The National Heart, Lung, and Blood Institute (NHLBI) convened a working group (WG) to develop a research agenda to enhance understanding and effectiveness of antithrombotic therapy. The WG brought together cardiologists, hematologists, interventionalists, clinical trialists, genetic epidemiologists, basic scientists, and other stakeholders to review a) coagulation, platelet activation and aggregation, and antithrombotic therapy; b) issues surrounding antithrombotic therapy failure – how to define it, how to predict and diagnose it, available tests and how to optimize them; c) the factors that affect the efficacy, safety, and predictability of antithrombotic therapies; d) how to optimize antithrombotic therapy, improve upon present interventions, and individually tailor therapy to increase efficacy and safety and to avoid failure; and e) the clinical applicability and cost-effectiveness of individually tailored antithrombotic therapy based on functional and genetic testing. The WG characterized and discussed challenges for guided antithrombotic therapy in four domains: therapeutic strategies, antithrombotic metrics, pharmacology and pharmacogenetics, and stakeholders’ roles. Overall, the WG identified and prioritized the most pressing clinical needs to focus future research and translational efforts. This report presents highlights of these reviews and a summary of suggested research directions.
I. GAPS OF KNOWLEDGE IN CLINICAL THROMBOSIS
There has been tremendous progress in the field of thrombosis in the past two decades.1-5 The ramifications on cardiovascular care have been profound. A greater appreciation of the central role of platelets in atherothrombosis and an increased understanding of the receptors involved in platelet activation and aggregation have led to pivotal randomized controlled trials (RCTs) of novel agents.6 Many of these agents have been associated with substantial reductions in adverse cardiovascular outcomes. Simultaneously, an appreciation of the complexity of the coagulation cascade and the artificiality of separating it from cellular and platelet interactions has promoted a deeper understanding of thrombosis, and consequently, identification of pharmacological targets to prevent thrombosis.7, 8
Excessive thrombosis is highly relevant to a variety of disease states. Atrial fibrillation, many forms of stroke, acute and chronic coronary artery disease (CAD), prosthetic heart valves, and venous thromboembolism are all large areas of cardiovascular medicine in which thrombosis is a major part of the pathology. In each of these areas, recent data have expanded our knowledge and clinical armamentarium, with more options available to clinicians than ever before. Nevertheless, large gaps in our knowledge persist in each of these areas.
1. Atrial Fibrillation
Atrial fibrillation is an important risk factor for stroke and is common in the elderly. Its prevalence in the United States is projected to exceed 5.5 million individuals (~1-2% of the population) by the year 2050.9 The 2011 American College of Cardiology Foundation (ACCF)/American Heart Association (AHA)/Heart Rhythm Society (HRS) Guidelines for the management of patients with atrial fibrillation recommend that the selection of the appropriate antithrombotic agent (including aspirin monotherapy) should be based upon the absolute risks of stroke and bleeding and the balance of risk and benefit for a given patient.10 Defining these parameters is therefore critical in optimizing outcomes for atrial fibrillation patients.
Currently, recommended therapy is aspirin in patients without risk factors for stroke; aspirin or warfarin in patients with one moderate risk factor; and warfarin in patients with one high risk factor or more than one moderate risk factor.11 Alternative prognostic models for thromboembolic risk include the CHADS2 (Cardiac failure, Hypertension, Age, Diabetes, Stroke [Doubled]) and CHA2DS2VASc risk scores.12, 13 Although CHA2DS2VASc may identify more patients who may benefit from anticoagulation,13 even better risk stratification among low-risk patients would provide clinical value, especially in the setting of newer anticoagulants that are associated with less major or intracranial bleeding compared with warfarin.14, 15 The validation in larger populations of the incremental utility of imaging data that are specific for thrombosis within the left atrium, such as atrial size, blood stasis, and appendage velocities, would be particularly useful. Development of better risk models and risk scores for bleeding on anticoagulant therapy, particularly the new oral anticoagulants, is also critical.16, 17
Warfarin therapy has several limitations, including a narrow therapeutic window; a wide variation in metabolism and numerous food and drug interactions; a requirement for regular laboratory monitoring and dose adjustment; and slow onset and offset of pharmacodynamic effects. Aspirin is less effective than warfarin for nonvalvular atrial fibrillation and is only recommended in patients with low risk for thromboembolism or contraindications for warfarin. Dual antiplatelet therapy with aspirin and clopidogrel is also inferior to warfarin in the setting of atrial fibrillation, though it is superior to aspirin alone.18, 19
The safety and efficacy of several new oral anticoagulants compared with warfarin have been examined in RCTs of non-valvular atrial fibrillation. In the open-label Randomized Evaluation of Long-term Anticoagulant Therapy (RE-LY) trial, the direct thrombin inhibitor dabigatran 150 mg twice daily significantly reduced the risk of stroke or systemic embolism with a similar risk of major bleeding as warfarin.20 A dose of 110 mg twice daily was also tested in RE-LY but is not approved in the US. Since dabigatran is cleared primarily by the kidneys, patients with chronic kidney disease may be at increased risk for bleeding. Based on pharmacokinetic data, the FDA approved a dose of 75 mg daily in patients with diminished renal function, though there are no large outcome data with this dose. Future research will need to determine the wisdom of approving doses on pharmacokinetic grounds as opposed to large clinical trial evaluation, especially in drugs that need to balance ischemic and thrombotic reduction with bleeding concerns.
Rivaroxaban and apixaban are oral Factor Xa inhibitors. In the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET-AF) trial, rivaroxaban 20 mg daily was non-inferior to warfarin according to the primary intent-to-treat analysis for the prevention of stroke or systemic embolism with similar rates of bleeding and superior to warfarin according to the per-protocol, as-treated analysis,14 while in the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial, apixaban 5 mg twice daily was superior to warfarin in preventing stroke or systemic embolism with less major bleeding.15 These randomized trials differed significantly in their designs, the baseline stroke risk in the populations studied (e.g., CHADS2 scores), and time within therapeutic range on warfarin. A comparative trial of the safety and efficacy of direct thrombin (Factor IIa) inhibitors versus Factor Xa inhibitors is needed. Exploratory pharmacogenomic analyses are required to identify more precisely patients in whom a particular agent would provide greatest benefit. The conclusions about dabigatran, rivaroxaban, and apixaban were derived from RCTs. Clinical trials and/or registry results in “all-comer” populations will be needed to prove superiority, non-inferiority, or inferiority to warfarin in actual clinical practice. Furthermore, “real-world” registries are required to assess bridging and reversal strategies for patients with different degrees of thromboembolic risk who need to interrupt therapy with one of the newer anticoagulants for planned or emergent procedures.21
Although the direct thrombin and Factor Xa inhibitors possess several advantages, the rates of major bleeding with these agents are not insignificant, particularly when considering that the duration of treatment is indefinite. In the ARISTOTLE trial, the rate of major bleeding according to the International Society of Thrombosis and Haemostasis criteria in patients randomly assigned to apixaban was 2.13% per year, and the rate of any bleeding event was 18.1% per year.15 Mechanical approaches to stroke prevention such as transcatheter left atrial appendage (LAA) occlusion may reduce the risk of stroke without exposing the patient to the long-term bleeding hazard of anticoagulation. In a randomized clinical trial involving 707 patients and 1065 patient-years of follow-up, LAA occlusion with the WATCHMAN device was non-inferior to warfarin for the prevention of stroke, systemic embolism, or cardiovascular death, at the cost of more frequent procedural complications,22 although procedural safety has improved with increased operator experience.23 The safety and efficacy of this strategy is being explored further in the Prospective Randomized Evaluation of the WATCHMAN LAA Closure Device In Patients with Atrial Fibrillation Versus Long Term Warfarin Therapy (PREVAIL) trial. Rigorous, prospective, and preferably randomized data compared with anticoagulant therapy are required to determine the relative safety and efficacy of other mechanical approaches to stroke prevention, such as surgical exclusion of the LAA and catheter-based LAA ligation. Furthermore, studies that compare clinical outcomes between LAA occlusion and newer anticoagulants would help ascertain the appropriate role of a mechanical approach within the current paradigm of pharmacological therapies. Comparative trials of different approaches to LAA occlusion would also be useful, as well as further research into the appropriate target populations, such as patients with atrial fibrillation and stroke while therapeutic on anticoagulation.
Another mechanical approach that may decrease thromboembolic consequences of atrial fibrillation is catheter ablation. While primarily used to treat patients with symptoms due to atrial fibrillation that are refractory to medical therapy, there may be an additional role in preventing stroke and systemic embolism, though this hypothesis remains controversial and unproven. Preliminary data appear mixed.24, 25 However, further randomized data are needed to determine any impact of catheter ablation on subsequent stroke risk and the durability of any effect.26
Coronary artery disease is common in patients with atrial fibrillation. Treatment of patients with atrial fibrillation who require dual antiplatelet therapy (DAPT) after acute coronary syndrome and/or percutaneous coronary intervention (PCI) is a common clinical dilemma. Triple therapy after PCI with warfarin and DAPT is associated with a substantial risk of bleeding; in ten observational studies involving 1349 patients on triple therapy, the pooled rate of major bleeding at 30 days was 2.2% (95% confidence interval, 0.7-3.7%).27 Current treatment recommendations are based on expert consensus and follow the general principle of considering the anticipated risk of bleeding and the risk of stent thrombosis for a particular individual.8 One current approach in patients with atrial fibrillation and a stent is to give one to three months of triple therapy, followed by an anticoagulant plus an antiplatelet agent. Delineation of the appropriate role, timing, and duration of triple therapy in these patients is required, particularly with the Factor Xa and direct thrombin inhibitors, for which clinical experience is limited. Furthermore, which patients ought to be on dual therapy with one antiplatelet agent and one anticoagulant or those who should only be on anticoagulation 12 months after a stent are all areas that require further research.
Gaps of knowledge in Atrial Fibrillation and Stroke
From the above review of data, it is quite clear that there have been significant advances in anticoagulation for atrial fibrillation. The major gaps that were identified are as follows:
The need for a comparative trial of oral direct thrombin inhibitors versus Factor Xa inhibitors, as well as a comparative trial of alternative Factor Xa inhibitors, including comparison of once daily versus twice daily regimens.
Strategies to improve adherence, which become even more important with therapies that do not require INR monitoring.
Study of mechanical versus pharmacological approaches to thrombus prevention in atrial fibrillation, as well as maintenance of sinus rhythm as a potential antithrombotic strategy, including with catheter ablation.
Better risk stratification at the lower end of the thromboembolic risk spectrum and potential incorporation of imaging modalities such as echocardiography and magnetic resonance imaging to risk stratification algorithms.
A registry of bridging strategies.
Development of novel oral anticoagulants that are not cleared by the kidneys and are safe during pregnancy.
Delineation of the role of triple antithrombotic therapy after stenting and/or acute coronary syndromes.
Development of a single point-of-care assay for antiplatelet drugs and anticoagulant agents that provides a global measure of thrombotic risk.
Study of atrial fibrillation in the setting of valvular heart disease
2. Non-Cardioembolic Ischemic Stroke
Carotid artery and brain imaging are performed using duplex ultrasonography, computed tomography (CT) angiography, magnetic resonance (MR) angiography, or catheter-based contrast angiography. While CT and MR imaging for screening patients at increased risk for ischemic stroke is becoming more sophisticated, the predictive value and cost effectiveness will need to be proven to move them from research tools to routine clinical practice.
Aspirin is recommended to prevent thromboembolic events in patients with carotid artery disease, whereas warfarin is recommended in patients with mechanical heart valves or atrial fibrillation.28, 29, 30 Optimal medical therapy also includes ACE inhibitors, beta blockers, statins, and control of risk factors to target levels. Carotid surgery and stenting increase blood flow to the brain and change the atherosclerotic substrate. Whereas there has been great interest in comparing carotid endarterectomy and stenting in asymptomatic patients, neither has been compared against optimal medical therapy in the contemporary era. Given that a significant proportion of revascularization procedures are performed in asymptomatic patients, the added benefit of carotid revascularization compared with optimal medical therapy alone needs to be shown to justify continuing this widespread practice.
Major efforts have been made to increase the public awareness of the signs and symptoms of stroke. More success is needed in promoting the importance of calling 911 to activate emergency medical services shortly after symptom onset and improving the emergency response system. Developing integrated regional stroke networks that will decrease barriers to rapid treatment is necessary to decrease the fragmentation of stroke care that currently exists in many communities. Implementing prehospital, emergency department and interhospital transfer acute stroke protocols is critically important. Improving time-to-treatment will require the same effort and outcomes research that has been pursued in ST-elevation myocardial infarction (STEMI).31 The ability to develop an imaging tool that could be used in the ambulance to make the diagnosis of stroke and facilitate prehospital activation of the Stroke Team would be a major advance and might allow the investigation of prehospital initiation of fibrinolytic therapy.
Stroke centers have the capability of rapidly performing CT or MR brain imaging and initiating intravenous fibrinolytic therapy in patients who meet treatment criteria. It is possible that bolus intravenous therapy with reteplase or tenecteplase has advantages over alteplase, particularly if prehospital therapy becomes possible. More research is needed comparing intravenous fibrinolytic therapy with selective intra-arterial fibrinolytic therapy. Selective catheter-based reperfusion is another rich topic for investigation.
In patients with a history of non-cardioembolic ischemic stroke, antiplatelet drugs are recommended for the prevention of recurrent ischemic stroke. Aspirin monotherapy, clopidogrel monotherapy, and the combination of aspirin and dipyridamole are recommended options for initial therapy.29 Although combining aspirin and clopidogrel is contraindicated for routine secondary prevention of stroke, limited duration combination therapy is indicated in patients with recent acute coronary syndrome and/or vascular stenting.
Prasugrel is contraindicated in patients with a history of stroke or transient ischemic attack (TIA), and ticagrelor is contraindicated in patients with a history of intracerebral hemorrhage. However, intracranial bleeding risk might be lower for patients with a history of TIA compared with stroke. It is possible that prasugrel or ticagrelor monotherapy could be superior agents for ischemic stroke prevention in patients with a history of TIA. Determining whether there are differences in response to antithrombotic therapy between TIA and stroke could increase treatment options for TIA. Dabigatran, rivaroxaban, and apixaban are anticoagulant options that also deserve evaluation. There have been no trials of treatment options in patients who have recurrent events on antithrombotic therapy. Options include adding or switching agents.
More information is needed to prove that aspirin actually prevents ischemic stroke in primary prevention. It is not known whether prasugrel or ticagrelor are superior to aspirin or clopidogrel monotherapy for primary prevention of ischemic stroke. Nor is it known whether aspirin 81 mg daily is as effective as or safer than aspirin 325 mg daily for cerebrovascular disease.
Gaps of knowledge in Ischemic Stroke
Ischemic stroke is an area that is ripe for improvement. Critical areas of research were identified:
A randomized controlled trial of optimal medical therapy alone versus revascularization for asymptomatic carotid artery stenosis, potentially incorporating better brain and/or carotid imaging for risk stratification.
Differentiate etiology of stroke and TIA more precisely, as well as antithrombotic management, respectively.
Expand the role of regional pre-hospital diagnosis/treatment of ischemic stroke.
Evaluate the potential role of stroke imaging in the ambulance.
Catheter-directed therapy trial for ischemic stroke, as has been done with STEMI.
Determination of what to do with patients who experience recurrent events on treatment; should one add or switch anti-thrombotic agents?
3. Acute & Chronic CAD-“Smarter” Anti-Thrombotics
Hemostasis and Thrombosis
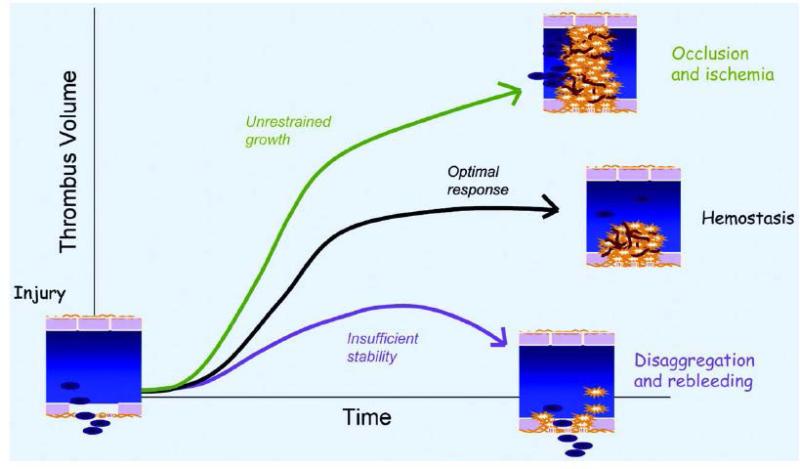
Platelets are critical in the initiation, progression, and thrombotic complications of atherosclerosis.32, 33, 34 While new antiplatelet agents (e.g., prasugrel, ticagrelor) have been developed to further reduce ischemic events when added to aspirin therapy, these therapeutic advances are associated with an increase in severe bleeding.35, 36, 37 Given the association between bleeding and increased mortality,38 balancing the efficacy and safety of new antiplatelet agents and multi-drug antithrombotic regimens has taken on even greater importance. It is in this context that there is great interest in emerging experimental data from animal models raising the intriguing possibility that physiologic hemostasis and pathologic thrombosis might represent two mechanistically different processes (Figure 1),39 thereby permitting the targeting of thrombosis, but not hemostasis (i.e., decreased bleeding risk). Sachs and Nieswandt have cataloged 34 genetically modified mouse strains studied in vivo for thrombus formation (typically ferric chloride or photochemical carotid injury or laser-induced injury of the cremaster microvasculature) and hemostasis (i.e., tail bleeding time).40 These mouse strains broadly cover the complex interplay involving a large number of platelet surface receptors, signaling pathways, and enzymatic cascades that mediate platelet tethering and adhesion, platelet activation, and platelet aggregation and thrombus propagation. Interestingly, only 6 out of 34 targets—namely, platelet P2X1, CD150, Gas6, CD40L, and coagulation factors XI and XII—have been reported to regulate thrombosis, but not hemostasis.
Figure 1.
Hemostatic and thrombotic responses. Platelet and fibrin accumulation after vascular injury is intended to limit further blood loss without compounding the original injury by occluding blood flow. In this context, the optimal hemostatic response is one that is large enough to be stable, but not so large that it occludes the vascular lumen, producing downstream ischemia and further tissue damage (from Brass LF, Zhu L, Stalker TJ. Translational therapeutics at the platelet vascular interface: A CME-certified activity. Novel therapeutic targets at the platelet vascular interface. Arterioscler Thromb Vasc Biol 2008; 28:s43-s50; reprinted with permission).39
Inflammation and Thrombosis
New lines of research suggest that thrombosis and hemorrhage may also be uncoupled at the interface of pathways controlling thrombosis and inflammation. Leukocyte-platelet interactions induce bi-directional signals that amplify pro-inflammatory and pro-thrombotic cellular responses.41 One remarkable example is spleen tyrosine kinase (Syk), a 72 kDa signaling protein with kinase and scaffolding activities. Several in vitro lines of evidence suggest that inflammatory and thrombotic signaling pathways converge on Syk. In platelets, phosphorylation of Syk has been reported following activation by multiple receptors.42, 43 Syk is also a mediator of signaling events induced by high shear stress.42, 44, 45, 46, 47, 48 In leukocytes, Syk promotes the recruitment of these cells to both inflamed and injured blood vessels.49, 50 Recent studies using a novel and highly selective pharmacologic inhibitor of Syk (PRT060318; 2-((1R,2S)-2-aminocyclohexylamino)-4-(m-tolylamino)pyrimidine-5-carboxamide), coupled with genetic experiments, demonstrate that Syk inhibition ameliorates both the acute and chronic responses to vascular injury without affecting hemostasis.51 PRT060318 strongly inhibited arterial thrombosis in vivo in multiple animal species while having minimal impact on bleeding. Furthermore, leukocyte-platelet dependent responses to vascular injury were markedly inhibited by PRT060318. Dose-response studies will be required to establish whether a strategy aimed at targeting the kinase activity of Syk in a chronic setting is feasible and safe. The therapeutic potential of Syk may exemplify a new class of anti-atherothrombotic agents that target the interface between thrombosis and inflammation.
Gaps of knowledge in Acute & Chronic CAD - Antithrombotic
The knowledge base in acute and chronic CAD has grown exponentially. Remaining potentially high yield targets for research include:
Identification of molecular targets that dissociate bleeding and thrombosis.
Development of a polypill for secondary prevention, which may ensure better adherence (although at the risk of greater non-adherence to all included medications).
Evaluating different dosing strategies of reversible agents to prevent myocardial infarction during non-cardiac surgery.
Personalized care with genetic and/or point-of-care assays.
Development of better animal models of bleeding and thrombosis that are organ-specific (brain, heart, gastrointestinal, etc.).
Development of better non-animal models for thrombosis.
4. Prosthetic Heart Valves and Thromboembolism
Consensus guidelines recommend the addition of aspirin 75 to 100 mg once daily to vitamin K antagonists (VKA) for all patients with mechanical heart valves and those patients with biological valves who have risk factors for thromboembolism.52 Clinical evidence in support of this recommendation is provided by a review of 11 RCTs involving 2,428 patients.53 The combination of aspirin plus a VKA resulted in a 42% relative risk reduction in thromboembolic events. Beneficial effects were offset by an increase in major bleeding (odds ratio of 1.66), an apparent dose-dependent effect seen with aspirin that has led to the recommendation for the use of low dose aspirin plus a VKA.
The beneficial effects associated with the combination of aspirin plus a VKA for the prevention of thromboembolic events identifies an important gap in our knowledge. Tests have been developed and implemented to quantify effects of anticoagulants and antiplatelet agents, yet no single test has been developed to provide a global assessment of anti-thrombotic effects. Such a test may be useful to identify patients at greater risk of thromboembolic events and, conversely, those patients at greater risk of bleeding complications. The availability of such a test would be particularly useful to guide therapy in patients who require interruption in their anti-thrombotic therapy and in patients who are likely to have different anti-thrombotic requirements.
Pregnancy in association with a prosthetic heart valve entails substantial risk to both the mother and fetus.54 Our gap in knowledge is perhaps greatest in this situation. It has long been believed that both warfarin and heparin pose risks to the mother and fetus.55 More recent data suggest that the risk of embryopathy with warfarin, especially at doses of 5 mg daily or less, may have been overestimated.56 Changes in volume status and the apparent volume of distribution of the VKA, combined with changes in the synthesis of coagulation factors, add to the complexity and mandate regular testing. A global assessment of anti-thrombotic effects would be particularly useful to develop effective anti-thrombotic strategies in pregnant women who have prosthetic heart valves. More data are needed as to the optimal anticoagulation approach for the duration of pregnancy.
New direct-acting anticoagulants inhibit coagulation Factor Xa or thrombin (Factor IIa). The more consistent anti-thrombotic effects of these agents may be useful in patients with prosthetic heart valves. The dire consequences of valve thrombosis present serious challenges to investigating new regimens; however, preliminary studies in vitro suggest that dabigatran may prevent valve thrombosis as effectively as heparin.57 Once again, a global assessment of anti-thrombotic effects that is sensitive to the effects of all anticoagulants would aid in development. Ideally, comparative testing of different direct-acting anticoagulants would indicate whether inhibiting thrombin or Factor Xa is preferable for preventing thromboembolic events.
Patients with prosthetic valves require periodic interruption of anti-thrombotic therapy. By limiting the duration during which the patient is not anticoagulated, the thromboembolic risk is reduced. The rapid onset of action of direct-acting anticoagulants shortens the interval during which the patient is not anticoagulated. The availability of an agent that could reverse the drug's effect could also shorten this interval. In addition, such an agent would be useful when spontaneous major bleeding occurs.
The risk of thromboembolism is influenced not only by valve type (mechanical or bioprosthetic) but also by associated conditions such as atrial fibrillation and systolic heart failure. The development of new anticoagulants, new antiplatelet agents, and new methods to implant aortic valves (transcatheter replacement) has created important gaps in our knowledge regarding the most effective anti-thrombotic therapy. Additional clinical trials are needed. The success of these trials would be enhanced by the availability of an accurate and precise measure of global anti-thrombotic effects.
Gaps of knowledge in Prosthetic Heart Valves and Thromboembolism
Progress in anti-thrombotic therapy for prosthetic valvular heart disease has been relatively slow, in part because of the dire consequences of valve thrombosis. Important areas to address are:
Anticoagulation bridging for invasive procedures: who, when, how?
Role of new direct thrombin and Factor Xa inhibitors in pregnancy.
Role of acute and chronic antithrombotic therapy in transcatheter valve replacement.
Testing direct thrombin and Factor Xa inhibitors for artificial valves and a comparative trial of direct thrombin and Factor Xa inhibitors.
Antidotes for direct thrombin and Factor Xa inhibitors.
5. Venous Thromboembolism
There has been considerable progress in the prevention, diagnosis, and treatment of venous thromboembolism (VTE) in recent years. The risk factors for VTE have been better defined, and the armamentarium of drugs for the prevention and treatment of VTE has expanded with the introduction of new oral anticoagulants that target Factor Xa or thrombin. Nonetheless, several challenges remain.58
First, anticoagulants have long been the mainstay for primary and secondary prevention of VTE. However, there has been resurgence in the use of antiplatelet drugs for this indication; many orthopedic surgeons routinely use aspirin in place of anticoagulants for thromboprophylaxis after hip or knee replacement surgery,59 and a recent study demonstrated a 40% reduction in recurrent VTE when aspirin was compared with placebo for secondary prevention in patients with unprovoked VTE who had been treated with a 6-month course of conventional anticoagulation.60 Aspirin is easier to administer than warfarin or injectable anticoagulants. Building on these findings, there is a need for studies to compare aspirin with the new oral anticoagulants for primary and secondary VTE prevention.
Second, while it is clear that patients with VTE in the setting of a transient risk factor, such as surgery or trauma, have a low risk of recurrent VTE after a 3-month course of anticoagulant therapy, this is not the case with unprovoked VTE. Unprovoked VTE is a chronic disease; the risk of recurrence when anticoagulant therapy is stopped in such patients is about 10% at 1 year and 30% at 5 years even after a 2-year course of anticoagulation.61 Because of this high risk of recurrence, some experts recommend long-term anticoagulation, which is not only inconvenient for patients and costly for the healthcare system but also places patients at risk for bleeding. These concerns identify several unmet needs. These include (a) the need for a global test or testing strategy that identifies patients at high risk for recurrent VTE so that these patients can be targeted for extended anticoagulant therapy, (b) trials comparing anticoagulants (including the new anticoagulants) with aspirin for secondary prevention, and (c) trials to determine whether lower doses of the newer anticoagulants will have similar efficacy, but better safety, than full doses for secondary VTE prevention.
Third, special populations of patients with VTE continue to pose challenges. These include pregnant women and patients with cancer. There are no licensed oral anticoagulants for prevention or treatment of VTE in pregnancy.62 Based on preclinical assessments in animals, at least two of the new oral anticoagulants appear to be safe in pregnancy (dabigatran and apixaban); clinical studies are needed to evaluate this possibility. Patients with VTE in the setting of cancer are particularly difficult to manage and are often given extended treatment with low-molecular-weight heparin (LMWH), which necessitates a daily subcutaneous injection. Studies are needed to determine whether the new oral anticoagulants are as effective and safe as LMWH in this setting. Finally, certain subsets of patients receiving systemic combination chemotherapy for treatment of cancer are at high risk for VTE. Preliminary studies indicate that prophylaxis with injectable anticoagulants lowers this risk.63,64 Better risk stratification schemes are needed to identify high-risk patients.65 In addition, to streamline thromboprophylaxis in this vulnerable patient population, the new oral anticoagulants need to be compared with injectable agents.66
Fourth, advances in our understanding of the molecular mechanisms of coagulation have enabled the identification of thrombophilic conditions that predispose patients to VTE. Although one or more of these abnormalities can be identified in 40% to 50% of patients with hereditable forms of VTE, at least half of the patients with VTE do not have recognized abnormalities, and many patients with thrombophilia do not suffer VTE. Genome-wide association studies (GWAS) have failed to identify new genetic defects in VTE.67 Therefore, we need to utilize other approaches to identify novel genetic factors leading to thrombophilia, such as whole genome analysis and deep sequencing. In addition, more information is required about the influence of environmental factors and/or modifier genes on the phenotype in these patients.
Finally, there is increased emphasis on personalized medicine. Point-of-care genotyping can identify common polymorphisms that influence warfarin metabolism and average dose requirements.68 Incorporation of this information into warfarin-dosing algorithms may streamline achievement of therapeutic levels of anticoagulation.69 Studies are ongoing to determine whether genotyping improves patient outcome. With the move to new oral anticoagulants, there is a need for additional genetic studies to determine whether a patient-centered approach can be used to optimize the efficacy and safety of these agents.
Gaps of knowledge in Venous Thromboembolism
The main challenges in VTE are:
Definition of the role of aspirin and/or other antiplatelet agents in primary and secondary prevention in at-risk patients.
Prediction of recurrent thrombosis and determination of optimal duration of antithrombotic therapy.
Management of special patient populations, including pregnant women, patients with VTE in the setting of cancer, and patients with thrombophilia.
Genetic and functional point-of-care testing to identify risk and personalize treatment of VTE.
Prioritizing Future Directions in Clinical Thrombosis
As discussed above, there are several knowledge gaps in thrombosis as it pertains to cardiovascular medicine. Atrial fibrillation, stroke, acute and chronic CAD, prosthetic heart valves, and VTE all have important knowledge deficits and unmet clinical needs. The highest priority studies in our opinion are summarized below:
Comparative trials of evolved oral direct thrombin inhibitors and Factor Xa inhibitors in patients with atrial fibrillation.
Mechanical versus pharmacological approaches for stroke reduction and maintenance of sinus rhythm in atrial fibrillation.
Optimal medical therapy versus revascularization for asymptomatic carotid stenosis, with consideration of better brain and/or carotid imaging for risk stratification.
Catheter directed therapy trial for stroke (as has been done with STEMI).
Identification of molecular targets that dissociate thrombosis from bleeding, to the extent possible.
Anticoagulation bridging for invasive procedures: who, when, how?
Predictors of recurrent VTE and determination of appropriate length of therapy.
As is apparent, these studies would encompass RCTs, translational work, and basic investigation. The knowledge generated would have an enormous public health impact by addressing fundamental unresolved clinical issues for some of the most prevalent cardiovascular conditions worldwide.
II. PERSONALIZED ANTITHROMBOTIC THERAPY
Warfarin therapy has long been guided by a functional assay, the international normalized ratio (INR) of the prothrombin time. Although there has been interest in the use of genetic assays (VKORC1 and CYP2C9) to guide warfarin dosing 70 (see section on pharmacogenetics), the field appears to be moving in a different direction: namely, the use of newer anticoagulant drugs, either direct thrombin inhibitors (e.g., dabigatran) or factor Xa inhibitors (e.g., rivaroxaban, apixaban, edoxaban) that do not require routine coagulation monitoring.71
In contrast, because of the variation in response to antiplatelet therapy, there is great interest in the use of functional and/or genetic assays to guide such treatment.72,73 Possible mechanisms of aspirin and clopidogrel “resistance,” hyporesponsiveness, or high on-treatment platelet reactivity include: bioavailability (non-adherence, underdosing, poor absorption [enteric-coated aspirin]); interference (NSAIDs/aspirin, atorvastatin/clopidogrel, PPIs/clopidogrel); single nucleotide polymorphisms (CYP2C19*2, ABCB1 for clopidogrel); platelet interactions with other cells (endothelial cells and monocytes synthesize thromboxane A2, as well as its precursor, prostaglandin H2, both of which may be taken up by platelets, thereby bypassing the COX-1 inhibition by aspirin); platelet function (accelerated platelet turnover, with introduction into the bloodstream of newly formed, drug-unaffected platelets; stress-induced COX-2 production in platelets; underlying platelet hyperreactivity).68
1. Role of Platelet Function Testing
Platelet function tests for measuring the response to aspirin include those with thromboxane as the end point (serum thromboxane B2, urinary 11-dehydro thromboxane B2), arachidonic acid as the stimulus (turbidometric platelet aggregometry, impedance platelet aggregometry, the VerifyNow Aspirin assay, the thromboelastogram [TEG] PlateletMapping system, the Impact cone and plate(let) analyzer, flow cytometric assays of platelet surface P-selectin, platelet surface activated αIIbβ3 [GPIIb/IIIa], and/or leukocyte-platelet aggregates) and others (e.g., PFA-100).74,75 Platelet function tests for measuring the response to clopidogrel utilize adenosine diphosphate (ADP) as the stimulus and include: phosphorylation of vasodilator-stimulated phosphoprotein (VASP) measured by flow cytometry, turbidometric platelet aggregometry, impedance platelet aggregometry, the VerifyNow P2Y12 assay, the TEG PlateletMapping system, the Impact cone and plate(let) analyzer, flow cytometric assays of platelet surface P-selectin, platelet surface activated GPIIb/IIIa, and/or leukocyte-platelet aggregates).74,75 PGE1 can be used to better focus the test on the P2Y12-mediated (i.e., clopidogrel-dependent) pathway of platelet signaling, and this reagent is included in the commercially available VASP assay (BioCytex, Marseilles, France) and VerifyNow P2Y12 assay (Accumetrics, San Diego, CA). Point-of-care assays (also referred to as point-of-service assays) have potential advantages over laboratory-based tests. For example, point-of-care assays can inform immediate decision-making about the type and dose of antiplatelet therapy in the interventional cardiology suite. A rigorous definition of a point-of-care assay is one that meets all of the following criteria: use at or near the patient bedside; easy to use without special skills; no sample processing; no pipetting; and a rapid readout. The only currently available test that meets these criteria is the VerifyNow assay.
The concept of guiding antiplatelet therapy based on platelet function testing can be considered in three steps. First, is there inter-patient response variability to the antiplatelet agent as judged by a specific platelet function test? The answer to this first question is yes for both aspirin76 and clopidogrel.77 Second, in patients on antiplatelet therapy, is hyporesponsiveness in a platelet function test predictive of major adverse cardiovascular events (MACE) (when other risk factors are accounted for)? The answer to this second question is also yes for both aspirin78 and clopidogrel79 with stronger evidence for clopidogrel.73 Third, and most important, does guided antiplatelet therapy based on the results of platelet function testing decrease MACE? The answer to this third question is unknown for aspirin and, based on very limited data, possibly yes for clopidogrel.73,80,81 However, the data are currently insufficient to recommend platelet function testing to guide antiplatelet therapy in the clinical setting.
Compared with clopidogrel, newer P2Y12 antagonists (e.g., prasugrel and ticagrelor) produce greater and more consistent inhibition of ADP-dependent platelet function and decrease MACE to a greater extent.82,83 Nevertheless, there is still inter-patient variability in on-treatment platelet reactivity with these antiplatelet agents, albeit less than with clopidogrel.84,85 With these newer P2Y12 antagonists, the relationship between high on-treatment platelet reactivity and MACE, and the role (if any) of guided therapy, are unknown.
Randomized controlled trials are needed to address the hypothesis that modification of antiplatelet therapy in individual patients based on platelet function testing reduces MACE without increasing bleeding. There have been no such RCTs of personalized aspirin therapy. Because aspirin is not patent-protected, such trials are unlikely to be funded by industry. In contrast, there have been several RCTs of personalized clopidogrel therapy. Small, short-term studies of adjusted clopidogrel loading doses according to the VASP phosphorylation index showed a decreased MACE rate in PCI patients with high on-treatment platelet reactivity.80,81 The GRAVITAS study showed that, among patients with VerifyNow-defined high on-treatment platelet reactivity (P2Y12 reaction units [PRU] >230) after PCI and implantation of drug-eluting stents, doubling the dose of clopidogrel compared with standard-dose clopidogrel did not reduce the incidence of death from cardiovascular causes, nonfatal myocardial infarction, or stent thrombosis.86 However, the GRAVITAS study had limitations, including a low MACE rate and only a fixed doubling of the clopidogrel dose whereas the small, but successful VASP studies specifically tailored the clopidogrel dose to up to 4 times baseline.80,81 Furthermore, in a post-hoc analysis of the GRAVITAS study in which the PRU cut-off at randomization or during follow-up was reduced to <208 PRU, there was a lower risk of MACE, even after adjustment for other predictors.87 The RECLOSE 2 ACS study, which had similar limitations to GRAVITAS, also did not show benefit to guided clopidogrel therapy based on ADP-induced platelet aggregation.88 The ARCTIC trial (http://clinicaltrials.gov/ct2/show/NCT00827411) is in progress.
Future Directions in Platelet Function Testing
Important issues to be resolved in future randomized trials include:
Study populations with a sufficiently high risk of MACE to provide appropriate statistical power.
Selection of the appropriate platelet function test(s), ideally those that can identify both risk of thrombosis and risk of bleeding.
Optimal time(s) to measure platelet function, because platelet function has been shown to vary over time and high on-treatment platelet reactivity is associated with acute coronary syndromes68 – multiple measurements may therefore be needed to optimally tailor antiplatelet therapy (analogous to what is currently done with LDL cholesterol, for example).
Identification of optimal cutoff values for the platelet function test(s).
Integration of the result of the platelet function test(s) with the results of genetic tests (see section on pharmacogenetics).
Development of algorithms that combine clinical, procedural, and demographic data
Although the focus of this research field has been on clopidogrel, questions remain about the non-COX-1-dependent effects of aspirin and their correlation with clinical outcomes.78,89
Non-adherence plays a potentially critical role: if a patient does not take an antiplatelet drug, a platelet function test will record the patient as being non-responsive to the drug. Behavioral studies of prescribers and patients with regard to adherence and adoption of new drugs and monitoring strategies are therefore needed as well as implementation of targeted interventions to enhance adherence by patients, with a focus on reversible drugs that require more than once-a-day therapy (e.g., ticagrelor).
See also the final bullet in the section on Future Directions in Genetic Testing.
2. Role of Genetic Testing
Whereas rare Mendelian genetic disorders of platelet biology and hemostasis have been recognized for more than a century, the influence of more common genetic variants on platelet biology, hemostasis, the predilection for thrombosis, and the response to antithrombotic therapies has only recently been appreciated.
Studies from the Framingham Heart Study (FHS) demonstrated that heritable factors accounted for 20-30% of the overall variance in platelet aggregation in response to agonists such as epinephrine.90 A subsequent genome wide association study (GWAS) in two cohorts (FHS and Genetic Study of Atherosclerosis Risk) identified associations of seven loci with platelet aggregation near or within GP6, PEAR1, ADRA2A, PIK3CG, JMJD1C, MRVI1 and SHH.91 In addition, a recent large GWAS has reported 68 genomic loci associated with platelet count and volume, underscoring the role of genetic factors in megakaryopoesis and platelet formation.92
In terms of antiplatelet therapies, aspirin irreversibly inhibits cyclooxygenase-1 and 2 (COX-1 and 2). In platelets, COX-1 catalyzes the conversion of arachidonic acid to PGG2 and PGH2, resulting in downstream synthesis of TXA2. Variability in aspirin-mediated antiplatelet effects has been noted, with potential associations with adverse cardiovascular outcomes.93 Genetic polymorphisms may play a role in individual response to aspirin, but consistent, validated associations remain to be established.94,95,96
Clopidogrel, an oral thienopyridine, is a prodrug, approximately 85% of which is metabolized by esterases in the gut to form an inactive carboxylic acid derivative. The remaining 15% is biotransformed into the active compound by hepatic metabolism involving two sequential cytochrome P450-mediated steps, with CYP2C19 involved in both steps.97 The active thiol metabolite then irreversibly binds the P2Y12 ADP receptor, leading to partial inhibition of ADP-dependent platelet activation and aggregation. Variability in clopidogrel response has been observed: on-treatment platelet reactivity in clopidogrel-treated patients approximates a bell-shaped distribution, and individuals with high on-treatment platelet reactivity have higher rates of ischemic events, including stent thrombosis.98
In a study of healthy Amish persons given clopidogrel for 7 days, the platelet response to clopidogrel was highly heritable (h2 = 0.73). A GWAS identified 13 single-nucleotide polymorphisms (SNPs) on chromosome 10q24 within the CYP2C18–CYP2C19–CYP2C9– CYP2C8 region that were associated with a diminished response to clopidogrel.99 The lead SNP was in strong linkage disequilibrium with the CYP2C19*2 variant, which involves a single base pair mutation of G→A at position 681, creating an aberrant splice site that leads to the synthesis of truncated non-functional CYP2C19 protein.100 This locus accounted for 12% of the variation in platelet aggregation to ADP. The *2 variant is carried by approximately 30% of whites, 40% blacks, and 55% of East Asians.101 Compared with noncarriers, carriers of at least one copy of the CYP2C19*2 allele or other loss-of-function CYP2C19 variants have approximately 30% lower levels of active clopidogrel metabolite and approximately 25% less platelet inhibition.102
Moreover, among patients with acute coronary syndromes and planned PCI treated with clopidogrel, carriers of at least one copy of a CYP2C19 loss-of-function variant had a 50% increase in the risk of cardiovascular death, MI, or stroke and a three-fold increased risk of stent thrombosis.102 These observations have been seen in a total of 9 clinical studies involving patients treated with clopidogrel predominantly for PCI.103 Based on the totality of the data to date, both heterozygotes and homozygotes appear to be at increased risk. The clopidogrel label has been updated by the U.S. Food and Drug Administration (FDA) to include a boxed warning about the impact of genetics on the response to clopidogrel. A recent meta-analysis showed a less robust association, but the study was questionable with regard to its relevance to current clinical use since it included non-stented patient populations in which clopidogrel has modest or no efficacy, did not restrict analyses to events while patients were on clopidogrel, and included non-cardiovascular outcomes unaffected by clopidogrel.104
Other variants have been found that have been associated with the pharmacologic and/or clinical response to clopidogrel. The ABCB1 gene (also known as MDR1) encodes for the xenobiotic efflux p-glycoprotein pump involved in intestinal absorption of clopidogrel. In some but not all studies, the 3435TT genotype has been associated with an increased rate of cardiovascular events in the setting of treatment with clopidogrel therapy after an acute myocardial infarction.105,106,107 The PON1 gene encodes for paraoxonase-1, an esterase synthesized in the liver and associated with HDL cholesterol in the blood. Although one study found the PON1 Q192R polymorphism to affect variability in clopidogrel efficacy and to confer increased risks for definite stent thrombosis,108 these observations have not been replicated in multiple subsequent studies.109,110
The CLOVIS-2 and ELEVATE-TIMI 56 trials have demonstrated, respectively, that tripling the loading and tripling the maintenance doses of clopidogrel in patients with poor antiplatelet responses to standard doses can achieve on-treatment platelet reactivity comparable to that observed with standard dosing in wild-type, responsive patients.111,112 Alternately, prasugrel and ticagrelor are third-generation ADP receptor antagonists whose efficacy is not influenced by CYP2C19 loss-of-function genetic variants.107,113
In terms of anticoagulants, warfarin interrupts hepatic vitamin K recycling by inhibiting vitamin K epoxide reductase and vitamin K quinine reductase. Warfarin is ingested as a combination of active R- and S-warfarin enantiomers, with the S-enantiomer about 3-5 times more potent. S-warfarin is inactivated by CYP2C9-mediated hydrolysis; the less active R-enantiomer is metabolized by CYP1A2 and CYP3A4 enzymes. GWAS studies have confirmed the association between genetic polymorphisms in VKORC1 and CYP2C9 and warfarin dosing variability.114,115 CYP2C9 and VKORC1 genotype information account for ~40% of the variability in warfarin dosing. In 2007, the U.S. FDA updated the warfarin label to note that VKORC1 and CYP2C9 variants may influence warfarin dosage requirement. In 2010, the label was further updated with inclusion of a pharmacogenetic-guided dosing scheme for initiation of warfarin therapy (factoring in the presence or absence of VKORC1-1369 G->A and CYP2C9*2 or CYP2C9*3).
Algorithms incorporating a patient's genotype, demographic factors, and co-medications have been developed in an attempt to improve prediction of initial warfarin dosing; these algorithms provide the greatest benefit in patients requiring extremes of warfarin dosage (≤21 mg per week or ≥49 mg per week).116 Several small studies have suggested that genotype-based warfarin dosing can result in smaller and fewer dose adjustments117 however, no impact on clinical outcomes has yet been demonstrated in prospective studies. Several large-scale prospective studies are underway to further elucidate whether genotype-guided algorithms improve clinical outcomes. Novel anticoagulants, including dabigatran, rivaroxaban, and apixaban, have emerged as alternatives to warfarin that are not influenced by these pharmacogenetic interactions.
Future Directions in Genetic Testing
Five areas of study for the genetics of antithrombotic therapy are recommended:
Identify and characterize the full spectrum of genetic variants associated with platelet biology, hemostasis, and thrombosis phenotypes as well as the pharmacokinetic and pharmacodynamic responses to antiplatelet and anticoagulant therapies. Such characterization could be accomplished by GWAS and/or whole exome sequencing. Quantitative phenotypes such as platelet aggregation and coagulation parameters permit adequate statistical power in studies of relatively smaller numbers of individuals. More challenging is attempting to apply such approaches to clinical outcomes as the sample sizes required rise steeply. One cost-efficient approach would be to support ancillary genetic studies in existing or planned clinical studies.
Perform functional validation and characterization of discovered genetic loci. Understanding the mechanisms underlying observed associations between loci and the aforementioned phenotypes will be important for both furthering our knowledge of basic biology and developing drug targets. Such investigation will likely require a combination of cell-based and animal-based models as well as ex vivo and in vivo human studies.
Characterize second-order interactions. Acknowledging the complexity of the biology of thrombosis and antithrombotic therapy, both gene-gene and gene-environment interactions will need to be defined. Data already suggest that the clinical implications of certain pharmacogenetic interactions (such as CYP2C19 and clopidogrel) depend on the patient's clinical status (e.g., whether they have undergone PCI or not). Screening methods for these second-order interactions could include both wet laboratory and bioinformatics approaches.
Bring genetic testing into clinical practice. For genetics to be readily incorporated into clinical practice, genotyping must move from research laboratories and send-out tests to rapid-turnaround or even rapid point-of-care (POC) testing. To that end, there have been several recent successful demonstrations of POC testing for genotyping for CYP2C19 loss-of-function alleles. Down the road, comprehensive hemostasis/thrombosis arrays or even sequencing may be readily available. As genome-wide genetic data become increasingly available to clinicians, support for integrating such information into clinical practice will be needed.
Determine the utility of platelet function and genetic testing in clinical practice. Studies will need to be done in appropriately sized cohorts with sufficient statistical power for major adverse cardiovascular outcomes. For evidence-based use, data from platelet function and genetic testing will need to be integrated with clinical characteristics. Both thrombotic and bleeding outcomes should be examined. For platelet function testing, as results change over time, multiple measurements may be needed to optimally tailor therapy. Moreover, the optimal cutoff values will need to be determined. Although a dedicated RCT comparing clinical outcomes in patients who undergo testing versus those who do not would represent the highest level of evidence, such a trial would need to be exceedingly large in order to be adequately powered for clinical outcomes. Thus, one objective is to identify a range of more practical trial designs. For example, one such design, that is analogous to what has been done for other high-risk features such as diabetes or an elevated troponin, is to use platelet function testing or genotyping to define subgroups within RCTs of therapies. Moreover, testing might be used to identify high-risk subgroups for enrollment into trials (somewhat akin to what is done in oncology).
III. THE STAKEHOLDER'S ROLES
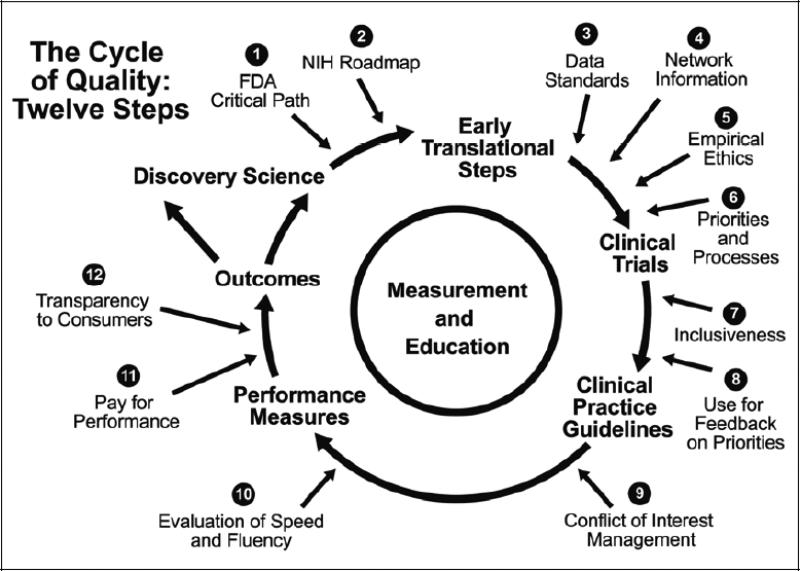
Because antithrombotic therapy is critical to the treatment of the world's leading causes of death and disability (coronary artery disease, stroke, and atrial fibrillation), many stakeholders have an interest in developing new therapies and in understanding existing therapies better. Further, as information becomes increasingly available, policymakers are envisioning the global therapeutic development enterprise in a much more sophisticated manner than in the past (Figure 2).118
Figure 2.
The cycle of quality. Adapted from: Califf RM, Harrington RA, Madre LK, Peterson ED, Roth D, Schulman KA. Curbing the cardiovascular disease epidemic: aligning industry, government, payers, and academics. Health Aff (Millwood). 2007;26:62-74; reprinted with permission.
In an ideal system, adequate investment in preclinical thrombosis research would lead to conceptual advances. These advances would in turn be translated into novel molecules capable of inhibiting or enhancing selective elements of the coagulation system in a well-defined manner. Following preclinical proof-of-concept evaluation, early-stage clinical trials would be performed to winnow out unfavorable approaches. Ultimately, approaches that showed the greatest promise in early studies would be selected for testing in trials designed and powered to provide a clear estimate of the balance of risk and benefit associated with the use of such treatments in clinical practice. Definitive trial results would be translated into clinical practice guidelines, and the most clear-cut results would inform performance indicators once it became clear that a given therapy should or should not be given in a particular clinical circumstance. Finally, the sum of this information and knowledge would be stored in databases and knowledge repositories to drive a system of continuous learning.
Although we have yet to attain this ideal in practice, multiple societal entities can help move us closer to achieving an ideal learning health system, and the field of antithrombotic therapy represents an important possible prototype for such a system. One distinctive element of an ideal learning health system is that multiple stakeholders must be involved: pharmaceutical, biotechnology, and device companies (i.e., the “medical products industry”); academic health and science systems (AHASs); medical practitioners and providers; voluntary health organizations/patient advocacy groups (VHO/PAGs); and government agencies. Further, while opportunities to develop therapies with high-quality evidence are expanding, so too is the organizational complexity of biomedical research as it evolves into a truly global enterprise.
1. The Medical Products Industry
The medical products industry develops drugs and companion diagnostics along a continuum that reaches from the preclinical arena through clinical trials and marketing. However, the industry has been under economic and regulatory pressures for some time, and many believe that its current financial models are not sustainable due to the enormous costs of bringing new treatments to market.119Although the particulars of the cost estimates can be debated (including the cost of all developmental failures and the opportunity costs of the delay on return on investment),120 the view that the cost of developing new therapies has escalated is largely undisputed.
Moreover, as the risks associated with treatments have become better understood, the evidentiary standards of regulatory agencies have become stricter, further increasing the cost and risk of development and marketing. Many have expressed worry about a diminution in both venture capital for cardiovascular drug development and investment in large pharmaceutical companies at later stages of drug development. Nevertheless, given the enormous market occasioned by vascular disease as a cause of death and disability in the economically developed world, the reward for developing a successful drug can be significant. Accordingly, the medical products industry likely will continue to invest in new therapies, and, it is hoped, in an increasing number of personalized treatments in which companion diagnostics can be used to determine which patients will achieve the best results with specific drugs or combinations of drugs. However, as companies face escalating costs and the uncertainties of a crowded market, the need for public-private partnerships to stimulate development is likely to prove increasingly critical.
An important development in the medical products industry is the reduction in fundamental research and development due to the financial pressures described above. Shrinking corporate research and development budgets mean that the NIH and academia will have to take on added responsibility to fill the pipeline with discoveries that will lead to new and better drugs. One countervailing trend of note is the increasing willingness of pharmaceutical companies to enter into creative agreements with academia in the preclinical development arena.
Because the medical products industry appears to have limited interest in comparative effectiveness research, government and academia have to fill this important need. Given the large number of patients who need to be studied in comparative effectiveness studies and the high cost of these studies, it will be critical to form partnerships that can identify the most important questions to be answered and can conduct studies efficiently. The Patient-Centered Outcomes Research Institute (PCORI)121 represents a major effort by the U.S. government to create a partnership in which the medical products industry can contribute to the design of comparative effectiveness studies without having to fund those studies.
2. Academic Health and Science Systems
Over the past few years, academic institutions—typically medical schools with affiliated hospitals—have evolved into entities with an array of institutions needed to support integrated health systems, or “academic health and science systems” (AHASs).122 AHASs produce the scientists and clinicians who will develop and use antithrombotic agents. As pharmaceutical and device companies continue to pare down their research and development budgets, there is a corresponding growth of interest in forging novel relationships with AHASs. Although these relationships hold great promise for joint efforts in laboratory research, numerous issues regarding financial conflicts of interest and the not-for-profit status of American academic centers await resolution. Effective consulting by knowledgeable academics to provide input and guidance into corporate decision-making will likewise be critical to success. AHASs will also likely play a key role in conducting clinical trials as the field seeks more efficient methods in this arena.
3. Medical Practitioners and Providers
Medical practitioners will ultimately use therapies developed by the medical products industry and AHASs and conduct necessary clinical trials. Professional societies representing the cardiovascular medicine community, including the American College of Cardiology (ACC), the American Heart Association (AHA), and the European Society of Cardiology (ESC) all play central roles in developing clinical practice guidelines and performance measures. These groups are also developing and implementing registries that integrate clinical quality assessment and post-marketing surveillance. 123,124,125
The medical products industry needs to address the remaining health needs of the population as defined by the providers who prescribe their products. There is general agreement among providers that we need antithrombotics with better risk-benefit profiles as well as a better understanding of how combinations can be used together in practice. Given the financial constraints enumerated above, there will be a persistent gap between the available evidence and the needed evidence until providers form more efficient research groups, using methods such as integration of registries and electronic health records into randomized trials. These essential studies may range from intensive study groups focused on biological mechanisms to global trials networks and health–system-based post-marketing surveillance efforts.
Providers also have an important role as advocates for continued development by the medical products industry and by government. Given that typical drug development processes do not clarify which alternatives are best for particular groups or individual patients, provider groups must advocate for funding for comparative effectiveness studies from industry and/or government. Such arguments, however, will be more persuasive if providers have developed more cost-efficient methods for performing these trials.
4. Patient Advocacy Groups/Voluntary Health Organizations
Patient advocacy groups/voluntary health organizations (PAG/VHOs) are emerging as major forces in therapeutic development. In diseases such as cancer, HIV-AIDS, multiple myeloma, and cystic fibrosis, PAG/VHOs have become a significant source for funding research and are exerting influence in shaping policies related to research and the evaluation and regulation of drugs and devices. For example, the Cystic Fibrosis Foundation invests in product development and funds a research network that performs clinical trials. In the arena of thrombosis, however, PAG/VHOs are less focused because so many different diseases are involved. For example, the AHA is a potent advocacy organization that funds its own research, but its broad mandate limits direct influence on antithrombotic drug development. Sustained focus on the profound impact of thrombosis on human health is needed to ensure sufficient investment for developing more effective therapies.
5. Government Agencies
The National Institutes of Health
Multiple government agencies are involved in antithrombotic drug development, with the National Institutes of Health (NIH) in general and the National Heart, Lung, and Blood Institute (NHLBI) in particular funding much of the discovery science and applied basic science that produces new ideas about antithrombotic drugs. Defining optimal strategies for investing money allocated to the NHLBI is of vital interest to all other constituents and is the primary subject of this report. The urgent need for NIH investment in research into the basic biology of thrombosis is only heightened by the reductions in fundamental research and development spending by the medical products industry noted above. At the same time, support for the NIH is under stress, with flat budgets that actually represent a decline in scientific purchasing power. The NIH will need to carefully craft strategies for maximum leverage, applying public money in key areas where investment from the private sector is less likely.
AHRQ
The Agency for Healthcare Research and Quality (AHRQ) is charged with improving the quality, safety, efficiency, and effectiveness of health care for all Americans. This broad mission includes significant efforts to aggregate research data across studies, but the agency's ability to conduct primary research is limited by its relatively small budget.
CMS, VA, and DOD
The Centers for Medicare and Medicaid Services (CMS), the Department of Veterans Affairs (VA), and the Department of Defense (DOD) provide services that use antithrombotic therapies. In the case of the VA and the DOD, a modest but significant research budget is allocated to focus on research pertaining to the health needs of the military and veterans. The creation of incentives aimed at stimulating research into new treatments, and the development of evidence to guide more effective use of therapies, are in the direct interest of these agencies and their beneficiaries.
FDA
The U.S. FDA regulates the development and appropriate use of antithrombotic drugs in the United States. Its policies and guidelines control the direction that preclinical and clinical studies take as biological concepts are translated into useful products. But as the number of available antithrombotics has grown, issues such as noninferiority trials, joint approval of new therapeutics with companion diagnostic assays, the use of biomarkers and putative surrogates, simplification of trials, and regulation of combination therapies have emerged as critical limiting or enhancing factors, depending upon specific FDA precedents. The relatively new field of regulatory science creates opportunities for systematically analyzing the regulatory process related to evaluation and approval of antithrombotic therapy, with the goal of streamlining the process. The development of academic units focused on regulatory science would increase the likelihood of success and provide a cadre of experts who could spearhead evidence-based regulatory science advances.
6. Globalization
Until recently, the development of new drugs and accompanying devices largely within North America, Europe and Japan. Now, however, clinical trials—including research funded by the U.S. government—are performed globally,126,127and the largest future markets are in Asia and the BRICK (Brazil, Russia, India, China, Korea) countries. Much of the research and development enterprise formerly centered in the U.S. and Western Europe is becoming globalized as well. Accordingly, as strategies for developing antithrombotics are devised, consideration must be given to parallel funding agencies, professional groups, PAG/VHOs, and government agencies on a global scale. An effective global approach that would rapidly accumulate evidence about the benefits and risks of new and competing therapies is a key public health priority for the world's 7 billion people, given that approximately 17 to 24 million persons are expected to die each year from cardiovascular diseases in the coming decades.128
7. Conflict of interest
In order to produce a successful system for developing new antithrombotic therapies, while also maintaining the ability to understand how to most effectively use the old therapies, conflicts of interest will be engendered across the spectrum of interactions. While the conflict between the driving profit motive of industry and the not-for-profit sector has been emphasized in the past and deserves continued emphasis129,130, it should also be noted that government agencies charged with reimbursement may not favor new, more expensive therapies, journals receive advertising and have their own biases and the press is under increasing pressure to deliver sensational news as the mainstream press has downsized. The complexity of this situation is best handled by transparency about both financial and non-financial conflict of interest and instituting rational and explicit management plans, including having open discussion about the issues engendering potential bias.
8. Pulling It Together
The complexity of the task of developing and optimizing antithrombotic therapy is very challenging. There are four key areas to consider in antithrombotic development: 1) basic research into the biology of thrombosis, 2) preclinical development, 3) definitive clinical trials, and 4) comparative effectiveness. The NHLBI has a special role to play in funding research that explores the basic biology of thrombosis. Because there are no other major societal entities focused on this issue, sustained support is crucial, and in this arena the traditional peer-review system works well for identifying and promoting the best science. However, preclinical development is one area where aggressive partnership-building efforts could enable the NHLBI to significantly catalyze more effective preclinical translational efforts. Continued work on preclinical models, including animal models and in silico experiments, as well as investigation into the systems biology of thrombosis, are key priorities in preclinical development. The NHLBI's recently created mechanisms for translational units also should remain a high priority in order to attract more industry funding in this area.
The U.S. clinical trials enterprise is bogged down by low throughput and high costs. Recent NHLBI workshops131 have suggested creative approaches to addressing this issue. In the field of thrombosis, intensive studies of human systems biology are needed, as are more efficient mechanisms for conducting large trials. Collaboration with the newly created National Center for Advancing Translational Sciences (NCATS) 132 will be critical in these regards, but NCATS is a general support system, and specific efforts aimed at furthering thrombosis research will be essential.
Finally, the areas of comparative effectiveness and the assessment of antithrombotic combinations require leadership from NHLBI. Head-to-head comparisons and evaluation of combination therapies have not been emphasized by industry because of the economic risk and the complexity of regulatory review. Over the years, the NHLBI has conducted many landmark trials that have examined marketed therapies and evaluated their value for patients. These efforts should continue and focus on antithrombotic therapy, but must now accommodate a larger number of potential partners for funding.
Future Directions of NHLBI and Other Stakeholders
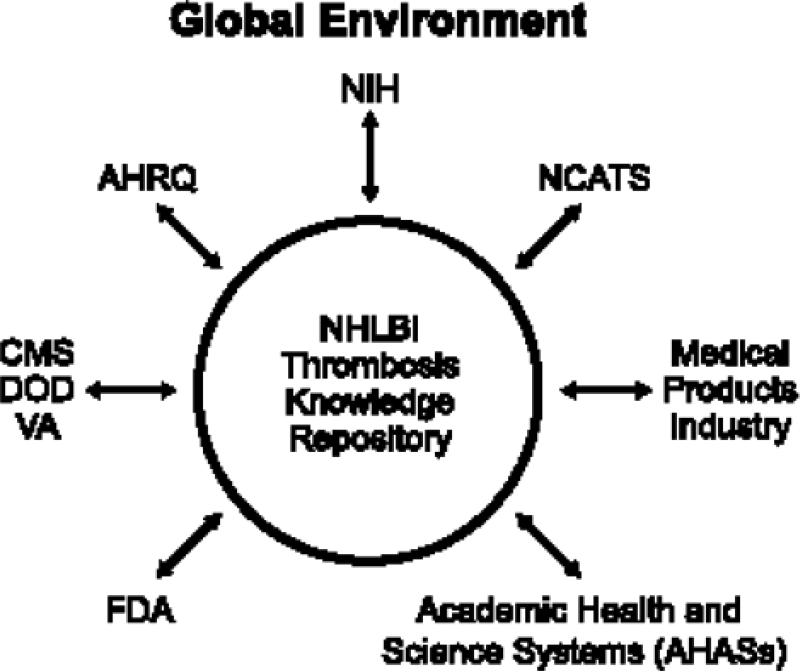
The potential role for NHLBI as a convener in this field is shown in Figure 3. Because of funding limitations, collaboration across traditional lines will be necessary. Given the NHLBI's transparency and the degree of public trust reposed in it, the agency should be constantly engaged in thrombosis research, bringing together the many interested parties to fund important research through creative partnerships. In many cases, the NHLBI can aggregate information and serve as the neutral ground for interpretation, thus setting the direction of research without having to provide the funding; in other cases, the research should be funded directly by public sources. The specific recommendations in this report on trials for antithrombotic agents represent an example of an effort that could be expanded to other fields.
Much of the accretion of knowledge in this field is stored in digital media. Given the accelerating rate at which these data are accumulating, there is a pressing need for a knowledge repository (encompassing precompetitive and publicly available information) that could be used and contributed to by all interested parties.
Figure 3.
The potential role of the National Heart, Lung, and Blood Institute as a convener of therapeutic development efforts in atherothrombosis research.
Footnotes
Working Group Chair: Valentin Fuster, MD, PhD; Subgroup Chairs: Deepak L. Bhatt, MD, MPH, Robert M. Califf, MD, Barry S. Coller, MD, Alan D. Michelson, MD, Marc S. Sabatine, MD, MPH; Members:
Dominick J. Angiolillo, MD, PhD, Eric R. Bates, MD, David J. Cohen, MD, MSc, Bruce Furie, MD, Jean-Sebastien Hulot, MD, PhD, Kenneth G. Mann, PhD, Jessica L. Mega, MD, MPH, Kiran Musunuru, MD, PhD, MPH, Christopher J. O'Donnell, MD, MPH, Matthew J. Price, MD, David J. Schneider, MD, Daniel I. Simon, MD, Jeffrey I. Weitz, MD, Marlene S. Williams, MD, NHLBI Staff: Ahmed A.K. Hasan, MD, PhD, W. Keith Hoots, MD, Yves D. Rosenberg, MD, MPH
Disclosures: Dr. Angiolillo received honoraria for lectures / speakers bureau from Bristol Myers Squibb; Sanofi-Aventis; Eli Lilly Co; Daiichi Sankyo, Inc; Abbott Vascular; Astra Zeneca; consulting fees from Bristol Myers Squibb; Sanofi-Aventis; Eli Lilly Co; Daiichi Sankyo, Inc.; The Medicines Company; Portola; Novartis; Medicure; Accumetrics; Arena Pharmaceuticals; Abbott Vascular; Astra Zeneca; Merck; Evolva; research grants (paid to Institution) from Bristol Myers Squibb; Sanofi-Aventis; GlaxoSmithKline; Otsuka; Eli Lilly Co; Daiichi Sankyo, Inc., The Medicines Company; Portola; Accumetrics; Schering-Plough; Astra-Zeneca; Eisai. Dr. Bates serves as consultant for AstraZeneca, Eli Lilly, and Sanofi-Aventis. Dr. Bhatt reports Advisory Board: Medscape Cardiology; Board of Directors: Boston VA Research Institute, Society of Chest Pain Centers; Chair: American Heart Association Get With The Guidelines Science Subcommittee; Honoraria: American College of Cardiology (Editor, Clinical Trials, Cardiosource), Duke Clinical Research Institute (clinical trial steering committees), Slack Publications (Chief Medical Editor, Cardiology Today Intervention), WebMD (CME steering committees); Research Grants: Amarin, AstraZeneca, Bristol-Myers Squibb, Eisai, Ethicon, Medtronic, Sanofi Aventis, The Medicines Company; Unfunded Research: PLx Pharma, Takeda. Dr. Califf reports receiving research grants that partially support his salary from Amylin, Johnson & Johnson (Scios), Merck, Novartis Pharma, Schering Plough, Bristol-Myers Squibb Foundation, Aterovax, Bayer, Roche, and Lilly; all grants are paid to Duke University. Dr Califf also consults for TheHeart.org, Johnson & Johnson (Scios), Kowa Research Institute, Nile, Parkview, Orexigen Therapeutics, Pozen, Servier International, WebMD, Bristol-Myers Squibb Foundation, AstraZeneca, Bayer-OrthoMcNeil, BMS, Boerhinger Ingelheim, Daiichi Sankyo, GlaxoSmithKline, Li Ka Shing Knowledge Institute, Medtronic, Merck, Novartis, sanofi-aventis, XOMA, and University of Florida; all income from these consultancies is donated to nonprofit organizations, with the majority going to the clinical research fellowship fund of the Duke Clinical Research Institute. Dr Califf holds equity in Nitrox LLC. Financial disclosure information for Dr. Califf is also publicly available at https://www.dcri.org/about-us/conflict-of-interest. Dr. Cohen has received research grants from Eli Lilly, Astra-Zeneca, Daiichi-Sanko, Medtronic, Edwards Lifesciences, Abbott Vascular, and Boston Scientific, and honoraria from Eli Lilly, Medtronic, and St. Jude. Dr. Coller has royalty interests in abciximab (Centocor) and the VerifyNow Assays (Accumetrics) and currently serves as an unpaid advisor to Pfizer and Merck. As a result, Dr. Coller's roles in the workshop and in writing this review were confined to providing scientific comments, without offering any specific diagnostic or therapeutic recommendations. Dr. Furie has been the principal investigator on a research grant to the Marine Biological Laboratory from Pfizer and a consultant to Eleven Biotherapeutics. Dr. Hasan has no disclosure. Dr. Hoots has no disclosure. Dr. Hulot receives research grants (significant) from Biotronik and Medco Research Institute, and honoraria (significant) from Biotronik and Medco Health Solutions. Dr. Mann serves as a consultant to Daiichi-Sankyo, Merck, Baxter, GTI, Alnylam and T2 Biosystems. He has a current NIH PO1-HL046703 and a prior grant and consultant with Johnson & Johnson. He is the Chairman of the Board of Haematologic Technologies, Inc. Dr. Mega receives research grants (significant) through Brigham and Women's Hospital from Eli Lilly, Daiichi Sankyo, Johnson & Johnson, Bayer, and Bristol-Myers Squibb/Sanofi-Aventis; honoraria from AstraZeneca, Merck, Bayer Healthcare, Bristol-Myers Squibb, and Sanofi-Aventis; and research supplies from Accumetrics and Nanosphere. Dr. Michelson has been the principal investigator or coinvestigator on research grants to Children's Hospital Boston from GLSynthesis, Lilly and Takeda and on advisory boards for Lilly and Sanofi-Aventis. Dr. Musunuru has no disclosure. Dr. O’Donnell has no disclosure. Dr. Price receives research grants (significant) from Bristol-Myers Squibb/Sanofi-Aventis, Quest Diagnostics, and Accumetrics, and honoraria (significant) from Daiichi-Sankyo/Eli Lilly & Co., AstraZeneca, Accumetrics, W.L. Gore, and (moderate) from Boston Scientific, Johnson &
Johnson, Bristol-Myers Squibb/Sanofi-Aventis, St. Jude Medical, Medicure, and Medtronic. Dr. Rosenberg has no disclosure. Dr. Sabatine receives research grants (significant) through Brigham and Women's Hospital from Abbott, Amgen, AstraZeneca, Bristol-Myers Squibb, Daiichi-Sankyo, Nanosphere, Roche, Sanofi-Aventis, and Takeda, honoraria from Amgen, Sanofi-Aventis (significant) and Bristol-Myers Squibb/Sanofi-Aventis. Dr. Schneider has received research grants from Johnson & Johnson, Bayer Pharmaceuticals, Bristol-Myers Squibb, Sanofi Aventis, and The Medicines Company and honoraria from Johnson & Johnson, Sanofi Aventis, Bristol Myers Squibb, The Medicines Company, Astra Zeneca, and Regado Bioscience. Dr. Simon has served on the scientific advisory board and/or consulting for Cordis/Johnson & Johnson (significant), Daiichi-Sankyo, Medtronic Vascular, Merck, and Portola. This work was supported in part by National Institutes of Health HL57506 MERIT Award to Dr. Simon. Dr. Weitz receives honoraria (significant) from Boehringer-Ingelheim, Merck, Bayer Pharmaceuticals, Janssen Pharmaceuticals, Bristol-Myers Squibb, Pfizer, and Daiichi-Sankyo. Dr. Williams has no disclosure.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yousuf O, Bhatt DL. The evolution of antiplatelet therapy in cardiovascular disease. Nat Rev Cardiol. 2011;8:547–559. doi: 10.1038/nrcardio.2011.96. [DOI] [PubMed] [Google Scholar]
- 2.Sakhuja R, Yeh RW, Bhatt DL. Antiplatelet agents in acute coronary syndromes. Curr Probl Cardiol. 2010;35:123–170. doi: 10.1016/j.cpcardiol.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Sakhuja R, Yeh RW, Bhatt DL. Anticoagulant agents in acute coronary syndromes. Curr Probl Cardiol. 2011;36:127–168. doi: 10.1016/j.cpcardiol.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Kolandaivelu K, Bhatt DL. Antiplatelet therapy in coronary heart disease prevention. Cardiol Clin. 2011;29:71–85. doi: 10.1016/j.ccl.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Desai NR, Bhatt DL. The state of periprocedural antiplatelet therapy after recent trials. JACC Cardiovasc Interv. 2010;3:571–583. doi: 10.1016/j.jcin.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Meadows TA, Bhatt DL. Clinical aspects of platelet inhibitors and thrombus formation. Circ Res. 2007;100:1261–1275. doi: 10.1161/01.RES.0000264509.36234.51. [DOI] [PubMed] [Google Scholar]
- 7.Depta JP, Bhatt DL. Atherothrombosis and atrial fibrillation: Important and often overlapping clinical syndromes. Thromb Haemost. 2010;104:657–663. doi: 10.1160/TH10-05-0332. [DOI] [PubMed] [Google Scholar]
- 8.Faxon DP, Eikelboom JW, Berger PB, Holmes DR, Jr., Bhatt DL, Moliterno DJ, Becker RC, Angiolillo DJ. Antithrombotic therapy in patients with atrial fibrillation undergoing coronary stenting: A north american perspective: Executive summary. Circ Cardiovasc Interv. 2011;4:522–534. doi: 10.1161/CIRCINTERVENTIONS.111.965186. [DOI] [PubMed] [Google Scholar]
- 9.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults. JAMA: The Journal of the American Medical Association. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 10.Fuster V, Rydén LE, Cannom DS, Crijns HJ, Curtis AB, Ellenbogen KA, Halperin JL, Kay GN, Le Huezey JY, Lowe JE, Olsson SB, Prystowsky EN, Tamargo JL, Wann LS. American College of Cardiology Foundation/American Heart Association Task Force. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123:e269–367. doi: 10.1161/CIR.0b013e318214876d. [DOI] [PubMed] [Google Scholar]
- 11.Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting stroke: Results from the national registry of atrial fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 12.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 13.Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: A comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41:2731–2738. doi: 10.1161/STROKEAHA.110.590257. [DOI] [PubMed] [Google Scholar]
- 14.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 15.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al- Khalidi HR, Ansell J, Atar D, Avezum A, Bahit MC, Diaz R, Easton JD, Ezekowitz JA, Flaker G, Garcia D, Geraldes M, Gersh BJ, Golitsyn S, Goto S, Hermosillo AG, Hohnloser SH, Horowitz J, Mohan P, Jansky P, Lewis BS, Lopez-Sendon JL, Pais P, Parkhomenko A, Verheugt FW, Zhu J, Wallentin L. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 16.Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro heart survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- 17.Fang MC, Go AS, Chang Y, Borowsky LH, Pomernacki NK, Udaltsova N, Singer DE. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (anticoagulation and risk factors in atrial fibrillation) study. J Am Coll Cardiol. 2011;58:395–401. doi: 10.1016/j.jacc.2011.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly S, Pogue J, Hart R, Pfeffer M, Hohnloser S, Chrolavicius S, Pfeffer M, Hohnloser S, Yusuf S. Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomized controlled trial. Lancet. 2006;367:1903–1912. doi: 10.1016/S0140-6736(06)68845-4. [DOI] [PubMed] [Google Scholar]
- 19.Connolly SJ, Pogue J, Hart RG, Hohnloser SH, Pfeffer M, Chrolavicius S, Yusuf S. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009;360:2066–2078. doi: 10.1056/NEJMoa0901301. [DOI] [PubMed] [Google Scholar]
- 20.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 21.Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate / clinical perspective. Circulation. 2011;124:1573–1579. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 22.Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: A randomized non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 23.Reddy VY, Holmes D, Doshi SK, Neuzil P, Kar S. Safety of percutaneous left atrial appendage closure: Results from the watchman left atrial appendage system for embolic protection in patients with af (protect af) clinical trial and the continued access registry. Circulation. 2011;123:417–424. doi: 10.1161/CIRCULATIONAHA.110.976449. [DOI] [PubMed] [Google Scholar]
- 24.Chao TF, Lin YJ, Tsao HM, Tsai CF, Lin WS, Chang SL, Lo LW, Hu YF, Tuan TC, Suenari K, Li CH, Hartono B, Chang HY, Ambrose K, Wu TJ, Chen SA. CHADS(2) and CHA(2)DS(2)-Vasc scores in the prediction of clinical outcomes in patients with atrial fibrillation after catheter ablation. J Am Coll Cardiol. 2011;58:2380–2385. doi: 10.1016/j.jacc.2011.08.045. [DOI] [PubMed] [Google Scholar]
- 25.Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ, Jr., Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ. HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: Recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) task force on catheter and surgical ablation of atrial fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace. 2007;9:335–379. doi: 10.1093/europace/eum120. [DOI] [PubMed] [Google Scholar]
- 26.Viles-Gonzalez JF, Fuster V, Halperin J, Calkins H, Reddy VY. Rhythm control for management of patients with atrial fibrillation: balancing the use of antiarrhythmic drugs and catheter ablation. Clin Cardiol. 2011;34:23–9. doi: 10.1002/clc.20857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paikin JS, Wright DS, Crowther MA, Mehta SR, Eikelboom JW. Triple antithrombotic therapy in patients with atrial fibrillation and coronary artery stents. Circulation. 2010;121:2067–2070. doi: 10.1161/CIRCULATIONAHA.109.924944. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein LB, Adams R, Alberts MJ, Appel LJ, Brass LM, Bushnell CD, Culebras A, Degraba TJ, Gorelick PB, Guyton JR, Hart RG, Howard G, Kelly-Hayes M, Nixon JV, Sacco RL. Primary prevention of ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council. Stroke. 2006;37:1583–1633. doi: 10.1161/01.STR.0000223048.70103.F1. [DOI] [PubMed] [Google Scholar]
- 29.Adams RJ, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Sacco RL, Schwamm LH. Update to the AHA/ASA recommendations for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2008;39:1647–52. doi: 10.1161/STROKEAHA.107.189063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, Cates CU, Creager MA, Fowler SB, Friday G, Hertzberg VS, McIff EB, Moore WS, Panagos PD, Riles TS, Rosenwasser RH, Taylor AJ. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Circulation. 2011;124:e54–130. doi: 10.1161/CIR.0b013e31820d8c98. [DOI] [PubMed] [Google Scholar]
- 31.Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, Olson DM, Hernandez AF, Peterson ED, Schwamm LH. Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: Patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation. 2011;123:750–758. doi: 10.1161/CIRCULATIONAHA.110.974675. [DOI] [PubMed] [Google Scholar]
- 32.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 34.Sherman CT, Litvack F, Grundfest W, Lee M, Hickey A, Chaux A, Kass R, Blanche C, Matloff J, Morgenstern L, Ganz W, Swan HJC, Forrester J. Coronary angioscopy in patients with unstable angina pectoris. N Engl J Med. 1986;315:913–919. doi: 10.1056/NEJM198610093151501. [DOI] [PubMed] [Google Scholar]
- 35.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 36.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 37.Tricoci P, Huang Z, Held C, Moliterno DJ, Armstrong PW, Van de Werf F, White HD, Aylward PE, Wallentin L, Chen E, Lokhnygina Y, Pei J, Leonardi S, Rorick TL, Kilian AM, Jennings LH, Ambrosio G, Bode C, Cequier A, Cornel JH, Diaz R, Erkan A, Huber K, Hudson MP, Jiang L, Jukema JW, Lewis BS, Lincoff AM, Montalescot G, Nicolau JC, Ogawa H, Pfisterer M, Prieto JC, Ruzyllo W, Sinnaeve PR, Storey RF, Valgimigli M, Whellan DJ, Widimsky P, Strony J, Harrington RA, Mahaffey KW. Thrombin-receptor antagonist vorapaxar in acute coronary syndromes. N Engl J Med. 2012;366:20–33. doi: 10.1056/NEJMoa1109719. [DOI] [PubMed] [Google Scholar]
- 38.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–782. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 39.Brass LF, Zhu L, Stalker TJ. Translational therapeutics at the platelet vascular interface: A CME-certified activity. Novel therapeutic targets at the platelet vascular interface. Arterioscler Thromb Vasc Biol . 2008;28:s43–s50. doi: 10.1161/ATVBAHA.107.161026. [DOI] [PubMed] [Google Scholar]
- 40.Sachs UJ, Nieswandt B. In vivo thrombus formation in murine models. Circ Res. 2007;100:979–991. doi: 10.1161/01.RES.0000261936.85776.5f. [DOI] [PubMed] [Google Scholar]
- 41.McEver RP. Adhesive interactions of leukocytes, platelets, and the vessel wall during hemostasis and inflammation. Thromb Haemost. 2001;86:746–756. [PubMed] [Google Scholar]
- 42.Watson SP, Auger JM, McCarty OJ, Pearce AC. GPVI and integrin alphaIIb beta3 signaling in platelets. J Thromb Haemost. 2005;3:1752–1762. doi: 10.1111/j.1538-7836.2005.01429.x. [DOI] [PubMed] [Google Scholar]
- 43.Gibbins JM. Platelet adhesion signalling and the regulation of thrombus formation. J Cell Sci. 2004;117:3415–3425. doi: 10.1242/jcs.01325. [DOI] [PubMed] [Google Scholar]
- 44.Feng S, Lu X, Resendiz JC, Kroll MH. Pathological shear stress directly regulates platelet alphaIIbbeta3 signaling. Am J Physiol Cell Physiol. 2006;291:C1346–1354. doi: 10.1152/ajpcell.00559.2005. [DOI] [PubMed] [Google Scholar]
- 45.Yanaga F, Poole A, Asselin J, Blake R, Schieven GL, Clark EA, Law CL, Watson SP. Syk interacts with tyrosine-phosphorylated proteins in human platelets activated by collagen and cross-linking of the Fc gamma-IIA receptor. Biochem J. 1995;311:471–478. doi: 10.1042/bj3110471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibbins J, Asselin J, Farndale R, Barnes M, Law CL, Watson SP. Tyrosine phosphorylation of the Fc receptor gamma-chain in collagen-stimulated platelets. J Biol Chem. 1996;271:18095–18099. doi: 10.1074/jbc.271.30.18095. [DOI] [PubMed] [Google Scholar]
- 47.Poole A, Gibbins JM, Turner M, van Vugt MJ, van de Winkel JG, Saito T, Tybulewicz VL, Watson SP. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. Embo J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD, Theakston RD, Schweighoffer E, Zitzmann N, Morita T, Tybulewicz VL, Ozaki Y, Watson SP. A novel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 49.Abbal C, Lambelet M, Bertaggia D, Gerbex C, Martinez M, Arcaro A, Schapira M, Spertini O. Lipid raft adhesion receptors and Syk regulate selectin-dependent rolling under flow conditions. Blood. 2006;108:3352–3359. doi: 10.1182/blood-2006-04-013912. [DOI] [PubMed] [Google Scholar]
- 50.Mocsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10:387–402. doi: 10.1038/nri2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andre P, Morooka T, Sim D, Abe K, Lowell C, Nanda N, Delaney S, Siu G, Yan Y, Hollenbach S, Pandey A, Gao H, Wang Y, Nakajima K, Parikh SA, Shi C, Phillips D, Owen W, Sinha U, Simon DI. Critical role for Syk in responses to vascular injury. Blood. 2011;118:5000–5010. doi: 10.1182/blood-2011-06-360743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Jr, Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O'Gara PT, O'Rourke RA, Otto CM, Shah PM, Shanewise JS. 2006 Writing Committee Members; American College of Cardiology/American Heart Association Task Force. 2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118:e523–661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 53.Little SH, Massel DR. Antiplatelet and anticoagulation for patients with prosthetic heart valves (Review). The Cochrane Library. 2006;3:1–23. doi: 10.1002/14651858.CD003464. [DOI] [PubMed] [Google Scholar]
- 54.Elkayam U, Bitar F. Valvular heart disease and pregnancy: part II: prosthetic valves. J Am Coll Cardio.l. 2005;46:403–10. doi: 10.1016/j.jacc.2005.02.087. [DOI] [PubMed] [Google Scholar]
- 55.Chan WS, Anand S, Ginsberg JS. Anticoagulation of pregnant women with mechanical heart valves: a systematic review of the literature. Arch Intern Med. 2000;160:191–6. doi: 10.1001/archinte.160.2.191. [DOI] [PubMed] [Google Scholar]
- 56.Castellano JM, Narayan R, Vaishnava P, Fuster V. Anticoagulation during pregnancy in patients with a prosthetic heart valve. J Thorac Cardiovasc Surg. 2012 doi: 10.1038/nrcardio.2012.69. in press. [DOI] [PubMed] [Google Scholar]
- 57.Maegdefessel L, Linde T, Krapiec F, Hamilton K, Steinseifer U, van Ryn J, Raaz U, Buerke M, Werdan K, Schlitt A. In vitro comparison of dabigatran, unfractionated heparin, and low-molecular-weight heparin in preventing thrombus formation on mechanical heart valves. Thromb Res. 2010;126:e196–200. doi: 10.1016/j.thromres.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Weitz JI. Thromb Res. Vol. 123. Suppl 4: 2009. Unanswered questions in venous thromboembolism. pp. S2–S10. [DOI] [PubMed] [Google Scholar]
- 59.Struijk-Mulder MC, Etterna HB, Verheyen CC, Buller HR. Comparing consensus guidelines on thromboprophylaxis in orthopedic surgery. J Thromb Haemost. 2010;8:678–83. doi: 10.1111/j.1538-7836.2009.03728.x. [DOI] [PubMed] [Google Scholar]
- 60.Becattini C, Agnelli G, Poggio R, Eichinger S, Bucherini E, Silingardi M, Bianchi M, Moia M, Ageno W, Vandelli MR, Grandone E, Siragusa S, Salvi R, Ria L, Zanatt N, Poli D, Camporese G, Salvi A, Santi R, Cimminiello C, Scannapieco G, Barillari G, De Gaudenzi E, Cappelli R, Minno G, Frausini G, Bova C, Prandoni P. Aspirin after oral anticoagulants for prevention of recurrence in patients with unprovoked venous thromboembolism: The WARFASA study. Blood (ASH Annual Meeting Abstracts) 2011;118:543. [Google Scholar]
- 61.Kearon C. Four topics in venous thromboembolism. Circulation. 2003;107:I22–I30. doi: 10.1161/01.CIR.0000078464.82671.78. [DOI] [PubMed] [Google Scholar]
- 62.Weitz JI. Prevention and treatment of venous thromboembolism during pregnancy. Catheter Cardiovasc Interv. 2009;74(Suppl 1):S22–S26. doi: 10.1002/ccd.21994. [DOI] [PubMed] [Google Scholar]
- 63.Agnelli G, Gussoni G, Bianchini C, Verso M, Mandala M, Cavanna L, Barni S, Labianca R, Buzzi F, Scambia G, Passalacqua R, Ricci S, Gasparini G, Lorusso V, Bonizzoni E, Tonato M. Nadroparin for the prevention of thromboembolic events in ambulatory patients with metastatic or locally advanced solid cancer receiving chemotherapy: a randomized, placebo-controlled, double-blind study. Lancet Onco.l. 2009;10:943–49. doi: 10.1016/S1470-2045(09)70232-3. [DOI] [PubMed] [Google Scholar]
- 64.Verso M, Gussoni G, Agnelli G. Prevention of venous thromboembolism in patients with advanced lung cancer receiving chemotherapy: a combined analysis of the PROTECHT and TOPIC-2 studies. J Thromb Haemost. 2010;8:1649–51. doi: 10.1111/j.1538-7836.2010.03901.x. [DOI] [PubMed] [Google Scholar]
- 65.Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–07. doi: 10.1182/blood-2007-10-116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weitz JI. Emerging anticoagulants for the treatment of venous thromboembolism. Thromb Haemost. 2006;96:274–84. doi: 10.1160/TH06-05-0234. [DOI] [PubMed] [Google Scholar]
- 67.Germain M, Saut N, Greliche N, Dina C, Lambert JC, Perret C, Cohen W, Oudot-Mellakh T, Anton G, Alessi MC, Zelenika D, Cambien F, Tiret L, Bertrand M, Dupuy AM, Letenneur L, Lathrop M, Emmerich J, Amouyel P, Tregouet DA, Morange PE. Genetics of venous thrombosis: insights from a new genome wide association study. PloS One. 2011;6:e25581. doi: 10.1371/journal.pone.0025581. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stehle S, Kirchheiner J, Lazar A, Fuhr U. Pharmacogenetics of oral anticoagulants: a basis for dose individualization. Clin Pharmacokinet. 2008;47:565–94. doi: 10.2165/00003088-200847090-00002. [DOI] [PubMed] [Google Scholar]
- 69.Epstein RS, Moyer TP, Aubert RE, O'Kane DJ, Xia F, Verbrugge RR, Gage BF, Teagarden JR. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness Study). J Am Coll Cardiol. 2010;55:2804–12. doi: 10.1016/j.jacc.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 70.Carlquist JF, Anderson JL. Using pharmacogenetics in real time to guide warfarin initiation. A clinician update. Circulation. 2011;124:2554–9. doi: 10.1161/CIRCULATIONAHA.111.019737. [DOI] [PubMed] [Google Scholar]
- 71.Sakhuja R, Yeh RW, Bhatt DL. Anticoagulant agents in acute coronary syndromes. Current Problems in Cardiology. 2011;36:127–68. doi: 10.1016/j.cpcardiol.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 72.Cattaneo M. The clinical relevance of response variability to antiplatelet therapy. Hematology 2011 (American Society of Hematology Education Program Book) 2011:70–5. doi: 10.1182/asheducation-2011.1.70. [DOI] [PubMed] [Google Scholar]
- 73.Bonello L, Tantry US, Marcucci R, Blindt R, Angiolillo DJ, Becker R, Bhatt DL, Cattaneo M, Collet JP, Cuisset T, Gachet C, Montalescot G, Jennings LK, Kereiakes D, Sibbing D, Trenk D, Van Werkum JW, Paganelli F, Price MJ, Waksman R, Gurbel PA. Working Group on High On-Treatment Platelet Reactivity. Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol. 2010;56:919–33. doi: 10.1016/j.jacc.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 74.Michelson AD. Antiplatelet therapies for the treatment of cardiovascular disease. Nature Reviews Drug Discovery. 2010;9:154–69. doi: 10.1038/nrd2957. [DOI] [PubMed] [Google Scholar]
- 75.Harrison P, Frelinger AL, III, Furman MI, Michelson AD. Measuring antiplatelet drug effects in the laboratory. Thrombos Res. 2007;120:323–36. doi: 10.1016/j.thromres.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 76.Frelinger AL, Li Y, Linden MD, Tarnow I, Barnard MR, Fox ML, Michelson AD. Aspirin 'resistance': role of pre-existent platelet reactivity and correlation between tests. Journal of Thrombosis & Haemostasis. 2008;6:2035–44. doi: 10.1111/j.1538-7836.2008.03184.x. [DOI] [PubMed] [Google Scholar]
- 77.Hochholzer W, Trenk D, Frundi D, Blanke P, Fischer B, Andris K, Bestehorn HP, Büttner HJ, Neumann FJ. Time dependence of platelet inhibition after a 600-mg loading dose of clopidogrel in a large, unselected cohort of candidates for percutaneous coronary intervention. Circulation. 2005;111:2560–4. doi: 10.1161/01.CIR.0000160869.75810.98. [DOI] [PubMed] [Google Scholar]
- 78.Frelinger AL, 3rd, Li Y, Linden MD, Barnard MR, Fox ML, Christie DJ, Furman MI, Michelson AD. Association of cyclooxygenase-1-dependent and -independent platelet function assays with adverse clinical outcomes in aspirin-treated patients presenting for cardiac catheterization. Circulation. 2009;120:2586–96. doi: 10.1161/CIRCULATIONAHA.109.900589. [DOI] [PubMed] [Google Scholar]
- 79.Marcucci R, Gori AM, Paniccia R, Giusti B, Valente S, Giglioli C, Buonamici P, Antoniucci D, Abbate R, Gensini GF. Cardiovascular death and nonfatal myocardial infarction in acute coronary syndrome patients receiving coronary stenting are predicted by residual platelet reactivity to ADP detected by a point-of-care assay: a 12-month follow-up. Circulation. 2009;119:237–42. doi: 10.1161/CIRCULATIONAHA.108.812636. [DOI] [PubMed] [Google Scholar]
- 80.Bonello L, Camoin-Jau L, Armero S, Com O, Arques S, Burignat-Bonello C, Giacomoni MP, Bonello R, Collet F, Rossi P, Barragan P, Dignat-George F, Paganelli F. Tailored clopidogrel loading dose according to platelet reactivity monitoring to prevent acute and subacute stent thrombosis. American Journal of Cardiology. 2009;103:5–10. doi: 10.1016/j.amjcard.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 81.Bonello L, Camoin-Jau L, Arques S, Boyer C, Panagides D, Wittenberg O, Simeoni MC, Barragan P, Dignat-George F, Paganelli F. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol. 2008;51:1404–11. doi: 10.1016/j.jacc.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 82.Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM. TRITON-TIMI 38 Investigators. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 83.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, PLATO Investigators. Freij A, Thorsén M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 84.Michelson AD, Frelinger AL, 3rd, Braunwald E, Downey WE, Angiolillo DJ, Xenopoulos NP, Jakubowski JA, Li Y, Murphy SA, Qin J, McCabe CH, Antman EM, Wiviott SD. TRITON-TIMI 38 Investigators. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. Eur Heart J. 2009;30:1753–63. doi: 10.1093/eurheartj/ehp159. [DOI] [PubMed] [Google Scholar]
- 85.Gurbel PA, Bliden KP, Butler K, Antonino MJ, Wei C, Teng R, Rasmussen L, Storey RF, Nielsen T, Eikelboom JW, Sabe-Affaki G, Husted S, Kereiakes DJ, Henderson D, Patel DV, Tantry US. Response to ticagrelor in clopidogrel nonresponders and responders and effect of switching therapies: the RESPOND study. Circulation. 2010;121:1188–99. doi: 10.1161/CIRCULATIONAHA.109.919456. [DOI] [PubMed] [Google Scholar]
- 86.Price MJ, Berger PB, Teirstein PS, Tanguay JF, Angiolillo DJ, Spriggs D, Puri S, Robbins M, Garratt KN, Bertrand OF, Stillabower ME, Aragon JR, Kandzari DE, Stinis CT, Lee MS, Manoukian SV, Cannon CP, Schork NJ, Topol EJ. GRAVITAS Investigators. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 87.Price MJ, Angiolillo DJ, Teirstein PS, Lillie E, Manoukian SV, Berger PB, Tanguay JF, Cannon CP, Topol EJ. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the Gauging Responsiveness with a VerifyNow P2Y12 assay: Impact on Thrombosis and Safety (GRAVITAS) trial. Circulation. 2011;124:1132–7. doi: 10.1161/CIRCULATIONAHA.111.029165. [DOI] [PubMed] [Google Scholar]
- 88.Parodi G, Marcucci R, Valenti R, Gori AM, Migliorini A, Giusti B, Buonamici P, Gensini GF, Abbate R, Antoniucci D. High residual platelet reactivity after clopidogrel loading and long-term cardiovascular events among patients with acute coronary syndromes undergoing PCI. JAMA. 2011;306:1215–23. doi: 10.1001/jama.2011.1332. [DOI] [PubMed] [Google Scholar]
- 89.Gurbel PA, Bliden KP, DiChiara J, Newcomer J, Weng W, Neerchal NK, Gesheff T, Chaganti SK, Etherington A, Tantry US. Evaluation of dose-related effects of aspirin on platelet function: results from the Aspirin-Induced Platelet Effect (ASPECT) study. Circulation. 2007;115:3156–64. doi: 10.1161/CIRCULATIONAHA.106.675587. [DOI] [PubMed] [Google Scholar]
- 90.O'Donnell CJ, Larson MG, Feng D, Sutherland PA, Lindpaintner K, Myers RH, D'Agostino RA, Levy D, Tofler GH. Genetic and environmental contributions to platelet aggregation: the Framingham heart study. Circulation. 2001;103:3051–3056. doi: 10.1161/01.cir.103.25.3051. [DOI] [PubMed] [Google Scholar]
- 91.Johnson AD, Yanek LR, Chen MH, Faraday N, Larson MG, Tofler G, Lin SJ, Kraja AT, Province MA, Yang Q, Becker DM, O'Donnell CJ, Becker LC. Genome-wide meta-analyses identifies seven loci associated with platelet aggregation in response to agonists. Nat Genet. 2010;42:608–613. doi: 10.1038/ng.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gieger C, Radhakrishnan A, Cvejic A, Tang W, Porcu E, Pistis G, Serbanovic-Canic J, Elling U, Goodall AH, Labrune Y, Lopez LM, Magi R, Meacham S, Okada Y, Pirastu N, Sorice R, Teumer A, Voss K, Zhang W, Ramirez-Solis R, Bis JC, Ellinghaus D, Gogele M, Hottenga JJ, Langenberg C, Kovacs P, O'Reilly PF, Shin SY, Esko T, Hartiala J, Kanoni S, Murgia F, Parsa A, Stephens J, van der Harst P, Ellen van der Schoot C, Allayee H, Attwood A, Balkau B, Bastardot F, Basu S, Baumeister SE, Biino G, Bomba L, Bonnefond A, Cambien F, Chambers JC, Cucca F, D'Adamo P, Davies G, de Boer RA, de Geus EJ, Doring A, Elliott P, Erdmann J, Evans DM, Falchi M, Feng W, Folsom AR, Frazer IH, Gibson QD, Glazer NL, Hammond C, Hartikainen AL, Heckbert SR, Hengstenberg C, Hersch M, Illig T, Loos RJ, Jolley J, Tee Khaw K, Kuhnel B, Kyrtsonis MC, Lagou V, Lloyd-Jones H, Lumley T, Mangino M, Maschio A, Mateo Leach I, McKnight B, Memari Y, Mitchell BD, Montgomery GW, Nakamura Y, Nauck M, Navis G, Nothlings U, Nolte IM, Porteous DJ, Pouta A, Pramstaller PP, Pullat J, Ring SM, Rotter JI, Ruggiero D, Ruokonen A, Sala C, Samani NJ, Sambrook J, Schlessinger D, Schreiber S, Schunkert H, Scott J, Smith NL, Snieder H, Starr JM, Stumvoll M, Takahashi A, Tang WH, Taylor K, Tenesa A, Lay Thein S, Tonjes A, Uda M, Ulivi S, van Veldhuisen DJ, Visscher PM, Volker U, Wichmann HE, Wiggins KL, Willemsen G, Yang TP, Hua Zhao J, Zitting P, Bradley JR, Dedoussis GV, Gasparini P, Hazen SL, Metspalu A, Pirastu M, Shuldiner AR, Joost van Pelt L, Zwaginga JJ, Boomsma DI, Deary IJ, Franke A, Froguel P, Ganesh SK, Jarvelin MR, Martin NG, Meisinger C, Psaty BM, Spector TD, Wareham NJ, Akkerman JW, Ciullo M, Deloukas P, Greinacher A, Jupe S, Kamatani N, Khadake J, Kooner JS, Penninger J, Prokopenko I, Stemple D, Toniolo D, Wernisch L, Sanna S, Hicks AA, Rendon A, Ferreira MA, Ouwehand WH, Soranzo N. New gene functions in megakaryopoiesis and platelet formation. Nature. 2011;480:201–208. doi: 10.1038/nature10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eikelboom JW, Hirsh J, Weitz JI, Johnston M, Yi Q, Yusuf S. Aspirin-resistant thromboxane biosynthesis and the risk of myocardial infarction, stroke, or cardiovascular death in patients at high risk for cardiovascular events. Circulation. 2002;105:1650–1655. doi: 10.1161/01.cir.0000013777.21160.07. [DOI] [PubMed] [Google Scholar]
- 94.Undas A, Brummel K, Musial J, Mann KG, Szczeklik A. Pl(A2) polymorphism of beta(3) integrins is associated with enhanced thrombin generation and impaired antithrombotic action of aspirin at the site of microvascular injury. Circulation. 2001;104:2666–2672. doi: 10.1161/hc4701.099787. [DOI] [PubMed] [Google Scholar]
- 95.Lev EI, Patel RT, Guthikonda S, Lopez D, Bray PF, Kleiman NS. Genetic polymorphisms of the platelet receptors P2Y(12), P2Y(1) and GP IIIa and response to aspirin and clopidogrel. Thomb Res. 2007;119:355–360. doi: 10.1016/j.thromres.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 96.Herrera-Galeano JE, Becker DM, Wilson AF, Yanek LR, Bray P, Vaidya D, Faraday N, Becker LC. A novel variant in the platelet endothelial aggregation receptor-1 gene is associated with increased platelet aggregability. Arterioscler Thromb Vasc Biol. 2008;28:1484–1490. doi: 10.1161/ATVBAHA.108.168971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Caplain H, Donat F, Gaud C, Necciari J. Pharmacokinetics of clopidogrel. Semin Thromb Hemost. 1999;25:25–28. [PubMed] [Google Scholar]
- 98.Gurbel PA, Tantry US. Drug insight: Clopidogrel nonresponsiveness. Nat Clin Pract Cardiovasc Med. 2006;3:387–395. doi: 10.1038/ncpcardio0602. [DOI] [PubMed] [Google Scholar]
- 99.Shuldiner AR, O'Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, Pollin TI, Post W, Parsa A, Mitchell BD, Faraday N, Herzog W, Gurbel PA. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302:849–857. doi: 10.1001/jama.2009.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.de Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–15422. [PubMed] [Google Scholar]
- 101.Desta Z, Zhao X, Shin JG, Flockhart DA. Clinical significance of the cytochrome P450 2C19 genetic polymorphism. Clin Pharmacokinet. 2002;41:913–958. doi: 10.2165/00003088-200241120-00002. [DOI] [PubMed] [Google Scholar]
- 102.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias W, Braunwald E, Sabatine MS. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 103.Mega JL, Simon T, Collet JP, Anderson JL, Antman EM, Bliden K, Cannon CP, Danchin N, Giusti B, Gurbel P, Horne BD, Hulot JS, Kastrati A, Montalescot G, Neumann FJ, Shen L, Sibbing D, Steg PG, Trenk D, Wiviott SD, Sabatine MS. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306:2704–2714. doi: 10.1001/jama.2011.1880. [DOI] [PubMed] [Google Scholar]
- 105.Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, Steg PG, Ferrières J, Danchin N, Becquemont L. French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 106.Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, Antman EM, Braunwald E, Sabatine MS. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376:1312–1319. doi: 10.1016/S0140-6736(10)61273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wallentin L, James S, Storey RF, Armstrong M, Barratt BJ, Horrow J, Husted S, Katus H, Steg PG, Shah SH, Becker RC. PLATO investigators. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 108.Bouman HJ, Schömig E, van Werkum JW, Velder J, Hackeng CM, Hirschhäuser C, Waldmann C, Schmalz HG, ten Berg JM, Taubert D. Paraoxonase-1 is a major determinant of clopidogrel efficacy. Nat Med. 2011;17:110–116. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 109.Sibbing D, Koch W, Massberg S, Byrne RA, Mehilli J, Schulz S, Mayer K, Bernlochner I, Schömig A, Kastrati A. No association of paraoxonase-1 Q192R genotypes with platelet response to clopidogrel and risk of stent thrombosis after coronary stenting. Eur Heart J. 2011;32:1605–1613. doi: 10.1093/eurheartj/ehr155. [DOI] [PubMed] [Google Scholar]
- 110.Trenk D, Hochholzer W, Fromm MF, Zolk O, Valina CM, Stratz C, Neumann FJ. Paraoxonase-1 Q192R polymorphism and antiplatelet effects of clopidogrel in patients undergoing elective coronary stent placement. Circ Cardiovasc Genet. 2011;4:429–436. doi: 10.1161/CIRCGENETICS.111.960112. [DOI] [PubMed] [Google Scholar]
- 111.Collet JP, Hulot JS, Anzaha G, Pena A, Chastre T, Caron C, Silvain J, Cayla G, Bellemain-Appaix A, Vignalou JB, Galier S, Barthelemy O, Beygui F, Gallois V, Montalescot G. High doses of clopidogrel to overcome genetic resistance: the randomized crossover CLOVIS-2 (Clopidogrel and Response Variability Investigation Study 2). JACC Cardiovasc Interv. 2011;4:392–402. doi: 10.1016/j.jcin.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 112.Mega JL, Hochholzer W, Frelinger AL, 3rd, Kluk MJ, Angiolillo DJ, Kereiakes DJ, Isserman S, Rogers WJ, Ruff CT, Contant C, Pencina MJ, Scirica BM, Longtine JA, Michelson AD, Sabatine MS. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA. 2011;306:2221–2228. doi: 10.1001/jama.2011.1703. [DOI] [PubMed] [Google Scholar]
- 113.Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, Walker JR, Antman EM, Macias WL, Braunwald E, Sabatine MS. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation. 2009;119:2553–2560. doi: 10.1161/CIRCULATIONAHA.109.851949. [DOI] [PubMed] [Google Scholar]
- 114.Takeuchi F, McGinnis R, Bourgeois S, Barnes C, Eriksson N, Soranzo N, Whittaker P, Ranganath V, Kumanduri V, McLaren W, Holm L, Lindh J, Rane A, Wadelius M, Deloukas P. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009;5:e1000433. doi: 10.1371/journal.pgen.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cooper GM, Johnson JA, Langaee TY, Feng H, Stanaway IB, Schwarz UI, Ritchie MD, Stein CM, Roden DM, Smith JD, Veenstra DL, Rettie AE, Rieder MJ. A genome- wide scan for common genetic variants with a large influence on warfarin maintenance dose. Blood. 2008;112:1022–1027. doi: 10.1182/blood-2008-01-134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klein TE, Altman RB, Eriksson N, Gage BF, Kimmel SE, Lee MT, Limdi NA, Page D, Roden DM, Wagner MJ, Caldwell MD, Johnson JA. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med. 2009;360:753–764. doi: 10.1056/NEJMoa0809329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Anderson JL, Horne BD, Stevens SM, Grove AS, Barton S, Nicholas ZP, Kahn SF, May HT, Samuelson KM, Muhlestein JB, Carlquist JF. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116:2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 118.Califf RM, Peterson ED, Gibbons RJ, Garson A, Jr, Brindis RG, Beller GA, Smith SC., Jr. American College of Cardiology; American Heart Association. Integrating quality into the cycle of therapeutic development. J Am Coll Cardiol. 2002;40:1895–901. doi: 10.1016/s0735-1097(02)02537-8. [DOI] [PubMed] [Google Scholar]
- 119.DiMasi JA, Hansen RW, Grabowski HG. The price of innovation: new estimates of drug development costs. J Health Econ. 2003;22:151–185. doi: 10.1016/S0167-6296(02)00126-1. [DOI] [PubMed] [Google Scholar]
- 120.Light DW, Warburton R. Demythologizing the high costs of pharmaceutical research. Biosocieties. 2011;6:34–50. Available at: http://www.palgrave-journals.com/biosoc/journal/v6/n1/abs/biosoc201040a.html (accessed January 31, 2012) [Google Scholar]
- 121.Patient-Centered Outcomes Research Institute website About us. Available at: http://www.pcori.org/about/ (accessed January 31, 2012)
- 122.Dzau VJ, Ackerly DC, Sutton-Wallace P, Merson MH, Williams RS, Krishnan KR, Taber RC, Califf RM. The role of academic health science systems in the transformation of medicine. Lancet. 2010;375:949–53. doi: 10.1016/S0140-6736(09)61082-5. [DOI] [PubMed] [Google Scholar]
- 123.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–5. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 124.Smaha LA. American Heart Association. The American Heart Association Get With the Guidelines program. Am Heart J. 2004;148(5 Suppl):S46–8. doi: 10.1016/j.ahj.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 125.Flynn MR, Barrett C, Cosío FG, Gitt AK, Wallentin L, Kearney P, Lonergan M, Shelley E, Simoons ML. The Cardiology Audit and Registration Data Standards (CARDS), European data standards for clinical cardiology practice. Eur Heart J. 2005;26:308–13. doi: 10.1093/eurheartj/ehi079. [DOI] [PubMed] [Google Scholar]
- 126.Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM, Schulman KA. Ethical and scientific implications of the globalization of clinical research. N Engl J Med. 2009;360:816–23. doi: 10.1056/NEJMsb0803929. [DOI] [PubMed] [Google Scholar]
- 127.Kim ES, Carrigan TP, Menon V. International participation in cardiovascular randomized controlled trials sponsored by the National Heart, Lung, and Blood Institute. J Am Coll Cardiol. 2011;58:671–6. doi: 10.1016/j.jacc.2011.01.066. [DOI] [PubMed] [Google Scholar]
- 128.World Health Organization Fact Sheet No. 317 Cardiovascular diseases (CVDs) 2011 Sep; Available at: http://www.who.int/mediacentre/factsheets/fs317/en/index.html (accessed January 31, 2012)
- 129.New NIH policy on. COI: http://www.nih.gov/about/ethics_COI.htm (accessed April 30, 2012)
- 130.IOM report on COI. http://www.iom.edu/Activities/Workforce/ConflictOfInterest.aspx (accessed April 30, 2012)
- 131.Probstfield JL, Frye RL. Strategies for recruitment and retention of participants in clinical trials. JAMA. 2011;306:1798–9. doi: 10.1001/jama.2011.1544. [DOI] [PubMed] [Google Scholar]
- 132.Collins FS. Reengineering translational science: The time is right. Sci Transl Med. 2011;3:90cm17. doi: 10.1126/scitranslmed.3002747. [DOI] [PMC free article] [PubMed] [Google Scholar]