Abstract
Background
Abnormal tibiofemoral contact stress and aberrant kinematics may influence the progression of osteoarthritis in the ACL-deficient and the ACL-reconstructed knee. However, relationships between contact stress and kinematics following ACL reconstruction are poorly understood. Therefore, we posed the following research questions: (1) How do ACL deficiency and reconstruction affect kinematics of and contact stress in the tibiofemoral joint? (2) What kinematic differences are associated with abnormal contact stress following ACL reconstruction?
Hypothesis/Purpose
Center-center ACL reconstruction will not restore knee kinematics and contact stress. Correlations will exist between abnormal contact stress and aberrant kinematics following ACL reconstruction will exist.
Study Design
Controlled laboratory study
Methods
Clinical tests of anterior and rotational stability were simulated on eleven cadaveric knees using an industrial robot. Tests were conducted with the ACL intact, sectioned, and after single bundle ACL reconstruction using a quadrupled hamstring autograft with tunnels drilled through the center of the native footprints. Kinematics were recorded during the tests. Contact stress was continuously recorded from a stress transducer fixed to the tibial plateau and mean contact stress was calculated regionally.
Results
ACL deficiency resulted in increased mean contact stress in the posterior sectors of the medial and lateral compartments under anterior and rotational loads, respectively. Reconstruction reduced stress in these locations; however contact stress abnormalities remained. On average, kinematics were overconstrained following ACL reconstruction (≤1.8mm and ≤2.6° in all directions). However, combinations of overconstrained and underconstrained motions in ab/adduction and medial-lateral translation in response to combined moments, and axial rotation, anterior-posterior and medial-lateral translation in response to an anterior load were associated with abnormal mean contact stress.
Conclusions
ACL reconstruction reduces high stresses generated in the posterior compartment of the ACL-deficient knee. Abnormal contact stress following ACL reconstruction is related to multiplanar variations in knee kinematics.
Clinical Relevance
Clinical measures of multiplanar kinematics may help to better characterize the quality of ACL reconstruction. Such measures may help identify those at increased risk of long-term joint degeneration following this surgery.
Keywords: ACL reconstruction, kinematics, contact stress, osteoarthritis
INTRODUCTION
ACL reconstruction improves stability of the ACL-deficient knee, and may mitigate subsequent damage to the medial meniscus35. However, it does not consistently protect the joint from osteoarthritis (OA) progression over the long-term21, 27, 28. The high number of sports-related ACL injuries in young individuals, and the lack of effective treatment options for knee OA in this population46 makes this an emerging problem.
Mechanical factors, such as abnormal tibiofemoral kinematics and aberrant articular contact stress following ACL rupture and reconstruction likely contribute to the onset and progression of OA5, 10. Moreover, the relative movement of the tibiofemoral articulating surfaces, i.e., arthrokinematics, remains abnormal after ACL injury including anterior and medial shift of the contact location on the medial compartment of the tibia during a lunge25. However, these data do not describe the direct effect of ACL injury and reconstruction on the magnitude and distribution of the mechanical loads borne by the articulating surfaces, despite their potentially important role in OA pathogenesis. Such data can only be obtained either directly using stress transducers or indirectly using computational stress analysis. For example, single bundle ACL reconstruction resulted in increased mean contact stress on the medial and lateral compartments near full extension in response to an axial load in an in vitro model32. However, the ability of ACL reconstruction to restore contact mechanics under more complicated loading conditions, including the pivot shift exam are not well characterized, despite its importance as a clinical surrogate for knee function, its strong association with clinical outcome, and its potential to be an OA risk factor18, 22, 24.
Although ACL reconstruction can restore knee stability on average, less attention has been devoted to the impact of inter-individual differences in knee motions following ACL reconstruction. Small inter-subject variations in rotations and translations may influence articular contact stress, which could play a role in OA progression4, 12. In fact, the severity of OA progression following ACL injury may be associated with the level of instability42. However, relationships between abnormal contact stress and altered knee kinematics following ACL injury and reconstruction are poorly understood.
Understanding how inter-subject variations in knee kinematics impact contact stress could help identify specific knee motions to assess the quality of ACL reconstruction. This would provide a basis to enhance current clinical exams, which have thus far been devised primarily on the basis of detecting ACL injury11.
The failure of ACL reconstruction to completely restore knee joint mechanics, the recognition of increased OA risk with ACL injury, and the potential for failure of vertically oriented grafts motivates current clinical efforts to improve surgical technique2, 13. This includes placement of the tunnels of the tibia and femur of the ACL reconstruction within the footprints of the native ACL insertions, i.e., center-center reconstruction6, 43. Unfortunately, little biomechanical data is available characterizing the ability of this technique to restore both tibiofemoral kinematics and regional contact stress.
Therefore, we posed the following research questions: (1) How do ACL deficiency and reconstruction affect kinematics of and contact stress in the tibiofemoral joint? (2) What kinematic differences are associated with abnormal contact stress following ACL reconstruction?
METHODS
Cadaveric knee joints (n=11) were utilized for this Institutional Review Board (IRB)-approved study. Sample size was based on previously work32 reporting mean contact stress on the tibial plateau, which was the primary outcome measure in the present work. In the previous work, mean contact stress of 3.7 ± 0.4MPa was generated in response to an axial load of 1kN at full extension with the ACL intact. Thus, nine specimens were required to detect differences of 15% with 80% power and α = 0.05 using a repeated measures study design.
Fresh-frozen knees were thawed at room temperature 36 hours before testing. Average specimen age was 38 ± 12.5 ranging from 21 to 58 years old. Nine males and two females were utilized with six right legs and five left legs. Specimens were sectioned at the midshaft of the tibia-fibula and femur, leaving approximately 25cm of the shaft of each bone. The soft tissues surrounding the joint including capsular structures were left intact. Specimens were excluded if any gross joint abnormality, instability, or cartilage degeneration was observed visually through an anterior medial arthrotomy. A carpenter screw was drilled across the tibia and fibula proximally to stabilize the tibiofibular joint. The tibial and the femoral shafts were potted in bonding cement (Bondo/3M, Atlanta, Georgia). Two carpenter screws were drilled transversely in each shaft to ensure fixation between bone and cement.
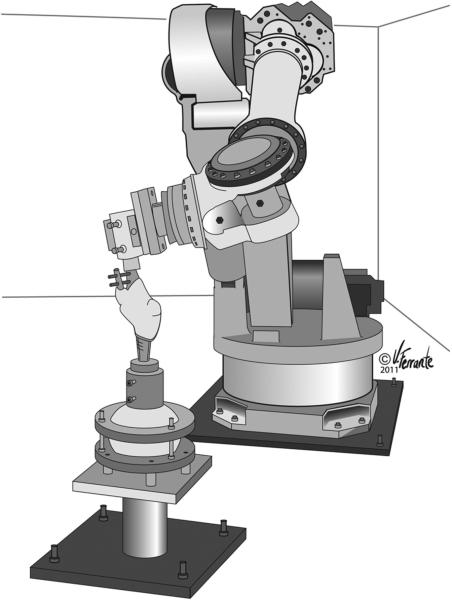
The knees were loaded using a six-degrees-of-freedom robotic arm (ZX165U; Kawasaki) with ±0.3mm repeatability1 (Fig. 1). A universal force-moment sensor (Delta; ATI, Apex, NC resolution: Fx = Fy = 1/8N, Fz = 1/4N, Tx = Ty = Tz = 10/1333Nm) mounted to the end of the robotic arm measured the forces acting across the knee joint. The potted femur was secured to a pedestal that was fixed to the floor. The tibia was attached to the robotic arm through a custom fixture. The specimen was aligned in full extension. Subsequently, anatomic landmarks were identified using a 3D digitizer (accuracy: 0.23mm) (MicroScribe; Immersion, San Jose, California) to define reference frames that describe motion of the tibia relative to the femur.
Rotations and translations of the tibia relative to the fixed femur were expressed using the convention described by Grood and Suntay15. The medial and lateral femoral epicondyles defined the orientation of the flexion-extension axis. This axis was directed laterally and medially for right and left specimens, respectively. The bisection of this axis was assigned to be the origin of the femoral coordinate system. The long axis of the tibia was directed distally and defined internal-external rotation. Its orientation was defined by the most distal point on the center of the tibial shaft, and the bisection of the distal insertions of the medial and lateral collateral ligaments. The origin of the tibial coordinate system was assigned to be coincident with the origin of the femoral coordinate system at full extension. The common perpendicular of the flexion/extension axis and the internal/external rotation axis faced posteriorly and defined ad/abduction. Translations were expressed as the projection of the vector defined by the origins of the tibial and femoral coordinate systems onto each anatomic direction described above.
Force feedback algorithms were used to determine the position and orientation of the tibia that minimized the difference between the current and the targeted load to a resultant force ≤5N and a resultant moment ≤0.5Nm14, 36. Testing was begun by determining the path of passive flexion of the intact knee from full extension to 90° of flexion in 1° increments of flexion. To assess anterior stability, a 134N anterior force was applied at 0, 15, 30, 60 and 90° flexion. We tested at these angles because the posterolateral bundle of the ACL is the primary restraint to anterior forces at 0, 15 and 30° flexion, while the anteromedial bundle is the primary restraint to anterior forces at 60 and 90° flexion37. To assess rotational stability combined moments of 8 and 4Nm in abduction and internal rotation, respectively, were applied at 5, 15 and 30° flexion20. We tested at these angles because anterior translation is highest with ACL deficiency under these combined moments between full extension and 30° flexion20. The position and orientation of the knee as found during the passive flexion path served as the starting points for the application of loads45. Net knee motions in all directions were calculated for each loading condition, each flexion angle, and with the ACL intact, sectioned, and reconstructed. Net knee motion was defined as the change in knee position between the maximum applied load and the intial reference position along the path of passive flexion. The order of testing between the ACL deficient and reconstructed states was selected at random. ACL sectioning or reconstruction was performed through a medial parapatellar arthrotomy to allow direct visualization of the ACL anatomy. The arthrotomy was sutured closed after both procedures.
The native ACL was preconditioned by determining the motion required to achieve 134N anterior load at 30° flexion, and repeating this motion for ten cycles. Similarly, the medial collateral structures were preconditioned by determining the motion required to apply the combined moments at 15° flexion. This motion was then also repeated for ten cycles.
Single bundle ACL reconstruction was performed after resecting the native ACL by drilling in the center of the ACL footprints (Fig. 2)6. A quadrupled semitendinosus and gracilis autograft measuring 9cm in length was prepared using an endobutton and 15mm loop. Graft material was harvested from each specimen and used only for that specimen. The diameter of the femoral tunnel was chosen to accommodate the size of the graft harvested from each specimen. The diameter of the tibial tunnel was drilled one mm larger then the femoral tunnel to account for increased graft diameter after suturing the tendons together. The femoral tunnel was made by first drilling a guide pin into the center of the native femoral ACL footprint through the medial parapatellar arthrotomy in a “medial portal equivalent” approach. It was drilled to a depth of 32mm. The Endobutton (Smith & Nephew, Inc., Andover, Massachusetts) drill bit was used to drill through the cortex. Adjustments to the tunnel depth were then made as needed. An ACL tibial drill guide set to 55° was positioned in the center of the tibial ACL footprint, adjacent to the anterior horn of the lateral meniscus. The graft was shuttled into position and the endobutton was deployed for femoral fixation. The knee was cycled twenty times. With the knee held in neutral rotation and 20° flexion44, the sutures from the graft were tied around a cortical screw and washer, which had been placed in the tibia and fixed under 89N (20lbs) of pretension. A 10 × 25mm biointerference screw was placed in the tibial tunnel for supplemental fixation. Similar to the native ACL, the reconstructed ACL was preconditioned by determining the motion required to achieve 134N anterior translation at 30° flexion, and then cycling ten times.
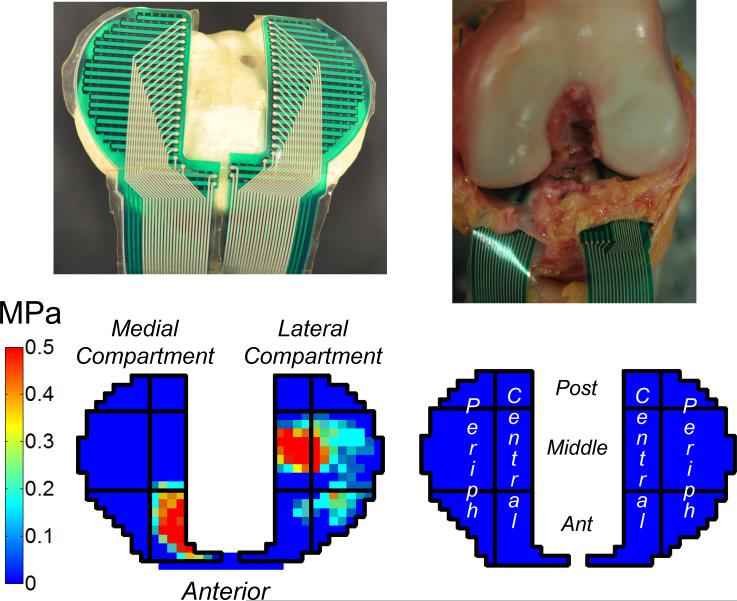
After determining knee motions for each loading condition and each state of the ACL, a stress transducer (4010N, Tekscan, South Boston, Massachusetts) was slid beneath the menisci and sutured in place so that it remained fixed to the tibial plateau (Fig. 3). All kinematic pathways were replayed, and the contact stresses in the medial and lateral compartments of the tibia were recorded. To assess spatial variation in contact stress patterns on the tibial plateau, the area of the stress transducer was divided into six sectors in each compartment (anterior, middle, or posterior in anterior-posterior direction, and central or peripheral in medial-lateral direction) (Fig. 3). The mean contact stress in each sector was calculated at the position corresponding to the maximum applied external load for each loading condition. Mean contact stress was chosen as a representative measure for the distribution of load at the articulating surface.
The stress transducer was calibrated prior to testing by loading it to 20% and 80% of the maximum expected load and then fitting these data with a two-parameter power function. The calibration accuracy was tested by loading the sensor in an MTS loading system (MTS Systems, Eden Prairie, Minnesota) with a Instron Controller (8500; Instron, Norwood, Massachusetts) and a 444.8N (100-lb) load cell (Interface, Scottsdale, Arizona) after calibration. Repeatability of sensor measurements over the course of testing was assessed by repeating a subset of motions (n=14) in a subset of specimens (n=6). These data were presented as mean and standard deviation of the percent change in the total force measured by the sensor at the maximum applied external load across the repeated measurements.
Mean contact stress was compared across ACL intact, deficient and reconstructed conditions on a sector-by-sector basis using generalized estimating equations (GEE)16. This technique is suitable for data that are not normally distributed. A separate analysis was performed for each applied load at each flexion angle. Similarly, ML and AP translations, and axial rotation, and ab/adduction were compared across each condition of the ACL, at each applied load, and at each flexion angle using GEE. Statistical significance for all comparisons was set at p<0.05. Means, standard deviations and 95% confidence intervals were calculated for all outcomes.
Associations between kinematics and sectors where mean contact stress remained abnormal following ACL reconstruction were assessed using multiple linear regression. The differences between the intact and ACL reconstructed conditions were used in this regression model for both kinematic and contact stress measures. Regression coefficients with p<0.05 were reported along with their 95% confidence intervals and the coefficient of determination (r2).
RESULTS
Our first reconstruction was performed by a different surgeon than the others and was considered a pilot test; therefore, these data were excluded. Graft fixation failed at the tibia in another specimen; therefore, these data were also excluded from the analysis. Graft diameter ranged between 7 and 9mm on the femur. The sutured tibial side of the graft was 1mm larger than the femoral side in all cases.
The ACL sectioning increased medial and anterior translations and internal rotation compared to the intact condition in response to combined moments (Table 1). With an anterior load, ACL sectioning increased anterior translation at all flexion angles tested (Table 2).
Table 1.
Translations and rotations of the intact, ACL-deficient and ACL-reconstructed knee in response to combined abduction and internal rotation moments at 5, 15, and 30° flexion.
| Flexion (°) | Translations (mm) | Rotations (°) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Medial | Anterior | Internal | Abduction | |||||||||
| Intact | Deficient | Reconstructed | Intact | Deficient | Reconstructed | Intact | Deficient | Reconstructed | Intact | Deficient | Reconstructed | |
| 5 | 1.4 ± 0.5 | 3.7 ± 1.3*§ | 0.6 ± 0.9* | 0.2 ± 1.4 | 5.3 ± 2.0*§ | −1.1 ± 1.8* | 9.8 ± 2.4 | 13.7 ± 2.0*§ | 7.2 ± 2.1* | 1.2 ± 0.5 | 2.3 ± 0.5*§ | 1.1 ± 0.5 |
| (1.0,1.7) | (2.9, 4.5) | (0.0, 1.2) | (−0.6, 1.1) | (4.1, 6.5) | (−2.3, 0.1) | (8.3, 11.3) | (12.5, 15.0) | (5.8, 8.4) | (0.8, 1.5) | (2.0, 2.7) | (0.8, 1.5) | |
| 15 | 1.8 ± 0.9 | 4.7 ± 1.7*§ | 1.3 ± 0.8 | 1.5 ± 2.4 | 7.4 ± 3.0*§ | −0.2 ± 2.3* | 15.9 ± 4.5 | 19.0 ± 4.0*§ | 13.6 ± 4.6* | 1.7 ± 1.0 | 3.9 ± 1.1*§ | 1.5 ± 0.8 |
| (1.3, 2.3) | (3.8, 5.7) | (0.7, 1.8) | (0.1, 2.8) | (5.7, 9.0) | (−1.6, 1.3) | (13.4, 18.4) | (16.7, 21.2) | (11.1, 16.0) | (1.0, 2.3) | (3.2, 4.6) | (1.0, 2.0) | |
| 30 | 2.8 ± 1.5 | 5.6 ± 2.4*§ | 2.4 ± 1.4 | 2.5 ± 3.3 | 7.3 ± 4.2*§ | 1.5 ± 3.1 | 20.4 ± 6.9 | 22.3 ± 6.9*§ | 19.2 ± 7.0* | 2.5 ± 1.4 | 4.9 ± 1.8*§ | 2.3 ± 1.3* |
| (1.9, 3.6) | (4.2, 6.9) | (1.5, 3.2) | (0.7, 4.4) | (5.0, 9.7) | (−0.4, 3.5) | (16.5, 24.2) | (18.4, 26.1) | (15.2, 22.7) | (1.7, 3.3) | (3.9, 5.9) | (1.4, 2.8) | |
Values are expressed as mean ± standard deviation; 95% confidence intervals are in parentheses.
p<0.05 compared with the intact knee.
p<0.05 compared with the ACL-reconstructed knee.
Table 2.
Anterior translation of the intact, ACL-deficient and ACL-reconstructed knee in response to 134N anterior force at 0, 15, 30, 60, and 90° flexion.
| Flexion Angle | Anterior Translation (mm) |
||
| Intact | Deficient | Reconstructed | |
| 0° | 4.6 ± 0.5 | 13.1 ± 2.1*§ | 3.1 ± 1.2* |
| (4.3, 4.9) | (11.9, 14.3) | (2.3, 3.8) | |
| 15° | 6.5 ± 1.4 | 18.9 ± 2.8*§ | 4.7 ± 1.6* |
| (5.7, 7.3) | (17.3, 20.5) | (3.7, 5.7) | |
| 30° | 7.4 ± 1.5 | 20.3 ± 3.6*§ | 6.3 ± 1.7* |
| (6.6, 8.3) | (18.3, 22.4) | (5.2, 7.3) | |
| 60° | 6.5 ± 2.3 | 16.2 ± 5.5*§ | 6.6 ± 2.0 |
| (5.2, 7.7) | (13.0, 19.3) | (5.4, 7.8) | |
| 90° | 5.2 ± 2.2 | 12.2 ± 4.2*§ | 5.2 ± 2.1 |
| (4.0, 6.5) | (9.8, 14.6) | (3.9, 6.5) | |
Values are expressed as mean ± standard deviation; 95% confidence intervals are in parentheses.
p<0.05 compared with the intact knee.
p<0.05 compared with the ACL-reconstructed knee.
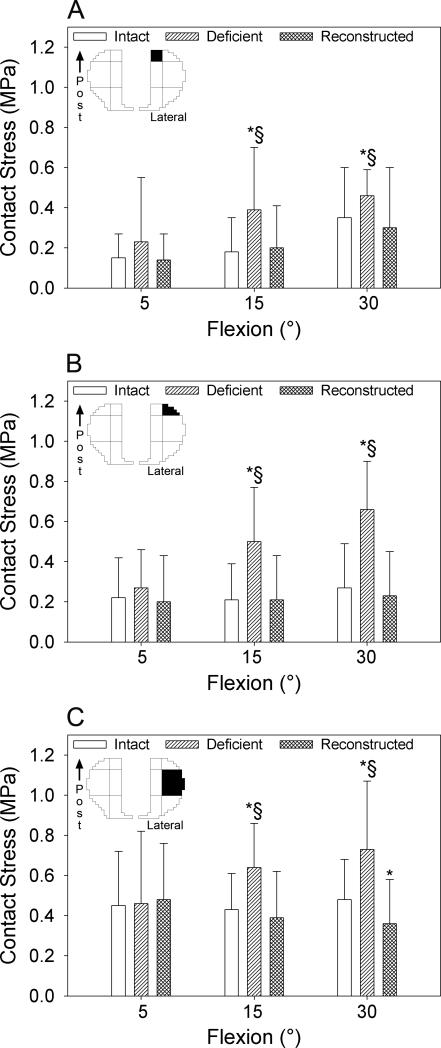
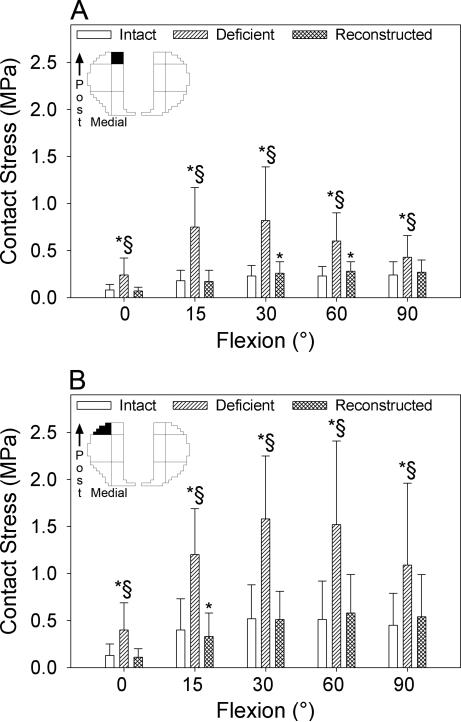
With combined moments, sectioning the ACL increased mean contact stress beyond intact levels in the posterior central (Fig. 4A), posterior peripheral (Fig. 4B), and middle peripheral (Fig. 4C) sectors of the lateral compartment at 15 and 30° flexion. With an anterior load, sectioning the ACL increased mean contact stress in the posterior central (Fig. 5A) and the posterior peripheral (Fig. 5B) sectors of the medial compartment across all flexion angles at which the knee was loaded.
The ACL reconstruction decreased mean medial translation beyond intact levels by 0.8mm at 5° flexion in response to combined moments, while decreasing mean anterior translation by 1.3 and 1.7mm at 5 and 15° flexion, respectively (Table 1). Following reconstruction, mean internal rotation also decreased by 2.6, 2.3 and 1.2° at 5, 15 and 30° flexion, respectively, compared to the intact knee. In response to an anterior load, ACL reconstruction decreased mean anterior translation by 1.5, 1.8 and 1.1mm relative to the intact knee at 0, 15, and 30° flexion, respectively (Table 2).
The ACL reconstruction restored mean contact stress in the posterior central and posterior peripheral sectors of the lateral compartment at 15 and 30° flexion to within 15% of their intact magnitudes in response to combined moments (Fig. 4A, B). However, ACL reconstruction led to a 25% decrease in mean contact stress in the middle peripheral sector of the lateral compartment at 30° flexion relative to the intact condition (Fig. 4C, Table 3). ACL reconstruction reduced mean contact stress in the posterior central sector of the medial compartment to within 13% of the intact magnitudes at all flexion angles except for 60° in response to an anterior load. Here, it remained elevated by 20% (Fig. 5A). ACL reconstruction also restored mean contact stress in the posterior peripheral sector of the medial compartment relative to the ACL intact condition at 0, 30, 60 and 90° flexion. However, it was 18% less than intact levels in this sector at 15° (Fig. 5B, Table 3).
Table 3.
Sectors where mean contact stress remained abnormal following ACL reconstruction (p<0.05) in response to combined abduction and internal rotation moments or in response to an anterior load
| Applied Load | Flexion | Compartment | Sector | Contact Stress (MPa) | ||
|---|---|---|---|---|---|---|
| Intact | Reconstructed | P | ||||
| Abduction, Internal | 5° | Lateral | middle, central | 0.29 ± 0.13 | 0.44 ± 0.10 | 0.004 |
| (0.21, 0.34) | (0.37, 0.50) | |||||
| 30° | Lateral | middle, peripheral | 0.48 ± .20 | 0.36 ± 0.22 | 0.002 | |
| (0.37, 0.59) | (0.23, 0.50) | |||||
| Anterior | 15° | Lateral | anterior, peripheral | 0.09 ± 0.10 | 0.18 ± 0.19 | 0.022 |
| (0.03, 0.14) | (0.07, 0.30) | |||||
| Lateral | middle, peripheral | 0.21 ± 0.15 | 0.31 ± 0.13 | 0.008 | ||
| (0.10, 0.28) | (0.22, 0.39) | |||||
| Medial | posterior, peripheral | 0.40 ± 0.33 | 0.33 ± 0.25 | 0.017 | ||
| (0.22, 0.57) | (0.18, 0.49) | |||||
| 30° | Medial | anterior, peripheral | 0.13 ± 0.08 | 0.17 ± 0.11 | 0.011 | |
| (0.07, 0.16) | (0.10, 0.24) | |||||
| Medial | anterior, central | 0.04 ± 0.04 | 0.06 ± 0.05 | 0.043 | ||
| (0.02, 0.06) | (0.03, 0.09) | |||||
| Medial | middle, central | 0.09 ± 0.15 | 0.15 ± 0.23 | 0.045 | ||
| (−0.003, 0.16) | (0.01, 0.29) | |||||
| Medial | posterior, central | 0.23 ± 0.11 | 0.26 ± 0.12 | 0.027 | ||
| (0.16, 0.28) | (0.19, 0.33) | |||||
| 60° | Medial | posterior, central | 0.23 ± 0.10 | 0.28 ± 0.10 | 0.010 | |
| (0.17, 0.28) | (0.22, 0.34) | |||||
Values are expressed as mean ± standard deviation; 95% confidence intervals are in parentheses. Abduction, internal = combined abduction and internal rotation moments.
After ACL reconstruction, ten sectors experienced abnormal mean contact stress across all loading conditions and flexion angles (Table 3). Abnormal contact stress was associated with aberrant kinematics in four of these ten sectors with r2>0.5 and p<0.05 (Table 4). Specifically, with combined abduction and internal rotation moments, mean contact stress increased by 52% at 5° flexion in the middle central sector of the lateral compartment after ACL reconstruction. This was associated with decreased medial translation and increased abduction (r2=0.66) (Table 4). Mean contact stress in the middle peripheral sector of the lateral compartment increased beyond intact levels by 48% with an anterior load at 15° flexion following reconstruction. This was associated with increased internal rotation (r2=0.59) (Table 4). Mean contact stress increased by 13% in the posterior central sector of the medial compartment with an anterior load at 30° flexion following reconstruction. This was associated with increased medial and anterior translations, and decreased internal rotation (r2=0.85) (Table 4). Mean contact stress in the anterior peripheral sector of the medial compartment increased by 31% with an anterior load following reconstruction. This was associated with decreased internal rotation (r2=0.53) (Table 4). The assessment of stress transducer repeatability revealed changes of 3.3±3.5% (max: 9.6%, min: −3.5%) in total contact force at the maximum applied load.
Table 4.
Multiple linear regressions relating kinematics and mean contact stress for sectors exhibiting abnormal contact stress following ACL reconstruction.
| Compartment Sector Applied Load Flexion Angle | Lateral middle, central Abduction, Internal 5° | Lateral middle, peripheral Anterior 15° | Medial posterior, central Anterior 30° | Medial anterior, peripheral Anterior 30° | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | p | r2 | β | p | r2 | β | p | r2 | β | p | r2 | ||
| Coefficient | Med (kPa/mm) | −140 | 0.022 | 0.66 | 0.59 | 58 | 0.001 | 0.85 | 0.53 | ||||
| (−223, −57) | (40, 75) | ||||||||||||
| Ant (kPa/mm) | 24 | 0.028 | |||||||||||
| (8,39) | |||||||||||||
| Int (kPa/°) | 37 | 0.009 | −25 | 0.001 | −13 | 0.016 | |||||||
| (17, 58) | (−34, −17) | (−21, −5) | |||||||||||
| Abd (kPa/°) | 343 | 0.034 | |||||||||||
| (118, 574) | |||||||||||||
Compartment, sector, applied load, and flexion angle where abnormal stress was observed are identified in the first four rows. Applied loads were: combined abduction and internal rotation moments (Abduction, Internal), and anterior force (Anterior). ° = regression coefficients in units of mm per kilopascal (kPa) or degress per kPa, for translations or rotations, respectively. 95% confidence intervals for the regression coefficients are in parentheses. Med=Medial translation; Ant=anterior translation; Int=internal rotation; Abd=abduction. r2 = adjusted coefficient of determination. A dash (-) indicates p°0.05. Using the first column of results as an example, after reconstruction, contact stress in the middle, central sector of the lateral compartment in response to combined abduction and internal rotation moments (abduction, internal) at 5° flexion increased on average by 140kPa (p=0.022) and 343kPa (p=0.034) for every 1mm decrease in medial translation and 1° increase in abduction, respectively. The coefficient of determination (r2=0.66) indicates that differences in ab/adduction and medial/lateral translation following reconstruction explained 66% of the variation in mean contact stress.
DISCUSSION
ACL reconstruction decreased the high posterior contact stresses observed in the ACL-deficient knee (Figs. 4, 5); however, contact stress abnormalities remained even after the reconstruction (Table 3). We found that the inability of ACL reconstruction to restore contact stress was related to residual differences in multiplanar kinematics (Table 4). Specifically, abnormal contact stress following reconstruction was associated with: 1) combinations of overconstrained and underconstrained motions even though knee motions were slightly overconstrained on average; and 2) multiplanar differences in ML and AP translations, ab/adduction, and axial rotation (Table 4, β coefficients). These results indicate that surgeons should strive to restore the multiplanar constraint of the native ACL to achieve more normal tibiofemoral contact stresses.
The associations that we found between abnormal contact stress and multiplanar kinematics support development of quantitative, objective clinical tools to measure the multi-degree of freedom response of the knee. This includes measurement of primary motions (i.e., motions in the directions of the applied loads) and coupled motions (i.e., motions other than in the directions of the applied loads). Instruments capable of measuring these multiplanar motions would enhance examination of the ACL-reconstructed knee in the clinic, and may help identify stability patterns that expose a patient to increased OA risk. Our data specifically identify ab/adduction and medial/lateral translation in addition to anterior translation and axial rotation to be important factors in explaining abnormal contact stress following reconstruction. Unfortunately, current clinical assessments of knee stability are limited in their ability to quantify these complex motions. They consist of single degree of freedom measures of AP translation11 (e.g., KT-1000) or axial rotation30, 34, 39, and subjective assessment of rotational stability (i.e., pivot shift)23.
Since abnormal articular contact stress was associated with small inter-subject variations in multiplanar rotations and translations following center-center reconstruction (Tables 3, 4), surgical variables in addition to tunnel placement may need to be individualized based on patient-specific factors to better restore multiplanar kinematics and contact stress. Surgical variables may include tunnel location, graft stiffness, shape, fixation angle and pretension, and bundle arrangement, while patient factors could range from morphology of both the articular surface and the ACL insertion to knee laxity. Understanding the multifactorial impact of these variables on knee kinematics and contact stress could help identify the most appropriate treatment for each patient. Although placement of graft tunnels within the anatomic footprints may be an important first step, it is probably not the only determinant of knee performance despite current focus on this topic6, 43.
Our findings of contact stress abnormalities following ACL reconstruction (Table 3; Figs. 4, 5) corroborate studies of in vivo arthrokinematics that report increased cartilage deformation in both compartments17. Our kinematic data also agree with previous work reporting altered axial rotations during gait in the presence of ACL-deficiency and reconstruction3-5, 9, 38, 40. The current results also reveal the important role that abnormal frontal plane motions (ML translation, ab/adduction)26, 40 have in explaining aberrant contact stress patterns in the ACL-reconstructed knee. However, the loading conditions in our study simulate clinical exams, not those of ambulation. Thus, any link between our results and OA pathomechanics must be made with caution. In vivo studies are needed to identify what differences in contact stress or kinematics described here, if any, best correlate with OA progression. On the other hand, our cadaver model compliments in vivo gait analysis by providing the ability to assess the relationship between knee kinematics and direct measures of contact stress under well-controlled loading conditions. Furthermore, the combined moments that we applied simulate the pivot shift exam20. In addition to its strong correlation with clinical outcome22-24, this exam may be a surrogate for knee performance during challenging functional activities because it loads both the ligamentous constraints19 as well as the articular surfaces, as shown by the contact stresses generated on the lateral compartment in this study. Although the contact stress magnitudes that we measured were relatively small (≤2.0MPa), they would probably be amplified during gait since reaction forces at the knee joint can reach 2.5 times body weight33.
The shape of the articulating surfaces may help explain the relationships that we found between increased stress and abnormal kinematics following reconstruction (Table 4). For example, increased abduction and decreased medial translation in response to combined moments might exacerbate impingement of the lateral femoral condyle on the lateral aspect of the tibial spine. This could explain why elevated stresses developed centrally on the lateral compartment under these loading conditions. Furthermore, the conforming shape of the medial compartment could explain the association of abnormal anteromedial and posteromedial stresses with altered translations and rotations in response to anterior loads. High levels of conformity would allow subtle kinematic changes to lead to large shifts in contact location3. Overall, this line of reasoning suggests the need for further study of articular morphology and its relationship with abnormal contact stress following ACL reconstruction.
Our finding of increased posterior contact stresses in the ACL-deficient knee probably indicates increased loading of the menisci (Figs. 4, 5). This agrees with previous laboratory data35, and supports a mechanical pathway for clinical findings of meniscal damage. The decreased posterior contact stress that we observed after ACL reconstruction supports use of this surgery to unload the menisci.
Our finding of overconstraint near full extension with an anterior load following ACL reconstruction is corroborated by a similar biomechanical study of a laterally oriented graft48. The overconstraint may be explained by inability of the graft to restore the length-tension patterns of the posterior lateral bundle of the native ACL, which bears load at full extension37. It may also be related to the flexion angle at which the graft was fixed. Fixing the graft in flexion may abnormally load the graft as the knee is extended. The inability of center-to-center reconstruction to restore kinematics and articular contact stress is not surprising since grafts do not mimic the length-tension patterns, shape, collagen fiber architecture31 and material properties8 of the native ligament.
Center-center ACL reconstruction controlled anterior translation at full extension better than reconstruction of the anteromedial bundle alone47. Center-center graft placement also behaved similarly to a double bundle reconstruction by controlling coupled anterior translation under combined moments and anterior translation with an anterior load47, 48. Since double bundle reconstruction also has the propensity to overconstrain knee motions29, the center-center approach may be a less surgically demanding alternative6.
The size of the tendons harvested from the ipsilateral knee of each specimen dictated graft diameter. Although this reflects clinical practice, differing graft diameters between specimens may have contributed to variations in kinematics and contact stress. However, previous researchers found no increase in knee stability with a 50% increase in graft diameter from six to nine mm7. Small differences in tunnel location across specimens may also have contributed to variations in kinematics and contact stress. However, the same surgeon performed all reconstructions while directly visualizing the ACL footprints through an anterior arthrotomy to help standardize this variable.
This in vitro model does not account for graft remodeling, which may lead to increased knee motion with time41. Nevertheless, this does not impact our finding that multiplanar kinematic differences are associated with abnormal contact stress after ACL reconstruction. The smallest change in contact stress reported in this study (16%, Table 3) was about five times greater, on average, than the variation observed in our assessment of sensor repeatability (3.3±3.5%). The worst case sensor error (9.6%) in our repeatability assessment was still 40% less than the smallest reported change in contact stress. Therefore, we deemed the repeatability of the stress transducers to be adequate for this work.
Our results emphasize the need for ACL reconstruction to restore the multiplanar constraint provided by the native ligament to achieve more normal tibiofemoral contact stresses. These multiplanar motions should be assessed clinically to better characterize the quality of ACL reconstruction, and to develop treatments that may more consistently delay OA progression.
What is known about this subject
ACL reconstruction results in residual abnormalities in knee kinematics, on average. However, the relationships between these residual abnormalities and important mechanical factors in OA progression including altered contact stress at the tibiofemoral joint are not well understood.
What this study adds to existing knowledge
Altered contact stress following ACL reconstruction is associated with complex combinations of multiplanar motions. In particular, differences in medial-lateral translation and ab/adduction between intact and reconstructed knees correlated with stress abnormalities during a simulated pivot shift exam. Differences in axial rotation and medial-lateral translation between intact and reconstructed knees in response to an anterior load also correlated with stress abnormalities. These data suggest that clinicians should strive to restore these motions with ACL reconstruction. These motions should also be assessed clinically to better characterize the quality of this surgery. The work also supports ACL reconstruction as a meniscus-unloading operation, since the posterior aspect of the medial and lateral compartments had decreased mean contact stress after surgery in comparison to ACL-deficiency.
Figure 1.
Robotic system with knee specimen prepared for testing. Illustration reproduced with permission from Ferrante Medical Media.
Figure 2.
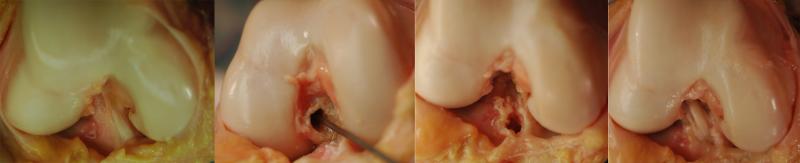
Comparison of the native ACL (A), and the location of the tibial and femoral tunnels (B and C, respectively) for center-center ACL reconstruction (D).
Figure 3.
Contact stress generated on the tibial plateau was measured using a stress-transducer (top left). It was slid underneath the menisci and sutured in place on the tibial plateau (top right). Representative two-dimensional plot of contact stress on the tibial plateau (bottom left). Each compartment was divided into six sectors (bottom right), containing central and peripheral columns (Periph=Peripheral) and anterior, middle and posterior rows (Post=Posterior, Ant=Anterior). Mean contact stress was calculated in each sector at the position of the knee corresponding to the maximum applied load.
Figure 4.
Mean contact stress generated in the posterior central (A), posterior peripheral (B), and middle peripheral (C) sectors of the lateral compartment of the tibial plateau in response to combined abduction and internal rotation moments applied at 5, 15 and 30° flexion with the ACL intact (white), deficient (diagonal lines) and reconstructed (crosshatched lines). Whiskers indicate one standard deviation. The top left image in each graph identifies the sector (filled in black) of the contact stress map that the data refers to. Post=posterior; *p<0.05 compared with the intact knee; §p<0.05 compared with the ACL-reconstructed knee.
Figure 5.
Mean contact stress generated in the posterior central (A), and posterior peripheral (B) sectors of the medial compartment of the tibial plateau in response to an anterior load applied at full extension, 15, 30, 60 and 90° flexion with the ACL intact (white), deficient (diagonal lines) and reconstructed (crosshatched lines). Whiskers indicate one standard deviation. The top left images identify the sectors (filled in black) of the contact stress map that the data in the bar graphs refer to. Post=posterior; *p<0.05 compared with the intact knee; §p<0.05 compared with the ACL-reconstructed knee.
References
- 1.C series controller and operations and programming manual. Kawasaki Robotics, Training and Documentation Department; Wixom: 1999. [Google Scholar]
- 2.Anderson AF, Snyder RB, Lipscomb AB., Jr. Anterior cruciate ligament reconstruction. A prospective randomized study of three surgical methods. The American journal of sports medicine. 2001;29(3):272–279. doi: 10.1177/03635465010290030201. [DOI] [PubMed] [Google Scholar]
- 3.Andriacchi TP, Briant PL, Bevill SL, Koo S. Rotational changes at the knee after ACL injury cause cartilage thinning. Clinical orthopaedics and related research. 2006;442:39–44. doi: 10.1097/01.blo.0000197079.26600.09. [DOI] [PubMed] [Google Scholar]
- 4.Andriacchi TP, Koo S, Scanlan SF. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. The Journal of bone and joint surgery. American volume. 2009;91(Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 6.Bedi A, Altchek DW. The “footprint” anterior cruciate ligament technique: an anatomic approach to anterior cruciate ligament reconstruction. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2009;25(10):1128–1138. doi: 10.1016/j.arthro.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Bedi A, Maak T, Musahl V, et al. Effect of tunnel position and graft size in single-bundle anterior cruciate ligament reconstruction: an evaluation of time-zero knee stability. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2011;27(11):1543–1551. doi: 10.1016/j.arthro.2011.03.079. [DOI] [PubMed] [Google Scholar]
- 8.Butler DL, Guan Y, Kay MD, Cummings JF, Feder SM, Levy MS. Location-dependent variations in the material properties of the anterior cruciate ligament. J Biomech. 1992;25(5):511–518. doi: 10.1016/0021-9290(92)90091-e. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Medicine and science in sports and exercise. 2008;40(2):215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 10.Chen CT, Bhargava M, Lin PM, Torzilli PA. Time, stress, and location dependent chondrocyte death and collagen damage in cyclically loaded articular cartilage. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2003;21(5):888–898. doi: 10.1016/S0736-0266(03)00050-0. [DOI] [PubMed] [Google Scholar]
- 11.Daniel DM, Malcom LL, Losse G, Stone ML, Sachs R, Burks R. Instrumented measurement of anterior laxity of the knee. The Journal of bone and joint surgery. American volume. 1985;67(5):720–726. [PubMed] [Google Scholar]
- 12.Frank CB, Beveridge JE, Huebner KD, et al. Complete ACL/MCL deficiency induces variable degrees of instability in sheep with specific kinematic abnormalities correlating with degrees of early osteoarthritis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30(3):384–392. doi: 10.1002/jor.21549. [DOI] [PubMed] [Google Scholar]
- 13.Freedman KB, D'Amato MJ, Nedeff DD, Kaz A, Bach BR., Jr. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. The American journal of sports medicine. 2003;31(1):2–11. doi: 10.1177/03635465030310011501. [DOI] [PubMed] [Google Scholar]
- 14.Fujie H, Mabuchi K, Woo SL, Livesay GA, Arai S, Tsukamoto Y. The use of robotics technology to study human joint kinematics: a new methodology. Journal of biomechanical engineering. 1993;115(3):211–217. doi: 10.1115/1.2895477. [DOI] [PubMed] [Google Scholar]
- 15.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three- dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 16.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. American journal of epidemiology. 2003;157(4):364–375. doi: 10.1093/aje/kwf215. [DOI] [PubMed] [Google Scholar]
- 17.Hosseini A, Van de Velde S, Gill TJ, Li G. Tibiofemoral cartilage contact biomechanics in patients after reconstruction of a ruptured anterior cruciate ligament. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30(11):1781–1788. doi: 10.1002/jor.22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonsson H, Riklund-Ahlstrom K, Lind J. Positive pivot shift after ACL reconstruction predicts later osteoarthrosis: 63 patients followed 5-9 years after surgery. Acta Orthop Scand. 2004;75(5):594–599. doi: 10.1080/00016470410001484. [DOI] [PubMed] [Google Scholar]
- 19.Kanamori A, Woo SL, Ma CB, et al. The forces in the anterior cruciate ligament and knee kinematics during a simulated pivot shift test: A human cadaveric study using robotic technology. Arthroscopy. 2000;16(6):633–639. doi: 10.1053/jars.2000.7682. [DOI] [PubMed] [Google Scholar]
- 20.Kanamori A, Zeminski J, Rudy TW, Li G, Fu FH, Woo SL. The effect of axial tibial torque on the function of the anterior cruciate ligament: a biomechanical study of a simulated pivot shift test. Arthroscopy. 2002;18(4):394–398. doi: 10.1053/jars.2002.30638. [DOI] [PubMed] [Google Scholar]
- 21.Kessler MA, Behrend H, Henz S, Stutz G, Rukavina A, Kuster MS. Function, osteoarthritis and activity after ACL-rupture: 11 years follow-up results of conservative versus reconstructive treatment. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2008;16(5):442–448. doi: 10.1007/s00167-008-0498-x. [DOI] [PubMed] [Google Scholar]
- 22.Kocher MS, Steadman JR, Briggs KK, Sterett WI, Hawkins RJ. Relationships between objective assessment of ligament stability and subjective assessment of symptoms and function after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32(3):629–634. doi: 10.1177/0363546503261722. [DOI] [PubMed] [Google Scholar]
- 23.Lane CG, Warren R, Pearle AD. The pivot shift. J Am Acad Orthop Surg. 2008;16(12):679–688. doi: 10.5435/00124635-200812000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Leitze Z, Losee RE, Jokl P, Johnson TR, Feagin JA. Implications of the pivot shift in the ACL-deficient knee. Clin Orthop Relat Res. 2005;(436):229–236. doi: 10.1097/01.blo.0000160026.14363.22. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88(8):1826–1834. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Papannagari R, DeFrate LE, Yoo JD, Park SE, Gill TJ. The effects of ACL deficiency on mediolateral translation and varus-valgus rotation. Acta orthopaedica. 2007;78(3):355–360. doi: 10.1080/17453670710013924. [DOI] [PubMed] [Google Scholar]
- 27.Li RT, Lorenz S, Xu Y, Harner CD, Fu FH, Irrgang JJ. Predictors of radiographic knee osteoarthritis after anterior cruciate ligament reconstruction. The American journal of sports medicine. 2011;39(12):2595–2603. doi: 10.1177/0363546511424720. [DOI] [PubMed] [Google Scholar]
- 28.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 29.Markolf KL, Park S, Jackson SR, McAllister DR. Anterior-posterior and rotatory stability of single and double-bundle anterior cruciate ligament reconstructions. The Journal of bone and joint surgery. American volume. 2009;91(1):107–118. doi: 10.2106/JBJS.G.01215. [DOI] [PubMed] [Google Scholar]
- 30.Mayr HO, Hoell A, Bernstein A, et al. Validation of a measurement device for instrumented quantification of anterior translation and rotational assessment of the knee. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2011;27(8):1096–1104. doi: 10.1016/j.arthro.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 31.Mommersteeg TJ, Kooloos JG, Blankevoort L, Kauer JM, Huiskes R, Roeling FQ. The fibre bundle anatomy of human cruciate ligaments. Journal of anatomy. 1995;187( Pt2):461–471. [PMC free article] [PubMed] [Google Scholar]
- 32.Morimoto Y, Ferretti M, Ekdahl M, Smolinski P, Fu FH. Tibiofemoral joint contact area and pressure after single- and double-bundle anterior cruciate ligament reconstruction. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2009;25(1):62–69. doi: 10.1016/j.arthro.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 33.Mundermann A, Dyrby CO, D'Lima DD, Colwell CW, Jr., Andriacchi TP. In vivo knee loading characteristics during activities of daily living as measured by an instrumented total knee replacement. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26(9):1167–1172. doi: 10.1002/jor.20655. [DOI] [PubMed] [Google Scholar]
- 34.Musahl V, Bell KM, Tsai AG, et al. Development of a simple device for measurement of rotational knee laxity. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2007;15(8):1009–1012. doi: 10.1007/s00167-007-0317-9. [DOI] [PubMed] [Google Scholar]
- 35.Papageorgiou CD, Gil JE, Kanamori A, Fenwick JA, Woo SL, Fu FH. The biomechanical interdependence between the anterior cruciate ligament replacement graft and the medial meniscus. The American journal of sports medicine. 2001;29(2):226–231. doi: 10.1177/03635465010290021801. [DOI] [PubMed] [Google Scholar]
- 36.Prisk VR, Imhauser CW, O'Loughlin PF, Kennedy JG. Lateral ligament repair and reconstruction restore neither contact mechanics of the ankle joint nor motion patterns of the hindfoot. The Journal of bone and joint surgery. American volume. 2010;92(14):2375–2386. doi: 10.2106/JBJS.I.00869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sakane M, Fox RJ, Woo SL, Livesay GA, Li G, Fu FH. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 1997;15(2):285–293. doi: 10.1002/jor.1100150219. [DOI] [PubMed] [Google Scholar]
- 38.Scanlan SF, Chaudhari AM, Dyrby CO, Andriacchi TP. Differences in tibial rotation during walking in ACL reconstructed and healthy contralateral knees. Journal of biomechanics. 2010;43(9):1817–1822. doi: 10.1016/j.jbiomech.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shultz SJ, Shimokochi Y, Nguyen AD, Schmitz RJ, Beynnon BD, Perrin DH. Measurement of varus-valgus and internal-external rotational knee laxities in vivo--Part I: assessment of measurement reliability and bilateral asymmetry. J Orthop Res. 2007;25(8):981–988. doi: 10.1002/jor.20397. [DOI] [PubMed] [Google Scholar]
- 40.Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clin Orthop Relat Res. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 41.Tashman S, Kolowich P, Collon D, Anderson K, Anderst W. Dynamic function of the ACL-reconstructed knee during running. Clinical orthopaedics and related research. 2007;454:66–73. doi: 10.1097/BLO.0b013e31802bab3e. [DOI] [PubMed] [Google Scholar]
- 42.Tochigi Y, Vaseenon T, Heiner AD, et al. Instability dependency of osteoarthritis development in a rabbit model of graded anterior cruciate ligament transection. The Journal of bone and joint surgery. American volume. 2011;93(7):640–647. doi: 10.2106/JBJS.J.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Eck CF, Lesniak BP, Schreiber VM, Fu FH. Anatomic single- and double-bundle anterior cruciate ligament reconstruction flowchart. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2010;26(2):258–268. doi: 10.1016/j.arthro.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 44.Williams RJ, 3rd, Hyman J, Petrigliano F, Rozental T, Wickiewicz TL. Anterior cruciate ligament reconstruction with a four-strand hamstring tendon autograft. Surgical technique. The Journal of bone and joint surgery. American volume. 2005;87(Suppl 1)(Pt1):51–66. doi: 10.2106/JBJS.D.02805. [DOI] [PubMed] [Google Scholar]
- 45.Woo SL, Kanamori A, Zeminski J, Yagi M, Papageorgiou C, Fu FH. The effectiveness of reconstruction of the anterior cruciate ligament with hamstrings and patellar tendon . A cadaveric study comparing anterior tibial and rotational loads. The Journal of bone and joint surgery. American volume. 2002;84-A(6):907–914. doi: 10.2106/00004623-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Wright TM, Maher SA. Current and novel approaches to treating chondral lesions. J Bone Joint Surg Am. 2009;91(Suppl 1):120–125. doi: 10.2106/JBJS.H.01390. [DOI] [PubMed] [Google Scholar]
- 47.Yagi M, Wong EK, Kanamori A, Debski RE, Fu FH, Woo SL. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30(5):660–666. doi: 10.1177/03635465020300050501. [DOI] [PubMed] [Google Scholar]
- 48.Yamamoto Y, Hsu WH, Woo SL, Van Scyoc AH, Takakura Y, Debski RE. Knee stability and graft function after anterior cruciate ligament reconstruction: a comparison of a lateral and an anatomical femoral tunnel placement. Am J Sports Med. 2004;32(8):1825–1832. doi: 10.1177/0363546504263947. [DOI] [PubMed] [Google Scholar]