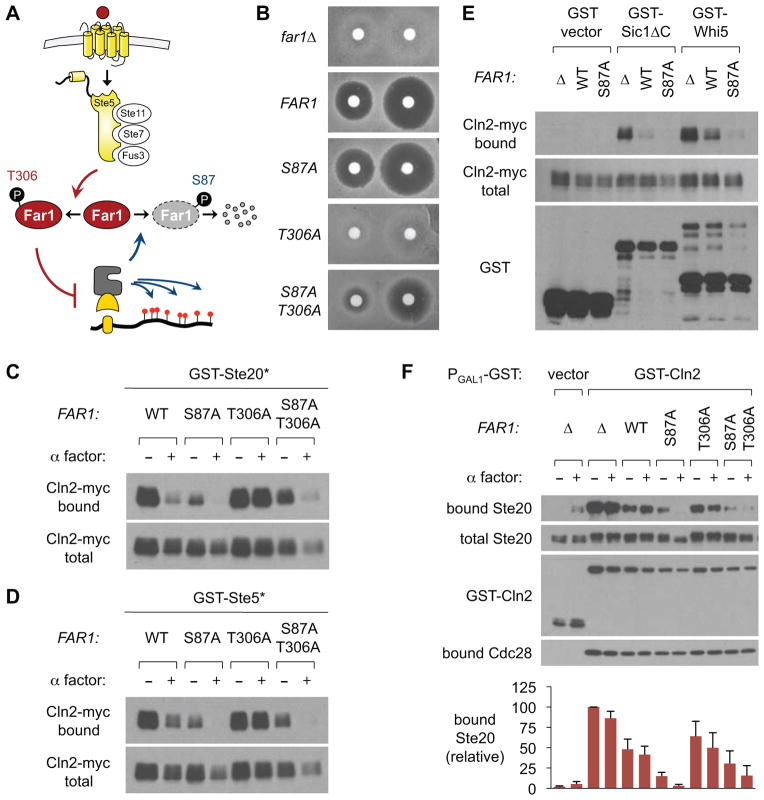
Next, we tested the role of regulatory phosphorylation sites in Far1 (Figure 2A): phosphorylation at residue T306 by the MAPK Fus3 promotes Far1 function, whereas phosphorylation at residue S87 by Cdk triggers its degradation [5]. We introduced non-phosphorylatable Ala mutations at these sites, tested previously in an N-terminal fragment of Far1 [5], into full-length Far1 expressed from the native FAR1 locus. As expected, the T306A mutant was defective at pheromone arrest whereas the S87A mutant remained functional (Figure 2B); the S87A T306A double mutant showed an intermediate phenotype, indicating that T306 phosphorylation is not absolutely required if Far1 is stabilized by the S87A mutation. When we tested Cln2-substrate binding in these strains, we observed several notable features (Figures 2C, 2D). First, the T306A mutation blocked the ability of pheromone to disrupt Cln2-substrate interactions, whereas the S87A mutation increased this disruptive effect. Second, this increased potency of the Far1-S87A mutant was evident even in the absence of pheromone. Third, the S87A mutation partially suppressed the defect of the T306A mutation, consistent with the arrest phenotypes. (Note that the effect of pheromone in the S87A T306A double mutant cannot be due to Far1 activation by phosphorylation at T306, and instead it may reflect elevated FAR1 transcription [2].) The ability of Far1-S87A to reduce Cln2-substrate binding even without pheromone was unanticipated, but it may imply that the unmodified wild-type protein is partially active (rather than inactive) and that this activity becomes more evident in the S87A mutant due to higher protein levels or presence in a greater fraction of cells (see below). Overall, the binding results mirror the G1 arrest phenotypes, implying that interference with Cln2-substrate docking relates to the arrest function of Far1. In further support of this view, we found that Far1 (especially Far1-S87A) also disrupted binding of Cln2 to the G1/S regulators Sic1 and Whi5 (Figures 2E, S2A), which are Cdk substrates with Cln1/2 docking sites similar to those in Ste5 and Ste20 [6, 7].
Figure 2. Far1 inhibition of docking correlates with G1 arrest ability.
(A) Pheromone triggers phosphorylation of Far1 at T306, which promotes G1 arrest, whereas Cdk phosphorylates Far1 at S87, which promotes its degradation [5].
(B) The indicated FAR1 strains were tested for pheromone arrest. Cell lawns were overlaid with disks containing 20 μL of α factor (20 or 100 μM), and incubated at 30 °C for 2 days.
(C, D) Binding of Cln2-myc to GST-Ste20* or GST-Ste5* was analyzed (as in Figure 1) using strains with different FAR1 alleles, in the presence or absence of pheromone.
(E) Far1 disrupts Cln2 binding to Sic1 and Whi5. Sic1ΔC (residues 1–214) lacks its Cdk-inhibitor domain but includes its Cln1/2 docking site [6, 7]. Pheromone was omitted from these assays because it affected GST-Whi5 levels. Also see Figure S2A.
(F) Far1 disrupts binding of GST-Cln2 to full-length Ste20. Strains harbored a PGAL1-GST-CLN2 plasmid or GST vector, plus V5-tagged Ste20. To reduce effects of pheromone on Cln2 levels, we used a truncated Cln2 (residues 1–372), which lacks its destabilizing C-terminus [35]. Cells were induced with galactose ± pheromone; bound complexes were captured and analyzed by anti-V5, anti-GST, and anti-Cdc28 blots. Graphs quantify relative levels of Ste20 binding (mean ± SEM; n = 3). Also see Figure S2B–E.
We confirmed these findings via reciprocal assays in which a GST-Cln2 fusion was used to co-precipitate full-length substrates (Ste20 and Ste5). Binding of each substrate to GST-Cln2 required their docking sequences (Figures S2B, S2C) and was strongest in far1Δ cells, weakest in FAR1-S87A cells, and intermediate in FAR1-WT cells (Figure 2F, S2). This trend was seen even without pheromone treatment, further reinforcing the notion that unmodified Far1 is partially active. Importantly, Far1 did not affect binding of Cln2 to its partner Cdk molecule, Cdc28 (Figures 2F, S2E). The effect of pheromone was less evident in these experiments than when using the previous (reverse) procedure, perhaps because chronic Cln2 expression can induce Far1 degradation, counteracting its activation by pheromone. Overall, however, the results confirm the disruptive effect of Far1 and argue that it blocks interactions between intact Cln2-Cdk complexes and their substrates.
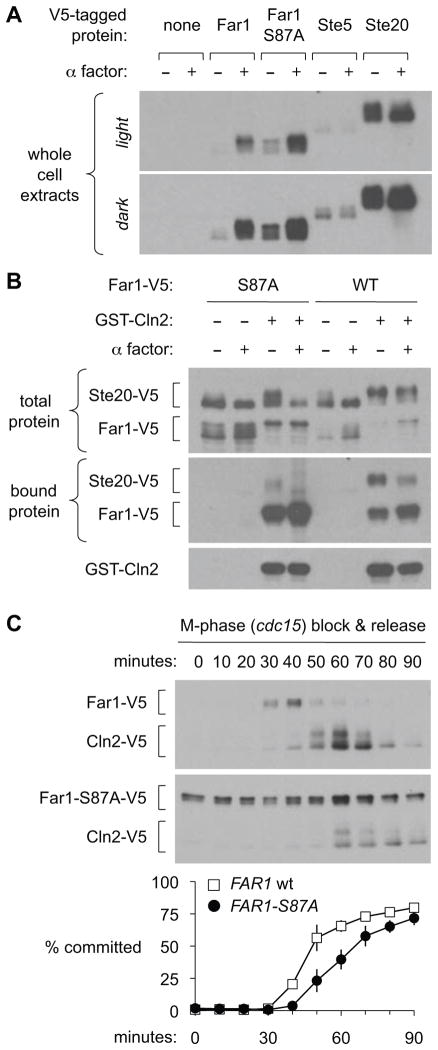
Because Far1 binds Cln-Cdk complexes [3, 5, 8], we asked if Far1 and substrates bind Cln2 competitively, and if Far1 outcompetes substrates via higher concentration or affinity. First, we compared their concentrations by marking Far1 and substrates with the same epitope tag (3xV5). Far1 levels ranged between those of Ste20 and Ste5, depending on whether it had been induced by pheromone or stabilized by the S87A mutation (Figure 3A). Next, we used cells that simultaneously expressed V5-tagged forms of Far1 and Ste20 to compare their binding to Cln2 (Figure 3B). The results suggest that Far1 binds Cln2 more favorably, as total Far1-WT was much less abundant than Ste20 and yet it bound Cln2 at equal or greater levels. Similarly, Far1-S87A was comparably abundant to Ste20 yet showed disproportionally greater binding to Cln2. When comparing WT and S87A forms of Far1, the increased Cln2 binding to the S87A mutant was accompanied by reduced binding to Ste20, implying that Far1 competes with Ste20. Indeed, pheromone caused increased Cln2-Far1 binding and reduced Cln2-Ste20 binding. Collectively, these results suggest that Far1 binds Cln2 in a way that is mutually exclusive with Cln2-substrate docking, and that the preferential binding of Far1 allows it to outcompete substrates.
Figure 3. Far1 outcompetes substrates for binding to Cln2.
(A) Far1, Ste5, and Ste20 were tagged with the identical 3xV5 tag to compare protein levels. Far1 (WT or S87A) was expressed from its native genomic locus; Ste5 and Ste20 were expressed from their native promoters on low copy number plasmids. Whole cell extracts were prepared and equivalent amounts of total protein were analyzed by SDS-PAGE and anti-V5 blots.
(B) Strains with V5-tagged Far1 or Far1-S87A harbored a V5-Ste20 plasmid plus galactose-inducible GST-Cln2 or GST vector. Cells were induced with galactose with or without pheromone, and then binding of Far1 to GST-Cln2 was assayed.
(C) Strains with V5-tagged Far1 and Cln2 were synchronized by arrest in mitosis (using a cdc15-2 mutant). At various times after release, aliquots were taken to assess protein levels and then treated with pheromone to assess whether they could still arrest in G1 or had passed Start (committed). Signal levels in the two blots are directly comparable, as all steps were performed in parallel using equal protein loading. Graphs show mean ± SEM (n = 4–6). See Figure S3 for additional tests.
We reasoned that Far1 should be in excess of Cln2 in order to effectively outcompete Cln2-substrate interactions. Therefore, we compared their levels as cells approached the critical point of cell cycle commitment, or “Start”. Using synchronous cultures in which both Far1 and Cln2 had the same epitope tag, we monitored protein levels and the ability of cells to arrest in G1 in response to pheromone (Figures 3C, S3). Far1 was generally in large excess over Cln2 as cells approached Start, and a sharp increase in Cln2 corresponded to the first appearance of committed cells. It did not seem that Cln2 must reach peak levels or exceed Far1 for cells to pass Start, but rather it only must begin to accumulate. This pattern fits previous findings that Start occurs simultaneous with CLN2 promoter firing [11], and is reminiscent of the mammalian cell restriction point occurring at very low levels of cyclin E [12, 13]. Thus, it may be necessary for Far1 to substantially exceed cyclin levels to prevent Start, whereas cyclin levels may not need to exceed Far1 to pass Start, perhaps because the positive feedback loop governing Cln1/2 expression [14] makes them destined to overwhelm Far1 once their expression begins. In accord with recent work [11], the Far1-S87A mutant caused mild delays in commitment and Cln2 expression, though to varying degrees (Figures 3C, S3). Notably, the Far1-S87A protein was not strongly over-expressed compared to peak levels of Far1-WT, but it was present over a broader range of the cell cycle (Figures 3C, S3). Hence, the increased inhibitory activity of Far1-S87A seen in preceding experiments (using asynchronous cultures) may primarily reflect an increase in the fraction of cells expressing Far1 rather than in its concentration.
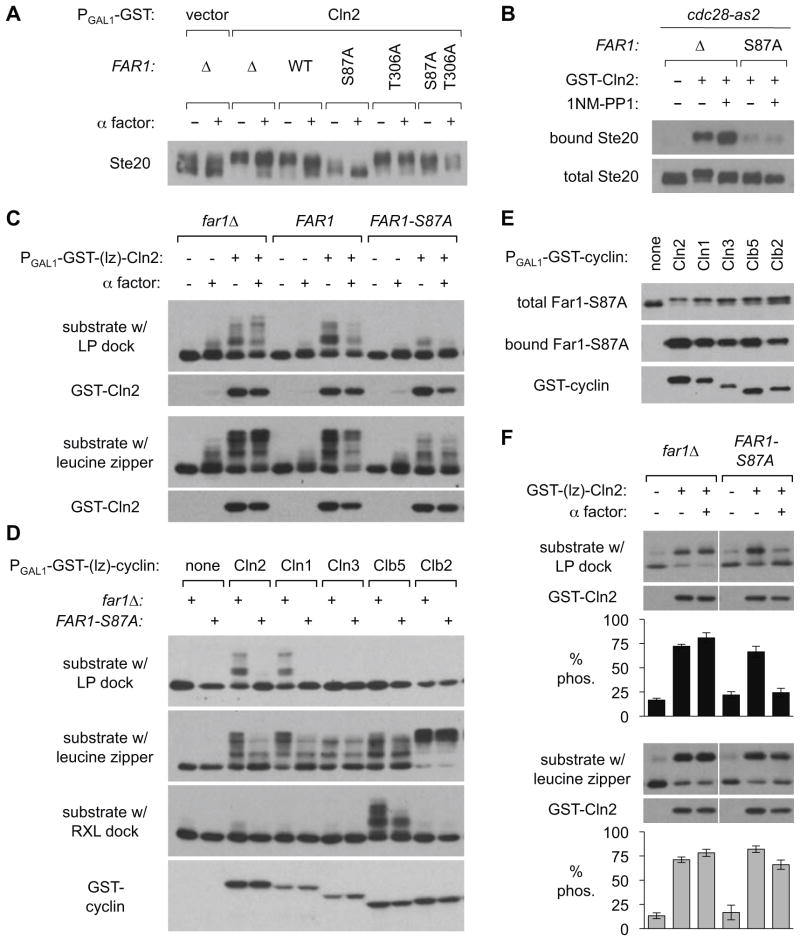
Cdk phosphorylation of both Ste20 and Ste5 alters their electrophoretic mobility [6, 15–17]. By using extended electrophoresis to better resolve Ste20 forms, we found that pheromone and Far1 inhibited Cln2-driven phosphorylation (Figures 4A, S4A). Specifically, Cln2 expression in far1Δ cells converted Ste20 to its slowest mobility, phosphorylated form. Pheromone had no effect in far1Δ cells, but it reduced Ste20 phosphorylation in FAR1-WT cells. In FAR1-S87A cells, Ste20 phosphorylation was reduced even without pheromone treatment, and pheromone caused a further reduction. The T306A mutant was ineffectual, while the S87A T306A double mutant showed a result intermediate between the two single mutants. Collectively, these results suggest parallel effects of Far1 on substrate docking and substrate phosphorylation by Cln2-Cdk. Chemical inhibition of Cdk activity was not sufficient to reduce Cln2-Ste20 binding, and Far1-S87A was equally disruptive with and without Cdk inhibition (Figure 4B), suggesting that reduced binding causes reduced phosphorylation rather than vice-versa. Notably, to our knowledge these results provide the first demonstration that pheromone and Far1 reduce phosphorylation of Cdk substrates in vivo (see Discussion). In contrast, we saw no reduction in phosphorylation of the Cln1/2 C-termini (Figure S4B) or Far1 itself (Figure 3B, top), indicating that Far1 does not inhibit all Cln-Cdk phosphorylation events equally.
Figure 4. Effects of Far1 and pheromone on Cdk phosphorylation in vivo using substrates with native and artificial docking interactions.
(A) Extracts of cells harboring V5-Ste20 and galactose-inducible GST-Cln2, as in Figure 2F, were analyzed by extended electrophoresis to resolve the extent of Ste20 phosphorylation triggered by Cln2 expression. See Figure S4A for replicates.
(B) Cdk inhibition alone does not disrupt Cln2-Ste20 binding. Strains with a drug-sensitive Cdk (cdc28-as2) harbored V5-tagged Ste20 plus galactose-inducible GST-Cln2 or GST vector. Cells were induced with galactose either with or without the ATP analog 1-NM-PP1 (15 μM), and GST fusions were captured. Total and bound Ste20 were analyzed by anti-V5 blots.
(C) The indicated FAR1 strains harbored a plasmid expressing GST-Cln2 with an attached leucine half-zipper (lz), plus a plasmid expressing an HA-tagged Cdk substrate with either the matching half-zipper or an LP-type Cln1/2 docking site (see Figure S4Eii). Cultures were pre-incubated for 2 hr ± α factor (0.1 μM), and then induced with galactose for 2 hr. Substrate phosphorylation [6] and GST-Cln2 expression was monitored by anti-HA and anti-GST blots, respectively. Figure S4C shows that leucine zipper binding is resistant to pheromone and Far1.
(D) FAR1-S87A and far1Δ strains co-expressing GST-(lz)-cyclins with an HA-tagged substrate (Figure S4Eii) were induced with galactose for 2.5 hr. Levels of GST-(lz)-cyclins were monitored in each experiment; one representative anti-GST blot is shown. Note that, aside from effects of Far1, these results confirm that cyclin docking drives substrate use, because switching the docking site alters which cyclins are effective, as seen previously [6].
(E) GST-(lz)-cyclin plasmids were introduced into a strain with V5-tagged Far1-S87A. Cultures were induced with galactose for 1.5 hr and then association of Far1 with the GST-tagged cyclin was assayed.
(F) Strains co-expressed GST-(lz)-Cln2 with HA-tagged substrates that each show only two mobility forms (see Figure S4Eiii), which makes it easier to quantify phosphorylation. Cultures were pre-incubated with α factor (0.1 μM, 30 min.), and then induced with galactose (40 min). Graphs (mean ± SEM, n = 3–4) show the signal in the upper band as a percentage of the total signal (% phos.).
Finally, we asked if the ability of Far1 to disrupt substrate phosphorylation is due to inhibition of Cln2-substrate docking, or Cln2-Cdk kinase activity, or a combination of both. To address this point, we compared Cdk substrates with and without Cln2 docking motifs. Using an approach to be elaborated elsewhere (S.B. and P.M.P., in preparation), we replaced a native docking interaction with a foreign leucine zipper (Figure S4Eii), thereby allowing phosphorylation of a single substrate to be driven by either a native cyclin docking site or an artificial linkage. Then, we analyzed phosphorylation driven by different cyclins, and the effects of Far1. When the substrate harbored a native “LP”-type Cln2 docking site, its phosphorylation was inhibited strongly by Far1-S87A, but when the leucine zipper was used, the degree of inhibition was substantially reduced, though not eliminated (Figures 4C, 4D). These results imply that Far1 inhibits substrate docking strongly, with a residual effect on some non-docking function such as Cdk kinase activity. This residual effect might also signify a reduction in kinase processivity mediated by the Cks1 subunit of the Cdk complex [18–20], though it was still evident when the role of Cks1 was blocked (by changing threonine phosphorylation sites to serine; Figure S4D). It is also notable that this residual effect was only seen with the G1/S cyclins Cln1 and Cln2 (Figure 4D), even though Far1-S87A could bind all cyclins (Figure 4E). Interestingly, however, when the substrate contained an “RXL” docking motif favored by S-phase cyclins such as Clb5 [6, 21, 22], Far1- S87A could mildly inhibit Clb5-driven phosphorylation (Figure 4D), again suggesting that docking-dependent phosphorylation is more susceptible to inhibition by Far1. To help quantify the extent of phosphorylation, we performed related experiments using a variant substrate with only two electrophoretic mobility forms: unphosphorylated and phosphorylated (Figure 4F). Here, pheromone treatment of Far1-S87A cells almost completely reversed phosphorylation driven by the native Cln2 docking site (Figure 4F, top), but had only a mild effect when the leucine zipper was used (Figure 4F, bottom). Collectively, these results suggest that Far1 can reduce Cdk phosphorylation of substrates irrespective of docking, but substrates that require docking are especially sensitive to Far1. Therefore, Far1 may engage cyclin-Cdk complexes in a way that simultaneously disrupts both substrate recognition and kinase activity.
Discussion
This study addresses long-standing uncertainties about how yeast pheromone signaling and the presumed CKI protein Far1 promote cell cycle arrest. Our findings reveal an unsuspected mode of Cdk regulation, in which an extracellular signal stimulates an inhibitory factor, Far1, to disrupt interactions of specific cyclin-Cdk complexes with substrates. Far1 appears to disrupt Cln2-substrate docking by binding Cln2 more favorably so that it outcompetes substrates, and mutant analyses suggest that this effect parallels its ability to mediate G1 arrest. Because Cln1/2 docking enhances substrate phosphorylation [6, 7], inhibition of docking by Far1 should contribute to reduced substrate phosphorylation in vivo, in addition to any effects of Far1 on Cdk activity per se. Indeed, our findings suggest that Far1 is a multi-mode inhibitor that separately disrupts both Cdk activity and substrate docking. This combined effect raises the possibility that Far1 has the strongest inhibitory effect on substrates that are most dependent on docking, which might include proteins with inherently poor (e.g., non-consensus) phosphorylation sites or those that must be phosphorylated at multiple positions. Similar themes could also apply to other kinases.
We find that the ability of Cln1/2-Cdk to phosphorylate substrates in vivo can be inhibited by pheromone and Far1. Remarkably, to our knowledge this is the first such demonstration. Although there are numerous prior examples in which Cdk substrates are unphosphorylated in pheromone-arrested cells, this can be explained by the fact that cyclins are not expressed in G1 phase, and hence it does not necessarily indicate that the kinase activity of cyclin-Cdk complexes is reduced. Here, by expressing cyclins independent of cell cycle position, we could detect regulation of phosphorylation by a set amount of Cln1/2-Cdk in vivo. Also, by linking different cyclins to substrates using a common leucine zipper, we could compare their sensitivity to Far1. Of note, compared to Cln1/2-Cdk, Cln3-Cdk seemed less susceptible to Far1 inhibition, which could underlie different roles for these cyclins in driving cell cycle re-entry after pheromone arrest [23].
Surprisingly, Far1 could partially interfere with Cln2-substrate binding and phosphorylation even without pheromone treatment. Prior findings implied that Far1 must be activated, because G1 arrest required pheromone-induced phosphorylation of Far1 at T306 [5] and FAR1 over-expression was not sufficient [24]. We suggest a new interpretation in which unphosphorylated Far1 is partially active but is less potent than when phosphorylated at T306. This view is supported by our binding and phosphorylation data as well as by the partial arrest observed in FAR1-S87A T306A cells, which shows that T306 phosphorylation is not absolutely essential. It is also relevant to findings that far1Δ cells show accelerated entry into the cell cycle [25] and that FAR1-S87A cells show a delay in Start [11]. Because Far1 is expressed only during a narrow pre-Start window of the cell cycle [26], the partially active state would normally be restricted to cells poised for G1 arrest, but detection of this state was enhanced when using the stabilized S87A mutant, which is expressed in a larger fraction of cells. This mutant also revealed that inhibitory effects of Far1 are at least partly independent of T306 phosphorylation. Yet, T306 phosphorylation makes Far1 a more potent inhibitor, likely via enhanced binding to Cdk complexes [5] and possibly via engaging the phospho-threonine binding pocket in Cks1 [19, 20].
The specific mechanism by which Far1 disrupts Cln2 docking is not yet known. Currently, there are no structural data on the Cln2-substrate binding interface, but the short docking motifs [6, 7] likely bind a peptide-recognition pocket on the cyclin, as with RXL motif recognition by S-phase cyclins [27]. Thus, Far1 could displace substrates either by having a higher affinity docking peptide or by interacting with a broader region of Cln2 in a way that obscures peptide recognition. The latter view may be favored by the fact that two separate parts of Far1 are required to bind Cln2 [3]. This view is also reminiscent of mammalian CKI proteins p21 and p27, whose RXL sequences contribute to cyclin binding and Cdk inhibition [28–30], but as only a small part of a much larger binding interface involving both the cyclin and Cdk subunits [31]. In fact, such multipartite interactions may have contributed to confusion about inhibitory mechanisms for both Far1 and p21/p27. Namely, complexes of p21 with cyclin-Cdk sometimes retained kinase activity [32, 33], leading to speculation that under such conditions the CKI might contact only the cyclin and not the Cdk [34], a notion later supported by p21 mutants that bind only the cyclin [29]. Analogous heterogeneity of Far1-Cln-Cdc28 complexes might explain why kinase inhibition was observed in some studies [4] but not others [5]. In addition, the use of generic substrates that do not require docking (e.g., histone H1) would have bypassed the ability of Far1 to regulate this step in either study.
We suggest that Far1 engages the cyclin-Cdk complex in a way that disrupts multiple distinct functions, including both substrate docking and kinase activity but perhaps also others such as Cks1-mediated processivity [18–20]. Multiple concerted effects may help ensure maximal inhibition. Thus, in future studies using in vitro assays, it will be important to compare substrates with a range of requirements, in order to dissect the effect of Far1 on total kinase activity, utilization of docking sites, kinase processivity, and multi-site phosphorylation. Investigation of these issues will further illuminate how differential regulation of distinct mechanistic steps in substrate phosphorylation can provide additional layers of control that fine-tune protein kinase networks.
Supplementary Material
Highlights.
Yeast pheromone signaling and Far1 inhibit docking of Cln1/2 cyclins to substrates
Far1 mutants that cannot disrupt docking are defective at mediating cell cycle arrest
Far1 outcompetes substrates for interaction with Cln2
Both Cln1/2-Cdk kinase activity and substrate recognition are inhibited by Far1
Acknowledgments
We thank Gustav Ammerer, Alejandro Colman-Lerner and Jan Skotheim for plasmids, Matt Winters for technical assistance, and Dan McCollum for comments on the manuscript. This work was supported by a grant from the National Institutes of Health (GM57769) to P.M.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bardwell L. A walk-through of the yeast mating pheromone response pathway. Peptides. 2005;26:339–350. doi: 10.1016/j.peptides.2004.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang F, Herskowitz I. Identification of a gene necessary for cell cycle arrest by a negative growth factor of yeast: FAR1 is an inhibitor of a G1 cyclin, CLN2. Cell. 1990;63:999–1011. doi: 10.1016/0092-8674(90)90503-7. [DOI] [PubMed] [Google Scholar]
- 3.Peter M, Gartner A, Horecka J, Ammerer G, Herskowitz I. FAR1 links the signal transduction pathway to the cell cycle machinery in yeast. Cell. 1993;73:747–760. doi: 10.1016/0092-8674(93)90254-n. [DOI] [PubMed] [Google Scholar]
- 4.Peter M, Herskowitz I. Direct inhibition of the yeast cyclin-dependent kinase Cdc28-Cln by Far1. Science. 1994;265:1228–1231. doi: 10.1126/science.8066461. [DOI] [PubMed] [Google Scholar]
- 5.Gartner A, Jovanovic A, Jeoung DI, Bourlat S, Cross FR, Ammerer G. Pheromone-dependent G1 cell cycle arrest requires Far1 phosphorylation, but may not involve inhibition of Cdc28-Cln2 kinase, in vivo. Mol Cell Biol. 1998;18:3681–3691. doi: 10.1128/mcb.18.7.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhaduri S, Pryciak PM. Cyclin-specific docking motifs promote phosphorylation of yeast signaling proteins by G1/S Cdk complexes. Curr Biol. 2011;21:1615–1623. doi: 10.1016/j.cub.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koivomagi M, Valk E, Venta R, Iofik A, Lepiku M, Morgan DO, Loog M. Dynamics of Cdk1 Substrate Specificity during the Cell Cycle. Mol Cell. 2011;42:610–623. doi: 10.1016/j.molcel.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tyers M, Futcher B. Far1 and Fus3 link the mating pheromone signal transduction pathway to three G1-phase Cdc28 kinase complexes. Mol Cell Biol. 1993;13:5659–5669. doi: 10.1128/mcb.13.9.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeoung DI, Oehlen LJ, Cross FR. Cln3-associated kinase activity in Saccharomyces cerevisiae is regulated by the mating factor pathway. Mol Cell Biol. 1998;18:433–441. doi: 10.1128/mcb.18.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valdivieso MH, Sugimoto K, Jahng KY, Fernandes PM, Wittenberg C. FAR1 is required for posttranscriptional regulation of CLN2 gene expression in response to mating pheromone. Mol Cell Biol. 1993;13:1013–1022. doi: 10.1128/mcb.13.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doncic A, Falleur-Fettig M, Skotheim JM. Distinct interactions select and maintain a specific cell fate. Mol Cell. 2011;43:528–539. doi: 10.1016/j.molcel.2011.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ekholm SV, Zickert P, Reed SI, Zetterberg A. Accumulation of cyclin E is not a prerequisite for passage through the restriction point. Mol Cell Biol. 2001;21:3256–3265. doi: 10.1128/MCB.21.9.3256-3265.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinsson HS, Starborg M, Erlandsson F, Zetterberg A. Single cell analysis of G1 check points-the relationship between the restriction point and phosphorylation of pRb. Exp Cell Res. 2005;305:383–391. doi: 10.1016/j.yexcr.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Skotheim JM, Di Talia S, Siggia ED, Cross FR. Positive feedback of G1 cyclins ensures coherent cell cycle entry. Nature. 2008;454:291–296. doi: 10.1038/nature07118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oehlen LJ, Cross FR. Potential regulation of Ste20 function by the Cln1-Cdc28 and Cln2-Cdc28 cyclin-dependent protein kinases. J Biol Chem. 1998;273:25089–25097. doi: 10.1074/jbc.273.39.25089. [DOI] [PubMed] [Google Scholar]
- 16.Wu C, Leeuw T, Leberer E, Thomas DY, Whiteway M. Cell cycle- and Cln2p-Cdc28p-dependent phosphorylation of the yeast Ste20p protein kinase. J Biol Chem. 1998;273:28107–28115. doi: 10.1074/jbc.273.43.28107. [DOI] [PubMed] [Google Scholar]
- 17.Strickfaden SC, Winters MJ, Ben-Ari G, Lamson RE, Tyers M, Pryciak PM. A mechanism for cell-cycle regulation of MAP kinase signaling in a yeast differentiation pathway. Cell. 2007;128:519–531. doi: 10.1016/j.cell.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koivomagi M, Valk E, Venta R, Iofik A, Lepiku M, Balog ER, Rubin SM, Morgan DO, Loog M. Cascades of multisite phosphorylation control Sic1 destruction at the onset of S phase. Nature. 2011;480:128–131. doi: 10.1038/nature10560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koivomagi M, Ord M, Iofik A, Valk E, Venta R, Faustova I, Kivi R, Balog ER, Rubin SM, Loog M. Multisite phosphorylation networks as signal processors for Cdk1. Nat Struct Mol Biol. 2013;20:1415–1424. doi: 10.1038/nsmb.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGrath DA, Balog ER, Koivomagi M, Lucena R, Mai MV, Hirschi A, Kellogg DR, Loog M, Rubin SM. Cks confers specificity to phosphorylation-dependent CDK signaling pathways. Nat Struct Mol Biol. 2013;20:1407–1414. doi: 10.1038/nsmb.2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilmes GM, Archambault V, Austin RJ, Jacobson MD, Bell SP, Cross FR. Interaction of the S-phase cyclin Clb5 with an “RXL” docking sequence in the initiator protein Orc6 provides an origin-localized replication control switch. Genes Dev. 2004;18:981–991. doi: 10.1101/gad.1202304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loog M, Morgan DO. Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature. 2005;434:104–108. doi: 10.1038/nature03329. [DOI] [PubMed] [Google Scholar]
- 23.Doncic A, Skotheim JM. Feedforward regulation ensures stability and rapid reversibility of a cellular state. Mol Cell. 2013;50:856–868. doi: 10.1016/j.molcel.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang F, Herskowitz I. Phosphorylation of FAR1 in response to alpha-factor: a possible requirement for cell-cycle arrest. Mol Biol Cell. 1992;3:445–450. doi: 10.1091/mbc.3.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberghina L, Rossi RL, Querin L, Wanke V, Vanoni M. A cell sizer network involving Cln3 and Far1 controls entrance into S phase in the mitotic cycle of budding yeast. J Cell Biol. 2004;167:433–443. doi: 10.1083/jcb.200405102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKinney JD, Chang F, Heintz N, Cross FR. Negative regulation of FAR1 at the Start of the yeast cell cycle. Genes Dev. 1993;7:833–843. doi: 10.1101/gad.7.5.833. [DOI] [PubMed] [Google Scholar]
- 27.Lowe ED, Tews I, Cheng KY, Brown NR, Gul S, Noble ME, Gamblin SJ, Johnson LN. Specificity determinants of recruitment peptides bound to phospho-CDK2/cyclin A. Biochemistry. 2002;41:15625–15634. doi: 10.1021/bi0268910. [DOI] [PubMed] [Google Scholar]
- 28.Adams PD, Sellers WR, Sharma SK, Wu AD, Nalin CM, Kaelin WG., Jr Identification of a cyclin-cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol. 1996;16:6623–6633. doi: 10.1128/mcb.16.12.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Saha P, Kornbluth S, Dynlacht BD, Dutta A. Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol. 1996;16:4673–4682. doi: 10.1128/mcb.16.9.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wohlschlegel JA, Dwyer BT, Takeda DY, Dutta A. Mutational analysis of the Cy motif from p21 reveals sequence degeneracy and specificity for different cyclin-dependent kinases. Mol Cell Biol. 2001;21:4868–4874. doi: 10.1128/MCB.21.15.4868-4874.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russo AA, Jeffrey PD, Patten AK, Massague J, Pavletich NP. Crystal structure of the p27Kip1 cyclin-dependent-kinase inhibitor bound to the cyclin A-Cdk2 complex. Nature. 1996;382:325–331. doi: 10.1038/382325a0. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Hannon GJ, Beach D. p21-containing cyclin kinases exist in both active and inactive states. Genes Dev. 1994;8:1750–1758. doi: 10.1101/gad.8.15.1750. [DOI] [PubMed] [Google Scholar]
- 33.Harper JW, Elledge SJ, Keyomarsi K, Dynlacht B, Tsai LH, Zhang P, Dobrowolski S, Bai C, Connell-Crowley L, Swindell E, et al. Inhibition of cyclin-dependent kinases by p21. Mol Biol Cell. 1995;6:387–400. doi: 10.1091/mbc.6.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan DO. Under arrest at atomic resolution. Nature. 1996;382:295–296. doi: 10.1038/382295a0. [DOI] [PubMed] [Google Scholar]
- 35.Lanker S, Valdivieso MH, Wittenberg C. Rapid degradation of the G1 cyclin Cln2 induced by CDK-dependent phosphorylation. Science. 1996;271:1597–1601. doi: 10.1126/science.271.5255.1597. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.