Abstract
OBJECTIVE
To determine if maternal plasma concentrations of placental growth factor (PlGF), soluble endoglin (sEng), and soluble vascular endothelial growth factor receptor-1 (sVEGFR-1) at 30–34 weeks can identify mothers at risk for preeclampsia (PE), stillbirth and small-for-gestational-age neonates (SGA).
STUDY DESIGN
A prospective cohort study included 1269 singleton pregnant women who had blood samples obtained at 30–34 weeks and delivered after 34 weeks of gestation. Plasma concentrations of PlGF, sEng, and sVEGFR-1 were determined by ELISA.
RESULTS
The prevalence of late (>34 weeks) PE, severe late PE, stillbirth and SGA was 3.2% (n=40), 1.8% (n=23), 0.4% (n=5) and 8.5% (n=108), respectively. A plasma concentration of PlGF/sEng <0.3 MoM was associated with severe late PE [adjusted odds ratio (aOR) 16]; addition of PlGF/sEng to clinical risk factors increased the area under the ROC curve (AUC) from 0.76 to 0.88 (p=0.03). The ratio of PlGF/sEng or PlGF/sVEGFR-1 in the third trimester outperformed those obtained in the first or second trimester and uterine artery Doppler velocimetry at 20–25 weeks for the prediction of severe late PE (comparison of AUC; each p≤0.02). Both PlGF/sEng and PlGF/sVEGFR-1 ratios achieved a sensitivity of 74% with a fixed false positive rate of 15% for the identification of severe late PE. A plasma concentration of PlGF/sVEGFR-1 <0.12 MoM at 30–34 weeks had a sensitivity of 80%, a specificity of 94%, and a likelihood ratio of a positive test of 14 for the identification of subsequent stillbirth. Similar findings (sensitivity 80% and specificity 93%) were observed in a separate case-control study. Integrating these biomarkers with clinical data did not improve the prediction of SGA.
CONCLUSIONS
Risk assessment for severe late PE and stillbirth in the third trimester is possible with the determination of maternal plasma concentrations of angiogenic and anti-angiogenic factors at 30–34 weeks of gestation.
Keywords: fetal death, placental growth factor (PlGF), soluble endoglin (sEng), soluble vascular endothelial growth factor receptor-1 (sVEGFR-1), severe preeclampsia, SGA
INTRODUCTION
Preeclampsia (PE), a leading cause of maternal and perinatal morbidity/mortality worldwide,1–4 affects 2–8% of all pregnancies and has a complex pathophysiology involving abnormal physiologic transformation of the spiral arteries,5–10 intravascular inflammation,11–13 endothelial cell dysfunction,14–20 excessive generation of thrombin,21–25 oxidative stress,26–29 and an anti-angiogenic state.30–38
PE may be classified as “early” or “late” according to gestational age at diagnosis or delivery, The gestational age cut-off most frequently used is 34 weeks.39 Early PE is associated with multisystemic involvement,40,41 a higher frequency of small for gestational age neonates (SGA),42 and placental vascular lesions of underperfusion.43 Since early PE is a frequent indication for preterm delivery, the condition is also associated with a higher rate of neonatal morbidity.44 In contrast, PE at term is associated with better neonatal outcomes than preterm PE. Although much emphasis has been focused on early PE, most (75%) cases of PE occur at, or near term.45,46,47 Consequently, late PE accounts for a substantial proportion of medically indicated preterm (34–36 weeks) births44,48–50 and severe maternal morbidity, including most cases of eclampsia,51–53 which is the form of the disease that accounts for most maternal deaths.54 Hence, identifying predictors of late PE is a health care priority.
Stillbirth, another obstetrical syndrome55,56 which may or may not be related to PE, affects more than 3 million women in the third trimester worldwide each year.57–59 The circumstances surrounding stillbirth vary depending upon socioeconomic conditions.60,61 In high-income countries, stillbirth is associated with fetal growth restriction or placental insufficiency, although in nearly half of the cases, the etiology is unknown.61 Intrapartum complications, PE and infection play a more important role in the etiology of stillbirth in low-income countries.62–64 Currently, there is no effective risk assessment tool for the detection of stillbirth at, or near term.60,65,66
An imbalance of angiogenic/anti-angiogenic factors has been implicated in the pathophysiology of PE,67–88 pregnancies with SGA neonates,89–92 stillbirth,93–95 and other obstetrical complications.96–102 Changes in the concentrations of the angiogenic factor, placental growth factor (PlGF), and anti-angiogenic factors, soluble vascular endothelial growth factor receptor (sVEGFR)-1 [also known as soluble fms-like tyrosine kinase-1 (sflt-1)] and soluble endoglin (sEng) in maternal circulation, precede the clinical diagnosis of PE,103–109 SGA90,91 and stillbirth.110 Most studies examining the value of these biomarkers, however, have focused on the prediction of PE and only on screening in the 1st 111–114 or 2nd trimesters.109,115–117 The results of such studies largely suggest that an imbalance between angiogenic and anti-angiogenic factors increases the likelihood of preterm PE at a higher magnitude than that in term PE.36,90,103,112,117–121 Yet, not all studies have arrived at the same conclusion.122–124 Thus far, no cohort studies have evaluated the diagnostic performance of these biomarkers in the third trimester for identifying the patient at risk for stillbirth at or near term or late-onset PE.
Recently, a new approach for screening of adverse pregnancy outcomes has been proposed to focus on the prevention of pregnancy complications at term. Such an approach would identify the more prevalent disease (e.g. preelcampsia at term) and predictive models could be applied to low-income settings, where the majority of maternal and perinatal death occurs.51,125
The objective of this study was to determine if maternal plasma concentrations of PlGF, sEng, sVEGFR-1 and their ratios at 30–34 weeks of gestation could be used to identify patients at risk for stillbirth, late PE, severe late PE, or SGA without PE.
METHODS
Study Design
We first designed a cohort study of women who had a venipuncture between 30–34 weeks of gestation, and outcome data to examine the value of PlGF, sVEGFR-1, and sEng in the identification of patients who subsequently developed late PE, severe late PE, stillbirth, and SGA. Subsequent to this cohort study, a case-control study was performed to determine if these biomarkers and their ratios could identify patients at risk for stillbirth at or near term in a different population.
Cohort Study
A prospective longitudinal cohort study was conducted between November 2003 and August 2006 to identify biological markers for the prediction of PE, SGA, and stillbirth. Patients were enrolled in the prenatal clinic of the Sotero del Rio Hospital, a tertiary care center in Santiago, Chile, and followed until delivery. Inclusion criteria were: 1) singleton gestation; and 2) 6 – 22 weeks of gestation. Exclusion criteria were: 1) preterm labor, preterm prelabor rupture of membranes, PE, or impaired fetal growth at the time of recruitment; 2) known major fetal anomaly or fetal demise; 3) active vaginal bleeding; and 4) serious medical illness (renal insufficiency, congestive heart disease, chronic respiratory insufficiency, or active hepatitis). At enrollment and each subsequent visit, patients underwent a venipuncture for the collection of maternal blood. The protocol consisted of collecting samples every 4 weeks until 24 weeks, and every 2 weeks thereafter until delivery.
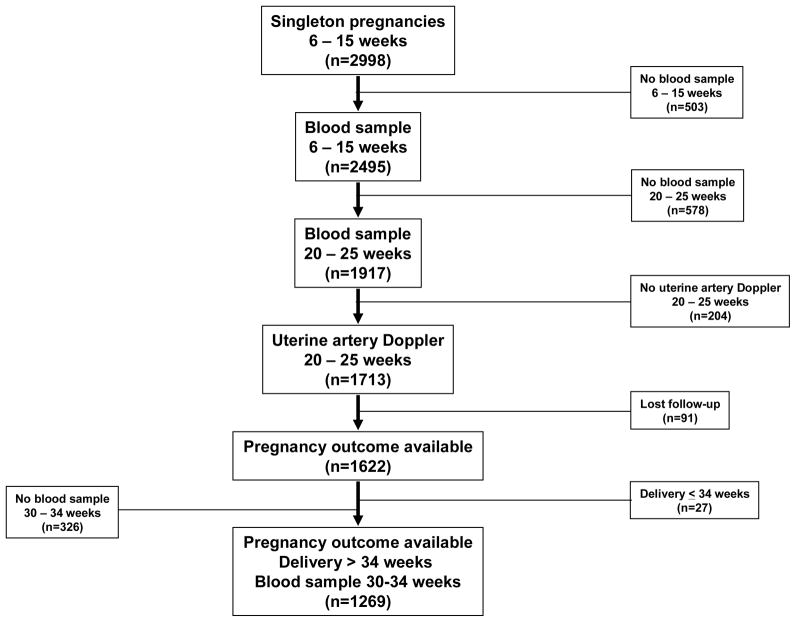
We have reported the diagnostic performance of angiogenic/anti-angiogenic factors at 6–15 weeks and 20–25 weeks of gestation, as well as uterine artery Doppler velocimetry (UADV) at 20–25 weeks of gestation in the prediction of PE from this cohort study.119 In summary, 2,998 consecutive women were enrolled during the study period mentioned above; 2,495 women had a plasma sample collected in early pregnancy. Of those, 1,917 women had an additional plasma sample obtained in the midtrimester. Subsequently, an additional 204 patients without results of UADV in the second trimester were excluded. Ninety-one patients were lost to follow-up; the remaining 1,622 patients had been included in a previous publication examining the role of angiogenic/anti-angiogenic factors at 6–15 weeks and 20–25 weeks of gestation.119 The current study involved a subset of this cohort, which excluded patients who delivered at or before 34 weeks of gestation (n=27), as well as those who did not have a plasma sample collected between 30–34 weeks of gestation (n=326) to examine the role of angiogenic/anti-angiogenic factors at 30–34 weeks for the identification of adverse pregnancy outcomes after 34 weeks of gestation.
All women provided written informed consent before participating in the study. The use of clinical and ultrasound data and collection and utilization of maternal blood for research purposes was approved by the Institutional Review Boards of the Sotero del Rio Hospital, Santiago, Chile (an affiliate of the Pontificia Catholic University of Santiago, Chile), and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS).
Outcomes of the study
The outcomes of the study included late PE, severe late PE, SGA without PE and stillbirth. PE was defined as new-onset hypertension that developed after 20 weeks of gestation and proteinuria. Hypertension was defined as systolic ≥140 and/or diastolic blood pressure ≥90 mm Hg, measured at two occasions, 4 hours to 1 week apart. Proteinuria was defined as a urine protein of ≥300 mg in a 24-hour urine collection, or two random urine specimens, obtained 4 hours to 1 week apart, showing ≥1+ by dipstick or one dipstick demonstrating ≥2+ protein. Late PE was defined as patients with PE who delivered after 34 weeks of gestation.39 Severe PE was diagnosed based on ACOG criteria.126 SGA was defined as a birthweight <10th percentile for gestational age according to the Chilean birthweight distribution of a Hispanic population.127 Stillbirth was defined as death of a fetus prior to delivery which is not a consequence of an induced termination of pregnancy (including intrapartum and antepartum stillbirth).128 Abnormal UADV was defined as the mean uterine artery Doppler pulsatility index > 1.45.129–130
Data quality
Customized case report forms and a perinatal database were generated. Data were extracted from medical records by trained research nurses. To account for misclassification, abstracters were trained, the data collection methods were verified, and data logic was monitored. Cases of uncertainty were resolved by iterative discussion among three of the authors. Gestational age at venipuncture and at delivery was based on best obstetrical estimates using last menstrual period and the earliest biometric measurements, which were performed at ≤20 weeks in 98.2% of cases.
Sample collection and immunoassays
Blood was obtained by venipuncture and collected into tubes containing EDTA. Samples were centrifuged and stored at −70°C. Maternal plasma concentrations of sVEGFR-1, PlGF, and sEng were determined by sensitive and specific immunoassays (R&D Systems, Minneapolis, MN, U.S.A) as previously described.119 The inter- and intra-assay coefficients of variation (CV) were: 1.4% and 3.9% for sVEGFR-1, 2.3% and 4.6% for sEng, and 6.02% and 4.8%, respectively, for PlGF. The sensitivity of the assays was 16.97 pg/ml for sVEGFR-1, 0.08 ng/ml for sEng, and 9.52 pg/ml for PlGF. The laboratory personnel performing the assays were blinded to the clinical information.
Statistical analysis
Differences in distributions of dichotomous and categorical variables were tested using Chi-square or Fisher’s Exact Test where appropriate; continuous parameters were compared by analysis of variance (ANOVA) or Friedman’s two-way nonparametric ANOVA test with Bonferroni correction for multiple comparisons depending on the distribution of data. Normality was assessed using the Kolmogorov-Smirnov test and visual plot inspection.
Quantile regression131 was used to calculate median analyte ratio concentrations (PlGF/sVEGFR-1, PlGF/sEng) conditional upon gestational age among uncomplicated pregnancies (n=886). Multiples of the Median (MoM) values were calculated for both analyte ratios for each patient. MoM cutoffs were calculated based on inspection of receiver operating characteristic (ROC) curves calculated for each outcome (stillbirth, late PE, severe late PE, and SGA without PE). Prognostic logistic regression models were constructed for each outcome, including the MoM cutoff and clinical risk factors. Covariables included in adjusted models were selected based on clinical knowledge. Model reduction was performed additionally based on the plausibility of regression coefficients, association with independent variables and the magnitude of change in the main effect parameter estimates.132 To account for potential model over-fitting, when van Houwelingen and le Cessie’s heuristic shrinkage estimator fell below 0.85 (indicator of instability), bootstrap estimated linear shrinkage factors and Firth’s penalized maximum likelihood estimation were used to calculate conservative estimates less likely to be affected by over-fitting.132,133
Diagnostic performance metrics were also calculated for each outcome. Paired sample non-parametric statistical techniques were used to compare area under the ROC curves (AUC) of models constructed using logistic regression for the identification of selected pregnancy outcomes.134 A McNemar’s test was also used to test for differences in sensitivity at a fixed false positive rate of 15%. A 5% threshold for type I error was used to determine statistical significance. Statistical analyses were performed using SAS version 9.3 (Cary, NC, U.S.A).
Case-Control Study for Stillbirth
Participants were identified from a cohort of 5,828 singleton pregnancies who were enrolled in a similar longitudinal protocol to that used in the Chilean cohort and another cross-sectional protocol from 2007 to 2009 at Hutzel Woman’s Hospital, Detroit, MI, USA. Stillbirth was defined as death of a fetus prior to delivery (which is not a consequence of an induced termination of pregnancy).128 In the longitudinal study, plasma samples were obtained from the first or early second trimester and at the time of each prenatal visit, scheduled every 4 weeks until 24 weeks, and every 2 weeks thereafter until delivery. In the cross-sectional study, patients were enrolled when they presented to the labor and delivery unit with a suspicion of spontaneous preterm labor or medically indicated preterm birth. Among 31 cases of stillbirth at ≥34 weeks of gestation, five had a plasma sample collected between 30–34 weeks of gestation and were included. Controls were identified from uncomplicated pregnancies who delivered an appropriate weight for gestational age neonate at term, and had a plasma sample collected between 30 and 34 weeks of gestation. Controls were matched to cases at a ratio of 6 to 1 on gestational age at venipuncture, parity, ethnicity, tobacco use and body mass index (BMI). Maternal plasma concentrations of sVEGFR-1, sEng and PlGF were determined by sensitive and specific immunoassays (R&D Systems, Minneapolis, MN, U.S.A) similar to those used in the Chilean cohort as described above.
All women provided written informed consent before participating in the study. The use of clinical and ultrasound data and collection and utilization of maternal blood for research purposes was approved by the Institutional Review Boards of the Wayne State University, Detroit, MI, U.S.A and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS).
Statistical Analysis for Case-Control Study
Differences among cases and controls were tested using the Chi-square, Fisher’s Exact or Mann-Whitney U tests where appropriate. AUC was calculated and sensitivities and specificities were determined using absolute value thresholds for each biomarker ratio derived from inspection of ROC curves.
RESULTS
The cohort study included 1,269 pregnant women (Figure 1). The prevalence of late PE, severe late PE, stillbirth and SGA without PE was 3.2% (n=40), 1.8% (n=23); 0.4% (n=5), and 8.5% (n=108), respectively. Among 23 patients who were diagnosed with severe PE, 6 experienced severe high blood pressure and severe proteinuria, 4 had severe high blood pressure, 4 had severe high blood pressure with severe proteinuria with SGA fetuses, 3 had SGA fetuses, 2 had severe headache with severe proteinuria, 2 had severe proteinuria, 1 had severe high blood pressure and the last one had severe proteinuria and pulmonary edema. Table 1 displays the demographic and obstetrical characteristics of patients with SGA, PE, stillbirth, other complications (see table note) and those without any of these complications (uncomplicated pregnancy). There were no significant differences in the mean gestational age at venous sampling or mean duration of sample storage among the four groups. The distribution of baseline characteristics did not significantly differ between patients included in the current study compared to the overall cohort (data not shown). Similarly, there were no significant differences in the risk of stillbirth or SGA between the entire cohort and sub-cohort. However, by design, participants in the sub-cohort were more likely to deliver after 34 weeks of gestation. Patients in this sub-cohort had a lower frequency of PE than those in the entire cohort (3.2% vs. 4.8%; p=0.03). There were three patients diagnosed with gestational hypertension prior to venipuncture at 30–34 weeks of gestation. However, none subsequently developed PE. The median MoM plasma concentration of PlGF/sVEGFR-1 and PlGF/sEng was significantly lower in patients with subsequent stillbirth, PE and SGA than those without these conditions (p<0.05 for each comparison; see Table 1).
Figure 1.
Flow diagram of patients enrolled in the study
Table 1.
Demographic and obstetrical characteristics of the study population
| Patient Characteristics | Uncomplicated Pregnancy (n=886) | SGA (n=108) | Preeclampsia (n=40) | Stillbirth (n=5) | Other complicatio ns (n=230) |
|---|---|---|---|---|---|
| Maternal age (years) | 26.2±5.9 | 26.5 ±7.1 | 23.6 ±5.4* | 27 ±10 | 28 ±6.5 |
| Tobacco use | 10.5% (93) | 18.50% (20) | 12.50% (5) | 0 | 12.2% (28) |
| Nulliparous | 40.2% (356) | 44.4% (48) | 70%* (28) | 40% (2) | 33.0 (76) |
| Multiparous with previous history of preeclampsia | 2.1% (19) | 0.9% (1) | 7.5%* (3) | 0 | 6.1% (14) |
| Multiparous without previous history of preeclampsia | 57.7% (511) | 54.60% (59) | 22.50%* (9) | 60% (3) | 60.9% (140) |
| Body mass index (Kg/m2) | 24.6 ± 4.2 | 24 ±4.3 | 27.4 ±8 | 22.4 ±1.6 | 27.3 ± 6 |
| GA at venipuncture (weeks) | 32.2 ± 1.1 | 32.2 ±1.1 | 32.2 ±1.2 | 32 ±0.9 | 32.2±1.2 |
| Storage time (years) | 6.8 ±0.7 | 6.8 ±0.8 | 6.9 ±1.1 | 6.6 ±0.6 | 6.8 ±0.7 |
| GA at delivery (weeks) | 39.6 ± 1.1 | 39.4 ±1.1 | 38.48* ±1.6 | 36.5* ±2.3 | 38.6± 1.7 |
| Birthweight (grams) | 3505± 399 | 2710*±230 | 3096* ±550 | 2896±642 | 3366±521 |
| PlGF/sVEGFR-1 MoM (median(IQR)) | 1.00 (0.51–1.83) | 0.53* (0.21–1.22) | 0.21* (0.08–0.50) | 0.08* (0.07–0.1) | 0.73 (0.33–1.27) |
| PlGF/sEng MoM (median(IQR)) | 1.00 (0.56–1.78) | 0.59* (0.26–1.10) | 0.27* (0.11–0.63) | 0.18* (0.1–0.3) | 0.74 (0.35–1.18) |
Value expressed as percent (number), mean ± standard deviation or median (interquartile range- IQR);
indicated significant difference (p<0.05) compared to combined ‘other + uncomplicated pregnancy’ categories;
Other complications include spontaneous preterm delivery (3%; n=38), chronic hypertension (2.2%; n=28), gestational hypertension (6.8%; n=86); gestational and pregestational diabetes (4.6%; n=58), placental abruption (0.4%; n=5), cholestasis of pregnancy (0.9%; n=12) and placenta previa (0.2%; n=3)
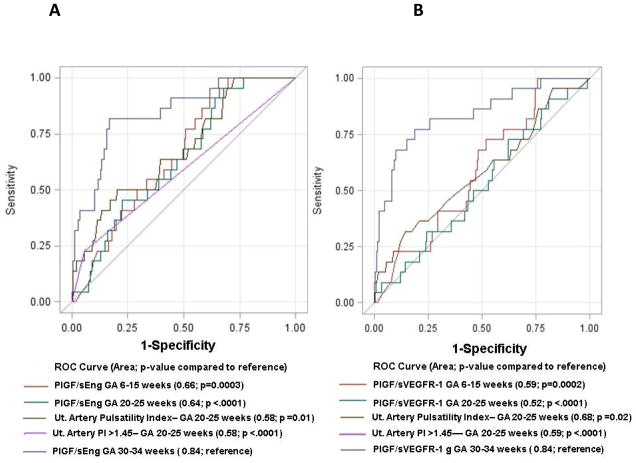
Table 2 displays the magnitude of association between abnormal biomarker profiles and late PE (overall and severe), SGA (birthweight <10%, <3%) as well as stillbirth. Patients with plasma PlGF/sEng or PlGF/sVEGFR-1 ratio concentrations <0.3 MoM were significantly more likely to develop late PE (adjusted odds ratio (aOR) 7.1; 95% confidence interval (CI) 3.6–13.8 and aOR 6.1; 95% CI 3.1–11.8; respectively) and severe late PE (aOR 16.1; 95% CI 5.8–44.6 and aOR 12.2; 95% CI 4.6–32; respectively) than those with MoM at or above the threshold (Table 2). The likelihood ratio of a positive test and sensitivity for either PlGF/sEng or PlGF/sVEGFR-1 ranged from 4.5–4.8 and 74%-78%, respectively; both had a specificity of 84% for the identification of patients with severe late PE (Table 3).
Table 2.
Likelihood (unadjusted and adjusted) of subsequent stillbirth, preeclampsia and small for gestational age neonate by PlGF/sVEGFR-1 and PlGF/sEng Multiples of the Median threshold
| Dependent Variable, Analyte Ratio & MOM Threshold | Others % (n/N) | Outcome % (n/N) | Unadjusted | Adjusted* | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| OR | 95% CI | OR | 95% CI | |||||
|
| ||||||||
| Preeclampsia (n=40) | ||||||||
| PlGF/sVEGFR- 1 <0.3 MOM | 16.2% (199/1229) | 57.5% (23/40) | 7.9 | 4.1 | 15.2 | 6.1 | 3.1 | 11.8 |
|
| ||||||||
| PlGF/sEng <0.3 MoM | 15.9% (196/1229) | 60.0% (24/40) | 5.9 | 1.9 | 18.7 | 7.1 | 3.6 | 13.8 |
|
| ||||||||
| Severe Preeclampsia (n=23) | ||||||||
| PlGF/sVEGFR- 1 <0.3 MOM | 16.5% (205/1246) | 73.9% (17/23) | 11.9 | 2.2 | 66 | 12.2 | .6 | 32 |
|
| ||||||||
| PlGF/sEng <0.3 MoM | 16.3% (202/1246) | 78.3% (18/23) | 11.7 | 2.1 | 64.6 | 16.1 | 5.8 | 44.6 |
|
| ||||||||
| SGA < 10th % (n=108) | ||||||||
| PlGF/sVEGFR- 1 <0.3 MOM | 15.8% (184/1161) | 35.2% (38/108) | 4.2 | 2.4 | 7.3 | 3 | 2 | 4.7 |
|
| ||||||||
| PlGF/sEng <0.3 MoM | 16.3% (189/1161) | 28.7% (31/108) | 3.6 | 2 | 6.4 | 2 | 1.3 | 3.1 |
|
| ||||||||
| SGA < 3rd % (n=23) | ||||||||
| PlGF/sVEGFR- 1 <0.3 MOM | 16.9% (210/1246) | 52.2% (12/23) | 6.9 | 2.4 | 19.4 | 5.5 | 2.3 | 13.1 |
|
| ||||||||
| PlGF/sEng <0.3 MoM | 16.8% (209/1246) | 47.8% (11/23) | 7 | 2.5 | 19.8 | 4.4 | 1.8 | 10.4 |
|
|
||||||||
| Stillbirth (n=5) | ||||||||
| PlGF/sVEGFR- 1 <0.12 MOM | 5.6% (71/1264) | 80% (4/5) | 20.1 | 4.8 | 84.3 | 23.1 | 5.6 | 95.4 |
|
|
||||||||
| PlGF/sEng <0.2 MoM | 10.8% (137/1264) | 60% (3/5) | 8.4 | 2 | 35.1 | 9.1 | 2.2 | 37.2 |
OR=Odds Ratio, CI= Confidence Interval, MoM= Multiples of the Median,
PlGF= placental growth factor, sEng= soluble endoglin, sVEGFR-1=soluble vascular endothelial growth factor receptor-1
ORs represent the likelihood of outcome in subjects with abnormal analyte ratio concentrations (above/below MOM cutoff) relative to patients with normal analyte ratio concentration MoM.
Medians were calculated among uncomplicated pregnancies (n=886) by quantile regression (PlGF/sVEGFR-1 Median= 1.8863 + (−0.0508 *gestational week); PlGF/sEng Median= 354.3280 + (−8.9791*week)), cutoffs were selected based on inspection of receiver operating characteristic curves.
Prediction of stillbirth adjusted for gestational age at venipuncture (continuous);
Prediction of preeclampsia and SGA adjusted for: maternal age (continuous), combined parity & history of preeclampsia, pre-pregnancy body mass index (continuous), tobacco use.
PlGF/sVEGFR-1 MoM cutoff <0.12 (or 5th–6th percentile of uncomplicated pregnancies) for stillbirth, <0.3 (or 17th percentile of uncomplicated pregnancies) for preeclampsia and SGA
PlGF/sEng MoM cutoff <0.2 (or 11th percentile of uncomplicated pregnancies) for stillbirth, <0.3 (or 17th percentile of uncomplicated pregnancies) for preeclampsia and SGA
Table 3.
Diagnostic performance of maternal plasma concentrations of angiogenic & anti-angiogenic factors for stillbirth and preeclampsia screening according to PlGF/sVEGFR-1 and PlGF/sEng Multiples of the Median threshold
| Diagnostic Performance Metrics | Preeclampsia | Stillbirth (n=5) | ||||
|---|---|---|---|---|---|---|
| Overall (n=40) | Severe (n=23) | |||||
| Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| PlGF/sVEGFR-1 | ||||||
| Sensitivity (%) | 58 | (40–73) | 74 | (52–90) | 80 | (28–100) |
| Specificity (%) | 83 | (82–86) | 84 | (81–86) | 94 | (93–96) |
| + predictive value (%) | 10 | (6–15) | 8 | (5–12) | 5 | (1–13) |
| − predictive value (%) | 98 | (97–99) | 99 | (99–100) | 100 | (99–100) |
| False + probability (%) | 16 | (14–18) | 16 | (14–19) | 6 | (4–7) |
| False − probability (%) | 43 | (27–59) | 26 | (10–48) | 20 | (0.5–72) |
| + likelihood ratio | 3.6 | (2.6–4.8) | 4.5 | (3.4–5.9) | 14.2 | (8.7–23.3) |
| − likelihood ratio | 0.5 | (0.4–0.7) | 0.3 | (0.2–0.6) | 0·2 | (0.04–1.22) |
| PlGF/sEng | ||||||
| Sensitivity (%) | 60 | (43–75) | 78 | (56–93) | 60 | (15–95) |
| Specificity (%) | 84 | (82–86) | 84 | (82–86) | 89 | (87–91) |
| + predictive value (%) | 11 | (7–16) | 8 | (5–13) | 2 | (0.4–6) |
| − predictive value (%) | 98 | (97–99) | 99 | (99–100) | 99 | (99–100) |
| False + probability (%) | 16 | (14–18) | 16 | (14–18) | 11 | (9–13) |
| False − probability (%) | 40 | (25–57) | 22 | (7–44) | 40 | (5–85) |
| + likelihood ratio | 3.8 | (2.8–5.0) | 4.8 | (3.8–6.2) | 5.5 | (2.7–11.5) |
| − likelihood ratio | 0.5 | (0.3–0.7) | 0.3 | (0.1–0.6) | 0.4 | (0.2–1.3) |
PlGF= placental growth factor, sEng= soluble endoglin, sVEGFR-1=soluble vascular endothelial growth factor receptor-1
MOM= Multiples of the Median. Medians were calculated among uncomplicated pregnancies (n=886) by quantile regression (PlGF/sVEGFR-1 Median= 1.8863 + (−0.0508 *gestational week); PlGF/sEng
Median= 354.3280 +(−8.9791*week)), cutoffs were selected based on inspection of receiver operating characteristic curves.
PlGF/sVEGFR-1 MoM cutoff <0.12 (or 5th–6th percentile of uncomplicated pregnancies) for stillbirth, <0.3 (or 17th percentile of uncomplicated pregnancies) for preeclampsia
PlGF/sEng MoM cutoff <0.2 (or 11th percentile of uncomplicated pregnancies) for stillbirth, <0.3 (or 17th percentile of uncomplicated pregnancies) for preeclampsia
The addition of the PlGF/sEng or PlGF/sVEGFR-1 ratio to the clinical risk factors increased the AUC from 0.76 to 0.88 and 0.86, respectively, for the prediction of severe late PE (p=0.03 and p=0.06). With a fixed false positive rate of 15%, both the PlGF/sEng ratio and PlGF/sVEGFR-1 ratios achieved a sensitivity of 74% in predicting severe PE. These biomarkers in the third trimester outperformed those obtained previously at 6–15 and 20–25 weeks of gestation, and UADV assessed at 20–25 weeks of gestation for the prediction of severe late PE (each p≤0.02; Figure 2). Further, the addition of the PlGF/sVEGFR-1 or the PlGF/sEng ratio measured in the 3rd trimester to clinical risk factors (age, BMI, combined parity and history of preeclampsia, and tobacco use) yielded significantly greater sensitivity at a fixed false positive rate of 15% compared to a model using the same biomarker ratios measured in the 2nd trimester, clinical risk factors, and abnormal UADV obtained at 20–25 weeks of gestation (74% vs. 50%; p=0.008 and p=0.03, respectively). The direction, magnitude and significance of these associations also persisted during sensitivity analyses performed excluding patients having a history of preeclampsia (n=37) based on their elevated a-priori risk in the current pregnancy.
Figure 2.
Comparison of receiver-operating characteristic curves for the identification of severe late preeclampsia using plasma concentrations of PlGF/sEng (A) or PlGF/sVEGFR-1 (B) Multiples of the Median at 6–15, 20–25 and 30–34 weeks of gestation and uterine artery Doppler velocimetry at 20–25 weeks of gestation
While patients with plasma PlGF/sVEGFR-1 or PlGF/sEng ratio concentrations <0.3 MoM were more likely to develop SGA without PE (aOR 2–3; Table 2), adding these biomarkers to demographic/perinatal data did not improve the AUC (0.64 vs. 0.62; p=0.2 and p=0.6; respectively). Subgroup analysis focusing on patients with severe SGA (birthweight <3rd centile; n=23) indicated that the adjusted odds ratio of patients with PlGF/sVEGFR-1 or PlGF/sEng ratio <0.3 MoM to develop severe SGA ranged from 4.4 to 5.5 (Table 2). However, the addition of these biomarkers to clinical risk factors did not significantly improve the AUC (p>0.05).
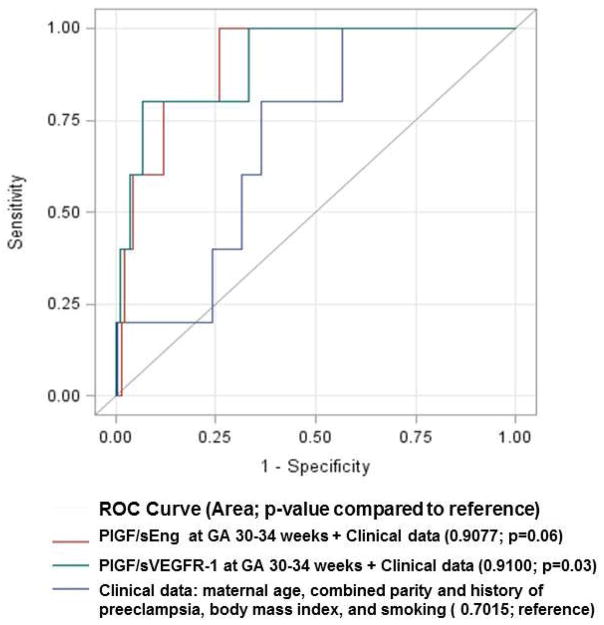
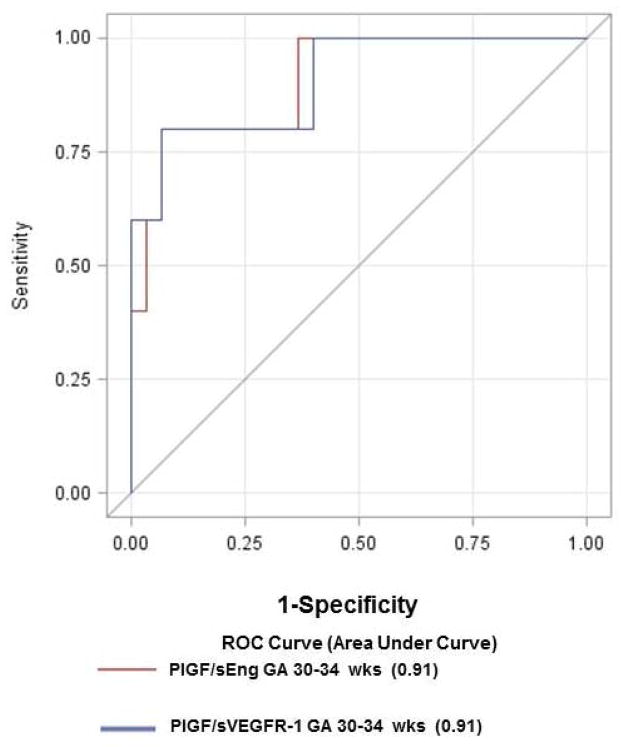
Patients with a PlGF/sVEGFR-1 ratio <0.12 MoM were significantly more likely to have a stillbirth than patients with a MoM ratio at or above the threshold [aOR 23.1; 95% CI 5.6–95.4]. This cut-off had a sensitivity of 80%, specificity of 94%, and a likelihood ratio of a positive test of 14.2 for the identification of a subsequent stillbirth after 34 weeks of gestation (Table 3). Compared to a model including only clinical data (maternal age, combined parity and history of preeclampsia, BMI and tobacco use), addition of the PlGF/sVEGFR-1 ratio or the PlGF/sEng ratio to clinical data increased the AUC from 0.7 to 0.91 (p=0.03 and p=0.06, respectively; Figure 3). The association between an abnormal ratio of angiogenic/anti-angiogenic factors and stillbirth at or near term was also observed in the subsequent case-control study performed in a different population (Table 4). A maternal plasma concentration of PlGF/sVEGFR-1 ratio ≤0.046 or PlGF/sEng ratio ≤11.7 pg/ng at 30–34 weeks had a sensitivity of 80% and a specificity of 93% for the identification of subsequent stillbirth (Figure 4).
Figure 3.
Comparison of receiver-operating characteristic curves for the identification of stillbirth using plasma concentrations of PlGF/sVEGFR-1 or PlGF/sEng Multiples of the Median at 30–34 weeks gestation in addition to clinical data compared to clinical data alone
Table 4.
Demographic and obstetrical characteristics of the population in the case-control study
| Normal Pregnancies (n=30) | Fetal Death (n=5) | p | |
|---|---|---|---|
| Maternal age (years) | 21.5 (19.8–23.2) | 26 (21.5–36.0) | 0.154 |
| Gestational age at venipuncture (weeks) | 32.9 (32.1–33.6) | 33.4 (32–33.7) | 0.493 |
| Body mass index (Kg/m2) | 26.1 (21.1–35.8) | 24.6 (17.9–44.5) | 0.873 |
| Nulliparity | 53.3%(16) | 60% (3) | 0.585 |
| Tobacco Use | 0 | 0 | -- |
| African American | 80% (24) | 80% (4) | 0.90 |
| Gestational age at delivery (weeks) | 39.5 (38.9–40.6) | 37.7 (34.7–38.9) | 0.047 |
| Birthweight (grams) | 3273 (3165–3478) | 2305 (1635–3360) | 0.030 |
| PlGF (pg/ml) | 646 (279–1108) | 97 (63–640) | 0.016 |
| sVEGFR-1 (pg/ml) | 2779 (1822–4349) | 6333 (3740–6908) | 0.016 |
| sEng (ng/ml) | 7.5 (5.7–10.1) | 23.8 (14.4–33.4) | 0.002 |
| PlGF/sVEGFR-1 ratio | 0.25 (0.09–0.5) | 0.02 (0.009–0.1) | 0.002 |
| PlGF/sEng ratio (pg/ng) | 96 (28–167) | 6.9 (2.3–28) | 0.002 |
Value expressed as median (interquartile range) or percentage (number);
PlGF= placental growth factor, sEng= soluble endoglin, sVEGFR-1=soluble vascular endothelial growth factor receptor-1
Figure 4.
Receiver-operating characteristic curves for the identification of subsequent stillbirth in case-control study participants using maternal plasma concentrations of PlGF/sVEGFR-1 or PlGF/sEng at 30–34 weeks of gestation. Area under the ROC curves = 0·91 for both biomarkers.
Table 5 displays the obstetrical events at delivery, gestational age at venipuncture, and placental pathology of each patient with stillbirth in the cohort and the case-control study. Among patients with a stillbirth in the cohort study, the interval from venipuncture to the diagnosis of stillbirth ranged from 2.2 to 6.1 weeks (median 4.5 weeks). One patient was diagnosed to have gestational diabetes mellitus and another had an abruptio placentae. Three patients had histologic placental lesions consistent with maternal underperfusion according to the criteria of the Society for Pediatric Pathology.135 Chronic chorioamnionitis and hyalinized avascular villi, consistent with fetal thrombotic vasculopathy, were observed in the other two cases. None of the cases included in the cohort study had a fetal autopsy performed. Of five cases included in the case-control study, two were diagnosed with diabetes mellitus, one was diagnosed with severe PE, one was diagnosed with chronic hypertension and another with Marfan’s syndrome. The interval from venipuncture to the diagnosis of stillbirth ranged from 2.4 to 5.4 weeks (median 4 weeks). All four cases of stillbirth included in the case-control study who had a plasma concentration of angiogenic/anti-angiogenic factor ratio below the above cut-off had lesions in the placenta suggestive of maternal underperfusion. Two stillbirths had a karyotype performed and they were 46 XY. Among four cases with available fetal autopsy results, one had lesions in the fetal brain consistent with acute hypoxic/ischemic damage in the grey matter.
Table 5.
Obstetrical events at delivery, gestational age at venipuncture, and placental pathology of patients with stillbirth
| Case | Obstetrical events at delivery | PlGF/ sVEGFR-1 ratio (MoM) | GA at venous sampling (weeks) | GA at delivery (weeks) | Birthweight in grams (percentile) | Placental lesions consistent with maternal underperfusion | Placenta pathology | Fetal Autopsy |
|---|---|---|---|---|---|---|---|---|
| Cohort study | ||||||||
| 1 | Normal blood pressure | 0.07 | 32 1/7 | 34 3/7 | 2200 (50%) | Yes | Diffuse chronic villitis, Persistent muscularization of basal plate arteries | Not Available |
| 2 | GDM non- compliance with care | 0.10 | 31 3/7 | 34 4/7 | 2280 (58%) | Yes | Increased syncytial knot | Not Available |
| 3 | Blood pressure 140/90, urine protein dipstick negative, placental abruption | 0.04 | 31 | 35 4/7 | 3000 (92%) | No | Chronic chorioamnionitis | Not Available |
| 4 | Normal blood pressure, decreased fetal movement, thick meconium stained amniotic fluid | 0.08 | 33 3/7 | 39 3/7 | 3650 (74%) | Yes | Increased intervillous fibrin, Prominent nucleated RBC, absence of physiologic change of the spiral arteries | Not Available |
| 5 | Normal blood pressure | 0.78 | 32 2/7 | 38 3/7 | 3350 (60.5%) | No | Hyalinized avascular villi, Fetal thrombotic vasculopathy | Not Available |
| Case-control study | ||||||||
|---|---|---|---|---|---|---|---|---|
| Case | Obstetrical events at delivery | PlGF/sVEGFR-1 ratio | GA at venous sampling (weeks) | GA at delivery (weeks) | Birthweight in grams (percentile) | Placental lesions consistent with maternal underperfusion | Placenta pathology | Fetal Autopsy |
| 1 | Gestational diabetes mellitus class A2 – poorly controlled glucose | 0.02 | 33 5/7 | 37 5/7 | 3620 (81.5%) | Yes | Microscopic chorionic pseudocysts in placental membranes | No congenital anomalies; No etiology found |
| 2 | Pre-gestational diabetes mellitus class B – poorly controlled glucose | 0.04 | 33 5/7 | 39 1/7 | 3100 (28.1%) | Yes | Recent villous infarction, persistent muscularization of basal plate arteries | Acute hypoxic/ischemic gray matter damage & Subarachnoid hemorrhage; No congenital anomalies |
| 3 | Severe preeclampsia | 0.01 | 31 3/7 | 34 2/7 | 2040 (15%) | Yes | Recent villous infarction | No congenital anomalies; No etiology found |
| 4 | Chronic hypertension | 0.005 | 32 4/7 | 35 | 1231 (1%) | Yes | Remote villous infarction, increased syncytial knots | Not available |
| 5 | Marfan’s syndrome | 0.19 | 33 3/7 | 38 4/7 | 2305 (1%) | No | Normal | No congenital anomalies; No etiology found |
GDM= gestational diabetes; GA= gestational age; MoM= Multiple of Median; PlGF= placental growth factor, sVEGFR-1=soluble vascular endothelial growth factor receptor-1
DISCUSSION
Principal findings
This is the first prospective cohort study evaluating the diagnostic performance of angiogenic/anti-angiogenic factors in the third trimester for the identification of patients with late PE, severe late PE, SGA without PE and stillbirth. The principal findings are: 1) a maternal plasma concentration of PlGF/sEng <0.3 MoM at 30–34 weeks was associated with late PE (aOR 7) and severe late PE (aOR 16). With a fixed false positive rate of 15%, both the PlGF/sEng and PlGF/sVEGFR-1 ratios achieved a sensitivity of 74% for the identification of severe late PE; 2) the ratio of PlGF/sEng or PlGF/sVEGFR-1 in the third trimester outperformed those obtained at 6–15 and 20–25 weeks of gestation and abnormal UADV obtained at 20–25 weeks of gestation for the identification of severe late PE (comparisons of AUC; each p≤0.02); and 3) a maternal plasma concentration of PlGF/sVEGFR-1 ratio <0.12 MoM at 30–34 weeks was significanlty associated with a subsequent stillbirth (aOR 23). This cut-off had a sensitivity of 80%, a specificity of 94%, and a likelihood ratio of a positive result of 14 for the identification of patients destined to have a stillbirth; and 4) while a low maternal plasma concentration of the PlGF/sVEGFR-1 and PlGF/Eng ratio was associated with a significant increase in the likelihood of developing SGA, these biomarkers did not improve the identification of SGA from the models using clinical factors alone.
Rationale for examining biomarkers in the third trimester
The rationale for using biomarkers in the third trimester, in addition to the first or second trimesters, includes: 1) testing performed closer to the event of interest or diagnosis usually yields better results than those performed earlier in gestation. Several studies on screening tests in the first or second trimester for conditions related to placental dysfunction (PE, SGA or fetal death) using either biochemical markers136–142 or UADV114,143–145 indicate that both are strongly associated with complications which develop earlier in pregnancy and therefore, temporally close to the assessment of biomarkers.125,146 Our findings that plasma concentrations of the ratio between angiogenic/anti-angiogenic factors outperformed those obtained in the first two trimesters for the identification of patients with late PE strongly support this view; 2) the risk for a prospective stillbirth increases after 34 weeks of gestation147 and, similarly, the prevalence of late-onset PE is much higher than that of early-onset disease;45,119 and 3) the strategy of testing at the beginning of the third trimester to assess the risk of disease or pregnancy complications could be considered for patients who did not receive earlier prenatal care or undergo testing.
A disadvantage of performing a screening test in the third trimester is that this may be too late to implement therapeutic interventions that can reverse the pathophysiological process responsible for the disease. However, the precise mechanisms of late-onset PE are unknown, and there is no effective intervention even if at-risk patients are identified in early gestation. Although a recent meta-analysis suggests that the administration of aspirin before 16 weeks of gestation may prevent PE,148 this strategy is not effective to prevent PE at term.149 Thus, a method to identify the patient at risk for late PE is needed, given that late PE accounts for the majority of severe maternal morbidity including eclampsia,47,52,53 especially in developing countries.51 Furthermore, our study which examined the diagnostic performance of biomarkers in the third trimester in a low-risk population is consistent with the recently proposed new approach for screening of adverse pregnancy outcomes that focuses on the prevention of pregnancy complications at term in low-risk, unselected populations.125 However, while an appreciable magnitude of the association was observed between these biomarkers in the third trimester and late severe PE (which outperformed the screening implemented in earlier gestations), further study is necessary to explain why some women with distinctly abnormal angiogenic/anti-angiogenic profiles have uncomplicated pregnancies. Alternatively, additional biomarkers would be needed to increase the likelihood ratio of a positive test to justify clinical utility.
Risk assessment for stillbirth: a neglected area of prenatal care
Our findings open new avenues for understanding the pathophysiology, risk assessment and prevention of stillbirth, a neglected area of prenatal care.57,61 In our cohort, there was no intra-partum stillbirth case. Among eight stillbirths identified with these biomarkers from the cohort and the case-control studies, seven had lesions in the placenta suggestive of maternal underperfusion.135 These lesions, although could be observed in 15% of uncomplicated pregnancy at term, have been shown to be more frequently found in PE (relative risk 2–3).43 Another patient with a stillbirth had chronic chorioamnionitis, a lesion associated with evidence of maternal anti-fetal rejection and fetal death.150 Although the precise mechanisms responsible for stillbirth are unknown, it appears that biomarkers investigated in this study may be able to identify a large fraction of stillbirths resulting from placental rather than non-placental related etiologies (such as cord accident, fetal thrombosis, or feto-maternal hemorrhage). This interpretation is consistent with the findings from a recent study which demonstrated an association between stillbirth at, or near term and UADV in the second trimester, indicating that an increase in impedance to blood flow to the placenta is one of the major risk factors for stillbirth at term.151 Moreover, since markers of placental dysfunction, such as high maternal serum alpha-fetoprotein or beta human chorionic gonadotropin are associated with an increased risk of unexplained stillbirth and other pregnancy complications such as PE or SGA,139–141,146,152–154 it is possible that a subset of unexplained stillbirth, PE, and SGA are different clinical manifestations of a similar placental response from insults at different gestational ages. Evidence in support of this hypothesis is that rats subjected to reduced utero-placental perfusion by applying clips to the abdominal aorta at different gestational ages had a different magnitude of change in the angiogenic/anti-angiogenic imbalance, fetal growth restriction and the severity of placental ischemia-induced systemic hypertension.155 Our findings of an association between abnormal angiogenic/anti-angiogenic factors and stillbirth, PE, and SGA without PE strengthen this hypothesis.
Limitations of the study
In order to facilitate comparison of the diagnostic performance of angiogenic/anti-angiogenic factors in the third trimester with that of these biomarkers in the first and second trimester as well as that of UADV in the second trimester, a number of patients without plasma samples in the first two intervals and those without UADV information were excluded from the cohort. However, the distribution of baseline characteristics (maternal age, body mass index, nulliparity, combined parity and previous PE) did not significantly differ between patients included in the current study compared to the overall cohort, and there were no significant differences in the risk of stillbirth or SGA between the full and sub-cohort. However, by design, participants in the sub-cohort were more likely to deliver after 34 weeks of gestation. Patients in the sub-cohort were also less likely to develop late PE (3.2% vs. 4.8%; p=0.03), indicating that our results for PE may have been biased toward the null hypothesis.
Another limitation is that the determination of plasma concentrations of these biomarkers was performed in stored samples. However, serum PlGF and sVEGFR-1 concentrations are stable for at least three years when stored at −80C,156 and the duration of sample storage did not differ significantly by pregnancy outcome. Therefore, this is unlikely to have introduced a bias into our study.
A limited number of patients with stillbirth were included in this cohort of a low-risk population. This is a common problem of cohort studies with rare outcomes. We have used statistical methods to address possible over-fitting of models and replicated our findings in a separate case-control study performed in a different population. Taken together, our results indicate that the ratio of maternal plasma concentrations of angiogenic/anti-angiogenic factors in the third trimester have value in risk assessment for stillbirth, given the high likelihood ratio of a positive test (14), although further study is necessary to both validate these findings and to determine generalizability.
A low positive predictive value, consistent with that observed in this study, is often invoked as a limitation of a test. However, it is noteworthy that positive predictive values are dependent on the prevalence of the disease, and will always be low when the condition under study is rare. This is the case of stillbirth, which had a prevalence of 0.4% in our study. Even if we had a test with 99% sensitivity and 99% specificity, the positive predictive value with this prevalence of disease would be 28%. Thus, most patients screened positive would not have the disease (false-positive). Yet, this scenario occurs daily in the practice of obstetrics (most patients undergoing midtrimester amniocentesis or chorionic villous sampling do not have aneuploidy).
Conclusions
Risk assessment for severe late PE and stillbirth in the third trimester may be possible with the determination of maternal plasma concentrations of angiogenic and anti-angiogenic factors at 30–34 weeks of gestation. Of interest, the StAmP trial, a randomized controlled trial to determine the effect of pravastatin on the changes of maternal angiogenic/anti-angiogenic factors concentrations in patients with a diagnosis of early-onset PE, is currently ongoing in the United Kingdom (http://www.birmingham.ac.uk/research/activity/index.aspx). Statins have the potential to reverse the abnormalities in angiogenic/anti-angiogenic factors152 that we have previously demonstrated in stillbirths of unknown etiology,100 and therefore, may represent an intervention with patients identified with the approach herein. Other proposed therapeutic interventions to reverse an anti-angiogenic state during pregnancy include the administration of VEGF 121157 or extracorporeal removal of sVEGFR-1.158 Accordingly, biomarkers investigated in this study in the third trimester may be useful as an additional tool for risk stratification in future interventional trials for the prevention of stillbirth and/or severe late PE at or near term. A specific clinical example is that patients at risk for stillbirth after being identified by the markers proposed herein can undergo intensive antepartum surveillance and deliver the fetus at or near term once the risks of prolonging pregnancy outweigh those of complications of prematurity.
Acknowledgments
Financial Support: This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
The authors would like to thank Professor Gordon C.S. Smith of Cambridge University for his advice and helpful discussions on the analytical methods of the data reported in this study.
Footnotes
Disclosure: The authors report no conflict of interest
Presented as an oral presentation at the 57th Annual Meeting of the Society for Gynecologic Investigation, March 21–24, 2012, San Diego, CA, USA
CONDENSATION: Risk assessment for severe late preeclampsia and stillbirth is possible with the determination of maternal plasma concentrations of angiogenic/anti-angiogenic factors at 30–34 weeks of gestation.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Semin Perinatol. 1988;12(4):302–23. [PubMed] [Google Scholar]
- 2.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308(5728):1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 3.Berg CJ, Callaghan WM, Syverson C, Henderson Z. Pregnancy-related mortality in the United States, 1998 to 2005. Obstet Gynecol. 2010;116(6):1302–9. doi: 10.1097/AOG.0b013e3181fdfb11. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Meikle S, Trumble A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens Pregnancy. 2003;22(2):203–12. doi: 10.1081/PRG-120021066. [DOI] [PubMed] [Google Scholar]
- 5.Brosens IA. Morphological changes in the utero-placental bed in pregnancy hypertension. Clin Obstet Gynaecol. 1977;4(3):573–93. [PubMed] [Google Scholar]
- 6.Brosens JJ, Pijnenborg R, Brosens IA. The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol. 2002;187(5):1416–23. doi: 10.1067/mob.2002.127305. [DOI] [PubMed] [Google Scholar]
- 7.Pijnenborg R, Anthony J, Davey DA, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98(7):648–55. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 8.Robertson WB, Brosens I, Dixon G. Maternal uterine vascular lesions in the hypertensive complications of pregnancy. Perspect Nephrol Hypertens. 1976;5:115–27. [PubMed] [Google Scholar]
- 9.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204(3):193–201. doi: 10.1016/j.ajog.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romero R, Kusanovic JP, Chaiworapongsa T, Hassan SS. Placental bed disorders in preterm labor, preterm PROM, spontaneous abortion and abruptio placentae. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):313–27. doi: 10.1016/j.bpobgyn.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sacks GP, Studena K, Sargent K, Redman CW. Normal pregnancy and preeclampsia both produce inflammatory changes in peripheral blood leukocytes akin to those of sepsis. Am J Obstet Gynecol. 1998;179(1):80–6. doi: 10.1016/s0002-9378(98)70254-6. [DOI] [PubMed] [Google Scholar]
- 12.Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 Pt 1):499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 13.Gervasi MT, Chaiworapongsa T, Pacora P, et al. Phenotypic and metabolic characteristics of monocytes and granulocytes in preeclampsia. Am J Obstet Gynecol. 2001;185(4):792–7. doi: 10.1067/mob.2001.117311. [DOI] [PubMed] [Google Scholar]
- 14.Friedman SA, Schiff E, Emeis JJ, Dekker GA, Sibai BM. Biochemical corroboration of endothelial involvement in severe preeclampsia. Am J Obstet Gynecol. 1995;172(1 Pt 1):202–3. doi: 10.1016/0002-9378(95)90113-2. [DOI] [PubMed] [Google Scholar]
- 15.Lyall F, Greer IA. The vascular endothelium in normal pregnancy and pre- eclampsia. Rev Reprod. 1996;1(2):107–16. doi: 10.1530/ror.0.0010107. [DOI] [PubMed] [Google Scholar]
- 16.Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. Am J Hypertens. 1991;4(8):700–8. doi: 10.1093/ajh/4.8.700. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JM. Endothelial dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):5–15. doi: 10.1055/s-2007-1016248. [DOI] [PubMed] [Google Scholar]
- 18.Taylor RN, de Groot CJ, Cho YK, Lim KH. Circulating factors as markers and mediators of endothelial cell dysfunction in preeclampsia. Semin Reprod Endocrinol. 1998;16(1):17–31. doi: 10.1055/s-2007-1016249. [DOI] [PubMed] [Google Scholar]
- 19.Chaiworapongsa T, Romero R, Yoshimatsu J, et al. Soluble adhesion molecule profile in normal pregnancy and pre-eclampsia. J Matern Fetal Neonatal Med. 2002;12(1):19–27. doi: 10.1080/jmf.12.1.19.27. [DOI] [PubMed] [Google Scholar]
- 20.Petrozella L, Mahendroo M, Timmons B, Roberts S, McIntire D, Alexander JM. Endothelial microparticles and the antiangiogenic state in preeclampsia and the postpartum period. Am J Obstet Gynecol. 2012;207(2):140–6. doi: 10.1016/j.ajog.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Boer K, ten Cate JW, Sturk A, Borm JJ, Treffers PE. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol. 1989;160(1):95–100. doi: 10.1016/0002-9378(89)90096-3. [DOI] [PubMed] [Google Scholar]
- 22.Chaiworapongsa T, Yoshimatsu J, Espinoza J, et al. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med. 2002;11(6):362–7. doi: 10.1080/jmf.11.6.362.367. [DOI] [PubMed] [Google Scholar]
- 23.Cadroy Y, Grandjean H, Pichon J, et al. Evaluation of six markers of haemostatic system in normal pregnancy and pregnancy complicated by hypertension or pre-eclampsia. Br J Obstet Gynaecol. 1993;100(5):416–20. doi: 10.1111/j.1471-0528.1993.tb15264.x. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JR, Walshe JJ, Darling MR, Norris L, Bonnar J. Hemostasis in the uteroplacental and peripheral circulations in normotensive and pre-eclamptic pregnancies. Am J Obstet Gynecol. 1998;179(2):520–6. doi: 10.1016/s0002-9378(98)70389-8. [DOI] [PubMed] [Google Scholar]
- 25.Hayashi M, Inoue T, Hoshimoto K, Negishi H, Ohkura T, Inaba N. Characterization of five marker levels of the hemostatic system and endothelial status in normotensive pregnancy and pre-eclampsia. Eur J Haematol. 2002;69(5–6):297–302. doi: 10.1034/j.1600-0609.2002.02691.x. [DOI] [PubMed] [Google Scholar]
- 26.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999;222(3):222–35. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 27.Vaughan JE, Walsh SW. Oxidative stress reproduces placental abnormalities of preeclampsia. Hypertens Pregnancy. 2002;21(3):205–23. doi: 10.1081/PRG-120015848. [DOI] [PubMed] [Google Scholar]
- 28.Myatt L, Kossenjans W, Sahay R, Eis A, Brockman D. Oxidative stress causes vascular dysfunction in the placenta. J Matern Fetal Med. 2000;9(1):79–82. doi: 10.1002/(SICI)1520-6661(200001/02)9:1<79::AID-MFM16>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Zhang GY, Wang J, Lu SL, Cao J, Sun LZ. A novel bridge between oxidative stress and immunity: the interaction between hydrogen peroxide and human leukocyte antigen G in placental trophoblasts during preeclampsia. Am J Obstet Gynecol. 2012;206(5):447–16. doi: 10.1016/j.ajog.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am J Obstet Gynecol. 1998;179(6 Pt 1):1539–44. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 31.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am J Obstet Gynecol. 2001;184(6):1267–72. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 32.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin Endocrinol Metab. 2003;88(5):2348–51. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 34.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am J Obstet Gynecol. 2003;188(1):177–82. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 35.Tsatsaris V, Goffin F, Munaut C, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88(11):5555–63. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 36.Chaiworapongsa T, Romero R, Espinoza J, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190(6):1541–7. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 37.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12(6):642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 38.Weissgerber TL, Roberts JM, Jeyabalan A, et al. Haptoglobin phenotype, angiogenic factors, and preeclampsia risk. Am J Obstet Gynecol. 2012;206(4):358. doi: 10.1016/j.ajog.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–8. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 40.Romero R, Vizoso J, Emamian M, et al. Clinical significance of liver dysfunction in pregnancy-induced hypertension. Am J Perinatol. 1988;5(2):146–51. doi: 10.1055/s-2007-999675. [DOI] [PubMed] [Google Scholar]
- 41.Romero R, Mazor M, Lockwood CJ, et al. Clinical significance, prevalence, and natural history of thrombocytopenia in pregnancy-induced hypertension. Am J Perinatol. 1989;6(1):32–8. doi: 10.1055/s-2007-999540. [DOI] [PubMed] [Google Scholar]
- 42.Long PA, Abell DA, Beischer NA. Fetal growth retardation and pre-eclampsia. Br J Obstet Gynaecol. 1980;87(1):13–8. doi: 10.1111/j.1471-0528.1980.tb04419.x. [DOI] [PubMed] [Google Scholar]
- 43.Ogge G, Chaiworapongsa T, Kusanovic JP, et al. Evidence that placental lesions are more frequent in early-onset than in late-onset preeclampsia. Reproductive Science. 2011;18(3):124A. doi: 10.1515/JPM.2011.098. Ref Type: Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sibai BM. Preeclampsia as a cause of preterm and late preterm (near-term) births. Semin Perinatol. 2006;30(1):16–9. doi: 10.1053/j.semperi.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365(9461):785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 46.Ales KL, Charlson ME. Epidemiology of preeclampsia and eclampsia. Am J Obstet Gynecol. 1991;165(1):238. doi: 10.1016/0002-9378(91)90267-u. [DOI] [PubMed] [Google Scholar]
- 47.Tuffnell DJ, Jankowicz D, Lindow SW, et al. Outcomes of severe pre- eclampsia/eclampsia in Yorkshire 1999/2003. BJOG. 2005;112(7):875–80. doi: 10.1111/j.1471-0528.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 48.McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. 2008;111(1):35–41. doi: 10.1097/01.AOG.0000297311.33046.73. [DOI] [PubMed] [Google Scholar]
- 49.Lubow JM, How HY, Habli M, Maxwell R, Sibai BM. Indications for delivery and short-term neonatal outcomes in late preterm as compared with term births. Am J Obstet Gynecol. 2009;200(5):e30–e33. doi: 10.1016/j.ajog.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 50.Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195(6):1557–63. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 51.Kenneth L, Hall DR, Gebhardt S, Grove D. Late onset preeclampsia is not an innocuous condition. Hypertens Pregnancy. 2010;29(3):262–70. doi: 10.3109/10641950902777697. [DOI] [PubMed] [Google Scholar]
- 52.Liu S, Joseph KS, Liston RM, et al. Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987–94. doi: 10.1097/AOG.0b013e31823311c1. [DOI] [PubMed] [Google Scholar]
- 53.Duley L, Gulmezoglu AM, Henderson-Smart DJ, Chou D. Magnesium sulphate and other anticonvulsants for women with pre-eclampsia. Cochrane Database Syst Rev. 2010;(11):CD000025. doi: 10.1002/14651858.CD000025.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moodley J. Maternal deaths associated with hypertensive disorders of pregnancy: a population-based study. Hypertens Pregnancy. 2004;23(3):247–56. doi: 10.1081/PRG-200030301. [DOI] [PubMed] [Google Scholar]
- 55.Romero R. Prenatal medicine: the child is the father of the man. Prenatal and Neonatal Medicine. 1996;1:8–11. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 56.Fretts RC. Etiology and prevention of stillbirth. Am J Obstet Gynecol. 2005;193(6):1923–35. doi: 10.1016/j.ajog.2005.03.074. [DOI] [PubMed] [Google Scholar]
- 57.Froen JF, Cacciatore J, McClure EM, et al. Stillbirths: why they matter. Lancet. 2011;377(9774):1353–66. doi: 10.1016/S0140-6736(10)62232-5. [DOI] [PubMed] [Google Scholar]
- 58.Lawn JE, Yakoob MY, Haws RA, Soomro T, Darmstadt GL, Bhutta ZA. 3. 2 million stillbirths: epidemiology and overview of the evidence review. BMC Pregnancy Childbirth. 2009;9 (Suppl 1):S2. doi: 10.1186/1471-2393-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhutta ZA, Yakoob MY, Lawn JE, et al. Stillbirths: what difference can we make and at what cost? Lancet. 2011;377(9776):1523–38. doi: 10.1016/S0140-6736(10)62269-6. [DOI] [PubMed] [Google Scholar]
- 60.Flenady V, Middleton P, Smith GC, et al. Stillbirths: the way forward in high- income countries. Lancet. 2011;377(9778):1703–17. doi: 10.1016/S0140-6736(11)60064-0. [DOI] [PubMed] [Google Scholar]
- 61.Lawn JE, Blencowe H, Pattinson R, et al. Stillbirths: Where? When? Why? How to make the data count? Lancet. 2011;377(9775):1448–63. doi: 10.1016/S0140-6736(10)62187-3. [DOI] [PubMed] [Google Scholar]
- 62.McClure EM, Saleem S, Pasha O, Goldenberg RL. Stillbirth in developing countries: a review of causes, risk factors and prevention strategies. J Matern Fetal Neonatal Med. 2009;22(3):183–90. doi: 10.1080/14767050802559129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menezes EV, Yakoob MY, Soomro T, Haws RA, Darmstadt GL, Bhutta ZA. Reducing stillbirths: prevention and management of medical disorders and infections during pregnancy. BMC Pregnancy Childbirth. 2009;9 (Suppl 1):S4. doi: 10.1186/1471-2393-9-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yakoob MY, Lawn JE, Darmstadt GL, Bhutta ZA. Stillbirths: epidemiology, evidence, and priorities for action. Semin Perinatol. 2010;34(6):387–94. doi: 10.1053/j.semperi.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 65.Smith GC. Predicting antepartum stillbirth. Clin Obstet Gynecol. 2010;53(3):597–606. doi: 10.1097/GRF.0b013e3181eb64a6. [DOI] [PubMed] [Google Scholar]
- 66.Hui D, Okun N, Murphy K, Kingdom J, Uleryk E, Shah PS. Combinations of maternal serum markers to predict preeclampsia, small for gestational age, and stillbirth: a systematic review. J Obstet Gynaecol Can. 2012;34(2):142–53. doi: 10.1016/S1701-2163(16)35157-X. [DOI] [PubMed] [Google Scholar]
- 67.Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1999;106(10):1019–22. doi: 10.1111/j.1471-0528.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 68.Ahmed A. New insights into the etiology of preeclampsia: identification of key elusive factors for the vascular complications. Thromb Res. 2011;127 (Suppl 3):S72–S75. doi: 10.1016/S0049-3848(11)70020-2. [DOI] [PubMed] [Google Scholar]
- 69.Luttun A, Carmeliet P. Soluble VEGF receptor Flt1: the elusive preeclampsia factor discovered? J Clin Invest. 2003;111(5):600–2. doi: 10.1172/JCI18015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bujold E, Romero R, Chaiworapongsa T, et al. Evidence supporting that the excess of the sVEGFR-1 concentration in maternal plasma in preeclampsia has a uterine origin. J Matern Fetal Neonatal Med. 2005;18(1):9–16. doi: 10.1080/14767050500202493. [DOI] [PubMed] [Google Scholar]
- 71.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195(1):201–7. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 72.Chaiworapongsa T, Romero R, Gotsch F, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med. 2008;21(1):41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaiworapongsa T, Romero R, Tarca AL, et al. A decrease in maternal plasma concentrations of sVEGFR-2 precedes the clinical diagnosis of preeclampsia. Am J Obstet Gynecol. 2010;202(6):550–10. doi: 10.1016/j.ajog.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benton SJ, Hu Y, Xie F, et al. Angiogenic factors as diagnostic tests for preeclampsia: a performance comparison between two commercial immunoassays. Am J Obstet Gynecol. 2011;205(5):469–8. doi: 10.1016/j.ajog.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 75.Garovic VD. The role of angiogenic factors in the prediction and diagnosis of preeclampsia superimposed on chronic hypertension. Hypertension. 2012;59(3):555–7. doi: 10.1161/HYPERTENSIONAHA.111.184192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ghosh SK, Raheja S, Tuli A, Raghunandan C, Agarwal S. Serum PLGF as a potential biomarker for predicting the onset of preeclampsia. Arch Gynecol Obstet. 2012;285(2):417–22. doi: 10.1007/s00404-011-1960-4. [DOI] [PubMed] [Google Scholar]
- 77.Chaiworapongsa T, Romero R, Savasan ZA, et al. Maternal plasma concentrations of angiogenic/anti-angiogenic factors are of prognostic value in patients presenting to the obstetrical triage area with the suspicion of preeclampsia. J Matern Fetal Neonatal Med. 2011;24(10):1187–207. doi: 10.3109/14767058.2011.589932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hagmann H, Thadhani R, Benzing T, Karumanchi SA, Stepan H. The promise of angiogenic markers for the early diagnosis and prediction of preeclampsia. Clin Chem. 2012;58(5):837–45. doi: 10.1373/clinchem.2011.169094. [DOI] [PubMed] [Google Scholar]
- 79.Molvarec A, Szarka A, Walentin S, Szucs E, Nagy B, Rigo J., Jr Circulating angiogenic factors determined by electrochemiluminescence immunoassay in relation to the clinical features and laboratory parameters in women with pre-eclampsia. Hypertens Res. 2010;33(9):892–8. doi: 10.1038/hr.2010.92. [DOI] [PubMed] [Google Scholar]
- 80.Rana S, Powe CE, Salahuddin S, et al. Angiogenic factors and the risk of adverse outcomes in women with suspected preeclampsia. Circulation. 2012;125(7):911–9. doi: 10.1161/CIRCULATIONAHA.111.054361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verlohren S, Stepan H, Dechend R. Angiogenic growth factors in the diagnosis and prediction of pre-eclampsia. Clin Sci (Lond) 2012;122(2):43–52. doi: 10.1042/CS20110097. [DOI] [PubMed] [Google Scholar]
- 82.Staff AC, Braekke K, Johnsen GM, Karumanchi SA, Harsem NK. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am J Obstet Gynecol. 2007;197(2):176. doi: 10.1016/j.ajog.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 83.Smith GC, Wear H. The perinatal implications of angiogenic factors. Curr Opin Obstet Gynecol. 2009;21(2):111–6. doi: 10.1097/GCO.0b013e328328cf7d. [DOI] [PubMed] [Google Scholar]
- 84.Weed S, Bastek JA, Anton L, Elovitz MA, Parry S, Srinivas SK. Examining the correlation between placental and serum placenta growth factor in preeclampsia. Am J Obstet Gynecol. 2012;207(2):140–6. doi: 10.1016/j.ajog.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 85.Maynard SE, Karumanchi SA. Angiogenic factors and preeclampsia. Semin Nephrol. 2011;31(1):33–46. doi: 10.1016/j.semnephrol.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46(5):1077–85. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 87.Karumanchi SA, Maynard SE, Stillman IE, Epstein FH, Sukhatme VP. Preeclampsia: a renal perspective. Kidney Int. 2005;67(6):2101–13. doi: 10.1111/j.1523-1755.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 88.Levine RJ, Karumanchi SA. Circulating angiogenic factors in preeclampsia. Clin Obstet Gynecol. 2005;48(2):372–86. doi: 10.1097/01.grf.0000160313.82606.d7. [DOI] [PubMed] [Google Scholar]
- 89.Chaiworapongsa T, Espinoza J, Gotsch F, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med. 2008;21(1):25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crispi F, Llurba E, Dominguez C, Martin-Gallan P, Cabero L, Gratacos E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;31(3):303–9. doi: 10.1002/uog.5184. [DOI] [PubMed] [Google Scholar]
- 91.Asvold BO, Vatten LJ, Romundstad PR, Jenum PA, Karumanchi SA, Eskild A. Angiogenic factors in maternal circulation and the risk of severe fetal growth restriction. Am J Epidemiol. 2011;173(6):630–9. doi: 10.1093/aje/kwq373. [DOI] [PubMed] [Google Scholar]
- 92.Gourvas V, Dalpa E, Konstantinidou A, Vrachnis N, Spandidos DA, Sifakis S. Angiogenic factors in placentas from pregnancies complicated by fetal growth restriction (review) Mol Med Report. 2012;6(1):23–7. doi: 10.3892/mmr.2012.898. [DOI] [PubMed] [Google Scholar]
- 93.Espinoza J, Chaiworapongsa T, Romero R, et al. Unexplained fetal death: another anti-angiogenic state. J Matern Fetal Neonatal Med. 2007;20(7):495–507. doi: 10.1080/14767050701413022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chaiworapongsa T, Romero R, Kusanovic JP, et al. Unexplained fetal death is associated with increased concentrations of anti-angiogenic factors in amniotic fluid. J Matern Fetal Neonatal Med. 2010;23(8):794–805. doi: 10.3109/14767050903443467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chaiworapongsa T, Kusanovic JP, Savasan ZA, et al. Fetal death: a condition with a dissociation in the concentrations of soluble vascular endothelial growth factor receptor-2 between the maternal and fetal compartments. J Matern Fetal Neonatal Med. 2010;23(9):960–72. doi: 10.3109/14767050903410664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bixel K, Silasi M, Zelop CM, et al. Placental origins of angiogenic dysfunction in mirror syndrome. Hypertens Pregnancy. 2012;31(2):211–7. doi: 10.3109/10641955.2011.638959. [DOI] [PubMed] [Google Scholar]
- 97.Espinoza J, Romero R, Nien JK, et al. A role of the anti-angiogenic factor sVEGFR-1 in the ‘mirror syndrome’ (Ballantyne’s syndrome) J Matern Fetal Neonatal Med. 2006;19(10):607–13. doi: 10.1080/14767050600922677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanter D, Lindheimer MD, Wang E, et al. Angiogenic dysfunction in molar pregnancy. Am J Obstet Gynecol. 2010;202(2):184–5. doi: 10.1016/j.ajog.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koga K, Osuga Y, Tajima T, et al. Elevated serum soluble fms-like tyrosine kinase 1 (sFlt1) level in women with hydatidiform mole. Fertil Steril. 2010;94(1):305–8. doi: 10.1016/j.fertnstert.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 100.Kusanovic JP, Romero R, Espinoza J, et al. Twin-to-twin transfusion syndrome: an antiangiogenic state? Am J Obstet Gynecol. 2008;198(4):382–8. doi: 10.1016/j.ajog.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Llurba E, Marsal G, Sanchez O, et al. Angiogenic and antiangiogenic factors before and after resolution of maternal mirror syndrome. Ultrasound Obstet Gynecol. 2011 doi: 10.1002/uog.10136. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 102.Rana S, Venkatesha S, DePaepe M, Chien EK, Paglia M, Karumanchi SA. Cytomegalovirus-induced mirror syndrome associated with elevated levels of circulating antiangiogenic factors. Obstet Gynecol. 2007;109(2 Pt2):549–52. doi: 10.1097/01.AOG.0000248538.03280.cf. [DOI] [PubMed] [Google Scholar]
- 103.Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern Fetal Neonatal Med. 2005;17(1):3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 104.Moore Simas TA, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet Gynecol. 2007;197(3):244–8. doi: 10.1016/j.ajog.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 105.Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21(5):279–87. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21(1):9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Unal ER, Robinson CJ, Johnson DD, Chang EY. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am J Obstet Gynecol. 2007;197(2):211–4. doi: 10.1016/j.ajog.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 108.Widmer M, Villar J, Benigni A, Conde-Agudelo A, Karumanchi SA, Lindheimer M. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstet Gynecol. 2007;109(1):168–80. doi: 10.1097/01.AOG.0000249609.04831.7c. [DOI] [PubMed] [Google Scholar]
- 109.Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol. 2005;193(3 Pt 2):984–9. doi: 10.1016/j.ajog.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 110.Romero R, Chaiworapongsa T, Erez O, et al. An imbalance between angiogenic and anti-angiogenic factors precedes fetal death in a subset of patients: results of a longitudinal study. J Matern Fetal Neonatal Med. 2010;23(12):1384–99. doi: 10.3109/14767051003681121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Smith GC, Crossley JA, Aitken DA, et al. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstet Gynecol. 2007;109(6):1316–24. doi: 10.1097/01.AOG.0000265804.09161.0d. [DOI] [PubMed] [Google Scholar]
- 112.Parra-Cordero M, Rodrigo R, Barja P, et al. Prediction of early and late pre-eclampsia from maternal characteristics, uterine artery Doppler and markers of vasculogenesis during the first trimester of pregnancy. Ultrasound Obstet Gynecol. 2012 doi: 10.1002/uog.12264. [DOI] [PubMed] [Google Scholar]
- 113.Di LG, Ceccarello M, Cecotti V, et al. First trimester maternal serum PIGF, free beta-hCG, PAPP-A, PP-13, uterine artery Doppler and maternal history for the prediction of preeclampsia. Placenta. 2012;33(6):495–501. doi: 10.1016/j.placenta.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 114.Poon LC, Akolekar R, Lachmann R, Beta J, Nicolaides KH. Hypertensive disorders in pregnancy: screening by biophysical and biochemical markers at 11–13 weeks. Ultrasound Obstet Gynecol. 2010;35(6):662–70. doi: 10.1002/uog.7628. [DOI] [PubMed] [Google Scholar]
- 115.Pedrosa AC, Matias A. Screening for pre-eclampsia: a systematic review of tests combining uterine artery Doppler with other markers. J Perinat Med. 2011;39(6):619–35. doi: 10.1515/jpm.2011.077. [DOI] [PubMed] [Google Scholar]
- 116.Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol. 2007;109(6):1368–74. doi: 10.1097/01.AOG.0000264552.85436.a1. [DOI] [PubMed] [Google Scholar]
- 117.Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol. 2006;195(1):255–9. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 118.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 119.Kusanovic JP, Romero R, Chaiworapongsa T, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22(11):1021–38. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355(10):992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 121.Ohkuchi A, Hirashima C, Matsubara S, et al. Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens Res. 2007;30(2):151–9. doi: 10.1291/hypres.30.151. [DOI] [PubMed] [Google Scholar]
- 122.Powers RW, Jeyabalan A, Clifton RG, et al. Soluble fms-Like tyrosine kinase 1 (sFlt1), endoglin and placental growth factor (PlGF) in preeclampsia among high risk pregnancies. PLoS One. 2010;5(10):e13263. doi: 10.1371/journal.pone.0013263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Srinivas SK, Larkin J, Sammel MD, et al. The use of angiogenic factors in discriminating preeclampsia: are they ready for prime time? J Matern Fetal Neonatal Med. 2010;23(11):1294–300. doi: 10.3109/14767051003677988. [DOI] [PubMed] [Google Scholar]
- 124.Kleinrouweler C, Wiegerinck M, Ris-Stalpers C, et al. Accuracy of circulating placental growth factor, vascular endothelial growth factor, soluble fms-like tyrosine kinase 1 and soluble endoglin in the prediction of pre-eclampsia: a systematic review and meta-analysis. BJOG. 2012 doi: 10.1111/j.1471-0528.2012.03311.x. [DOI] [PubMed] [Google Scholar]
- 125.Smith GC. Researching New Methods of Screening for Adverse Pregnancy Outcome: Lessons from Pre-eclampsia. PLoS Med. 2012;9(7):e1001274. doi: 10.1371/journal.pmed.1001274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99(1):159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 127.Gonzalez RP, Gomez RM, Castro RS, et al. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev Med Chil. 2004;132(10):1155–65. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 128.MacDorman MF, Kirmeyer S. The challenge of fetal mortality. NCHS Data Brief. 2009;(16):1–8. [PubMed] [Google Scholar]
- 129.Albaiges G, Missfelder-Lobos H, Lees C, Parra M, Nicolaides KH. One-stage screening for pregnancy complications by color Doppler assessment of the uterine arteries at 23 weeks’ gestation. Obstet Gynecol. 2000;96:559–64. doi: 10.1016/s0029-7844(00)00946-7. [DOI] [PubMed] [Google Scholar]
- 130.Yu CK, Khouri O, Onwudiwe N, Spiliopoulos Y, Nicolaides KH Fetal Medicine Foundation Second-Trimester Screening Group. Prediction of pre-eclampsia by uterine artery Doppler imaging: relationship to gestational age at delivery and small-for-gestational age. Ultrasound Obstet Gynecol. 2008;31:310–3. doi: 10.1002/uog.5252. [DOI] [PubMed] [Google Scholar]
- 131.Koenker R, Bassett G. Regression quantiles. Econometrica. 1978;46:36–50. [Google Scholar]
- 132.Steyerberg EW, Eijkemans MJ, Harrell FE, Jr, Habbema JD. Prognostic modelling with logistic regression analysis: a comparison of selection and estimation methods in small data sets. Stat Med. 2000;19(8):1059–79. doi: 10.1002/(sici)1097-0258(20000430)19:8<1059::aid-sim412>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 133.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 134.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 135.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation--a workshop report. Placenta. 2005;26 (Suppl A):S114–S117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 136.Jauniaux E, Gulbis B, Tunkel S, Ramsay B, Campbell S, Meuris S. Maternal serum testing for alpha-fetoprotein and human chorionic gonadotropin in high-risk pregnancies. Prenat Diagn. 1996;16(12):1129–35. doi: 10.1002/(SICI)1097-0223(199612)16:12<1129::AID-PD9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 137.Smith GC, Crossley JA, Aitken DA, et al. First-trimester placentation and the risk of antepartum stillbirth. JAMA. 2004;292(18):2249–54. doi: 10.1001/jama.292.18.2249. [DOI] [PubMed] [Google Scholar]
- 138.Spencer K, Yu CK, Cowans NJ, Otigbah C, Nicolaides KH. Prediction of pregnancy complications by first-trimester maternal serum PAPP-A and free beta-hCG and with second-trimester uterine artery Doppler. Prenat Diagn. 2005;25(10):949–53. doi: 10.1002/pd.1251. [DOI] [PubMed] [Google Scholar]
- 139.Dugoff L, Hobbins JC, Malone FD, et al. Quad screen as a predictor of adverse pregnancy outcome. Obstet Gynecol. 2005;106(2):260–7. doi: 10.1097/01.AOG.0000172419.37410.eb. [DOI] [PubMed] [Google Scholar]
- 140.Odibo AO, Sehdev HM, Stamilio DM, Macones GA. Evaluating the thresholds of abnormal second trimester multiple marker screening tests associated with intra-uterine growth restriction. Am J Perinatol. 2006;23(6):363–7. doi: 10.1055/s-2006-947724. [DOI] [PubMed] [Google Scholar]
- 141.Gagnon A, Wilson RD, Audibert F, et al. Obstetrical complications associated with abnormal maternal serum markers analytes. J Obstet Gynaecol Can. 2008;30(10):918–49. doi: 10.1016/S1701-2163(16)32973-5. [DOI] [PubMed] [Google Scholar]
- 142.Olsen RN, Woelkers D, Dunsmoor-Su R, Lacoursiere DY. Abnormal second-trimester serum analytes are more predictive of preterm preeclampsia. Am J Obstet Gynecol. 2012 doi: 10.1016/j.ajog.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 143.Papageorghiou AT, Yu CK, Bindra R, Pandis G, Nicolaides KH. Multicenter screening for pre-eclampsia and fetal growth restriction by transvaginal uterine artery Doppler at 23 weeks of gestation. Ultrasound Obstet Gynecol. 2001;18(5):441–9. doi: 10.1046/j.0960-7692.2001.00572.x. [DOI] [PubMed] [Google Scholar]
- 144.Papageorghiou AT, Leslie K. Uterine artery Doppler in the prediction of adverse pregnancy outcome. Curr Opin Obstet Gynecol. 2007;19(2):103–9. doi: 10.1097/GCO.0b013e32809bd964. [DOI] [PubMed] [Google Scholar]
- 145.Smith GC, Yu CK, Papageorghiou AT, Cacho AM, Nicolaides KH. Maternal uterine artery Doppler flow velocimetry and the risk of stillbirth. Obstet Gynecol. 2007;109(1):144–51. doi: 10.1097/01.AOG.0000248536.94919.e3. [DOI] [PubMed] [Google Scholar]
- 146.Smith GC, Shah I, White IR, Pell JP, Crossley JA, Dobbie R. Maternal and biochemical predictors of antepartum stillbirth among nulliparous women in relation to gestational age of fetal death. BJOG. 2007;114(6):705–14. doi: 10.1111/j.1471-0528.2007.01343.x. [DOI] [PubMed] [Google Scholar]
- 147.MacDorman MF, Kirmeyer S. Fetal and perinatal mortality, United States, 2005. Natl Vital Stat Rep. 2009;57(8):1–19. [PubMed] [Google Scholar]
- 148.Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet Gynecol. 2010;116(2 Pt 1):402–14. doi: 10.1097/AOG.0b013e3181e9322a. [DOI] [PubMed] [Google Scholar]
- 149.Roberge S, Villa P, Nicolaides K, et al. Early administration of low-dose aspirin for the prevention of preterm and term preeclampsia: a systematic review and meta-analysis. Fetal Diagn Ther. 2012;31(3):141–6. doi: 10.1159/000336662. [DOI] [PubMed] [Google Scholar]
- 150.Lee J, Romero R, Dong Z, et al. Unexplained fetal death has a biological signature of maternal anti-fetal rejection: chronic chorioamnionitis and alloimmune anti-human leucocyte antigen antibodies. Histopathology. 2011;59(5):928–38. doi: 10.1111/j.1365-2559.2011.04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Singh T, Leslie K, Bhide A, D’Antonio F, Thilaganathan B. Role of second-trimester uterine artery Doppler in assessing stillbirth risk. Obstet Gynecol. 2012;119(2 Pt 1):256–61. doi: 10.1097/AOG.0b013e318242ad81. [DOI] [PubMed] [Google Scholar]
- 152.Spencer K. Second-trimester prenatal screening for Down syndrome and the relationship of maternal serum biochemical markers to pregnancy complications with adverse outcome. Prenat Diagn. 2000;20(8):652–6. [PubMed] [Google Scholar]
- 153.Yaron Y, Cherry M, Kramer RL, et al. Second-trimester maternal serum marker screening: maternal serum alpha-fetoprotein, beta-human chorionic gonadotropin, estriol, and their various combinations as predictors of pregnancy outcome. Am J Obstet Gynecol. 1999;181(4):968–74. doi: 10.1016/s0002-9378(99)70334-0. [DOI] [PubMed] [Google Scholar]
- 154.Lepage N, Chitayat D, Kingdom J, Huang T. Association between second-trimester isolated high maternal serum maternal serum human chorionic gonadotropin levels and obstetric complications in singleton and twin pregnancies. Am J Obstet Gynecol. 2003;188(5):1354–9. doi: 10.1067/mob.2003.278. [DOI] [PubMed] [Google Scholar]
- 155.Banek CT, Bauer AJ, Gingery A, Gilbert JS. Timing of ischemic insult alters fetal growth trajectory, maternal angiogenic balance and markers of renal oxidative stress in the pregnant rat. Am J Physiol Regul Integr Comp Physiol. 2012 doi: 10.1152/ajpregu.00250.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Law LW, Sahota DS, Chan LW, Chen M, Lau TK, Leung TY. Effect of long-term storage on placental growth factor and fms-like tyrosine kinase 1 measurements in samples from pregnant women. J Matern Fetal Neonatal Med. 2010;23(12):1475–80. doi: 10.3109/14767051003678242. [DOI] [PubMed] [Google Scholar]
- 157.Li Z, Zhang Y, Ying MJ, et al. Recombinant vascular endothelial growth factor 121 attenuates hypertension and improves kidney damage in a rat model of preeclampsia. Hypertension. 2007;50(4):686–92. doi: 10.1161/HYPERTENSIONAHA.107.092098. [DOI] [PubMed] [Google Scholar]
- 158.Thadhani R, Kisner T, Hagmann H, et al. Pilot study of extracorporeal removal of soluble fms-like tyrosine kinase 1 in preeclampsia. Circulation. 2011;124(8):940–50. doi: 10.1161/CIRCULATIONAHA.111.034793. [DOI] [PubMed] [Google Scholar]