Abstract
Purpose
To determine the anatomic characteristics and pharmacokinetic properties of intravitreally placed bevacizumab and ranibizumab after pars plana lensectomy or pars plana vitrectomy and to compare these with nonoperated control eyes in a rabbit model.
Methods
Three groups of six Dutch-belted rabbits each underwent pars plana vitrectomy, pars plana lensectomy, or served as nonsurgical controls. Twelve days after surgery, 3 rabbits from each group underwent intravitreal injection in one eye with 1.25 mg/0.05 mL I-124–bevacizumab or 0.5 mg/0.05 mL I-124-ranibizumab. Serial imaging with PET/CT were obtained on Days 0, 2, 5, 7, 14, 21, 28, and 35. Measured radioactivity emission in becquerels/milliliter was used to calculate the half-lives for each agent.
Results
The intravitreally placed radiolabeled agents were contained within the vitreous cavity for the duration of the study. The average clearance half-lives with standard error for bevacizumab and ranibizumab after correction for radioactive decay were, respectively, 4.22 ± 0.07 days and 2.81 ± 0.05 days in unoperated eyes, 2.30 ± 0.09 days (P < 0.0001) and 2.13 ± 0.05 days (P < 0.0001) after vitrectomy, and 2.08 ± 0.07 days (P = 0.0001) and 1.79 ± 0.05 days (P < 0.0001) after lensectomy.
Conclusion
Intravitreal retention was longer for bevacizumab than ranibizumab within all study groups and was significantly reduced after vitrectomy and lensectomy for both agents. Consideration for more frequent intravitreal anti–vascular endothelial growth factor dosing regimens may be made for patients whose treated eyes have undergone previous vitrectomy or who are aphakic.
Keywords: bevacizumab, iodine-124, lensectomy, pharmacokinetics, positron emission tomography, ranibizumab, vitrectomy
Bevacizumab and ranibizumab suppress vascular endothelial growth factor (VEGF). They are the most commonly used agents for the treatment of the exudative form of age-related macular degeneration and macular edema secondary to central and branch retinal vein occlusion. Intravitreal injection of anti-VEGF agents is currently the most commonly performed procedure in the field of retina. Intravitreal treatments with bevacizumab and ranibizumab are most commonly performed at intervals of 4 weeks to 8 weeks.
The clinical question often arises whether the clearance rate of intravitreally placed anti-VEGF agents is more rapid if the treated eye has undergone previous lens or vitreous removal. The clearance rates of other intravitreally placed medications such as triamcinolone, amphoterecin B, ciprofloxacin, amikacin, ceftazidime, and vancomycin have been shown to be faster after vitrectomy and/or lensectomy.1–6 A similar finding would be expected with intravitreally placed bevacizumab and ranibizumab. To our knowledge, there are no previous pharmacokinetic studies on humans or animal models examining the pharmacokinetic properties of bevacizumab and ranibizumab after lens or vitreous removal.
A previous report demonstrated that the use of PET/CT imaging provides a novel approach to directly and noninvasively visualize I-124 bevacizumab and I-124 ranibizumab in the vitreous cavity and to determine their pharmacokinetic properties.7 The clearance half-lives for ranibizumab and bevacizumab (2.8 days and 4.2 days, respectively) compared favorably with previously reported findings using immunoassay techniques.7–9 In this study, PET/CT was used to image intravitreally placed I-124 radiolabeled bevacizumab and ranibizumab in a rabbit model after vitrectomy or lensectomy. The goals of our project were two-fold: first, to determine whether intravitreal anti-VEGF agents placed after vitrectomy or lensectomy remain within the vitreous cavity; and second, to study the pharmacokinetic properties of these two agents in eyes after vitrectomy and lensectomy and to compare these with nonoperated eyes.
Materials and Methods
In this laboratory investigation, bevacizumab (Avastin; Genentech, San Francisco, CA/Roche, Basel, Switzerland) and ranibizumab (Lucentis; Genentech, San Francisco, CA/Roche, Basel, Switzerland) were radiolabeled with I-124 (IBA Molecular, Dulles, VA) using a modified Iodogen method previously described by Zou et al.10 Radiochemical purity of I-124–labeled bevacizumab and I-124 ranibizumab was found to be 95% and 98%, respectively.
Eighteen male Dutch-belted rabbits (Myrtle's Rabbitry, Inc, Thompsons Station, TN) weighing 1.5 kg to 1.8 kg were used for this study. All experimental protocols were approved, and the procedures followed were in accordance with the ethical standards of the Institutional Animal Care and Use Committee at the Ohio State University. All treatments were conducted in agreement with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Six rabbits underwent pars plana vitrectomy, six rabbits underwent pars plana lensectomy, and six rabbits served as nonsurgical controls. The rabbits were anesthetized with 0.1 mL to 0.2 mL of xylazine (5 mg/mL) and 1.5 mL to 2.0 mL of ketamine hydrochloride (100 mg/mL) placed intramuscularly. An Alcon Accurus vitrector (Alcon Laboratories, Fort Worth, TX) was used, and all the procedures were performed on the left eye by one surgeon (J.C.). After the surgeries, the vitrectomized and lensectomized eyes were treated with topical tobradex ointment twice a day for 3 days and 7 days, respectively. The rabbits were monitored daily and by Day 7 all the operated eyes were absolutely quiet and the sclerotomy sites were white and closed. Twelve days after the surgical procedures, the operated eye of each anesthetized rabbit underwent an intravitreal injection with a 32-gauge needle 1 mm posterior to the limbus consisting of 0.05 mL containing 0.5 mg/0.05 mL I-124–labeled ranibizumab or 1.25 mg/0.05 mL I-124–labeled bevacizumab.
The anesthetized rabbits were then lightly secured to the scanner bed with elastic socks or gauze for imaging. The animals were imaged for 10 minutes in the micro-PET/CT (Inveon; Siemens Preclinical, Knoxville, TN), followed by an attenuation scan of 15 minutes. The micro-PET scans each resulted in a reconstructed volume with an effective pixel size of 0.78 mm, whereas the micro-CT had an effective pixel size of 0.099 mm. The scans were performed on Days 0, 2, 5, and 7 and then weekly until 1 week after the radiolabeled agent was undetectable. This occurred on Day 21 for ranibizumab and Day 28 for bevacizumab in the postsurgical eyes and on Day 28 and Day 35, respectively, in the nonsurgical eyes. To eliminate noise and provide a consistent range of emission, the range of radioactive emission was set at 10% to 75% for all the figures. Euthanasia was carried out after the last imaging session by intravenous injection of 3 mL of saturated KCl.
The radioactive units were becquerels/milliliter and these were modified with a correction factor to account for radioactive decay of I-124, which has a physical half-life of 4.18 days. The radioactive measurements were used to formulate the retention curves and to calculate the intravitreal clearance half-lives for each agent using the following formula for first order kinetics:
whereby T½ is the half life; T, elapsed time; [Drug]b, beginning amount; [Drug]e, ending amount.
Statistical analysis was performed using a general linear model to fit and compare the difference in clearance half-life between surgery groups and the control group. Dunnett–Hsu method for multiple comparisons was used to adjust for multiple hypothesis tests. All analyses were performed using SAS/STAT software, version 9.2 (SAS Institute, Inc, Cary, NC) under Windows XP system.
Results
Escape from the Vitreous Cavity
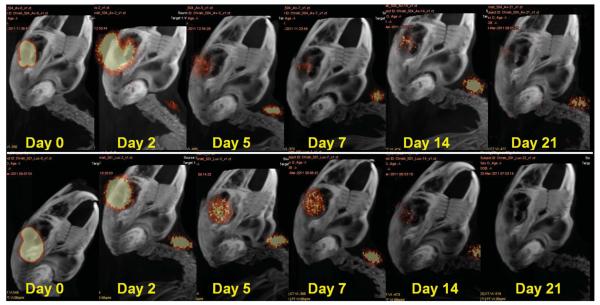
In all 18 rabbits, I-124 bevacizumab and I-124 ranibizumab were not detectable outside the vitreous cavity and the thyroid for the length of the study after intravitreal injection. None of the eyes developed evidence of endophthalmitis, uveitis, or other adverse events during the study. The 2 montages illustrate sequential postoperative images for each agent over time after vitrectomy (Figure 1) and after lensectomy (Figure 2).
Fig. 1.
Image montages illustrating clearance patterns of I-124 bevacizumab (top) and I-124 ranibizumab (bottom) within the vitreous cavity after pars plana vitrectomy in a rabbit model. Note the I-124 accumulation in the thyroid gland. Range of acquisition of radioactive emission is 10% to 75%.
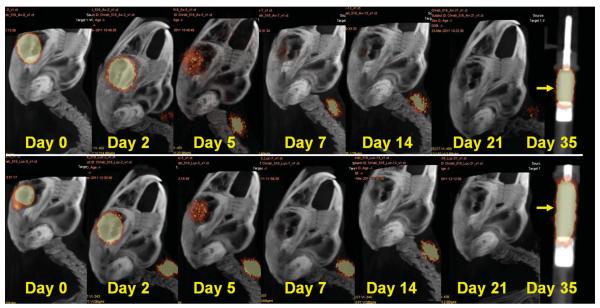
Fig. 2.
Image montages illustrating clearance patterns of I-124 bevacizumab (top) and I-124 ranibizumab (bottom) within the vitreous cavity after pars plana lensectomy in a rabbit model. Note the I-124 accumulation in the thyroid gland. A phantom containing I-124 bevacizumab (top) and I-124 ranibizumab (bottom) in a tuberculin syringe is easily discerned on Day 35 (arrow). Range of acquisition of radioactive emission is 10% to 75%.
Intravitreal levels of radioactivity (Bq/mL) are listed for each rabbit in Table 1. Radioactivity levels below 30 Bq/mL were considered compatible with background noise. I-124 counts were highly elevated throughout the study in one vitrectomized eye injected with I-124 bevacizumab (Subject 2) presumably because of the incarceration of I-124 bevacizumab within the lens, and the data from this subject were not used in the pharmacokinetic calculations. By graphic extrapolation of I-124 levels to the noise plane at 30 Bq/mL, average I-124 bevacizumab was detectable in the vitreous until Day 26 in unoperated eyes, Day 22 after vitrectomy, and Day 19 after lensectomy (Figure 3). Average I-124 ranibizumab was detectable in the vitreous until Day 21 in unoperated eyes, 19 days after vitrectomy and 13 days after lensectomy (Figure 3). Phantoms of I-124 ranibizumab and I-124 bevacizumab in tuberculin syringes were clearly visible on Day 35, indicating that the lack of positron emission in the vitreous cavity was the result of agent clearance rather than I-124 decay (Figure 2).
Table 1.
Intravitreal Radioactivity Levels (Bq/mL)
| Day | Bev. 1 | Bev. 2 | Bev. 3 | Ran. 1 | Ran. 2 | Ran. 3 |
|---|---|---|---|---|---|---|
| 0 | 175,370.0 | 205,250.0 | 178,970.0 | 167,730.0 | 116,000.0 | 164,540.0 |
| 2 | 848,61.5 | 106,740.0 | 89,862.3 | 89,780.3 | 50,111.7 | 69,510.8 |
| 5 | 312,47.2 | 383,12.8 | 337,22.5 | 210,29.2 | 140,30.5 | 196,04.7 |
| 7 | 155,23.8 | 198,24.4 | 170,06.6 | 5,933.5 | 5,946.1 | 8,350.8 |
| 14 | 1,549.1 | 1,929.9 | 1,695.3 | 470.6 | 275.7 | 454.5 |
| 21 | 174.5 | 44.3 | 161.4 | 31.6 | 16.7 | 31.1 |
| 28 | 17.2 | 23.8 | 15.9 | 4.9 | 8.2 | 9.1 |
| 35 | 7.6 | 5.1 | 9.1 | |||
| Day | Bev. 1-PPV | Bev. 2-PPV | Bev. 3-PPV | Ran. 1-PPV | Ran. 2-PPV | Ran. 3-PPV |
| 0 | 327,500.0 | 318,600.0 | 360,480.0 | 278,150.0 | 234,510.0 | 225,600 |
| 2 | 22,463.8 | 107,000.0 | 16,220.1 | 4,972.4 | 2,186.5 | 663.4 |
| 5 | 2,186.6 | 27,782.9 | 1,663.8 | 1,322.1 | 281.0 | 143.8 |
| 7 | 1,043.9 | 164,15.4 | 707.2 | 650.9 | 169.7 | 67.0 |
| 14 | 108.0 | 3,342.2 | 69.8 | 60.1 | 35.8 | 16.9 |
| 21 | 21.8 | 630.4 | 16.2 | 9.6 | 15.7 | 7.1 |
| 28 | 8.6 | 110.2 | 5.9 | |||
| 35 | 33.3 | |||||
| Day | Bev. 1-PPL | Bev. 2-PPL | Bev. 3-PPL | Ran. 1-PPL | Ran. 2-PPL | Ran. 3-PPL |
| 0 | 332,020.0 | 365,280.0 | 346,410.0 | 309,570.0 | 245,630.0 | 272,970.0 |
| 2 | 308,57.3 | 9,899.4 | 421,50.5 | 48,136.8 | 32,017.1 | 14,947.9 |
| 5 | 1,760.8 | 698.1 | 2,357.7 | 2,456.4 | 1,835.3 | 168.7 |
| 7 | 477.9 | 321.7 | 596.8 | 169.0 | 286.8 | 38.8 |
| 14 | 31.5 | 39.9 | 71.0 | 11.4 | 10.0 | 9.3 |
| 21 | 6.0 | 10.0 | 15.3 | 4.6 | 5.1 | 7.1 |
Bev., bevacizumab; PPL, pars plana lensectomy; PPV, pars plana vitrectomy; Ran., ranibizumab.
Fig. 3.
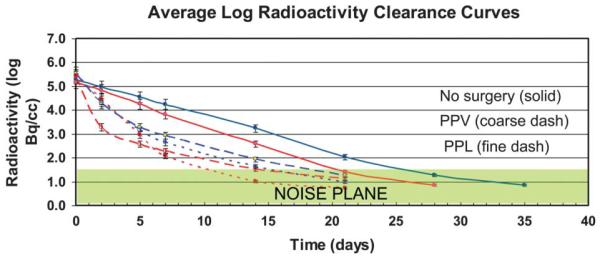
Graph demonstrating average log clearance curves for the two I-124–labeled agents in unoperated eyes (solid), after vitrectomy (coarse dashes), and after lensectomy (fine dashes). The units are corrected for I-124 radioactive decay. The noise plane indicates the level of background noise (<30 Bq/mL).
Accumulation of I-124 in the thyroid gland was detectable at lower emission thresholds in the non-operated subjects and was only visible until Day 14 in 5 of 6 subjects. By comparison, thyroid I-124 levels were greatly elevated and clearly visible on Day 21 in all postsurgical subjects (Table 2 and Figures 1 and 2).
Table 2.
Thyroid Radioactivity Levels (Bq/mL)
| Day | Bev. 1-Control | Bev. 2-Control | Bev. 3-Control | Ran. 1-Control | Ran. 2-Control | Ran. 3-Control |
|---|---|---|---|---|---|---|
| 2 | 429.4 | 490.6 | 351.3 | 475.0 | 410.0 | 464.9 |
| 5 | 311.4 | 388.5 | 272.8 | 321.0 | 416.0 | 367.6 |
| 7 | 224.0 | 323.4 | 217.3 | 207.1 | 297.1 | 294.4 |
| 14 | 77.2 | 113.8 | 71.1 | 43.5 | 71.8 | 59.1 |
| 21 | 33.6 | |||||
| Day | Bev. 1-PPV | Bev. 2-PPV | Bev. 3-PPV | Ran. 1-PPV | Ran. 2-PPV | Ran. 3-PPV |
| 2 | 2,267.8 | 1,176.5 | 2,490.6 | 2,225.4 | 2,406.3 | 1,144.7 |
| 5 | 2,268.2 | 1,618.5 | 2,799.8 | 1,424.6 | 1,278.0 | 656.7 |
| 7 | 1,463.8 | 940.2 | 2,084.6 | 852.2 | 792.6 | 379.4 |
| 14 | 430.4 | 235.0 | 605.3 | 152.4 | 162.2 | 52.0 |
| 21 | 89.2 | 63.0 | 91.3 | 27.9 | 35.2 | 8.7 |
| 28 | 21.3 | 17.3 | 32.4 | 10.7 | 6.1 | |
| 35 | 10.9 | |||||
| Day | Bev. 1-PPL | Bev. 2-PPL | Bev. 3-PPL | Ran. 1-PPL | Ran. 2-PPL | Ran. 3-PPL |
| 2 | 1,879.2 | 3,095.4 | 3,306.2 | 4,453.2 | 10,001.2 | 4,248.3 |
| 5 | 1,242.4 | 2,183.1 | 2,599.3 | 2,355.4 | 4,965.8 | 1,804.7 |
| 7 | 890.7 | 1,400.4 | 2,039.7 | 1,444.5 | 2,879.3 | 1,077.2 |
| 14 | 332.7 | 359.0 | 551.7 | 251.6 | 548.7 | 219.5 |
| 21 | 70.1 | 79.9 | 145.8 | 82.0 | 108.1 | 48.7 |
Bev., bevacizumab; PPL, pars plana lensectomy; PPV, pars plana vitrectomy; Ran., ranibizumab.
Pharmacokinetic Properties
The resultant clearance patterns were consistent within each group of subjects (Figure 3). For each subgroup, the clearance appeared to fit a 2-phase curve with an initial rapid distribution phase until Day 5 followed by a slower elimination phase. The average clearance half-lives with standard error and confidence intervals for bevacizumab and ranibizumab after correction for radioactive decay were found to be, respectively, 4.22 ± 0.07 days (4.04–4.40) and 2.81 ± 0.05 days (2.68–2.93) in unoperated eyes; 2.30 ± 0.09 days (2.07–2.52) (P < 0.0001) and 2.13 ± 0.05 days (2.01–2.25) (P < 0.0001) after vitrectomy; and 2.08 ± 0.07 days (1.90, 2.27) (P = 0.0001) and 1.79 ± 0.05 days (1.66, 2.91) (P < 0.0001) after lensectomy. In comparison with vitrectomy, lensectomy further reduced retention of bevacizumab (P = 0.007) and ranibizumab (P = 0.230).
Discussion
In this study, the clearance half-lives of intravitreally placed I-124 bevacizumab and I-124 ranibizumab were found to be significantly reduced after vitrectomy and lensectomy for both agents compared with nonsurgical control eyes. The clearance half-lives were longer for bevacizumab than ranibizumab in all three study groups. The increased intravitreal clearance rates of bevacizumab and ranibizumab after vitrectomy and lensectomy are consistent with the findings of other intravitreal agents reported in previous studies using different methodologies in a rabbit model. Schindler et al1 demonstrated that triamcinolone disappeared more rapidly in eyes that underwent combined vitrectomy and lensectomy (6.5 days) and vitrectomy only (16.8 days) compared with unoperated rabbit eyes (41 days).1 In other reports, the intravitreal agent clearances for normal phakic eyes and vitrectomized–lensectomized in rabbit model eyes were found to be 9.1 days and 1.4 days, respectively, for amphoterecin B2, 2.2 hours and 1 hour for ciprofloxacin,3 25.5 hours and 7.0 hours for amikacin,4 25.1 hours to 9.0 hours for vancomycin,5 and 13.8 hours and 4.7 hours for ceftazidime.6
The significant intravitreal clearance half-life reduction for both anti-VEGF agents after vitrectomy compared with nonsurgical controls was surprising because the vitrectomized rabbits retained their native lenses. Vitrectomy by itself creates a vitreous with lower viscosity that allows for increased convection that may help to disperse bevacizumab and ranibizumab faster than in nonvitrectomized eyes.11 However, it is unclear whether the route of escape is primarily anterior through the trabecular meshwork or posterior through the retina and choroid. The significant decrease in retention time after vitrectomy in the presence of an intact lens would indicate a more likely posterior outflow mechanism.
The addition of lensectomy further reduced the retention that was significant for bevacizumab (P = 0.007) but not significant for ranibizumab (P = 0.230). Although the cause for this difference between the two agents is unclear, the decreased retention time after lensectomy may indicate the addition of an anterior escape route despite preservation of the anterior capsule. Pflugfelder et al12 found that preservation of the anterior capsule slows the clearance rate of intravitreal agents and more accurately simulates a phakic model in a rabbit. Because the vitreous and the posterior lens capsule are usually preserved during cataract surgery, it seems plausible that the clearance half-life of intravitreal anti-VEGF therapy would not be greatly affected in pseudophakic patients. The rabbit is a challenging animal model for placement of an intraocular lens because of severe miosis and an exudative fibrinous response in young rabbits. Further studies using an animal model that more closely simulates pseudo-phakia are warranted to accurately examine the pharmacokinetic effects of intravitreal anti-VEGF agents in the presence of an intraocular lens implant.
A previously vitrectomized eye would be expected to be less responsive to standard intravitreal treatment intervals because of a more rapid clearance of the agent from the eye. A retrospective study by et al13 showed reduced efficacy of intravitreal bevacizumab for diabetic macular edema after previous pars plana vitrectomy by examining foveal thickness by OCT and visual acuity. The use of an intravitreal device with sustained release of drug would be a plausible solution in previously vitrectomized or aphakic eyes. In the CHAMPLAIN Study, intravitreal placement of a single dexamethasone implant (Ozurdex; Allergan, Inc, Irvine, CA) for the treatment of diabetic macular edema in previously vitrectomized eyes resulted in improvement of visual acuity and central retinal thickness that remained significantly improved throughout the 26-week study.14
The presence of posterior vitreous detachment is often seen in patients who are treated with anti-VEGF intravitreal therapy. The increased vitreous liquefaction present in a posterior vitreous detachment may partially simulate the increased clearance rates of intravitreally placed agents seen with vitrectomy. In an elegant study, Goldenberg et al15 demonstrated that bevacizumab was absorbed more quickly through the retina following microplasmin-induced posterior vitreous detachment compared with nontreated eyes in a rabbit model. Gad Elkareem et al16 demonstrated higher differential fluorescein diffusion rates in both plasmin and microplasmin compared with saline-treated vitreous cavities.
There was no evidence of bevacizumab and ranibizumab escape from the vitreous cavity into the central nervous system or elsewhere in any of the subjects during the length of the study. Once the anti-VEGF agent is absorbed into the blood stream, I-124 decouples from its substrate and is sequestered in the thyroid gland. Iodine-124 was detected in the thyroid in all groups, most prominently in postsurgical rabbits. It is unlikely that there was agent escape through the surgical sclerotomy sites; 23-gauge (0.57 mm) sclerotomy wounds are expected to be completely healed 12 days after the intraocular procedures. Previously, Hikichi et al17 demonstrated that 3.2 mm sclerotomy wounds in a rabbit model were completely healed both histologically and by ultrasound biomicroscopy by postoperative Day 7. Measurement of hematogenous agent escape is below PET/CT resolution thresholds and cannot be excluded based on our findings. We were, unfortunately, not able to directly measure ranibizumab and bevacizumab in the blood because the methodology is not available at our institution or any of our referral laboratories.
The novelty of integrated PET/CT imaging compared with immunoassay methods for studying the pharmacokinetic properties of intravitreally placed agents lies in its ability to directly and noninvasively visualize the labeled agent and to serially follow the same subject over time. The use of this method is particularly advantageous in studying postsurgical subjects because the variables of performing a larger number of surgeries for multiple time points are minimized. Although the number of rabbits studied per treatment group was small, serial measurements were obtained at 6 to 8 time points for each subject. Thus, the number of total measurements obtained by PET/CT compares favorably with those of intravitreal pharmacokinetic studies using immunoassay methods. Further studies that directly compare these two methodologies in the same laboratory could more definitively validate I-124 labeling as a method for determining pharmacokinetic properties of intravitreally placed agents.
There were limitations with this study. First, the use of a rabbit model has several inherent constraints. The vitreous cavity is significantly smaller than that of adult humans (about 1.5 cc vs. 4.5 cc) thereby increasing ocular drug concentration in comparison with the clinical setting. However, the rabbit vitreous volume is closer in size to that of an retinopathy of prematurity eye (1.6–2.5 mL)18,19 and may be used to more accurately simulate the intravitreal pharmacokinetic properties of these agents in this group of patients. The clearance half-lives of bevacizumab and ranibizumab have been found to be longer in adult humans than in rabbits, possibly because the larger vitreous volume results in lowered agent concentrations and increased diffusion time before clearance from the eye.20–23 Furthermore, rabbit retinas contain a tapetum lucidum and it is unclear how this affects agent absorption. Second, it remains uncertain whether decoupling of I-124 from the antibody occurs in the vitreous cavity. Anti-VEGF agents are not known to be metabolized while in the vitreous cavity. The clearance half-lives of intravitreal I-124 bevacizumab and I-124 ranibizumab measured by radioactive emission compare favorably with similar studies using immuno-assay methods in a rabbit model.7–9 If intravitreal decoupling occurred, the disparity of clearance half-lives measured with these two methods would be expected to be greater. Nevertheless, further studies examining possible I-124 decoupling from an antibody within the vitreous would help to further elucidate this issue. Third, any drug reentering the vitreous from the circulation would be dissociated from I-124 and therefore not detectable by PET. Bakri et al8 found this effect to be present in bevacizumab and not ranibizumab. The amount of intravitreal bevacizumab entering the fellow noninjected eye was found to be less than three orders of magnitude and this effect on the half-life would be expected to be negligible.
In conclusion, PET/CT imaging of intravitreally placed I-124 ranibizumab and I-124 bevacizumab revealed significantly faster clearance rates for both agents after vitrectomy and lensectomy compared with placebo nonsurgical controls in a rabbit model. Based on our findings, a more frequent treatment regimen of anti-VEGF therapy may be considered in nonresponding treated patients who have undergone previous vitrectomy or who are aphakic. Our described methodology may offer a novel approach for studying the anatomic and pharmacokinetic properties of intravitreally placed therapeutic agents in normal and post-surgical subjects.
Acknowledgments
Supported in part by Ohio Lions, Inc, Grove City, OH.
Footnotes
None of the authors have any conflicts of interest that pertain to the information presented in this manuscript.
References
- 1.Schindler RH, Chandler D, Thresher R, Machemer R. The clearance of intravitreal triamcinolone acetonide. Am J Ophthalmol. 1982;93:415–417. doi: 10.1016/0002-9394(82)90130-1. [DOI] [PubMed] [Google Scholar]
- 2.Doft BH, Weiskopf J, Nilsson-Ehle I, Wingard LB., Jr Amphotericin clearance in vitrectomized versus nonvitrectomized eyes. Ophthalmology. 1985;92:1601–1605. doi: 10.1016/s0161-6420(85)33838-1. [DOI] [PubMed] [Google Scholar]
- 3.Pearson PA, Hainsworth DP, Ashton P. Clearance and distribution of ciprofloxacin after intravitreal injection. Retina. 1993;13:326–330. doi: 10.1097/00006982-199313040-00010. [DOI] [PubMed] [Google Scholar]
- 4.Mandell BA, Meredith TA, Aguilar E, et al. Effects of inflammation and surgery on amikacin levels in the vitreous cavity. Am J Ophthalmol. 115:770–774. doi: 10.1016/s0002-9394(14)73646-3. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar HE, Meredith TA, el-Massry A, et al. Vancomycin levels after intravitreal injection. Effects inflammation surgery. Retina. 1995;15:428–432. [PubMed] [Google Scholar]
- 6.Shaarawy A, Meredith TA, Kincaid M, et al. Intraocular injection of ceftazidime. Effects inflammation surgery. Retina. 1995;15:433–438. [PubMed] [Google Scholar]
- 7.Christoforidis JB, Carlton MM, Knopp MV, Hinkle GH. PET/CT imaging of I-124-radiolabeled bevacizumab and ranibizumab after intravitreal injection in a rabbit model. Invest Ophthalmol Vis Sci. 2011;52:5899–5903. doi: 10.1167/iovs.10-6862. [DOI] [PubMed] [Google Scholar]
- 8.Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal bevacizumab (Avastin) Ophthalmology. 2007;114:855–859. doi: 10.1016/j.ophtha.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Bakri SJ, Snyder MR, Reid JM, et al. Pharmacokinetics of intravitreal ranibizumab (Lucentis) Ophthalmology. 2007;114:2179–2182. doi: 10.1016/j.ophtha.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Zou P, Povoski SP, Hall NC, et al. 124I-HuCC49deltaCH2 for TAG-72 antigen-directed positron emission tomography (PET) imaging of LS174T colon adenocarcinoma tumor implants in xenograft mice: preliminary results. World J Surg Oncol. 2010;8:65. doi: 10.1186/1477-7819-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gisladottir S, Loftsson T, Stefansson E. Diffusion characteristics of vitreous humour and saline solution follow the Stokes Einstein equation. Graefes Arch Clin Exp Ophthalmol. 2009;247:1677–1684. doi: 10.1007/s00417-009-1141-3. [DOI] [PubMed] [Google Scholar]
- 12.Pflugfelder SC, Hernandez E, Fliesler SJ, et al. Intravitreal vancomycin. Arch Ophthalmol. 1987;105:831–837. doi: 10.1001/archopht.1987.01060060117045. [DOI] [PubMed] [Google Scholar]
- 13.Yanyali A, Aytug B, Horozoglu F, et al. Bevacizumab (Avastin) for diabetic macular edema in previously vitrectomized eyes. Am J Ophthalmol. 2007;144:124–126. doi: 10.1016/j.ajo.2007.02.048. [DOI] [PubMed] [Google Scholar]
- 14.Boyer DS, Faber D, Gupta S, et al. Dexamethasone intravitreal implant for treatment of diabetic macular edema in vitrectomized patients. Retina. 2011;31:915–923. doi: 10.1097/IAE.0b013e318206d18c. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg DT, Giblin FJ, Cheng M, et al. Posterior vitreous detachment with microplasmin alters the retinal penetration of intravitreal bevacizumab (Avastin) in rabbit eyes. Retina. 2011;31:393–400. doi: 10.1097/IAE.0b013e3181e586b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gad Elkareem AM, Willikens B, Stassen JM, de Smet MD. Differential vitreous dye diffusion following microplasmin or plasmin pre-treatment. Curr Eye Res. 2010;35:235–241. doi: 10.3109/02713680903484259. [DOI] [PubMed] [Google Scholar]
- 17.Hikichi T, Yoshida A, Hasegawa T, et al. Wound healing of scleral self-sealing incision: a comparison of ultrasound biomicroscopy and histology findings. Graefes Arch Clin Exp Ophthalmol. 1998;236:775–778. doi: 10.1007/s004170050157. [DOI] [PubMed] [Google Scholar]
- 18.Darlow BA, Ells AL, Gilbert CE, et al. Are we there yet? Bevacizumab therapy for retinopathy of prematurity. Arch Dis Child Fetal Neonatal Ed. 2011 doi: 10.1136/archdischild-2011-301148. [DOI] [PubMed] [Google Scholar]
- 19.Meyer CH, Krohne TU, Holz FG. Intraocular pharmacokinetics after a single intravitreal injection of 1.5 mg versus 3.0 mg of bevacizumab in humans. Retina. 2011;31:1877–1884. doi: 10.1097/IAE.0b013e318217373c. [DOI] [PubMed] [Google Scholar]
- 20.Gaudreault J, Webb W, Van Hoy M, et al. Pharmacokinetics and retinal distribution of AMD rhufab V2 after intravitreal administration in rabbits. AAPS Pharm Sci. 1999;(suppl 1):3207. [Google Scholar]
- 21.Zhu Q, Ziemssen F, Henke-Fahle S, et al. Vitreous levels of bevacizumab and vascular endothelial growth factor- in patients with choroidal neovascularization. Ophthalmology. 2008;115:1750–1755. doi: 10.1016/j.ophtha.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Krohne TU, Eter N, Holz FG, Meyer CH. Intraocular pharmacokinetics of bevacizumab after a single intravitreal injection in humans. Am J Ophthlamol. 2008;146:508–512. doi: 10.1016/j.ajo.2008.05.036. [DOI] [PubMed] [Google Scholar]
- 23.Maurice DM, Mishima S. Ocular pharmacokinetics. In: MI Sears., editor. Pharmacology of the Eye. Springer-Verlag; New York, NY: 1984. pp. 19–116. [Google Scholar]