Abstract
Background
To determine whether the addition of molecular and imaging biomarkers to established clinical risk factors could help predict locoregional failure (LRF) after chemoradiation in human papillomavirus (HPV)-related(+) oropharyngeal cancer (OPC) and improve patient selection for locoregional treatment de-intensification.
Methods
HPV status was determined for 198 consecutive patients with Stage III/IV OPC treated with definitive chemoradiation from 5/2003–10/2010. The impact of pre-therapy epidermal growth factor receptor (EGFR) overexpression; imaging biomarkers including primary tumor and nodal maximum standardized uptake values on FDG-PET, gross tumor volumes, and matted nodes; and clinical factors on LRF (including residual disease at adjuvant neck dissection) was assessed.
Results
Primary tumors were HPV+ in 184 patients and HPV-negative in 14. EGFR overexpression was related to HPV-negative status and was univariately associated with LRF in the overall population, but was neither retained in the multivariate model after adjustment for HPV status, nor associated with LRF in HPV+ patients. Similarly, imaging biomarkers were univariately associated with LRF, but correlated with T-stage and/or N-stage and did not remain predictive in HPV+ patients after adjustment for T4- and N3-stages, which were the only significant predictors of LRF on multivariate analysis. Among HPV+ patients with non-T4- or N3-stages, only minimal smoking was associated with decreased LRF.
Conclusion(s)
The prognostic impact of EGFR overexpression and imaging biomarkers on LRF was predominantly related to their association with HPV-negative status and T- or N-stage, respectively. . Among HPV+ OPC patients treated with uniform chemoradiation, only T4-stage, N3-stage, and smoking contributed to risk-stratification for LRF.
Introduction
Increasing recognition of the favorable prognosis associated with human papillomavirus (HPV)-related (+) oropharyngeal cancer (OPC) and the morbidities associated with chemoradiotherapy has motivated efforts to reduce the intensity of multimodality therapy for patients with locally advanced HPV+ OPC [1–3]. Selection of appropriate patients for treatment de-intensification and the method by which treatment should be de-intensified, however, remain areas of ongoing controversy [2]. Recent studies have identified clinical factors such as smoking and advanced T-stage and N-stage as modifiers of survival and distant metastases in patients with HPV+ tumors [1, 4–6]. No published study, however, has explored how these factors specifically affect locoregional failure (LRF) in HPV+ OPC, an issue of relevance to patient selection for locoregional treatment modification. Furthermore, the accuracy of patient-reported smoking history is suboptimal; thus its use as a prognostic marker for treatment selection has potential uncertainty [7]. Additional objective markers to identify patients with HPV+ OPC who may be inappropriate candidates for de-escalated therapy due to increased risk of LRF, despite favorable clinical features, therefore remain highly desirable.
A number of imaging and molecular characteristics, including EGFR overexpression, tumor uptake of 18F-fluorodeoxyglucose (FDG) on pre-treatment positron emission tomography (PET), and gross tumor volume (GTV), have been identified as potential prognostic factors for both survival and LRF in head and neck squamous cell carcinoma (HNSCC) [8–12]. The impact of each of these risk factors on LRF in HPV+ OPC, however, has only been assessed as individual variables in small and/or heterogeneously treated patient cohorts [10, 13, 14]. We therefore hypothesized that the addition of EGFR overexpression, FDG-PET imaging, and radiographic tumor characteristics to traditional clinical prognostic factors may improve LRF risk-stratification of patients with HPV+ OPC.
Methods
Patients
This study was approved by the University of Michigan Institutional Review Board. Two-hundred thirty one consecutive patients with histologically confirmed, previously untreated AJCC stage III or IV oropharyngeal squamous cell carcinoma who received definitive radiotherapy and concomitant chemotherapy with curative intent at our institution from 5/2003–10/2010 were retrospectively identified. After excluding patients with unknown HPV status, those who had received induction chemotherapy, presented with distant metastases or synchronous HN cancer, underwent pre-radiotherapy neck dissection, a total of 198 patients were included.
Treatment
After staging consisting of clinical examination, direct laryngoscopy, contrast-enhanced computed tomography (CT) or FDG-PET with fused CT (PET/CT), and chest imaging, patients underwent CT-simulation in a 5-point thermoplastic mask. All patients received intensity-modulated radiation therapy (IMRT), with prescription doses of 70 Gy to the high-risk clinical target volume (CTV) consisting of the gross tumor volume (GTV) with tight margins, 59–64 Gy to intermediate-risk CTVs, and 56–59 Gy to low-risk CTVs. CTVs were uniformly expanded 3–5 mm to create planning target volumes. Nearly all patients (98%) received once-daily IMRT delivered over 35 fractions, while the remainder (2%) received twice-daily IMRT at 1.25 Gy per fraction. All patients received concurrent chemotherapy with RT, the large majority (96%) with weekly carboplatin (AUC 1) and paclitaxel (30 mg/m2) and the remainder (4%) with cisplatin-based regimens.
All patients were routinely seen in follow-up in the Departments of Radiation Oncology, Otolaryngology, and Hematology/Oncology, with clinical examination performed every 6–12 weeks and head and neck imaging (either contrast-enhanced CT or PET/CT) obtained at 3 months following completion of chemoradiation. Post-chemoradiotherapy neck management evolved over the study period; in earlier years, patients with advanced nodal disease at presentation often underwent planned adjuvant neck dissection after chemoradiation, while in later years patients were clinically and radiographically observed with adjuvant neck dissection performed only for clinical, radiographic, or scintigraphic suspicion of residual disease at 3–6 months after completion of chemoradiation.
HPV, p16, and EGFR Expression
HPV detection was performed using either multiplex polymerase chain reaction (PCR) MassArray following DNA extraction from a core tissue sample for 179 patients (90%) whose tumors were banked as part of a prospectively assembled tissue microarray as previously described [15, 16], or in-situ hybridization (ISH) for high-risk HPV using the INFORM HPV ISH assay (Ventana Medical Systems Inc., Tucson, AZ) with a cocktail directed against a subset of high-risk HPV genotypes (HPV 16, 18, 33, 35, 39, 45, 51, 52, 56, and 66) for 19 patients (10%) as part of routine clinical practice towards the end of the study period. ISH and immunohistochemical stains were performed on 4µm paraffin-embedded tissue block sections containing a representative sample of primary tumor; PCR was performed on DNA isolated from primary tumor cores using the QIAamp DNA FFPE Tissue Kit (Qiagen, Valencia, CA) with DNA concentration and purity confirmed via NanoDrop spectrophotometer (Thermo Scientific, Waltham, MA). Immunohistochemical staining for p16INK4a was performed per the manufacturer’s protocol (CINtec Histology Kit; MTM Laboratories, Heidelberg, Germany) and visualized using the ultraView polymer detection system (Ventana Medical Systems Inc., Tucson, AZ) on a Ventana Benchmark Ultra autostainer. p16 was considered positive when strong nuclear and cytoplasmic staining was present in > 75% of tumor cells..Performance characteristics for each of these HPV assays and p16 immunohistochemistry have been previously described[17]. HPV status was classified as positive if either HPV or p16-expression assays were positive. EGFR immunostaining was performed using primary antibody EGFR/31G7 (Zymed Laboratories, South San Francisco, CA). EGFR expression was scored as 1 (none), 2 (low), 3 (moderate), or 4 (high) intensity, and averaged from multiple cores per patient, as previously described [13, 16].
Tumor Volume and Imaging Characteristics
Primary and nodal gross tumor volumes (pGTV and nGTV, respectively) were calculated in milliliters (ml) from the contrast-enhanced simulation CT scan used for RT planning, using the UMplan treatment planning software. The presence of matted lymph nodes, defined as 3 nodes abutting one another with replacement of the intervening fat plane by extracapsular spread, was recorded as previously described [16].
SUVmax Measurement
Pre-therapy FDG-PET/CT studies were available for 140 (64%) patients. Per institutional protocol, patients fasted for >4–6 hours and had glucose levels <250 mg/dL prior to undergoing PET/CT. Sixty minutes following intravenous administration of 300 MBq (8 mCi) of FDG, sequential PET and CT imaging was performed on an integrated PET/CT scanner (Siemens Biograph T6; Siemens Medical Solutions, Hoffman Estates, IL, USA). Helical CT from skull vertex to mid-thigh was performed with 5 mm collimation, followed immediately by whole body PET at multiple overlapping bed positions and then by dedicated contrast-enhanced head and neck CT. Contrast-enhanced head and neck CT and attenuation-corrected FDG-PET images were co-registered and reviewed on a workstation using software with fusion capability (MedImage; MedView Pty, Canton, MI, USA) by 2 readers (one head and neck radiologist and one nuclear medicine physician) providing a single read per study. A region of interest was defined for each primary tumor and for cervical lymph nodes (LNs) displaying FDG uptake above background using the corresponding CT images for anatomic orientation. The maximum standardized uptake values (SUVmax) for the primary tumor and for the involved LN with the highest SUVmax on PET were retrospectively recorded.
Study Endpoints
LRF was defined as any persistent or recurrent squamous cell carcinoma above the clavicles as a component of first failure after completion of chemoradiation, including residual disease present on post-chemoradiotherapy neck dissection. Time to failure was measured from the first day of RT to either the date of histological confirmation of failure, or the date of conclusive imaging of recurrence if pathological confirmation was not obtained.
Patterns of Failure Analysis
Imaging studies and operative reports at the time of LRFs were reviewed and the location of LRF classified as within the GTV (i.e. high-risk CTV), intermediate-risk CTV, or low-risk CTV (defined as the LRF epicenter located inside the 95% prescription dose isodose line [IDL] for each target volume), marginal failure (LRF epicenter located between 20% and 95% IDLs), or out-of-field failure (LRF epicenter outside 20% IDL), as previously defined [18].
Statistical Analysis
Baseline characteristics of HPV+ and HPV-negative patients were compared using independent samples t-test, Welch’s test, or Chi-square test, as appropriate. Smoking status was determined retrospectively by review of the patient tobacco history documented by each multidisciplinary specialty (Head and Neck Surgery, Radiation Oncology, and Medical Oncology) in the electronic medical record. Patients were categorized as never, former (quit > 6 months prior to diagnosis), or current smokers, and number of pack-years were recorded. Spearman rank correlation (ρ) was used to test correlation between baseline characteristics. Rates of LRF, local failure (LF), regional failure (RF), and distant failure (DF) as first failure were estimated using Kaplan-Meier methods, with patients censored at date of last follow-up, treatment failure at another site, second primary tumor, or death. The log-rank test was used to compare rates of failure between patient groups. Univariate Cox regression was used to determine associations between co-variates and LRF for the entire population and HPV+ cohort. Variables associated with LRF with p<0.20 were entered into a stepwise regression model with entry and removal at the 5% and 10% significance level, respectively. For regression analyses, age, smoking pack-years, pGTV, nGTV, pSUVmax, and nSUVmax were treated as continuous variables, whereas categorical variables were dichotomized (EGFR expression: high vs. none/low/moderate; smoking status: current vs. never/former). T-stage (T4 vs. T1–3) and N-stage (N3 vs. N0-2c) were dichotomized as per the recently proposed risk-classifications for HPV+ OPC [5]. A two-sided p-value ≤0.05 was used to denote statistical significance for all analyses. All statistical analyses were performed using MedCalc (v12.2.1.0, MedCalc Software, Mariakerke, Belgium).
Results
Patient Characteristics
For the 198 patients with established HPV status, 184 (92.9%) were HPV+ and 14 (7.1%) were HPV-negative. HPV and p16 expression were concordant in 172/183 (94%) patients for whom both were determined. Discordant results occurred in 10 HPV+/p16-negative patients and 1 HPV-negative/p16+ non-smoker. Baseline characteristics for the entire cohort are shown in Table 1. Patients with HPV+ tumors were younger and had smaller pGTVs, less tobacco exposure, and lower EGFR over-expression compared with HPV-negative patients. EGFR expression positively correlated with HPV-negative tumors (Spearman’s rank correlation coefficient ρ=0.24,p=0.001) and pSUVmax (ρ=0.30,p=0.001), and trended toward correlation with T-stage (p=0.087), but not pGTV (p=0.41), smoking status (p=0.72), or smoking pack-years (p=0.99). pSUVmax correlated positively with T-stage (ρ=0.37,p<0.001) and pGTV (ρ=0.28,p=0.001), while nSUVmax correlated with N-stage (ρ=0.38,p<0.001), nGTV (ρ=0.69,p<0.001) and matted nodes (ρ=0.30,p<0.001).
Table 1.
Patient, Tumor, and Treatment Characteristics
| Characteristic | HPV Positive (n=184) |
HPV Negative (n=14) |
p-value (HPV+ vs. HPV-) |
|||
|---|---|---|---|---|---|---|
| Median | Range | Median | Range | |||
| Age (years) | 55 | 34 – 78 | 59 | 50–76 | 0.052 | |
| Pack-years | 5.5 | 0 – 140 | 34 | 0* – 68 | 0.001 | |
| Primary GTV† (ml) | 44.5 | 3.1 – 240.2 | 75.9 | 17.7 – 185.6 | 0.018 | |
| Nodal GTV† (ml) | 27.3 | 0 – 294.1 | 27.7 | 0 – 294.1 | 0.94 | |
| Primary Tumor SUVmax†† (n=139) | 11.7 | 3.1 – 32.2 | 10.5 | 4.5 – 18.7 | 0.49 | |
| Nodal SUVmax†† (n=140) | 9.1 | 0 – 37.3 | 8.9 | 5.8 – 18.6 | 0.61 | |
| N | % | N | % | |||
| Male Sex | 164 | 89.1% | 12 | 85.7% | 0.96 | |
| Smoking Status | ||||||
| Current | 51 | 27.7% | 13 | 92.9% | <0.0001 | |
| Former | 61 | 33.2% | 1 | 7.1% | ||
| Never | 72 | 39.7% | 0 | 0.0% | ||
| T-stage | ||||||
| T1 | 29 | 15.8% | 1 | 7.1% | 0.072 | |
| T2 | 72 | 39.1% | 2 | 14.3% | ||
| T3 | 34 | 18.5% | 6 | 42.9% | ||
| T4 | 49 | 26.6% | 5 | 35.7% | ||
| N-stage | ||||||
| N0 | 10 | 5.4% | 1 | 7.1% | 0.90 | |
| N1 | 13 | 7.1% | 2 | 14.3% | ||
| N2a | 15 | 8.2% | 0 | 0.0% | ||
| N2b | 77 | 41.8% | 6 | 42.9% | ||
| N2c | 45 | 24.5% | 2 | 14.3% | ||
| N3 | 24 | 13.0% | 3 | 21.4% | ||
| Matted Nodes Present | 32 | 17.4% | 5 | 35.7% | 0.18 | |
| EGFR‖ Expression (n=176) | ||||||
| None | 34 | 21.0% | 1 | 7.7% | 0.0031 | |
| Low | 41 | 25.3% | 0 | 0.0% | ||
| Moderate | 36 | 22.2% | 2 | 15.4% | ||
| High | 51 | 31.5% | 10 | 76.9% | ||
| Adjuvant Neck Dissection | 40 | 21.7% | 6 | 42.8% | 0.14 | |
HPV-negative patient with 0 pack-years was a heavy chewing tobacco user;
Gross Tumor Volume;
Maximum Standardized Uptake Value;
Epidermal Growth Factor Receptor
Forty (22%) HPV+ positive patients and 6 (43%) HPV-negative patients underwent post-chemoradiation neck dissection within 6 months of chemoradiation completion (p=0.14). Neck dissections were performed as planned adjuvant locoregional therapy in 23 (58%) HPV+ patients and 0 (0%) HPV-negative patients, for residual neck mass on clinical exam or CT in 10 (25%) HPV+ patients and 3 (50%) HPV-negative patients, and for PET/CT-based suspicion of residual disease in 7 (18%) HPV+ patients and 3 (50%) HPV-negative patients. Patients with N3 disease were more likely to undergo post-CRT neck dissection than those with N0-N2c disease (48% vs. 18%, respectively, p=0.002).
Locoregional Failure by HPV Status
At a median follow-up of 53.7 months (range 14.9–109.3) for living patients, treatment failure at any site occurred in 44/184 (23.9%) HPV+ patients and 9/14 (64.3%) HPV-negative patients (3-year failure 22.6% vs. 50.0%, hazard ratio [HR] 0.34, log-rank p=0.002). LRF as a component of first failure (including residual disease at neck dissection) occurred in 27 (14.7%) HPV+ and 7 (50.0%) HPV-negative patients. LRF consisted of LF in 6 (3.3%) HPV+ and 4 (28.6%) HPV-negative patients, and RF in 24 (13.0%) HPV+ and 3 (21.4%) HPV-negative patients. 3 HPV+ patients experienced synchronous LF and RF.
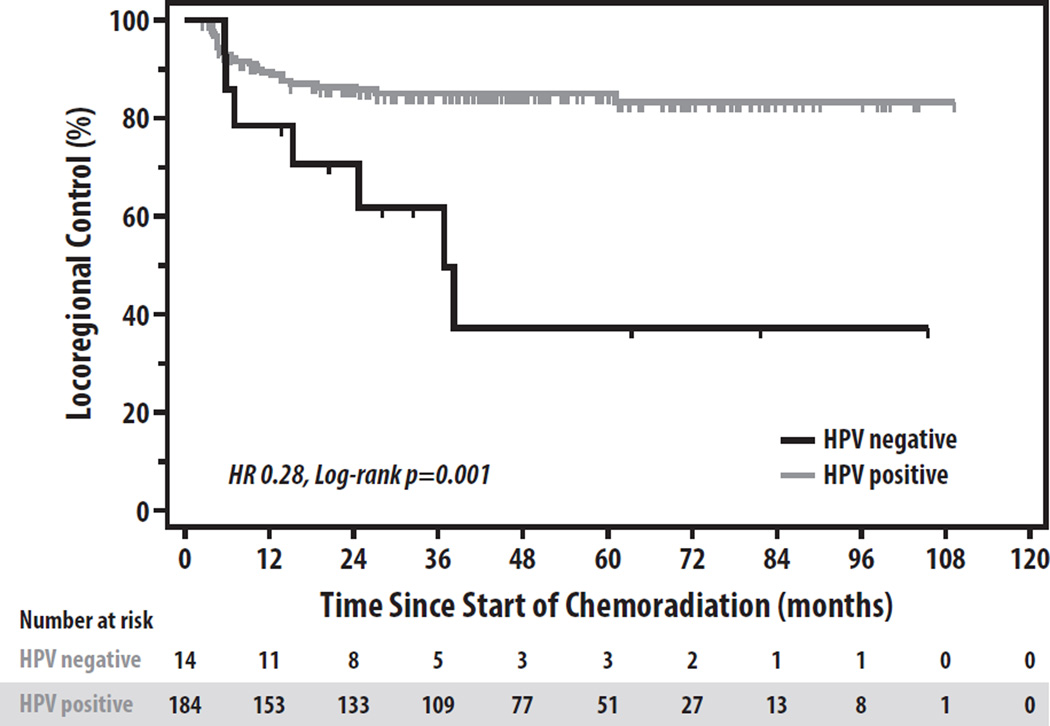
LRF was more common among HPV-negative patients than HPV+ patients (3-yr LRF 38.1% vs. 14.9%, HR 0.28, p=0.001) (Figure 1), primarily due to a higher rate of LF (3-yr LF 27.8% vs. 3.0%, HR 0.10, p<0.001). RF was similar between HPV-negative and HPV+ patients (3-yr RF 14.3% vs. 13.8%), though RFs in 50% (12/24) of HPV+ patients and 33% (1/3) of HPV-negative patients were due solely to residual disease on neck dissection within 6 months of completion of CRT. If residual disease at adjuvant neck dissection is not included as a RF event, LRF still remained more common in HPV-negative than HPV+ patients (3-yr LRF 31.2% vs. 7.8%, p<0.001). DF was similar between HPV+ and HPV-negative patients (3-yr DF 10.0% vs. 10.5%, p=0.42), while patients with HPV+ tumors had superior survival to those with HPV-negative disease (3-yr overall survival 88.9% vs. 52.4%, HR 0.24, p<0.001).
Figure 1.
Locoregional Control by HPV Status.
Patterns of Locoregional Failure
Almost all LRFs occurred within the GTV (i.e. within the 70 Gy IDL) in both the HPV+ (23/27; 85%) and HPV-negative (7/7; 100%) cohorts. All 4 LRFs outside the GTV were RFs, only one of which occurred within an elective CTV (failure in the contralateral jugulodigastric node within the 63 Gy IDL [intermediate-risk CTV] in a current smoker with a T3N0 base-of-tongue cancer). The 3 other LRFs which occurred outside the GTV consisted of a marginal recurrence (within the 50 Gy IDL) in the right thyroid lobe in a never-smoker with a T4N2c base-of-tongue primary and right-sided level 3 adenopathy, an out-of-field contralateral retropharyngeal node recurrence near the skull base in a never-smoker with a T4N0 base-of-tongue primary, and an out-of-field recurrence at the sternal notch in a former-smoker with a T4N2b base-of-tongue primary with gross extracapsular spread infiltrating the adjacent sternocleidomastoid muscle.
Predictors of Locoregional Failure in All Patients
Univariate and stepwise multivariate Cox regression models for the entire 198 patient cohort are shown in Tables 2 and 3, respectively. On univariate analysis, HPV status (HR 0.27,p=0.002), pack-years (HR 1.01,p=0.017), current smoking (HR 2.27,p=0.017), T4-stage (HR 2.09,p=0.035), N3-stage (HR 3.18,p=0.003), p16 overexpression (HR 0.41,p=0.028), high EGFR expression (HR 2.53,p=0.012), pGTV (HR 1.01,p=0.028), nGTV (HR 1.01,p=0.010), and matted nodes (HR 2.38,p=0.027) were all significantly associated with LRF, while pSUVmax (p=0.072) and nSUVmax (p=0.12) trended towards significance. On stepwise regression, HPV status (HR 0.42,p=0.046), N3 stage (HR 3.18,p=0.004), and pack-years (HR 1.01,p=0.038) independently predicted LRF.
Table 2.
Univariate Cox Regression for Locoregional Failure after Chemoradiation in the Overall and HPV-positive Oropharyngeal Cancer Cohorts. Co-variates with p<0.05 displayed in bold.
| ALL PATIENTS (N=198) | HPV+ COHORT (N=184) | |||
|---|---|---|---|---|
| Characteristic | HR (95% CI) | P | HR (95% CI) | P |
| Age | 0.2032 (0.03 to 1.47) | 0.117 | 0.99 (0.95 to 1.04) | 0.763 |
| HPV Status (positive vs. negative) | 0.27 (0.12 to 0.63) | 0.002 | - | - |
| Pack-Years | 1.01 (1.00 to 1.02) | 0.017 | 1.01 (1.00 to 1.02) | 0.160 |
| Smoking History (current vs. other) | 2.27 (1.16 to 4.42) | 0.017 | 1.65 (0.76 to 3.58) | 0.211 |
| T-stage (T4 vs. T1-3) | 2.09 (1.06 to 4.12) | 0.035 | 2.14 (0.99 to 4.59) | 0.053 |
| N-stage (N3 vs. N0-2c) | 3.18 (1.49 to 6.82) | 0.003 | 2.20 (1.07 to 4.55) | 0.033 |
| EGFR* Expression (high vs. other) (n=173) | 2.53 (1.23 to 5.19) | 0.012 | 1.71 (0.75 to 3.87) | 0.205 |
| Primary GTV† | 1.01 (1.00 to 1.01) | 0.028 | 1.00 (1.00 to 1.01) | 0.290 |
| Nodal GTV† | 1.01 (1.00 to 1.01) | 0.010 | 1.01 (1.00 to 1.01) | 0.010 |
| Matted Nodes (present vs. none) | 2.38 (1.11 to 5.12) | 0.027 | 2.46 (1.04 to 5.84) | 0.042 |
| Primary Tumor SUVmax†† (n=139) | 1.07 (0.99 to 1.15) | 0.072 | 1.08 (0.99 to 1.16) | 0.068 |
| Nodal SUVmax†† (n=140) | 1.05 (0.99 to 1.10) | 0.12 | 1.05 (0.99 to 1.11) | 0.083 |
Epidermal Growth Factor Receptor;
Gross Tumor Volume;
Maximum Standardized Uptake Value
Table 3.
Stepwise Multivariate Cox Regression for Locoregional Failure after Chemoradiation in the All Patients and the HPV+ Cohorts
| ALL PATIENTS (N=198) | ||
| Characteristic* | HR | P-value |
| Pack-Years | 1.01 (1.00 to 1.02) | 0.038 |
| N-stage (N3 vs. N0-2c) | 3.18 (1.44 to 6.98) | 0.004 |
| HPV Status (Positive vs. negative) | 0.42 (0.18 to 0.98) | 0.046 |
| HPV-POSITIVE COHORT (N=184) | ||
| Characteristic* | HR | P-value |
| T-stage (T4 vs. T1-3) | 2.17 (1.01 to 4.67) | 0.048 |
| N-stage (N3 vs. N0-2c) | 3.99 (1.75 to 9.11) | 0.001 |
Other characteristics which were statistically significant in the univariate models (Table 2) did not improve the multivariate model fit and were therefore omitted
Predictors of Locoregional Failure in HPV+ Patients
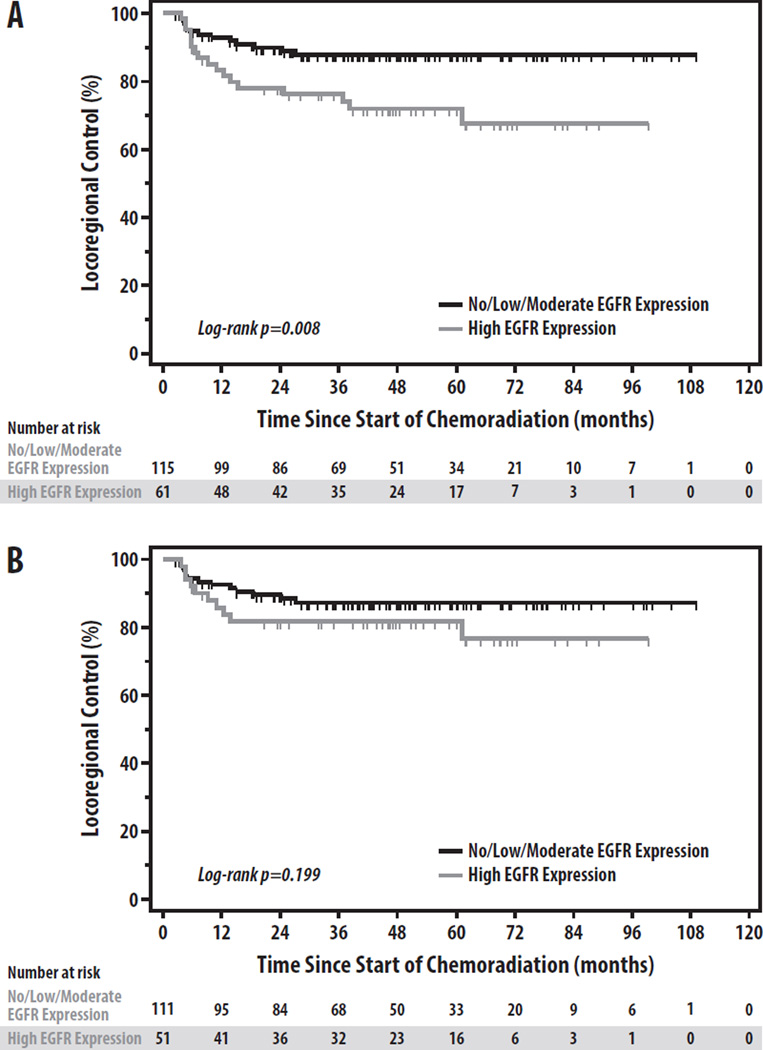
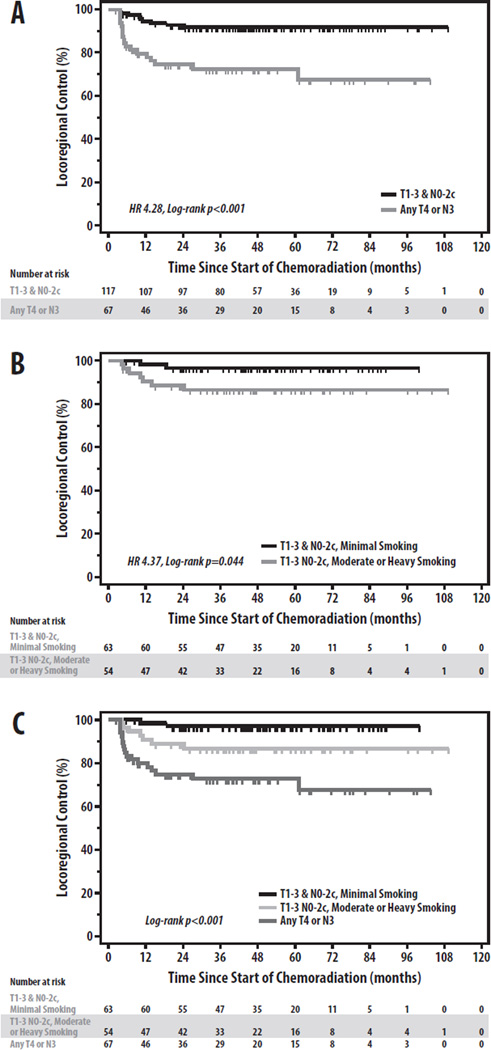
To further assess the relationship between these factors and LRF in HPV+ OPC, we restricted our analysis to the 184 HPV+ patients. On univariate analysis, N3 stage (p=0.001), nGTV (p=0.010), and matted nodes (p=0.042) were significantly associated with LRF, while T4 stage (p=0.053), pSUVmax (p=0.068), and nSUVmax (p=0.083) all trended toward significance (Table 2). Notably, whereas EGFR overexpression, pack-years, and pGTV had been univariately associated with LRF in the overall cohort, none of these factors were associated with LRF in the HPV+ subset (all p>0.15). LRF by EGFR expression in the overall and HPV+ cohorts is shown in Figure 2. Negative p16 expression in HPV+ patients, although infrequent, was similarly not associated with worse LRF (3-yr LRF 15.2% vs. 14.9%, respectively, p=0.63). On multivariate regression (Table 3), T4 stage (HR 2.17, p=0.048) and N3 stage (HR 3.99, p=0.001) were the only independent predictors of LRF (Figure 3a). Stratifying the HPV+ cohort into high-(T4 or N3) and low-risk (T1–3 and N0–2c) groups by these factors yields significant differences in LRF (3-yr LRF 27.5% vs. 8.0%, HR 4.28, p<0.001) (Figure 3a)[5].
Figure 2.
Locoregional Control by EGFR Overexpression in the (A) Overall and (B) HPV+ Cohorts
Figure 3.
Risk-Stratification for Locoregional Control in HPV-Related Oropharyngeal Cancer. Locoregional control stratified by (A) T- and N-stage in all HPV+ patients, (B) Smoking history (≥vs.< 10 pack-years) in T1-3 N0-2c patients, and (C) T-stage, N-stage, and Smoking history among all HPV+ patients.
Predictors of Locoregional Failure among T1-3 N0-2c HPV+ Patients
As our findings demonstrate that T1-3 N0-2c HPV+ patients represent a group at lower risk for LRF, we sought to determine whether any additional risk factors could be identified to further risk-stratify this sub-population (n=108). On univariate regression, only smoking pack-years (p=0.059) trended towards significance; no association with LRF was observed for any other clinical, molecular, or imaging factors (p>0.20 for all co-variates). Stratifying T1-3 N0-2c patients by minimal vs. moderate/heavy smoking (<or ≥10 pack-years) revealed significantly lower LRF in those with minimal smoking (3-yr LRF 3.3% vs. 13.6%, HR 4.37, log-rank p=0.04) (Figure 3b). Combining the regression analysis results for the HPV+ cohort suggests 3 distinct LRF risk groups in HPV+ OPC, with T4/N3 patients at highest risk (3-yr LRF 27.5%), T1-3 N0-2c moderate/heavy smokers at intermediate risk, and T1-3 N0-2c minimal smokers at lowest risk (p<0.001)(Figure 3c). Among non-N3 patients with ≥ 10 smoking pack-years, LRF did not differ between patients with N0-2a and N2b-c disease, irrespective of inclusion of those with only T1-3 stages (n=54: 3-yr LRF 17.0% vs. 11.6%, respectively; p=0.62) or all T-stages (n=76: 3-yr LRF 13.5% vs. 14.0%, respectively; p=0.72).
Discussion
The primary finding of our study is that in HPV+ OPC, the established clinical risk factors T4-stage, N3- stage, and smoking history were the only predictive factors for LRF. These criteria enabled patient stratification into low-, intermediate-, and high-risk groups. While this conclusion parallels those of Ang et al. and O’Sullivan et al., whose reports examined the endpoints DF and survival [1, 5], respectively, ours is the first study in HPV+ OPC to specifically investigate predictors of LRF. This has distinct implications for risk-adapted local-regional treatment de-intensification.
Our study attempted to identify whether EGFR expression, SUVmax, gross tumor volume, and imaging evidence of matted nodes could further refine risk-stratification in HPV+ OPC, based upon their prognostic value for LRF and survival in prior studies of HNSCC [8–12, 16]. Although several of these biomarkers were associated with LRF on univariate analysis, none remained significant on multivariate analysis in either the overall population or the HPV+ cohort. While EGFR overexpression was strongly associated with HPV-negative tumors, no statistically significant association with LRF was observed in HPV+ tumors, suggesting that EGFR overexpression may be a hallmark of HPV-negative tumors with little impact on LRF risk in HPV+ OPC. This finding is consistent with a few previous publications [10, 19, 20], but contrasts with other studies demonstrating inferior LRF and survival in HPV+ patients with EGFR overexpression [13, 21]. It is possible that much larger patient numbers would reveal a statistically significant relationship between EGFR overexpression and LRF in HPV+ patients, as a small non-significant trend was observed in our study (Figure 2B). However, even if found to be statistically significant in a larger cohort, EGFR overexpression appears unlikely to be a clinically useful biomarker for LRF risk-stratification in HPV+ OPC.
Our study additionally did not confirm SUVmax as an independent predictor of LRF in HPV+ OPC. While both pSUV and nSUV trended towards significance on univariate analysis, neither was retained in the multivariate model, likely due to their correlation with T-stage and N-stage (p<0.001 for both). Although earlier studies of FDG-PET in mixed HNSCC cohorts suggested that pSUVmax was predictive for LF and survival after radiotherapy [9, 22, 23], our findings are consistent with a recent prospective study demonstrating that pre-treatment SUVmax lacks predictive value in HPV+ OPC [14]. Similar findings have been shown in a recently published surgical series, in which SUVmax failed to predict for disease-specific survival after adjustment for co-variates including HPV status [24]. In contrast to SUVmax, which measures the maximal metabolic activity of a single pixel, more global measures such as metabolic tumor volume(MTV) [14] and mean SUV (SUVmean)[25] have recently been demonstrated as independent predictors of outcome in HNSCC. While the broad clinical applicability of such advanced PET metrics may be presently limited due to the sophisticated software applications required for their measurement, FDG-PET MTV and SUVmean may indeed prove useful in the future for risk-stratification if validated specifically in HPV+ OPC patients [14].
Although our study did not demonstrate a role for several putative molecular and radiographic biomarkers in predicting LRF in HPV+ OPC, our results importantly support a risk-classification for LRF in this population, in which T4 and N3 patients are high-risk, while patients with T1-3 N0-2c disease are stratified into intermediate- and low-risk groups based upon smoking history (Figure 3c). Interestingly, although we specifically investigated the endpoint of LRF, the resultant risk-classification bears striking resemblance to those proposed for DF by O’Sullivan et al. and for survival by Ang et al. [1, 5]. One important difference between our results and the latter analysis is the categorization of moderate/heavy smokers with N0-2a stages, who in the present analysis had a similar risk of LRF as moderate/heavy smokers with N2b-2c stages, supporting their inclusion in an intermediate-risk, rather than low-risk, group. This subtlety aside, the especially low rates of LRF (3.3% at 3-years) observed in minimal smokers with HPV+ T1-3 N0-2c tumors mirror those in these recent reports, and provide further support for considering the inclusion of such patients in protocols that reduce the intensity of both locoregional and systemic therapy [2, 3, 5]. The scarcity of failures in the electively-treated CTVs, which occurred in only one patient in our series, additionally suggests that elective CTVs may be rationale targets for radiotherapy dose de-escalation, a strategy which may translate into lower doses to adjacent organs-at-risk whose chemoradiation is associated with long-term morbidity [26, 27]. In contrast, the significantly higher 3-yr LRF estimates of 13.6% in T1-3 N0-2c moderate/heavy smokers and 27.5% in T4/N3 patients argue for excluding such patients from studies of chemoradiotherapy de-escalation.
The main strength of our study is that it represents the largest investigation of the combined prognostic impact of EGFR expression, FDG-PET SUVmax, and tumor volume on LRF in a uniformly-treated cohort of HPV+ OPC patients. Limitations of this study include its retrospective nature, small number of HPV-negative patients that reflect the changing epidemiology of OPC in the past decade, non-uniform use of post-chemoradiotherapy neck dissection, and limited number of LRF events, which may have limited the statistical power of our analysis. It is also noteworthy that we included the presence of residual disease at adjuvant neck dissection as a LRF event, which accounted for 50% of RFs in the HPV+ cohort. While this methodology contrasts with that employed by prior investigators, the inclusion of residual neck disease as an RF event provides the most conservative estimates of locoregional control after definitive chemoradiation, whereas the exclusion of these events as RFs could result in an overestimation of the ability of chemoradiotherapy to sterilize all locoregional disease, and thus potentially misinform patient risk-stratification in the design of future treatment de-intensification efforts. This methodological distinction may explain the somewhat higher rate of RF observed in our series compared with prior reports of chemoradiation for OPC [12, 28].
In summary, the putative biomarkers EGFR overexpression, SUVmax, pGTV, nGTV, and imaging evidence of matted nodes were unable to independently predict LRF in this cohort of HPV+ OPC patients treated with uniform chemoradiation, in whom only T4-stage, N3-stage, and smoking contributed to risk-stratification for LRF. These findings support careful, prospective clinical studies to reduce local-regional treatment intensity in HPV+ patients at lowest risk for LRF.
Acknowledgments
Supported in part by The University of Michigan Head and Neck Specialized Program of Research Excellence (SPORE): P50CA097248. The Molecular Basis of Head and Neck Cancer Biology, the NIH NIDCR R01 DE019126 Biomarkers to Guide Treatment and Improve Survival in Oral and Oropharyngeal Cancer, and by the Newman Family Research Fund. HMW was supported by the Cancer Biology Training Grant and the Eleanor Lewis Graduate Student Scholarship. JMV supported in part by the Woodworth Award for Excellence in Radiation Oncology Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial conflicts of interest to disclose.
Contributor Information
Jeffrey M. Vainshtein, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI.
Matthew E. Spector, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Jonathan B. McHugh, Department of Pathology, University of Michigan, Ann Arbor, MI.
Ka Kit Wong, Division of Nuclear Medicine, Department of Radiology, University of Michigan, Ann Arbor, MI.
Heather M. Walline, Doctoral Program in Cancer Biology, University of Michigan, Ann Arbor, MI.
Serena A. Byrd, University of Michigan School of Medicine, Ann Arbor, MI.
Christine M. Komarck, Department of Otolaryngology, University of Michigan, Ann Arbor, MI
Mohannad Ibrahim, Department of Radiology, University of Michigan, Ann Arbor, MI.
Matthew H. Stenmark, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI.
Mark E. Prince, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Carol R. Bradford, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Gregory T. Wolf, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Scott McLean, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Francis P. Worden, Division of Hematology Oncology, Department of Internal Medicine, University of Michigan Health System, Ann Arbor, MI.
Douglas B. Chepeha, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Thomas Carey, Department of Otolaryngology – Head and Neck Surgery, University of Michigan, Ann Arbor, MI.
Avraham Eisbruch, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI.
References
- 1.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quon H, Forastiere AA. Controversies in treatment deintensification of human papillomavirusassociated oropharyngeal carcinomas: should we, how should we, and for whom? J Clin Oncol. 2013;31:520–522. doi: 10.1200/JCO.2012.46.7746. [DOI] [PubMed] [Google Scholar]
- 3.Patel SC, Hackman T, Hayes DN, Chera BS. De-intensification of treatment for human papilloma virus associated oropharyngeal squamous cell carcinoma: A discussion of current approaches. Pract RadiatOncol. 2012;2:282–287. doi: 10.1016/j.prro.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 4.O'Sullivan B, Huang SH, Perez-Ordonez B, Massey C, Siu LL, Weinreb I, et al. Outcomes of HPV-related oropharyngeal cancer patients treated by radiotherapy alone using altered fractionation. RadiotherOncol. 2012;103:49–56. doi: 10.1016/j.radonc.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 5.O'Sullivan B, Huang SH, Siu LL, Waldron J, Zhao H, Perez-Ordonez B, et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J Clin Oncol. 2013;31:543–550. doi: 10.1200/JCO.2012.44.0164. [DOI] [PubMed] [Google Scholar]
- 6.Gillison ML, Zhang Q, Jordan R, Xiao W, Westra WH, Trotti A, et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. JClin Oncol. 2012;30:2102–2111. doi: 10.1200/JCO.2011.38.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warren GW, Arnold SM, Valentino JP, Gal TJ, Hyland AJ, Singh AK, et al. Accuracy of self-reported tobacco assessments in a head and neck cancer treatment population. Radiother Oncol. 2012;103:45–48. doi: 10.1016/j.radonc.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao KS, Ozyigit G, Blanco AI, Thorstad WL, Deasy JO, Haughey BH, et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: impact of tumor volume. Int J Radiat Oncol Biol Phys. 2004;59:43–50. doi: 10.1016/j.ijrobp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz DL, Rajendran J, Yueh B, Coltrera MD, Leblanc M, Eary J, et al. FDG-PET prediction of head and neck squamous cell cancer outcomes. Arch Otolaryngol Head Neck Surg. 2004;130:1361–1367. doi: 10.1001/archotol.130.12.1361. [DOI] [PubMed] [Google Scholar]
- 10.Hong A, Dobbins T, Lee CS, Jones D, Jackson E, Clark J, et al. Relationships between epidermal growth factor receptor expression and human papillomavirus status as markers of prognosis inoropharyngeal cancer. Eur J Cancer. 2010;46:2088–2096. doi: 10.1016/j.ejca.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 11.Ang KK, Berkey BA, Tu X, Zhang HZ, Katz R, Hammond EH, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 12.Lok BH, Setton J, Caria N, Romanyshyn J, Wolden SL, Zelefsky MJ, et al. Intensity-modulated radiation therapy in oropharyngeal carcinoma: effect of tumor volume on clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82:1851–1857. doi: 10.1016/j.ijrobp.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar B, Cordell KG, Lee JS, Worden FP, Prince ME, Tran HH, et al. EGFR, p16, HPV Titer, Bcl-xL andp53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J ClinOncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang C, Murphy JD, Khong B, La TH, Kong C, Fischbein NJ, et al. Validation that metabolic tumor volume predicts outcome in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2012;83:1514–1520. doi: 10.1016/j.ijrobp.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang AL, Hauff SJ, Owen JH, Graham MP, Czerwinski MJ, Park JJ, et al. UM-SCC-104: a new humanpapillomavirus-16-positive cancer stem cell-containing head and neck squamous cell carcinoma cell line. Head Neck. 2012;34:1480–1491. doi: 10.1002/hed.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spector ME, Gallagher KK, Light E, Ibrahim M, Chanowski EJ, Moyer JS, et al. Matted nodes: poor prognostic marker in oropharyngeal squamous cell carcinoma independent of HPV and EGFR status. Head Neck. 2012;34:1727–1733. doi: 10.1002/hed.21997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walline HM, Komarck C, McHugh JB, Byrd SA, Spector ME, Hauff SJ, et al. High-risk human papillomavirus detection in oropharyngeal, nasopharyngeal, and oral cavity cancers: comparison of multiple methods. JAMA Otolaryngol Head Neck Surg. 2013;139:1320–1327. doi: 10.1001/jamaoto.2013.5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eisbruch A, Marsh LH, Dawson LA, Bradford CR, Teknos TN, Chepeha DB, et al. Recurrences near base of skull after IMRT for head-and-neck cancer: implications for target delineation in high neck and for parotid gland sparing. Int J Radiat Oncol Biol Phys. 2004;59:28–42. doi: 10.1016/j.ijrobp.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 19.Reimers N, Kasper HU, Weissenborn SJ, Stutzer H, Preuss SF, Hoffmann TK, et al. Combined analysis of HPV-DNA, p16 and EGFR expression to predict prognosis in oropharyngeal cancer. Int J Cancer. 2007;120:1731–1738. doi: 10.1002/ijc.22355. [DOI] [PubMed] [Google Scholar]
- 20.Young RJ, Rischin D, Fisher R, McArthur GA, Fox SB, Peters LJ, et al. Relationship between epidermal growth factor receptor status, p16(INK4A), and outcome in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2011;20:1230–1237. doi: 10.1158/1055-9965.EPI-10-1262. [DOI] [PubMed] [Google Scholar]
- 21.Kong CS, Narasimhan B, Cao H, Kwok S, Erickson JP, Koong A, et al. The relationship between human papillomavirus status and other molecular prognostic markers in head and neck squamous cell carcinomas. Int J Radiat Oncol Biol Phys. 2009;74:553–561. doi: 10.1016/j.ijrobp.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Machtay M, Natwa M, Andrel J, Hyslop T, Anne PR, Lavarino J, et al. Pretreatment FDG-PET standardized uptake value as a prognostic factor for outcome in head and neck cancer. Head Neck. 2009;31:195–201. doi: 10.1002/hed.20942. [DOI] [PubMed] [Google Scholar]
- 23.Xie P, Li M, Zhao H, Sun X, Fu Z, Yu J. 18F-FDG PET or PET-CT to evaluate prognosis for head and neck cancer: a meta-analysis. J Cancer Res Clin Oncol. 2011;137:1085–1093. doi: 10.1007/s00432-010-0972-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joo YH, Yoo IR, Cho KJ, Park JO, Nam IC, Kim MS. Preoperative F-FDG PET/CT and high-risk HPV in patients with oropharyngeal squamous cell carcinoma. Head Neck. 2013 doi: 10.1002/hed.23296. [DOI] [PubMed] [Google Scholar]
- 25.Higgins KA, Hoang JK, Roach MC, Chino J, Yoo DS, Turkington TG, et al. Analysis of pretreatment FDG-PET SUV parameters in head-and-neck cancer: tumor SUVmean has superior prognostic value. Int JRadiat Oncol Biol Phys. 2012;82:548–553. doi: 10.1016/j.ijrobp.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 26.Eisbruch A, Kim HM, Feng FY, Lyden TH, Haxer MJ, Feng M, et al. Chemo-IMRT of oropharyngeal cancer aiming to reduce dysphagia: swallowing organs late complication probabilities and dosimetric correlates. Int J Radiat Oncol Biol Phys. 2011;81:e93–e99. doi: 10.1016/j.ijrobp.2010.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;45:577–587. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 28.Garden AS, Dong L, Morrison WH, Stugis EM, Glisson BS, Frank SJ, et al. Patterns of Disease Recurrence Following Treatment of Oropharyngeal Cancer With Intensity Modulated Radiation Therapy. Int J Radiat Oncol Biol Phys. 2012 doi: 10.1016/j.ijrobp.2012.08.004. [DOI] [PubMed] [Google Scholar]