Abstract
We have determined the volume and location of hippocampal tissue required for normal acquisition of a spatial memory task. Ibotenic acid was used to make bilateral symmetric lesions of 20-100% of hippocampal volume. Even a small transverse block (minislab) of the hippocampus (down to 26% of the total) could support spatial learning in a water maze, provided it was at the septal (dorsal) pole of the hippocampus. Lesions of the septal pole, leaving 60% of the hippocampi intact, caused a learning deficit, although normal electrophysiological responses, synaptic plasticity, and preserved acetylcholinesterase staining argue for adequate function of the remaining tissue. Thus, with an otherwise normal brain, hippocampal-dependent spatial learning only requires a minislab of dorsal hippocampal tissue.
Full text
PDF


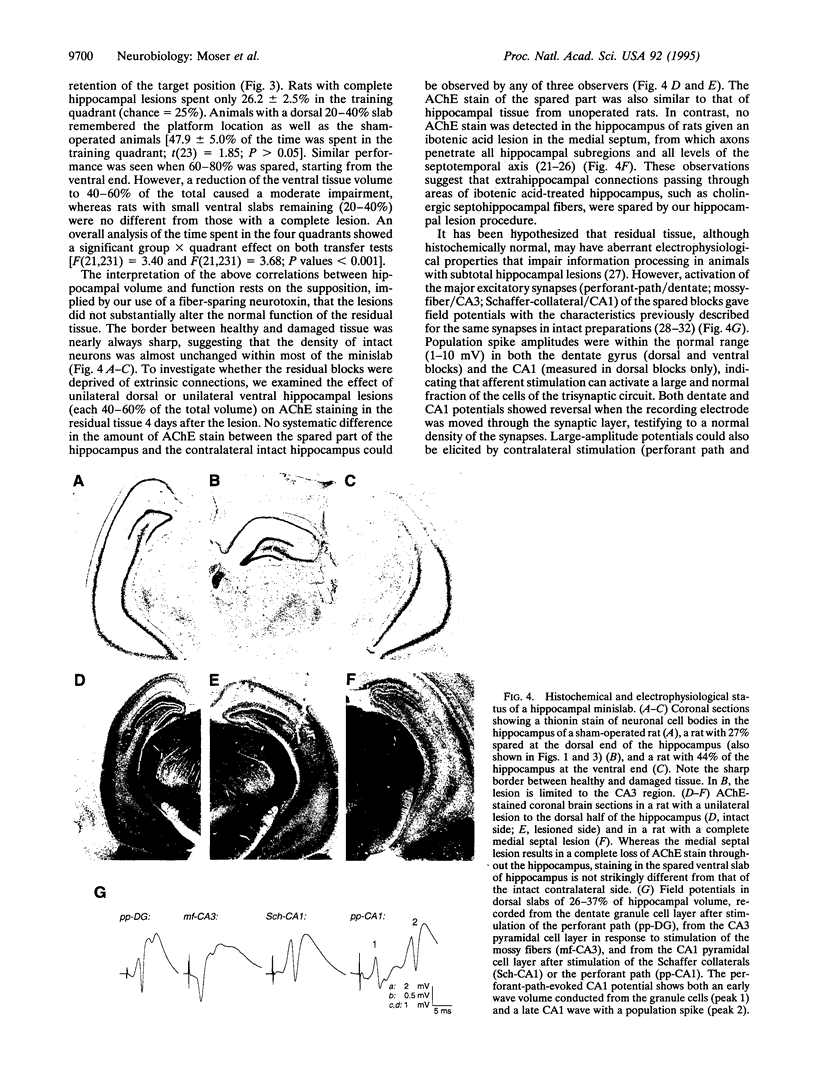

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P. Interhippocampal impulses. II. Apical dendritic activation of CAI neurons. Acta Physiol Scand. 1960 Mar 18;48:178–208. doi: 10.1111/j.1748-1716.1960.tb01858.x. [DOI] [PubMed] [Google Scholar]
- ANDERSEN P. Interhippocampal impulses. III. Basal dendritic activation of CA3 neurons. Acta Physiol Scand. 1960 Mar 18;48:209–230. doi: 10.1111/j.1748-1716.1960.tb01857.x. [DOI] [PubMed] [Google Scholar]
- Amaral D. G., Witter M. P. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31(3):571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Andersen P., Bliss T. V., Skrede K. K. Lamellar organization of hippocampal pathways. Exp Brain Res. 1971;13(2):222–238. doi: 10.1007/BF00234087. [DOI] [PubMed] [Google Scholar]
- Andersen P., Holmqvist B., Voorhoeve P. E. Entorhinal activation of dentate granule cells. Acta Physiol Scand. 1966 Apr;66(4):448–460. doi: 10.1111/j.1748-1716.1966.tb03223.x. [DOI] [PubMed] [Google Scholar]
- Bachevalier J., Mishkin M. Mnemonic and neuropathological effects of occluding the posterior cerebral artery in Macaca mulatta. Neuropsychologia. 1989;27(1):83–105. doi: 10.1016/0028-3932(89)90092-4. [DOI] [PubMed] [Google Scholar]
- Beckstead R. M. Afferent connections of the entorhinal area in the rat as demonstrated by retrograde cell-labeling with horseradish peroxidase. Brain Res. 1978 Aug 25;152(2):249–264. doi: 10.1016/0006-8993(78)90254-8. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deacon T. W., Eichenbaum H., Rosenberg P., Eckmann K. W. Afferent connections of the perirhinal cortex in the rat. J Comp Neurol. 1983 Oct 20;220(2):168–190. doi: 10.1002/cne.902200205. [DOI] [PubMed] [Google Scholar]
- Freund T. F., Antal M. GABA-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature. 1988 Nov 10;336(6195):170–173. doi: 10.1038/336170a0. [DOI] [PubMed] [Google Scholar]
- Frotscher M., Léránth C. Cholinergic innervation of the rat hippocampus as revealed by choline acetyltransferase immunocytochemistry: a combined light and electron microscopic study. J Comp Neurol. 1985 Sep 8;239(2):237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Houser C. R., Crawford G. D., Barber R. P., Salvaterra P. M., Vaughn J. E. Organization and morphological characteristics of cholinergic neurons: an immunocytochemical study with a monoclonal antibody to choline acetyltransferase. Brain Res. 1983 Apr 25;266(1):97–119. doi: 10.1016/0006-8993(83)91312-4. [DOI] [PubMed] [Google Scholar]
- Insausti R., Amaral D. G., Cowan W. M. The entorhinal cortex of the monkey: II. Cortical afferents. J Comp Neurol. 1987 Oct 15;264(3):356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Ishizuka N., Weber J., Amaral D. G. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. J Comp Neurol. 1990 May 22;295(4):580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jarrard L. E. On the role of the hippocampus in learning and memory in the rat. Behav Neural Biol. 1993 Jul;60(1):9–26. doi: 10.1016/0163-1047(93)90664-4. [DOI] [PubMed] [Google Scholar]
- Jarrard L. E. On the use of ibotenic acid to lesion selectively different components of the hippocampal formation. J Neurosci Methods. 1989 Sep;29(3):251–259. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- Jung M. W., McNaughton B. L. Spatial selectivity of unit activity in the hippocampal granular layer. Hippocampus. 1993 Apr;3(2):165–182. doi: 10.1002/hipo.450030209. [DOI] [PubMed] [Google Scholar]
- Jung M. W., Wiener S. I., McNaughton B. L. Comparison of spatial firing characteristics of units in dorsal and ventral hippocampus of the rat. J Neurosci. 1994 Dec;14(12):7347–7356. doi: 10.1523/JNEUROSCI.14-12-07347.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C., Silver A. Confirmation from choline acetylase analyses of a massive cholinergic innervation to the rat hippocampus. J Physiol. 1967 Jul;191(1):215–224. doi: 10.1113/jphysiol.1967.sp008246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. R., Shute C. C. The cholinergic limbic system: projections to hippocampal formation, medial cortex, nuclei of the ascending cholinergic reticular system, and the subfornical organ and supra-optic crest. Brain. 1967 Sep;90(3):521–540. doi: 10.1093/brain/90.3.521. [DOI] [PubMed] [Google Scholar]
- Li X. G., Somogyi P., Ylinen A., Buzsáki G. The hippocampal CA3 network: an in vivo intracellular labeling study. J Comp Neurol. 1994 Jan 8;339(2):181–208. doi: 10.1002/cne.903390204. [DOI] [PubMed] [Google Scholar]
- Lomo T. Potentiation of monosynaptic EPSPs in the perforant path-dentate granule cell synapse. Exp Brain Res. 1971;12(1):46–63. [PubMed] [Google Scholar]
- Morris R. G. M., Schenk F., Tweedie F., Jarrard L. E. Ibotenate Lesions of Hippocampus and/or Subiculum: Dissociating Components of Allocentric Spatial Learning. Eur J Neurosci. 1990;2(12):1016–1028. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Garrud P., Rawlins J. N., O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982 Jun 24;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984 May;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Moser E., Moser M. B., Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993 Sep;13(9):3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R. U., Kubie J. L., Ranck J. B., Jr Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987 Jul;7(7):1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba T., Nakamura T., Grob D. Staining for nerve fiber and cholinesterase activity in fresh frozen sections. Am J Clin Pathol. 1967 Jan;47(1):74–77. doi: 10.1093/ajcp/47.1.74. [DOI] [PubMed] [Google Scholar]
- O'Keefe J., Conway D. H. Hippocampal place units in the freely moving rat: why they fire where they fire. Exp Brain Res. 1978 Apr 14;31(4):573–590. doi: 10.1007/BF00239813. [DOI] [PubMed] [Google Scholar]
- O'Keefe J., Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971 Nov;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976 Apr;51(1):78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O'Keefe J., Speakman A. Single unit activity in the rat hippocampus during a spatial memory task. Exp Brain Res. 1987;68(1):1–27. doi: 10.1007/BF00255230. [DOI] [PubMed] [Google Scholar]
- Olton D. S., Branch M., Best P. J. Spatial correlates of hippocampal unit activity. Exp Neurol. 1978 Feb;58(3):387–409. doi: 10.1016/0014-4886(78)90096-1. [DOI] [PubMed] [Google Scholar]
- Olton D. S., Walker J. A., Gage F. H. Hippocampal connections and spatial discrimination. Brain Res. 1978 Jan 13;139(2):295–308. doi: 10.1016/0006-8993(78)90930-7. [DOI] [PubMed] [Google Scholar]
- Paré D., Llinás R. Non-lamellar propagation of entorhinal influences in the hippocampal formation: multiple electrode recordings in the isolated guinea pig brain in vitro. Hippocampus. 1994 Aug;4(4):403–409. doi: 10.1002/hipo.450040403. [DOI] [PubMed] [Google Scholar]
- Quirk G. J., Muller R. U., Kubie J. L. The firing of hippocampal place cells in the dark depends on the rat's recent experience. J Neurosci. 1990 Jun;10(6):2008–2017. doi: 10.1523/JNEUROSCI.10-06-02008.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Room P., Groenewegen H. J. Connections of the parahippocampal cortex. I. Cortical afferents. J Comp Neurol. 1986 Sep 22;251(4):415–450. doi: 10.1002/cne.902510402. [DOI] [PubMed] [Google Scholar]
- Ruth R. E., Collier T. J., Routtenberg A. Topography between the entorhinal cortex and the dentate septotemporal axis in rats: I. Medial and intermediate entorhinal projecting cells. J Comp Neurol. 1982 Jul 20;209(1):69–78. doi: 10.1002/cne.902090107. [DOI] [PubMed] [Google Scholar]
- Sik A., Ylinen A., Penttonen M., Buzsáki G. Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science. 1994 Sep 16;265(5179):1722–1724. doi: 10.1126/science.8085161. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J. Quantitative histochemistry of acetylcholinesterase in rat hippocampal region correlated to histochemical staining. J Neurochem. 1970 Jun;17(6):739–750. doi: 10.1111/j.1471-4159.1970.tb03344.x. [DOI] [PubMed] [Google Scholar]
- Tamamaki N., Nojyo Y. Disposition of the slab-like modules formed by axon branches originating from single CA1 pyramidal neurons in the rat hippocampus. J Comp Neurol. 1990 Jan 22;291(4):509–519. doi: 10.1002/cne.902910403. [DOI] [PubMed] [Google Scholar]
- Wilson M. A., McNaughton B. L. Dynamics of the hippocampal ensemble code for space. Science. 1993 Aug 20;261(5124):1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Witter M. P., Groenewegen H. J., Lopes da Silva F. H., Lohman A. H. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33(3):161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Witter M. P., Van Hoesen G. W., Amaral D. G. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. J Neurosci. 1989 Jan;9(1):216–228. doi: 10.1523/JNEUROSCI.09-01-00216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]






