Abstract
The serum levels of several metabolites are significantly altered in schizophrenia patients. In this study, we performed a targeted analysis of 34 candidate metabolites in schizophrenia patients (n = 25) and compared them with those in age- and gender-matched healthy subjects (n = 27). Orthogonal partial least square-discriminant analysis revealed that complete separation between controls and patients was achieved based on these metabolites. We found that the levels of γ-glutamylcysteine (γ-GluCys), linoleic acid, arachidonic acid, D-serine, 3-hydroxybutyrate, glutathione (GSH), 5-hydroxytryptamine, threonine, and tyrosine were significantly lower, while D-lactate, tryptophan, kynurenine, and glutamate levels were significantly higher in schizophrenia patients compared to controls. Using receiver operating characteristics (ROC) curve analysis, the sensitivity, specificity, and the area under curve of γ-GluCys, a precursor of GSH, and D-lactate, a terminal metabolite of methylglyoxal, were 88.00%, 81.48%, and 0.8874, and 88.00%, 77.78%, and 0.8415, respectively. In addition, serum levels of D-lactate were negatively correlated with γ-GluCys levels in patients, but not in controls. The present results suggest that oxidative stress-induced damage may be involved in the pathogenesis of schizophrenia.
Introduction
Schizophrenia is a severe psychiatric disease that affects approximately 1% of the world’s population; it is comprised of positive and negative symptoms and cognitive deficits, with an onset during adolescence [1]. Optimal treatment thus requires early detection and suitable medication, which in turn require highly trained psychiatrists. Recently, metabonomic studies have revealed significant alterations in endogenous metabolites, including amino acids, polyunsaturated fatty acids (PUFAs), myo-inositol, and citrate, in the serum of schizophrenia patients [2]–[4]. These alterations reflect the impairment of systemic metabolism in peripheral tissues, and indicate that schizophrenia should be regarded as not only a dysfunction of the central nervous system (CNS), but also as a disorder of systemic metabolism. In previous studies, however, the discriminatory power of metabonomics was found to be heavily dependent on the capacity of the instruments used for analysis; consequently, several different metabolites have been proposed as crucial factors in the pathogenesis of schizophrenia.
In the present study, we focused on metabolites that have previously been suggested to be associated with schizophrenia. For example, the glutamate hypothesis of schizophrenia etiology suggests that endogenous D-serine is a crucial factor related to the hypofunction of the N-methyl-D-aspartate (NMDA) receptor [5]–[7]. Oxidative stress is also associated with schizophrenia [8], and a low level of the antioxidant glutathione (GSH) has been reported in schizophrenia patients [9]. Thus, we selected 34 target metabolites, many of which have been implicated in schizophrenia, and performed quantitative analyses of their levels in serum from schizophrenia patients. Subsequently, we applied a multivariate analysis between controls and the patients, and a correlation matrix approach to determine whether there was any association between these metabolites.
Methods
1. Participants
Table 1 lists the demographic features of the patients and controls that participated in this study. Twenty-five schizophrenia patients (11 men and 14 women) were clinically diagnosed by experienced doctors according to International Classification of Diseases (ICD)-10 criteria and recruited from Sumiyoshi Hospital (Koufu-shi) and the Nanko Clinic of Psychiatry (Shirakawa-shi), both located in Japan; the mean ± SD age of patients was 28.2±4.4 years. All were medicated outpatients, treated with olanzapine (60%; n = 15), aripiprazole (40%, n = 10), risperidone long acting injection (16%, n = 4), levomepromazine (8%; n = 2), blonanserin (8%; n = 2), paliperidone (8%; n = 2), quetiapine (4%; n = 1), fluphenazine (4%; n = 1), risperidone (4%; n = 1), or sulpiride (4%; n = 1). Eleven patients (44%) had taken more than 2 kinds of antipsychotics. Age-matched healthy human volunteers (n = 27), including 12 men and 15 women with a mean ± SD age of 26.5±5.6 years, comprised the control group.
Table 1. Characteristics of subjects.
| Controls | Patients | p value | |||
| Number of subjects | 27 | 25 | |||
| Gender (male/female) | 12/15 | 11/14 | 0.8065 | ||
| Age (year) | 26.5±5.6 | (18–37) | 28.2±4.4 | (21–35) | 0.251 |
| Onset age (year) | 21.4±4.9 | (15–30) | |||
| Duration of illness (month) | 84.5±57.8 | (1–240) | |||
| Chlorpromazine equivalent (mg) | 605±345 | (150–1455) | |||
| Diabetes mellitus (Y/N) | 0/27 | 0/25 | |||
| Smoker/nonsmoker | 1/26 | 10/15 | <0.001 | ||
| BMI (kg/m2) | 21.4±2.8 | (17.4–27.8) | 24±4.1 | (14.4–31.2) | 0.014 |
The comparison between 2 groups was performed using the χ2 test for gender difference and smoking, and the Student’s t-test for age and body mass index (BMI).
Ethics statement: Written informed consent was obtained from all subjects prior to participation, and the protocol was approved by the ethics committee in the Faculty of Pharmaceutical Sciences, Toho University (approval number 23-1, 24-6). The capacities of all patients to provide consent were established by psychiatrists with more than 10 years’ experience. In this study, there were no surrogate consent procedures required.
2. Blood sampling
Five milliliters of blood was drawn from the arm vein of participants at 11∶00 AM–12∶00 PM before lunch to avoid the effect of food intake wherever possible, and collected in Venoject-II AUTOSEP tubes (Terumo Corporation; Tokyo, Japan); samples were allowed to stand for 30 min at room temperature, consistent with a previous procedure for serum D-serine analysis [6], [10]. Tubes were then centrifuged at 1200×g for 15 min. The serum obtained was divided into aliquots of 100 µL each in screw-capped vials, which were stored at –80°C.
3. Determination of targeted metabolites in serum
A total of 34 target metabolites were examined: L-tryptophan (Trp), L-kynurenine (Kyn), kynurenic acid (KYNA), 5-hydroxytryptamine (5-HT), D-serine (D-Ser), D-lactate, L-lactate, 3-hydroxybutyrate (3-HB), GSH, γ-glutamylcysteine (GluCys), cysteine (Cys), cysteinylglycine (CysGly), L-amino acids (His, Arg, Gln, Ser, Asn, Glu, Gly, Pro, Thr, Ala, Leu, Ile, Val, Phe, Lys, and Tyr), fatty acids [linoleic acid (LA), arachidonic acid (AA), oleic acid, linolenic acid, and palmitoleic acid], and glucose. The serum levels of these metabolites were determined by using high-performance liquid chromatography (HPLC) methods. An HPLC with fluorescence detection method was used for quantification of L-amino acids [11]–[13], thiol compounds [14], D- and L-Ser [6], [15], D- and L-lactate [16], 3-HB [17], [18], fatty acids [19], and KYNA [20], [21].
Serum Trp and Kyn levels were determined using an HPLC with mass spectrometric detection method as described previously [22], and 5-HT levels were determined using HPLC-electrochemical detection [23]. Glucose levels were determined using a commercial kit (Wako; Osaka, Japan). These methods have already been validated at the serum level for each metabolite.
4. Statistical analyses
The non-parametric Mann-Whitney U-test (2-tailed) was performed for statistical analysis of metabolite concentrations, with p<0.05 considered significant. The Spearman rank correlation test was performed to determine statistical correlations between variables.
For multivariate analysis, the collected data were imported to SIMCA 13.0.3 software (Umetrics; Umea, Sweden), and an orthogonal partial least square-discrimination analysis (OPLS-DA) was carried out. The statistics, R 2 (cumulative) and Q 2 (cumulative), were calculated by OPLS-DA. R 2 and Q 2 represent explanatory variable estimating the goodness of fit of the model and predictor, which is the 7-fold cross-validated predictive ability, respectively. The statics range (minimum–maximum) is 0–1. Receiver operating characteristics (ROC) curve tests for metabolites were performed using GraphPad Prism 6 (GraphPad Software, Inc.; La Jolla, CA, USA).
Results
1. Subject characteristics
For the purpose of early detection of schizophrenia, young patients, aged 21–35 years, were enrolled in the present study. In addition, age- and gender-matched healthy subjects were enrolled as controls. As shown in Table 1, no significant difference in sex ratio or age was observed between controls and patients (p = 0.807 and p = 0.251, respectively).
2. Discrimination of controls and patients
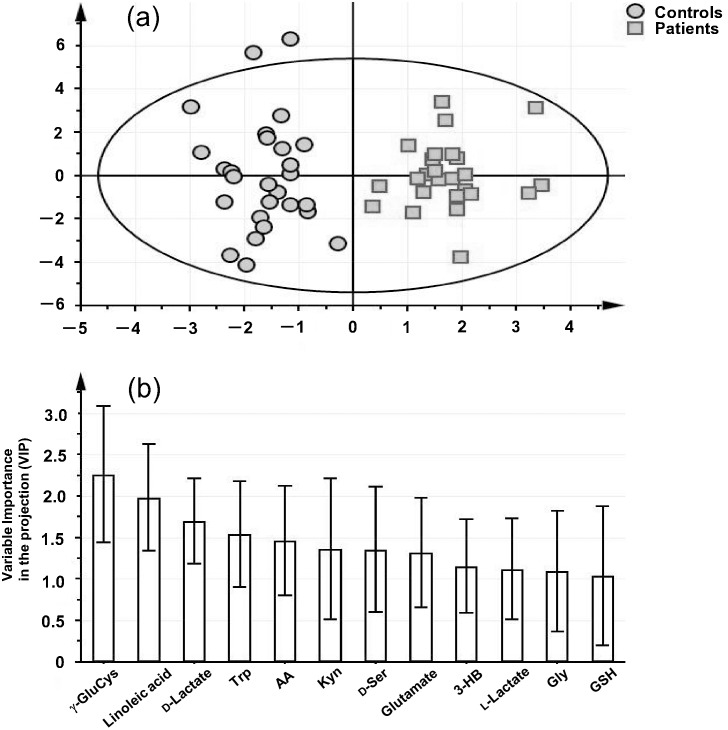
A total of 34 serum metabolites were quantified from controls and from schizophrenia patients. A multivariate analysis, OPLS-DA, was then carried out, and the results are shown in Fig. 1a. Clear separation between controls and patients was achieved with an R2 (cumulative) and a Q2 (cumulative) of 0.8606 and 0.7203, respectively. For Q2, a predictor calculated by cross-validation, a value above 0.4 indicates that the OPLS-DA model is reliable; these results showed that patients could be discriminated from controls using these serum metabolite levels. Among the 34 compounds tested, 12 had a variable importance in the projection (VIP; an indicator of discriminatory power) value above 1.0; these were γ-GluCys, LA, D-lactate, Trp, AA, Kyn, D-Ser, Glu, 3-HB, L-lactate, Gly, and GSH (Fig. 1b). The relatively large VIP values of γ-GluCys (2.26), LA (1.98), and D-lactate (1.70) showed that these metabolites were the main contributors to the patient-group separation in this study.
Figure 1. Score plot derived from orthogonal partial least square-discriminant analysis (OPLS-DA) based on the serum levels of 34 targeted metabolites in the subjects (a), and variable importance in the projection (VIP) data of the compounds with VIP values above 1.0 (b).
3. Comparison of serum levels between controls and patients
The serum level results are categorized into 5 separate tables (Tables S1, S2, S3, S4, and S5). The metabolites for which serum levels differed between controls and schizophrenia patients are listed in Table 2. With regard to serum amino acids, the levels of Glu (p = 0.0145), D-Ser (p = 0.00163), Tyr (p = 0.0469), and Thr (p = 0.0286) were significantly altered between patients and controls. Serum levels of D-lactate (p = 2.43×10−5) and 3-HB (p = 0.00438) were significantly different between the patient and control groups. The analysis of thiol compounds and fatty acids, respectively, revealed that levels of GSH (p = 0.0344), γ-GluCys (p = 1.75×10−6), LA (p = 1.61×10−5), and AA (p = 0.00149) were significantly altered in patients. With regard to Trp metabolites, serum levels of Trp (p = 0.00101), Kyn (p = 0.00568), and 5-HT (p = 0.0131), but not KYNA (p = 0.6145), were significantly altered.
Table 2. Serum levels of metabolites (µmol/L) that were differentially expressed between controls and schizophrenia patients.
| Compound | Levels in serum | ||
| Controls (n = 27) | Schizophrenia patients (n = 25) | p value* | |
| γ–Glutamylcysteine | 3.05±0.11 | 2.07±0.12 | 1.746E-06a |
| Linoleic acid | 264±17.0 | 159±12.0 | 1.610E-05a |
| D-Lactate | 7.26±0.481 | 13.1±1.42 | 2.426E-05a |
| L-Tryptophan | 81.3±3.36 | 101±4.48 | 0.001011a |
| Arachidonic acid | 55.4±4.48 | 38.5±2.18 | 0.001485 |
| L-Kynurenine | 1.74±0.121 | 2.35±0.162 | 0.005676 |
| D-Serine | 1.88±0.07 | 1.57±0.07 | 0.00163 |
| Glutamate | 35.5±3.20 | 71.3±12.3 | 0.0145 |
| 3-Hydroxybutyrate | 46.5±10.1 | 17.6±3.84 | 0.004378 |
| Glutathione | 3.67±0.241 | 3.03±0.149 | 0.03437 |
| 5-Hydroxytryptamine | 0.85±0.077 | 0.61±0.096 | 0.01308 |
| Threonine | 126±7.44 | 111±10.3 | 0.0286 |
| Tyrosine | 65.1±2.55 | 57.2±3.33 | 0.0469 |
Only p values below 0.05 are included in this table.
*Non-parametric Mann-Whitney U-test.
Significantly different following Bonferroni correction (<0.05/34).
In summary, the serum levels of 9 compounds, γ-GlyCys, LA, AA, D-Ser, 3-HB, GSH, 5-HT, Thr, and Tyr, were significantly decreased, whereas those of 4 compounds, D-lactate, Trp, Kyn, and Glu, were significantly increased in patients compared to controls.
When a Bonferroni correction was applied, levels of 4 metabolites (γ-GluCys, LA, D-lactate, and Trp) were still significantly different between control and patient groups, while the following metabolites were present at similar levels in both groups: AA, Kyn, D-Ser, Glu, 3-HB, GSH, 5-HT, Thr, and Tyr (Table 2).
Similar to a previous study [10], there was a higher number of smokers among enrolled schizophrenia patients than controls (Table 1). There were no significant differences in the serum levels of metabolites between smokers and non-smokers with the exception of γ-GluCys (p = 0.035) (Table S6). Regarding γ-GluCys, however, significant differences between control and patient groups were observed in both non-smoking (p = 5.71E-07) and smoking patients (p = 0.0152, Bonferroni test).
4. ROC curve analysis
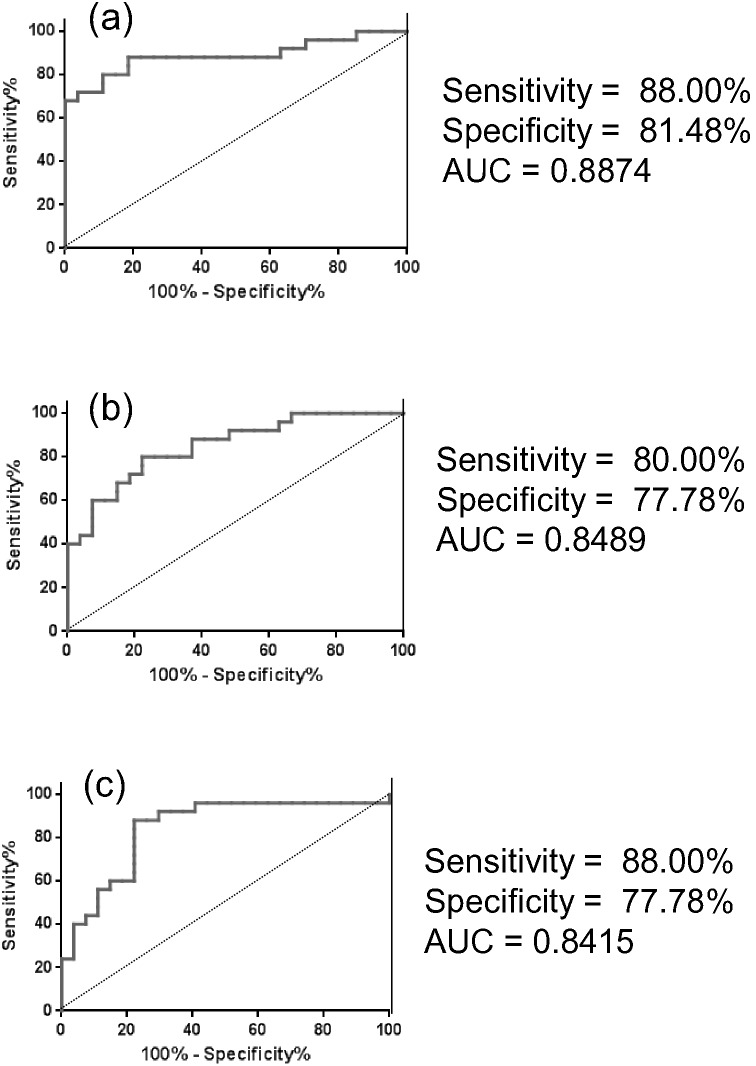
The ROC curves of serum levels of γ-GluCys, LA, and D-lactate, which displayed relatively large VIP values, are shown in Fig. 2. The AUC values for these metabolites were 0.8874, 0.8489, and 0.8415, respectively. The sensitivity and specificity at the cut-off points for γ-GluCys, LA, and D-lactate were 88.00 and 81.48%, 80.00 and 77.78%, and 88.00 and 77.78%, respectively.
Figure 2. Receiver operating characteristic (ROC) curve analyses of γ-GluCys (a), lenoleic acid (b), and D-lactate (c).
5. Correlations of the serum levels in controls and patients
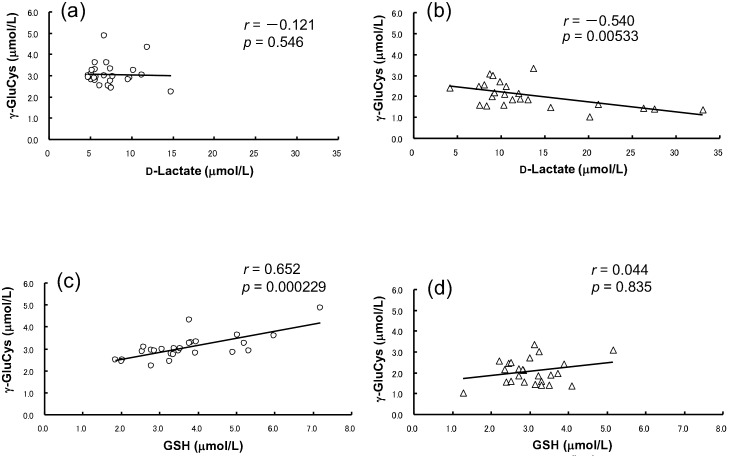
Among the 13 compounds that discriminated the control and patient groups (Table 2), a negative correlation between D-lactate and γ-GluCys levels was observed (p = 0.00533, r = –0.5400) in patients (Fig. 3b), but not in controls (Fig. 3a). A positive correlation between γ-GluCys and GSH was observed in controls (r = 0.652, p = 0.000229) (Fig. 3c), but not in patients (r = 0.044, p = 0.8351) (Fig. 3d).
Figure 3. Correlation plots of D-lactate and γ-GluCys serum levels in controls (a) and in patients with schizophrenia (b), and between GSH and γ-GluCys in controls (c) and in patients with schizophrenia (d).
Serum Tyr and 5-HT levels in patients were significantly correlated with daily chlorpromazine (CP) equivalents, and the levels of D-Ser, Glu, 3-HB, AA, and LA were positively correlated with duration of illness (Table 3).
Table 3. Correlations between serum levels of metabolites and CP equivalent (mg) or duration of illness (months).
| Tyr | 5-HT | ||||
| CP equivalent | r = –0.488 | r = 0.397 | |||
| (mg) | (p = 0.0133) | (p = 0.0491) | |||
| D-Ser | Glu | 3-HB | Arachidonic acid | Linoleic acid | |
| Duration of illness | r = 0.478 | r = 0.397 | r = 0.540 | r = 0.630 | r = 0.415 |
| (months) | (p = 0.0157) | (p = 0.0491) | (p = 0.0064) | (p = 0.0007) | (p = 0.0390) |
Discussion
Our data revealed that analysis of 34 serum metabolites was sufficient to discriminate between controls and schizophrenia patients by OPLS-DA (Fig. 1 (a)), and that the decreased levels of γ-GluCys and increased levels of D-lactate were the major contributors to this discrimination (Fig. 1 (b)). To the best of our knowledge, there have been no reports to date showing that serum levels of γ-GluCys or D-lactate are altered in schizophrenia patients. By analyses of ROC curves (Fig. 2), the serum levels of these metabolites revealed sufficient AUC values (0.8874 and 0.8415) to discriminate patients from controls. The sensitivity and selectivity values were also satisfactory (Fig. 2).
Oxidative stress may be involved in the pathogenesis of schizophrenia [8], [24]. On the other hand, carbonyl stress, such as the glycation and inactivation of proteins by the dicarbonyl compound methylglyoxal (MGO), is involved in diabetic complications [25], [26]. In the glyoxalase pathway, glyoxalase I converts MGO to S-lactoylglutathione, and subsequently, S-lactoylglutathione is converted to D-lactate by glyoxalase II [25] (Fig. S1). Increased d-lactate levels have been found in diabetes mellitus, likely due to enhanced MGO production [25]. Indeed, we previously reported a significant increase in the serum d-lactate level in diabetic patients [16]. Recently, it was reported that carbonyl stress was enhanced in a subpopulation of schizophrenia patients due to a decline in glyoxalase I activity [27]. Therefore, a reduction in glyoxalase I activity would be expected to lead to a lower serum D-lactate level. However, our current data show that the serum D-lactate level was significantly increased in patients. Although further experiments are required, we suggest that increased activity of glyoxalase II in patients may underlie the increased D-lactate level.
Another finding in the present study is that the serum γ-GluCys level was remarkably reduced in schizophrenia patients. γ-GluCys is a precursor of an endogenous antioxidant, GSH, and is produced from Glu and Cys by glutamatecysteine ligase (GCL; Fig. S2). GCL activity is a rate-limiting enzyme for GSH synthesis, and genetic polymorphisms in GCL significantly modulate schizophrenia risk [28], [29]. As shown in Table 2, the low GSH level observed in patients is consistent with previous reports [9], [30]. However, to the best of our knowledge, we are the first to report a decreased level of the GSH precursor γ-GluCys in the sera of schizophrenia patients. In addition, the level of Glu, which is a precursor of γ-GluCys, was significantly increased in patients (Table 2), suggesting that GCL activity might also be attenuated. The significant decrease in the GSH level of patients indicates that anti-oxidant defenses against reactive oxygen species (ROS) and reactive nitrogen species (RNS) may be compromised in schizophrenia. Interestingly, a significant negative correlation between D-lactate and γ-GluCys was observed specifically in the patients’ sera (Fig. 3b). Since S-lactoylglutathione is metabolized by glyoxalase II to release D-lactate and GSH (Fig. S2), elevated GSH levels would be expected in patient serum; however, we observed low serum GSH levels in patients (Table 2). In addition, a positive correlation between serum γ-GluCys and GSH levels was observed specifically in controls (Fig. 3c and d). Taken together, these data suggest that reduced GSH levels in schizophrenia patients may be due to the rapid consumption of GSH during its anti-ROS or -RNS activities.
Recently, an increased 3-HB level was observed in serum from schizophrenia patients in a metabonomics study [2]. Conversely, Cai et al. (2012) reported a decreased 3-HB level in the serum of patients. As shown in Table 2, we found that 3-HB levels were reduced in patient serum. As a ketone body, 3-HB is produced from acetoacetate, which in turn is derived from fatty acids (Fig. S3). 3-HB levels increase as a result of fasting and in diabetic subjects due to limited utilization of glucose; under these conditions, there is instead enhanced utilization of ketone bodies for energy production. A recent report showed that 3-HB-dependent increases in transcription of the oxidative stress resistance factors FOXO3A and MT2 constitute an anti-oxidant pathway [31]. Thus, depletion of endogenous 3-HB in patients may induce oxidative stress, which is implicated in schizophrenia etiology [8].
Regarding PUFAs, we found that serum AA and LA levels were significantly decreased in patients (Table 2). These results are consistent with previous reports of low PUFA levels in schizophrenia patients [32]. LA is initially obtained from the daily diet, and is then converted to AA by elongase and desaturase (Fig. S3). Considering these facts, it is likely that the reduction in PUFA levels in schizophrenia patients is caused by their excess oxidative stress. In addition, it has been reported that increased tissue expression of fatty acid binding protein (FABP) is closely associated with the etiology of schizophrenia [33], and both LA and AA are bound by FABP7 [33], [34]. Therefore, FABP7-dependent sequestering of circulating PUFAs in biological fluids might also contribute to reduced serum PUFA levels in patients.
In the present study, we examined Trp and its metabolites, Kyn, KYNA, and 5-HT, since the associated metabolic pathways produce several neuroactive substances [35], [36]. Our data revealed that serum Trp and Kyn levels were significantly increased in patients (Table 2). Trp is metabolized to Kyn by tryptophan 2,3-dioxygenase or indoleamine 2,3-dioxygenase in the Kyn pathway, and to 5-HT by tryptophan hydrolase (TH) and aromatic amino acid decarboxylase (AAD) in the serotonin pathway (Fig. S4) [37], [38]. Since only medicated patients were enrolled in the present study, our present results regarding serum Trp levels are consistent with the report of Xuan et al. [4], who found increased Trp levels in the serum of medicated patients. By contrast, the 5-HT level was significantly decreased in patients compared to controls (Table 2). Therefore, activities of TH or AAD might also be decreased in patients. 5-HT is further metabolized to melatonin, which possesses anti-oxidant activity [36], [39]. We speculate that the decreased level of 5-HT will lead to lower melatonin levels, and thus compromised antioxidant defenses, in patients.
Conversely, the serum KYNA level was not altered significantly between controls and patients (p = 0.6145), although increased KYNA levels in the post-mortem brain tissue [40] or cerebrospinal fluids [41] from schizophrenia patients have been reported. KYNA acts as an endogenous antagonist of the glycine site of the NMDA receptor. However, we found no alteration in the serum KYNA levels of patients, indicating that increased KYNA levels might occur only in the CNS. By contrast, we did observe a significant decrease in serum levels of D-serine, a co-agonist of the glycine site of the NMDA receptor [5], [42] (Table 2), which is consistent with previous reports [6], [10]. Genetic studies of schizophrenia show that the susceptibility gene G72 plays a role in regulation of D-amino acid oxidase (DAO), an enzyme that is upregulated in schizophrenia [43], [44]. Thus, high-level expression of DAO in several tissues, including the brain, liver, and kidney, may underlie the observed decreased serum D-serine level in patients.
In this study, we were only able to include medicated patients, as recruitment of drug-naïve patients was not possible. Therefore, we cannot exclude the potential effects of medication in the interpretation of our results. Serum Tyr and 5-HT levels were significantly correlated with daily CP, and the levels of both metabolites were significantly lower in patients. The negative correlation between the serum Tyr level and CP equivalent is likely due to a medication-induced effect. Tyr is produced from Phe by phenylalanine hydroxylase (PH), and is then converted to catecholamines (e.g., dopamine and noradrenaline) by several enzymes such as tyrosine hydrolase and dopa decarboxylase. Since the serum level of Phe was not altered (Table S1), the involvement of decreased activity of PH or enhanced enzyme activities of catecholamines biosynthesis might occur in patients.
By contrast, the serum 5-HT level was positively correlated with CP equivalents. 5-HT is sensitive to oxidation; thus, elevated ROS or RNS in patients could lead to non-enzymatic decomposition of 5-HT in vivo. The second generation antipsychotics (SGA) aripiprazole and quetiapine have indirect anti-oxidative effects via induction of the anti-oxidative enzyme, superoxide dismutase [45]. Thus, the positive correlation between 5-HT levels and CP equivalents may be due to the SGA-dependent suppression of 5-HT oxidation.
The serum levels of D-Ser, Glu, 3-HB, AA, and LA exhibited a significant positive correlation with duration of illness (Table 3). Since the serum Glu level is reportedly increased even in drug-naïve patients [46], the effect of medication on the Glu level might be negligible. Calcia et al. [10] reported that plasma D-Ser levels were lower in non-medicated than in medicated patients. Consistent with this, D-Ser levels were higher in patients with a longer history of recorded illness, suggesting that sustained medication use may increase D-Ser levels. Indeed, we observed that SGAs possess DAO-inhibitory activity in vitro (data not shown). Furthermore, the anti-oxidant effects of SGAs [45] may explain why prolonged treatment of patients restores the levels of 3-HB, AA, and LA, as each of these metabolites are sensitive to oxidative stress.
There are nonetheless limitations to the current study. In the present study, blood samples were not collected from subjects in the early morning in a fasting state. Therefore, future studies should be performed under this condition to exclude the possibility that feeding is a confounding variable. Next, we only studied relatively young patients (≤35 years old), and we therefore cannot extrapolate our findings to a broader patient range. In addition, all patients were medicated, and it is therefore important to gather information from drug-naïve patients in future studies.
Conclusion
The present finding that the serum levels of 13 metabolites may be differentially regulated in schizophrenia patients extends our knowledge of the pathophysiology of the disease. Building upon this study, future investigations could identify which metabolites are suitable biomarkers for the early detection and prognosis of schizophrenia and its associated treatment regimens.
Supporting Information
D-Lactate-associated biosynthetic and metabolic pathways. Rectangles denote the quantified compound, and bold red rectangles denote metabolites that were differentially altered in patients compared to controls. Red upward or downward arrows indicate that the level increased or decreased, respectively.
(TIF)
Relevant biosynthetic and metabolic pathways associated with GSH. Rectangles denote quantified compounds, and bold red rectangles denote the metabolites that were differentially altered in the serum of patients versus controls. Red upward or downward arrows indicate that the level increased or decreased, respectively.
(TIF)
Relevant biosynthetic and metabolic pathway of PUFAs. Rectangles denotes the compounds that were quantified, and bold red rectangles denote the metabolites that differed between patients and controls. Red downward arrow means that the level decreased.
(TIF)
Relevant metabolic pathway of Trp. Rectangle denotes compounds quantified and bold red rectangle denotes the compounds whose levels were altered in patients compared to healthy controls. Red upward or downward arrows indicate that the level increased or decreased, respectively.
(TIF)
Serum levels of amino acids (including D-serine) in controls and patients with schizophrenia.
(XLS)
Serum levels of glucose, D-lactate, L-lactate, and 3-hydroxybutyrate (3-HB) in controls and patients with schizophrenia.
(XLS)
Serum levels of thiol compounds in controls and patients with schizophrenia.
(XLS)
Serum levels of unsaturated fatty acids in controls and patients with schizophrenia.
(XLS)
Serum levels of Trp and its metabolites in controls and patients with schizophrenia.
(XLS)
Serum levels of metabolites (µmol/L) in smoking and non-smoking schizophrenia patients.
(XLS)
Acknowledgments
The authors thank emeritus Prof. C. Nishimura, Toho University, for his kind and helpful advice on the statistical analyses, Drs. H. Yamada and S. Iwasa, Toho University, for their valuable discussions during this research, Mr. H. Ohashi, Mr. K. Suzuki, Miss M. Koshikawa, and Miss M. Kume, Toho University, for their technical assistance, and Mr. T. Takaku of the Himorogi group for his kind management of blood sampling from patients. The authors also thank all the participants who provided blood samples.
Funding Statement
This research was financially supported by the Japan Society for the Promotion of Science, Grant-in-Aid for Scientific Research (C) Grant Numbers 23617027 and 25460224, and the Faculty of Pharmaceutical sciences, Toho University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Freedman R (2003) Schizophrenia. N Engl J Med 349: 1738–1749. [DOI] [PubMed] [Google Scholar]
- 2. Yang J, Chen T, Sun L, Zhao Z, Qi X, et al. (2013) Potential metabolite markers of schizophrenia. Mol Psychiatry 18: 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cai H-L, Li H-D, Yan X-Z, Sun B, Zhang Q, et al. (2012) Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naive schizophrenia patients after treatment with risperidone. J Proteome Res 11: 4338–4350. [DOI] [PubMed] [Google Scholar]
- 4. Xuan J, Pan G, Qiu Y, Yang L, Su M, et al. (2011) Metabolomic profiling to identify potential serum biomarkers for schizophrenia and risperidone action. J Proteome Res 10: 5433–5443. [DOI] [PubMed] [Google Scholar]
- 5. Schell M, Brady R, Molliver M, Snyder S (1997) D-Serine as a neuromodulator: Regional and developmental localizations in rat brain glia resemble NMDA receptors. J Neurosci 17: 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, et al. (2003) Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry 60: 572–576. [DOI] [PubMed] [Google Scholar]
- 7. Hashimoto K, Shimizu E, Iyo M (2005) Dysfunction of glia-neuron communication in pathophysiology of schizophrenia. Curr Psychiatry Rev 1: 151–163. [Google Scholar]
- 8. Bitanihirwe BKY, Woo T-UW (2011) Oxidative stress in schizophrenia: An integrated approach. Neurosci Biobehav Rev 35: 878–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Raffa M, Mechri A, Ben Othman L, Fendri C, Gaha L, et al. (2009) Decreased glutathione levels and antioxidant enzyme activities in untreated and treated schizophrenic patients. Prog Neuro-Psychopharmacol Biol Psychiatry 33: 1178–1183. [DOI] [PubMed] [Google Scholar]
- 10. Calcia Marilia A, Madeira C, Alheira Flavio V, Silva Thuany CS, Tannos Filippe M, et al. (2012) Plasma levels of D-serine in Brazilian individuals with schizophrenia. Schizophr Res 142: 83–87. [DOI] [PubMed] [Google Scholar]
- 11. Tomiya M, Fukushima T, Kawai J, Aoyama C, Mitsuhashi S, et al. (2006) Alterations of plasma and cerebrospinal fluid glutamate levels in rats treated with the N-methyl-D-aspartate receptor antagonist, ketamine. Biomed Chromatogr 20: 628–633. [DOI] [PubMed] [Google Scholar]
- 12. Tomiya M, Fukushima T, Watanabe H, Fukami G, Fujisaki M, et al. (2007) Alterations in serum amino acid concentrations in male and female schizophrenic patients. Clin Chim Acta 380: 186–190. [DOI] [PubMed] [Google Scholar]
- 13. Aoyama C, Santa T, Tsunoda M, Fukushima T, Kitada C, et al. (2004) A fully automated amino acid analyzer using NBD-F as a fluorescent derivatization reagent. Biomed Chromatogr 18: 630–636. [DOI] [PubMed] [Google Scholar]
- 14. Isokawa M, Funatsu T, Tsunoda M (2013) Fast and simultaneous analysis of biothiols by high-performance liquid chromatography with fluorescence detection under hydrophilic interaction chromatography conditions. Analyst (Cambridge, U K) 138: 3802–3808. [DOI] [PubMed] [Google Scholar]
- 15. Fukushima T, Kawai J, Imai K, Toyo’oka T (2004) Simultaneous determination of D- and L-serine in rat brain microdialysis sample using a column-switching HPLC with fluorimetric detection. Biomed Chromatogr 18: 813–819. [DOI] [PubMed] [Google Scholar]
- 16. Hasegawa H, Fukushima T, Lee J-A, Tsukamoto K, Moriya K, et al. (2003) Determination of serum D-lactic and L-lactic acids in normal subjects and diabetic patients by column-switching HPLC with pre-column fluorescence derivatization. Anal Bioanal Chem 377: 886–891. [DOI] [PubMed] [Google Scholar]
- 17. Tsai Y, Liao T, Lee J (2003) Identification of L-3-hydroxybutyrate as an original ketone body in rat serum by column-switching high-performance liquid chromatography and fluorescence derivatization. Anal Biochem 319: 34–41. [DOI] [PubMed] [Google Scholar]
- 18. Hsu WY, Kuo CY, Fukushima T, Imai K, Chen CM, et al. (2011) Enantioselective determination of 3-hydroxybutyrate in the tissues of normal and streptozotocin-induced diabetic rats of different ages. J Chromatogr B 879: 3331–3336. [DOI] [PubMed] [Google Scholar]
- 19. Prados P, Fukushima T, Santa T, Homma H, Tsunoda M, et al. (1997) 4-N,N-Dimethylaminosulfonyl-7-N-(2-aminoethyl)amino-benzofurazan as a new precolumn fluorescence derivatization reagent for carboxylic acids (fatty acids and drugs containing a carboxyl moiety) in liquid chromatography. Anal Chim Acta 344: 227–232. [Google Scholar]
- 20. Fukushima T, Mitsuhashi S, Tomiya M, Iyo M, Hashimoto K, et al. (2007) Determination of kynurenic acid in human serum and its correlation with the concentration of certain amino acids. Clin Chim Acta 377: 174–178. [DOI] [PubMed] [Google Scholar]
- 21. Mitsuhashi S, Fukushima T, Kawai J, Tomiya M, Santa T, et al. (2006) Improved method for the determination of kynurenic acid in rat plasma by column-switching HPLC with post-column fluorescence detection. Anal Chim Acta 562: 36–43. [Google Scholar]
- 22. Ohashi H, Iizuka H, Yoshihara S, Otani H, Kume M, et al. (2013) Determination of l-tryptophan and l-kynurenine in human serum by using LC-MS after derivatization with (R)-DBD-PyNCS. Int J Tryptophan Res 6: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hubbard KE, Wells A, Owens TS, Tagen M, Fraga CH, et al. (2010) Determination of dopamine, serotonin, and their metabolites in pediatric cerebrospinal fluid by isocratic high performance liquid chromatography coupled with electrochemical detection. Biomed Chromatogr 24: 626–631. [DOI] [PubMed] [Google Scholar]
- 24. Yao JK, Keshavan MS (2011) Antioxidants, redox signaling, and pathophysiology in schizophrenia: An integrative view. Antioxid Redox Signaling 15: 2011–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thornalley PJ (1994) Methylglyoxal, glyoxalases and the development of diabetic complications. Amino Acids 6: 15–23. [DOI] [PubMed] [Google Scholar]
- 26. Kalapos M (1999) Methylglyoxal in living organisms - Chemistry, biochemistry, toxicology and biological implications. Toxicol Lett 110: 145–175. [DOI] [PubMed] [Google Scholar]
- 27. Arai M, Yuzawa H, Nohara I, Ohnishi T, Obata N, et al. (2010) Enchanced carbonyl stress in a subpopulation of schizophrenia. Arch Gen Psychiatry 67: 589–597. [DOI] [PubMed] [Google Scholar]
- 28. Gysin R (2007) Impaired glutathione synthesis in schizophrenia: convergent genetic and functional evidence. Proc Natl Acad Sci U S A 104: 16621–16626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nichenarnetla SN, Ellison I, Calcagnotto A, Lazarus P, Muscat JE, et al. (2008) Functional significance of the GAG trinucleotide-repeat polymorphism in the gene for the catalytic subunit of gamma-glutamylcysteine ligase. Free Radical Biol Med 45: 645–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gawryluk JW, Wang J-F, Andreazza AC, Shao L, Young LT (2011) Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14: 123–130. [DOI] [PubMed] [Google Scholar]
- 31. Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, et al. (2013) Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science (Washington, DC, U S) 339: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ramos-Loyo J, Medina-Hernandez V, Estarron-Espinosa M, Canales-Aguirre A, Gomez-Pinedo U, et al. (2013) Sex differences in lipid peroxidation and fatty acid levels in recent onset schizophrenia. Prog Neuro-Psychopharmacol Biol Psychiatry 44: 154–161. [DOI] [PubMed] [Google Scholar]
- 33. Maekawa M, Owada Y, Yoshikawa T (2011) Role of polyunsaturated fatty acids and fatty acid binding protein in the pathogenesis of schizophrenia. Curr Pharm Des 17: 168–175. [DOI] [PubMed] [Google Scholar]
- 34. Iwayama Y, Hattori E, Maekawa M, Yamada K, Toyota T, et al. (2010) Association analyses between brain-expressed fatty-acid binding protein (FABP) genes and schizophrenia and bipolar disorder. Am J Med Genet, Part B 153B: 484–493. [DOI] [PubMed] [Google Scholar]
- 35. Myint AM (2012) Kynurenines: from the perspective of major psychiatric disorders. FEBS J 279: 1375–1385. [DOI] [PubMed] [Google Scholar]
- 36. Yao JK, Dougherty GG Jr, Reddy RD, Keshavan MS, Montrose DM, et al. (2010) Altered interactions of tryptophan metabolites in first-episode neuroleptic-naive patients with schizophrenia. Mol Psychiatry 15: 938–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moroni F (1999) Tryptophan metabolism and brain function: focus on kynurenine and other indole metabolites. Eur J Pharmacol 375: 87–100. [DOI] [PubMed] [Google Scholar]
- 38. Batabyal D, Yeh S-R (2007) Human tryptophan dioxygenase: A comparison to indoleamine 2,3-dioxygenase. J Am Chem Soc 129: 15690–15701. [DOI] [PubMed] [Google Scholar]
- 39. Reiter RJ, Tan DX, Jou MJ, Korkmaz A, Manchester LC, et al. (2008) Biogenic amines in the reduction of oxidative stress: Melatonin and its metabolites. Neuroendocrinol Lett 29: 391–398. [PubMed] [Google Scholar]
- 40. Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, et al. (2001) Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 50: 521–530. [DOI] [PubMed] [Google Scholar]
- 41. Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, et al. (2001) Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 313: 96–98. [DOI] [PubMed] [Google Scholar]
- 42. Schell M, Molliver M, Snyder S (1995) D-Serine, an endogenous synaptic modulator - localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA 92: 3948–3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chumakov I, Blumenfeld M, Guerassimenko O, Cavarec L, Palicio M, et al. (2002) Genetic and physiological data implicating the new human gene G72 and the gene for D-amino acid oxidase in schizophrenia. Proc Natl Acad Sci USA 99: 13675–13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R (2008) Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr Res 101: 76–83. [DOI] [PubMed] [Google Scholar]
- 45. Miljevic C, Nikolic-Kokic A, Nikolic M, Niketic V, Spasic MB, et al. (2013) Effect of atypical antipsychotics on antioxidant enzyme activities in human erythrocytes (in vitro study). Hum Psychopharmacol Clin Exp 28: 1–6. [DOI] [PubMed] [Google Scholar]
- 46. Tortorella A, Monteleone P, Fabrazzo M, Viggiano A, De Luca L, et al. (2001) Plasma concentrations of amino acids in chronic schizophrenics treated with clozapine. Neuropsychobiol 44: 167–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
D-Lactate-associated biosynthetic and metabolic pathways. Rectangles denote the quantified compound, and bold red rectangles denote metabolites that were differentially altered in patients compared to controls. Red upward or downward arrows indicate that the level increased or decreased, respectively.
(TIF)
Relevant biosynthetic and metabolic pathways associated with GSH. Rectangles denote quantified compounds, and bold red rectangles denote the metabolites that were differentially altered in the serum of patients versus controls. Red upward or downward arrows indicate that the level increased or decreased, respectively.
(TIF)
Relevant biosynthetic and metabolic pathway of PUFAs. Rectangles denotes the compounds that were quantified, and bold red rectangles denote the metabolites that differed between patients and controls. Red downward arrow means that the level decreased.
(TIF)
Relevant metabolic pathway of Trp. Rectangle denotes compounds quantified and bold red rectangle denotes the compounds whose levels were altered in patients compared to healthy controls. Red upward or downward arrows indicate that the level increased or decreased, respectively.
(TIF)
Serum levels of amino acids (including D-serine) in controls and patients with schizophrenia.
(XLS)
Serum levels of glucose, D-lactate, L-lactate, and 3-hydroxybutyrate (3-HB) in controls and patients with schizophrenia.
(XLS)
Serum levels of thiol compounds in controls and patients with schizophrenia.
(XLS)
Serum levels of unsaturated fatty acids in controls and patients with schizophrenia.
(XLS)
Serum levels of Trp and its metabolites in controls and patients with schizophrenia.
(XLS)
Serum levels of metabolites (µmol/L) in smoking and non-smoking schizophrenia patients.
(XLS)