Abstract
There is intense interest in the role of vitamin D in the development of asthma and allergies. However, studies differ on whether a higher vitamin D intake or status in pregnancy or at birth is protective against asthma and allergies. To address this uncertainty, the Vitamin D Antenatal Asthma Reduction Trial (VDAART) was developed. VDAART is a randomized, double-blind, placebo-controlled trial of vitamin D supplementation in pregnant women to determine whether prenatal supplementation can prevent the development of asthma and allergies in the women’s offspring. A secondary aim is to determine whether vitamin D supplementation can prevent the development of pregnancy complications, such as preeclampsia, preterm birth, and gestational diabetes. Women were randomized to the treatment arm of 4,000 IU/day of vitamin D3 plus a daily multivitamin that contained 400 IU of vitamin D3 or the placebo arm of placebo plus a multivitamin that contained 400 IU daily of vitamin D3. Women who were between the gestational ages of 10–18 weeks were randomized from three clinical centers across the United States – Boston Medical Center, Washington University in St. Louis, and Kaiser Permanente Southern California Region (San Diego, CA). Supplementation took place throughout pregnancy. Monthly monitoring of urinary calcium to creatinine ratio was performed in addition to medical record review for adverse events. Offspring are being evaluated quarterly through questionnaires and yearly during in-person visits until the 3rd birthday of the child. Ancillary studies will investigate neonatal T-regulatory cell function, maternal vaginal flora, and maternal and child intestinal flora.
Keywords: Vitamin D, asthma, allergy, randomized controlled trial, Deveopmental Origins, prenatal
Introduction
Asthma is one of the leading causes of morbidity in children with 90% of all cases diagnosed by age 6,1, 2 remains the most common chronic disease of childhood3, 4, and incurs significant healthcare costs.5, 6 Thus, preventing the development of this disorder would be of great public health importance. Because asthma and allergies have their roots very early in life,7–9 interventions may need to begin prenatally.
Vitamin D deficiency occurs worldwide,10, 11 and has been reported in many subgroups including healthy children, young adults (especially African Americans), and middle-aged and elderly adults.10, 11 Vitamin D status is defined by the circulating level of 25-hydroxyvitamin D3 (25(OH)D), measured from either plasma or serum.12, 13 Vitamin D deficiency exits despite fortification of foods in some Western countries and despite intake of multivitamins containing vitamin D. This suggests that as these countries adopt a western lifestyle, people spend more time indoors rather than participate in outdoor activities. For example, it is estimated that in the US alone, Americans spend an average of 93% of their time indoors14. Pregnant and lactating mothers and their neonates are at especially high risk for vitamin D deficiency15–18, with newborn plasma levels strongly correlated with maternal levels,19 reinforcing the fact that infant vitamin D status is dependent on maternal vitamin D status.
Vitamin D deficiency in pregnancy is likely to have significant import in relation to asthma and allergy development, as vitamin D has many recognized effects on the developing lung and immune system during the fetal and early post-natal periods. These effects have been reviewed elsewhere,20–23 and synthesized in Figure 1. We reported that higher maternal dietary vitamin D intakes in pregnancy had protective effects on wheezing phenotypes in young children in two separate cohorts,24, 25 with over 60% reduction in recurrent wheeze in young children born to mothers with the highest intakes of vitamin D. Because vitamin D intake in these studies was estimated from food frequency questionnaires (FFQ), rather than direct measurement of vitamin D status in the mothers, there may be residual confounding by diet quality, even after appropriate adjustment.
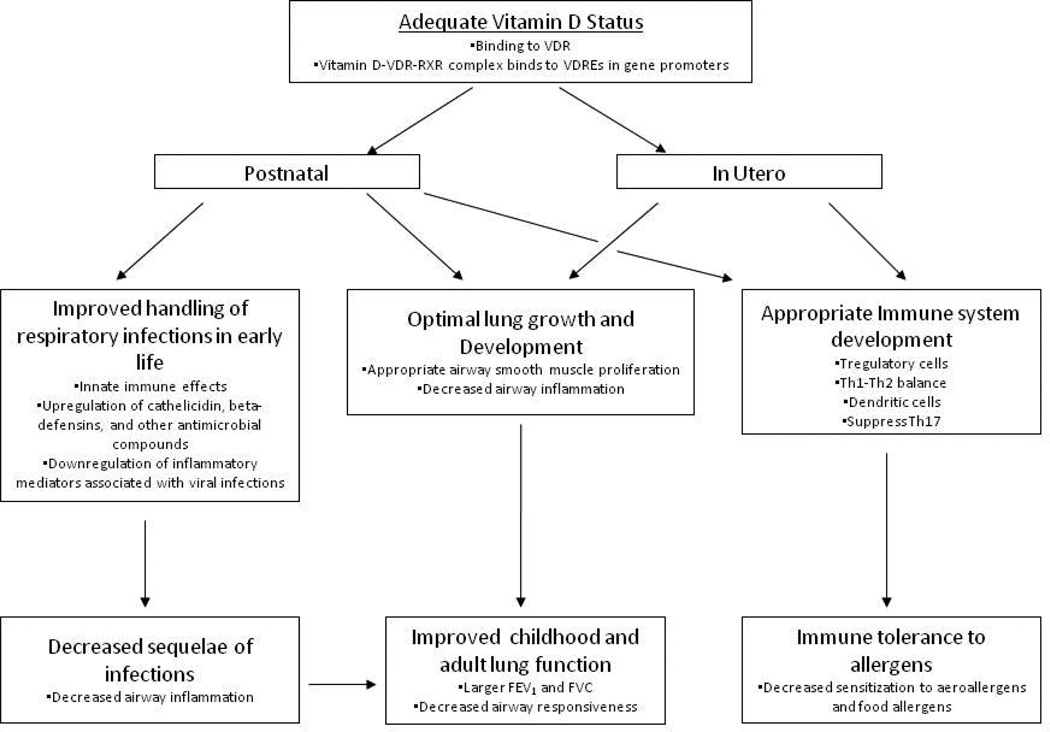
Figure 1. A paradigm for how adequate vitamin D status in pregnancy and in early life may prevent the development of asthma and allergies.
Vitamin D has been shown to affect in utero lung growth and development. Since the lung continues to develop post-natally, this vitamin D effect likely persists through this period. This paradigm also accounts for the effects of vitamin D on the developing immune system, on innate immune responses, and the anti-inflammatory effects. Reprinted with permission from Litonjua26.
Recent studies have tried to address the limitations of the previous FFQ-based epidemiologic studies by directly measuring vitamin D status. These studies measured 25(OH)D, the metabolite that defines vitamin D status, in either the pregnant mother or in cord blood. Results of these studies have been conflicting, with one showing an adverse effect on asthma development 27, and the others showing no effects28–30. These studies are still limited by having only one measure of 25(OH)D and the lengthy time interval between measurement of vitamin D levels and the outcomes. The disparities between studies measuring vitamin D status and those estimating vitamin D intake from FFQs are due to the fact that vitamin D levels reflect current status while estimates of intake from FFQs reflect chronic intakes31. Given the limitations of these observational studies, only a clinical trial of vitamin D supplementation in pregnancy could answer the question of whether adequate vitamin D status during pregnancy can prevent the development of asthma and allergies in children. To address these uncertainties, the Vitamin D Antenatal Asthma Reduction Trial (VDAART) was developed.
Materials and methods
A. Overview of study design (Figure 2)
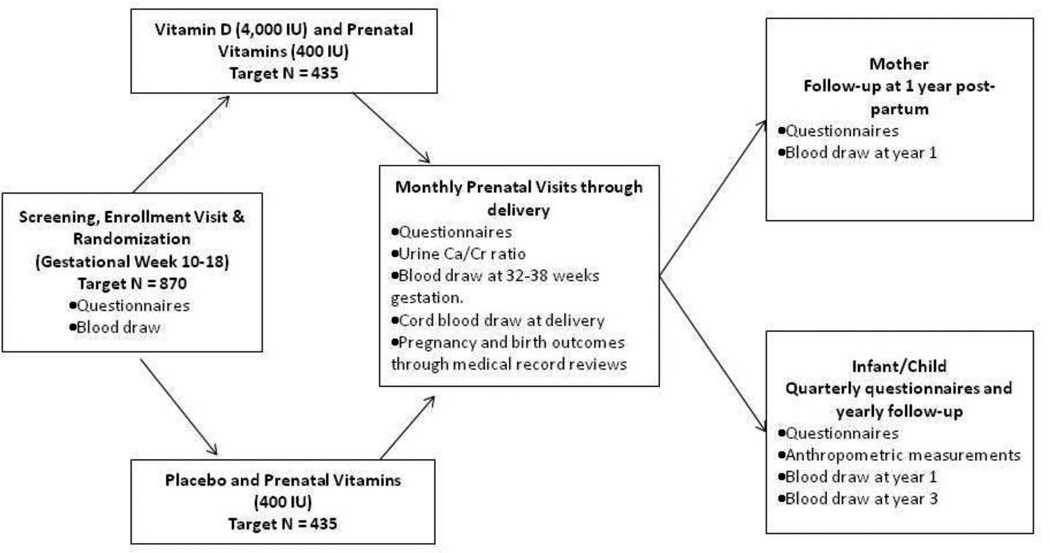
Figure 2. The Vitamin DAntenatal Asthma Reduction Trial (VDAART) design.
The primary hypothesis of VDAART is that vitamin D supplementation in pregnant women will prevent the development of asthma and allergies in their children. To address the role of vitamin D in the development of these disorders, we are conducting VDAART, a randomized, double-blind, placebo-controlled clinical trial among 881 pregnant women with either history of asthma or allergies in themselves or the biological father. Pregnant women, between 18 and 40 years of age and at an estimated gestational age between 10 and 18 weeks, were recruited at a scheduled obstetrical prenatal visit at three clinical centers: Boston Medical Center (Boston, MA), Washington University at Saint Louis (St. Louis, MO), and Kaiser Permanente Southern California Region (San Diego, CA). The Data Coordinating Center (DCC) is based at the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital, (Boston, MA). Participants were randomized to either vitamin D (cholecalciferol, 4,000 IU/day; equivalent to 100 mcg/day) or to placebo. All pregnant mother participants received prenatal vitamins containing 400 IU (10 mcg/day) of cholecalciferol; thus, the vitamin D arm received a total of 4,400 IU/day (110 mcg/day) and the placebo arm received 400 IU/day (10 mcg/day). The treatment period was the duration of the woman’s pregnancy, which was about 22 to 30 weeks for a 40-week pregnancy. Questionnaires were obtained at baseline and monthly thereafter during pregnancy and inquired about maternal health, new disease diagnoses, and non-study medications. Monthly urine samples were obtained and analyzed for urine calcium and urine creatinine levels. Maternal blood samples were obtained at baseline, at 32 to 38 weeks gestation, and at the child’s first yearly visit. At the monthly prenatal visits, adherence was assessed via download of data from MEMS® (Medication Events Monitoring Systems) caps,32 an electronic cap that recorded each time the pill bottle was opened. This had been shown to be superior to self reports33 or pill counts.34 Labor and delivery medical records were reviewed, and cord blood samples were obtained. Postnatally, quarterly (i.e. every 3 months) questionnaires, administered to the mother by telephone up to the child’s third birthday inquired about the health of the infant and child, specifically the occurrence of wheezing illnesses and asthma and allergy symptoms and diagnoses. Yearly in-person child visits obtained questionnaire data, determined anthropometric measurements, and collected blood (at the year 1 and year 3 visits). The novelty of VDAART is based on the intervention administered to women prenatally with the primary outcomes collected from the offspring postnatally. VDAART is therefore a primary prevention trial which tests the Developmental Origins of Health and Disease35 paradigm, that posits that environmental perturbations during the phase of developmental plasticity in early life either increase the risk for disease or improve the capacity of the individual to cope with its environment later in life.
B. Aims
VDAART has two primary aims: (1) to determine whether vitamin D supplementation in the pregnant mother is associated with reduced incidence of asthma (defined as a doctor’s diagnosis and/or recurrent wheeze) in the child during the first 3 years of life; and (2) to determine whether a vitamin D dose of 4,400 IU/day versus the standard 400 IU/day in pregnancy is sufficient to maintain mothers’ vitamin D levels in the range of ≥ 75 nmol/L (≥ 30 ng/ml) 25(OH)D. Secondary aims include: (1) determining whether vitamin D supplementation in the pregnant mother is associated in the newborn with reduced cord blood total IgE (immunoglobulin E) level and in the child at 3-years reductions in (a) allergic sensitization (specific food and aeroallergen IgE), (b) doctor’s diagnosis of eczema and (c) lower respiratory tract infections; (2) determining whether vitamin D supplementation in the pregnant mother is associated with improved vitamin D status in their offspring through measurement of 25(OH)D levels in cord blood (at delivery), and children’s blood (at 1 and 3 yrs of age); (3) determining whether sufficient vitamin D supplementation in the pregnant mother is associated with reduced incidence of preterm birth (birth <37 weeks gestation), preeclampsia, gestational hypertension, and/or Hemolytic anemia, Elevated Liver enzymes, Low Platelet count (HELLP syndrome) compared to the usual care VDAART control group.
C. Sponsor
VDAART (www.vdaart.com) is supported by The National Heart, Lung, and Blood Institute (NHLBI) and was registered at ClinicalTrials.gov (NCT00920621). This trial is funded via the UO1 mechanism, which is a cooperative agreement between the NHLBI and the investigators. As such, the NHLBI monitored the conduct of the trial and selected the 10-member Data and Safety Monitoring Board (DSMB). All communication between the investigators and the DSMB courses through the staff of the NHLBI. All manuscripts, including this current one, during the course of the trial are presented to the DSMB for approval prior to submission for peer review. VDAART was approved by the Institutional Review Boards (IRB) of the participating Clinical Centers and the Data Coordinating Center, with pregnant women signing informed consent at the first enrollment visit.
D. Intervention: Vitamin D supplement
VDAART is testing a total cholecalciferol (vitamin D3) dose of 4,400 IU/day vs 400 IU/day. VDAART was designed in 2008, and began recruitment in 2009, when the adequate intake (AI) for vitamin D was considered to be 200 IU/day for pregnant women36 (although most over-the-counter prenatal vitamins contained 400 IU of vitamin D3). However, multiple studies report that these recommendations result in a high degree of vitamin D deficiency in various adult populations37–39. One study supplemented 160 minority women in the United Kingdom with 800–1600 IU/day vitamin D throughout their pregnancies39. After vitamin D supplementation, blood 25(OH)D concentration doubled to 28 ± 16 nmol/L (11.2 ± 6.4 ng/ml) at term from 14.5 ± 2.2 nmol/L (5.8 ± 0.88 ng/ml) at the beginning of pregnancy. Therefore, many mothers who were deficient at the beginning of their pregnancy exhibited insufficient levels at term, despite supplementation with a higher than recommended dose of vitamin D. The dose of vitamin D for the VDAART trial was selected after a recent trial40 which showed that the 4400 IU/day dose most rapidly and consistently increased 25(OH)D levels in the participants from all ethnic groups, with a mean of about 112 nmol/L (44.8 ng/ml), and practically all participants achieved levels of at least 75 nmol/L (30 ng/ml). Additionally, the 4400 IU/day dose eliminated all seasonal variation in circulating 25(OH)D levels. There were no instances of adverse events from the 4400 IU/day dose, either in the mother or the infant, even in those mothers whose beginning 25(OH)D levels were > 75 nmol/L (>30 ng/ml).
E. Trial Eligibility
VDAART randomized 881 pregnant women. The original recruitment goal, on which power calculations were based, was 870.
Inclusion Criteria (applied to the pregnant women)
Maternal personal history of or biological father history of asthma, eczema, allergic rhinitis
Gestational age between 10 and 18 weeks at the time of randomization
Maternal age between 18 and 39 years
Not a current smoker (defined as not having smoked for at least 1 month prior to enrollment) and not a user of other nicotine products (e.g. nicotine patch) for at least 1 month prior to enrollment
English- or Spanish-speaking
Intent to participate for the full 4 years (through pregnancy and then until the 3rd birthday of the child)
Exclusion Criteria
Gestational age >18 weeks
Presence of chronic medical conditions: (i) hypertension on medications, (ii) diabetes mellitus, (iii) parathyroid disease, (iv) uncontrolled thyroid disease, v) kidney stones, and (vi) sarcoidosis
Intake of vitamin D supplements containing > 2,000 IU/day of vitamin D3
Multiple gestation pregnancy
Pregnancy achieved by assisted reproduction techniques (e.g. IUI, IVF)
Current use of illicit drugs (defined as any use in the past 6 months prior to enrollment)
Previously enrolled in VDAART for a prior pregnancy
Any major fetal anomalies detected prior to delivery
Patient Health Questionnaire (PHQ-9)41 depression scale ≥ 15
Any condition, in the opinion of the Clinical Center Principal Investigator, that would inhibit compliance with the study medications or prohibit long-term participation in the trial
F. Recruitment and Randomization; Participant Characteristics
Recruitment and randomization have been completed. The recruitment goal for the trial was 870 women, 290 subjects from each site. We estimated the rate of miscarriages and stillbirths at 8%,42 and the rate of dropouts at 18% by the end of the 3-year follow-up. As such, it was estimated that by the end of the trial, VDAART would have evaluated about 220 children per Clinical Center or a total of 660 children at age 3 years. Over the course of the recruitment period, we exceeded our goal and recruited and randomized a total of 881 women. The baseline characteristics of the population are presented in Table 1.
Table 1.
Baseline characteristics of VDAART participants
|
Treatment A (N=442) |
Treatment B (N=439) |
||
|---|---|---|---|
| Age, yrs, mean (sd) | 27.5 (5.5) | 27.2 (5.6) | |
| (min, max) | (18, 39.4) | (18, 39.5) | |
| Gestational age, weeks, mean (sd) | 14.1 (2.8) | 14.2 (2.7) | |
| (min, max) | (2.1, 19) | (9.7, 18.9) | |
| Mother | |||
| Asthma, n(%) | 191 (43) | 168 (38) | |
| allergic rhinitis, n(%) | 276 (62) | 284 (65) | |
| eczema, n(%) | 139 (31) | 141 (32) | |
| Father | |||
| asthma, n(%) | 109 (31) | 95 (22) | |
| allergic rhinitis, n(%) | 173 (39) | 192 (44) | |
| eczema, n(%) | 82 (19) | 64 (15) | |
| Race/ethnicity | |||
| African American, n(%) | 191 (43) | 193 (44) | |
| Caucasian Hispanic, n(%) | 59 (13) | 61 (14) | |
| Caucasian, non-Hispanic, n(%) | 115 (26) | 116 (26) | |
| Other, n(%) | 77 (17) | 69 (16) | |
| Education completed | |||
| less than high school, n(%) | 67 (15) | 42 (10) | |
| high school, technical school, n(%) | 123 (28) | 145 (33) | |
| some college, n(%) | 108 (24) | 105 (24) | |
| college graduate/Graduate school, n(%) |
144 (33) | 147 (33) | |
| Marital status | |||
| Married, n(%) | 192 (43) | 203 (46) | |
| divorced/separated, n(%) | 13 (3) | 10 (2) | |
| not married, n(%) | 237 (54) | 226 (51) | |
| Household income | |||
| <$30,000, n(%) | 133 (30) | 131 (30) | |
| $30 – 49,999, n(%) | 62 (14) | 58 (13) | |
| $50 – 74,999, n(%) | 50 (11) | 51 (12) | |
| $75 – 99,999, n(%) | 43 (10) | 39 (9) | |
| $100 – 149,999, n(%) | 35 (8) | 36 (8) | |
| >$150,000, n(%) | 18 (4) | 14 (3) | |
| refused/unknown, n(%) | 101 (23) | 110 (25) | |
F.1. Screening visit
The screening visit occurred at the subjects’ pre-natal visit between 10 weeks and 18 weeks gestation. Research staff obtained the schedules for all prenatal visits for the week, and scheduled visits were reviewed for potential subjects. During the visit, the research staff described the study, presented the potential participant with a written description of the study, and reviewed the eligibility criteria via a screening questionnaire and a study admissions criteria questionnaire. A Screening ID was assigned for these questionnaires. If the subject was eligible, did not meet any exclusion criteria, and expressed interest in the study, the subject was either enrolled at this visit or an enrollment visit was scheduled within one month.
F.2. Enrollment visit
A separate enrollment/randomization visit was scheduled within one month of the prenatal visit, but not later than the 18th week of gestation. In some instances, enrollment occurred at the time the subject was screened. At this visit, research staff reviewed study procedures and the consent form. Once consent was obtained, participants were assigned a Study ID and several questionnaires were administered, skin pigmentation was measured using the SmartProbe400® (IMS, Inc, Milford, CT), and blood was drawn, aliquoted, and stored for 25(OH)D measurements and other analytes for future studies. Participants were randomized to one of the treatment arms.
F.3. Randomization
Participants who were deemed eligible to participate were randomized by the DCC to either of the 2 trial arms. Only those who agreed, in principle, to follow-up until their child’s 3rd birthday were randomized. At the time of recruitment, the idea of a potential follow-up until the children turned 6 years of age was presented to the participants by the Clinical Center’s coordinator, acknowledging that funding and consent for the current portion of the study covered only the follow-up to 3 years.
Randomization was performed by the DCC using a system that automates the random assignment of treatment groups to Study ID numbers. The randomization scheme employed stratified permuted blocks with randomly varied block sizes of 4 and 6, and one block allocation list per stratum (study site and racial/ethnic group). That is, separate permuted block randomization lists were maintained for each racial/ethnic group (e.g., Caucasian/Non-Hispanics, Caucasian/Hispanic, African-American, and Other) within each study site.
F.4. Race/ethnicity and characteristics of the study population
The trial population had a significant proportion of Black/African American participants. As Table 1 shows, about 44% of the participants identified themselves as African American, 26% identified themselves as Caucasian, non-Hispanic, and about 14% identified themselves as Caucasian Hispanics; the rest identified themselves as Other or Mixed race. Based on the fact that two of the three clinical centers served inner-city populations, a large proportion of participants came from lower socio-economic strata, with about 43% reporting yearly household incomes < $50,000 (Table 1).
F.5. Follow-up visits
After enrollment, the research staff noted the subject’s scheduled obstetrical visits and made sure that urine samples were collected at each of these scheduled monthly clinical prenatal visits. Also at these monthly visits, a short maternal health questionnaire was administered, MEMS® cap information was downloaded, and study medication and prenatal vitamins were refilled. Additionally, the research staff conducted monthly reviews of electronic medical records to check for pregnancy complications. At 32–38 weeks gestation, in addition to the monthly routine, a blood draw, skin pigmentation determination, and a number of the questionnaires that were administered at the enrollment visit were repeated. At delivery, cord blood was collected and the research staff collected information regarding the type of delivery, birth weight, and other anthropometric measures. After delivery, the research staff made telephone calls every 3 months and inquired about the health and symptoms of the infant, medication use, the type and frequency of feeding of the child, and supplement use. The mother and child came in for 3 yearly follow-up visits, during which blood was drawn, skin pigmentation tests were performed, additional questionnaires were administered, and anthropometric measurements of the child were obtained. All questionnaires and measurements are summarized in Table 2.
Table 2.
Summary of measurements in VDAART
| Enrollment (10–18 weeks gestation) |
Monthly | Third Trimester (32–38 weeks gestation) |
Delivery | Quarterly | 6 Months after delivery |
Year 1 | Year 2 |
Year 3 |
|
|---|---|---|---|---|---|---|---|---|---|
| Study Admission Criteria Questionnaire |
x | ||||||||
| Maternal Questionnaire | x | ||||||||
| Food Questionnaire (Maternal) |
x | x | x | x | |||||
| Food Questionnaire (Child) |
x | x | x | ||||||
| Baseline Sun Exposure Questionnaire |
x | ||||||||
| Follow-Up Sun Exposure Questionnaire |
x | x (mother and child) |
x (child) |
x (child) |
|||||
| Monthly Maternal Questionnaire |
x | x | |||||||
| Medical Record Review (electronic and/or paper) |
x | x | x | ||||||
| Determination of skin pigmentation |
x | x | x (mother and child) |
x (child) |
|||||
| Blood draw | x1 (mother) |
x1 (mother) |
x2 (cord blood) |
x1,3 (mother and child) |
x4 (child) |
||||
| Urine5 | x | x | |||||||
| Patient Health Questionnaire |
x | x | x | x | x | ||||
| Hardships Questionnaire |
x | x | x | x | |||||
| Labor and Delivery Form |
x | ||||||||
| Quarterly Infant Follow-up Questionnaire | x | x | x | x | |||||
| In-person visit | x | x | x | x | x | ||||
| Anthropometric Measurements |
x (child) |
x (child) |
x (child) |
||||||
| MEMS information download |
x | x | x (after delivery) |
Mother: 25(OH)D, blood for DNA extraction and plasma (1 purple top tube) and gene expression studies (2 PAXgene tubes).
Cord blood: 25(OH)D, total IgE (2 tiger top red tubes for serum), and blood for DNA extraction (2 purple top tubes for buffy coats and plasma) and gene expression studies (2 PAXgene tubes).
Child: total and specific IgE 25(OH)D (1 purple top tube for plasma and buffy coat); Mother: 25(OH)D (1 purple top for plasma and buffy coat)
Child: total and specific IgE, 25(OH)D, and blood for DNA extraction (1 purple tube for plasma and buffy coat) and gene expression studies (1 PAXgene tube)
Urinary calcium, creatinine.
G. Assessment of outcomes
The primary endpoints are doctor’s diagnosis of asthma by age 3, recurrent wheezing at age 3, and allergic sensitization at age 3. Secondary endpoints include lower respiratory infections (LRI) and atopic eczema over the 3 years of the trial. The collection of 3-year outcomes is currently underway.
G.a. Doctor’s Diagnosis of Asthma
Doctor’s diagnosis of asthma will be defined as a positive response to the direct question at any time in the first three years of life. We have had extensive experience with this definition of asthma in early life43–45, and have shown that a doctor’s diagnosis correlates with indices of more severe disease46 and a doctor’s diagnosis with current wheeze is associated with airways responsiveness in the asthmatic range47. Recent reports have shown that recent symptoms may help identify young children with significant asthma48, thus, we will also use a more specific definition of doctor’s diagnosis plus symptoms or medication use in the past year. We have also used this composite definition in previous analyses and have found it to be a reliable marker for childhood asthma49, 50.
G.b. Recurrent Wheezing
Young children who have experienced asthma-like symptoms in the past year are likely to have more severe asthma. Moreover, children who experience more frequent (recurrent) wheeze in early life are more likely to develop persistent asthma51. For example, children with recurrent wheeze in the first year of life had a threefold increase in the odds of asthma at age 7 years52. We will define recurrent wheeze as parental reports of wheezing in either of the first two years of life and wheezing in the third year53, 54.
G.c. Allergic sensitization
Sensitization to common allergens can be detected by measuring serum IgE specific to those allergens. We will define sensitization to each allergen as a specific IgE level ≥ 0.35 IU/ml. Allergy will then be defined as sensitization to at least one of the 13 allergens included in the study.
G.d. Lower respiratory illnesses (LRI)
The occurrence of certain LRIs in early life are associated with the development of asthma in later childhood45. We will define LRI as a response to standard questions inquiring whether physician-diagnosed croup, bronchitis, bronchiolitis, or pneumonia has occurred in the child. Individual LRIs and a composite variable for any LRI will be investigated. As with the other early-life phenotypes above, we have had extensive experience with this variable in our previous analyses45, 49.
G.e. Eczema
Atopic eczema, the earliest common clinical manifestation of atopy, predicts atopic asthma in childhood.55, 56 Using questions adapted from the ISAAC questionnaire, we will define atopic eczema as parental report of both physician-diagnosed eczema and a persistent pruritic rash in a typical distribution.57 For confirmatory analyses, we will use a definition of eczema that also requires the presence of sensitization to at least one allergen or a total serum IgE above the geometric mean value. Since we will be assessing these outcomes every 3 months after the child is born, we will be able to determine the sequence of the diagnosis of eczema relative to the diagnosis of asthma.
H. Blood collection and assays
H.1. Blood collection
Blood collection during pregnancy, delivery, and the 1-year visits has been completed; blood collection at the 3-year visit is ongoing. Non-fasting blood samples were collected from the mothers at the enrollment visit, at the 32–38 week visit, and at the child’s 1-year visit. Maternal blood samples will be assayed for 25OHD levels, and aliquots will be stored for future analyses. The baseline blood sample will establish the initial level of 25OHD prior to beginning the study drug. Although the initial level is not an exclusion or inclusion criterion, we will be able to include initial level as a covariate in the analysis as it is likely that this plays a role in the levels achieved during pregnancy and may affect the outcomes in the children. The 32–38 week blood sample will allow us to confirm adherence and will inform us of the achieved levels for the study dose. The last maternal blood sample will give us an idea of the level of 25OHD in the mother in a non-pregnant state.
At delivery, cord blood was also collected from each infant. Cord blood samples will allow us to compare the levels in the newborns with the maternal 32–38 week levels. Additional blood samples on the children were collected at the 1-year visit, and the 3-year visits are ongoing. These samples will allow us to track 25OHD levels as the children are getting older. In addition to 25OHD levels in the children, total serum IgE will be measured from cord blood samples, and from year 3 samples from the children. In addition, allergen-specific food and aeroallergen IgE will be measured from the year 3 samples from the children. Atopy will then be defined as sensitization to at least one of the 13 allergens included in the study. Total serum IgE is increased in atopic individuals. For the statistical analysis, total serum IgE will be transformed to a log natural scale, in recognition of the distribution of total serum IgE in human populations58.
The types of blood samples collected and the tubes used for the study are shown in Table 2. Samples were collected at each clinical center, and shipped in dry ice to the DCC. For samples collected after hours, they were stored in refrigerators, and shipped to the DCC within 24 hours of collection. Samples collected in the same type of tubes were processed in the Channing Division of Network Medicine laboratory using the same standard protocols.
Purple top tubes. Tubes were kept cold at all times (i.e. at 4°C), before and during processing. Tubes were centrifuged at 2000 RPM at 4°C for 10 min. Plasma was then aliquoted into two 1.8 ml NUNC tubes. The remaining purple top vacutainer contents (buffy coat and red cell layers) were poured into a 50 ml centrifuge tube filled with 30 ml RBC lysis solution and mixed well. The tube was then capped, shaken vigorously and incubated on ice for 15 min. After this, the tube was centrifuged at 2000 RPM at 4°C for 10 min, leaving the WBC pellet. The supernatant was discarded and 5 ml of RBC lysis solution was added to thoroughly break up the pellet, then incubated on ice for another 5 min. After incubation, the tube was centrifuged at 2000 RPM at 4°C for 10 min. Supernatant was then discarded and at total of 1200 ul NE buffer was added to create a homogeneous mixture, and aliquoted into a NUNC tube. Both the plasma aliquots and the WBC aliquot were stored in −80°C freezers.
Tiger top tubes. Samples were kept cold before and during processing. The red top vacutainer was centrifuged at 2000 RPM at 4°C. Serum was then aliquoted into three 1.8 ml NUNC tubes, which were then stored in −80°C freezers.
PAXgene tubes. Contents of tubes were mixed gently immediately after collection. They were then shipped to the DCC and stored in −80°C freezers.
H.2. Blood assays
Serum or plasma assays of 25OHD will be performed at the Channing Laboratory in the DCC. Circulating 25(OH)D will be determined using the DiaSorin Liaison® machine, which uses a chemiluminescence immunoassay (CLIA)59, to determine plasma concentrations of 25(OH)D. For quality control, the laboratory uses US National Institute of Standards and Technology (NIST) level 1 SRM (Standard Reference Material) 972 Vitamin D in Human Serum, in each run. Total IgE will be measured by the UniCAP 250 system (Phadia AB in Portage, MI), with samples measured in duplicate. Serum from each child will also be assayed for IgE to 13 allergens (dust mite [D. farinae], cockroach [Bla g], cat dander, dog dander, rye grass pollen, ragweed pollen, Alternaria tenuis, Aspergillus, wheat, soybean, egg white, milk, and peanut) using the UniCAP system (Phadia AB in Portage, MI ). For specific IgE, the enzyme-immunoassay is based on the sandwich technique, utilizing a solid phase (allergen-impregnated discs) for separation. Sensitization to common allergens can be detected by measuring serum IgE specific to those allergens. We will define sensitization to each allergen as a specific IgE level ≥ 0.35 IU/ml.
I. Manufacture, distribution, and quality of study medications
The study medications, including all prenatal vitamins, the vitamin D capsules, and the placebo capsules were manufactured by Tishcon Corp. (www.tishcon.com), a leading manufacturer and marketer of vitamins, related dietary and herbal supplements, and private label non-prescription (over-the-counter) pharmaceuticals. It is one of 12 soft gelatin capsule manufacturers in the United States. The pills and capsules were sent in bulk from Tishcon to the DCC. The DCC contracted with the Brigham and Women’s Hospital Pharmacy to package and label the pills in bottles. The vitamin D bottles and the placebo pill bottles were given a letter label from A to F, with 3 letters corresponding to the vitamin D pills and 3 letters corresponding to the placebo. Clinical Center investigators and staff were blinded to the treatment code. Once packaged and labeled, the pill bottles were shipped to the respective Clinical Centers. Each participant received 2 bottles – one bottle contained the standard prenatal vitamins with 400 IU vitamin D, and was labeled accordingly, and the other bottle contained the intervention pill containing 4,000 IU vitamin D or a placebo, and labeled “Study Drug A” through “Study Drug F.”
A few months into the trial, each of the three clinical centers were requested to retrieve 1 capsule from random bottles of each letter label (i.e. 1 capsule from bottle A, 1 from bottle B, etc), for a total of 3 capsules from each of the 6 letter codes. These eighteen capsules were then sent to Heartland Assays, Inc. (Ames, IA), where the vitamin D3 content of each capsule was analyzed using the procedure described by Phillips et al.60 Staff at Heartland Assays, Inc. were blinded to the treatment code. Briefly, individual capsules were weighed and dissolved in 5 ml of methanolic KOH. The sample was spiked with an internal standard of [3H]-vitamin D3 to estimate recovery losses. After 60 mins at 60°C, 1 volume of H2O was added and the mixture extracted 3 times with 1 volume of hexane/ethyl acetate (85/15). The extract was collected and dried under vacuum. The dried extract was re-suspended in 1.0 ml of hexane/methylene chloride 90/10 v/v), applied to a solid-phase extraction cartridge (0.5 g and eluted with methylene chloride/2-propanol (99.8/0.2). The eluate was dried and re-suspended in hexane/methylene chloride/alcohols (85/15/0.2) and applied to Agilent HPLC ZORBAX SIL column (5µ, 0.9 × 25 cm, Agilent Technologies, Santa Clara, CA). The alcohols consisted of 2/1 (v/v) isopropanol/methanol). The vitamin D fraction was collected, dried and the residue re-suspended in hexane/alcohols (99.5/0.5) and applied to an HPLC ZORBAX NH2 column (5µ, 0.0.45 × 25 cm, Agilent Technologies, Santa Clara, CA). The vitamin D fraction was collected and dried for final purification.
The final purification for vitamin D by HPLC was achieved using a Vydac ODS column (Vydac part #201TP54, Chrom Tech, Inc, Apple Valley, MN) using a mobile phase of acetonitrile/methylene chloride (65/35), wi9th uv detection at 265 nm. Vitamin D3 was quantified by comparison of the UV peak areas with standards. Samples were collected for quantitation of [3H]-Vitamin D3. Vitamin D3 was quantified by comparison of the UV peak areas at 265 nm with known standards, using the recovered [3H]-Vitamin D3 to correct for losses. Results of the assays were sent to the DCC, where the means of the concentration of vitamin D3 in the vitamin D pills and the placebo pills were calculated. The mean (sd) concentration of cholecalciferol from the 9 vitamin D capsules was 4150.5 IU (109.7) while that for the 9 placebo capsules was 2.8 IU (0.7). None of the vitamin D capsules had content < 4000 IU of cholecalciferol, and none had content of > 4,400 IU of cholecalciferol.
J. Assessment of compliance - Medication Events Monitoring Systems (MEMS®)
The primary measure of compliance with pill-taking was monitoring via the MEMS® system – a pill bottle equipped with special cap that includes an electronic microchip that stores the date and time of each opening of bottle. The MEMS® is manufactured by Aardex LTD (http://www.aardexgroup.com). Each monitor recorded the time and date of each opening and closing of the container through integrated microcircuitry. The MEMS® stores up to 3800 medication events in non-volatile EEPROM memory, allows wireless data transfer, fits standard pharmacy bottles, provides 36 months battery life, and is water-resistant and CE marked. Monitors were designed to be used by one patient with one drug. A reader allowed transferring the dose timing data from the MEMS® to a MS-Windows based computer. The MEMS® monitors have demonstrated reliability and validity and have been widely used with great success in clinical trial research61, including NHLBI sponsored studies in childhood asthma62, 63 and asthma clinical research sponsored by industry64.
Data from the MEMS® system was downloaded at the monthly in-person prenatal visits and after delivery. The data from the pre-natal follow-up visits was used to provide feedback to the participant, reinforcing those subjects with good adherence and providing extra teaching and encouragement for those with suboptimal adherence. The data from both pre-natal follow-up and post-natal downloads was used to derive a summary adherence measure, to be used as a covariate in our analyses and also for secondary analyses that exclude any women who have taken less than 50% of the prescribed study medication. In addition to the information obtained from the MEMS® system, we will have 25(OH)D levels from baseline and at the 3rd trimester visits, and these data will provide us with excellent compliance information for this trial.
K. Analysis plan and statistical power
K.1. Analysis plan
This is a randomized, double blinded, multi-center, placebo controlled, clinical trial of prenatal vitamin D exposure as a preventive measure to reduce the risk of asthma and recurrent wheeze in childhood. The primary outcome is doctor-diagnosed asthma and recurrent wheeze at 3 year of age. Secondary outcomes include allergic sensitization, doctor’s diagnosis of eczema, and lower respiratory infection (LRI), at three years of age.
The primary analytic strategy will use the intention-to-treat paradigm. All analyses will occur in a framework of continuous quality assurance and verification. Preliminary statistical analysis will be used to describe the univariate distributions of key measures of interest to detect outliers or data anomalies that need to be addressed by data editing or sequestration of doubtful entries. Additionally, these analyses will examine whether any important risk factors for childhood asthma are imbalanced across treatment arms, as well as assess whether differential loss to follow-up is present across treatment groups. Detailed blinded codebooks will be provided to investigators as data accumulate to facilitate efficient substantive verification of basic distributional summaries and joint distributions of measured factors.
K.2. Statistical power
The trial was designed to detect a 25% reduction in asthma and recurrent wheeze incidence at 3 years in the vitamin D supplemented (4400 IU) group. Based on the observational results in previous birth cohorts, we estimated that the incidence of asthma and/or recurrent wheeze in the standard dosing group (400 IU) will be 40–50%. The original power calculations were based on a recruitment target of 870 pregnant women. We anticipated an 8% miscarriage rate42 and 17–18% loss-to-follow-up among remaining subjects over the study period, resulting in an estimated sample size of 660 subjects (330 per arm) at the end of the study period. Each power estimate was based on 10,000 simulations. For our group sequential design, we plan to use a Lan-Demets spending function with three equally spaced looks (with respect to the amount of information collected) and O’Brien-Fleming type bounds. The software program LANDEM (http://www.biostat.wisc.edu/landemets/)65 was used to calculate the stopping boundaries, which were |z|=3.7103, 2.5114, and 1.993 at the first, second, and third looks, respectively.
We estimated that there will be excellent power (91%) to detect a 25% reduction in asthma, assuming a 50% incidence of asthma/recurrent wheeze in the 400 IU dosing group. Even if the children in this study had a lower incidence of asthma and recurrent wheeze than previous cohorts (i.e., 40–45%), we still had good power (77–84%) to detect a 25% reduction in asthma and/or recurrent wheeze (Table 3).
Table 3.
Power to detect a 20–30% reduction in asthma and recurrent wheeze for 660 patients in a multi-site study*
| Percent reduction in Asthma and/or Recurrent Wheeze in 4400 IU group |
|||
|---|---|---|---|
|
Mean Incidence of Asthma and/or Recurrent Wheeze in 400 IU group |
30% | 25% | 20% |
| 0.50 | 0.974 | 0.906 | 0.726 |
| 0.45 | 0.950 | 0.837 | 0.653 |
| 0.40 | 0.901 | 0.773 | 0.574 |
18% LTF and 8% miscarriage rate
L. Trial monitoring
An external Data Safety and Monitoring Board (DSMB), composed of vitamin D metabolism experts, early life asthma experts, obstetricians, an ethicist and a statistician has been appointed by the NHLBI. The DSMB convened prior to the study initiation to review the protocol and to examine and comment upon “shell” reports that were used to illustrate data accumulation as the study proceeded. After the initiation of the trial, the DSMB has met biannually to review recruitment, adherence, adverse events and severe adverse events between treatment groups and other issues deemed to be pertinent.
All study subjects were monitored for hypervitaminosis D, the only known toxicity of which is hypercalcemia. Monitoring occurred through monthly measurement of urinary calcium (Ca) to creatinine (Cr) ratios. Previous studies of vitamin D supplementation in healthy subjects and patients with multiple sclerosis 66, 67 had identified a urinary Ca/Cr ratio of < 1.0 (when urinary Ca and Cr were reported in mmol/L) or < 0.37 (when urinary Ca and Cr were reported in mg/dl) as safe values not related to elevated serum Ca values. However, the pregnant state may, independently of vitamin D supplementation, be a state of calciuria 68, 69 and the urinary Ca/Cr ratio in non-pregnant subjects may not apply for pregnant women. Thus, we performed an analysis of urinary Ca/Cr results from a recently reported vitamin D supplementation trial in pregnancy (data kindly supplied by BDH).40 Based on descriptive statistics and histogram of urine Ca:Cr ratio measures of 672 untreated (control group) pregnant females in that trial, a 95% bootstrap confidence interval was computed for the 99th percentile of the distribution of this ratio. The lower bound of this confidence interval was 1.55 (urinary Ca and Cr in mmol/L) or 0.55 (urinary Ca and Cr in mg/dl). Therefore, operationally, we defined caution by urinary calcium/creatinine (Ca/Cr) ratio ≥ 1.55 (when urinary Ca and Cr are reported in mmol/L) or the equivalent ratio ≥ 0.55 (when urinary Ca and Cr are reported in mg/dl) from maternal urine samples that were obtained monthly starting 1 month after randomization. Whenever any patient exceeded the caution limit, the study medication was temporarily discontinued and a specific evaluation was initiated. After repeat testing and a detailed case review was performed by the clinical center and reviewed by the DSMB, an informed decision was made about continued participation of the study drug. The plan was if the elevated levels in the subject were attributed to the study drug, the participant was to be withdrawn from supplementation but would continue to be monitored and followed in the intent-to-treat paradigm. No participants were withdrawn due to hypercalcemia.
The occurrence of adverse events (AE) and serious adverse events (SAE) was monitored by each Clinical Center through the monthly maternal health questionnaire and the monthly obstetrical medical record review. (Table 4 shows events considered to be either AEs or SAEs). Additionally, hypercalciuria was monitored with the monthly urinary calcium excretion as detailed above. If a SAE was discovered, the Clinical Center staff, under the direction of the Clinical Center PI, completed a SAE form and determined whether this event was likely to have been related to the study medication and alerted the DCC who informed the DSMB Chair. In addition, the DCC prepared annual reports of all SAEs and AEs for the FDA and the IRB at each center. AEs were also monitored via this mechanism. All adverse events were reviewed by the entire DSMB at quarterly intervals, by e-mail, in between the regularly scheduled semi-annual meetings. SAEs and AEs were reported by masked treatment groups. In addition, all SAEs and AEs were reported by ethnicity and gender (for events among the infants), and with information about the expected rate of occurrence of these events in a general population of pregnant women (or infants). The Director of NHLBI, with advice from the DCC and the DSMB, had the sole power to stop the study in the event of an unanticipated statistically and clinically significant difference between arms in rates of serious adverse events, whether or not these are attributable to the study treatment. The definition of statistical significance for study stopping will be adjusted alpha = 0.01.
Table 4.
Pre-defined Serious Adverse Events (SAEs) and Adverse Events (AEs)
|
M. Ancillary studies
Three ancillary studies have been approved by the DCC,DSMB, and appropriate IRBs. While much is known regarding the mechanisms for how vitamin D influences bone health, there is less known regarding the mechanisms involved for other outcomes, particularly in pregnancy and in fetal programming. Thus, significant effort was placed in collecting specimens and performing studies that would provide insight into potential mechanisms of vitamin D in these settings. Funding for these studies was secured separately from the parent grant.
M.1. Intestinal microbiome
Increasing evidence implicates early infant intestinal flora as essential for immune tolerance development and associates alterations in this flora with atopic diseases.70–72 Furthermore, vitamin D deficiency may be related to abnormal intestinal flora73, 74. The fecal flora ancillary will investigate early infant intestinal flora in relation to maternal vitamin D status and child atopic disease. Maternal stool samples were collected during the third trimester and infant stool samples were collected at 3–6 months and 1 year; collection at 3 years of age is ongoing. Both culture and 16S rRNA sequencing will be performed and analyses will include determining the relationship between maternal vitamin D status and both maternal and infant intestinal flora, understanding the stability of child flora over time, and evaluating the relationship of infant flora to atopic diseases. This ancillary study (R01HL108818, VDAART Flora Ancillary Study, PI: Gold, DR) is funded through the Ancillary Studies in Clinical Trials (RFA HL14-0024) mechanism.
M.2. Vaginal Flora
The vaginal flora ancillary study, restricted to the Washington University Clinical Center, will evaluate whether vitamin D supplementation during pregnancy favorably impacts vaginal flora and decreases the risk of preterm delivery. Bacterial vaginosis, characterized by vaginal flora disruption, is a common cause of vaginal inflammation and may play a role in preterm delivery and adverse pregnancy outcomes.75, 76 Cross-sectional studies have found associations between vitamin D levels and disruption of vaginal flora.77, 78 This ancillary study will analyze cultures from self-collected vaginal swabs performed at randomization and between 26–34 weeks gestation by women who consented. Data will be analyzed using Nugent score (which shows extent of vaginal disruption)79, evaluated for change in distribution of bacterial species, and analyzed in association with pregnancy outcomes such as preterm delivery. This ancillary study is funded through institutional funds from Washington University School of Medicine.
M.3. Neonatal immune study
Vitamin D is an important modulator of immunity. The active form of vitamin D enhances the frequency of two distinct populations of T regulatory cells (Treg), IL-10 secreting80 and Foxp3+ Treg cells.81 These Treg maintain immune homeostasis in the respiratory environment 82. Their functional impairment is seen in allergy and asthma in young children and cord blood82–88. Vitamin D may have further benefit in asthma by enhancing responsiveness to corticosteroids88. In the cord blood T-regulatory ancillary study, the hypothesis is that maternal vitamin D status influences immunity in the neonate, specifically the frequency and function of Foxp3+Treg and IL-10-Treg cells. The study will evaluate how maternal vitamin D status alters immune cell composition, including effector and regulatory T cell frequencies; allergen-induced cytokine production; the inducibility of Foxp3+Treg versus IL-10-Treg; and the responsiveness to corticosteroids for induction of IL-10. This ancillary study (R01HL101390, Vitamin D Supplementation in Pregnancy: Impact on Neonatal Immune Phenotype, PI: O’Connor, G) is funded through the Ancillary Studies in Clinical Trials (RFA-HL-09-001) mechanism.
M.4. Other ancillary studies
Other ancillary studies to elucidate mechanisms are planned. We collected whole blood RNA samples from the mothers, cord blood, and are now collecting them at the age 3 yr blood draw, using PAXgene™ tubes. Along with these samples, we have stored white blood cells to allow for DNA extraction. Genomic and epigenomic studies are planned with these samples, for participants with the appropriate consent. Finally, stored serum and plasma samples will allow for other future studies (e.g. metabolomics).
Discussion
VDAART attempts to answer the question of whether vitamin D supplementation to women during pregnancy can prevent the development of asthma and allergies in their children, testing the Developmental Origins of Health and Disease paradigm.89, 90 This trial is unique in that the intervention is occurring in women (albeit pregnant women, and therefore, the intervention is also affecting the growing fetus), with the intervention over approximately 5 to 8 months, and the primary outcome not occurring until up to 3 years after the intervention ends. Most intervention trials in pregnancy investigate maternal outcomes or immediate birth outcomes. Several trials have investigated maternal supplementation of nutrients to assess childhood outcomes in countries with high rates of malnutrition.91–93 A few trials have investigated the prevention of asthma and allergies in children,94–97 although, to our knowledge, none of the published studies have started the intervention as early as 10 weeks gestation. The timing of initiation of the intervention is an important issue, as lung development begins as early as the end of 4 weeks post-fertilization, and progresses through childhood.98 It remains unclear, however, what the critical time period of exposure is for the development of asthma. While we are of the opinion that the ideal design would have been to initiate vitamin D supplementation prior to fertilization, that design is impractical. We, therefore, opted to recruit women as early in their pregnancy as possible, and that had implications with regard to accounting for potential miscarriages in the power calculations.
Another strength of VDAART is the intervention dose of vitamin D selected for pregnant women. This dose was selected because it had been shown to achieve a 25OHD level of at least 80 nmol/L (32 ng/ml) in at least 80% of pregnant women at the end of pregnancy.40 More importantly, this treatment dose is not associated with adverse events attributable to the vitamin D dose. Adherence to the intervention is always an issue in clinical trials. VDAART assessed adherence using the MEMS® system, with monitoring of adherence occurring on a monthly basis during the trial. Because a biomarker is available, we will also be able to assess adherence during the analysis period by measuring the change in 25OHD levels between randomization and the 32–38 week period. Finally, VDAART will have sufficient power to detect effects of the intervention on the study outcomes.
While there is much known about the mechanisms of vitamin D in bone health, not as much is known regarding the role of vitamin D in other outcomes. Our group, therefore, strove to obtain additional funding to perform ancillary studies to elucidate potential mechanisms of vitamin D in pregnancy and fetal immunology. We have been successful in obtaining funding for ancillary studies related to the microbiome and neonatal immune phenotype. These studies are novel and will potentially clarify additional mechanisms by which vitamin D may be operating in pregnancy and in fetal programming.
Some limitations exist. First, there is a possibility that starting the intervention at 10 to 18 weeks may be too late to see any effects on the outcomes, as 25OHD levels do not reach a steady state until a few weeks after initiation of daily dosing. For example, the lung arises at approximately 4 to 6 weeks gestation, and aleveolarization begins at approximately 20 weeks gestation.99 However, as stated above, recruiting women prior to conception would be an impractical design. Given that 25OHD blood levels were not specified at trial entry, we expect variation in these levels in early pregnancy. Thus, we should be able to evaluate the effects of baseline levels on the outcomes of the trial, as secondary analyses. VDAART was designed as a prenatal and not a post-natal intervention study. Since lung development continues after birth, the possibility exists that VDAART may miss a critical developmental phase during the postnatal period. We chose to recruit subjects with a high risk of having an asthmatic or allergic child. The main reason for this is since the risk of asthma or allergies is increased in these families, we would not need to recruit as many participants to obtain a sufficient number of outcomes. While, on one hand, this may somewhat decrease the generalizability of our findings, on the other hand, we are targeting a population that would benefit the most out of this intervention. Finally, VDAART was funded to evaluate the children only through age 3. Since the definitive diagnosis of asthma is difficult prior to the age of 6, the 3-year child follow-up may be too short. We plan to apply for additional funding to follow the children to age 6 years.
The prenatal effect of vitamin D on the development of asthma in children is of great interest to the research community, but studies have reported disparate findings. Studies that have estimated vitamin D intake during pregnancy using food frequency questionnaires have shown a protective effect of higher vitamin D intake on either wheeze, allergy outcomes, or asthma.24, 25, 100, 101 On the other hand, studies measuring vitamin D status either at one point in pregnancy or in cord blood, have not shown this protective effect.27–29, 102 Given these conflicting findings a definitive, novel, prospective, randomized, vitamin D supplementation study, represented by VDAART, is needed to address this important public health issue.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yunginger JW, Reed CE, O'Connell EJ, Melton LJ, 3rd, O'Fallon WM, Silverstein MD. A community-based study of the epidemiology of asthma. Incidence rates, 1964–1983. Am Rev Respir Dis. 1992;146(4):888–894. doi: 10.1164/ajrccm/146.4.888. [DOI] [PubMed] [Google Scholar]
- 2.King ME, Mannino DM, Holguin F. Risk factors for asthma incidence. A review of recent prospective evidence. Panminerva Med. 2004;46(2):97–110. [PubMed] [Google Scholar]
- 3.Mannino DM, Homa DM, Akinbami LJ, Moorman JE, Gwynn C, Redd SC. Surveillance for asthma--United States, 1980–1999. MMWR Surveill Summ. 2002;51(1):1–13. [PubMed] [Google Scholar]
- 4.CDC. Asthma prevalence, health care use, and mortality, 2002. Hyattsville, MD: US Department of Health and Human Services, CDC, National Center for Health Statistics; 2004. [Google Scholar]
- 5.Smith DH, Malone DC, Lawson KA, Okamoto LJ, Battista C, Saunders WB. A national estimate of the economic costs of asthma. Am J Respir Crit Care Med. 1997;156(3 Pt 1):787–793. doi: 10.1164/ajrccm.156.3.9611072. [DOI] [PubMed] [Google Scholar]
- 6.Adams PF, Hendershoot GE, Marano MA. Current Estimates from the National Health Interview Survey, 1996. Hyattsville, MD: National Center for Health Statistics; 1999. Vital Health Stat Ser 10. [PubMed] [Google Scholar]
- 7.De Luca G, Olivieri F, Melotti G, Aiello G, Lubrano L, Boner AL. Fetal and early postnatal life roots of asthma. J Matern Fetal Neona. 2010;23(Suppl 3):80–83. doi: 10.3109/14767058.2010.509931. [DOI] [PubMed] [Google Scholar]
- 8.Henderson AJ, Warner JO. Fetal origins of asthma. Semin Fetal Neonatal Med. 2012;17(2):82–91. doi: 10.1016/j.siny.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R. Prenatal factors and the development of asthma. Curr Opin Pediatr. 2008;20(6):682–687. doi: 10.1097/MOP.0b013e3283154f26. [DOI] [PubMed] [Google Scholar]
- 10.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 11.Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76(1):187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 12.Hollis BW. Assessment of vitamin D status and definition of a normal circulating range of 25-hydroxyvitamin D. Curr Opin Endocrinol Diabetes Obes. 2008;15(6):489–494. doi: 10.1097/MED.0b013e328317ca6c. [DOI] [PubMed] [Google Scholar]
- 13.Hollis BW, Wagner CL, Drezner MK, Binkley NC. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J Steroid Biochem Mol Biol. 2007;103(3–5):631–634. doi: 10.1016/j.jsbmb.2006.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.US Environmental Protection Agency. Report to Congress on indoor air quality, volume II: assessment and control of indoor air pollution, Report No. EPA 400-1-89-001C. Washington, DC: EPA; 1989. [Google Scholar]
- 15.Hollis BW, Wagner CL. Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nutr. 2004;79(5):717–726. doi: 10.1093/ajcn/79.5.717. [DOI] [PubMed] [Google Scholar]
- 16.Hollis BW, Wagner CL. Vitamin D deficiency during pregnancy: an ongoing epidemic. Am J Clin Nutr. 2006;84(2):273. doi: 10.1093/ajcn/84.1.273. [DOI] [PubMed] [Google Scholar]
- 17.Hollis BW, Wagner CL. Nutritional vitamin D status during pregnancy: reasons for concern. Cmaj. 2006;174(9):1287–1290. doi: 10.1503/cmaj.060149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez PA, Idrisa A, Bobzom DN, Airede A, Hollis BW, Liston DE, et al. Calcium and vitamin D status of pregnant teenagers in Maiduguri, Nigeria. J Natl Med Assoc. 1997;89(12):805–811. [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JM, Smith JR, Philipp BL, Chen TC, Mathieu J, Holick MF. Vitamin d deficiency in a healthy group of mothers and newborn infants. Clin Pediatr (Phila) 2007;46(1):42–44. doi: 10.1177/0009922806289311. [DOI] [PubMed] [Google Scholar]
- 20.Rehan VK, Torday JS. Vitamin D and Lung Development in Early Life. In: Litonjua AA, editor. Vitamin D and the Lung: Mechanisms and Disease Associations. New York: Springer-Humana Press; 2012. pp. 41–58. [Google Scholar]
- 21.Vazirnia A, Liu PT. Vitamin D and the Innate Immune Response. In: Litonjua AA, editor. Vitamin D and the Lung: Mechanisms and Disease Associations. New York: Springer - Humana Press; 2012. pp. 59–84. [Google Scholar]
- 22.Urry Z, Dimeloe S, Hawrylowicz CM. Vitamin D and Regulatory T Cells. In: Litonjua AA, editor. Vitamin D and the Lung: Mechanisms and Disease Associations. New York: Springer - Humana Press; 2012. pp. 85–102. [Google Scholar]
- 23.Adorini L, Laverny G, Penna G. Dendritic Cell Modulation by the Vitamin D System. In: Litonjua AA, editor. Vitamin D and the Lung: Mechanisms and Disease Associations. New York: Springer - Humana Press; 2012. pp. 103–126. [Google Scholar]
- 24.Camargo J CA, Rifas-Shiman SL, Litonjua AA, Rich-Edwards JW, Weiss ST, Gold DR, et al. Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at age 3 years. Am J Clin Nutr. 2007;85:788–795. doi: 10.1093/ajcn/85.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devereux G, Litonjua AA, Turner S, Craig L, McNeill G, Martindale S, et al. Maternal vitamin D intake during pregnancy and early childhood wheezing. Am J Clin Nutr. 2007;85:853–859. doi: 10.1093/ajcn/85.3.853. [DOI] [PubMed] [Google Scholar]
- 26.Litonjua AA. Vitamin D deficiency as a risk factor for childhood allergic disease and asthma. Curr Opin Allergy Clin Immunol. 2012;12(2):179–185. doi: 10.1097/ACI.0b013e3283507927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, et al. Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr. 2007 doi: 10.1038/sj.ejcn.1602680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camargo CA, Jr, Ingham T, Wickens K, Thadhani R, Silvers KM, Epton MJ, et al. Cord-blood 25-hydroxyvitamin D levels and risk of respiratory infection, wheezing, and asthma. Pediatrics. 2011;127(1):e180–e187. doi: 10.1542/peds.2010-0442. [DOI] [PubMed] [Google Scholar]
- 29.Morales E, Romieu I, Guerra S, Ballester F, Rebagliato M, Vioque J, et al. Maternal Vitamin D Status in Pregnancy and Risk of Lower Respiratory Tract Infections, Wheezing, and Asthma in Offspring. Epidemiology. 2012;23(1) doi: 10.1097/EDE.0b013e31823a44d3. [DOI] [PubMed] [Google Scholar]
- 30.Rothers J, Wright AL, Stern DA, Halonen M, Camargo CA., Jr Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. The Journal of allergy and clinical immunology. 2011;128(5):1093–1099. e5. doi: 10.1016/j.jaci.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Litonjua AA. Dietary factors and the development of asthma. Immunol Allergy Clin North Am. 2008;28(3):603–629. ix. doi: 10.1016/j.iac.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. http://www.mwvaardex.com/Products/DataCollection/MEMSCap/index.htm.
- 33.Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med. 2001;134(10):968–977. doi: 10.7326/0003-4819-134-10-200105150-00011. [DOI] [PubMed] [Google Scholar]
- 34.Namkoong K, Farren CK, O'Connor PG, O'Malley SS. Measurement of compliance with naltrexone in the treatment of alcohol dependence: research and clinical implications. J Clin Psychiatry. 1999;60(7):449–453. doi: 10.4088/jcp.v60n0706. [DOI] [PubMed] [Google Scholar]
- 35.Gillman MW. Developmental origins of health and disease. N Engl J Med. 2005;353(17):1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Food and Nutrition Board. Institute of Medicine. Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 37.Lehtonen-Veromaa M, Mottonen T, Irjala K, Karkkainen M, Lamberg-Allardt C, Hakola P, et al. Vitamin D intake is low and hypovitaminosis D common in healthy 9- to 15-year-old Finnish girls. Eur J Clin Nutr. 1999;53(9):746–751. doi: 10.1038/sj.ejcn.1600844. [DOI] [PubMed] [Google Scholar]
- 38.Vieth R, Cole DE, Hawker GA, Trang HM, Rubin LA. Wintertime vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr. 2001;55(12):1091–1097. doi: 10.1038/sj.ejcn.1601275. [DOI] [PubMed] [Google Scholar]
- 39.Datta S, Alfaham M, Davies DP, Dunstan F, Woodhead S, Evans J, et al. Vitamin D deficiency in pregnant women from a non-European ethnic minority population--an interventional study. BJOG. 2002;109(8):905–908. doi: 10.1111/j.1471-0528.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- 40.Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL. Vitamin D supplementation during pregnancy: double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res. 2011;26(10):2341–2357. doi: 10.1002/jbmr.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroenke K, Spitzer RL, Williams JB, Lowe B. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345–359. doi: 10.1016/j.genhosppsych.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 42.Wang X, Chen C, Wang L, Chen D, Guang W, French J. Conception, early pregnancy loss, and time to clinical pregnancy: a population-based prospective study. Fertil Steril. 2003;79(3):577–584. doi: 10.1016/s0015-0282(02)04694-0. [DOI] [PubMed] [Google Scholar]
- 43.Litonjua AA, Carey VJ, Burge HA, Weiss ST, Gold DR. Parental history and the risk for childhood asthma. Does mother confer more risk than father? Am J Respir Crit Care Med. 1998;158(1):176–181. doi: 10.1164/ajrccm.158.1.9710014. [DOI] [PubMed] [Google Scholar]
- 44.Litonjua AA, Rifas-Shiman SL, Ly NP, Tantisira KG, Rich-Edwards JW, Camargo J CA, et al. Maternal antioxidant intake in pregnancy and wheezing illnesses at 2 years of age. Am J Clin Nutr. 2006;84:903–911. doi: 10.1093/ajcn/84.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ramsey CD, Gold DR, Litonjua AA, Sredl DL, Ryan L, Celedon JC. Respiratory illnesses in early life and asthma and atopy in childhood. J Allergy Clin Immunol. 2007;119(1):150–156. doi: 10.1016/j.jaci.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 46.Weiss ST, Tager IB, Speizer FE, Rosner B. Persistent wheeze: its relation to respiratory illness, cigarette smoking, and level of pulmonary function in a population sample of children. Am Rev Respir Dis. 1980;122:697–707. doi: 10.1164/arrd.1980.122.5.697. [DOI] [PubMed] [Google Scholar]
- 47.Weiss ST, Tager IB, Weiss JW, Munoz A, Speizer FE, Ingram RH. Airways responsiveness in a population sample of adults and children. Am Rev Respir Dis. 1984;129(6):898–902. doi: 10.1164/arrd.1984.129.6.898. [DOI] [PubMed] [Google Scholar]
- 48.Galant SP, Morphew T, Amaro S, Liao O. Current asthma guidelines may not identify young children who have experienced significant morbidity. Pediatrics. 2006;117(4):1038–1045. doi: 10.1542/peds.2005-1076. [DOI] [PubMed] [Google Scholar]
- 49.Celedon JC, Litonjua AA, Ryan L, Weiss ST, Gold DR. Day care attendance, respiratory tract illnesses, wheezing, asthma and total serum IgE level in early childhood. Arch Pediatr Adolesc Med. 2002;156:241–245. doi: 10.1001/archpedi.156.3.241. [DOI] [PubMed] [Google Scholar]
- 50.Celedon JC, Wright RJ, Litonjua AA, Sredl D, Ryan L, Weiss ST, et al. Day care attendance in early life, maternal history of asthma, and asthma at the age of 6 years. Am J Respir Crit Care Med. 2003;167(9):1239–1243. doi: 10.1164/rccm.200209-1063OC. [DOI] [PubMed] [Google Scholar]
- 51.Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4 Pt 1):1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- 52.Ly NP, Gold DR, Weiss ST, Celedon JC. Recurrent wheeze in early childhood and asthma at age 7 years among children at risk for atopy. Proc Am Thoracic Soc. 2005;2:A700. doi: 10.1542/peds.2005-2271. [DOI] [PubMed] [Google Scholar]
- 53.Park JH, Gold DR, Spiegelman DL, Burge HA, Milton DK. House dust endotoxin and wheeze in the first year of life. Am J Respir Crit Care Med. 2001;163(2):322–328. doi: 10.1164/ajrccm.163.2.2002088. [DOI] [PubMed] [Google Scholar]
- 54.Gold DR, Burge HA, Carey VJ, Milton DK, Platts-Mills T, Weiss ST. Predictors of repeated wheeze in the first year of life. The relative roles of cockroach birth weight, acute lower respiratory illness, and maternal smoking. Am J Respir Crit Care Med. 1999;160(1):227–236. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- 55.Gehring U, Bolte G, Borte M, Bischof W, Fahlbusch B, Wichmann HE, et al. Exposure to endotoxin decreases the risk of atopic eczema in infancy: a cohort study. J Allergy Clin Immunol. 2001;108(5):847–854. doi: 10.1067/mai.2001.119026. [DOI] [PubMed] [Google Scholar]
- 56.Bergmann RL, Edenharter G, Bergmann KE, Forster J, Bauer CP, Wahn V, et al. Atopic dermatitis in early infancy predicts allergic airway disease at 5 years. Clin Exp Allergy. 1998;28:965–970. doi: 10.1046/j.1365-2222.1998.00371.x. [DOI] [PubMed] [Google Scholar]
- 57.Phipatanakul W, Celedon JC, Raby BA, Litonjua AA, Milton DK, Sredl D, et al. Endotoxin exposure and eczema in the first year of life. Pediatrics. 2004;114(1):13–18. doi: 10.1542/peds.114.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barbee RA, Halonen M, Lebowitz M, Burrows B. Distribution of IgE in a community population sample: correlations with age, sex, and allergen skin test reactivity. J All Clin Immun. 1981;68:106–111. doi: 10.1016/0091-6749(81)90167-6. [DOI] [PubMed] [Google Scholar]
- 59.Ersfeld DL, Rao DS, Body JJ, Sackrison JL, Jr, Miller AB, Parikh N, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37(10):867–874. doi: 10.1016/j.clinbiochem.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 60.Phillips KM, Byrdwell WC, Exler J, Harnly JM, Holden JM, Holick MF, et al. Development and validation of control materials for the measurement of vitamin D3 in selected US foods. J Food Compos Anal. 2008;21:527–534. [Google Scholar]
- 61.Riekert KA, Rand CS. Electronic monitoring of medication adherence: When is high-tech best? J Clin Psychol Med Settings. 2002;9:25–34. [Google Scholar]
- 62.Sorkness CA, Lemanske RF, Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long-term comparison of 3 controller regimens for mild-moderate persistent childhood asthma: The Pediatric Asthma Controller Trial. The Journal of allergy and clinical immunology. 2007;119(1):64–72. doi: 10.1016/j.jaci.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 63.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. The Journal of allergy and clinical immunology. 2005;115(2):233–242. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 64.Rand C, Bilderback A, Schiller K, Edelman JM, Hustad CM, Zeiger RS. for The MIAMI Study Research Group. Adherence with montelukast or fluticasone in a long-term clinical trial: results from the Mild Asthma Montelukast Versus Inhaled Corticosteroid (MIAMI) study. J Allergy Clin Immunol. 2007 doi: 10.1016/j.jaci.2006.12.664. in Press. [DOI] [PubMed] [Google Scholar]
- 65.Reboussin DM, DeMets DL, Kim KM, Lan KK. Computations for group sequential boundaries using the Lan-DeMets spending function method. Control Clin Trials. 2000;21(3):190–207. doi: 10.1016/s0197-2456(00)00057-x. [DOI] [PubMed] [Google Scholar]
- 66.Kimball SM, Ursell MR, O'Connor P, Vieth R. Safety of vitamin D3 in adults with multiple sclerosis. Am J Clin Nutr. 2007;86(3):645–651. doi: 10.1093/ajcn/86.3.645. [DOI] [PubMed] [Google Scholar]
- 67.Vieth R, Chan PC, MacFarlane GD. Efficacy and safety of vitamin D3 intake exceeding the lowest observed adverse effect level. Am J Clin Nutr. 2001;73(2):288–294. doi: 10.1093/ajcn/73.2.288. [DOI] [PubMed] [Google Scholar]
- 68.Gertner JM, Coustan DR, Kliger AS, Mallette LE, Ravin N, Broadus AE. Pregnancy as state of physiologic absorptive hypercalciuria. Am J Med. 1986;81(3):451–456. doi: 10.1016/0002-9343(86)90298-6. [DOI] [PubMed] [Google Scholar]
- 69.Seely EW, Brown EM, DeMaggio DM, Weldon DK, Graves SW. A prospective study of calciotropic hormones in pregnancy and post partum: reciprocal changes in serum intact parathyroid hormone and 1,25-dihydroxyvitamin D. American journal of obstetrics and gynecology. 1997;176(1 Pt 1):214–217. doi: 10.1016/s0002-9378(97)80039-7. [DOI] [PubMed] [Google Scholar]
- 70.McLoughlin RM, Mills KH. Influence of gastrointestinal commensal bacteria on the immune responses that mediate allergy and asthma. The Journal of allergy and clinical immunology. 2011;127(5):1097–1107. doi: 10.1016/j.jaci.2011.02.012. quiz 108-9. [DOI] [PubMed] [Google Scholar]
- 71.Ly NP, Litonjua A, Gold DR, Celedon JC. Gut microbiota, probiotics, and vitamin D: interrelated exposures influencing allergy, asthma, and obesity? The Journal of allergy and clinical immunology. 2011;127(5):1087–1094. doi: 10.1016/j.jaci.2011.02.015. quiz 95-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weiss ST. Bacterial components plus vitamin D: the ultimate solution to the asthma (autoimmune disease) epidemic? The Journal of allergy and clinical immunology. 2011;127(5):1128–1130. doi: 10.1016/j.jaci.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lagishetty V, Misharin AV, Liu NQ, Lisse TS, Chun RF, Ouyang Y, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010;151(6):2423–2432. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mai V, McCrary QM, Sinha R, Glei M. Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutr J. 2009;8:49. doi: 10.1186/1475-2891-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goffinet F, Maillard F, Mihoubi N, Kayem G, Papiernik E, Cabrol D, et al. Bacterial vaginosis: prevalence and predictive value for premature delivery and neonatal infection in women with preterm labour and intact membranes. Eur J Obstet Gynecol Reprod Biol. 2003;108(2):146–151. doi: 10.1016/s0301-2115(02)00423-2. [DOI] [PubMed] [Google Scholar]
- 76.Leitich H, Bodner-Adler B, Brunbauer M, Kaider A, Egarter C, Husslein P. Bacterial vaginosis as a risk factor for preterm delivery: a meta-analysis. American journal of obstetrics and gynecology. 2003;189(1):139–147. doi: 10.1067/mob.2003.339. [DOI] [PubMed] [Google Scholar]
- 77.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139(6):1157–1161. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Davis LM, Chang SC, Mancini J, Nathanson MS, Witter FR, O'Brien KO. Vitamin D insufficiency is prevalent among pregnant African American adolescents. J Pediatr Adolesc Gynecol. 2010;23(1):45–52. doi: 10.1016/j.jpag.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 79.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Urry Z, Xystrakis E, Richards DF, McDonald J, Sattar Z, Cousins DJ, et al. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J Clin Invest. 2009;119(2):387–398. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Penna G, Roncari A, Amuchastegui S, Daniel KC, Berti E, Colonna M, et al. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+Foxp3+ regulatory T cells by 1,25-dihydroxyvitamin D3. Blood. 2005;106(10):3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- 82.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol. 2005;5(4):271–283. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 83.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106(12):R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haddeland U, Karstensen AB, Farkas L, Bo KO, Pirhonen J, Karlsson M, et al. Putative regulatory T cells are impaired in cord blood from neonates with hereditary allergy risk. Pediatr Allergy Immunol. 2005;16(2):104–112. doi: 10.1111/j.1399-3038.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 85.Hartl D, Koller B, Mehlhorn AT, Reinhardt D, Nicolai T, Schendel DJ, et al. Quantitative and functional impairment of pulmonary CD4+CD25hi regulatory T cells in pediatric asthma. The Journal of allergy and clinical immunology. 2007;119(5):1258–1266. doi: 10.1016/j.jaci.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 86.Schaub B, Liu J, Hoppler S, Haug S, Sattler C, Lluis A, et al. Impairment of T-regulatory cells in cord blood of atopic mothers. The Journal of allergy and clinical immunology. 2008;121(6):1491–1499. e1–13. doi: 10.1016/j.jaci.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 87.Smith M, Tourigny MR, Noakes P, Thornton CA, Tulic MK, Prescott SL. Children with egg allergy have evidence of reduced neonatal CD4(+)CD25(+)CD127(lo/-) regulatory T cell function. The Journal of allergy and clinical immunology. 2008;121(6):1460–1466. 6 e1–6 e7. doi: 10.1016/j.jaci.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 88.Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116(1):146–155. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gillman MW. Developmental origins of health and disease. New Engl J Med. 2005;353(17):1848–1850. doi: 10.1056/NEJMe058187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Duijts L. Fetal and infant origins of asthma. Eur J Epidemiol. 2012;27(1):5–14. doi: 10.1007/s10654-012-9657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Prado EL, Alcock KJ, Muadz H, Ullman MT, Shankar AH. Maternal multiple micronutrient supplements and child cognition: a randomized trial in Indonesia. Pediatrics. 2012;130(3):e536–e546. doi: 10.1542/peds.2012-0412. [DOI] [PubMed] [Google Scholar]
- 92.Persson LA, Arifeen S, Ekstrom EC, Rasmussen KM, Frongillo EA, Yunus M. Effects of prenatal micronutrient and early food supplementation on maternal hemoglobin, birth weight, and infant mortality among children in Bangladesh: the MINIMat randomized trial. Jama. 2012;307(19):2050–2059. doi: 10.1001/jama.2012.4061. [DOI] [PubMed] [Google Scholar]
- 93.Christian P, Stewart CP, LeClerq SC, Wu L, Katz J, West KP, Jr, et al. Antenatal and postnatal iron supplementation and childhood mortality in rural Nepal: a prospective follow-up in a randomized, controlled community trial. Am J Epidemiol. 2009;170(9):1127–1136. doi: 10.1093/aje/kwp253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mihrshahi S, Peat JK, Webb K, Tovey ER, Marks GB, Mellis CM, et al. The childhood asthma prevention study (CAPS): design and research protocol of a randomized trial for the primary prevention of asthma. Control Clin Trials. 2001;22(3):333–354. doi: 10.1016/s0197-2456(01)00112-x. [DOI] [PubMed] [Google Scholar]
- 95.Ou CY, Kuo HC, Wang L, Hsu TY, Chuang H, Liu CA, et al. Prenatal and postnatal probiotics reduces maternal but not childhood allergic diseases: a randomized, double-blind, placebo-controlled trial. Clin Exp Allergy. 2012;42(9):1386–1396. doi: 10.1111/j.1365-2222.2012.04037.x. [DOI] [PubMed] [Google Scholar]
- 96.Palmer DJ, Sullivan T, Gold MS, Prescott SL, Heddle R, Gibson RA, et al. Effect of n-3 long chain polyunsaturated fatty acid supplementation in pregnancy on infants' allergies in first year of life: randomised controlled trial. Bmj. 2012;344:e184. doi: 10.1136/bmj.e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brunekreef B, Smit J, de Jongste J, Neijens H, Gerritsen J, Postma D, et al. The prevention and incidence of asthma and mite allergy (PIAMA) birth cohort study: design and first results. Pediatr Allergy Immunol. 2002;13(Suppl 15):55–60. doi: 10.1034/j.1399-3038.13.s.15.1.x. [DOI] [PubMed] [Google Scholar]
- 98.Burri PH. Fetal and postnatal development of the lung. Annu Rev Physiol. 1984;46:617–628. doi: 10.1146/annurev.ph.46.030184.003153. [DOI] [PubMed] [Google Scholar]
- 99.Shi W, Bellusci S, Warburton D. Lung development and adult lung diseases. Chest. 2007;132(2):651–656. doi: 10.1378/chest.06-2663. [DOI] [PubMed] [Google Scholar]
- 100.Erkkola M, Kaila M, Nwaru BI, Kronberg-Kippila C, Ahonen S, Nevalainen J, et al. Maternal vitamin D intake during pregnancy is inversely associated with asthma and allergic rhinitis in 5-year-old children. Clin Exp Allergy. 2009;39(6):875–882. doi: 10.1111/j.1365-2222.2009.03234.x. [DOI] [PubMed] [Google Scholar]
- 101.Miyake Y, Sasaki S, Tanaka K, Hirota Y. Dairy food, calcium and vitamin D intake in pregnancy, and wheeze and eczema in infants. Eur Respir J. 2010;35(6):1228–1234. doi: 10.1183/09031936.00100609. [DOI] [PubMed] [Google Scholar]
- 102.Rothers J, Wright AL, Stern DA, Halonen M, Camargo CA., Jr Cord blood 25-hydroxyvitamin D levels are associated with aeroallergen sensitization in children from Tucson, Arizona. J Allergy Clin Immunol. 2011;128(5):1093–1099. e5. doi: 10.1016/j.jaci.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]