Abstract
Objectives
Determine if the behavior of cancer stem cells (CSC) is affected by HPV status.
Study Design
An in vitro and in vivo analysis of HPV and CSC
Setting
University Laboratory
Subjects
Head and neck cell lines
Subjects and Methods
We isolated CSC from HPV(+) and HPV(-) cell lines. Two HPV(-) cell lines underwent lentiviral transduction of E6/E7. Chemoresistence was determined using colony formation assays. Native HPV(+) and HPV-E6/E7-transduced cells were compared for lung colonization after tail vein injection in NOD/SCID mice.
Results
The proportion of CSC is not significantly different in HPV(+) or HPV(-) HNSCC cell lines. HNSCC CSC are more resistant to cisplatin than the non-CSC, however there were no significant differences between HPV(+) and HPV(-) cells. HPV(-) cancer cells yielded low colony formation after cell sorting. After transduction with HPV E6/E7, increased colony formation was observed in both CSC and non-CSC. Results from tail vein injections yielded no differences in development of lung colonies between HPV E6/E7 transduced cells vs. the non-transduced cells.
Conclusions
HPV status does correlate with the proportion of CSC present in HNSCC. HPV(+) cells and those transduced with HPV E6/E7 have a greater clonogenicity than HPV(-) cells. HNSCC CSC are more resistant to cisplatin than non-CSC. This suggests that common chemotherapeutic agents may shrink tumor bulk by eliminating non-CS, while CSC have mechanisms that facilitate evasion of cell death. HPV status does not affect CSC response to cisplatin therapy, suggesting that other factors explain the better outcomes for patients with HPV(+) cancer.
Keywords: Head and Neck Cancer, HPV, Cancer Stem Cells, ALDH, Chemotherapy
Introduction
Treatment for advanced head and neck squamous cell carcinoma (HNSCC) has continued to yield poor outcomes. Cancer stem cells (CSC) have been identified in solid tumors and implicated in tumor resistance to therapy1-3. HNSCC CSCs have been identified using the cell surface marker CD44 and aldehyde dehydrogenase activity (ALDH)4-7. Both markers are useful in isolating cells that exhibit the characteristics of CSC: the ability to give rise to new tumors, to self-renew and to recapitulate the original tumor heterogeneity. CD44-expressing cells in HNSCC have an increased metastatic potential, predict tumor aggressiveness and have increased resistance to agents that induce apoptosis and to irradiation8-10.
While studies in other solid tumors have identified ALDHhigh cells as a reliable marker for chemoresistance in cancer cells11, 12, this property has not yet been explored in HNSCC. The intracellular enzyme ALDH functions to protect cells by oxidizing toxic metabolites13,14. Chemoresistance is not unexpected in these cells, since ALDH activity also protects against chemotherapeutic agents like cisplatin and carboplatinum15.
The recognition of high-risk human papillomaviruses (hrHPV) as important etiologic factors in oropharyngeal cancer has led to a significant interest in the role that hrHPV plays in development, progression and response to therapy in HNSCC. The rise in the incidence of oropharyngeal tumors due to HPV has challenged investigators to explain why HPV-related tumors have favorable patient outcomes. The high-risk HPV oncogenes E6 and E7 derail normal cell cycle pathways through interactions with the critical cell cycle regulators TP53 and retinoblastoma protein (Rb). The papillomaviruses that give rise to squamous cell carcinomas of both the cervix and the head and neck only infect the cells that reside in the basal layer of the mucosa, where normal stem cells exist, and as recently reported where ALDHhigh expressing cells co-localize6.
Using HNSCC cell lines derived from HPV(+) HNSCC and HPV(-) cell lines transduced with E6 and E7, we evaluated the role of HPV in HNSCC and CSC behavior. Our objectives were to determine: 1) if CSC are more resistant to chemotherapy; 2) if HPV status change CSC behavior; 3) if the proportion of CSCs varies in the presence of E6/E7; 4) if the presence of these oncogenes effect the tumorigenicity of the CSC.
Materials and Methods
IRBMED approval and informed consent was obtained from patients enrolled in the University of Michigan Head and Neck SPORE. The use of animals was reviewed and approved by the University of Michigan's Committee for the Humane Use and Care of Animals.
Cell Culture
Six head and neck cancer cell lines were utilized: UD-SCC-2, UM-SCC-12, UM-SCC-29, UM-SCC-38, UM-SCC-47 and UM-SCC-74B. UD-SCC-2 and UM-SCC-47 are HPV(+) cell lines. Cells were cultured in standard conditions including Dulbecco's Modified Eagle Medium (DMEM; Invitrogen) with 10% fetal bovine serum (FBS). Mycoplasma testing was performed using Myco Alert Mycoplasma Testing Kit (Lonza, Rockland ME). The identity of the cells lines was confirmed by Profiler Plus (Invitrogen) genotyping at 10 polymophic STR loci.
Lentivirus Production and Transduction
UM-SCC-29 and UM-SCC-38 underwent lentiviral transduction: Lentivirus packaging vectors (pMDL-RRE, pRSV-REV, pC1-VSVG) were co-transfected with pLentilox-HPV E6/E7-IRES-puromycin or pLentiloxEV-Luciferase proviral plasmid into 293T cells. Supernatants were collected after 72 hours, pelleted and resuspended (~1×107 TU/ml). Cell lines were seeded on a 6 well plate (~4×105 cells/well) one day prior to lentiviral transduction with lentivirusluciferase (labeled now as UM-SCC-29-Luc and UM-SCC-38-Luc). Cell media was changed to 1.2 ml of fresh complete media, 0.3 ml of 10x virus and 8 μg/ml Polybrene (Sigma) were added. The cell media was changed to complete media 24 hours after transduction. The cells were expanded and grown for 3-4 weeks then subjected to transduction with lentivirus-HPV E6/E7 IRES-puromycin under the same conditions as above (cell lines now named UM-SCC-29-E6/E7 and UM-SCC-38-E6/E7).
RNA Extraction and Reverse Transcription Polymerase Chain Reaction
RNA was isolated by phenol-chloroform extraction with TRIzol (Invitrogen). cDNA was synthesized using the Reverse Transcription System Kit (Promega) according to the manufacturer's protocol. Primers used to detect E6 and E7 are listed in Table 1. The HPV-16 containing CaSki cervical cancer cell line was used as a positive control for presence of oncogenes E6 and E7.
Table 1.
Primers used for HPV RT-PCR
| Primer | Sequence | Product length | Annealing Temperature | Cycle number |
|---|---|---|---|---|
| HPV16 E6 F# | ATGCACCAAAAGAGAACTG | 476bp | 55? | 35 |
| HPV16 E6 R# | TTACAGCTGGGTTTCTCTAC | 476bp | 55? | 35 |
| HPV16 E7 F | ACCGGTCGATGTATGTCTTGTTG | 360bp | 55? | 35 |
| HPV16E7R | CCGTACCCTCTTCCCCATTG | 360bp | 55? | 35 |
Also amplifies the alternate transcripts E6*I and E6*II
Flow Cytometry and Cell Sorting
CSC identification and isolation were performed using the enzymatic reaction involving aldehyde dehydrogenase (ALDH) using the ALDEFLUOR kit (StemCell Technologies). Two samples were used for controls: an ALDH-inhibited control achieved by adding diethylaminobenzaldehyde 50 mmol/l and a cell viability control with 4’, 6-diamidino-2-phenylindole (DAPI; BD Pharmigen). Flow cytometry gates were set using the inhibited control and the cell viability sample. An equal number of the ALDHlow expressing cells and ALDHhigh expressing cells were collected.
Multicellular Spheroid Formation
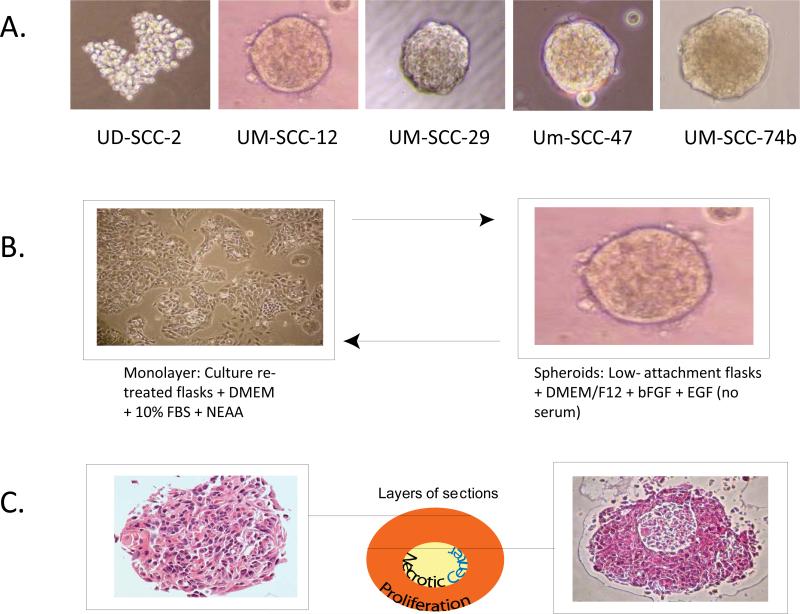
Cells were suspended in DMEM/F12 (Invitrogen) without serum. Additives included, 20 ng/μL basic fibroblast growth factor, 10 ng/μL epidermal growth factor and the stem cell growth supplement B-27 without retinyl acetate (GIBCO). Cells were plated in 6-well ultra-low attachment culture plates (Corning). To determine if spheroids were able to recapitulate the original monolayer phenotype, spheroids were placed back into standard culture plates with complete media containing 10% FBS. For the limiting dilution experiments, three cell lines (UM-SCC-47, UM-SCC 29, and UM-SCC 74B) were diluted to a ratio of 1 cell per 10 μL and plated at various densities using 96-well ultra-low attachment culture plates. Cells were observed for spheroid growth for >30 days.
Evaluating Spheroid Architecture
The cell line UM-SCC-12 was grown in spheroid-promoting conditions for 7 days at a cell density of 5 × 105 cells in ultra-low adherence plates. Spheroids were fixed in 70% ethanol. Histogel (Thermo Scientific) was used to resuspend the fixed pellet. The pellet was then embedded, sectioned and stained with hematoxylin and eosin.
Colony Formation Assays
CSC and non-CSC populations were plated at a cell density of 250 cells per 2.0 cm2 for cell lines: UD-SCC-2, UM-SCC-38-Luc, UM-SCC-47, and UM-SCC-38-E6/E7. For UM-SCC-29-Luc and UM-SCC-29-E6/E7, 500 cells per 2.0 cm2 were plated. Cells were allowed to attach overnight and then treated with cisplatin. Cisplatin (Cis-diammineplatinum(II) dischloride, DDP) (Sigma-Aldrich) was dissolved in sterile 0.9% NaCl to achieve a stock concentration of 3.33 mM. Complete DMEM was used to dilute the stock concentration of cisplatin to desired doses. Treatment with drug lasted for 12 hours. Cultures were observed for 7-14 days (depending on growth rate differences) to allow for untreated cells to reach >50 cells per colony. They were fixed and stained with crystal violet in 20% methanol. Plating efficiency (PE) was calculated by dividing the number of colonies formed in the no treatment group by the number of cells seeded Survival fraction (SF) was determined by colonies formed after treatment divided by the number of cells seeded multiplied by the plating efficiency. The student T-test determined statistical significance.
Tail Vein Injections
Non-obese diabetic severe combined immunodeficient (NOD/SCID) mice were injected and assessed for lung lesions by bioluminescent imaging. Four mice were injected for each cell line: UM-SCC-29-Luc, UM-SCC-29-E6/E7, UM-SCC-38-Luc and UM-SCC-38/E6/E7. Cells were suspended in phosphate-buffered saline (PBS, Gibco) for 50,000 cell and 25,000 cell injections. Mice without positive bioimaging of lung tumors were euthanized and the lungs sectioned to confirm the absence of squamous cell carcinoma.
Bioluminescence Imaging
Animals with tumor cell injections were imaged with the Xenogen IVIS-200 imaging system. Treated mice were given intraperitoneal luciferin before being imaged.
Results
E6/E7 Transduction
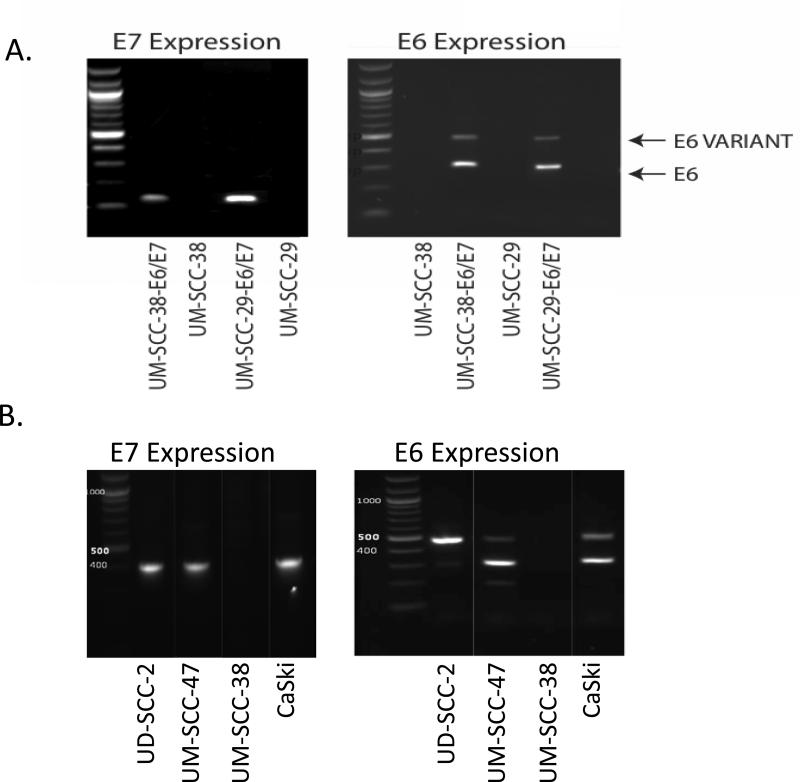
Transduction of E6 and E7 oncogenes was verified by comparing cDNA from UM-SCC-38-E6/E7 and UM-SCC-29-E6/E7 to E6/E7 containing CaSki cell line by RT-PCR (Figure 1A). The HPV(+) cell lines, UD-SCC-2 and UM-SCC-47, displayed E6 and E7; the HPV(-) cell lines, UM-SCC-29 and UM-SCC-38, did not (Figure 1B). E6 appeared as two bands for both cell lines representing a full length E6 gene product (476 base-pairs) and an internally spliced variant E6* (297 base-pairs). The amplicon size for E7 was 400 base-pairs.
Figure 1.
A. Transduction of E6 and E7 oncogenes into UMSCC-38 and UMSCC-29. B.UD-SCC-2 and UM-SCC-47, display E6 and E7,the HPV(-) cell line, UM-SCC-38, does not.
Proportion of ALDHhigh cells
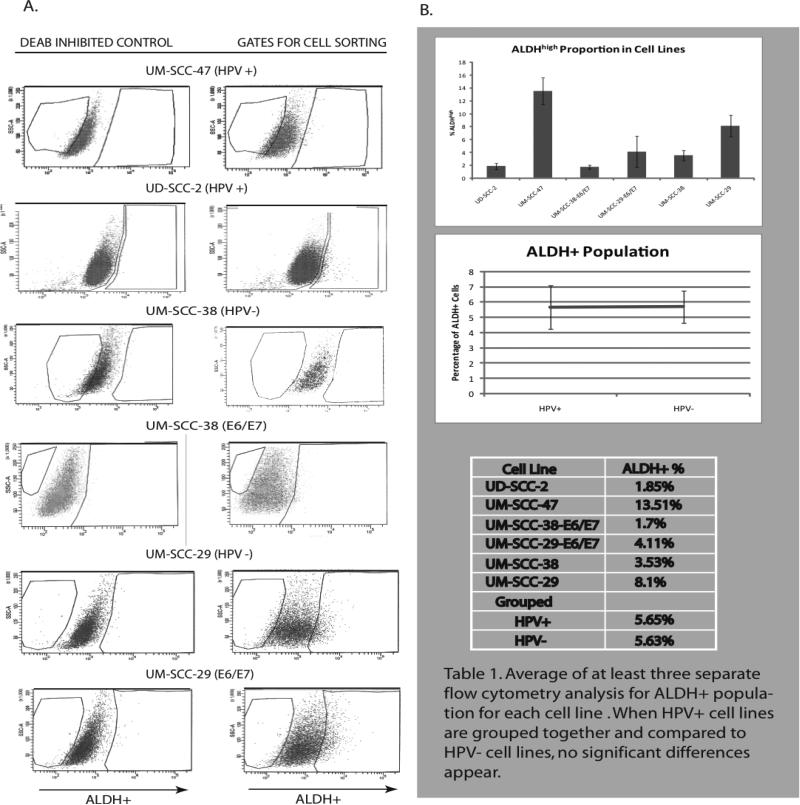
Six cell lines were analyzed and sorted for ALDHhigh populations in at least three separate trials (Figure 2). All cell lines had ALDH activity when analyzed by FACS. The average ALDHhigh population was 1.85% (SE=0.48%) in UD-SCC-2, 13.51% (SE=2.09%) in UM-SCC-47. 1.70% (SE=-0.29%) in UM-SCC-38-E6/E7, 4.11% (SE=2.42%) in UM-SCC-29-E6/E7, 3.52% (SE=0.81%) in UM-SCC-38 and 8.10% (SE=1.65%) in UM-SCC-29 (Figure 2B). When HPV(+)cell lines were averaged, including those cell lines transfected with E6/E7, the average ALDHhigh population was 5.65% (SE=1.42%) and HPV(-) average was 5.64% (SE=1.06%). There were no significant differences between the two groups (p=0.91). However, the proportion of ALDHhigh cells decreased after E6 and E7 transfection, from 3.52% to 1.7% and 8.10% to 4.11% for UM-SCC-38 and UM-SCC-29 respectively.
Figure 2.
Flow cytometry A. Left - Control ALDH activity after inhibition with DEAB. Right - ALDHhigh population. B. Percent ALDHhigh in in cell lines.
Chemoresistance
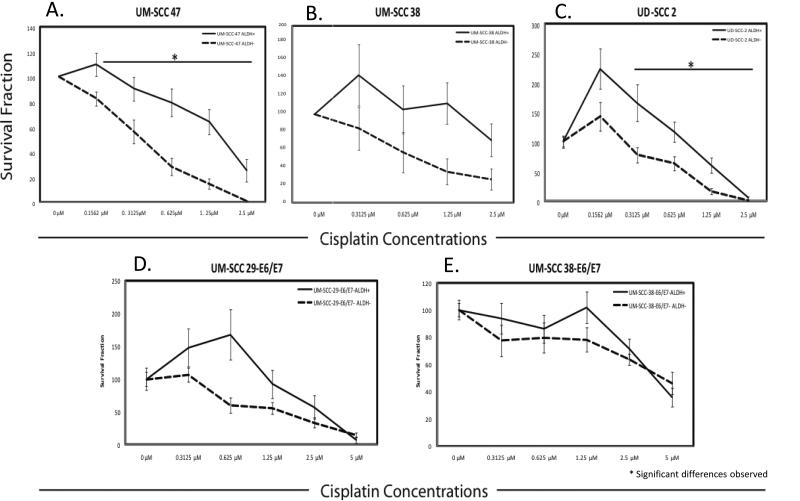
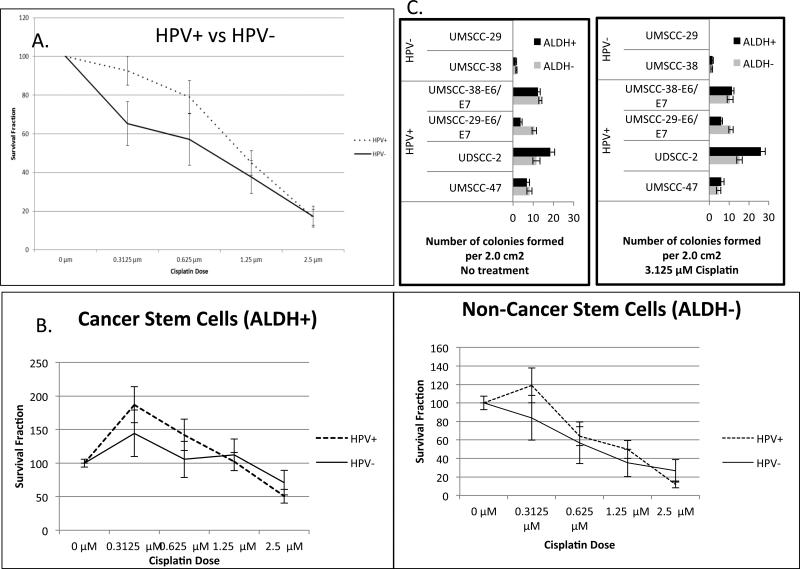
Multiple cell lines (both HPV(+), HPV(-) and E6/E7 transduced) were treated with graded concentrations of cisplatin and assessed for changes in colony formation. For HPV(+) cell lines, UM-SCC-47 and UD-SCC-2, there was a statistically significant higher survival fraction in the ALDHhigh cell population compared to the ALDHlow population at cisplatin concentrations: 0.3125, 0.625, 1.25, 2.5 μM (p<0.03) (Figure 3A & 3C). For the HPV(-) cell line UM-SCC-38, only at 1.25 μM cisplatin concentration did the ALDHhigh cells have a significantly higher survival fraction than the ALDHlow cell population (p<0.01) (Figure 3B). In the transduced cell line UM-SCC-29-E6/E7 there was a significantly higher survival fraction of ALDHhigh cells only at 0.625 μM cisplatin concentration (p<0.01) (Figure 3D). No significant differences in survival were evident between the ALDHhigh and ALDHlow cells for the transduced cell line UM-SCC-38-E6/E7 at any cisplatin concentration (Figure 3E). Overall there were no significant differences observed in the cisplatin sensitivity between the HPV(+) and HPV(-) cell lines (Figure 4A). Additionally, there were no significant differences in survival fraction of the ALDHhigh and ALDHlow cell subpopulations sorted from HPV(+) and HPV(-) cell lines (Figure 4B).
Figure 3.
Comparison of survival fraction of ALDH+ (ALDHhigh) and ALDH- (ALDHlow) cells sorted from cel lines and exposed to cisplatin at varuous concentrations
Figure 4.
A and B. Comparison of survival fraction between HPV(+) and HPV(-) cell lines at various doses of cisplatin. C. Colony formation assay.
Clonogenicity
The HPV(-) cell line UM-SCC-29, formed no colonies (Figure 4C). The HPV(-) cell line UM-SCC-38 had very low clonogenicity in all experimental groups. Each of the naturally infected HPV(+) cell lines, exhibited greater colony formation ability. In UD-SCC-2, there were 18.35 (SE=2.28) and 11.68 (SE=1.78) colonies for ALDHhigh and ALDHlow, respectively. In UM-SCC-47, there were 6.86 (SE=1.35) and 8.19 (SE=1.21) colonies in the ALDHhigh and ALDHlow, respectively. UM-SCC-29 and UM-SCC-38 both exhibited increased in clonogenicity when the cell lines were transfected with E6/E7. For UM-SCC-29-E6/E7, there were 3.86 (SE=0.68) and 10.53 (SE=1.1) colonies formed for ALDHhigh and ALDHlow cells, respectively. Likewise, there was increased clonogenicity in UM-SCC-38-E6/E7 with 12.38 (SE=1.11) average colonies formed for ALDHhigh and 13.5 (SE=0.80) average colonies for ALDHlow relative to the parental cells.
Self-renewal
UD-SCC-2, UM-SCC-12, UM-SCC-47, UM-SCC-38, UM-SCC-29 and UM-SCC-74B were tested for their ability to grow as spheroids. All cell lines, with the exception of UD-SCC-2, were able to form compact spheroids (Figure 5). UD-SCC-2 formed loose cellular aggregates that did not reflect true spherical geometry. UM-SCC-47 and UM-SCC-74B were tested using limiting dilution experiments to determine if single cells were able to form spheroids. No spheroids were observed to form below cell densities of 2500 cells/100 μL in 96 well plates suggesting that spheroid formation does not represent clonal growth, but rather aggregation. Spheroids were able to revert back to monolayer cell morphology when plated back in standard culture conditions (Figure 5). When spheroids are fixed, sectioned and stained, their morphology more closely resembles tumors than do monolayer cells.
Figure 5.
A. Spheroid formation in a series of cell lines. B. Spheroids are able to revert back to monolayer cell morphology. C. Spheroids closely mimic primary tumor morphology.
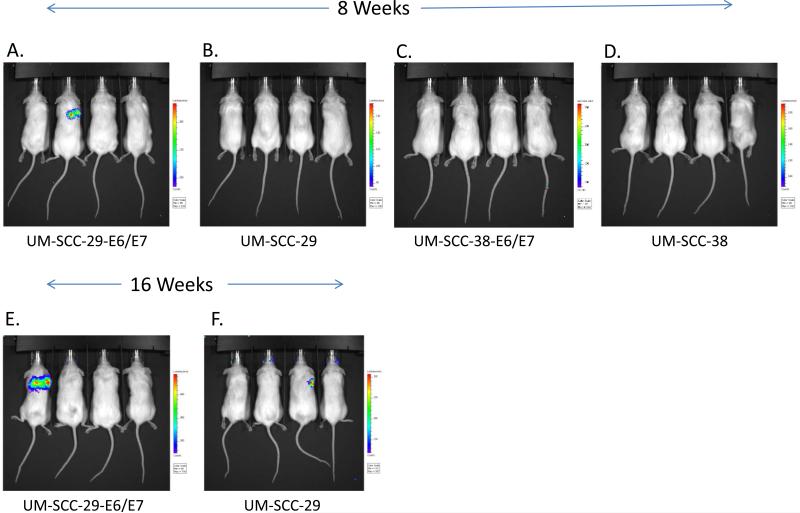
Tail Vein Injections
A total of 8 mice were injected with each cell line. Four mice in each group were injected with the control transfected cell lines (UM-SCC-29-Luc and UM-SCC-38-Luc) and four mice with the E6/E7 transfected cell lines (UM-SCC-29-E6/E7 and UM-SCC-38-E6/E7) (Figure 6). In the UM-SCC-29-E6/E7 tail vein injections 1 out of 4 mice grew lung lesions with 5 x 104 cells after 8 weeks and progressed at 16 weeks. For UM-SCC-29-Luc, there were no lesions at 8 weeks, but lung lesions appeared in 1 out of 4 mice at 16 weeks. UM-SCC-38-E6/E7 and UM-SCC-38-Luc did not produce lung lesions up to 16 weeks. The E6/E7 status of the cell line did not alter the ability of the cells to produce lung colonies.
Figure 6.
Tail vein injections in non-obese diabetic severe combined immunodeficient mice at 8 and 16 weeks. Bioluminescence reveals lung colonies
Discussion
Over the last twenty years, numerous treatment strategies have been suggested for patients with advanced head and neck cancers. Platinum-based therapy is often included in treatment arms. Cisplatin resistance in HNSCC is a major obstacle in spite of escalating doses. One explanation for this observation is that the cells surviving chemotherapy acquire resistance after multiple rounds of treatment. The cancer stem cell hypothesis suggests that treatment failures occur due to ineffective killing of cancer stem cells16. Our results indicate that the HNSCC CSC fraction, as identified by high ALDH expression, represents a very small subset of tumor cells that have increased cell survival after treatment with cisplatin. Four of the 5 cell lines we tested demonstrated increased cell survival in CSCs with exposure to at least one concentration of cisplatin. Cancers from other tumor sites have revealed similar findings, showing inherent chemoresistence in ALDHhigh expressing cells15. In breast cancer cell lines, Tanei et al. observed that ALDHhigh cells were more resistant to sequential treatment with paclitaxel and epirubicin-based chemotherapy than their ALDHlow counterparts17. They also observed that primary tumors had increased expression of ALDH1 cells after neoadjuvant chemotherapy, indicating a high rate of CSC survival after treatment. In colorectal cancer, inhibition ALDH1 appears to play a role in sensitizing cells to cyclophosphamide18. Our results are the first to show chemoresistance in HNSCC CSCs using ALDH as a marker to identify the CSC population. Multiple mechanisms have been proposed to explain this resistance in other cancers, including the involvement of the ALDH enzyme itself14.
Expression of CSC markers correlates with worse prognosis and advanced tumor stage in HNSCC and other tumors10,19. Conversely the presence of HPV-16 has proven to be a favorable prognostic indicator in patients with oropharyngeal cancer20,21. While our data showed no differences in the proportion of ALDHhigh expressing cells between HPV(+) and HPV(-) cell lines, we did observe a decrease in CSC proportion when our two HPV(-) cell lines were transduced with the HPV-16 oncogenes E6 and E7. It is tempting to suggest that this may play a role in changing tumor behavior, but further studies to test this finding are needed.
Currently, to our knowledge, there are only six HNSCC HPV(+) cell lines previously described22-25. Generally, these cell lines are not representative of the profile of the patients with HPV-associated oropharyngeal cancer, as many come from tumors that exhibit aggressive biological behavior. To better elucidate the effect of HPV status on CSC, more studies must be performed using primary tumor samples, and HPV(+) cell lines that reflect the patient population where HPV-associated improved outcomes are seen.
Although we did find that HNSCC cancer cells with high ALDH expression were more resistant to cisplatin therapy, we did not find differences between HPV(+) and HPV(-) cell lines, including our E6/E7 transduced cell lines. This suggests that HPV status alone does not directly correlate with cisplatin sensitivity. Additionally, our results indicate that transduction of E6 and E7 does not alter cisplatin sensitivity. In one study of HNSCC, only a modest increase in HPV(+) HNSCC cell line sensitivity was demonstrated between HPV(+) and HPV(-) cell lines in vitro; but in immunocompetent mice, HPV(+) tumors responded better to radiation and cisplatin26. As we did not identify any differences in cisplatin sensitivity in vitro between HPV(+) cancer cells and E6 and E7 transfected cancer cells, it suggests that HPV(+) HNSCC cells by themselves lack intrinsic properties that make them more sensitive to cisplatin. These findings indicate that response to therapy in HNSCC associated with HPV likely involves a complex interplay between therapeutic agents, the antigenic properties of the cancer cells and the host immune system26, 27.
The presence of transfected and integrated E6/E7 had an impact on cancer cell clonogenicity. After transfection, there was an enhanced ability of the cancer cells to form colonies. UM-SCC-29-Luc underwent anoikis (where cells undergo programmed cell death when detached from extracellular matrix) after cell sorting and culture in suspension, but when transformed with oncogenes E6/E7 tumor cells were able to resist anoikis and proliferate. Moreover, we found that our two naturally infected HPV(+) cell lines formed more colonies than our HPV(-) cell lines. Expression of E6 and E7 induces immortalization in several cell types by inactivating the apoptotic regulators TP53 and Rb28-30. Silencing of E6 and E7 results in apoptosis in HPV(+) HNSCC cell lines31. The precise biologic mechanisms by which E6 and E7 induce these changes are not fully understood.
Culture in suspension is a method by which the CSC populations can be enriched. When cells are grown in serum-free media and plated on non-adherent flasks, floating spheroids and cellular aggregates form. This technique has been tested in various malignancies including glioblastomas, breast cancers and colon cancers32-34. Focus on spheroid cultivation in HNSCCs has been limited. Our spheroid model in HNSCC did not demonstrate the behavior of self-renewal. We based our conditions on work done with glioma spheroids, where additives enhanced cell conditions that fostered self-renewal for those stem cells32. We speculate that for HNSCC CSC self-renewal, suitable microenvironments that promote single CSC maintenance and expansion may still need to be worked out. Krishnamurthy et al. recently demonstrated that CSC survival and self-renewal requires a niche that involves cell signaling initiated by factors secreted by endothelial cells6.
Nevertheless, we have demonstrated that the spheroids cultivated as a result of cellular aggregation represent a unique three-dimensional model that more closely represents the in vivo tumor structure than that of cells grown under adherent culture conditions. We postulate that this model may provide a more accurate method to evaluate anti-cancer drug efficacy in an in vitro environment35.
Conclusions
Cancer stem cells in head and neck tumors are more resistant to cisplatin than non-CSC. This finding suggests that although cisplatin may shrink the tumor it may be less effective in eradicating the CSCs, leaving this potent population of cancer cells behind. HPV status does not appear to have a significant effect on the proportion of cancer stem cells present in HNSCC or their sensitivity to cisplatin therapy. This indicates that other factors, possibly immune-regulated mechanisms, may be responsible for the more favorable outcomes of patients with HPV associated HNSCC.
Acknowledgements
This project was made possible by funding from the University of Michigan Comprehensive Cancer Center, the American Academy of Otolaryngology-HNS Percy Memorial Award, the Kirschstein National Research Service Award (NRSA) in Advanced Research Training in Otolaryngology: 5 T32 DC005356, and a gift from Patricia Korican of Korican Real Estate. Additional support was from NIDCR 1 R01-DE019126, NCI P50 CA97248 (Head and Neck SPORE), NIDCD P30 DC05188 (KHRI Research Center Core grant) and NCI P30 CA46592 (Cancer Center Core Grant). None of the funders had any role in the design, conduct, or interpretation of the above-mentioned experiments. The principal investigators in this study had full access to all data and take responsibility for the integrity of the data and the accuracy of the data analysis. Thank you to the Flow Cytometry Core and the Vector Core at the University of Michigan for their help in acquiring this data.
Footnotes
Presented at the American Academy of Otolaryngology Annual Meeting in Washington DC, Sept 2012
References
- 1.Rich JN. Cancer stem cells in radiation resistance. Cancer Res. 2007;67(19):8980–4. doi: 10.1158/0008-5472.CAN-07-0895. [DOI] [PubMed] [Google Scholar]
- 2.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 3.Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009;125(10):2323–31. doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 4.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104(3):973–8. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clay MR, Tabor M, Owen JH, et al. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32(9):1195–201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishnamurthy S, Dong Z, Vodopyanov D, et al. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70(23):9969–78. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen YC, Chen YW, Hsu HS, et al. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385(3):307–13. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 8.Davis SJ, Divi V, Owen JH, et al. Metastatic potential of cancer stem cells in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2010;136(12):1260–6. doi: 10.1001/archoto.2010.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chikamatsu K, Ishii H, Takahashi G, et al. Resistance to apoptosis-inducing stimuli in CD44+ head and neck squamous cell carcinoma cells. Head Neck. 2011 doi: 10.1002/hed.21732. [DOI] [PubMed] [Google Scholar]
- 10.Joshua B, Kaplan MJ, Doweck I, et al. Frequency of cells expressing CD44, a Head and Neck cancer stem cell marker: Correlation with tumor aggressiveness. Head Neck. 2011 doi: 10.1002/hed.21699. [DOI] [PubMed] [Google Scholar]
- 11.Awad O, Yustein JT, Shah P, et al. High ALDH activity identifies chemotherapy-resistant Ewing's sarcoma stem cells that retain sensitivity to EWS-FLI1 inhibition. PLoS One. 2010;5(11):e13943.12. doi: 10.1371/journal.pone.0013943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landen CN, Jr., Goodman B, Katre AA, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9(12):3186–99. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monroe MM, Anderson EC, Clayburgh DR, Wong MH. Cancer stem cells in head and neck squamous cell carcinoma. J Oncol. 2011;2011:762780. doi: 10.1155/2011/762780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4(6):697–720. doi: 10.1517/17425250802102627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alison MR, Guppy NJ, Lim SM, Nicholson LJ. Finding cancer stem cells: are aldehyde dehydrogenases fit for purpose? J Pathol. 2010;222(4):335–44. doi: 10.1002/path.2772. [DOI] [PubMed] [Google Scholar]
- 16.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 17.Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res. 2009;15(12):4234–41. doi: 10.1158/1078-0432.CCR-08-1479. [DOI] [PubMed] [Google Scholar]
- 18.Dylla SJ, Beviglia L, Park IK, et al. Colorectal cancer stem cells are enriched in xenogeneic tumors following chemotherapy. PLoS One. 2008;3(6):e2428. doi: 10.1371/journal.pone.0002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu R, Wang X, Chen GY, et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N Engl J Med. 2007;356(3):217–26. doi: 10.1056/NEJMoa063994. [DOI] [PubMed] [Google Scholar]
- 20.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128–37. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100(4):261–9. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 22.Ferris RL, Martinez I, Sirianni N, et al. Human papillomavirus-16 associated squamous cell carcinoma of the head and neck (SCCHN): a natural disease model provides insights into viral carcinogenesis. Eur J Cancer. 2005;41(5):807–15. doi: 10.1016/j.ejca.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Steenbergen RD, Hermsen MA, Walboomers JM, et al. Integrated human papillomavirus type 16 and loss of heterozygosity at 11q22 and 18q21 in an oral carcinoma and its derivative cell line. Cancer Res. 1995;55(22):5465–71. [PubMed] [Google Scholar]
- 24.Tang AL, Hauff SJ, Owen JH, et al. UM-SCC-104: A New human papillomavirus-16-positive cancer stem cell-containing head and neck squamous cell carcinoma cell line. Head Neck. 2012;34(10):1480–91. doi: 10.1002/hed.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner JC, Graham MP, Kumar B, et al. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010;32(4):417–26. doi: 10.1002/hed.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135(11):1137–46. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 27.Williams R, Lee DW, Elzey BD, Anderson ME, Hostager BS, Lee JH. Preclinical models of HPV+ and HPV− HNSCC in mice: an immune clearance of HPV+ HNSCC. Head Neck. 2009;31(7):911–8. doi: 10.1002/hed.21040. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Salas LM, Cullinan AE, Siwkowski A, Hampel A, DiPaolo JA. Inhibition of HPV-16 E6/E7 immortalization of normal keratinocytes by hairpin ribozymes. Proc Natl Acad Sci U S A. 1998;95(3):1189–94. doi: 10.1073/pnas.95.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munger K, Phelps WC, Bubb V, Howley PM, Schlegel R. The E6 and E7 genes of the human papillomavirus type 16 together are necessary and sufficient for transformation of primary human keratinocytes. J Virol. 1989;63(10):4417–21. doi: 10.1128/jvi.63.10.4417-4421.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wazer DE, Liu XL, Chu Q, Gao Q, Band V. Immortalization of distinct human mammary epithelial cell types by human papilloma virus 16 E6 or E7. Proc Natl Acad Sci U S A. 1995;92(9):3687–91. doi: 10.1073/pnas.92.9.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rampias T, Sasaki C, Weinberger P, Psyrri A. E6 and e7 gene silencing and transformed phenotype of human papillomavirus 16-positive oropharyngeal cancer cells. J Natl Cancer Inst. 2009;101(6):412–23. doi: 10.1093/jnci/djp017. [DOI] [PubMed] [Google Scholar]
- 32.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 33.Vermeulen L, Todaro M, de Sousa Mello F, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci U S A. 2008;105(36):13427–32. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charafe-Jauffret E, Ginestier C, Iovino F, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69(4):1302–13. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kunz-Schughart LA, Freyer JP, Hofstaedter F, Ebner R. The use of 3-D cultures for high-throughput screening: the multicellular spheroid model. J Biomol Screen. 2004;9(4):273–85. doi: 10.1177/1087057104265040. [DOI] [PubMed] [Google Scholar]