Abstract
We used functional magnetic resonance imaging (fMRI) to examine responses within auditory cortical fields during the passive listening of pure tone (PT) and frequency modulated (FM) stimuli in 7 early blind (EB), 5 late blind (LB) and 6 sighted control (SC) individuals. Subjects were scanned using a “sparse sampling” imaging technique while listening to PT and FM sounds presented at either low (400 Hz) or high (4kHz) center frequencies. When high tones were directly compared to low tones, the resulting activation maps showed a general tonotopic organization within the superior and middle temporal lobes at statistically significant thresholds for the SC and LB groups while the EB group showed a comparable tonotopic organization but only at statistically non-significance thresholds. A contrast of all tonal stimuli to a quiet baseline similarly revealed significantly less signal volume in the EB than in either the LB or SC groups. These results suggest that EB does not alter inherent patterns of tonotopic organization but rather, under low-demand listening conditions, results in a more efficient processing of simple auditory stimuli within the early stages of the auditory hierarchy. While these effects must be interpreted cautiously due to the small sample sizes, they indicate that functional responses in auditory cortical areas are altered by visual deprivation and that intramodal auditory plasticity may underlie previously reported auditory advantages observed in the blind.
Keywords: Blind, Auditory Cortex, fMRI, Plasticity
Early-onset blindness (EB) leads to functional changes of normal cortical organization and reported superior auditory abilities are believed to be a direct result of such plastic alterations [1, 10, 37]. For example, the now well established finding that auditory stimulation evokes electrophysiological and hemodynamic responses within visual occipital cortices in the EB (i.e. cross-modal plasticity) [2, 4, 22, 32] has been hypothesized to lead to enhanced sound localization [10, 17], greater temporal [37, 38] and spectral [7] processing.
Alterations in auditory cortical fields due to blindness have received less consideration despite the critical importance placed on audition in the blind. To date, no fMRI study has specifically focused on signal alterations within auditory cortex of he blind. A few event related potential (ERP) studies have examined the effects of blindness on auditory areas demonstrating electrophysiological changes around the core and belt regions of auditory cortex [8, 18, 19, 20]. For instance, one magnetoencephalography (MEG) study reported an expansion of tonotopic fields around the primary auditory cortex in response to low and high frequency bursts in a combined sample of individuals with acquired or late-onset blindness (LB) and EB peers [8].
One possible mechanism underlying functional alterations within auditory cortical fields is use-dependent plasticity, a phenomenon that molds cortical response properties as a function of behaviorally relevant stimulation [16, 43]. Studies with the EB suggest that the establishment of cross-modal plasticity (including the engagement of visual cortex by tactile stimulation – see ref 33) is developmentally regulated, revolving around the loss of sight prior to the closure of critical periods [33, 6, 3] (but this is controversial see [40, 4, 5]). In contrast, use-dependent plasticity, which relies on mechanisms not tightly coupled to developmental influences, is capable of altering cortical properties throughout life [14]. Use-dependent plastic effects have been observed throughout sensory cortex in adult SC humans and animals including both auditory core and belt regions [15, 28, 13]. Reported functional changes include refinement/degradation and expansion of the primary frequency bands [16, 30] and in nearly all cases depends on focused and sustained attention to various characteristics of an auditory stream [25, 12].
In the current study, we conducted an fMRI investigation focusing specifically on regional signal changes within the temporal lobes of blind and sighted subjects collected during a passive listening task. Our goal was to establish whether blindness alters the patterns of functional organization within auditory cortex (by contrasting activity between blind and sighted listeners) and whether possible adaptations stem from use-dependent plasticity (by contrasting activity between LB and EB). Blind (EB: N=7& LB: N=5, see Table 1) and sighted control (SC: N=6, mean age 45.4, range 36–54) subjects consented and participated in the study. All subjects had normal hearing as measured with a pitch discrimination task (data not shown). EB was considered an age of onset of less than 1 year and no memory of vision. None of the blind individuals had any reported light sensitivity. Subjects were excluded if they had been previously diagnosed with a neurological or psychiatry disorder and/or abused alcohol or drugs within the past five years. All procedures were approved by the OHSU institutional review board (IRB) and adhered to the principles of the Declaration of Helsinki.
Table 1.
Participant Characterisitics
| Early Blind | ||||||
| Sub# | Cause of Blindness | Gender | Age | Handedness | Light Sensitivity | Age of onset |
| EB1 | ROP | F | 53 | R | no | 0 |
| EB2 | ROP | F | 49 | R | no | 0 |
| EB3 | ROP; detached retinas | F | 50 | R | no | 0 |
| EB4 | ROP | M | 54 | R | no | 0 |
| EB5 | ROP | F | 49 | R | no | 0 |
| EB6 | Unknown… | M | 51 | R | no | 0 |
| EB7 | micro-opthalmia | F | 22 | R | no | 0 |
| Avg | 46.9 | 0 | ||||
| Late Blind | ||||||
| Sub# | Cause of Blidness | Gender | Age | Handedness | Light Sensitivity | Age of onset |
| LB1 | Glaucoma | M | 58 | R | no | 6 |
| LB2 | diabetic retinopathy | F | 66 | R | no | 53 |
| LB3 | diabetes | M | 40 | R | no | 24 |
| LB4 | retinal detachment | M | 48 | R | no | 32 |
| LB5 | glass explosion | M | 48 | R | no | 12 |
| Avg | 52 | 25.4 | ||||
The stimuli consisted of pure tones (PT) and frequency modulated tones (FM) generated in Matlab (Mathworks, Inc., Natick, MA). The PT stimuli were produced at frequencies between 0.4 – 0.48 kHz for the low frequency (LO) set and at frequencies between 4.0 kHz – 4.8 kHz for the high frequency (HI) set. The FM stimuli were created using the same LO and HI frequency ranges as the PT stimuli but each tone was frequency modulated at 10% of its center frequency. Each tone stimulus was 750 ms in duration (50 ms rise/fall time) and presented at 75 – 78 dB SPL. Stimulus amplitudes were calibrated and tested prior to scanning using a digital audiometer (UEI DSM 100 digital sound meter, Beaverton, OR).
During the silent interval (8.3 sec) of each repetition time (TR), a train of 11 tones presented with a 150 ms inter-stimulus interval was drawn from one of the five stimulus sets (PT-HI, FM-HI, PT-LO, FM-LO and a Silent baseline [BASE] condition). Each stimulus condition was chosen randomly without replacement and each of the five conditions were presented 15 times per scan. Subjects underwent six functional scans. To monitor attention during the task, subjects were instructed to press a button whenever they detected a PT condition following an FM condition.
Scanning was carried out on a 1.5 Tesla GE Horizon scanner equipped with a standard transmit-receive headcoil. The functional data was acquired with an EPI-BOLD sequence with “sparse sampling” in order to reduce the stimulation of auditory cortex by scanner noise during presentation of the stimuli [39, 11]. Eight trans-axial slices (5 mm thick, no skip) positioned parallel to the lateral sulcus of the left hemisphere were collected from each participant to sample from all of the temporal lobes bilaterally. The functional data were acquired with a TR of 9900 ms, with the volume acquisition occurring in the last 1600 ms, leaving 8300 ms of relative quiet. The other scan parameters were set as follows: TE = 45 ms, flip angle = 90°, matrix = 642, field of view = 24 cm2. There were 64 volumes per scan and a six functional scans were acquired for each subject.
fMRI data were processed using Brain Voyager v4.9 (Brain Innovation, Maastricht, The Netherlands). The functional scans were motion-corrected using a six-parameter rigid-body correction and re-aligned to the first volume of the first functional scan. A linear fit was applied to the time course of each voxel for each fMRI scan to remove intensity drifts across the scan. The data were then spatially smoothed using a 4 mm (FWHM) Gaussian kernel. The fMRI scans for each subject were aligned with the high-resolution SPGR anatomical scan and then transformed into a Talairach coordinate system and then interpolated into 1.0 mm3 voxels. The fMRI data were analyzed in two stages. First, a general linear model approach was used to generate statistical parametric maps for each subject’s data. Each scan was normalized using z-scores. Contrasts of interest were run on individual datasets to identify intensity peaks that were subsequently mapped in Talairach coordinates in order to capture the spatial variability of activity in the SC, EB and LB groups. Additionally, group analyses were conducted by creating composite brain images for each group. The data for each group were analyzed using a random effects model and planned contrasts were generated using a cut-off threshold of P < 0.005 corrected for multiple comparisons using a Bonferonni correction.
Three planned comparisons were carried out on the stimulus conditions: the PT and FM conditions were directly compared (PT-FM), the high frequency stimulation versus low frequency stimulation were contrasted (HI-LO) and PT and FM stimulation conditions were combined and compared to the quiet baseline (All-Base). In the PT-FM condition, few subjects showed significant signal difference between the two stimulus conditions and no voxels showed significant differences between the two conditions at the group level in either the EB or LB samples. Therefore, these analyses were not pursued. No responses were observed in the occipital lobe of any subject, likely reflecting the relatively passive nature of the task components associated with the period of fMRI signal acquisition.
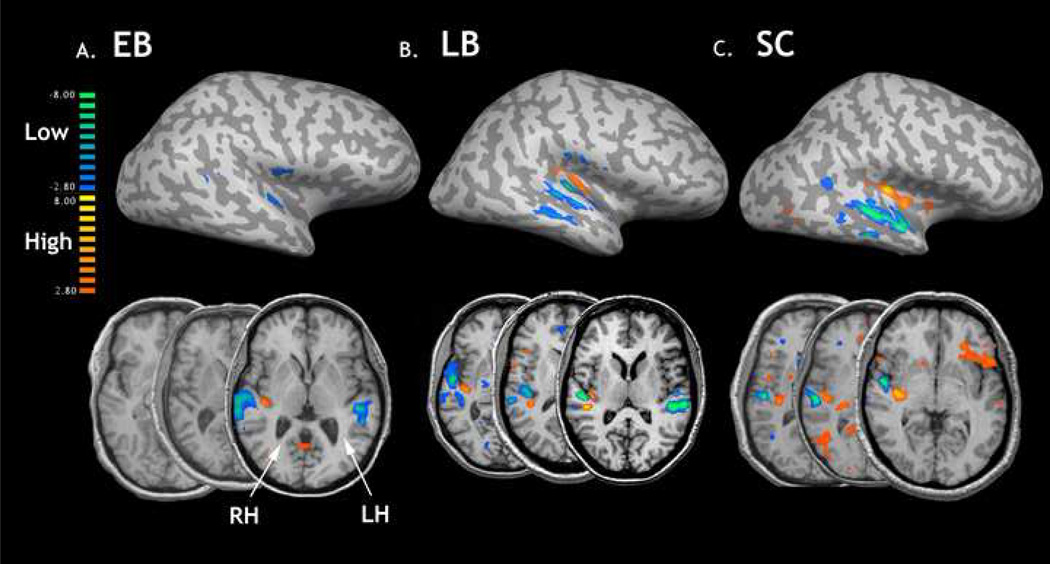
The contrast of high and low frequencies collapsed across PT and FM conditions showed regions that responded differentially to the 0.4 – 0.48 kHZ and 4.0 – 4.8 kHz tones. Despite previous reports of significant morphological heterogeneity throughout the core and belt regions of auditory cortex in sighted individuals [27, 34], we sought to establish averaged group maps of frequency-specific organization. This was done to investigate gross, tonotopic differences between groups. The random-effects model contrasting high and low frequencies revealed consistent patterns of activation throughout the superior, middle and transverse temporal cortices, with high frequency stimulation producing signal localized to the caudomedial extent of temporal lobe relative to the laterally localized low frequency response bands. This pattern was generally consistent in both SC and LB groups (Fig 1). However, there was an overall weaker response in the EB group throughout both hemispheres, with a net effect including a lack of significant high frequency activity under a corrected threshold of P < 0.005 (Fig 1a). Lowering the statistical threshold to a P < 0.2 did result in a gross group tonotopic pattern in the EB group within the left hemisphere (data not shown).
Figure 1.
BOLD responses from the high>low contrast in the blind and sighted listeners. The top row reveals the averaged group maps for all three groups generated using a high-low contrast, collapsing across both PT and FM conditions, displayed in the right hemisphere on an unfolded brain. The statistical threshold was set at P > 0.005, with a bonferroni correction for multiple comparisons. The orange and yellow activity represents regions responding to high tones while the blue and green activity are regions showing a greater response to low tonal stimuli. The bottom row shows three representative subjects from each group using a threshold of p > 0.05, uncorrected and shown in radiological convention.
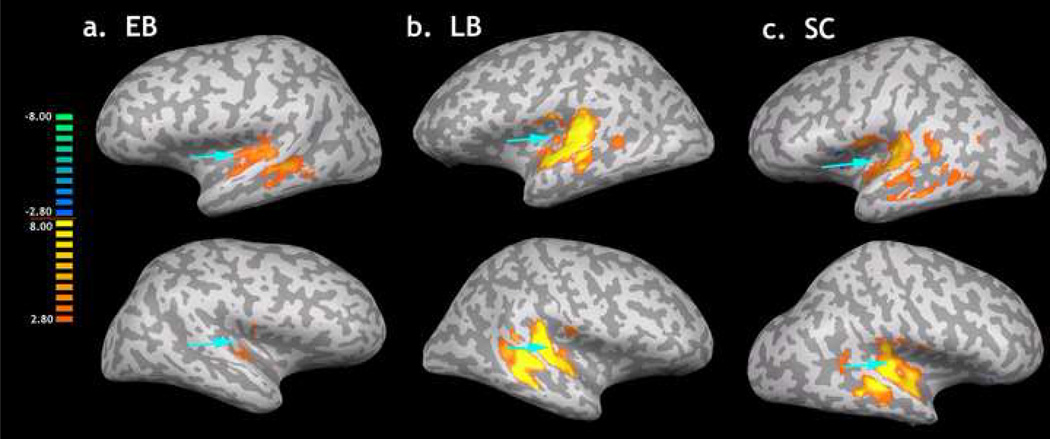
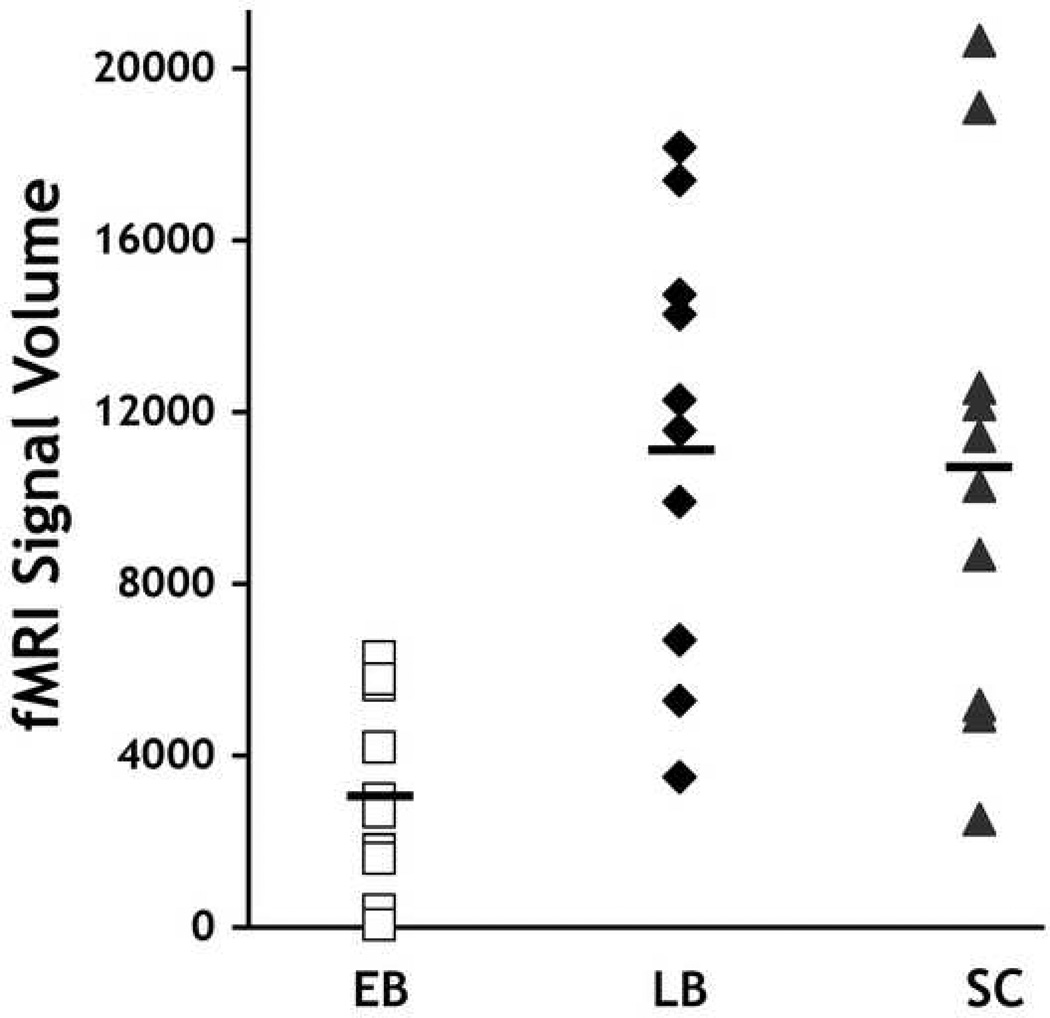
Collapsing across all tonal stimuli and comparing responses to silence (All-Base) resulted a similar discrepancy with EB appearing to have substantially less signal compared to the LB and SC groups. Figure 2 illustrates the extent of activity in and around auditory cortical fields for the all three groups produced by this contrast using a random effects model applied to each group (P < 0.005, corrected). To quantify this between-group discrepancy, we calculated the total number of significantly active voxels for each group. A repeated measures analysis of variance (ANOVA) with group as a between subjects factor and hemisphere as a within subjects effect revealed a reliable difference among groups in the number of active voxels in the temporal lobes (see Fig 3) [F(2,14) F = 10.613, P < 0.002], no reliable main effect of hemisphere [F(1,14) = 0.135, P = 0.719], and no reliable interaction between group and hemisphere: F(2,14) = 1.293, P = 0.305. Post Hoc contrasts using Tukey’s correction for multiple comparisons revealed that the EB groups had significantly fewer active voxels than either the LB (P = 0.001) or the SC group (P = 0.037). However the LB and SC groups did not differ from each other (P = 0.279). This indicates a substantial decrease in signal within auditory cortex in the EB subjects.
Figure 2.
The results of the All>Base contrast within auditory cortex in the left (top row) and right (bottom row) hemispheres of the A) EB, B) LB and C) SC subjects. Each brain (shown on an unfolded representative brain) reveals the areas within auditory cortex in which significant signal change (P < 0.005, corrected) was detected. Blue arrowheads point to the anatomical location of primary auditory cortex in each brain. The legend color bar shows the corresponding t-values.
Figure 3.
Total fMRI volume of All>Base contrast within auditory cortical regions across blind and sighted subjects. The total number of significantly active voxels within the temporal lobes at the 0.005 significance level are shown for each EB, LB and SC subject (one point for each hemisphere). Black bars represent group means.
Improved auditory abilities in the blind have been widely documented and the present findings indicate that it is likely that plastic effects also occur within auditory regions of the temporal lobe [8]. The current study is an initial step toward characterizing the effects of blindness in auditory cortex. We demonstrate a substantially less BOLD signal response to pure and frequency modulated tonal stimuli under low demand listening conditions in EB individuals compared to both LB and SC subjects, who did not differ from each other.
A rough high-to-low frequency gradient along the medial to lateral axis of the temporal gyri was observed in SC and LB groups and in the EB group at a lower statistical threshold, despite previously reported individual morphological variability [27] and the existence of multiple tonotopic fields [39, 34]. These data suggest that at a gross level, blindness, either early or acquired does not appear to alter the frequency mapping inherent to the core and possibly belt regions of the auditory cortex. However, these findings do not rule out alterations in tonotopy detectable at higher temporal resolution [8].
The reduced hemodynamic response to auditory stimulation in the EB group throughout the superior and middle temporal planes in both the high-low and the all-base contrasts may reflect differences in the EB subjects’ response to stimulation under low demand listening conditions. One hypothesis is that the decreased volume and intensity of signal detected in the EB group likely reflects greater processing efficiency within the first stages of auditory cortical analysis relative to late blind and sighted counterparts. This hypothesis is supported by electrophysiological studies revealing shorter latencies of early-evoked potentials (such as the Pa, Nb and N100) originating from electrodes placed over core and belt areas of auditory cortex in the EB [31, 18, 19, 20] and reduced metabolic responsiveness in a PET study of auditory localization [30]. Increased efficiency within temporal cortex during auditory perception appears to be a function of ‘loss-of-sight’ occurring before the closure of critical periods within development rather than a non-specific use-dependent mechanism. Our sample of LB individuals reported a mean duration of blindness of 26 years and an average age of blindness onset of 25 years (i.e. well after the development of full visual maturity – see table 1). The lack of statistical differences of volume and intensity of BOLD activity between LB and SC groups suggests that the loss of vision during critical developmental periods is necessary for the development of the decreased fMRI responses observed within auditory cortex. However, we acknowledge that the small sample size likely resulted in an under-powered analysis.
One possible explanation for this finding stems from differences in the neural systems involved in top-down attentional control in the EB. A series of recent reports have led to the hypothesis that occipital, ‘visual’ cortical regions in the EB contribute to and even expand top-down attentional networks [9, 37, 41 and 31] despite the findings of significant grey and white matter atrophy of various occipital loci [26] including regions along the medial occipital wall [21]. It has been proposed that the downstream consequence of this cross-modal plasticity is a more widespread preparatory attentional effect within auditory cortical regions [37]. The observed relative hypo-activity in EB stands in contrast to their greater event-related signal change in planum temporale when anticipating a complex auditory discrimination [37]. In that study subjects were presented with two auditory cues, one signaling a discrimination trial and another cue that signaled no-trial would occur. On a subset of trials where subjects received a cue to expect a discrimination trial, no stimuli were presented, providing a measure of preparatory activity. EB subjects showed highly robust responses relative to the no-trial condition [33]. Thus, under active listening conditions, EB subjects show more robust top-down modulation of auditory activity than SC individuals; an effect that likely arises through intact or maintained cortico-cortico connections between occipital and temporal lobes in the EB rather than degraded geniculocalcarine fibers [35, 36]. In this context, the current results provide a dissociation of BOLD activity within auditory cortex yielding the hypothesis that EB results in a more pronounced modulation of cortical activity as a function of expected discrimination demands relative to normal, sighted individuals. Here we show that the use of low demand listening conditions resulted in low demands on the perceptual processing of the stimuli. This adaptation would be particularly beneficial in situations when competing streams of sounds have to be segregated into relevant and meaningless categories without the aiding influence of visual cues.
The current results demonstrate that blindness early in life significantly alters BOLD responses within the first stages of cortical processing of the auditory pathway. Because auditory functions critically rely on the integrity of the temporal lobes [c.f. 44], auditory perceptual advantages that have been reported in the blind are most likely influenced by intramodal changes within this cortex. Given the overwhelming focus on the function of visual cortical regions in the blind human and animal literature, future studies are needed to determine if the current functional differences are rooted in anatomical, morphological or micro-structural alterations throughout auditory cortex. Additional studies will need to address the interplay between occipital and temporal cortex in the blind in order to delineate the contributions of various plastic changes on the auditory perceptual advantages that surface in blind individuals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Amedi A, et al. Early 'visual' cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci. 2003;6:758–766. doi: 10.1038/nn1072. [DOI] [PubMed] [Google Scholar]
- 2.Bavelier D, et al. Cross-modal plasticity: where and how? Nat Rev Neurosci. 2002;3:443–452. doi: 10.1038/nrn848. [DOI] [PubMed] [Google Scholar]
- 3.Berardi N, et al. Critical periods during sensory development. Curr Opin Neurobiol. 2000;10:138–145. doi: 10.1016/s0959-4388(99)00047-1. [DOI] [PubMed] [Google Scholar]
- 4.Burton H. Visual cortex activity in early and late blind people. J Neurosci. 2003;23:4005–4011. doi: 10.1523/JNEUROSCI.23-10-04005.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton H, et al. Visual cortex activation in late-onset, Braille naive blind individuals: an fMRI study during semantic and phonological tasks with heard words. Neurosci Lett. 2006;392:38–42. doi: 10.1016/j.neulet.2005.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen LG, et al. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- 7.Doucet ME, et al. Blind subjects process auditory spectral cues more efficiently than sighted individuals. Exp Brain Res. 2005;160:194–202. doi: 10.1007/s00221-004-2000-4. [DOI] [PubMed] [Google Scholar]
- 8.Elbert T, et al. Expansion of the tonotopic area in the auditory cortex of the blind. J Neurosci. 2002;22:9941–9944. doi: 10.1523/JNEUROSCI.22-22-09941.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg A, et al. Orienting auditory spatial attention engages frontal eye fields and medial occipital cortex in congenitally blind humans. Neuropsychologia. 2007;45:2307–2321. doi: 10.1016/j.neuropsychologia.2007.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gougoux F, et al. A functional neuroimaging study of sound localization: visual cortex activity predicts performance in early-blind individuals. PLoS Biol. 2005;3:e27. doi: 10.1371/journal.pbio.0030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall DA, et al. "Sparse" temporal sampling in auditory fMRI. Hum Brain Mapp. 1999;7:213–223. doi: 10.1002/(SICI)1097-0193(1999)7:3<213::AID-HBM5>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irvine DR. Auditory cortical plasticity: does it provide evidence for cognitive processing in the auditory cortex? Hear Res. 2007;229:158–170. doi: 10.1016/j.heares.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irvine DR, et al. Plasticity of spectral processing. Int Rev Neurobiol. 2005;70:435–472. doi: 10.1016/S0074-7742(05)70013-1. [DOI] [PubMed] [Google Scholar]
- 14.Keuroghlian AS, et al. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82:109–121. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Kilgard MP, et al. Plasticity of temporal information processing in the primary auditory cortex. Nat Neurosci. 1998;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kilgard MP, et al. Sensory input directs spatial and temporal plasticity in primary auditory cortex. J Neurophysiol. 2001;86:326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- 17.Lessard N, et al. Early-blind human subjects localize sound sources better than sighted subjects. Nature. 1998;395:278–280. doi: 10.1038/26228. [DOI] [PubMed] [Google Scholar]
- 18.Manjunath NK, et al. Shorter latencies of components of middle latency auditory evoked potentials in congenitally blind compared to normal sighted subjects. Int J Neurosci. 1998;95:173–181. doi: 10.3109/00207459809003339. [DOI] [PubMed] [Google Scholar]
- 19.Naveen KV, et al. Differences between congenitally blind and normally sighted subjects in the P1 component of middle latency auditory evoked potentials. Percept Mot Skills. 1998;86:1192–1194. doi: 10.2466/pms.1998.86.3c.1192. [DOI] [PubMed] [Google Scholar]
- 20.Naveen KV, et al. Middle latency auditory evoked potentials in congenitally blind and normal sighted subjects. Int J Neurosci. 1997;90:105–111. doi: 10.3109/00207459709000630. [DOI] [PubMed] [Google Scholar]
- 21.Pan WJ, et al. Progressive atrophy in the optic pathway and visual cortex of early blind Chinese adults: A voxel-based morphometry magnetic resonance imaging study. Neuroimage. 2007;37:212–220. doi: 10.1016/j.neuroimage.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 22.Pascual-Leone A, et al. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 23.Piche M, et al. Auditory responses in the visual cortex of neonatally enucleated rats. Neuroscience. 2007;145:1144–1156. doi: 10.1016/j.neuroscience.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 24.Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polley DB, et al. Perceptual learning directs auditory cortical map reorganization through top-down influences. J Neurosci. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ptito M, et al. Alterations of the visual pathways in congenital blindness. Exp Brain Res. 2008;187:41–49. doi: 10.1007/s00221-008-1273-4. [DOI] [PubMed] [Google Scholar]
- 27.Rademacher J, et al. Probabilistic mapping and volume measurement of human primary auditory cortex. Neuroimage. 2001;13:669–683. doi: 10.1006/nimg.2000.0714. [DOI] [PubMed] [Google Scholar]
- 28.Rauschecker JP. Cortical map plasticity in animals and humans. Prog Brain Res. 2002;138:73–88. doi: 10.1016/S0079-6123(02)38072-5. [DOI] [PubMed] [Google Scholar]
- 29.Raz N, et al. V1 activation in congenitally blind humans is associated with episodic retrieval. Cereb Cortex. 2005;15:1459–1468. doi: 10.1093/cercor/bhi026. [DOI] [PubMed] [Google Scholar]
- 30.Recanzone GH, et al. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roder B, et al. Event-related potentials during auditory and somatosensory discrimination in sighted and blind human subjects. Brain Res Cogn Brain Res. 1996;4:77–93. [PubMed] [Google Scholar]
- 32.Roder B, et al. Speech processing activates visual cortex in congenitally blind humans. Eur J Neurosci. 2002;16:930–936. doi: 10.1046/j.1460-9568.2002.02147.x. [DOI] [PubMed] [Google Scholar]
- 33.Sadato N, et al. Critical period for cross-modal plasticity in blind humans: a functional MRI study. Neuroimage. 2002;16:389–400. doi: 10.1006/nimg.2002.1111. [DOI] [PubMed] [Google Scholar]
- 34.Schonwiesner M, et al. Is it tonotopy after all? Neuroimage. 2002;17:1144–1161. doi: 10.1006/nimg.2002.1250. [DOI] [PubMed] [Google Scholar]
- 35.Shimony JS, et al. Diffusion tensor imaging reveals white matter reorganization in early blind humans. Cereb Cortex. 2006;16:1653–1661. doi: 10.1093/cercor/bhj102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shu N, et al. Abnormal diffusion of cerebral white matter in early blindness. Hum Brain Mapp. 2008 doi: 10.1002/hbm.20507. http://dx.doi.org/10.1002/hbm.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens AA, et al. Preparatory activity in occipital cortex in early blind humans predicts auditory perceptual performance. J Neurosci. 2007;27:10734–10741. doi: 10.1523/JNEUROSCI.1669-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stevens AA, et al. Auditory perceptual consolidation in early-onset blindness. Neuropsychologia. 2005;43:1901–1910. doi: 10.1016/j.neuropsychologia.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Talavage TM, et al. Frequency-dependent responses exhibited by multiple regions in human auditory cortex. Hear Res. 2000;150:225–244. doi: 10.1016/s0378-5955(00)00203-3. [DOI] [PubMed] [Google Scholar]
- 40.Voss P, et al. A positron emission tomography study during auditory localization by late-onset blind individuals. Neuroreport. 2006;17:383–388. doi: 10.1097/01.wnr.0000204983.21748.2d. [DOI] [PubMed] [Google Scholar]
- 41.Weaver KE, et al. Attention and sensory interactions within the occipital cortex in the early blind: an fMRI study. J Cogn Neurosci. 2007;19:315–330. doi: 10.1162/jocn.2007.19.2.315. [DOI] [PubMed] [Google Scholar]
- 42.Weeks R, et al. A positron emission tomographic study of auditory localization in the congenitally blind. J Neurosci. 2000;20:2664–2672. doi: 10.1523/JNEUROSCI.20-07-02664.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weinberger NM. Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci. 2004;5:279–290. doi: 10.1038/nrn1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zatorre RJ, et al. Spatial localization after excision of human auditory cortex. J Neurosci. 2001;21:6321–6328. doi: 10.1523/JNEUROSCI.21-16-06321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]