Abstract
BACKGROUND
In airway epithelia, the kinetics of recombinant adeno-associated virus (AAV) transgene expression is slow. This has negative practical implications for research and for translation into therapy. The DNA minor groove-binding agent Hoechst-33342 has been shown to enhance AAV transgene expression. Here we investigate the mechanism of Hoechst related augmentation of AAV-mediated transgene expression.
METHODS
We investigated the effect of Hoechst-33342 on HT1080, COS-7, mouse and human airway epithelia transduced with different AAV serotypes encoding eGFP. We exposed cells to increasing concentrations of Hoechst-33342 at different time points. We evaluated the effect on second strand DNA synthesis using a self-complementary GFP transgene. We also investigated the effect on expression from transfected plasmids with and without AAV2 ITRs.
RESULTS
We found that Hoechst-33342 significantly accelerated AAV transgene expression. This effect was seen for all serotypes and could not be explained by increased virus binding or internalization. Hoechst-33342 only had an effect when the treatment was given during or after transduction, even 120 days post-transduction, suggesting an effect on transgene expression regulation. Hoechst-33342 increased transgene expression when cells were transduced with an AAV expressing self-complementary eGFP, but no effect on cells transduced with AAV when the promoter was RSV. Finally, Hoechst-33342 increases gene expression from transfected plasmids regardless of the presence of AAV2 ITRs.
CONCLUSIONS
Hoechst dramatically augments and accelerates AAV-mediated transgene expression in airway epithelia without altering AAV-mediated gene transfer. Hoechst activation of the CMV promoter is seen in plasmids, but is drastically enhanced in the context of AAV.
Keywords: AAV, Hoechst, Transgene, Expression, CMV Promoter
INTRODUCTION
Adeno-associated viruses (AAV) are single stranded DNA viruses belonging to the dependovirus genus of the parvovirus family [1]. They have an approximately 4.7 kilobase genome containing inverted terminal repeats and three open reading frames, rep, cap, and aap. AAVs are non-pathogenic and have been studied as gene therapy vectors for multiple genetic diseases, including cystic fibrosis [2–5].
Gene therapy clinical trials for cystic fibrosis with the most studied serotype, AAV2, demonstrated safety, but suboptimal efficiency for treatment in the airways [5–7]. AAV5, which binds to receptors distinct from AAV2, has increased binding to the apical surface and increased transduction of airway epithelia in vitro, indicating that viral binding is a rate limiting step for successful transduction of airway epithelia by AAV [8, 9]. We have previously used directed evolution of AAV in well differentiated human airway epithelia to discover a novel AAV with increased binding and internalization in human airway epithelia compared to AAV5, suggesting the virus had sequentially overcome two different rate limiting steps (binding and internalization) [10, 11]. Others have shown different rate limiting steps for AAV transduction of airway epithelia, including intracellular trafficking and second strand synthesis [12, 13].
Hoechst is a bisbenzimide minor groove DNA-binding chemical [14]. It is commonly used for fluorescent staining of nuclei of live or dead cells for use in flow cytometry or microscopy. Because Hoechst dyes bind DNA, they can disrupt DNA replication during cell division and are known mutagens. Li et al., showed that Hoechst can improve AAV-mediated transgene expression in multiple cell types which could potentially be valuable for the use of AAV in in vivo models to accelerate transgene expression [15]. Here we investigated the effect of Hoechst on AAV-mediated transgene expression in human airway epithelia, and have identified a novel limitation for recombinant AAV, where transgene expression from the CMV promoter is increased by Hoechst treatment.
MATERIALS AND METHODS
Recombinant AAV
All recombinant AAV vectors were packaged and purified with iodixanol gradient centrifugation as previously described produced by the University of Iowa Gene Transfer Vector Core [16]. DNase-resistant genomic titers were determined by quantitative PCR.
Well-Differentiated Cultures of Airway Epithela
Human airway or mouse epithelial cells were isolated from trachea and bronchi, seeded onto collagen coated, semi-permeable membranes (Millipore), and grown at the air-liquid interface as previously described [17]. All experiments with human and mouse airway epithelia were carried out in accordance with the University of Iowa Institutional Review Board and the University of Iowa Institutional Animal Care and Use Committee.
AAV Transduction
Human and mouse airway epithelia were apically inoculated with 105 viral genomes per cell (vg/cell) of indicated AAV serotypes diluted in EMEM for 4 hours at 37°C. HT1080 cells and COS-7 cells were cultured as previously described [15] and were inoculated for 4 hours with the indicated AAV serotypes for 4 hours at 37 °C at the doses indicated. Hoechst-33342 was purchased from Invitrogen (catalog number H1399). Hoechst-33342 was diluted in the culture media for each cell type at the indicated concentrations. Hoechst treatment occurred at the time of viral inoculation unless indicated otherwise.
Plasmid Transfection
Transfection of plasmid DNA into COS-7 cells was done using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. Hoechst treatment occurred at the time of transfection and was maintained in the cell culture media for the entire duration of the experiment. β-galactosidase per milligram of total protein was measured 72 hours after transfection as previously described (Galacto-Light; Tropix, Inc., Bedford, Mass) [18]. The plasmids were generously provided by Lynda Ostedgaard [19]. The plasmid pCMV5–β-gal was modified to contain truncated CMV promoters or the full CMV promoter as previously described [19]. To determine the effect of AAV inverted terminal repeats (ITRs) on Hoechst-activated gene expression, plasmids encoding the CMV promoter driving GFP expression, with or without AAV2 ITRs flanking the reporter gene and promoter were used for electroporation in COS-7 cells by standard methodologies. Briefly, 10 million cells were mixed with 2 µg of plasmid DNA in 400 µl of cytomix (120mM KCl, 0.15mM CaCl2, 10mM K2HPO4, 10mM KH2PO4, 25mM HEPES, 2mM EGTA, 5mM MgCl2, 2mM ATP and glutathione) and put in an electroporation cuvette (Bio-Rad Laboratories, Hercules, CA) for 30 minutes on ice. After electroporation, cells were seeded onto 24 well tissue culture plates and treated with Hoechst for 24 hours post-transfection. Flow cytometry was performed to determine the percentage of GFP positive cells 72 hours post-transfection.
RESULTS
Hoechst increases transduction of human airway epithelia by AAV
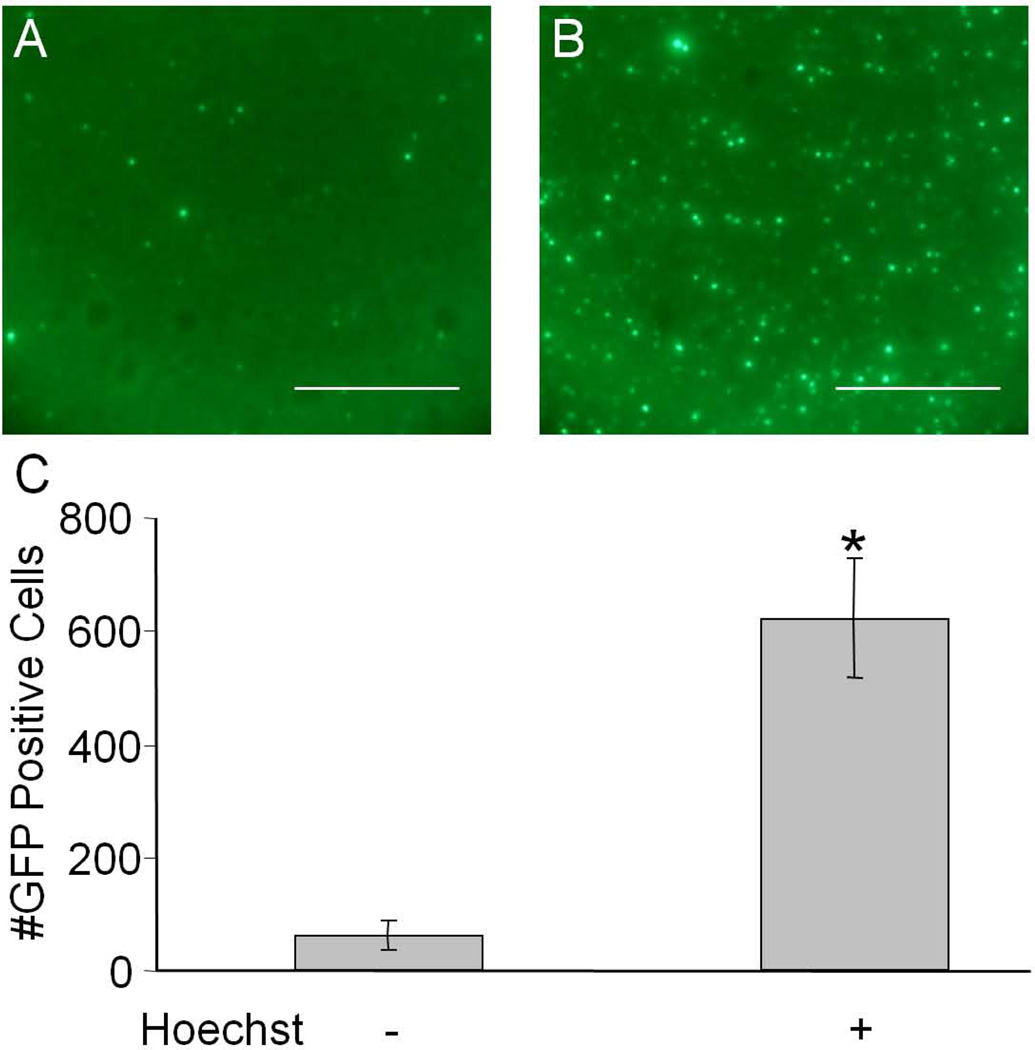
Human airway epithelia (HAE) represent an in vitro model that recapitulates many of the in vivo barriers to viral-mediated gene transfer in the lung. As in many tissues in vivo, AAV-mediated transduction of HAE has a significant lag time prior to transgene expression. Li et al showed that Hoechst treatment of non-polarized cells accelerated the time course of transgene expression. We first wanted to investigate if Hoechst would alter the time course of transgene expression or alternatively increase AAV-mediated transduction of human airway epithelia. We transduced well-differentiated cultures of human airway epithelia, from the apical surface, with recombinant AAV1 encoding GFP under the control of the CMV promoter. Cells were treated with basolateral media containing 10 µM Hoechst-33342 at the time of viral inoculation. Figure 1A and 1B show representative images of epithelia 7 days post-transduction in the presence or absence of Hoechst-33342. A 10-fold increase in the number of cells expressing GFP was observed when cells were treated with Hoechst (Figure 1c). These data suggest that, similar to Li et al, Hoechst increases AAV transgene expression and that increased numbers of cells were transduced.
Figure 1.
Human airway epithelia were transduced with 105 vg/cell with AAV5-CMV-eGFP and treated with 0 µM or 10 µM Hoechst-33342 and fluorescent images were taken 7 days later (A and B respectively). Scale bar = 200 µm. The number of GFP positive cells per field of view was quantified and shown in C. (*p<0.01)
The effect of Hoechst is dose dependent and serotype independent
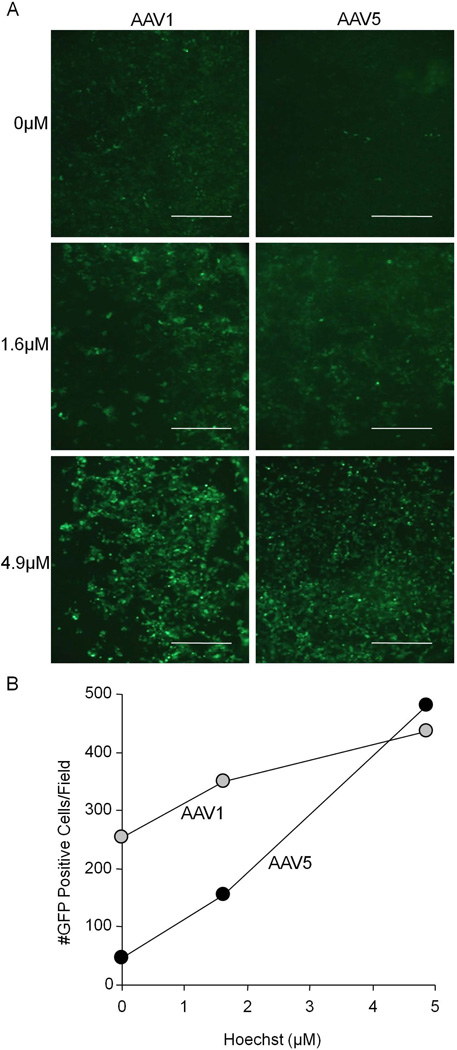
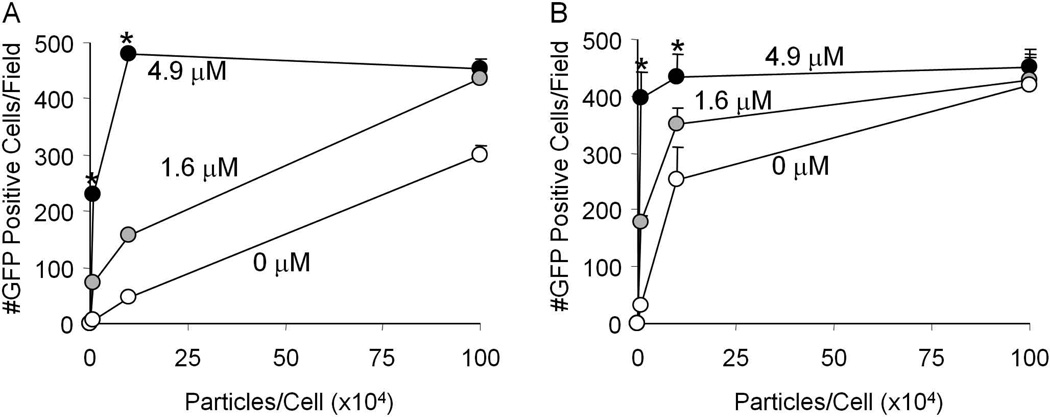
We investigated the effect of increasing concentrations of Hoechst on transgene expression and the relationship to the serotype and multiplicity of infection (MOI). We transduced HT1080 cells with AAV1 or AAV5 at an MOI of 105 vg/cell, and treated with increasing concentrations of Hoechst. As seen in figure 2A and 2B, Hoechst treatment results in increased transgene expression that was dose dependent for both AAV1 and AAV5. Although no toxicity was observed at Hoechst concentrations up to 10 µM, higher concentrations demonstrated cell death (data not shown). We then investigated the effect of Hoechst on transgene expression when we varied the MOI. Figure 3A (AAV1) and 3B (AAV5) show that the effect of Hoechst was more evident with lower amounts of virus. These data suggest that Hoechst has an effect that is dose dependent and AAV serotype independent. Hoechst treatment is able to increase the number of GFP positive cells to the level equivalent to 100 times more virus in the absence of Hoechst.
Figure 2.
HT1080 cells were transduced with 105 vg/cell of either AAV1 or AAV5 in the presence of 0 µM, 1.6 µM, or 4.9 µM Hoechst-33342. Representative images are shown from 48 hours post-transduction in A. Scale bar = 200 µm. The number of GFP positive cells per field of view is shown in B.
Figure 3.
HT1080 cells were transduced with increasing amounts of AAV1 (A) or AAV5 (B) and treated with 0 µM, 1.6 µM, or 4.9 µM Hoechst-33342. Scale bar = 200 µm. The number of GFP positive cells per field of view is shown. (*p<0.05)
The effect of Hoechst is seen after gene transfer
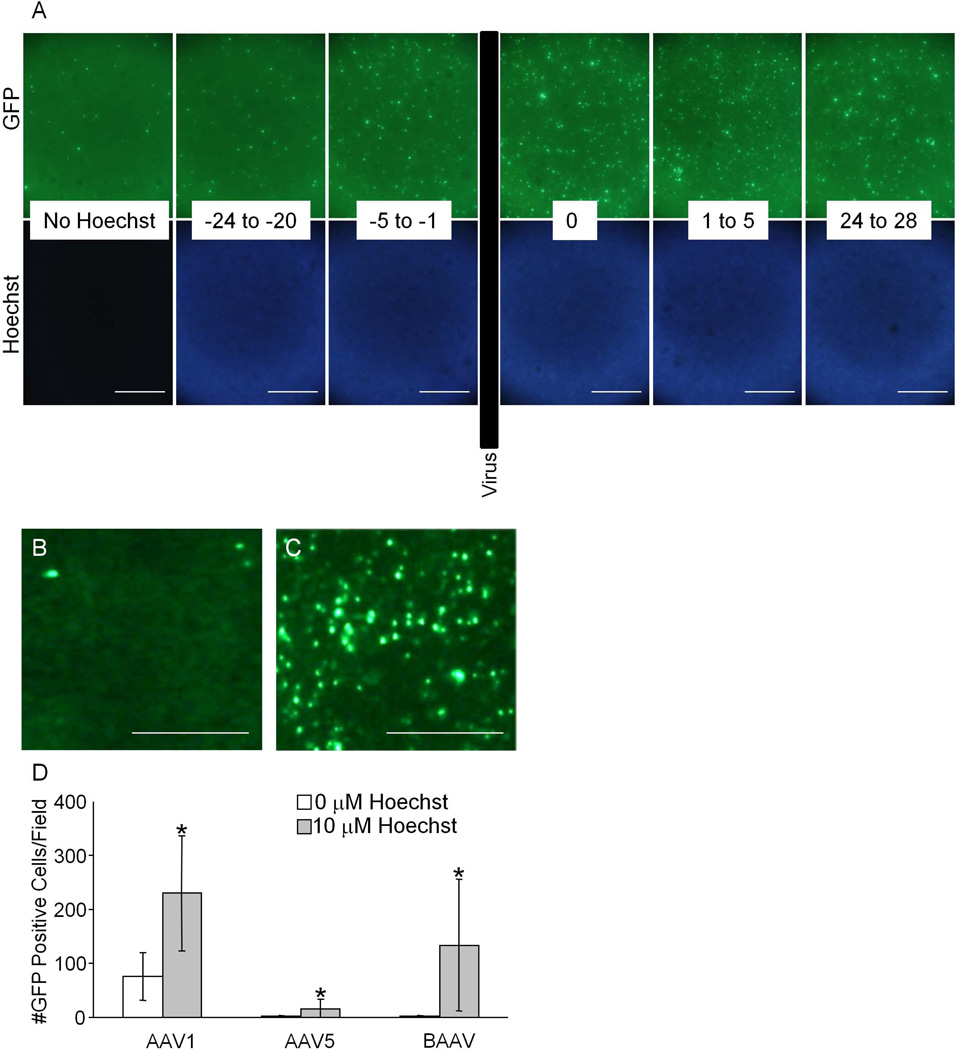
Li et al., suggested that Hoechst-33342 affects the cell cycle, which in turn affects the ability of AAV the transduce the cells. Considering the low baseline level of mitosis observed in polarized airway epithelia, we speculated that the effect of treatment with Hoechst would not be due to altering the airway epithelial cell cycle, and would instead facilitate viral DNA conversion and/or transgene expression. We applied Hoechst before or after transduction with AAV to investigate if the effect of Hoechst is dependent on the cell cycle. We hypothesized that if the mechanism behind the Hoechst-33342-mediated increase in AAV transduction was due to an effect on the cell cycle, then the effect would be preserved if Hoechst-33342 was applied to the cells prior to AAV, but not after AAV was applied to the cells. Briefly, Hoechst was applied for 4 hours at different intervals during the transduction of airway epithelial cells with AAV1 from time 0 to 4 hours. Figure 4A shows that Hoechst has minimal to no effect when applied to HAE prior to AAV transduction, whereas the effect was identical when applied 24 hours after the transduction. Note the staining of the nucleus was identical in all epithelia. These results suggest that Hoechst does not alter cells before transduction and does not alter the number of cells taking up AAV, but instead affects an acute step of transduction. Moreover, these data suggest that treatment after a significant period of time may trigger or augment expression from latent AAV.
Figure 4.
Human airway epithelia were treated with Hoechst-33342 for 4 hour intervals at different times relative to inoculation with AAV1 (A). “No Hoechst” indicates the cells were transduced with AAV1 but not treated with Hoechst. “−24 to −20” indicates the Hoechst was applied 24 hours prior and removed 20 hours prior to the addition of virus. “−5 to −1” indicates the Hoechst was applied 5 hours prior and removed 1 hour prior to the addition of virus. “0” indicates the Hoechst was applied and removed at the same time as the virus. “1 to 5” indicates the Hoechst was applied 1 hour after and removed 5 hours after the virus was removed. “24 to 28” indicates the Hoechst was applied 24 hours after and removed 28 hours after the virus was removed. Scale bar = 200 µm. B shows a mouse airway epithelial culture 120 days after transduction with bAAV before being treated with Hoechst-33342. C shows the same epithelium 24 hours after treatment with 10 µM Hoechst-33342. Scale bar = 50 µm. The numbers of GFP positive cells in mouse airway epithelia transduced with AAV1, AAV5, or bAAV pre- and 24 hours post-Hoechst treatment (D). (*p<0.05)
The effect of Hoechst is seen months after gene transfer
AAV infected airway epithelial cultures can survive for long periods of time (up to several months). We asked whether Hoechst-33342 would have an effect on AAV-mediated transgene expression in cultures transduced with AAV after a significant period of time. Mouse airway epithelia were transduced with 105 vg/cell of AAV1, AAV5, or bovine AAV (bAAV), and 120 days later were treated with 10 µM Hoechst-33342. Figure 4B–D shows the significant increase in the number of GFP positive cells observed for each AAV serotype, with the greatest increase occurring in the bAAV-transduced epithelium. Representative images of the effect of Hoechst on bAAV-GFP transduced epithelia are shown immediately prior to Hoechst treatment (5B) and 24 hours post-Hoechst treatment (5C). The ability of Hoechst to have an effect when applied after transduction points to three alternative possibilities: altered AAV intracellular trafficking, altered double strand formation/concatemerization of the DNA, or an effect on transcription.
Hoechst increases self-complementary AAV-mediated transgene expression
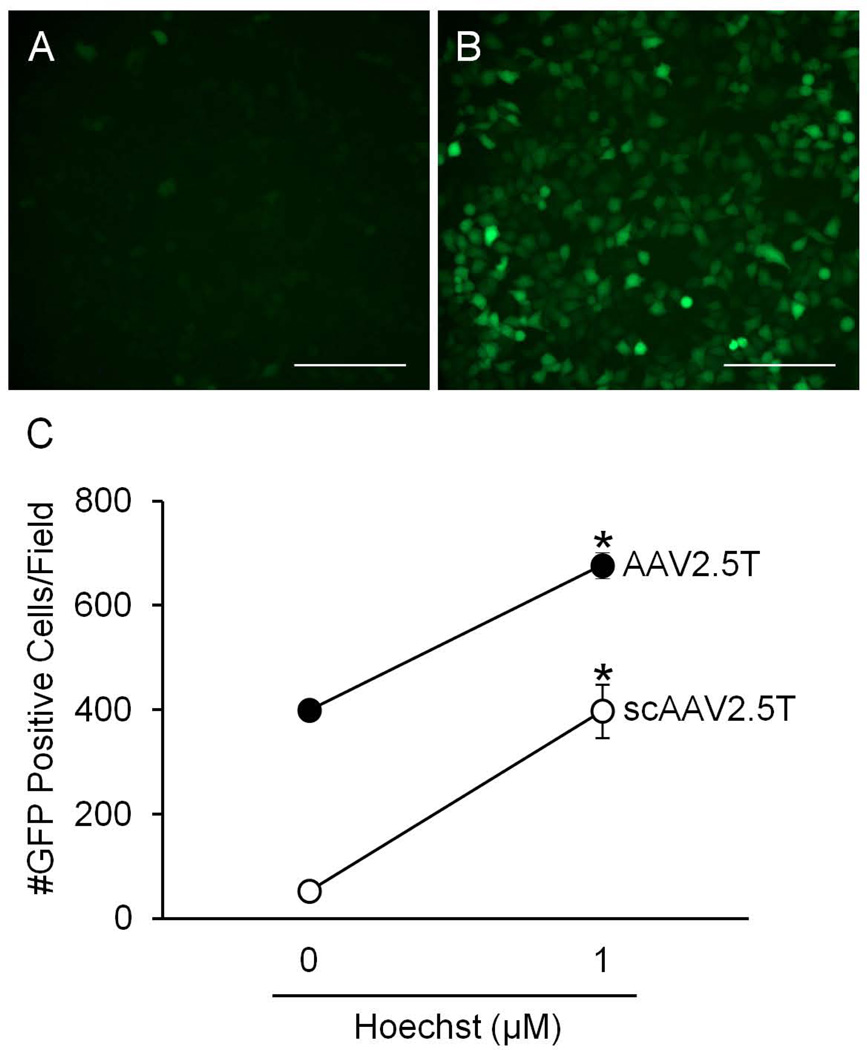
In order to investigate if the effect of Hoechst is due to augmenting the formation of double stranded DNA once the viral genome is delivered to the nucleus, we studied the effect of Hoechst on 2 different viruses: AAV2.5T [10] with a non-self-complementary or a self-complementary genome encoding the CMV promoter and an eGFP transgene [20]. HT1080 cells were transduced with 105 vg/cell of these viruses and treated with 0 µM or 1 µM Hoechst-33342 at the time of transduction. Few GFP positive cells were observed in epithelia treated with AAV2.5T-scGFP in the absence of Hoechst (Figure 5A). In contrast, numerous GFP positive cells were readily observed in epithelia treated with AAV2.5T-scGFP in the presence of Hoechst (Figure 5B). A similar increase was observed with AAV2.5T carrying the non-self complementary GFP genome (Figure 5C) demonstrating that similar to single stranded AAV, Hoechst improved expression of self-complementary AAV. These data suggest that Hoechst is not affecting second strand synthesis.
Figure 5.
HT1080 cells were transduced with self-complementary or non-self-complementary AAV2.5T-CMV-eGFP and treated with 0 µM or 1 µM Hoechst-33342. 48 hours later, GFP expression was determined by fluorescent microscopy. Representative images from cells transduced with the self-complementary AAV2.5T and treated with (A) 0 µM Hoechst or (B) 1 µM Hoechst. Scale bar = 200 µm. C shows the number of GFP positive cells per field of view for each virus. (*p<0.01)
Hoechst does not affect AAV encoding the RSV promoter
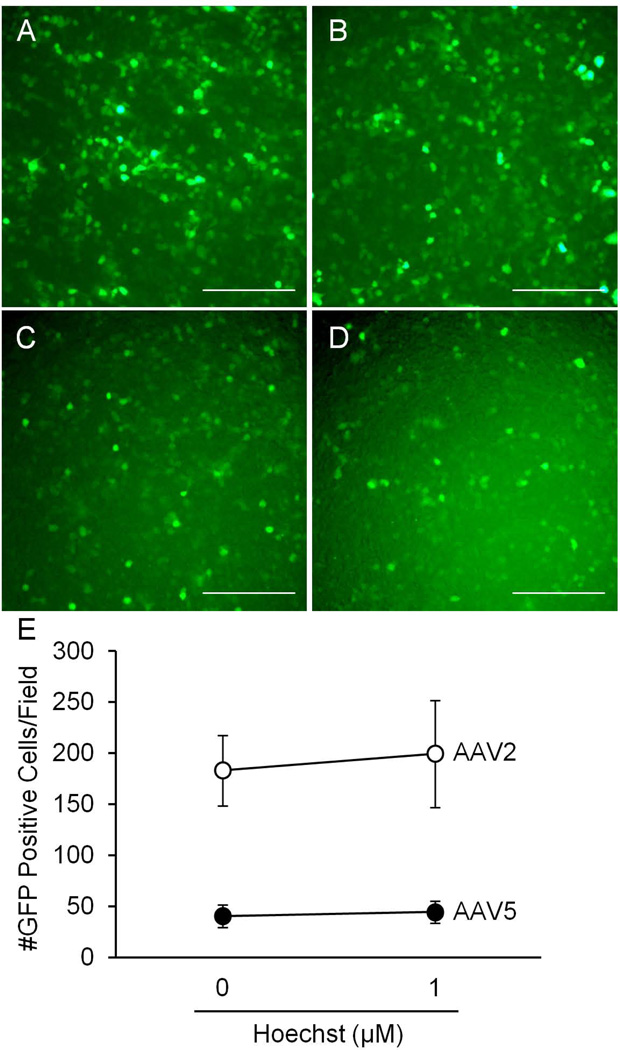
We next investigated whether Hoechst was promoter specific, and asked whether Hoechst treatment would have an effect on the RSV promoter in the context of AAV. We transduced HT1080 cells with 105 vg/cell of AAV2 or AAV5 encoding the RSV promoter driving the eGFP transgene. 0 µM or 1 µM Hoechst was applied at the time of transduction. Hoechst-33342 had no effect on RSV-promoted GFP expression for AAV2 and AAV5 (Figure 6). These data suggest that the effect of Hoechst may be due to activation of the CMV promoter, and that transcription may be a limitation in AAV-mediated gene transfer to human airway epithelia.
Figure 6.
HT1080 cells were transduced with either AAV2 or AAV5 encoding the RSV promoter and the eGFP transgene and treated with 0 µM or 1 µM Hoechst-33342. 48 hours later, GFP expression was determined by fluorescent microscopy. A and B are representative images of cells transduced with AAV2 and treated with 0 µM and 1 µM Hoechst-33342 respectively. C and D are representative images of cells transduced with AAV5 and treated with 0 µM and 1 µM Hoechst-33342 respectively. Scale bar = 200 µm. E shows the number of GFP positive cells per field of view for each virus.
Hoechst increases expression driven by the CMV promoter
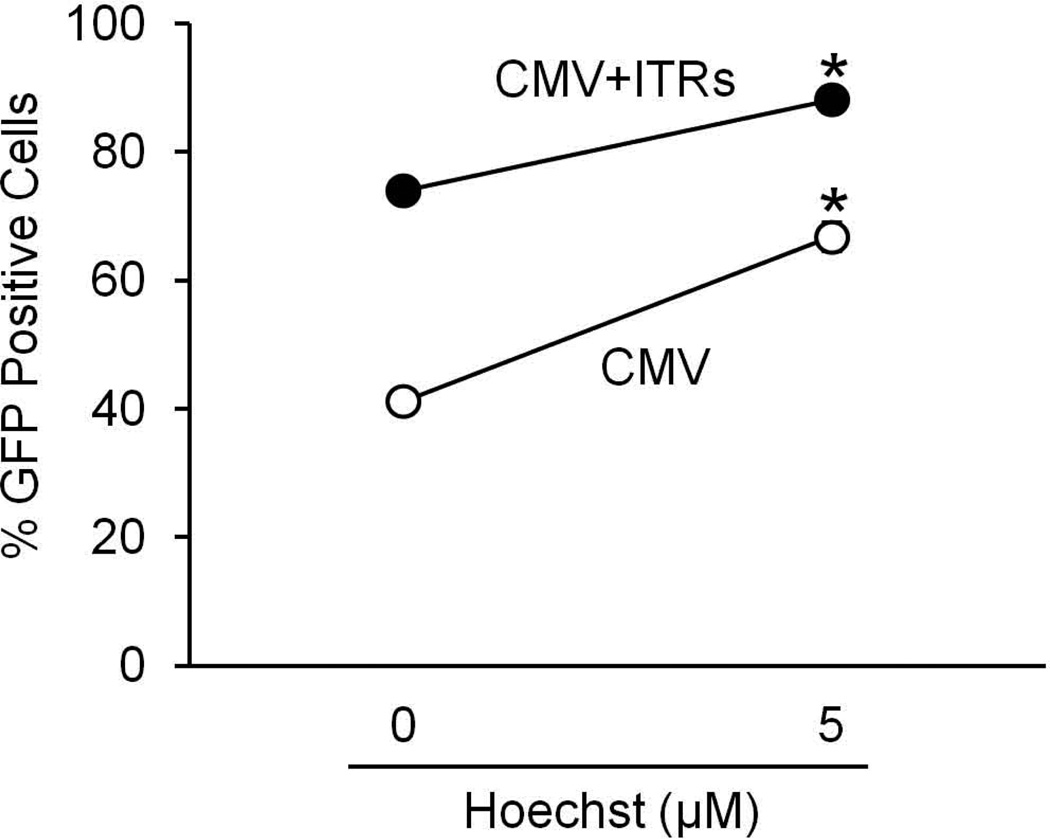
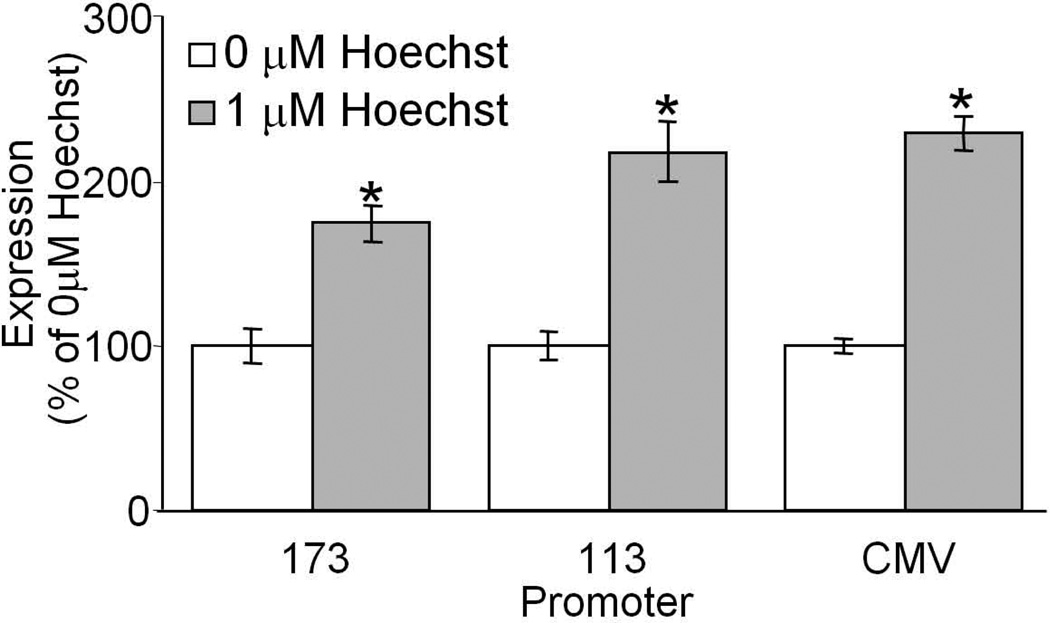
To determine the effect of Hoechst on the promoter, and whether the effect of Hoechst requires the inverted terminal repeats from AAV, we asked whether Hoechst-33342 would have an effect on gene expression from transfected plasmid DNA with or without AAV ITRs. Plasmids encoding the CMV promoter driving expression of GFP with or without the ITRs of AAV2 flanking the construct in the presence or absence of 5 µM Hoechst-33342 were transfected into COS-7 cells, and the cells were evaluated for GFP expression by flow cytometry 3 days after transfection (Figure 7). We found that the ITRs were not required for the Hoechst-mediated increase in gene expression, suggesting that there is an effect on CMV promoter activity. We then asked whether there was a minimal promoter unit required for increased gene expression and if the effect was dependent on the reporter gene. To test this COS-7 cells were transfected with plasmids encoding the full CMV promoter or truncated CMV promoters each driving expression of the β-galactosidase gene, in the presence or absence of 1 µM Hoechst-33342. Similar increases in transduction were observed in the presence of 1 µM Hoechst (Figure 8). This suggests that Hoechst has an effect on the CMV promoter that is not limited to just AAV-mediated transgene expression, is not transgene-dependent, and that Hoechst will likely have a similar effect in many cell types.
Figure 7.
COS-7 cells were transfected with plasmids containing the CMV promoter driving GFP expression with or without AAV2 ITRs flanking the promoter/gene. The cells were treated with 0 µM or 5 µM Hoechst-33342 at the time of transfection, and evaluated for GFP expression by flow cytometry 3 days later. (*p<0.01)
Figure 8.
COS-7 cells were transfected with plasmids containing the complete CMV promoter or truncated CMV promoters, each controlling expression of the β-galactosidase gene. These plasmids lacked the ITRs of AAV. The cells were treated with 0 µM or 1 µM Hoechst-33342 at the time of transfection, and evaluated for β-galactosidase activity 3 days later. (*p<0.001)
DISCUSSION
Despite identifying novel AAV serotypes that bind to the apical surface of airway epithelia, AAV-mediated gene transfer efficiency remains suboptimal for respiratory gene therapy. In this study we found that Hoechst improves the efficiency of AAV-mediated transgene expression in human airway epithelia by activating the CMV promoter. Work by Walters et al [8] and Halbert et al [21] suggest that binding is a rate limiting step for AAV2, while others have found that intracellular trafficking and second strand synthesis are rate limiting steps [12, 13]. We have also previously found that binding and internalization are sequential rate limiting steps, which an evolved virus (AAV2.5T) overcomes [10, 11]. Our work here suggests that transgene expression is an important limitation for recombinant AAV as well.
A practical limitation of using AAV for in vitro and in vivo experiments is the delay in transgene expression. We, and others have found maximal transgene expression after one month in airway epithelial cultures [10]. This not only delays experiments, but also affects the interpretation of the results depending on when assays are prepared and the sensitivity of the readout. Li et al have shown that Hoechst increases transgene expression and moreover, suggested the mechanism was due to a direct effect on the cell cycle. We were particularly interested in Hoechst for human airway epithelia because it accelerates expression from several weeks to days. The effect in human airway epithelia could not be explained by the results of Li et al., since primary airway epithelia are not mitotically active [22] and because Hoechst does not increase AAV-mediated transgene expression when airway epithelial cells are treated 24 hours before being inoculated with AAV. Additionally, the ability of Hoechst to increase transgene expression from transfected plasmid DNA and the lack of an effect by Hoechst on AAV-mediated transgene expression when under the control of the RSV promoter also suggest that the effect is not due to an altered cell cycle distribution. Zhang et al showed Hoechst-33342 increases luciferase gene expression in transfected BC3H-1 monocytes [14]. Others have shown that Hoechst increases transcription factor binding to the TATA-box-binding element in vitro [23–25]. These data support the conclusion that the effect of Hoechst on AAV-mediated transgene expression is due to activation of the CMV promoter.
Hemophilia gene therapy trials using AAV2 to target gene transfer to the liver demonstrate a delay of up to six weeks in transgene expression that is related to inefficient uncoating of the viral genome inside the nucleus [26]. In airway epithelia and liver, Duan et al. showed that AAV2 is delayed in endosomal processing and trafficking to the nucleus due to ubiquitination and proteosomal degradation of the capsid [12]. The increase in transgene expression with self-complementary AAV and the lack of an effect on AAV-mediated transgene expression with the RSV promoter indicate that Hoechst does not affect intracellular trafficking, nuclear uncoating, or single strand to double strand DNA conversion.
In summary, temporal activation of the CMV promoter is an important limitation in the transduction of multiple cell types by recombinant AAV. The effect of Hoechst is more pronounced when the CMV promoter is in the context of AAV, but Hoechst is able to increase expression from transfected plasmid DNA encoding a gene driven by the CMV promoter. These data have practical applications for experimental design and interpretation, and may lead to interventions that improve AAV-mediated transgene expression, and thus improve gene therapy efficacy.
ACKNOWLEDGMENTS
We especially want to thank Phil Karp, Pamella Hughes, Lynda Ostedgaard, Tatiana Rokhlina and David Stoltz for their help and suggestions. We appreciate the support of the Gene Transfer Vector Core [supported by the Roy J. Carver Charitable Trust, the NHLBI, CFF and NIDDK (DK54759)], the In Vitro Cell Models Core [supported by the NHLBI, CFF and NIDDK (DK54759)] and the Morphology Core of the Gene Therapy Center [supported by the NIDDK (DK 54759)] at the University of Iowa. This work was supported by the National Heart Lung and Blood Institute.
Footnotes
CONFLICTS OF INTEREST STATEMENT
We have no conflicts of interest to report.
References
- 1.Fields BN, Knipe DM, Howley PM. Fields virology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 2.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manno CS, Chew AJ, Hutchison S, et al. AAV-mediated factor I× gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 4.Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66:290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moss RB, Milla C, Colombo J, et al. Repeated aerosolized AAV-CFTR for treatment of cystic fibrosis: a randomized placebo-controlled phase 2B trial. Hum Gene Ther. 2007;18:726–732. doi: 10.1089/hum.2007.022. [DOI] [PubMed] [Google Scholar]
- 6.Griesenbach U, Ferrari S, Geddes DM, et al. Gene therapy progress and prospects: cystic fibrosis. Gene Ther. 2002;9:1344–1350. doi: 10.1038/sj.gt.3301791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griesenbach U, Alton EW. Current status and future directions of gene and cell therapy for cystic fibrosis. BioDrugs. 2011;25:77–88. doi: 10.2165/11586960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Zabner J, Seiler M, Walters R, et al. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol. 2000;74:3852–3858. doi: 10.1128/jvi.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walters RW, Yi SM, Keshavjee S, et al. Binding of adeno-associated virus type 5 to 2,3-linked sialic acid is required for gene transfer. J Biol Chem. 2001;276:20610–20616. doi: 10.1074/jbc.M101559200. [DOI] [PubMed] [Google Scholar]
- 10.Excoffon KJ, Koerber JT, Dickey DD, et al. Directed evolution of adeno-associated virus to an infectious respiratory virus. Proc Natl Acad Sci U S A. 2009;106:3865–3870. doi: 10.1073/pnas.0813365106. DOI: 0813365106 [pii]10.1073/pnas.0813365106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickey DD, Excoffon KJ, Koerber JT, et al. Enhanced sialic acid-dependent endocytosis explains the increased efficiency of infection of airway epithelia by a novel adeno-associated virus. J Virol. 2011;85:9023–9030. doi: 10.1128/JVI.05154-11. DOI: JVI.05154-11 [pii]10.1128/JVI.05154-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan D, Yue Y, Yan Z, et al. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher KJ, Gao GP, Weitzman MD, et al. Transduction with recombinant adeno-associated virus for gene therapy is limited by leading-strand synthesis. J Virol. 1996;70:520–532. doi: 10.1128/jvi.70.1.520-532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Kiechle FL. Hoechst 33342 alters luciferase gene expression in transfected BC3H-1 myocytes. Arch Pathol Lab Med. 2003;127:1124–1132. doi: 10.5858/2003-127-1124-HALGEI. DOI: AS2298 [pii]10.1043/1543-2165(2003)127<1124:HALGEI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 15.Li L, Yang L, Kotin RM. The DNA minor groove binding agents Hoechst 33258 and 33342 enhance recombinant adeno-associated virus (rAAV) transgene expression. J Gene Med. 2005;7:420–431. doi: 10.1002/jgm.681. [DOI] [PubMed] [Google Scholar]
- 16.Urabe M, Ding C, Kotin RM. Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther. 2002;13:1935–1943. doi: 10.1089/10430340260355347. [DOI] [PubMed] [Google Scholar]
- 17.Karp PH, Moninger T, Weber SP, et al. In: An in vitro model of differentiated human airway epithelia: methods and evaluation of primary cultures. Wise C, editor. Totowa, NJ: Humana Press, Inc.; 2002. pp. 115–137. [DOI] [PubMed] [Google Scholar]
- 18.Ashbourne Excoffon KJ, Moninger T, Zabner J. The coxsackie B virus and adenovirus receptor resides in a distinct membrane microdomain. J Virol. 2003;77:2559–2567. doi: 10.1128/JVI.77.4.2559-2567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostedgaard LS, Rokhlina T, Karp PH, et al. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc Natl Acad Sci U S A. 2005;102:2952–2957. doi: 10.1073/pnas.0409845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 21.Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (AAV6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of AAV2 vectors. J Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang G, Davidson BL, Melchert P, et al. Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J Virol. 1998;72:9818–9826. doi: 10.1128/jvi.72.12.9818-9826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Kiechle FL. Hoechst 33342 induces apoptosis and alters tata box binding protein/DNA complexes in nuclei from BC3H-1 myocytes. Biochem Biophys Res Commun. 1998;248:18–21. doi: 10.1006/bbrc.1998.8906. DOI: S0006-291X(98)98906-9 [pii]10.1006/bbrc.1998.8906. [DOI] [PubMed] [Google Scholar]
- 24.Kiechle FL, Zhang X. Apoptosis: biochemical aspects and clinical implications. Clin Chim Acta. 2002;326:27–45. doi: 10.1016/s0009-8981(02)00297-8. DOI: S0009898102002978 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Zhang X, Kiechle FL. Hoechst 33342-induced apoptosis is associated with intracellular accumulation of E2F-1 protein in BC3H-1 myocytes and HL-60 cells. Arch Pathol Lab Med. 2001;125:99–104. doi: 10.5858/2001-125-0099-HIAIAW. [DOI] [PubMed] [Google Scholar]
- 26.Thomas CE, Storm TA, Huang Z, et al. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78:3110–312. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]