Abstract
Background and aims
Several studies have found that brief interventions (BIs) for drug misuse have superior effectiveness to no-treatment controls. However, many health centers do not provide BIs for drug use consistently due to insufficient behavioral health staff capacity. Computerized BIs for drug use are a promising approach, but their effectiveness compared with in-person BIs has not been established. This study compared the effectiveness of a computerized brief intervention (CBI) to an in-person brief intervention (IBI) delivered by a behavioral health counselor.
Methods
Two-arm randomized clinical trial, conducted in two health centers in New Mexico, USA. Participants were 360 adult primary care patients with moderate-risk drug scores on the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST) who were randomly assigned on a 1:1 basis to a computerized brief intervention (CBI) or to an in-person brief intervention (IBI) delivered by a behavioral health counselor. Assessments were conducted at baseline and 3-month follow-up, and included the ASSIST and drug testing on hair samples.
Results
The IBI and CBI conditions did not differ at 3 months on global ASSIST drug scores (b=−1.79; 95% CI=−4.37,0.80) or drug-positive hair tests (OR=.97; 95% CI= 0.47,2.02). There was a statistically significant advantage of CBI over IBI in substance-specific ASSIST scores for marijuana (b=−1.73; 95% CI= −2.91,−0.55; Cohen’s d=.26; p=.004) and cocaine (b= −4.48; 95% CI= −8.26,−0.71; Cohen’s d=.50; p=.021) at 3 months.
Conclusions
Computerized brief intervention can be an effective alternative to in-person brief intervention for addressing moderate drug use in primary care.
Keywords: brief intervention, computer technology, substance abuse
Introduction
To address the international need for drug abuse intervention programs, the World Health Organization (WHO) developed the Alcohol, Smoking, and Substance Involvement Screening Tests (ASSIST) for use in primary care settings (1). Subsequently, a multi-site randomized clinical trial (RCT) conducted in four countries to evaluate a brief intervention (BI) for primary care patients with moderate drug use problems found that the group assigned to a staff-delivered BI had significantly reduced ASSIST scores at 3 months compared to the group assigned to a delayed-intervention control (2).
An RCT in the US found that peer educator-delivered BI for drug misuse can be effective in reducing heroin and cocaine use among primary care patients (3), while a trial in Germany found that counselor-delivered BI was effective in reducing prescription drug misuse in non-treatment-seeking hospital patients (4). Over the past decade, the U.S. government has supported the implementation of screening, brief intervention, and referral to treatment (SBIRT) services in medical settings (5), and SBIRT for drugs and alcohol is an important part of the US drug control strategy (6). A multi-site, pre-post evaluation of this effort showed that SBIRT could be widely implemented and that patients reported reductions in alcohol and drug use and associated problems (5).
There are several barriers to delivering BIs in primary care. Medical providers may have insufficient time for screening and prevention activities (7) and may not be inclined to discuss substance use (8). Behavioral health counselors (BHCs) can provide BIs but this may not fit within the budgets of primary care organizations.
The use of technology to provide psychosocial interventions is rapidly growing, as is evidence for its efficacy in a range of settings. Computerized BIs (CBIs) may have certain advantages over in-person BIs, including low cost, reliability, little need for training, a relatively small time commitment from providers (9), as well as the potential for greater patient disclosure of substance use and associated problems (10). Moreover, reviews and meta-analyses have found support for the efficacy of technology-delivered interventions for substance use and other health behaviors (11–14). At least three RCTs found support for CBIs specifically for drug use. Ondersma et al. (15) found a CBI to be more effective than a no-intervention control condition in reducing drug use among post-partum women (15)(16). Likewise, Gilbert et al. (17) found positive results for a CBI for drug use among HIV-positive adults.
Despite such evidence, the relative effectiveness of in-person v. computer-delivered BI for illicit drug use has not been previously tested. The present study was designed to address this gap by comparing CBI with a BI delivered by a behavioral health counselor (henceforth called in-person BI, or IBI) for primary care patients with illicit drug misuse.
Methods
Research Design and Hypotheses
This was a parallel, two-arm RCT that compared a CBI to an IBI delivered by a behavioral health counselor for moderate-risk drug use among 360 adult primary care patients who were not seeking drug treatment. Drug problems were assessed at baseline and at 3-month follow-up by repeated administration of the ASSIST and drug testing of hair samples. We hypothesized that CBI would be superior to IBI in reducing drug use problems at 3-month follow-up based on the premise that patients who are not seeking drug treatment services may be more comfortable completing a self-directed CBI than talking with a counselor. Computerized assessment has been shown to prompt increased disclosure of sensitive risk behaviors compared to face-to-face interviews (10, 18, 19). Moreover, the CBI would deliver appropriate content reliably, whereas the counselor may be less reliable due to competing demands at the clinic. We also hypothesized that the effect of CBI would be moderated by participants’ computer experience, such that CBI would be most effective for participants with greater computer experience.
Study Sites
The study was conducted between June 2010 and December 2012 at two community health centers in New Mexico (NM) in collaboration with the Sangre de Cristo Community Health Partnership (SDCCHP), a non-profit organization that had provided IBIs throughout New Mexico (NM) for 4 years prior to the trial (5, 20–22).
Eligibility Criteria
Primary care or dental patients ages 18 and older were eligible if they had a substance-specific risk score on the ASSIST between 4 and 26 (moderate-risk) for any illicit drug category, including nonmedical use of prescription drugs. Scores over 4 indicate at least weekly use, or less frequent use with the endorsement of drug-related problems. Because the CBI was not designed to provide referral-to-treatment or to address severe problems, individuals were excluded if they scored as high-risk on the ASSIST for alcohol or any drug except tobacco. Individuals were excluded if they had: not used illicit drugs within the past 3 months; drug treatment within the past year; an IBI at the clinic within the past month; or plans to move out of NM in the next year.
Recruitment
Research assistants (RAs) recruited patients in the clinics’ waiting areas and screened for eligibility in a private office. Screened patients were informed that their responses would not be shared with the clinic. Those eligible were offered informed consent and the study was approved by the Friends Research Institute and a local Institutional Review Board (IRB).
Assessment Procedure
The RA administered the following brief assessment battery, which was limited to approximately 15 minutes in duration, in an attempt to limit assessment reactivity (2, 23).
Participant Characteristics Questionnaire included age, gender, marital and employment status, and level of education.
ASSIST, which takes 5–10 minutes to complete, gauges patterns of use and problems related to tobacco, alcohol, non-medical prescription drug and illicit drug use (1, 24, 25). It was administered at baseline and three-month follow-up. The primary outcome measure was the Global Continuum of Illicit Drug Risk (GCIDR) score. Substance-specific risk scores were examined as secondary outcomes among those participants who reported moderate-risk use of each respective substance at baseline, following the approach of the WHO’s RCT of BI (2). The GCIDR captures risks accounting for polydrug use or switching drugs, whereas the substance-specific scores examine the risk by drug type.
Hair Testing for Drug Use. The RA collected 3.8 cm of scalp hair (or leg or under arm hair if scalp hair was insufficient) at baseline and follow-up, corresponding to a 90-day period, which aligns with the time frame covered by the ASSIST. Samples were sent to a certified independent laboratory for analysis of marijuana, cocaine, amphetamine/methamphetamine, and opioids (including morphine, heroin metabolite, and codeine, but not oxycodone testing because the latter was not available at the laboratory at the time of the study) by radioimmunoassay with gas chromatography/mass spectrometry (GC/MS) confirmation.
Computer Experience was assessed at baseline using the 6-item general competence subscale of the Computer Understanding and Experience (CUE) questionnaire (26) which asks respondents to rate agreement with several statements on a 5-point Likert scale (e.g.“I know what email is”, 71% strongly agree; 25% agree); “I am good at using computers”, 33% strongly agree; 27% agree). The CUE was found to be an internally consistent measure of computer experience, with good construct validity, and a lack of correlation with age or enrollment in a 4-year college (26). The CUE general competence subscale had high internal consistency in the current sample (Cronbach’s α = .85). The mean score on the CUE was 20.9 (SD=5.8).
Random Assignment
Within site, participants were randomly assigned to either CBI or IBI using a block randomization procedure such that for each successive block of four participants, two were assigned randomly to each study condition. Numbered, opaque envelopes containing assignments were prepared for each site by the Program Manager. After completing the baseline assessment (during which both participants and the RAs were blind to condition, although it is theoretically possible that the latter might have deduced the assignment), RAs opened the appropriate envelope to reveal the assigned condition. The RA escorted participants assigned to IBI to the BHC, or gave a tablet computer with headphones to those assigned to CBI. Participants received $20 for their baseline interview after they completed their assigned intervention.
Three-month follow-up assessments consisted of a repeat ASSIST and hair sample collection. Most interviews were conducted at the clinic, although 12 were conducted by phone. Participants were paid $20 for this interview.
Interventions
In-person Brief Intervention (IBI) condition was the standard of care at each clinic for several years. The IBI was delivered by a BHC who functioned as part of the health care team. Both clinics had a masters-level BHC on-site who had received extensive training in motivational interviewing (27) and ongoing clinical supervision from a PhD-level psychologist at SDCCHP, and both had multiple years of experience providing alcohol and drug BIs at the clinics prior to the study. The IBI was based on standard BI and motivational interviewing techniques, with interventions taking 14 minutes, on average, based on counselor report. Counselors were informed that the study was focused on drugs, but that they could also discuss alcohol as needed. They reported doing so in 67% of the interventions.
Computerized Brief Intervention (CBI) was created using an intervention authoring tool developed by Ondersma and colleagues (9) who have reported good acceptability and preliminary efficacy of interventions designed with this platform (9, 15, 16, 28, 29). This software uses a talking, animated narrator and synchronous interactivity to replicate many aspects of a traditional BI, including feedback, empathic reflection, and personalization. The content of the intervention was developed by the investigators in collaboration with SDCCHP leadership to mirror the content of IBI. This content incorporated evidence-based intervention strategies, including tailored content based on motivation to change, self-efficacy, and gender-specific normative feedback for each substance derived from national survey data (30–32). (The BHCs were provided with the same normative data for the IBI). The intervention was self-directed and emphasized participants’ choice throughout. Participants could complete up to two substance-specific intervention modules. Participants selected the drug on which they wanted to focus at the beginning of the intervention. The animated narrator asked questions and provided feedback. Participants were shown different content depending on their self-reported level of motivation to cut down or quit drug use, as well as their level of confidence that they could reduce their drug use. After completing the intervention, participants were given the option to complete an additional module for another substance. The focus of the study was on illicit drug use, but participants could select the alcohol module after completing one drug module. One quarter of participants elected to complete two modules (25%), and 16% completed the alcohol module. The CBI, as timed by the software, took an average 7 minutes to complete. Orienting participants to the CBI (e.g., navigation instructions) took an additional 2–3 minutes.
Statistical Analysis
Mean differences in GCIDR and substance-specific ASSIST scores at 3 months were examined using linear regression, adjusting for the baseline value of the respective outcome. Drug-positive hair test results were examined using logistic regression, adjusting for the baseline hair test result. For cases with missing follow-up information, the baseline value was substituted. For a two-group comparison of means, assuming α=.05, two-tailed, the trial’s N of 360 had 80% power to detect an effect size in the population of d=.296, corresponding to the small-to-medium range. To test the secondary hypothesis of differential effects as a function of participant computer experience, the models for the primary outcomes were extended to include an interaction between Condition and the CUE score. [We initially conducted an alternative analysis focused on change rather than endpoint; however, we determined that an analysis of outcome controlling for baseline value was more appropriate given that the significant Condition by Time interaction for the GCIDR ASSIST score (p= .04) was entirely driven by a non-significant baseline difference between IBI and CBI (mean score= 30.0 [SD=14.8] vs. 33.1 [SD=19.9], respectively); and the IBI and CBI conditions were nearly identical at follow-up (mean score= 28.6 [SD=15.3] vs. 28.8 [SD=17.8], respectively)].
Results
Participants
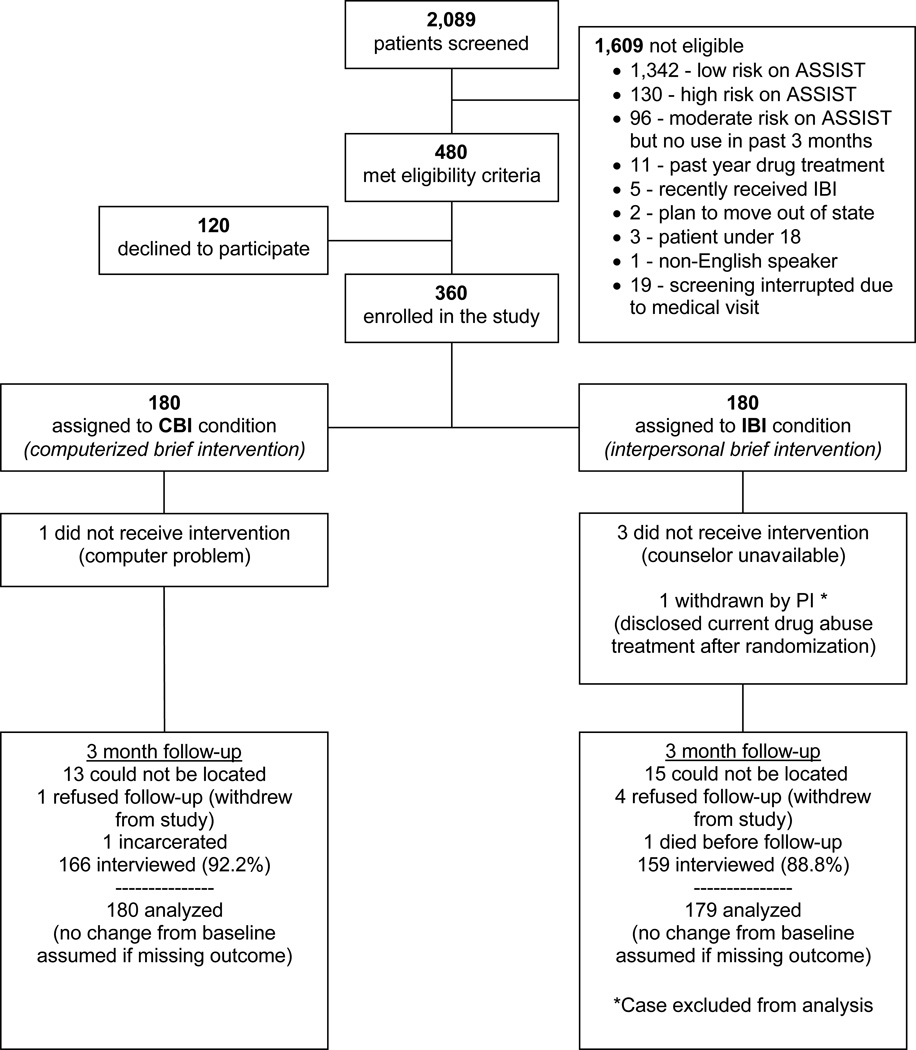
Study screening, enrollment, and follow-up details are shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 1). Enrolled participants had mean GCIDR scores that were about 5 points higher compared to patients who screened eligible but declined study enrollment. There were 360 study participants of whom 180 were randomly assigned to CBI and 180 to IBI. One participant was excluded following randomization after revealing ongoing buprenorphine treatment for opioid dependence, leaving an analysis sample of 359.
Figure 1. Consolidated Standards of Reporting Trials (CONSORT) Diagram.
Note: Percentages are relative to the number of participants assigned to each condition.
A total of 332 of 359 participants (93%) were located at 3-month follow-up. Five participants were located but refused to continue participation, one participant was incarcerated, and one non-study related death occurred prior to follow-up. The remaining 325 individuals completed the 3-month follow-up interview (91% of the full baseline sample).
Participant characteristics by condition are shown in Table 1. Participants were 46% female, 90% White, and 47% Hispanic ethnicity, with a mean age of 36.2 years (SD=14.6). Less than a quarter were currently married (22%), and the majority were not employed (59%), while only 21.7% reported full-time employment. Most reported finishing high school or equivalent education (78%). There was good representation across the sites, with 60% of the sample recruited at the larger clinic.
Table 1.
Participant baseline characteristics (N=359)
| In-Person (N=179) |
Computer BI (N= 180) |
|
|---|---|---|
| Background Characteristics | ||
| Female gender, % (n) | 45.80 (82) | 46.10 (83) |
| White race, % (n) | 89.9 (161) | 90.00 (162) |
| Hispanic Ethnicity, % (n) | 47.49 (85) | 46.11 (83) |
| Age, mean (SD) [range 18–85] | 35.69 (14.57) | 36.54 (14.73) |
| Currently Married, % (n) | 22.35 (40) | 22.22 (40) |
| Not employed, % (n) | 57.54 (103) | 59.44 (107) |
| Employed Part Time, % (n) | 20.11 (36) | 19.44 (35) |
| Employed Full Time, % (n) | 22.35 (40) | 21.11 (38) |
| ≥ High school education, % (n) | 78.21 (140) | 77.22 (139) |
| CUE score, mean (SD) [range 6–30] | 20.64 (5.85) | 21.11 (5.76) |
| Baseline ASSIST Scores, mean (SD) | ||
| Global ASSSIT score | 30.00 (14.77) | 33.11 (19.85) |
| Alcohol score | 8.07 (6.75) | 7.38 (6.81) |
| Marijuana score | 9.91 (5.94) | 9.76 (6.31) |
| Cocaine score | 2.32 (5.32) | 2.49 (5.33) |
| Amphetamines score | 1.13 (3.42) | 1.96 (4.95) |
| Sedatives score | 1.29 (3.70) | 1.84 (4.71) |
| Opioids score | 2.17 (5.02) | 3.28 (6.61) |
| Positive Hair Tests (% positive) | ||
| Marijuana (N=306) | 49.67 (75) | 43.87 (68) |
| Cocaine (N=314) | 20.65 (32) | 18.87 (30) |
| (Meth)Amphetamines (N=314) | 7.10 (11) | 8.81 (14) |
| Opioids (N= 311) | 2.60 (4) | 2.55 (4) |
Notes: Groups do not differ at baseline for any of the variables above (p>.05, two-tailed) using independent samples t tests (continuous variables) and χ2 tests of independence (categorical variables). N=359, except as noted for hair testing.
Baseline Substance Use
As shown in Table 1, marijuana was by far the most prevalent drug used in the sample. At baseline, 88% of participants scored in the moderate-risk range for marijuana use. The percentages of participants with moderate-risk use of other substances were as follows: 28% for alcohol, 20% for opioids, 18% for cocaine, 12% for sedatives, and 11% for amphetamines or methamphetamines. Use of hallucinogens and inhalants was rare, with only 6% and 2% scoring in the moderate-risk range, respectively. About half of participants (48%) scored as moderate-risk on more than one substance (including alcohol). Excluding alcohol, 32% scored as moderate-risk on more than one drug.
Usable hair testing data were obtained on 88% of the baseline sample and 75% of the follow-up sample. Missing data were due to participant refusal, insufficient hair for sampling, the conduct of 12 follow-up interviews by phone, and collected samples that could not be analyzed due to insufficient quantity. There were no significant differences between conditions in obtaining hair samples (p= .99 at baseline; p= .76 at follow-up), and direct refusals to provide samples (12 at baseline; 14 at follow-up) were split evenly across conditions. Overall concordance between hair testing results and self-reported past 3-month substance use at baseline (negative-negative and positive-positive matches) ranged from 57.8% for marijuana to 86.3% for amphetamines.
Global Continuum of Illicit Drug Risk ASSIST Scores and Hair Testing
Table 2 shows mean ASSIST scores by condition. GCIDR scores for the pooled sample decreased from baseline to follow-up by about 3 points, but CBI and IBI conditions were not significantly different at 3-month follow-up (b=−1.79; 95% CI= −4.37,0.80; Cohen’s d =.09; p=.175). Table 3 shows the hair test results at 3 month follow-up. There was virtually no change in the overall prevalence of drug-positive hair tests from baseline to 3 months (62% positive at both baseline and follow-up). There were no differences between conditions in drug-positive hair tests at 3-month follow-up (Adjusted OR= .97; 95% CI=.47,2.02; p=.94).
Table 2.
ASSIST scores at 3 months (N=359)
| In-Person BI | Computer BI | Mean Difference | p-value | |
|---|---|---|---|---|
| mean (SE) | mean (SE) | (95% CI) | ||
| ASSIST Scores | ||||
| Global | ||||
| Global ASSIST score (N=359) | 29.56 (.93) | 27.77 (.93) | −1.79 (−4.37 – 0.80) | .175 |
| Substance-Specific1 | ||||
| Alcohol (n=101) | 13.74 (1.00) | 11.28 (1.22) | −2.47 (−5.62 – 0.69) | .124 |
| Marijuana (n=314) | 11.04 (.43) | 9.31 (.43) | −1.73 (−2.91 – −0.55) | .004 |
| Cocaine (n=66) | 11.02 (1.39) | 6.54 (1.27) | −4.48 (−8.26 – −0.71) | .021 |
| Amphetamines (n=40) | 8.97 (2.38) | 6.35 (1.94) | −2.62 (−8.88 – 3.65) | .403 |
| Sedatives (n=43) | 6.07 (1.48) | 4.19 (1.20) | −1.88 (−5.73 – 1.98) | .331 |
| Opioids (n=72) | 7.99 (1.41) | 6.56 (1.26) | −1.43 (−5.23 – 2.37) | .455 |
Substance-specific ASSIST scores are restricted to participants scoring as moderate risk for the substance at baseline.
Table 3.
Drug-positive hair tests at 3 months (N=359)
| In-Person BI | Computer BI | Odds Ratio | Adjusted Odds Ratio1 | |
|---|---|---|---|---|
| % (n) | % (n) | (95% CI) | (95% CI) | |
| Hair Tests | ||||
| Any drug (n=314) | 64.52 (100) | 58.49 (93) | .78 (.49 – 1.22) | .97 (.47 – 2.02) |
| Marijuana (n=306) | 48.34 (73) | 43.23 (67) | .81 (.52 – 1.28) | .94 (.46 – 1.94) |
| Cocaine (n=314) | 20.65 (32) | 19.50 (31) | .93 (.54 – 1.62) | 1.19 (.31 – 4.56) |
| Amphetamines (n=314) | 7.74 (12) | 8.81 (14) | 1.15 (.51 – 2.57) | .88 (.22 – 3.46) |
| Opiates (n=311) | 1.95 (3) | 2.55 (4) | 1.32 (.29 – 5.98) | 1.67 (.22 – 12.88) |
Adjusts for the hair test result at baseline. Sample is restricted to participants with a baseline hair test result to allow for adjustment.
Differential Effectiveness by Computer Experience
The analysis examining an interaction between Condition and Computer Experience revealed no significant differential effectiveness of CBI vs. IBI based on computer experience in terms of either Global scores (p=.82) or drug-positive hair tests (p=.44). There was no correlation between the CUE score and global ASSIST scores for either the IBI (r= −.02; p=.81) or CBI condition (r=-.02;p=.78).
Substance-Specific Risk Scores
Substance-specific analyses were conducted for alcohol (n=101), marijuana (n=314), cocaine (n=66), amphetamines or methamphetamines (n=40), sedatives (n=43), and opioids (n=72), but not for moderate-risk hallucinogen or inhalant use as there were insufficient cases to analyze these substances separately. At 3 months, participants in the CBI condition had significantly lower mean marijuana risk scores than participants in the IBI condition (b=−1.73; 95% CI=−2.91,0.55; Cohen’s d=.26; p=.004). Likewise, participants in the CBI condition had significantly lower cocaine scores at 3 months than their IBI counterparts (b=−4.48; 95% CI=−8.26,−0.71; Cohen’s d =.50; p=.021). Mean differences between study conditions were non-significant for the other substances examined.
Discussion
This study was the first randomized clinical trial to compare a computerized vs. in-person brief intervention for primary care patients with moderate drug use. We found no significant differences between computer and counselor-delivered brief interventions in the primary outcome measures of Global ASSIST Scores and hair drug testing results. This finding supports the potential utility of computer-delivered BIs as an alternative to in-person BI. While health centers may encourage their medical staff to conduct BIs themselves, obstacles such as time constraints, lack of interest, or inadequate training can undermine consistent delivery of behavioral health interventions (7). Current findings suggest that a computerized BI may be a useful tool in clinics that have insufficient resources to hire counselors or inadequate provider time to consistently deliver BIs for drug use.
In contrast to the primary outcomes, the computer-delivered BI outperformed the counselor-delivered BI on several substance-specific ASSIST risk scores examined as secondary outcomes. CBI compared to IBI produced lower ASSIST scores for marijuana and cocaine risks, with effect sizes in the small-to-medium range. However, these findings for marijuana and cocaine were not corroborated by hair testing results. This failure is not necessarily inconsistent with the self-report findings because the ASSIST scores are weighted more heavily on substance-related problems than frequency of use. Furthermore, hair testing in studies of individuals with moderate or intermittent use may be of limited utility due to missing data (e.g., results were only available for 88% of participants at baseline and 75% at follow-up) and lower sensitivity (as in the study by Bernstein and colleagues (3), in which a number of participants who reported drug use had negative hair tests at baseline, a phenomenon also reflected in the current study).
Although this study provides evidence of similar outcomes for computerized BI and in-person BI, and perhaps an advantage of computerized intervention for some drugs, its findings should not be interpreted to suggest that counselor interventions are not useful. There are many circumstances in which a counselor will be preferable to even highly advanced computer intervention systems, including the need to provide drug abuse counseling of a complexity beyond that of a BI, to refer patients with substance use disorders to specialty drug treatment, and to deliver mental health treatment.
This study had a number of strengths, including a large sample size, two study sites, random assignment, and the use of both self-report and biological outcomes. Its study design was fashioned in part to mirror the multi-site international study by Humeniuk and colleagues (2), in that its inclusion criteria included the same moderate risk ASSIST score range, the Global ASSIST score was the primary outcome measure, individual substance ASSIST scores served as secondary outcome measures, the BI incorporated motivational interviewing techniques, and outcomes were measured at baseline and 3-month follow-up. Unlike previous studies in which the clinician that delivered the BI was responsible for collecting follow-up data on drug use (2, 5, 21, 33), in the present study, the research assistants were distinct from the counselors. Furthermore, the study’s counselors were masters-level clinicians with extensive training in motivational interviewing and BI, years of experience at the sites delivering the intervention, and were supervised by a PhD-level clinician who worked for the treatment agency. Thus, the in-person BI likely represents as high a level of quality as achievable for counselor-delivered BIs in community health practices.
Nevertheless, there were a number of limitations to the study. First, IBI sessions were not recorded and coded for fidelity. We did not monitor fidelity of IBI directly because doing so would have risked changing the counselors’ normal practice and subverting the goals of the study, which were to compare a computerized BI to an in-person BI as delivered in a real-world clinical setting by experienced BHCs. Second, there were only two counselors, and it is not possible to draw conclusions about the extent to which their performance was representative of other counselors or other healthcare environments. Third, the study did not include a no-intervention control condition, and hence it is not possible to determine how participants’ substance use risks may have changed in the absence of a BI. Fourth, hair testing may be of limited utility among participants with only moderate drug use, as evidenced by the high number of baseline-negative results and limited concordance with self-report. Finally, the follow-up time frame of 3 months is relatively short.
Despite these limitations, the study’s findings provide support for the use of computer-delivered BIs for individuals with moderate-risk drug use. On average, participants receiving the computer-delivered BI did as well (or in some analyses better) than individuals receiving the counselor-delivered BI. Importantly, in none of the analyses did participants in the counselor-delivered BI have superior outcomes. Given the likely expansion of primary care centers in the US under its health care reform (34) and the considerable global barriers to hiring BHCs or delivering BIs via primary care providers, the use of computer-delivered BIs appears to be a promising approach.
Acknowledgments
The study was supported through National Institute on Drug Abuse (NIDA) Grant No. R01DA13669 (PI Schwartz). NIDA or the National Institutes of Health hand no role in the design and conduct of the study; data acquisition, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Declarations of interest and source of funding:
No financial disclosures were reported by Drs. Schwartz, Gryczynksi, Mitchell, O’Grady, Gonzales, Moseley, or Peterson. Dr. Ondersma is part owner of Interva, Inc., which markets the intervention authoring tool that was used to develop the intervention for this study.
Clinical Trials Registration: Clinicaltrials.gov NCT01131520
References
- 1.WHO ASSIST Working Group. The Alcochol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction. 2002;97:1183–1194. doi: 10.1046/j.1360-0443.2002.00185.x. [DOI] [PubMed] [Google Scholar]
- 2.Humeniuk R, Ali R, Babor T, Souza-Formigoni ML, De Lacerda RB, Ling W, et al. A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction. 2012;107:957–966. doi: 10.1111/j.1360-0443.2011.03740.x. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein J, Bernstein E, Tassiopoulos K, Heeren T, Levenson S, Hingson R. Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend. 2005;77:49–59. doi: 10.1016/j.drugalcdep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Zahradnik A, Otto C, Crackau B, Lohrmann I, Bischof G, John U, et al. Randomized controlled trial of a brief intervention for problematic prescription drug use in non-treatment-seeking patients. Addiction. 2009;104:109–117. doi: 10.1111/j.1360-0443.2008.02421.x. [DOI] [PubMed] [Google Scholar]
- 5.Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW. Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend. 2009;99:280–295. doi: 10.1016/j.drugalcdep.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saitz R. Candidate performance measures for screening for, assessing, and treating unhealthy substance use in hospitals: advocacy or evidence-based practice? Ann Intern Med. 2010;153:40–43. doi: 10.7326/0003-4819-153-1-201007060-00008. [DOI] [PubMed] [Google Scholar]
- 7.Yarnall KS, Pollak KI, Ostbye T, Krause KM, Michener JL. Primary care: is there enough time for prevention? Am J Public Health. 2003;93:635–641. doi: 10.2105/ajph.93.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beich A, Gannik D, Malterud K. Screening and brief intervention for excessive alcohol use: qualitative interview study of the experiences of general practitioners. BMJ. 2002;325:870. doi: 10.1136/bmj.325.7369.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ondersma SJ, Chase SK, Svikis DS, Schuster CR. Computer-based brief motivational intervention for perinatal drug use. J Subst Abuse Treat. 2005;28:305–312. doi: 10.1016/j.jsat.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newman JC, Des Jarlais DC, Turner CF, Gribble J, Cooley P, Paone D. The differential effects of face-to-face and computer interview modes. Am J Public Health. 2002;92:294–297. doi: 10.2105/ajph.92.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore BA, Fazzino T, Garnet B, Cutter CJ, Barry DT. Computer-based interventions for drug use disorders: a systematic review. J Subst Abuse Treat. 2011;40:215–223. doi: 10.1016/j.jsat.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portnoy DB, Scott-Sheldon LA, Johnson BT, Carey MP. Computer-delivered interventions for health promotion and behavioral risk reduction: a meta-analysis of 75 randomized controlled trials: 1988–2007. Prev Med. 2008;47:3–16. doi: 10.1016/j.ypmed.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riper H, Spek V, Boon B, Conijn B, Kramer J, Martin-Abello K, et al. Effectiveness of E-self-help interventions for curbing adult problem drinking: a meta-analysis. J Med Internet Res. 2011;13:e42. doi: 10.2196/jmir.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rooke S, Thorsteinsson E, Karpin A, Copeland J, Allsop D. Computer-delivered interventions for alcohol and tobacco use: a meta-analysis. Addiction. 2010;105:1381–1390. doi: 10.1111/j.1360-0443.2010.02975.x. [DOI] [PubMed] [Google Scholar]
- 15.Ondersma SJ, Svikis DS, Schuster CR. Computer-based brief intervention a randomized trial with postpartum women. Am J Prev Med. 2007;32:231–238. doi: 10.1016/j.amepre.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ondersma SJ, Svikis DS, Thacker LR, Beatty JR, Lockhart N. Computer-delivered screening and brief intervention (e-SBI) for postpartum drug use: A randomized trial. J Subst Abuse Treat. 2014;46:52–59. doi: 10.1016/j.jsat.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gilbert P, Ciccarone D, Gansky SA, Bangsberg DR, Clanon K, Mcphee SJ, et al. Interactive "Video Doctor" counseling reduces drug and sexual risk behaviors among HIV-positive patients in diverse outpatient settings. PLoS One. 2008;3:e1988. doi: 10.1371/journal.pone.0001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Des Jarlais DC, Paone D, Milliken J, Turner CF, Miller H, Gribble J, et al. Audio-computer interviewing to measure risk behaviour for HIV among injecting drug users: a quasi-randomised trial. Lancet. 1999;353:1657–1661. doi: 10.1016/s0140-6736(98)07026-3. [DOI] [PubMed] [Google Scholar]
- 19.Perlis TE, Des Jarlais DC, Friedman SR, Arasteh K, Turner CF. Audio-computerized self-interviewing versus face-to-face interviewing for research data collection at drug abuse treatment programs. Addiction. 2004;99:885–896. doi: 10.1111/j.1360-0443.2004.00740.x. [DOI] [PubMed] [Google Scholar]
- 20.Gonzales A, Westerberg VS, Peterson TR, Moseley A, Gryczynski J, Mitchell SG, et al. Implementing a Statewide Screening, Brief Intervention, and Referral to Treatment (SBIRT) Service in Rural Health Settings: New Mexico SBIRT. Subst Abus. 2012;33:114–123. doi: 10.1080/08897077.2011.640215. PMCID: PMC3325793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gryczynski J, Mitchell SG, Peterson TR, Gonzales A, Moseley A, Schwartz RP. The relationship between services delivered and substance use outcomes in New Mexico's Screening, Brief Intervention, Referral and Treatment (SBIRT) Initiative. Drug Alcohol Depend. 2011;118:152–157. doi: 10.1016/j.drugalcdep.2011.03.012. PMCID: PMC21482039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell SG, Gryczynski J, Peterson T, Gonzales A, Moseley A, Schwartz RP. Screening, brief intervention, and referral to treatment (SBIRT) for substance use in a school-based program: Services and outcomes. American Journal on Addictions. 2012;21:S5–S13. doi: 10.1111/j.1521-0391.2012.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mccambridge J, Kypri K. Can simply answering research questions change behaviour? Systematic review and meta analyses of brief alcohol intervention trials. PLoS One. 2011;6:e23748. doi: 10.1371/journal.pone.0023748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, et al. Validation of the Alcohol, Smoking And Substance Involvement Screening Test (ASSIST) Addiction. 2008;103:1039–1047. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- 25.Newcombe DA, Humeniuk RE, Ali R. Validation of the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): report of results from the Australian site. Drug Alcohol Rev. 2005;24:217–226. doi: 10.1080/09595230500170266. [DOI] [PubMed] [Google Scholar]
- 26.Potosky D, Bobko P. The computer understanding and experience scale: A self-report measure of computer experience. Computers in Human Behavior. 1998;14:337–348. [Google Scholar]
- 27.Miller W, Rollnick S. Motivational Interviewing. New York: Guilford Press; 2002. [Google Scholar]
- 28.Ondersma SJ, Svikis DS, Lam PK, Connors-Burge VS, Ledgerwood DM, Hopper JA. A randomized trial of computer-delivered brief intervention and low-intensity contingency management for smoking during pregnancy. Nicotine Tob Res. 2012;14:351–360. doi: 10.1093/ntr/ntr221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzilos GK, Sokol RJ, Ondersma SJ. A randomized phase I trial of a brief computer-delivered intervention for alcohol use during pregnancy. J Womens Health (Larchmt) 2011;20:1517–1524. doi: 10.1089/jwh.2011.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larimer ME, Kaysen DL, Lee CM, Kilmer JR, Lewis MA, Dillworth T, et al. Evaluating level of specificity of normative referents in relation to personal drinking behavior. J Stud Alcohol Drugs Suppl. 2009:115–121. doi: 10.15288/jsads.2009.s16.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lewis MA, Neighbors C. Optimizing personalized normative feedback: the use of gender-specific referents. J Stud Alcohol Drugs. 2007;68:228–237. doi: 10.15288/jsad.2007.68.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neighbors C, Labrie JW, Hummer JF, Lewis MA, Lee CM, Desai S, et al. Group identification as a moderator of the relationship between perceived social norms and alcohol consumption. Psychol Addict Behav. 2010;24:522–528. doi: 10.1037/a0019944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell SG, Gryczynski J, O'grady KE, Schwartz RP. SBIRT for adolescent drug and alcohol use: Current status and future directions. J Subst Abuse Treat. 2013;44:463–472. doi: 10.1016/j.jsat.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.OFFICE of the Legislative Counsel for the U.S. House of Representatives. Patient Protection and Affordable Care Act. 2010 [Google Scholar]